Mechanical, Barrier, Antioxidant and Antimicrobial Properties of Alginate Films: Effect of Seaweed Powder and Plasma-Activated Water
Abstract
1. Introduction
2. Results and Discussion
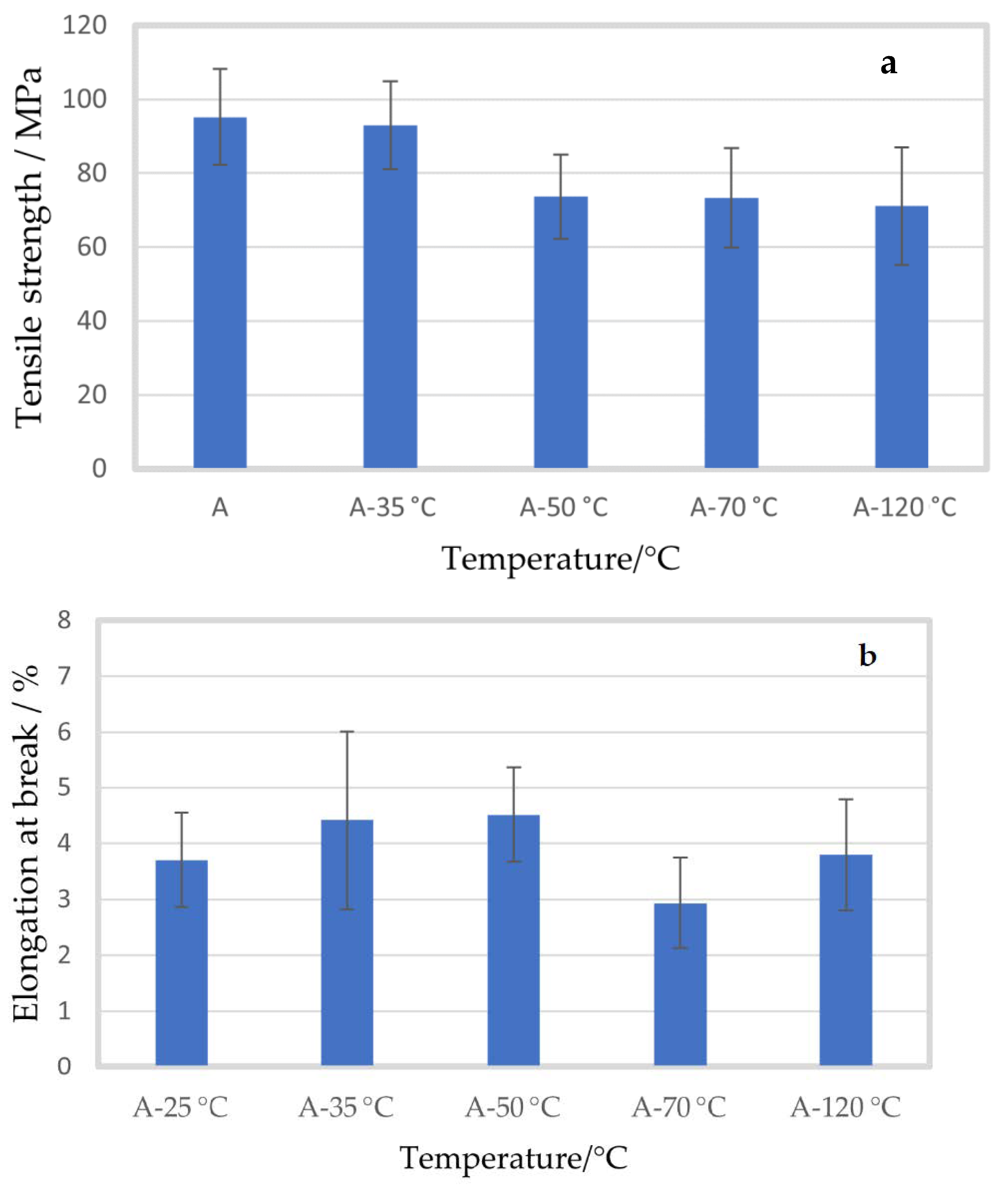
2.1. Effect of Temperature
Mechanical Properties
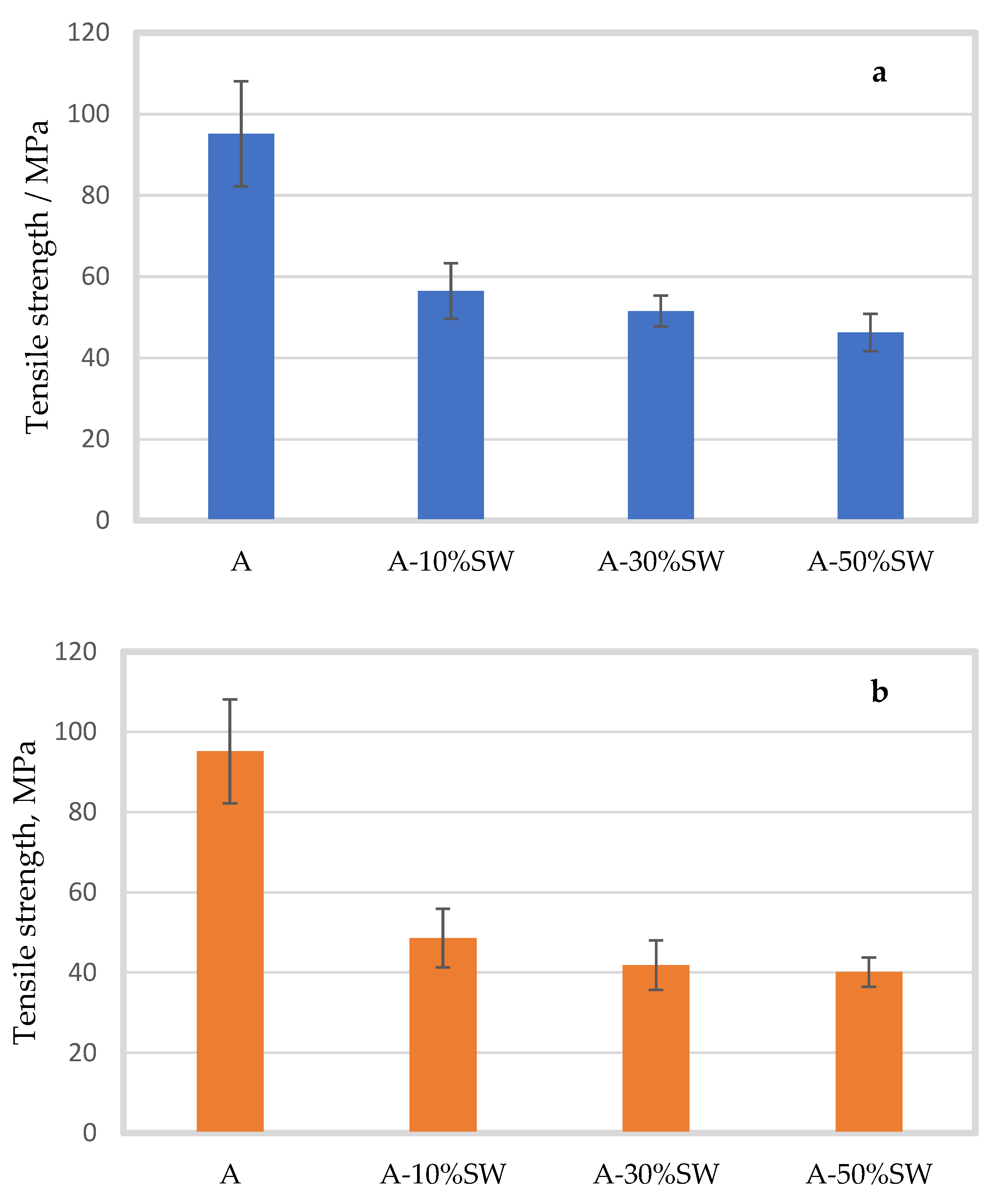
