Tellurium-Modified Nucleosides, Nucleotides, and Nucleic Acids with Potential Applications
Abstract
1. Introduction
2. Discussion
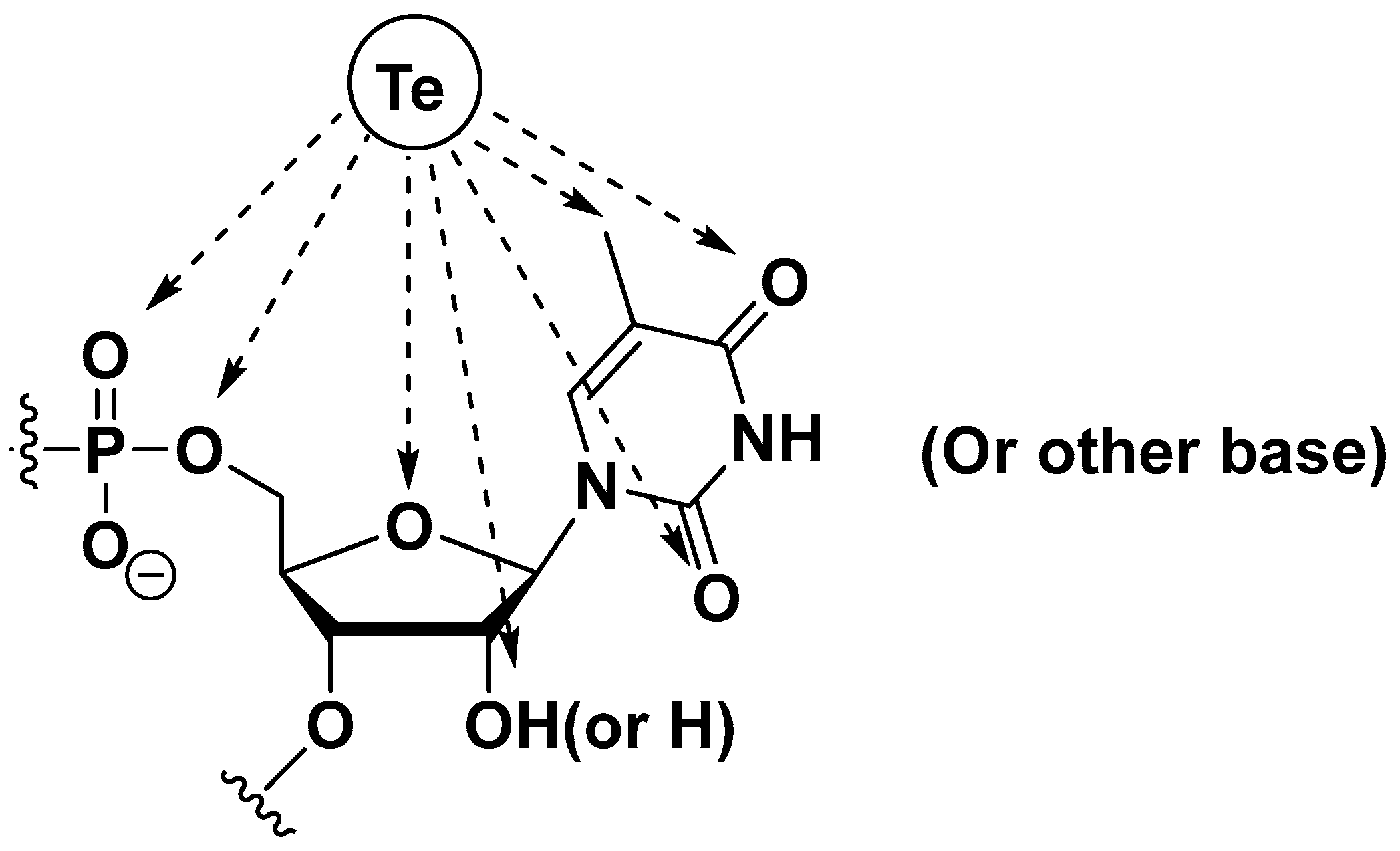
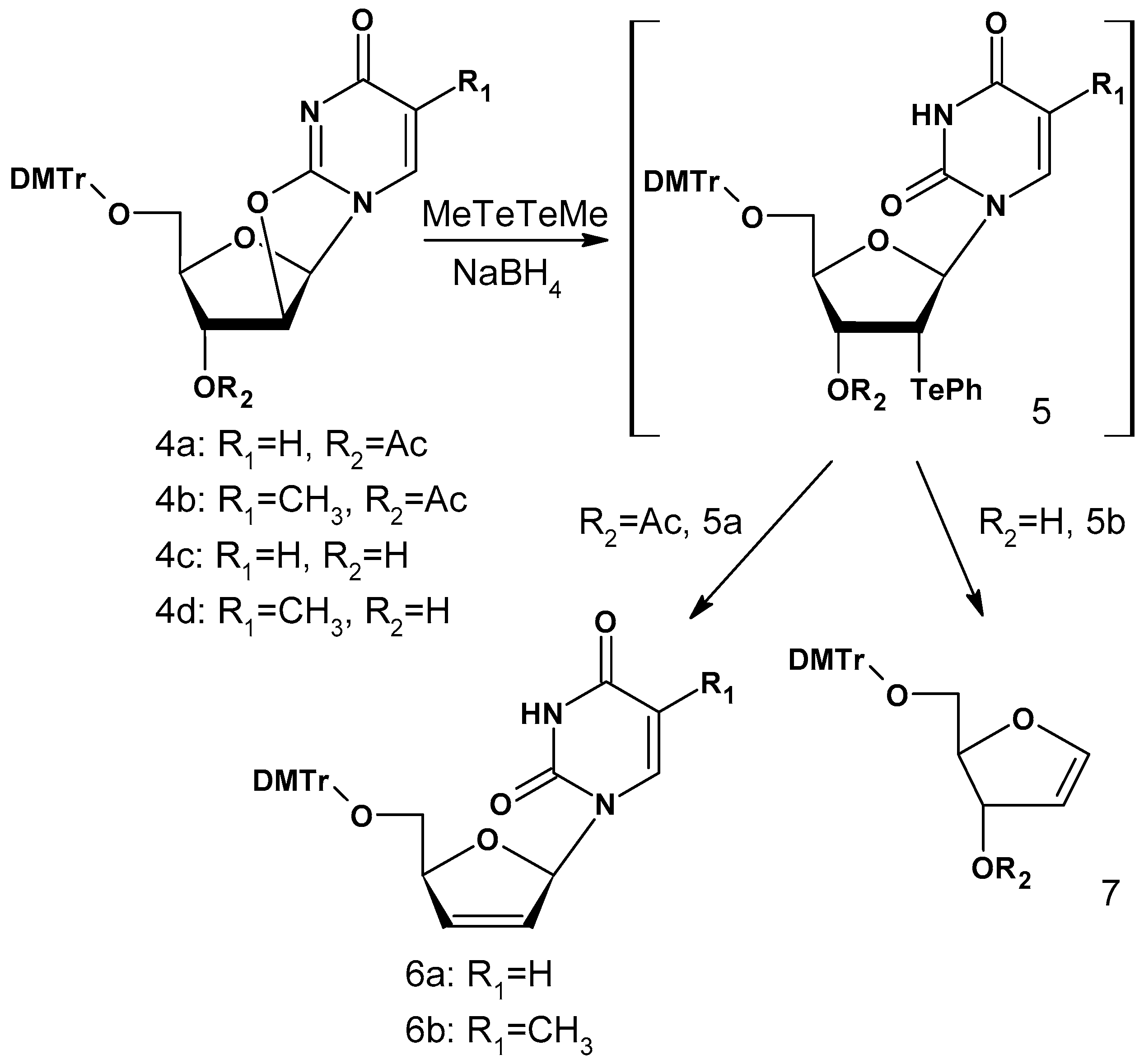
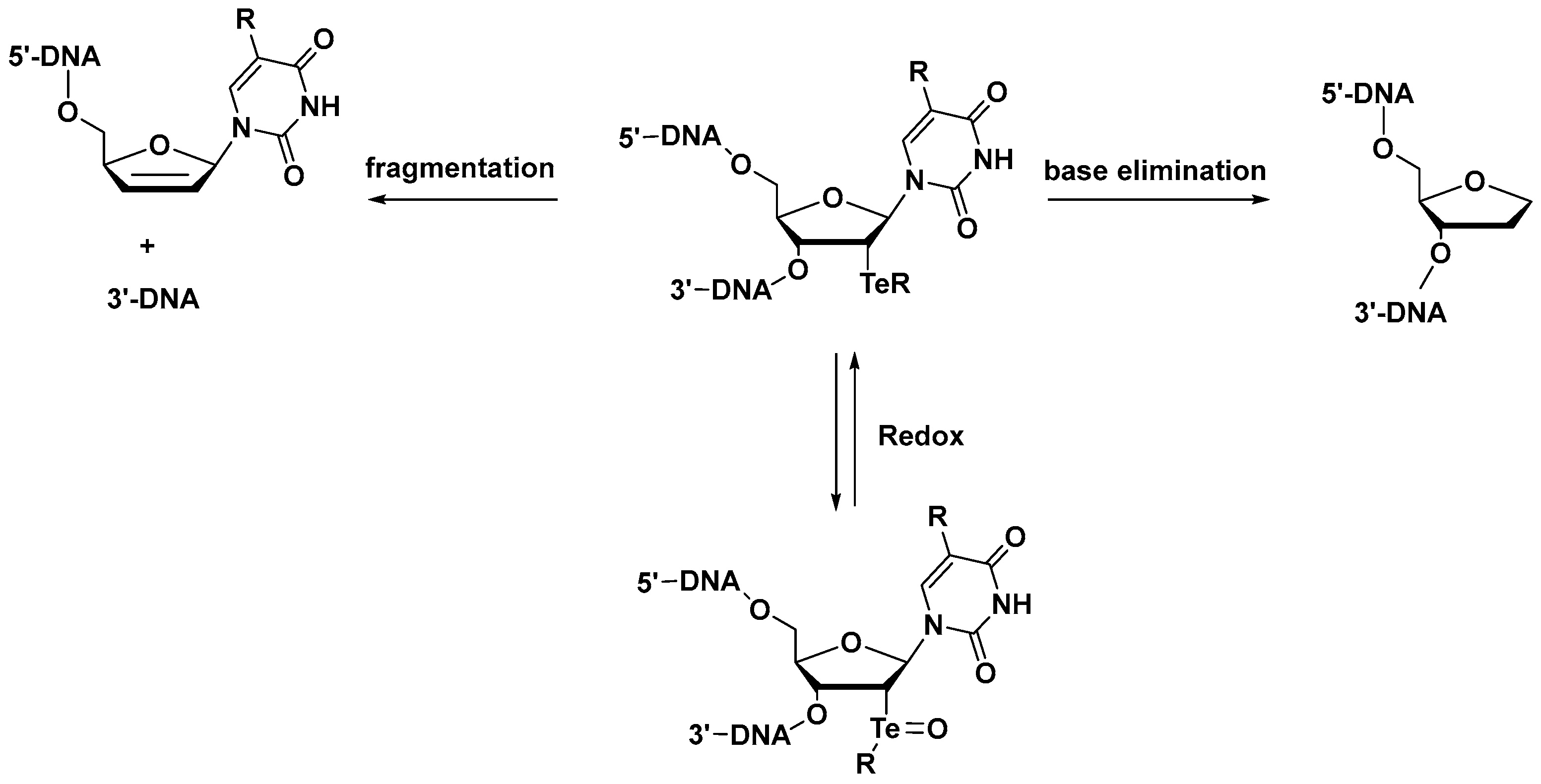
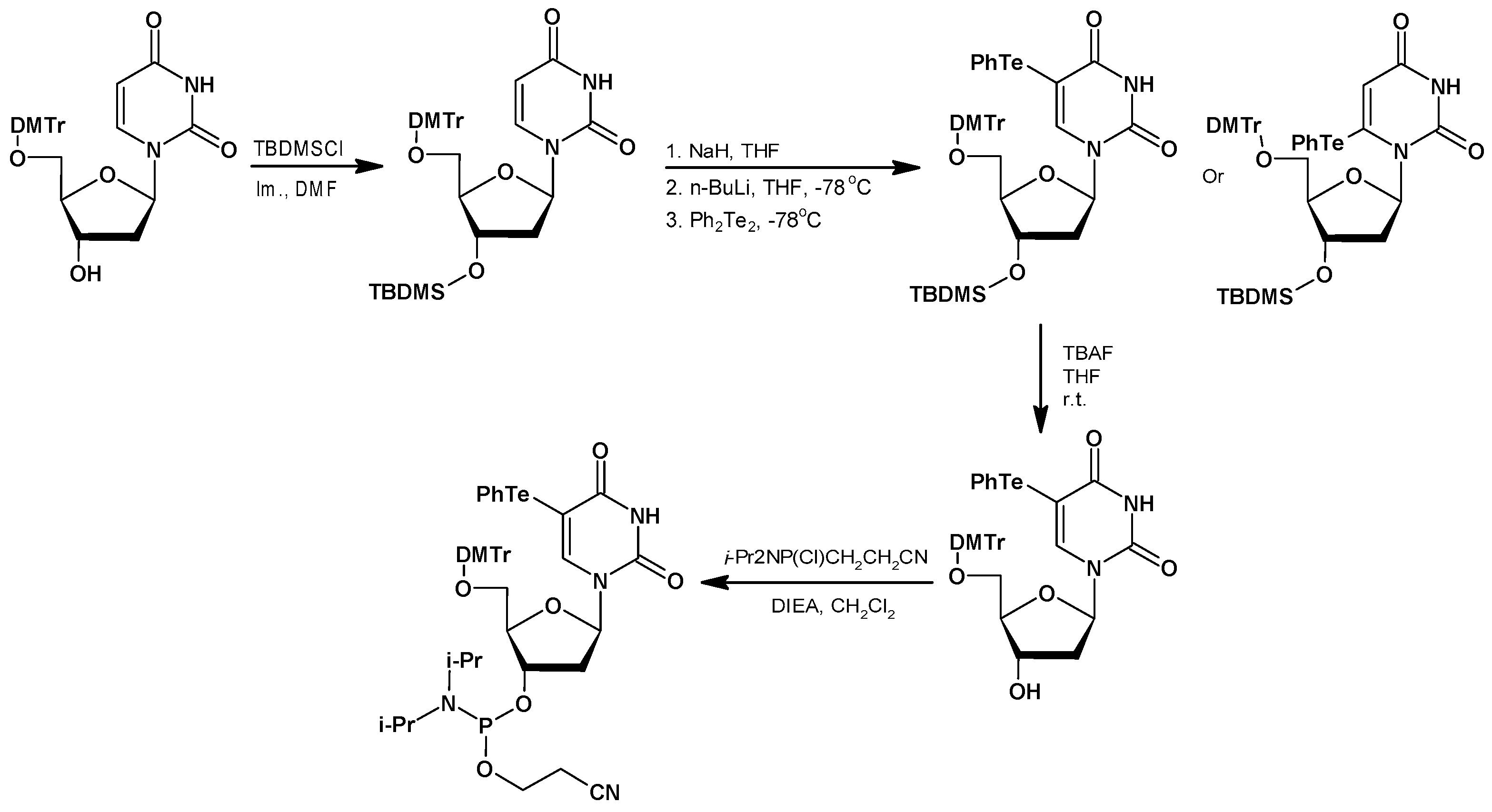
Te Modifications in Nucleoside, Nucleotide, and Nucleic Acids
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Watson, J.D.; Crick, F.H. Molecular structure of nucleic acids. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef]
- Blount, K.F.; Uhlenbeck, O.C. The structure-function dilemma of the hammerhead ribozyme. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Non–coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2001, 2, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.N. The Biochemistry of the Nucleic Acids; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- van Kasteren, P.B.; van Der Veer, B.; van den Brink, S.; Wijsman, L.; de Jonge, J.; van den Brandt, A.; Molenkamp, R.; Reusken, C.B.; Meijer, A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J. Clin. Virol. 2020, 128, 104412. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, P.; Hu, C.; Mao, H. Detecting the coronavirus (COVID-19). ACS Sens. 2020, 5, 2283–2296. [Google Scholar] [CrossRef]
- Scheler, O.; Glynn, B.; Kurg, A. Nucleic acid detection technologies and marker molecules in bacterial diagnostics. Expert Rev. Mol. Diagn. 2014, 14, 489–500. [Google Scholar] [CrossRef]
- Hartman, M.R.; Ruiz, R.C.; Hamada, S.; Xu, C.; Yancey, K.G.; Yu, Y.; Han, W.; Luo, D. Point-of-care nucleic acid detection using nanotechnology. Nanoscale 2013, 5, 10141–10154. [Google Scholar] [CrossRef]
- O’Connor, L.; Glynn, B. Recent advances in the development of nucleic acid diagnostics. Expert Rev. Med. Devices 2010, 7, 529–539. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef]
- El Wahed, A.A.; Patel, P.; Maier, M.; Pietsch, C.; Rüster, D.; Böhlken-Fascher, S.; Kissenkötter, J.; Behrmann, O.; Frimpong, M.; Diagne, M.M. Suitcase Lab for rapid detection of SARS-CoV-2 based on recombinase polymerase amplification assay. Anal. Chem. 2021, 93, 2627–2634. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, J.; Zhang, S.; Hu, B.; Hu, L.; Huang, Z. High-quality RT-PCR with chemically modified RNA controls. Talanta 2021, 224, 121850. [Google Scholar] [CrossRef] [PubMed]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Rossi, J.J. RNA-based therapeutics: Current progress and future prospects. Chem. Biol. 2012, 19, 60–71. [Google Scholar] [CrossRef]
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46, 1584–1600. [Google Scholar] [CrossRef]
- Stein, C.A.; Castanotto, D. FDA-approved oligonucleotide therapies in 2017. Mol. Ther. 2017, 25, 1069–1075. [Google Scholar] [CrossRef]
- Deleavey, G.F.; Damha, M.J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef]
- Crooke, S.T.; Vickers, T.A.; Liang, X.-H. Phosphorothioate modified oligonucleotide–protein interactions. Nucleic Acids Res. 2020, 48, 5235–5253. [Google Scholar] [CrossRef]
- Du, Q.; Carrasco, N.; Teplova, M.; Wilds, C.J.; Egli, M.; Huang, Z. Internal Derivatization of Oligonucleotides with Selenium for X-ray Crystallography Using MAD. J. Am. Chem. Soc. 2002, 124, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.H.; Parkinson, G.N. Crystallographic studies of quadruplex nucleic acids. Methods 2007, 43, 252–263. [Google Scholar] [CrossRef]
- Egli, M.; Pallan, P.S. Crystallographic studies of chemically modified nucleic acids: A backward glance. Chem. Biodivers. 2010, 7, 60–89. [Google Scholar] [CrossRef]
- Ennifar, E. Nucleic Acid Crystallography; Springer: New York, NY, USA, 2016. [Google Scholar]
