Development of Ultrahigh Permeance Hollow Fiber Membranes via Simple Surface Coating for CO2/CH4 Separation
Abstract
1. Introduction
2. Results and Discussion
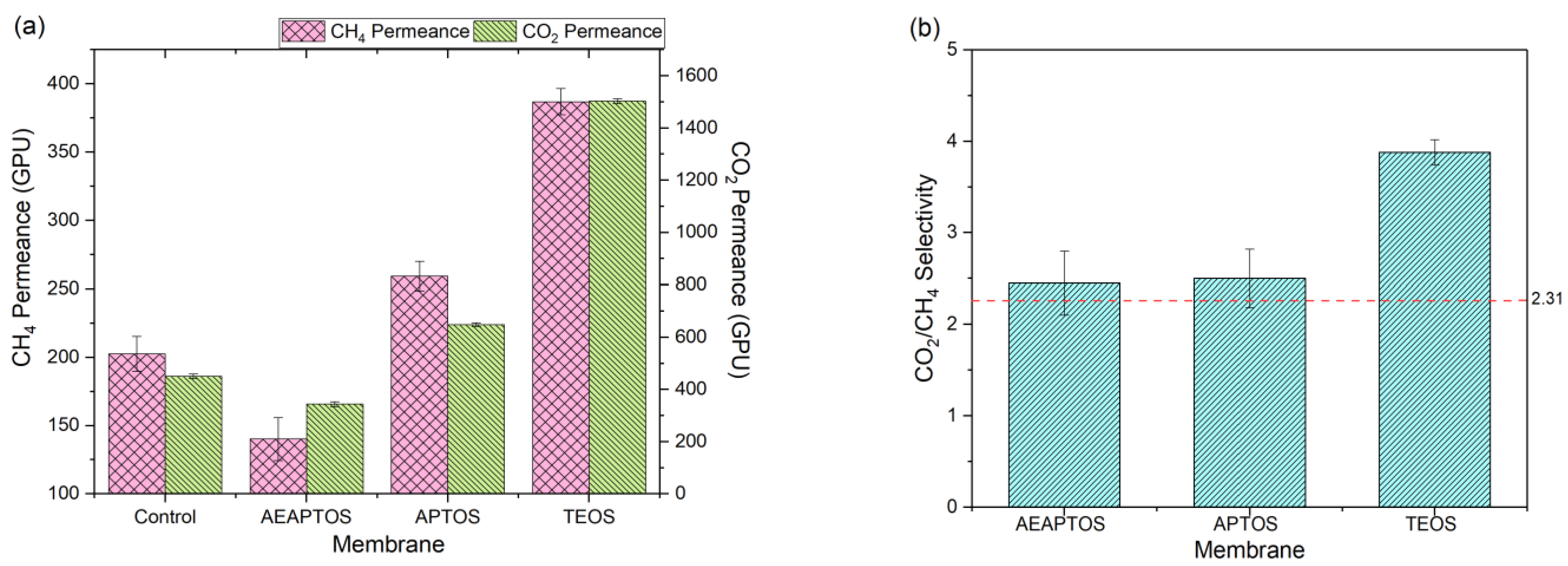
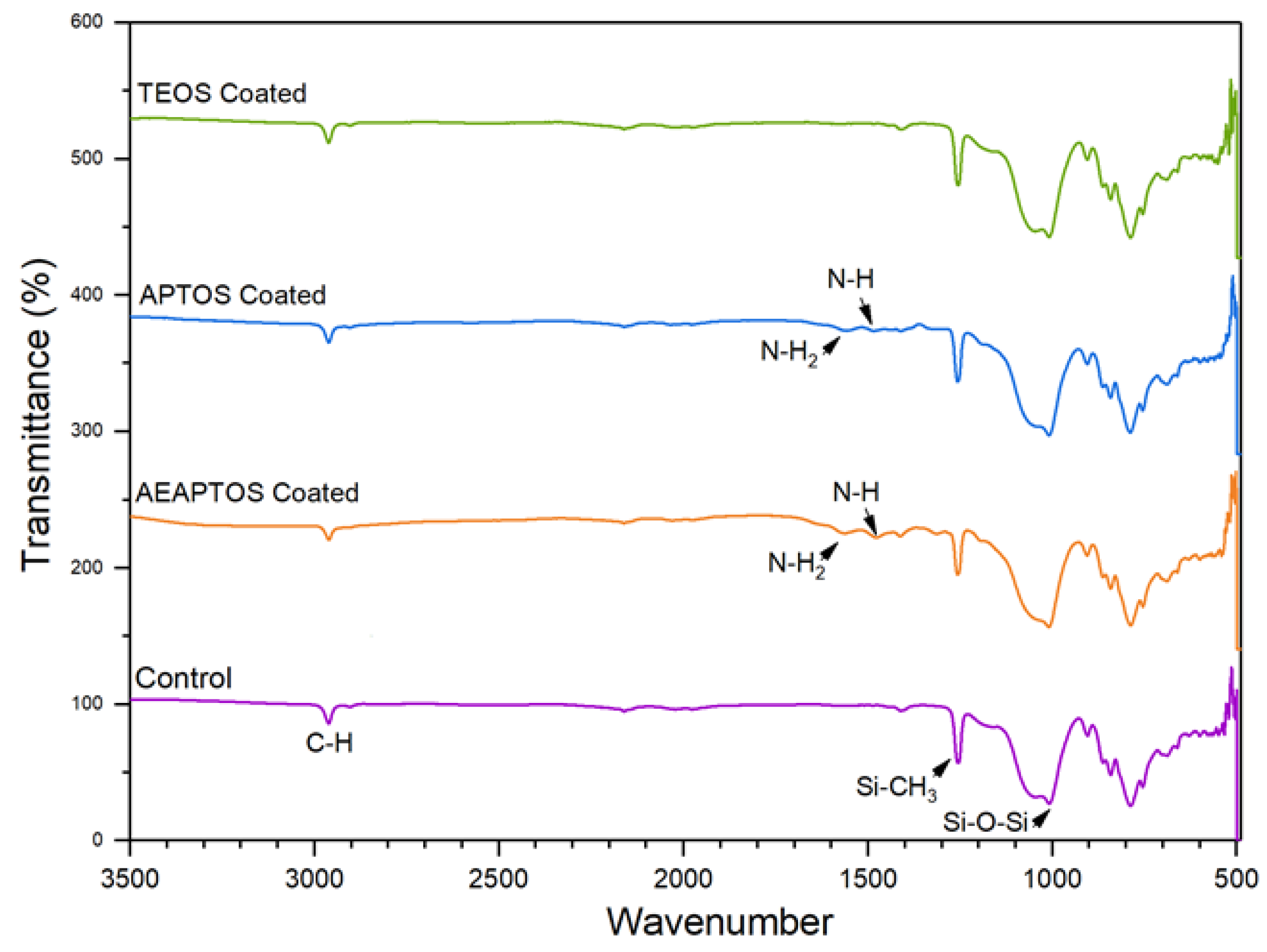
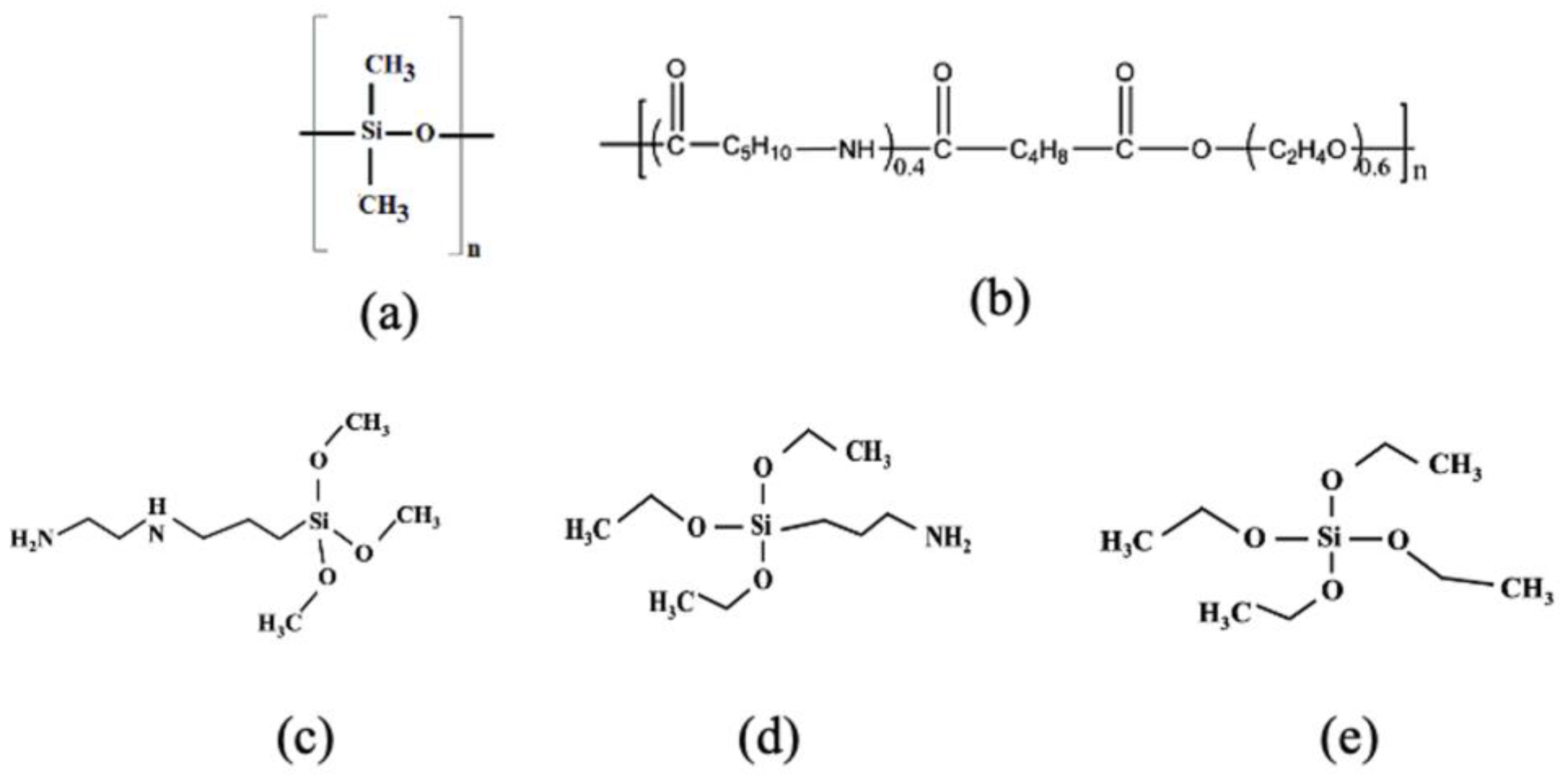
2.1. Effects of Silane Coating on Membrane Properties
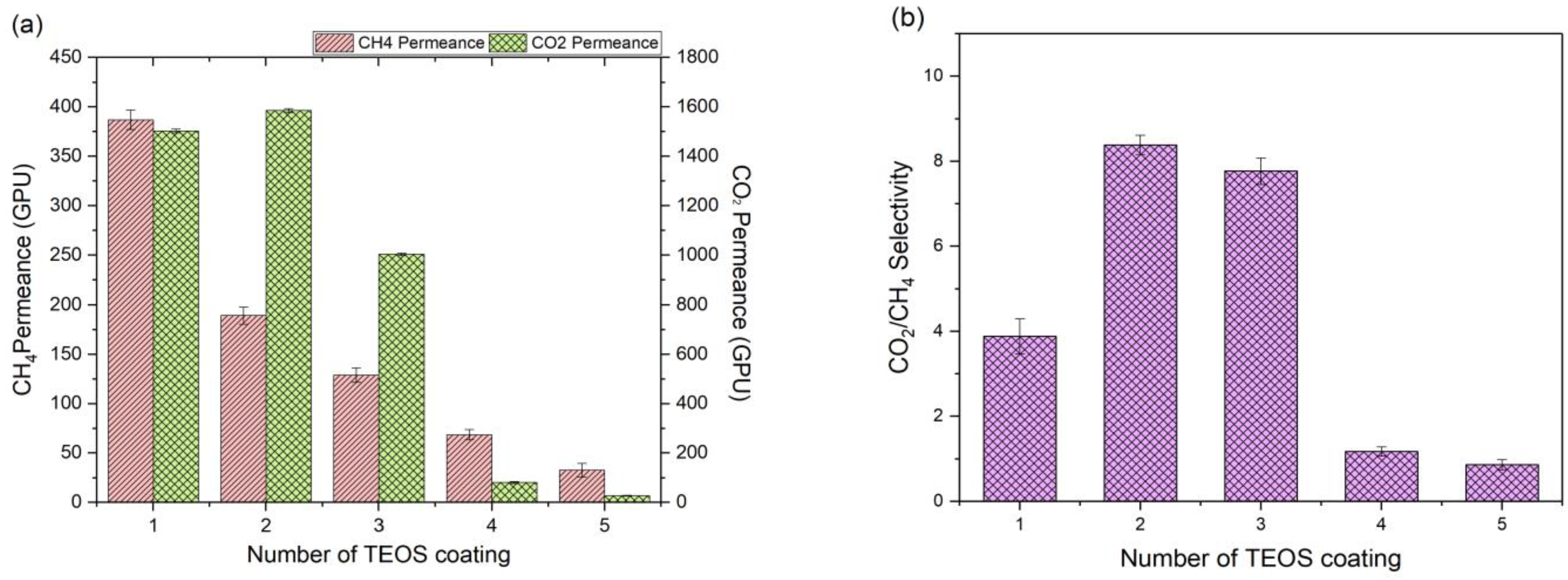
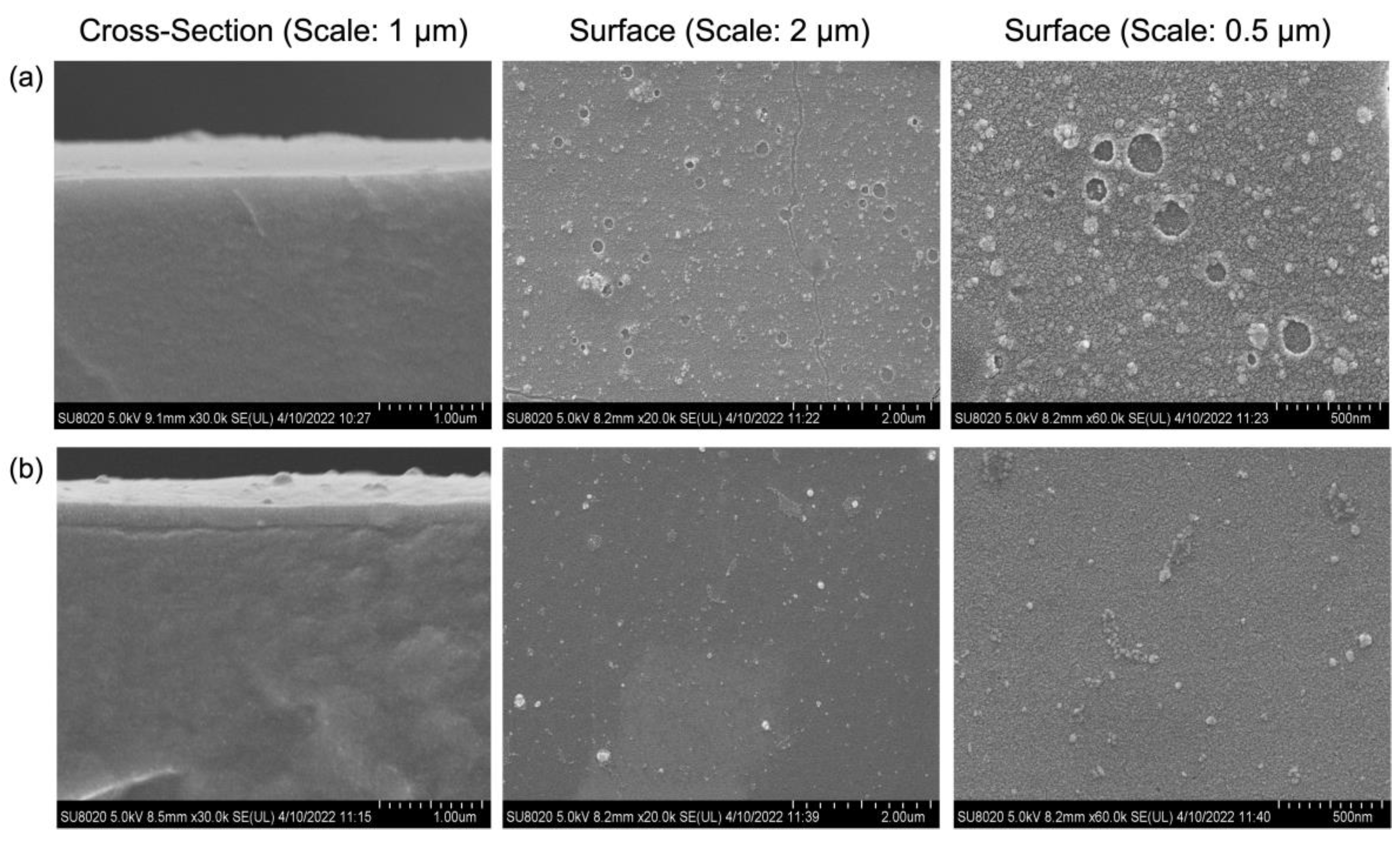
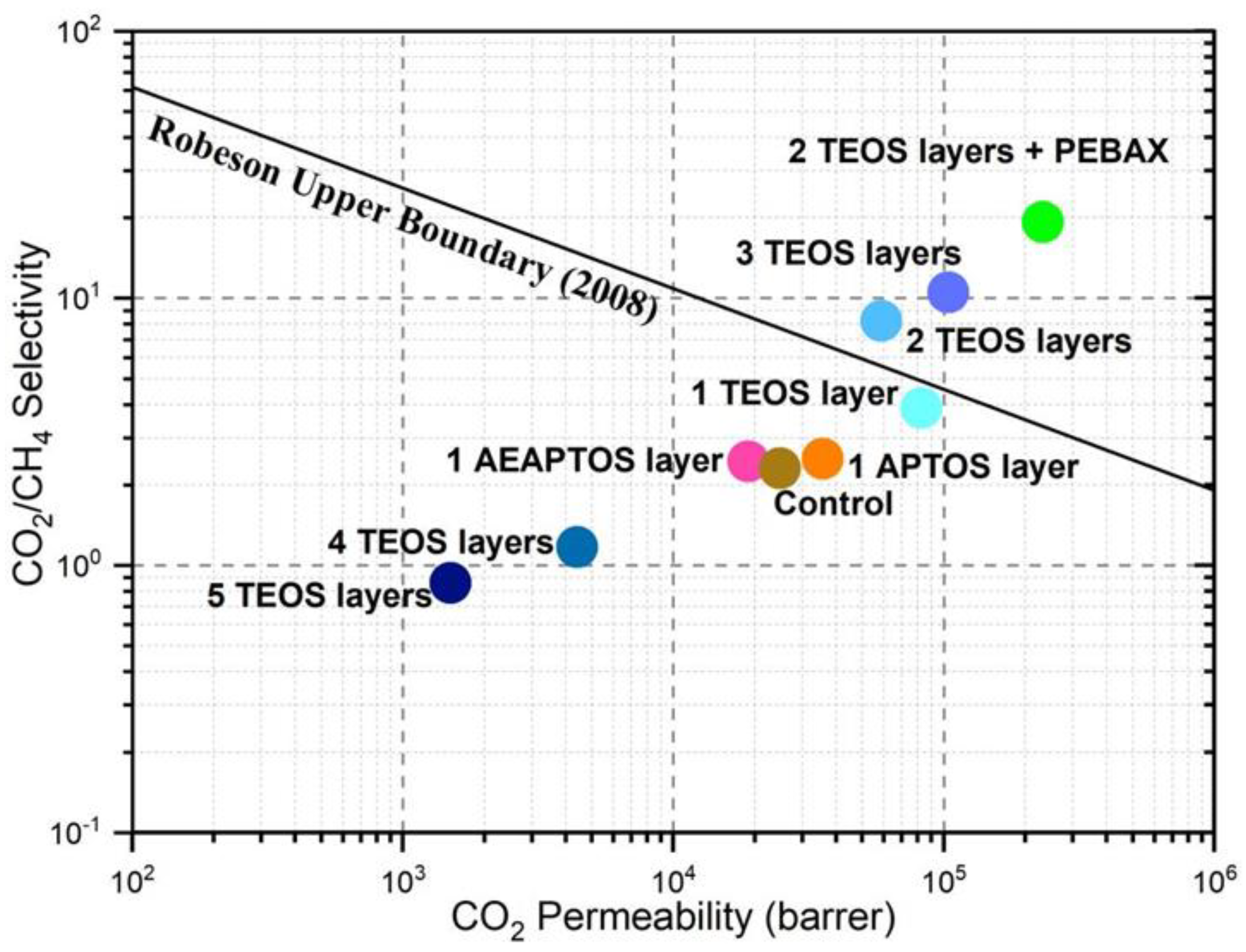
2.2. Effects of TEOS Coating Layers on Membrane Properties
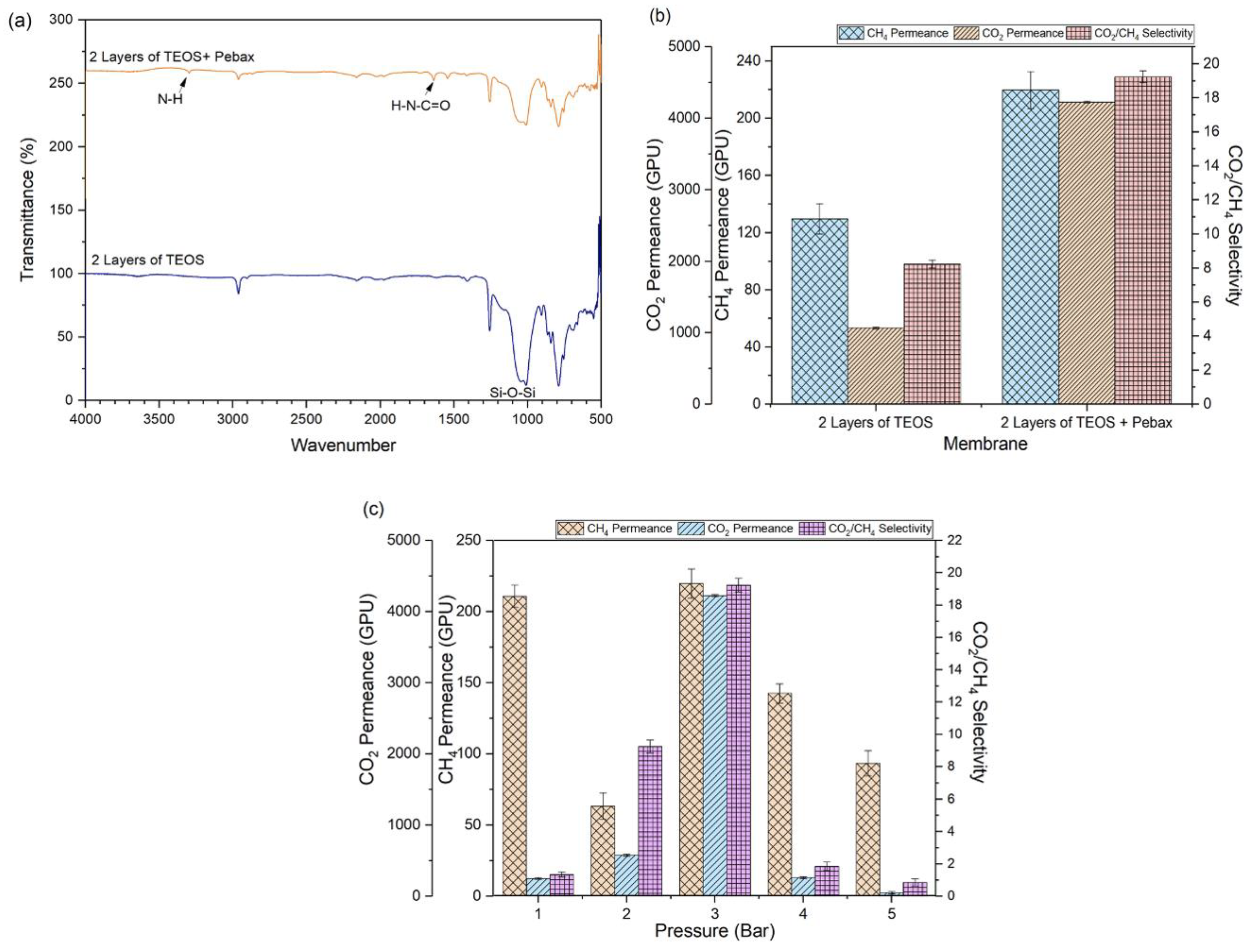
2.3. Effects of Pebax Coating on TEOS-Modified Membrane
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Membrane Surface Coating
3.2.1. Single Layer Coating
3.2.2. Multilayer Coating
3.2.3. Pebax Coating
3.3. Membrane Gas Separation Performance Evaluation
3.4. Membrane Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jankowski, A.; Grabiec, E.; Nocoń-Szmajda, K.; Marcinkowski, A.; Janeczek, H.; Wolińska-Grabczyk, A. Polyimide-based