2.2. Effect of Seaweed Concentration and Particle Size
2.2.1. Mechanical Properties
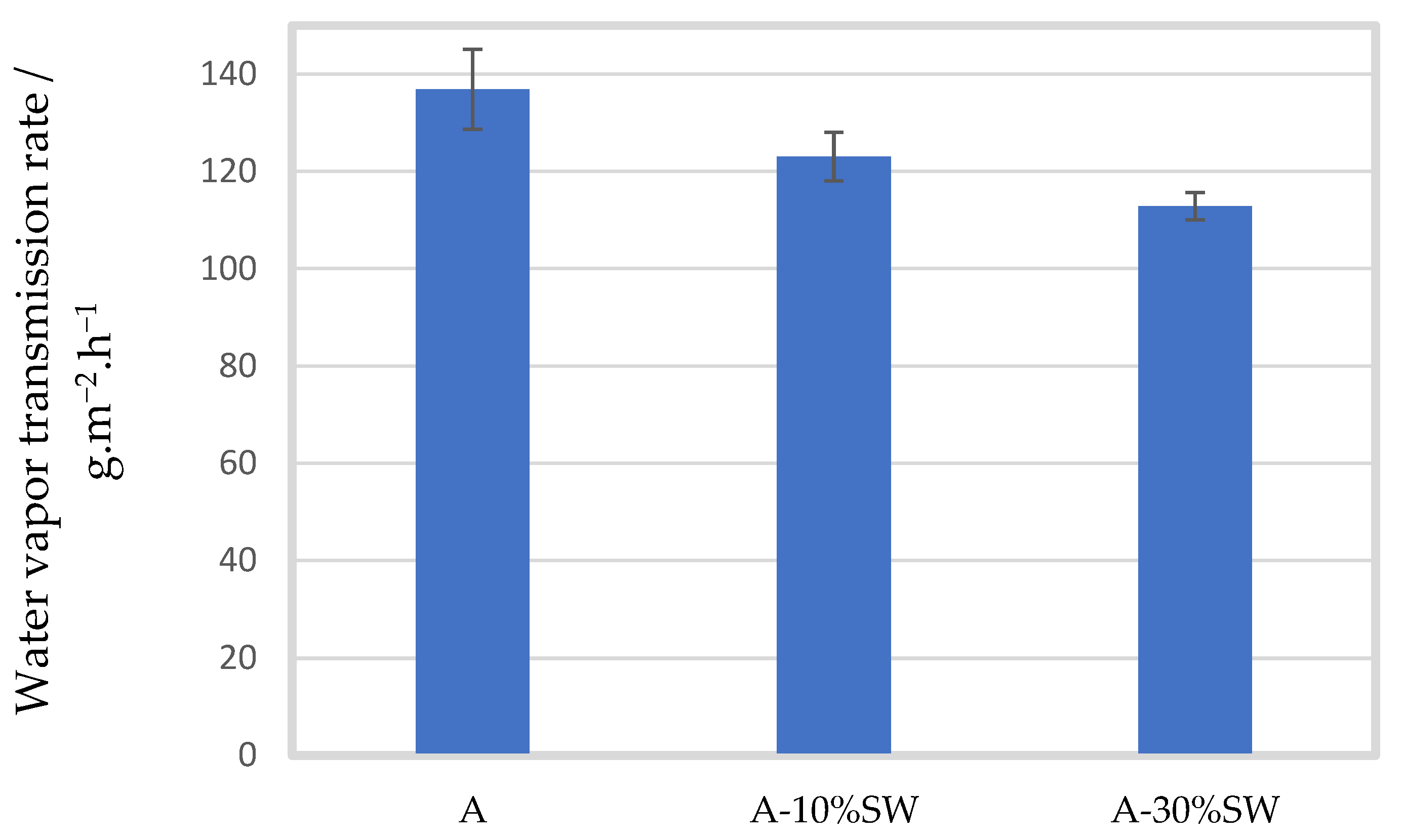
2.2.2. Barrier Properties
2.2.3. Antioxidant Properties
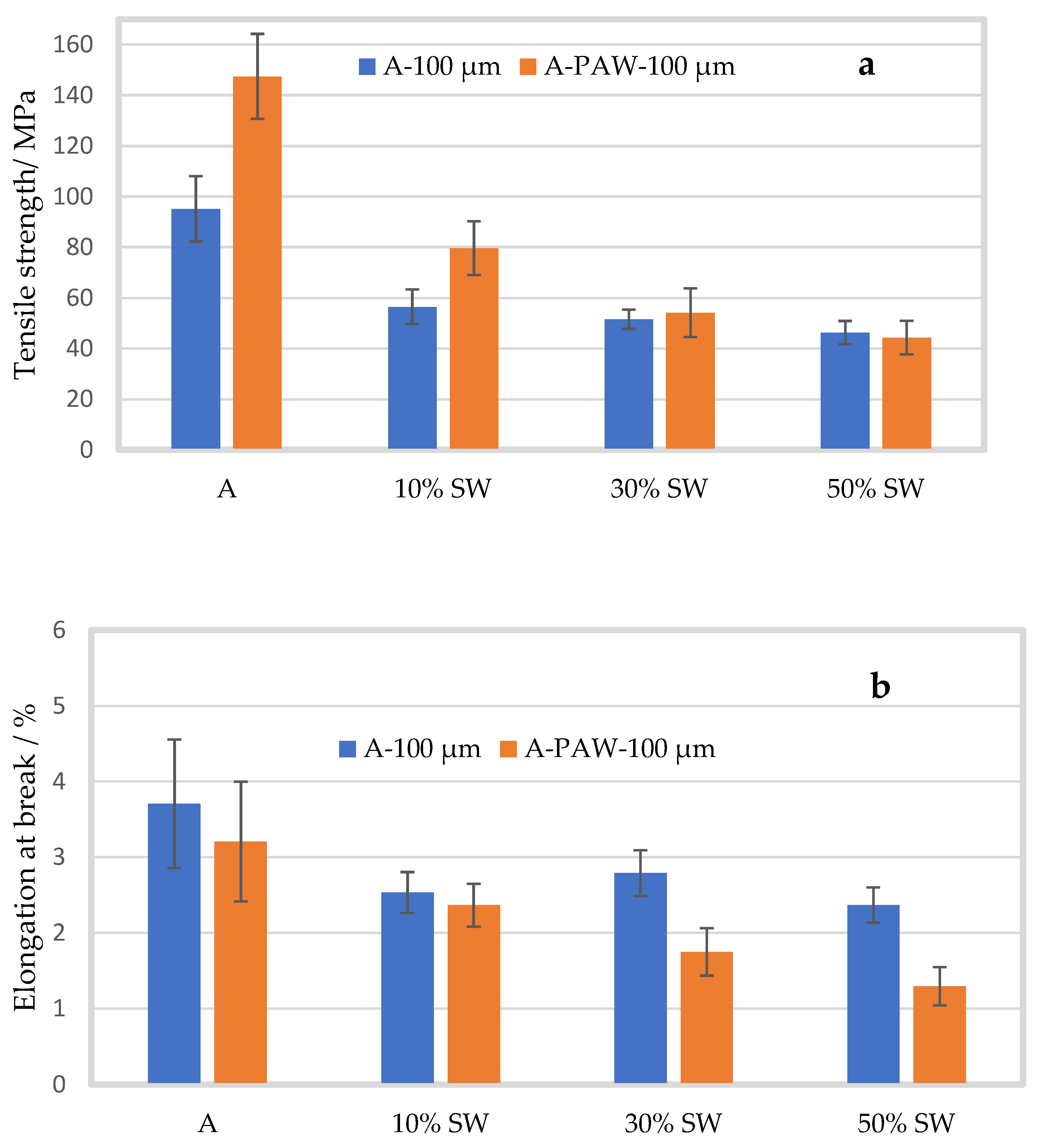
2.3. Effect of Plasma Activated Water
2.3.1. Mechanical Properties
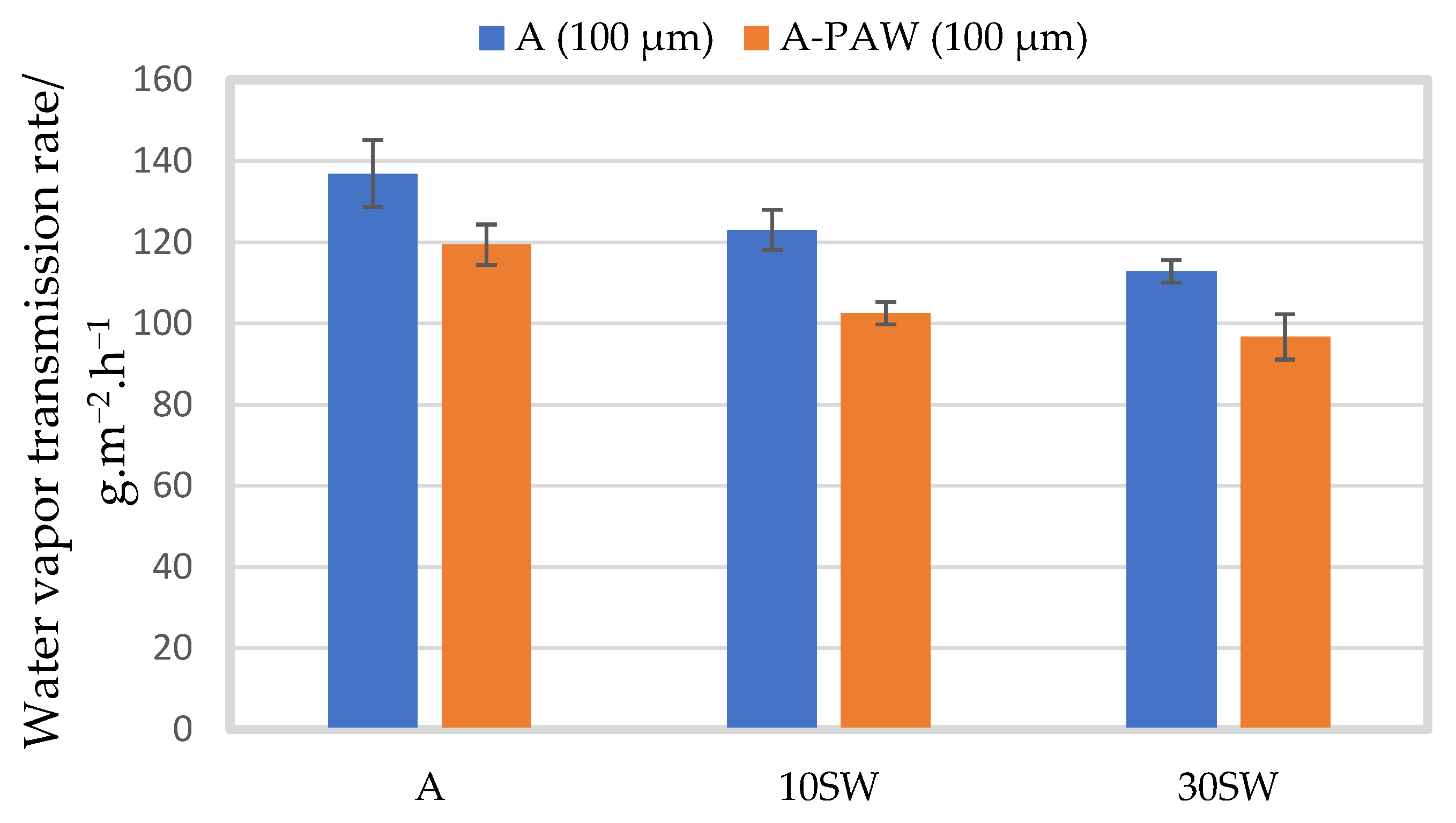
2.3.2. Barrier Properties