- Park, S.; Okamura, I.; Sakashita, S.; Yum, J.H.; Acharya, C.; Gao, L.; Sugiyama, H. Development of DNA Metalloenzymes Using a Rational Design Approach and Application in the Asymmetric Diels–Alder Reaction. ACS Catal. 2015, 5, 4708–4712. [Google Scholar] [CrossRef]
- Roelfes, G.; Feringa, B.L. DNA-Based Asymmetric Catalysis. Angew. Chem. Int. Ed. 2005, 44, 3230–3232. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, N.; Skiredj, A.; Mansot, J.; Leblanc, K.; Vasseur, J.J.; Beniddir, M.A.; Evanno, L.; Poupon, E.; Smietana, M.; Arseniyadis, S. DNA-Templated [2+2] Photocycloaddition: A Straightforward Entry into the Aplysinopsin Family of Natural Products. Angew. Chem. Int. Ed. 2018, 57, 11786–11791. [Google Scholar] [CrossRef] [PubMed]
- Amirbekyan, K.; Duchemin, N.; Benedetti, E.; Joseph, R.; Colon, A.; Markarian, S.A.; Bethge, L.; Vonhoff, S.; Klussmann, S.; Cossy, J. Design, synthesis, and binding affinity evaluation of hoechst 33,258 derivatives for the development of sequence-specific DNA-based asymmetric catalysts. ACS Catal. 2016, 6, 3096–3105. [Google Scholar] [CrossRef]
- Ward, W.L.; Plakos, K.; DeRose, V.J. Nucleic acid catalysis: Metals, nucleobases, and other cofactors. Chem. Rev. 2014, 114, 4318–4342. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Baillet, J.; Desvergnes, V.; Hamoud, A.; Latxague, L.; Barthélémy, P. Lipid and nucleic acid chemistries: Combining the best of both worlds to construct advanced biomaterials. Adv. Mater. 2018, 30, 1705078. [Google Scholar] [CrossRef]
- Ge, Z.; Gu, H.; Li, Q.; Fan, C. Concept and development of framework nucleic acids. J. Am. Chem. Soc. 2018, 140, 17808–17819. [Google Scholar] [CrossRef]
- Dong, Y.; Yao, C.; Zhu, Y.; Yang, L.; Luo, D.; Yang, D. DNA functional materials assembled from branched DNA: Design, synthesis, and applications. Chem. Rev. 2020, 120, 9420–9481. [Google Scholar] [CrossRef]
- Cutler, J.I.; Auyeung, E.; Mirkin, C.A. Spherical nucleic acids. J. Am. Chem. Soc. 2012, 134, 1376–1391. [Google Scholar] [CrossRef]
- Restifo, N.; Ying, H.; Hwang, L.; Leitner, W. The promise of nucleic acid vaccines. Gene Ther. 2000, 7, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Geall, A.J.; Mandl, C.W.; Ulmer, J.B. RNA: The new revolution in nucleic acid vaccines. Semin. Immunol. 2013, 25, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Deering, R.P.; Kommareddy, S.; Ulmer, J.B.; Brito, L.A.; Geall, A.J. Nucleic acid vaccines: Prospects for non-viral delivery of mRNA vaccines. Expert Opin. Drug Deliv. 2014, 11, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 vaccine pipeline: An overview. Curr. Trop. Med. Rep. 2020, 7, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Xiong, H.; Veedu, R.N.; Diermeier, S.D. Recent advances in oligonucleotide therapeutics in oncology. Int. J. Mol. Sci. 2021, 22, 3295. [Google Scholar] [CrossRef]
- Singh, J.; Ripp, A.; Haas, T.M.; Qiu, D.; Keller, M.; Wender, P.A.; Siegel, J.S.; Baldridge, K.K.; Jessen, H.J. Synthesis of modified nucleoside oligophosphates simplified: Fast, pure, and protecting group free. J. Am. Chem. Soc. 2019, 141, 15013–15017. [Google Scholar] [CrossRef]
- Knouse, K.W.; Justine, N.; Schmidt, M.A.; Zheng, B.; Vantourout, J.C.; Kingston, C.; Mercer, S.E.; Mcdonald, I.M.; Olson, R.E.; Zhu, Y. Unlocking P (V): Reagents for chiral phosphorothioate synthesis. Science 2018, 361, 1234–1238. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, S.K.; Krishnamurthy, P.M.; Alterman, J.F.; Haraszti, R.A.; Khvorova, A.; Prasad, A.K.; Watts, J.K. Synthesis and biological properties of triazole-linked locked nucleic acid. Chem. Commun. 2017, 53, 8906–8909. [Google Scholar] [CrossRef]