membrane materials for CO2 separation: A comparison of segmented and aromatic (Co)polyimides. Membranes 2021, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Chawla, M.; Saulat, H.; Masood Khan, M.; Mahmood Khan, M.; Rafiq, S.; Cheng, L.; Iqbal, T.; Rasheed, M.I.; Farooq, M.Z.; Saeed, M.; et al. Membranes for CO2/CH4 and CO2/N2 Gas Separation. Chem. Eng. Technol. 2020, 43, 184–199. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Fabrication of silanated zeolite T/6FDA-durene composite membranes for CO2/CH4 separation. J. Clean. Prod. 2017, 166, 1043–1058. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53(Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Aboukeila, H.; Yan, J.; Ansaloni, L.; Mineart, K.P.; Giacinti Baschetti, M.; Spontak, R.J.; Deng, L. Highly CO2-permeable membranes derived from a midblock-sulfonated multiblock polymer after submersion in water. NPG Asia Mater. 2019, 11, 53. [Google Scholar] [CrossRef]
- Haider, B.; Dilshad, M.R.; Atiq ur Rehman, M.; Akram, M.S.; Kaspereit, M. Highly permeable innovative PDMS coated polyethersulfone membranes embedded with activated carbon for gas separation. J. Nat. Gas Sci. Eng. 2020, 81, 103406. [Google Scholar] [CrossRef]
- Li, G.; Knozowska, K.; Kujawa, J.; Tonkonogovas, A.; Stankevicius, A.; Kujawski, W.K. Fabrication of Polydimethysiloxane (PDMS) Dense Layer on Polyetherimide (PEI) Hollow Fiber Support for the Efficient. Polymer 2021, 13, 756. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Badieh, M.M.S.; Vatanpour, V. Effect of Coating Method on Gas Separation by PDMS / PES Membrane. Polym. Eng. Sci. 2013, 53, 1878–1885. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Fachrurrazi, Z.G.; Izwanne, M.N.; Ismail, A.F. Asymmetric hollow fiber membrane coated with polydimethylsiloxane—Metal organic framework hybrid layer for gas separation. Sep. Purif. Technol. 2015, 146, 85–93. [Google Scholar] [CrossRef]