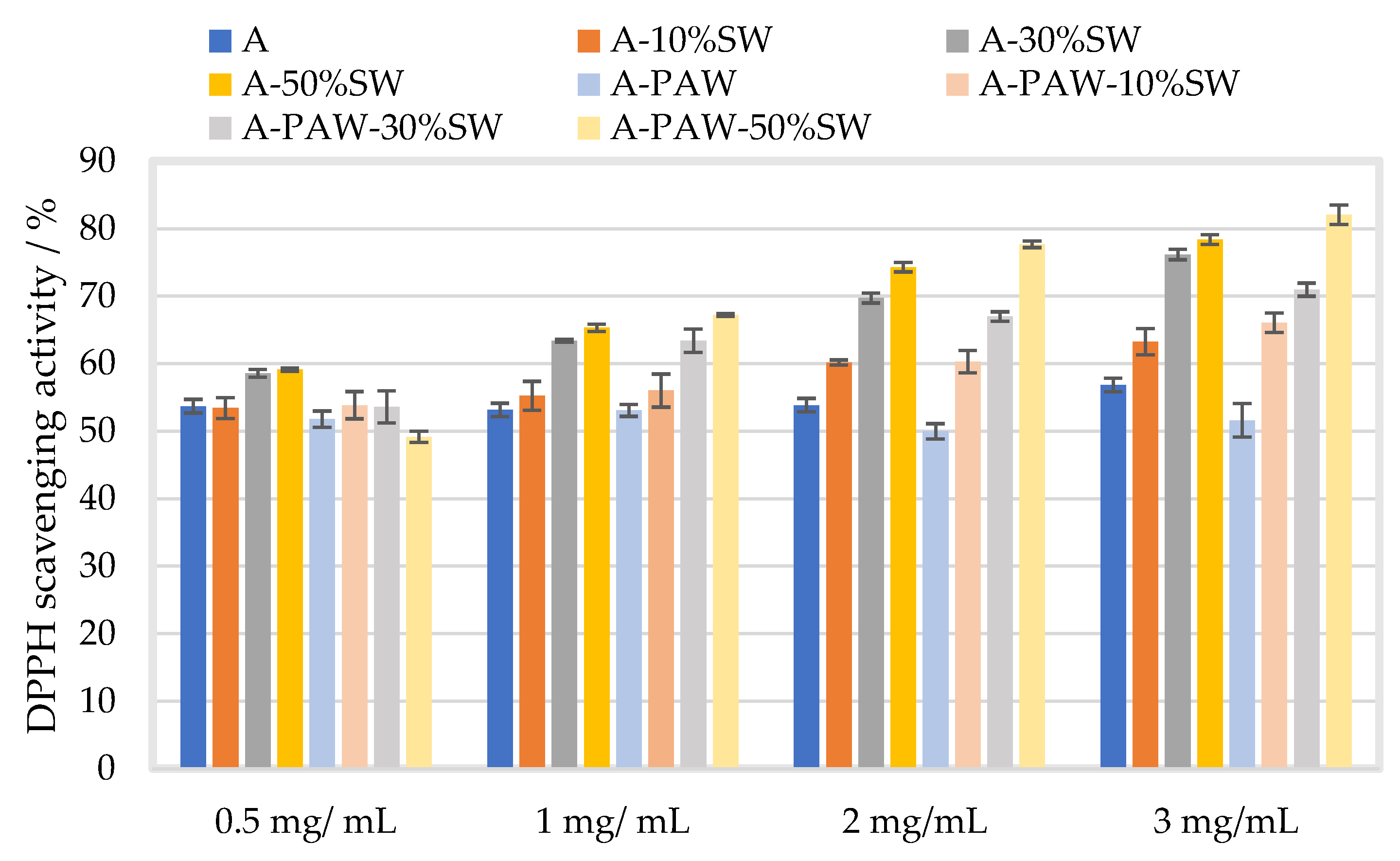
2.3.3. Antioxidant Properties
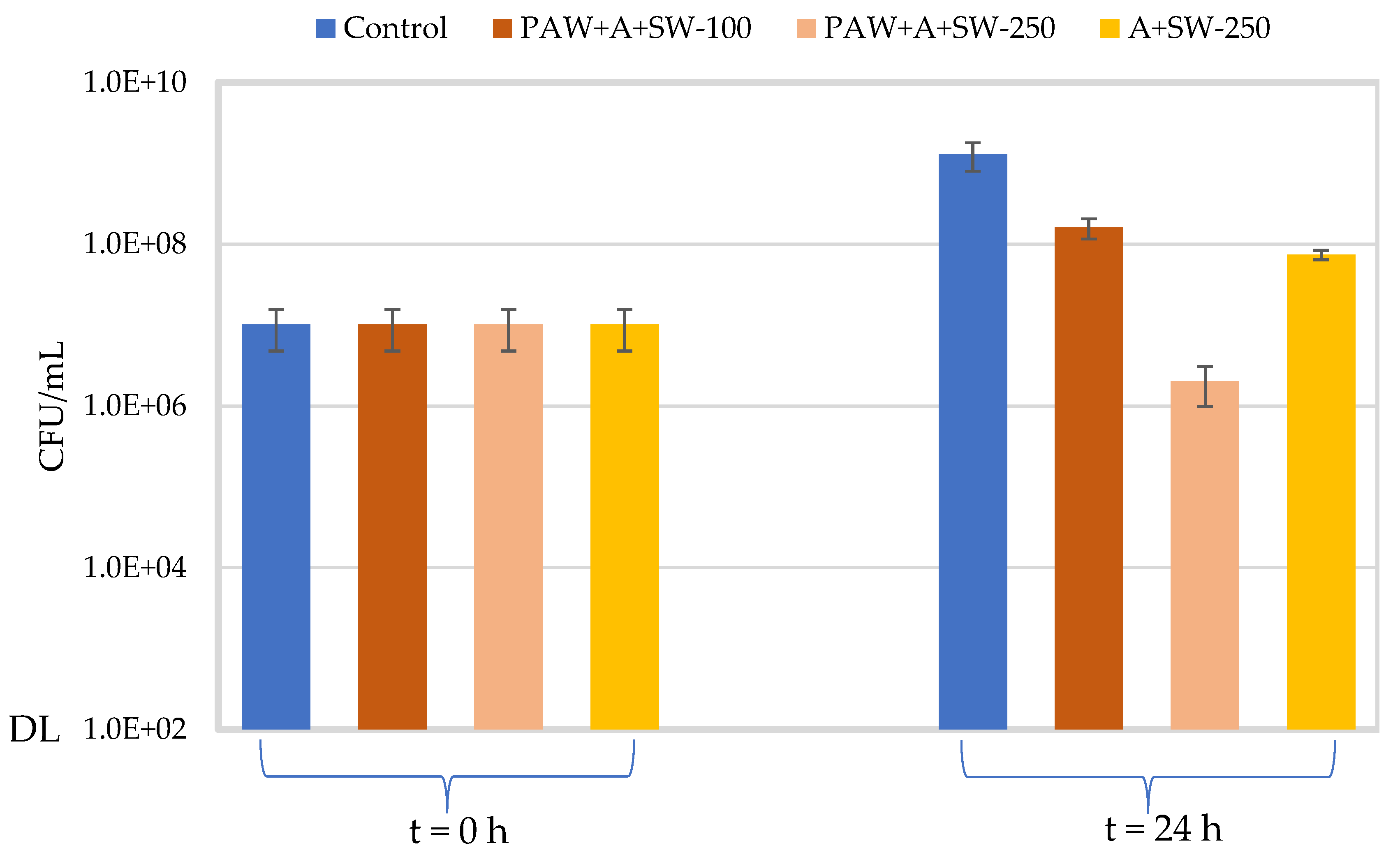
2.4. Antimicrobial Properties
3. Materials and Methods
3.1. Materials
3.2. Film Preparation
3.3. Seaweed Addition
3.4. Mechanical Properties
3.5. Antioxidant Activity
3.6. Barrier Properties
3.7. PAW Production
3.8. Antimicrobial Studies
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2019, 90, 162–171. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Annual Production of Plastics Worldwide from 1950 to 2020. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 1 March 2022).
- Parker, L. Fast Facts about Plastic Pollution. 2018. Available online: https://www.nationalgeographic.com/science/article/plastics-facts-infographics-ocean-pollution (accessed on 1 March 2022).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Marine Plastic Pollution. 2021. Available online: https://www.iucn.org/resources/issues-briefs/marine-plastic-pollution (accessed on 1 March 2022).