- Paul, S.; Caruthers, M.H. Synthesis of phosphorodiamidate morpholino oligonucleotides and their chimeras using phosphoramidite chemistry. J. Am. Chem. Soc. 2016, 138, 15663–15672. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sergueeva, Z.A.; Dobrikov, M.; Shaw, B.R. Nucleoside and oligonucleoside boranophosphates: Chemistry and properties. Chem. Rev. 2007, 107, 4746–4796. [Google Scholar] [CrossRef]
- Eckstein, F. Phosphorothioate oligodeoxynucleotides: What is their origin and what is unique about them? Antisense Nucleic Acid Drug Dev. 2000, 10, 117–121. [Google Scholar] [CrossRef]
- Eckstein, F. Phosphorothioate analogs of nucleotides. Acc. Chem. Res. 1979, 12, 204–210. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Sun, S.; Yan, W.; Zhang, C.; Luo, D.; Deng, H.; Hu, L.R.; Huang, Z. Synthesis of Selenium-Triphosphates (dNTPαSe) for More Specific DNA Polymerization. Angew. Chem. 2019, 131, 7917–7921. [Google Scholar] [CrossRef]
- Liu, C.; Cozens, C.; Jaziri, F.; Rozenski, J.; Marechal, A.; Dumbre, S.; Pezo, V.; Marlière, P.; Pinheiro, V.B.; Groaz, E. Phosphonomethyl oligonucleotides as backbone-modified artificial genetic polymers. J. Am. Chem. Soc. 2018, 140, 6690–6699. [Google Scholar] [CrossRef]
- Sawamoto, H.; Arai, Y.; Yamakoshi, S.; Obika, S.; Kawanishi, E. Synthetic method for 2′-amino-LNA bearing any of the four nucleobases via a transglycosylation reaction. Org. Lett. 2018, 20, 1928–1931. [Google Scholar] [CrossRef]
- Mitsuoka, Y.; Yamamoto, T.; Kugimiya, A.; Waki, R.; Wada, F.; Tahara, S.; Sawamura, M.; Noda, M.; Fujimura, Y.; Kato, Y. Triazole-and Tetrazole-Bridged Nucleic Acids: Synthesis, Duplex Stability, Nuclease Resistance, and in Vitro and in Vivo Antisense Potency. J. Org. Chem. 2017, 82, 12–24. [Google Scholar] [CrossRef]
- Rozners, E. Recent advances in chemical modification of peptide nucleic acids. J. Nucleic Acids 2012, 2012, 518162. [Google Scholar] [CrossRef]
- Kaur, H.; Babu, B.R.; Maiti, S. Perspectives on chemistry and therapeutic applications of Locked Nucleic Acid (LNA). Chem. Rev. 2007, 107, 4672–4697. [Google Scholar] [CrossRef]
- Ichikawa, E.; Kato, K. Sugar-modified nucleosides in past 10 years, a review. Curr. Med. Chem. 2001, 8, 385–423. [Google Scholar] [CrossRef] [PubMed]
- Summerton, J.; Weller, D. Morpholino antisense oligomers: Design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997, 7, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Wengel, J. Locked vs. unlocked nucleic acids (LNA vs. UNA): Contrasting structures work towards common therapeutic goals. Chem. Soc. Rev. 2011, 40, 5680–5689. [Google Scholar] [CrossRef]
- Oh, J.; Shin, J.; Unarta, I.C.; Wang, W.; Feldman, A.W.; Karadeema, R.J.; Xu, L.; Xu, J.; Chong, J.; Krishnamurthy, R. Transcriptional processing of an unnatural base pair by eukaryotic RNA polymerase II. Nat. Chem. Biol. 2021, 17, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.C.; Hashimoto, K.; Zhang, Y.; Feldman, A.W.; Dien, V.T.; Karadeema, R.J.; Adhikary, R.; Ledbetter, M.P.; Krishnamurthy, R.; Romesberg, F.E. New codons for efficient production of unnatural proteins in a semisynthetic organism. Nat. Chem. Biol. 2020, 16, 570–576. [Google Scholar] [CrossRef]
- Zhou, A.X.-Z.; Sheng, K.; Feldman, A.W.; Romesberg, F.E. Progress toward eukaryotic semisynthetic organisms: Translation of unnatural codons. J. Am. Chem. Soc. 2019, 141, 20166–20170. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.W.; Romesberg, F.E. Expansion of the genetic alphabet: A chemist’s approach to synthetic biology. Acc. Chem. Res. 2018, 51, 394–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Ptacin, J.L.; Fischer, E.C.; Aerni, H.R.; Caffaro, C.E.; San Jose, K.; Feldman, A.W.; Turner, C.R.; Romesberg, F.E. A semi-synthetic organism that stores and retrieves increased genetic information. Nature 2017, 551, 644–647. [Google Scholar] [CrossRef]
