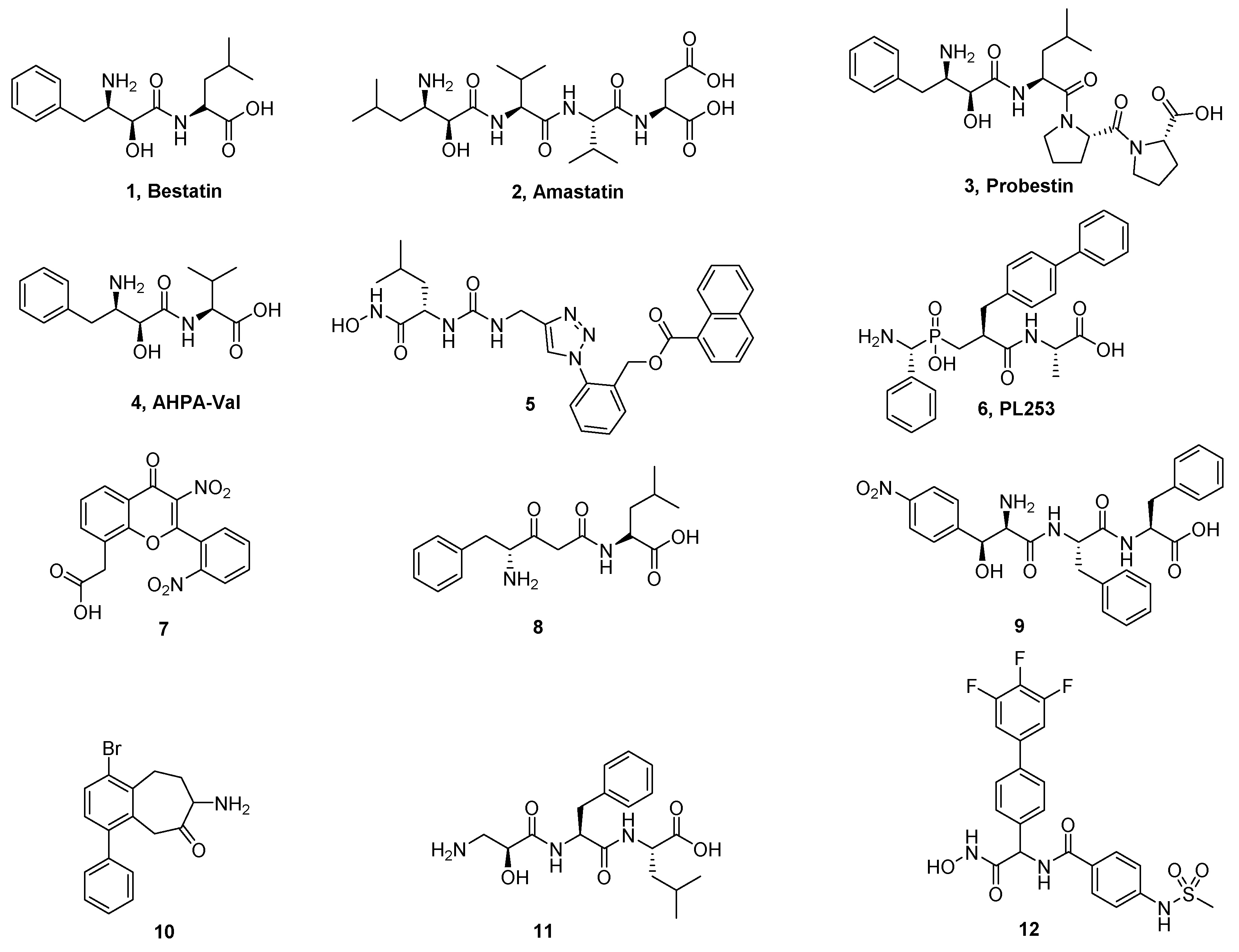
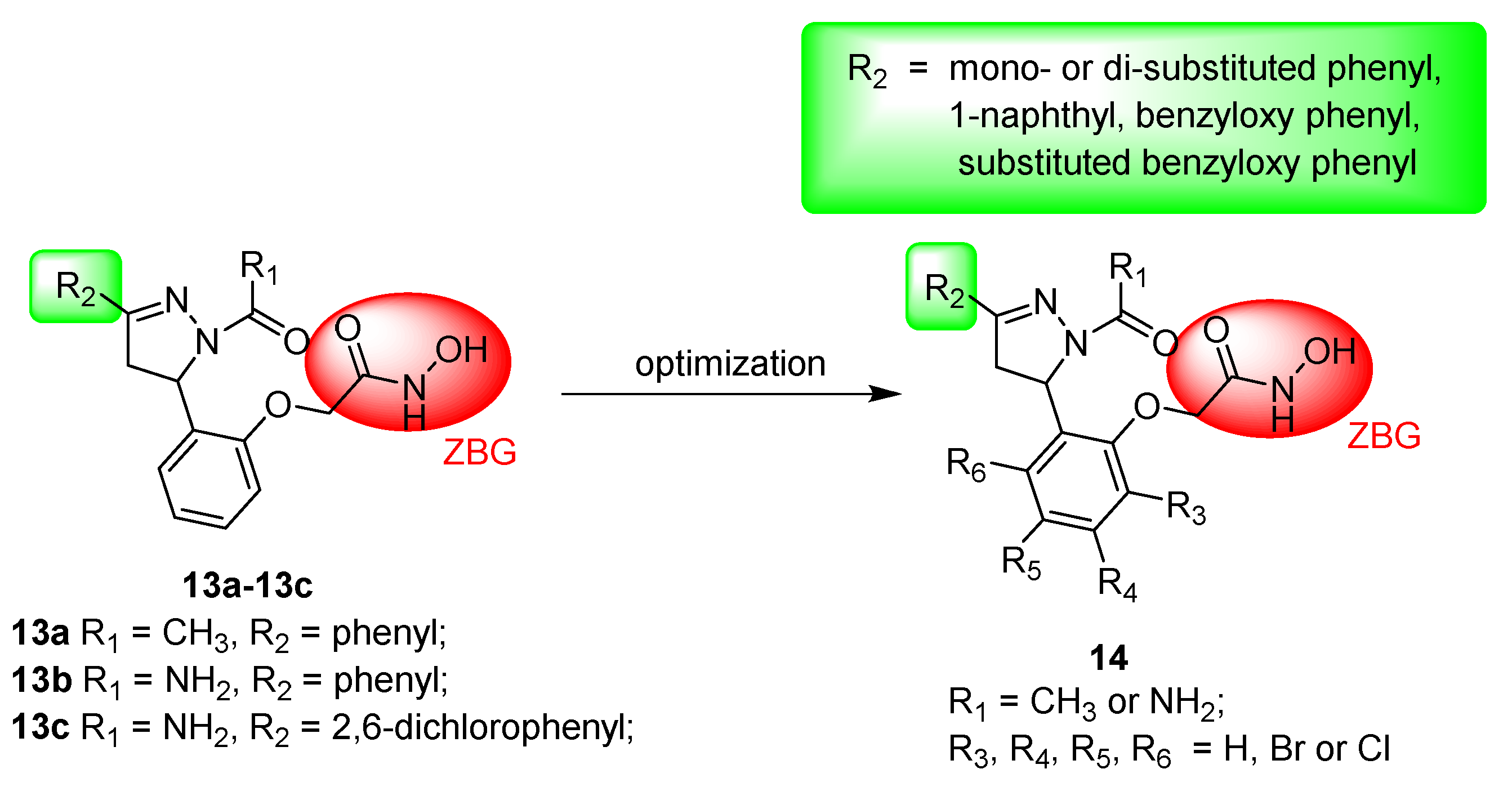
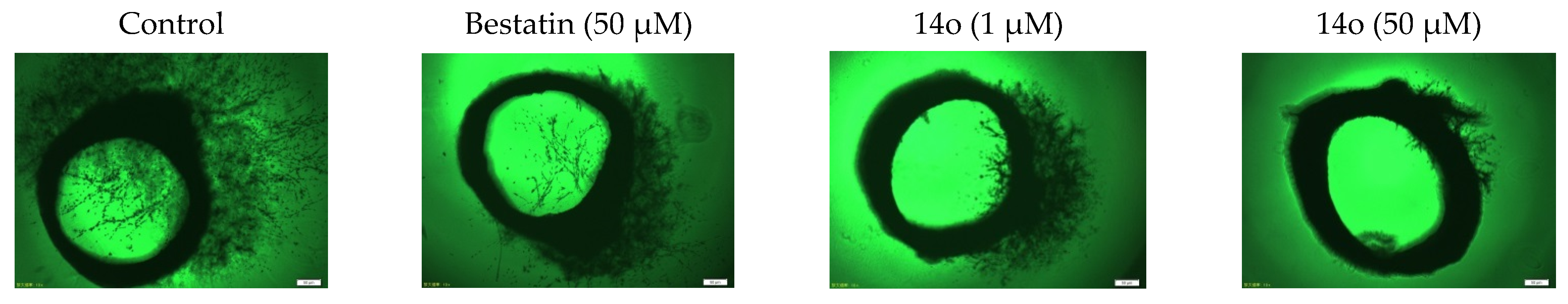
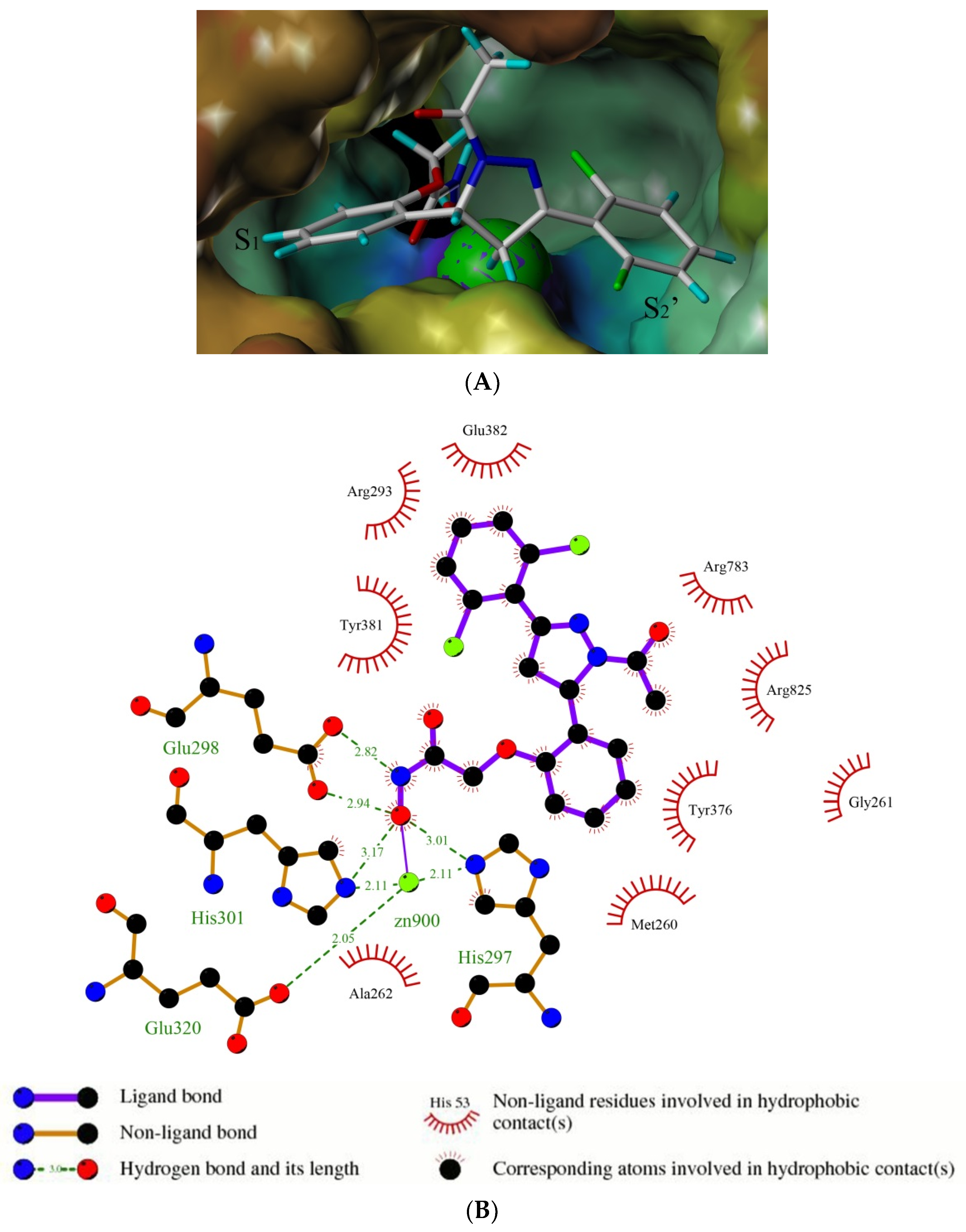
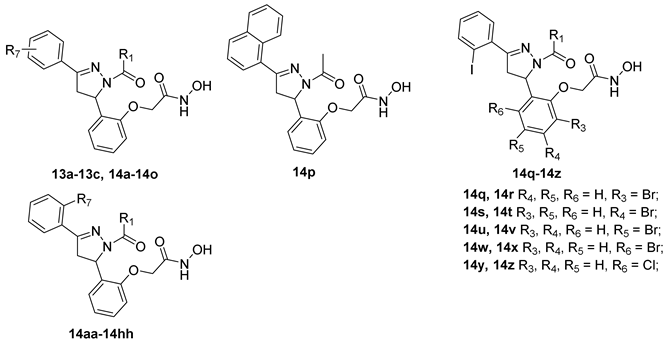
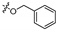
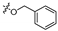
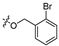
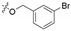
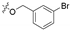
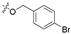
3.1.6. General Procedure for Synthesis of Compounds 14a–14z and 14aa–14hh