- Roslan, R.A.; Lau, W.J.; Lai, G.S.; Zulhairun, A.K.; Yeong, Y.F.; Ismail, A.F.; Matsuura, T. Impacts of Multilayer Hybrid Coating on PSF Hollow Fiber Membrane for Enhanced Gas Separation. Membranes 2020, 10, 335. [Google Scholar] [CrossRef]
- Moradi, M.R.; Pourafshari Chenar, M.; Noie, S.H. Using PDMS coated TFC-RO membranes for CO2/N2 gas separation: Experimental study, modeling and optimization. Polym. Test. 2016, 56, 287–298. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Characterization and Performance Evaluation of PDMS/PSF Membrane for CO2/CH4 Separation under the Effect of Swelling. Procedia Eng. 2016, 148, 176–183. [Google Scholar] [CrossRef][Green Version]
- Kheirtalab, M.; Abedini, R.; Ghorbani, M. A novel ternary mixed matrix membrane comprising polyvinyl alcohol (PVA)-modified poly (ether-block-amide)(Pebax®1657)/graphene oxide nanoparticles for CO2 separation. Process Saf. Environ. Prot. 2020, 144, 208–224. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved operational stability of Pebax-based gas separation membranes with ZIF-8: A comparative study of flat sheet and composite hollow fibre membranes. J. Memb. Sci. 2017, 524, 266–279. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, S. Mixed-matrix membranes incorporated with porous shape-persistent organic cages for gas separation. J. Colloid Interface Sci. 2017, 490, 29–36. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, J.; Li, Y.; Liu, J.; Zhou, J.; Cen, K. In-situ grafting to improve polarity of polyacrylonitrile hollow fiber-supported polydimethylsiloxane membranes for CO2 separation. J. Colloid Interface Sci. 2018, 510, 12–19. [Google Scholar] [CrossRef]
- Wang, Y.; Low, Z.; Kim, S.; Zhang, H.; Chen, X.; Hou, J.; Seong, J.G.; Lee, Y.M.; Simon, G.P.; Davies, C.H.J.; et al. Functionalized boron nitride nanosheets: A thermally rearranged polymer nanocomposite membrane for hydrogen separation. Angew. Chem. 2018, 130, 16288–16293. [Google Scholar] [CrossRef]