- Hoshika, S.; Leal, N.A.; Kim, M.-J.; Kim, M.-S.; Karalkar, N.B.; Kim, H.-J.; Bates, A.M.; Watkins, N.E.; SantaLucia, H.A.; Meyer, A.J. Hachimoji DNA and RNA: A genetic system with eight building blocks. Science 2019, 363, 884–887. [Google Scholar] [CrossRef]
- Biondi, E.; Benner, S.A. Artificially expanded genetic information systems for new aptamer technologies. Biomedicines 2018, 6, 53. [Google Scholar] [CrossRef]
- Hoshika, S.; Chen, F.; Leal, N.A.; Benner, S.A. Artificial Genetic Systems: Self-Avoiding DNA in PCR and Multiplexed PCR. Angew. Chem. 2010, 122, 5686–5689. [Google Scholar] [CrossRef]
- Yang, Z.; Le, J.T.; Hutter, D.; Bradley, K.M.; Overton, B.R.; McLendon, C.; Benner, S.A. Eliminating primer dimers and improving SNP detection using self-avoiding molecular recognition systems. Biol. Methods Protoc. 2020, 5, bpaa004. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chan, K.M.; Kool, E.T. Fluorescent nucleobases as tools for studying DNA and RNA. Nat. Chem. 2017, 9, 1043–1055. [Google Scholar] [CrossRef]
- Tsao, Y.-Y.T.; Wooley, K.L. Synthetic, functional thymidine-derived polydeoxyribonucleotide analogues from a six-membered cyclic phosphoester. J. Am. Chem. Soc. 2017, 139, 5467–5473. [Google Scholar] [CrossRef]
- Song, J.; Yi, C. Chemical modifications to RNA: A new layer of gene expression regulation. ACS Chem. Biol. 2017, 12, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Tolle, F.; Brändle, G.M.; Matzner, D.; Mayer, G. A versatile approach towards nucleobase-modified aptamers. Angew. Chem. Int. Ed. 2015, 54, 10971–10974. [Google Scholar] [CrossRef]
- Gottfried, A.; Weinhold, E. Sequence-specific covalent labelling of DNA. Biochem. Soc. Trans. 2011, 39, 623–628. [Google Scholar] [CrossRef]
- Schulz, D.; Holstein, J.M.; Rentmeister, A. A chemo-enzymatic approach for site-specific modification of the RNA cap. Angew. Chem. Int. Ed. 2013, 52, 7874–7878. [Google Scholar] [CrossRef]
- Weisbrod, S.H.; Marx, A. Novel strategies for the site-specific covalent labelling of nucleic acids. Chem. Commun. 2008, 44, 5675–5685. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Farzan, V.M.; Aparin, I.O.; Veselova, O.A.; Podkolzin, A.T.; Shipulin, G.A.; Korshun, V.A.; Zatsepin, T.S. Cy5/BHQ dye–quencher pairs in fluorogenic qPCR probes: Effects of charge and hydrophobicity. Anal. Methods 2016, 8, 5826–5831. [Google Scholar] [CrossRef]
- Malek-Adamian, E.; Guenther, D.C.; Matsuda, S.; Martínez-Montero, S.l.; Zlatev, I.; Harp, J.; Burai Patrascu, M.; Foster, D.J.; Fakhoury, J.; Perkins, L. 4′-C-Methoxy-2′-deoxy-2′-fluoro modified ribonucleotides improve metabolic stability and elicit efficient RNAi-mediated gene silencing. J. Am. Chem. Soc. 2017, 139, 14542–14555. [Google Scholar] [CrossRef] [PubMed]
- Abou Assi, H.; El-Khoury, R.; González, C.; Damha, M.J. 2′-Fluoroarabinonucleic acid modification traps G-quadruplex and i-motif structures in human telomeric DNA. Nucleic Acids Res. 2017, 45, 11535–11546. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montero, S.; Deleavey, G.F.; Dierker-Viik, A.; Lindovska, P.; Ilina, T.; Portella, G.; Orozco, M.; Parniak, M.A.; González, C.; Damha, M.J. Synthesis and properties of 2′-deoxy-2′, 4′-difluoroarabinose-modified nucleic acids. J. Org. Chem. 2015, 80, 3083–3091. [Google Scholar] [CrossRef]
- Martinez-Montero, S.; Deleavey, G.F.; Martín-Pintado, N.; Fakhoury, J.F.; Gonzalez, C.; Damha, M.J. Locked 2′-deoxy-2′, 4′-difluororibo modified nucleic acids: Thermal stability, structural studies, and siRNA activity. ACS Chem. Biol. 2015, 10, 2016–2023. [Google Scholar] [CrossRef]