- Abdul Khalil, H.P.S.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Nurul Fazita, M.R.; Mohamad Haafiz, M.K.; Nurmiati, Z.; Paridah, M.T. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Sellimi, S.; Younes, I.; Ben Ayed, H.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT—Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-Y.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, N.; Sonea, I.; Walshb, J.L.; Sivertsvika, M.; Noriega Fernández, E. Effect of citric acid and plasma activated water on the functional properties of sodium alginate for potential food packaging applications. Food Packag. Shelf Life 2021, 29, 100733. [Google Scholar] [CrossRef]
- Puscaselu, R.; Gutt, G.; Amariei, S. Rethinking the Future of Food Packaging: Biobased Edible Films for Powdered Food and Drinks. Molecules 2019, 24, 3136. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT—Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial activity of alginate/clay nanocomposite films enriched with essential oils against three common foodborne pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Hamedi, H.; Kargozari, M.; Shotorbani, P.M.; Mogadam, N.B.; Fahimdanesh, M. A novel bioactive edible coating based on sodium alginate and galbanum gum incorporated with essential oil of Ziziphora persica: The antioxidant and antimicrobial activity, and application in food model. Food Hydrocoll. 2017, 72, 35–46. [Google Scholar] [CrossRef]
- Vatsos, I.N.; Rebours, C. Seaweed extracts as antimicrobial agents in aquaculture. J. Appl. Phycol. 2015, 27, 2017–2035. [Google Scholar] [CrossRef]
- Hasan, M.; Lai, T.K.; Chong, E.; Gopakumar, D.; Rizal, S.; Hossain, M.; Fazita, M.R.N.; Haafiz, M.; Paridah, M.T.; Khalil, H. Organic and inorganic fillers’ role on the amelioration of Kappaphycus spp.-based biopolymer films’ performance. BioResources 2019, 14, 9198–9213. [Google Scholar]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Kadam, S.U.; Pankaj, S.K.; Tiwari, B.K.; Cullen, P.J.; O’Donnell, C.P. Development of biopolymer-based gelatin and casein films incorporating brown seaweed Ascophyllum nodosum extract. Food Packag. Shelf Life 2015, 6, 68–74. [Google Scholar] [CrossRef]
- Hassan, M.M.; Mueller, M.; Wagners, M.H. Exploratory study on seaweed as novel filler in polypropylene composite. J. Appl. Polym. Sci. 2008, 109, 1242–1247. [Google Scholar] [CrossRef]
- Stévant, P.; Rebours, C.; Chapman, A.A. Seaweed aquaculture in Norway: Recent industrial developments and future perspectives. Aquac. Int. 2017, 25, 1373–1390. [Google Scholar] [CrossRef]
- Govaerts, F.; Olsen, S.O. Exploration of seaweed consumption in Norway using the norm activation model: The moderator role of food innovativeness. Food Qual. Prefer. 2022, 99, 104511. [Google Scholar] [CrossRef]
- SINTEF. About the Centre. Available online: https://www.sintef.no/en/ocean/initiatives/norwegian-seaweed-technology-center/ (accessed on 1 April 2022).
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of seaweed on mechanical, thermal, and biodegradation properties of thermoplastic sugar palm starch/agar composites. Int. J. Biol. Macromol. 2017, 99, 265–273. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Freile-Pelegrín, Y.; Encinas, J.C.; Ríos-Soberanis, C.R.; Quintana-Owen, P. Biocomposites based on poly(lactic acid) and seaweed wastes from agar extraction: Evaluation of physicochemical properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Bulota, M.; Budtova, T. Valorisation of macroalgae industrial by-product as filler in thermoplastic polymer composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 271–277. [Google Scholar] [CrossRef]