2-(2-(1-Acetyl-3-(2-bromophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14a)
A solution of potassium hydroxide (15.00 g, 267.86 mmol) in anhydrous methanol (50 mL) was dropwise added into a solution of hydroxylamine hydrochloride (12.54 g, 180.43 mmol) in anhydrous methanol (50 mL) at 0 °C. After stirring at 0 °C for 40 min, the mixture was filtered and the filtrate was used for the transformation of the methyl ester group to a hydroxamate group. Compound 20a (1.00 g, 2.32 mmol) was dissolved in the above solution (10 mL) and the mixture was stirred at 25 °C for 0.5 h. Then the mixture was poured into water (100 mL) and 10% H3PO4 solution was added to neutralize the excess base. The formed precipitate was filtered off and dried under vacuum to give 0.54 g of compound 14a as a white solid. Yield: 54%, mp: 162–164 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 9.04 (s, 1H), 7.81 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.45–7.41 (m, 1H), 7.24–7.20 (m, 1H), 7.10 (d, J = 8.4 Hz, 1H), 7.03–6.96 (m, 2H), 6.94–6.86 (m, 2H), 5.81 (dd, J = 11.7 Hz, J = 4.3 Hz, 1H), 4.62–4.54 (m, 2H), 3.88 (dd, J = 18.6 Hz, J = 11.7 Hz, 1H), 3.79 (s, 3H), 3.14 (dd, J = 18.6 Hz, J = 4.3 Hz, 1H), 2.32 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.25, 164.76, 155.12, 154.77, 134.51, 132.62, 131.73, 131.47, 129.88, 128.89, 128.37, 126.02, 121.65, 121.14, 112.69, 66.53, 55.37, 44.23, 22.24; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H19BrN3O4: 432.0559, found: 432.0548.
Compounds 14b–14z and 14aa–14hh were prepared following the procedure described for the compound 14a.