- Jones, A.; Woodhouse, D. Bromination of nucleic acids and their derivatives. Nature 1959, 183, 1603–1605. [Google Scholar] [CrossRef]
- Ennifar, E.; Carpentier, P.; Ferrer, J.-L.; Walter, P.; Dumas, P. X-ray-induced debromination of nucleic acids at the Br K absorption edge and implications for MAD phasing. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 1262–1268. [Google Scholar] [CrossRef]
- Egli, M. Nucleic acid crystallography: Current progress. Curr. Opin. Chem. Biol. 2004, 8, 580–591. [Google Scholar] [CrossRef]
- Ukale, D.; Lönnberg, T. Organomercury Nucleic Acids: Past, Present and Future. ChemBioChem 2021, 22, 1733. [Google Scholar] [CrossRef]
- Zhang, W.; Szostak, J.W.; Huang, Z. Nucleic acid crystallization and X-ray crystallography facilitated by single selenium atom. Front. Chem. Sci. Eng. 2016, 10, 196–202. [Google Scholar] [CrossRef]
- Hendrickson, W.A. Determination of Macromolecular Structures from Anomalous Diffraction of. Science 1991, 254, 5028. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, W.A. Synchrotron crystallography. Trends Biochem. Sci. 2000, 25, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, W.A.; Horton, J.R.; LeMaster, D.M. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): A vehicle for direct determination of three-dimensional structure. EMBO J. 1990, 9, 1665. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Synthesis of Selenium and Tellurium Modified Nucleic Acids For DNA Crystallization, Structure and Function Studies. Ph.D. Thesis, Georgia State University, Atlanta, GA, USA, 2020. [Google Scholar]
- Sheng, J.; Huang, Z. Selenium Derivatization of Nucleic Acids for Phase and Structure Determination in Nucleic Acid X-ray Crystallography. Int. J. Mol. Sci. 2008, 9, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Huang, Z. Selenium Derivatization of Nucleic Acids for X-Ray Crystal-Structure and Function Studies. Chem. Biodivers. 2010, 7, 753–785. [Google Scholar] [CrossRef] [PubMed]
- Salon, J.; Sheng, J.; Jiang, J.; Chen, G.; Caton-Williams, J.; Huang, Z. Oxygen replacement with selenium at the thymidine 4-position for the Se base pairing and crystal structure studies. J. Am. Chem. Soc. 2007, 129, 4862–4863. [Google Scholar] [CrossRef]
- Sun, H.; Sheng, J.; Hassan, A.E.; Jiang, S.; Gan, J.; Huang, Z. Novel RNA base pair with higher specificity using single selenium atom. Nucleic Acids Res. 2012, 40, 5171–5179. [Google Scholar] [CrossRef]
- Salon, J.; Chen, G.; Portilla, Y.; Germann, M.W.; Huang, Z. Synthesis of a 2’-Se-uridine Phosphoramidite and Its Incorporation into Oligonucleotides for Structural Study. Org. Lett. 2005, 7, 5645–5648. [Google Scholar] [CrossRef]
- Salon, J.; Jiang, J.; Sheng, J.; Gerlits, O.O.; Huang, Z. Derivatization of DNAs with selenium at 6-position of guanine for function and crystal structure studies. Nucleic Acids Res. 2008, 36, 7009–7018. [Google Scholar] [CrossRef]
- Salon, J.; Gan, J.; Abdur, R.; Liu, H.; Huang, Z. Synthesis of 6-Se-guanosine RNAs for structural study. Org. Lett. 2013, 15, 3934–3937. [Google Scholar] [CrossRef]
- Tram, K.; Wang, X.; Yan, H. Facile synthesis of oligonucleotide phosphoroselenoates. Org. Lett. 2007, 9, 5103–5106. [Google Scholar] [CrossRef] [PubMed]
- Abdur, R.; Gerlits, O.O.; Gan, J.; Jiang, J.; Salon, J.; Kovalevsky, A.Y.; Chumanevich, A.A.; Weber, I.T.; Huang, Z. Novel complex MAD phasing and RNase H structural insights using selenium oligonucleotides. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Caton-Williams, J.; Huang, Z. Synthesis and DNA-polymerase incorporation of colored 4-selenothymidine triphosphate for polymerase recognition and DNA visualization. Angew. Chem. Int. Ed. 2008, 47, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E.; Sheng, J.; Zhang, W.; Huang, Z. High fidelity of base pairing by 2-selenothymidine in DNA. J. Am. Chem. Soc. 2010, 132, 2120–2121. [Google Scholar] [CrossRef]