- Hoque, M.; McDonagh, C.; Tiwari, B.K.; Kerry, J.P.; Pathania, S. Effect of Cold Plasma Treatment on the Packaging Properties of Biopolymer-Based Films: A Review. Appl. Sci. 2022, 12, 1346. [Google Scholar] [CrossRef]
- Okyere, A.Y.; Rajendran, S.; Annor, G.A. Cold plasma technologies: Their effect on starch properties and industrial scale-up for starch modification. Curr. Res. Food Sci. 2022, 5, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; RBlundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Oh, Y.A.; Roh, S.H.; Min, S.C. Cold plasma treatments for improvement of the applicability of defatted soybean meal-based edible film in food packaging. Food Hydrocoll. 2016, 58, 150–159. [Google Scholar] [CrossRef]
- Simon, S.; Salgado, B.; Hasan, M.I.; Sivertsvik, M.; Fernández, E.N.; Walsh, J.L. Influence of Potable Water Origin on the Physicochemical and Antimicrobial Properties of Plasma Activated Water. Plasma Chem. Plasma Process. 2022, 42, 377–393. [Google Scholar] [CrossRef]
- Sharmin, N.; Pang, C.; Sone, I.; Walsh, J.L.; Fernández, C.G.; Sivertsvik, M.; Fernández, E.N. Synthesis of Sodium Alginate–Silver Nanocomposites Using Plasma Activated Water and Cold Atmospheric Plasma Treatment. Nanomaterials 2021, 11, 2306. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, T.; Sun, W.; Ren, X. The depolymerization of sodium alginate by oxidative degradation. Pharm. Dev. Technol. 2012, 17, 763–769. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.W.; Lotfali, E.; Bagheri, R.; Pircheraghi, G. Alginate-based multifunctional films incorporated with sulfur quantum dots for active packaging applications. Colloids Surf. B Biointerfaces 2022, 215, 112519. [Google Scholar] [CrossRef]
- Augusto, A.; Dias, J.R.; Campos, M.J.; Alves, N.M.; Pedrosa, R.; Silva, S.F.J. Influence of Codium tomentosum Extract in the Properties of Alginate and Chitosan Edible Films. Foods 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Jiang, B.; Li, S.; Wu, Y.; Song, J.; Chen, S.; Li, X.; Sun, H. Preparation and characterization of natural corn starch-based composite films reinforced by eggshell powder. CyTA—J. Food 2018, 16, 1045–1054. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of sugar palm-derived cellulose reinforcement on the mechanical and water barrier properties of sugar palm starch biocomposite films. BioResources 2016, 11, 4134–4145. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, D.; Lu, J.; Li, T.; Jiao, C.; Li, S.; Lu, P.; Zhang, Z. Preparation and Properties of Biocomposite Films Based on Poly(vinyl alcohol) Incorporated with Eggshell Powder as a Biological Filler. J. Polym. Environ. 2020, 28, 2020–2028. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Agar-based antioxidant composite films incorporated with melanin nanoparticles. Food Hydrocoll. 2019, 94, 391–398. [Google Scholar] [CrossRef]
- Şen, M. Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isot. 2011, 69, 126–129. [Google Scholar] [CrossRef]