- Ostwal, M.; Singh, R.P.; Dec, S.F.; Lusk, M.T.; Way, J.D. 3-Aminopropyltriethoxysilane functionalized inorganic membranes for high temperature CO2/N2 separation. J. Memb. Sci. 2011, 369, 139–147. [Google Scholar] [CrossRef]
- Isanejad, M.; Mohammadi, T. Effect of amine modification on morphology and performance of poly (ether-block-amide)/fumed silica nanocomposite membranes for CO2/CH4 separation. Mater. Chem. Phys. 2018, 205, 303–314. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, C.; Goh, K.; Wang, R. A facile direct spray-coating of Pebax ® 1657: Towards large-scale thin-film composite membranes for efficient CO2/N2 separation A facile direct spray-coating of Pebax ® 1657: Towards large-scale thin-film composite membranes for efficient CO2/N2. J. Memb. Sci. 2021, 638, 119708. [Google Scholar] [CrossRef]
- He, Q.; Chen, W.; Wang, P.; Dou, X. Silicalite-1/PDMS Hybrid Membranes on Porous PVDF Supports: Preparation, Structure and Pervaporation Separation of Dichlorobenzene Isomers. Polymers 2022, 14, 1680. [Google Scholar] [CrossRef]
- Zhuang, G.L.; Wu, C.F.; Wey, M.Y.; Tseng, H.H. Impacts of green synthesis process on asymmetric hybrid PDMS membrane for efficient CO2/N2 separation. Membranes 2021, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Wold, C.; Uliyanchenko, E.; Weidner, S.; Falkenhagen, J. Characterization of copolymers of polycarbonate and polydimethylsiloxane by 2D chromatographic separation, MALDI-TOF mass spectrometry, and FTIR spectroscopy. Int. J. Polym. Anal. Charact. 2020, 25, 553–564. [Google Scholar] [CrossRef]
- Ye, S.; Wang, S.; Lin, L.; Xiao, M.; Meng, Y. CO2 derived biodegradable polycarbonates: Synthesis, modification and applications. Adv. Ind. Eng. Polym. Res. 2019, 2, 143–160. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef]
- Data Sheet of PDMSXA–2500, PermSelect®, USA. Available online: https://www.permselect.com/products/pdmsxa-2500 (accessed on 25 November 2022).