- Chen, C.; Fang, Z.; Huang, Z. 2′-β-Selenium Atom on Thymidine to Control β-Form DNA Conformation and Large Crystal Formation. Cryst. Growth Des. 2022, 22, 3601–3604. [Google Scholar] [CrossRef]
- Lin, L.; Sheng, J.; Huang, Z. Nucleic acid X-ray crystallography via direct selenium derivatization. Chem. Soc. Rev. 2011, 40, 4591–4602. [Google Scholar] [CrossRef]
- Caton-Williams, J.; Huang, Z. Biochemistry of selenium-derivatized naturally occurring and unnatural nucleic acids. Chem. Biodivers. 2008, 5, 396–407. [Google Scholar] [CrossRef]
- Adenis, C.; Langer, V.; Lindqvist, O. Reinvestigation of the structure of tellurium. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 941–942. [Google Scholar] [CrossRef]
- Ba, L.A.; Döring, M.; Jamier, V.; Jacob, C. Tellurium: An element with great biological potency and potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef]
- Petragnani, N.; Stefani, H.A. Tellurium in Organic Synthesis, 2nd ed.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Caldwell, R.S.; Fan, H. Optical properties of tellurium and selenium. Phys. Rev. 1959, 114, 664. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, R.; Khan, K.; Tareen, A.K.; Liu, X.; Liang, W.; Zhang, Y.; Ma, C.; Guo, Z.; Luo, X. Two-dimensional tellurium: Progress, challenges, and prospects. Nano-Micro Lett. 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Brumaghim, J.L. Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium; American Chemical Society: Washington, DC, USA, 2013. [Google Scholar]
- Lippolis, V.; Santi, C. Selenium & tellurium chemistry at the beginning of the 3rd millennium: A celebration of ICCST. New J. Chem. 2019, 43, 11032–11033. [Google Scholar]
- Moroder, L. Isosteric replacement of sulfur with other chalcogens in peptides and proteins. J. Peptide Sci. 2005, 11, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Ibers, J. Tellurium in a twist. Nat. Chem. 2009, 1, 508. [Google Scholar] [CrossRef]
- Rosman, K.J.R.; Taylor, P.D.P. Isotopic compositions of the elements 1997. Pure Appl. Chem. 1998, 70, 217–235. [Google Scholar] [CrossRef]
- Saito, S.; Zhang, J.; Tanida, K.; Takahashi, S.; Koizumi, T. A Systematic 125Te NMR Study of Organotellurium Compounds: The Effect of Oxidation States and Substituents. Tetrahedron 1999, 55, 2545–2552. [Google Scholar] [CrossRef]
- Li, G.M.; Zingaro, R.A.; Segi, M.; Reibenspies, J.H.; Nakajima, T. Synthesis and structure of telluroamides and selenoamides. The first crystallographic study of telluroamides. Organometallics 1997, 16, 756–762. [Google Scholar] [CrossRef]
- Beckmann, J.; Hesse, M.; Poleschner, H.; Seppelt, K. Formation of Mixed-Valent Aryltellurenyl Halides RX2TeTeR. Angew. Chem. Int. Ed. 2007, 46, 8277–8280. [Google Scholar] [CrossRef]
- Miyasato, M.; Minoura, M.; Akiba, K.Y. Cleavage of Tellurium–Carbon Bonds of Hexavalent Organotellurium Compounds by Potassium Graphite. Angew. Chem. Int. Ed. 2001, 40, 2674–2676. [Google Scholar] [CrossRef]
- Housecroft, C.; Sharpe, A. Inorganic Chemistry, Pearson Education Limited, 4th ed.; Pearson: London, UK, 2012. [Google Scholar]
- Mutoh, Y.; Murai, T.; Yamago, S. Telluration of seleno-and chloroiminium salts leading to various telluroamides, and their structure and NMR properties. J. Organomet. Chem. 2007, 692, 129–135. [Google Scholar] [CrossRef]