- Khajouei, R.A.; Keramat, J.; Hamdami, N.; Ursu, A.V.; Delattre, C.; Laroche, C.; Gardarin, C.; Lecerf, D.; Desbrières, J.; Djelveh, G.; et al. Extraction and characterization of an alginate from the Iranian brown seaweed Nizimuddinia zanardini. Int. J. Biol. Macromol. 2018, 118, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. Effect of processing conditions on phytochemical constituents of edible Irish seaweed Himanthalia elongata. J. Food Process. Preserv. 2012, 36, 348–363. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, L. Sodium alginate/carboxymethyl cellulose films containing pyrogallic acid: Physical and antibacterial properties. J. Sci. Food Agric. 2017, 97, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Charoux, C.M.G.; Patange, A.D.; Hinds, L.M.; Simpson, J.C.; O’Donnell, C.P.; Tiwari, B.K. Antimicrobial effects of airborne acoustic ultrasound and plasma activated water from cold and thermal plasma systems on biofilms. Sci. Rep. 2020, 10, 17297. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, X.; Yang, W.; Zhang, Y.; Ye, Z.; Hu, S.-H.; Ye, C.; Li, Y.; Lan, Y.; Shen, J.; et al. In vitro antimicrobial effects and mechanism of air plasma-activated water on Staphylococcus aureus biofilm. Plasma Processes Polym. 2020, 17, 1900270. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Rhimou, B.; Hassane, R.; Martínez, J.; Bourgougnon, N. The antibacterial potential of the seaweeds (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean Coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372. [Google Scholar]
- Val, A.; Platas, G.; Basilio, A.; Cabello, A.; Gorrochategui, J.; Suay, I.; Vicente, F.; Portillo, E.; Jiménez del Río, M.; Reina, G.G.; et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain). Int. Microbiol. 2001, 4, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, D.L.; Gerwick, W.H.; Velez, S.M.; Alexander, E.; Guevara, P. Antibiotic activity of lipid-soluble extracts from Caribbean marine algae. Hydrobiologia 1987, 151, 463–469. [Google Scholar] [CrossRef]



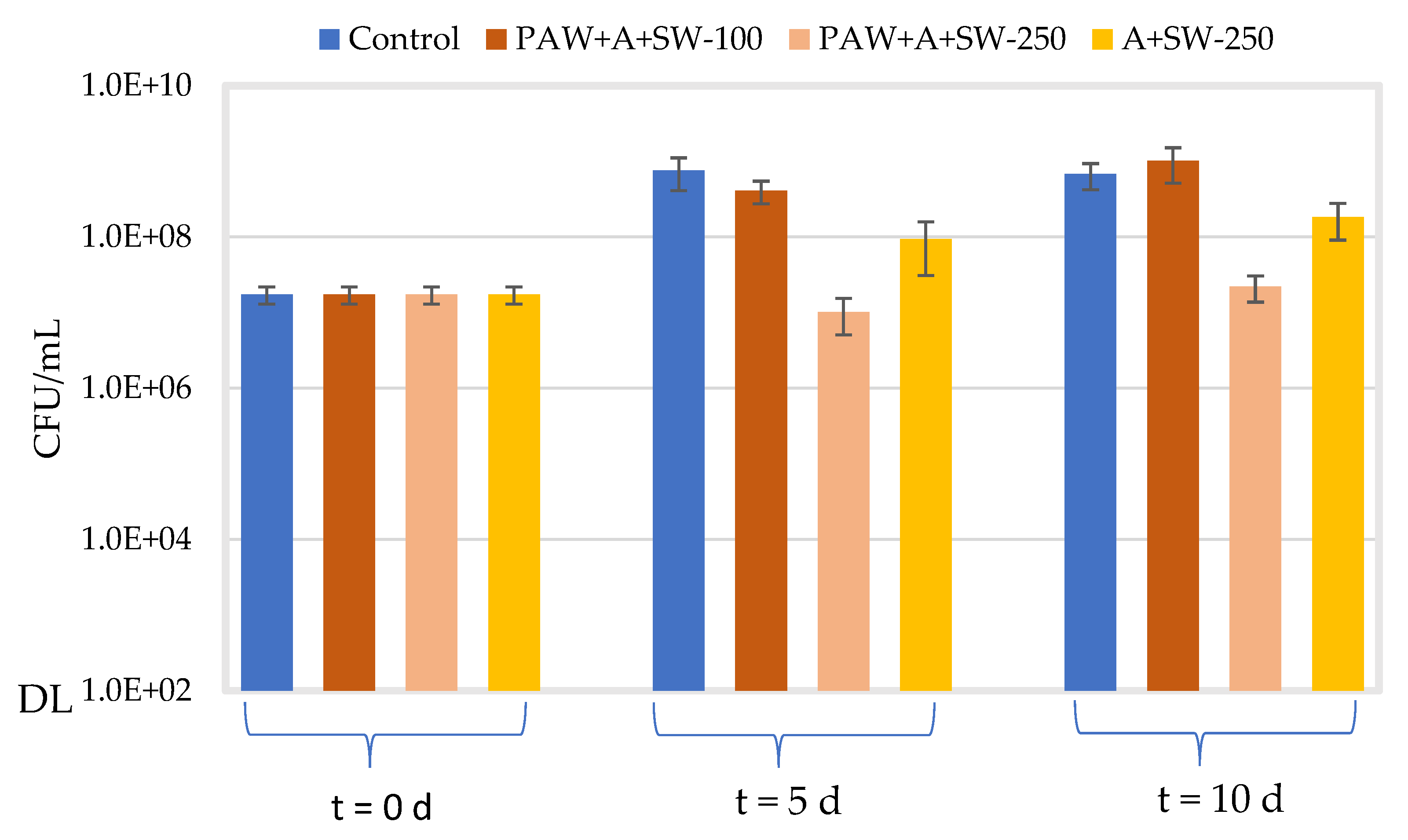
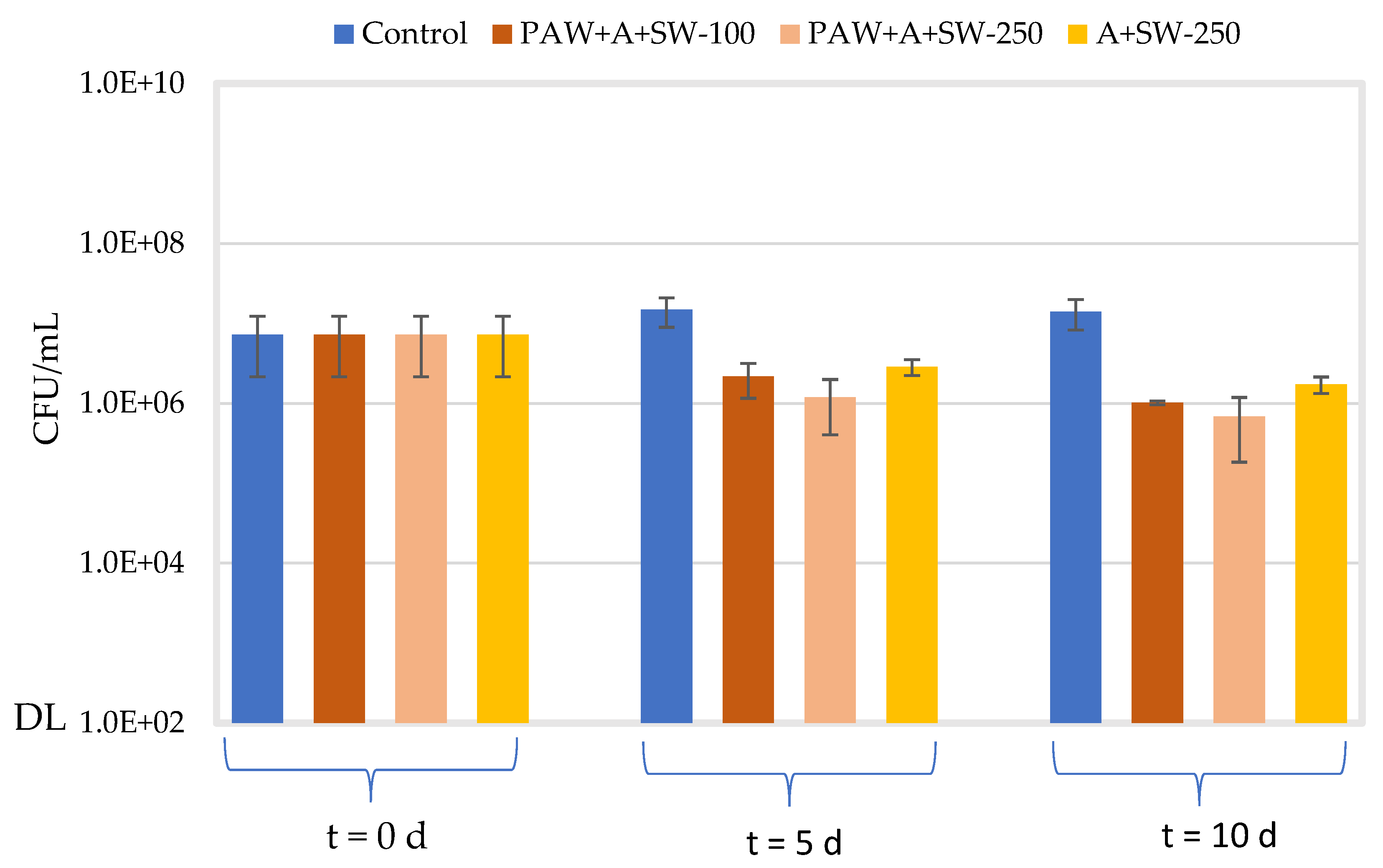
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dysjaland, H.; Sone, I.; Noriega Fernández, E.; Sivertsvik, M.; Sharmin, N. Mechanical, Barrier, Antioxidant and Antimicrobial Properties of Alginate Films: Effect of Seaweed Powder and Plasma-Activated Water. Molecules 2022, 27, 8356. https://doi.org/10.3390/molecules27238356
Dysjaland H, Sone I, Noriega Fernández E, Sivertsvik M, Sharmin N. Mechanical, Barrier, Antioxidant and Antimicrobial Properties of Alginate Films: Effect of Seaweed Powder and Plasma-Activated Water. Molecules. 2022; 27(23):8356. https://doi.org/10.3390/molecules27238356
Chicago/Turabian StyleDysjaland, Hege, Izumi Sone, Estefanía Noriega Fernández, Morten Sivertsvik, and Nusrat Sharmin. 2022. "Mechanical, Barrier, Antioxidant and Antimicrobial Properties of Alginate Films: Effect of Seaweed Powder and Plasma-Activated Water" Molecules 27, no. 23: 8356. https://doi.org/10.3390/molecules27238356
APA StyleDysjaland, H., Sone, I., Noriega Fernández, E., Sivertsvik, M., & Sharmin, N. (2022). Mechanical, Barrier, Antioxidant and Antimicrobial Properties of Alginate Films: Effect of Seaweed Powder and Plasma-Activated Water. Molecules, 27(23), 8356. https://doi.org/10.3390/molecules27238356