- George, V.G.; Dionysios, S.K.; Konstantinos, G.M.; Andreas, A.S.; Evangelos, P.K.; Evangelos, P.F. Graphene nanoplatelets based polyimide/Pebax dual-layer mixed matrix hollow fiber membranes for CO2/CH4 and He/N2 separations. Int. J. Greenh. Gas Control. 2022, 114, 103588. [Google Scholar] [CrossRef]
- Roslan, R.A.; Lau, W.J.; Zulhairun, A.K.; Goh, P.S.; Ismail, A.F. Improving CO2/CH4 and O2/N2 separation by using surface-modified polysulfone hollow fiber membranes. J. Polym. Res. 2020, 27, 119. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Knozowska, K.; Kujawa, J. The Effects of PEI Hollow Fiber Substrate Characteristics on PDMS/PEI Hollow Fiber Membranes for CO2/N2 Separation. Membranes 2021, 11, 56. [Google Scholar] [CrossRef]
- Qian, Q.; Wright, A.M.; Lee, H.; Dincă, M.; Smith, Z.P. Low-Temperature H2S/CO2/CH4 Separation in Mixed-Matrix Membranes Containing MFU-4. Chem. Mater. 2021, 33, 6825–6831. [Google Scholar] [CrossRef]


| Membrane | Coating Material | Operating Pressure (Bar) | CO2 Permeance (GPU or Barrer) | CO2/CH4 Selectivity | Year [Ref.] |
|---|---|---|---|---|---|
| Co-polyimide/graphene | Graphene/Pebax | 6 | 3.3 GPU | 28.6 | 2022 [27] |
| Polysulfone | PDMS/Pebax | 5 | 39.56 GPU | 34.28 | 2020 [28] |
| Polysulfone | PDMS/Pebax/Graphene oxide | 5 | 28.08 GPU | 52.57 | 2020 [10] |
| Polyetherimide | PDMS | 2 | 190 GPU | ~9.8 | 2021 [29] |
| Polyvinylidene fluoride | ZIF-8/Pebax | 2 | ~350 GPU | ~12 | 2017 [14] |
| a Mixed matrix membrane | - | 2 | 1042 barrer | 25 | 2021 [30] |
| Silicone | TEOS/Pebax | 3 | 4150 GPU or 2.3 × 105 barrer | ~19 | Our Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, N.; Wong, K.C.; Lau, W.J.; Khoo, Y.S.; Yeong, Y.F.; Othman, N.H.; Goh, P.S.; Ismail, A.F. Development of Ultrahigh Permeance Hollow Fiber Membranes via Simple Surface Coating for CO2/CH4 Separation. Molecules 2022, 27, 8381. https://doi.org/10.3390/molecules27238381
Said N, Wong KC, Lau WJ, Khoo YS, Yeong YF, Othman NH, Goh PS, Ismail AF. Development of Ultrahigh Permeance Hollow Fiber Membranes via Simple Surface Coating for CO2/CH4 Separation. Molecules. 2022; 27(23):8381. https://doi.org/10.3390/molecules27238381
Chicago/Turabian StyleSaid, Noresah, Kar Chun Wong, Woei Jye Lau, Ying Siew Khoo, Yin Fong Yeong, Nur Hidayati Othman, Pei Sean Goh, and Ahmad Fauzi Ismail. 2022. "Development of Ultrahigh Permeance Hollow Fiber Membranes via Simple Surface Coating for CO2/CH4 Separation" Molecules 27, no. 23: 8381. https://doi.org/10.3390/molecules27238381
APA StyleSaid, N., Wong, K. C., Lau, W. J., Khoo, Y. S., Yeong, Y. F., Othman, N. H., Goh, P. S., & Ismail, A. F. (2022). Development of Ultrahigh Permeance Hollow Fiber Membranes via Simple Surface Coating for CO2/CH4 Separation. Molecules, 27(23), 8381. https://doi.org/10.3390/molecules27238381