- Kuhn, N.; Henkel, G.; Kratz, T. Beiträge zur Chemie des Imidazols, III. 2-Telluroimidazoline—Stabile Tellurocarbonyl-Verbindungen. Chem. Ber. 1993, 126, 2047–2049. [Google Scholar] [CrossRef]
- Webber, D.H.; Brutchey, R.L. Photolytic preparation of tellurium nanorods. Chem. Commun. 2009, 38, 5701–5703. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Assoud, A.; Mayasree, O.; Kleinke, H. Solid state polyselenides and polytellurides: A large variety of Se–Se and Te–Te interactions. Molecules 2009, 14, 3115–3131. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, W.S.; Wachhold, M. Discrete Crown-Shaped Te8 Rings in Cs3 Te22. Angew. Chem. Int. Ed. Engl. 1995, 34, 450–451. [Google Scholar] [CrossRef]
- Deiseroth, H.J.; Wagener, M.; Neumann, E. (AgI) 2Te6 and (AgI) 2Se6: New Composite Materials with Cyclic Te6 and Se6 Molecules Stabilized in the “Solid Solvent” AgI. Eur. J. Inorg. Chem. 2004, 2004, 4755–4758. [Google Scholar] [CrossRef]
- Günther, A.; Isaeva, A.; Baranov, A.I.; Ruck, M. Neutral Tellurium Rings in the Coordination Polymers [Ru (Te9)](InCl4)2, [Ru (Te8)] Cl2, and [Rh (Te6)] Cl3. Chem. A Eur. J. 2011, 17, 6382–6388. [Google Scholar] [CrossRef]
- Jeske, J.; du Mont, W.W.; Jones, P.G. Synthesis of a Triiodide-Like Pentamesityl-tritellurium Cation by Addition of Dimesityltelluride to the Remarkably Electrophilic Trimesitylditelluronium Ion. Angew. Chem. Int. Ed. Engl. 1997, 36, 2219–2221. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakanishi, W. Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, 2nd ed.; Devillanova, F.A., du Mont, W.-W., Eds.; RSC Publishing: London, UK, 2013; Volume 2. [Google Scholar]
- Lin, T.P.; Gabbaï, F.P. Telluronium Ions as σ-Acceptor Ligands. Angew. Chem. Int. Ed. 2013, 52, 3864–3868. [Google Scholar] [CrossRef]
- Zhao, H.; Gabbaï, F.P. A bidentate Lewis acid with a telluronium ion as an anion-binding site. Nat. Chem. 2010, 2, 984–990. [Google Scholar] [CrossRef]
- Knight, F.R.; Arachchige, K.S.A.; Randall, R.A.; Bühl, M.; Slawin, A.M.; Woollins, J.D. Exploring hypervalency and three-centre, four-electron bonding interactions: Reactions of acenaphthene chalcogen donors and dihalogen acceptors. Dalton Trans. 2012, 41, 3154–3165. [Google Scholar] [CrossRef]
- Bühl, M.; Knight, F.R.; Křístková, A.; Malkin Ondík, I.; Malkina, O.L.; Randall, R.A.; Slawin, A.M.; Woollins, J.D. Weak Te, Te Interactions through the Looking Glass of NMR Spin–Spin Coupling. Angew. Chem. 2013, 125, 2555–2558. [Google Scholar] [CrossRef][Green Version]
- Knight, F.R.; Diamond, L.; Athukorala Arachchige, K.S.; Sanz Camacho, P.; Randall, R.A.M.; Ashbrook, S.E.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. Conformational dependence of through-space tellurium-tellurium spin-spin coupling in peri-substituted bis (tellurides). Chem. A Eur. J. 2014, 21, 3613–3627. [Google Scholar] [CrossRef]
- Ramadan, S.E.; Razak, A.; Ragab, A.M.; El-Meleigy, M. Incorporation of tellurium into amino acids and proteins in a tellurium-tolerant fungi. Biol. Trace Elem. Res. 1989, 20, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.O.; Lewinski, K.; Kunkle, M.; Odom, J.D.; Dunlap, R.B.; Lebioda, L.; Hatada, M. Bio-incorporation of telluromethionine into buried residues of dihydrofolate reductase. Nat. Struct. Biol. 1994, 1, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N.; Karnbrock, W.; Steinbacher, S.; Humm, A.; Prade, L.; Neuefeind, T.; Moroder, L.; Huber, R. Bioincorporation of Telluromethionine into Proteins: A Promising New Approach for X-ray Structure Analysis of Proteins. J. Mol. Biol. 1997, 270, 616–623. [Google Scholar] [CrossRef]
- Bijelic, A.; Rompel, A. Ten good reasons for the use of the tellurium-centered Anderson–Evans polyoxotungstate in protein crystallography. Acc. Chem. Res. 2017, 50, 1441–1448. [Google Scholar] [CrossRef]
- Yang, F.; Wong, K.-H.; Yang, Y.; Li, X.; Jiang, J.; Zheng, W.; Wu, H.; Chen, T. Purification and in vitro antioxidant activities of tellurium-containing phycobiliproteins from tellurium-enriched Spirulina platensis. Drug Des. Dev. Ther. 2014, 8, 1789. [Google Scholar]
- Sheng, J.; Hassan, A.E.A.; Huang, Z. New Telluride-Mediated Elimination for Novel Synthesis of 2’,3’-Didehydro-2’.3’-dideoxynucleosides. J. Org. Chem. 2008, 73, 3725–3729. [Google Scholar] [CrossRef]
- Sheng, J.; Hassan, A.E.A.; Huang, Z. Synthesis of the First Tellurium-Derivatized Oligonucleotides for Structural and Functional Studies. Chem. Eur. J. 2009, 15, 10210–10216. [Google Scholar] [CrossRef]
- Jiang, J.; Sheng, J.; Carrasco, N.; Huang, Z. Selenium derivatization of nucleic acids for crystallography. Nucleic Acids Res. 2006, 35, 477–485. [Google Scholar] [CrossRef]
- Salon, J.; Sheng, J.; Gan, J.; Huang, Z. Synthesis and crystal structure of 2′-Se-modified guanosine containing DNA. J. Org. Chem. 2010, 75, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Salon, J.; Gan, J.; Huang, Z. Synthesis and crystal structure study of 2′-Se-adenosine-derivatized DNA. Sci. China Chem. 2010, 53, 78–85. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Carrasco, N.; Buzin, Y.; Tyson, E.; Halpert, E.; Huang, Z. Selenium derivatization and crystallization of DNA and RNA oligonucleotides for X-ray crystallography using multiple anomalous dispersion. Nucleic Acids Res. 2004, 32, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Karino, N.; Ueno, Y.; Matsuda, A. Synthesis and properties of oligonucleotides containing 5-formyl-2′-deoxycytidine: In vitro DNA polymerase reactions on DNA templates containing 5-formyl-2′-deoxycytidine. Nucleic Acids Res. 2001, 29, 2456–2463. [Google Scholar] [CrossRef]
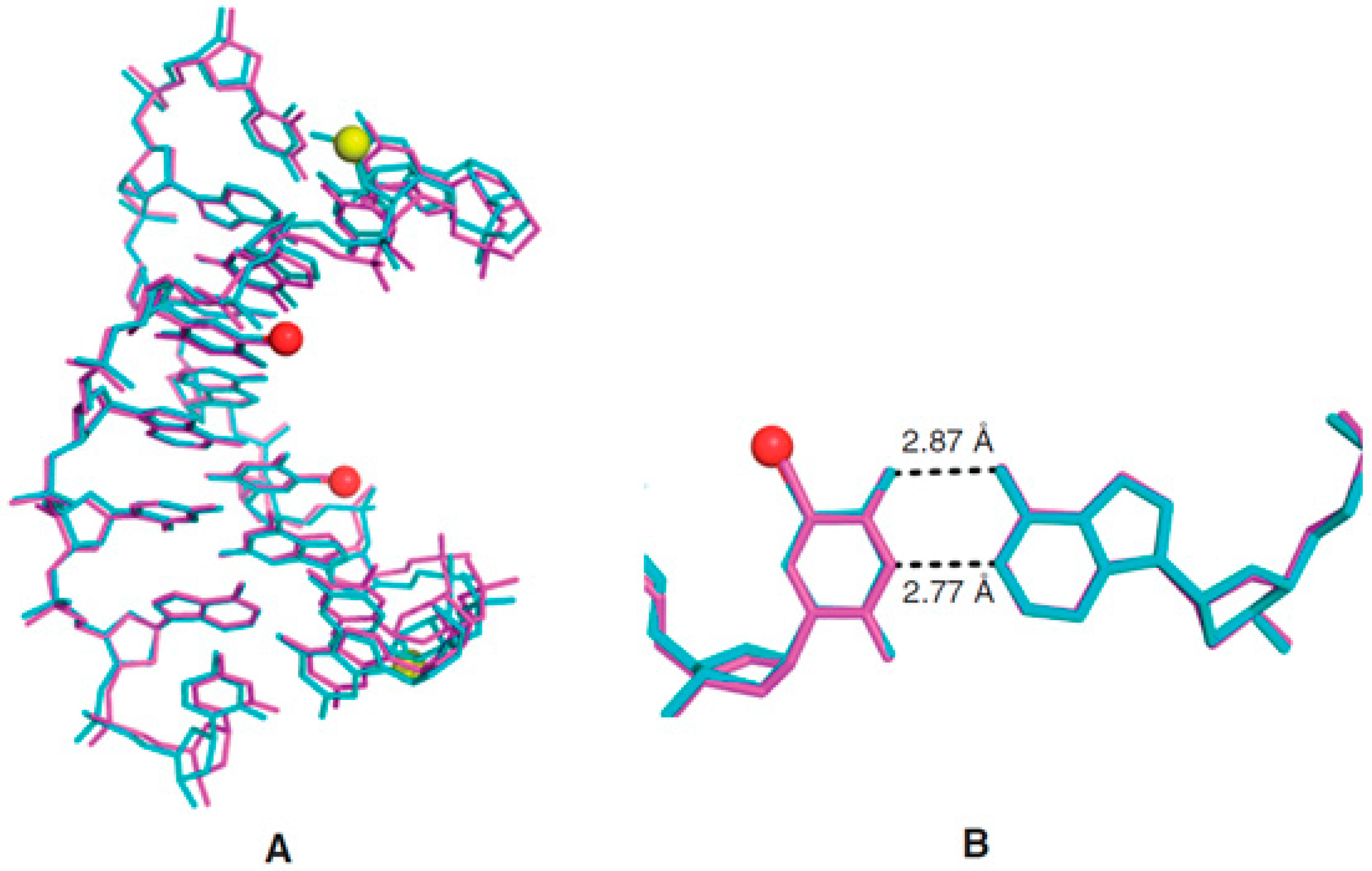
- Sheng, J.; Hassan, A.E.A.; Zhang, W.; Zhou, J.; Xu, B.; Soares, A.S.; Huang, Z. Synthesis, structure and imaging of oligodeoxyribonucleotides with tellurium-nucleobase derivatization. Nucleic Acids Res. 2011, 39, 3962–3971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jain, S.; Zon, G.; Sundaralingam, M. Base only binding of spermine in the deep groove of the A-DNA octamer d (GTGTACAC). Biochemistry 1989, 28, 2360–2364. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Pirillo, J.; De Simone, B.C.; Russo, N. Photophysical properties prediction of selenium-and tellurium-substituted thymidine as potential UVA chemotherapeutic agents. Theor. Chem. Acc. 2016, 135, 1–5. [Google Scholar] [CrossRef]
- Pirillo, J.; Mazzone, G.; Russo, N.; Bertini, L. Photophysical properties of S, Se and Te-substituted deoxyguanosines: Insight into their ability to act as chemotherapeutic agents. J. Chem. Inf. Model. 2017, 57, 234–242. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Huang, Z. Tellurium-Modified Nucleosides, Nucleotides, and Nucleic Acids with Potential Applications. Molecules 2022, 27, 8379. https://doi.org/10.3390/molecules27238379
Chen C, Huang Z. Tellurium-Modified Nucleosides, Nucleotides, and Nucleic Acids with Potential Applications. Molecules. 2022; 27(23):8379. https://doi.org/10.3390/molecules27238379
Chicago/Turabian StyleChen, Cen, and Zhen Huang. 2022. "Tellurium-Modified Nucleosides, Nucleotides, and Nucleic Acids with Potential Applications" Molecules 27, no. 23: 8379. https://doi.org/10.3390/molecules27238379
APA StyleChen, C., & Huang, Z. (2022). Tellurium-Modified Nucleosides, Nucleotides, and Nucleic Acids with Potential Applications. Molecules, 27(23), 8379. https://doi.org/10.3390/molecules27238379