2-(2-(1-Acetyl-3-(3-bromophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14b)
White solid, yield: 55%, mp: 152–154 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H), 9.04 (s, 1H), 7.91 (t, J = 1.8 Hz, 1H), 7.76 (d, J = 7.8 Hz, 1H), 7.66 (dd, J = 7.8 Hz, J = 2.0 Hz, 1H), 7.42 (t, J = 7.9 Hz, 1H), 7.26–7.20 (m, 1H), 6.98 (d, J = 8.3 Hz, 1H), 6.91 (d, J = 4.5 Hz, 2H), 5.85 (dd, J = 11.8 Hz, J = 4.6 Hz, 1H), 4.62 (d, J = 14.2 Hz, 1H), 4.55 (d, J = 14.2 Hz, 1H), 3.80 (dd, J = 18.2 Hz, J = 11.8 Hz, 1H), 3.21 (dd, J = 18.2 Hz, J = 4.6 Hz, 1H), 2.36 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.07, 164.78, 154.73, 154.28, 134.01, 133.32, 131.40, 129.95, 129.34, 128.88, 126.05, 125.84, 122.57, 121.63, 112.72, 66.67, 55.62, 41.37, 22.21; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H19BrN3O4: 432.0559, found: 432.0549.
2-(2-(1-Acetyl-3-(4-bromophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14c)
White solid, yield: 55%, mp: 202–204 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.75 (s, 1H), 9.04 (s, 1H), 7.71–7.65 (m, 4H), 7.26–7.20 (m, 1H), 6.98 (d, J = 8.3 Hz, 1H), 6.91 (d, J = 4.5 Hz, 2H), 5.85 (dd, J = 11.8 Hz, J = 4.6 Hz, 1H), 4.62 (d, J = 14.2 Hz, 1H), 4.54 (d, J = 14.2 Hz, 1H), 3.81 (dd, J = 18.1 Hz, J = 11.8 Hz, 1H), 3.19 (dd, J = 18.1 Hz, J = 4.6 Hz, 1H), 2.35 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.96, 164.75, 154.73, 154.66, 132.23, 130.89, 129.99, 128.97, 128.86, 125.87, 124.09, 121.63, 112.71, 66.61, 55.59, 41.41, 22.19; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H19BrN3O4: 432.0559, found: 432.0563.
2-(2-(1-Acetyl-3-(2-methoxyphenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14d)
White solid, yield: 52%, mp: 170–172 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 9.04 (s, 1H), 7.81 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.45–7.41 (m, 1H), 7.24–7.20 (m, 1H), 7.10 (d, J = 8.4 Hz, 1H), 7.03–6.96 (m, 2H), 6.94–6.86 (m, 2H), 5.81 (dd, J = 11.7 Hz, J = 4.3 Hz, 1H), 4.62–4.54 (m, 2H), 3.88 (dd, J = 18.6 Hz, J = 11.7 Hz, 1H), 3.79 (s, 3H), 3.14 (dd, J = 18.6 Hz, J = 4.3 Hz, 1H), 2.32 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.82, 164.77, 158.45, 154.94, 154.64, 132.17, 130.42, 129.09, 128.72, 125.62, 121.68, 121.08, 120.57, 112.85, 112.62, 66.52, 56.18, 54.87, 44.94, 22.20; HRMS (AP-ESI) m/z [M+H]+ calcd for C20H22N3O5: 384.1559, found: 384.1552.
2-(2-(1-Acetyl-3-(3-methoxyphenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14e)
White solid, yield: 57%, mp: 170–172 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 9.06 (s, 1H), 7.40–7.32 (m, 2H), 7.28–7.20 (m, 2H), 7.06–6.98 (m, 2H), 6.94–6.89 (m, 2H), 5.85 (dd, J = 11.7 Hz, J = 4.4 Hz, 1H), 4.63 (d, J = 14.3 Hz, 1H), 4.56 (d, J = 14.3 Hz, 1H), 3.84–3.77 (m, 4H), 3.20 (dd, J = 18.1 Hz, J = 4.4 Hz, 1H), 2.36 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.90, 164.78, 159.83, 155.47, 154.70, 132.98, 130.37, 130.12, 128.82, 125.74, 121.65, 119.51, 116.56, 112.69, 112.03, 66.65, 55.68, 55.30, 41.65, 22.17; HRMS (AP-ESI) m/z [M+H]+ calcd for C20H22N3O5: 384.1559, found: 384.1552.
2-(2-(1-Acetyl-3-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14f)
White solid, yield: 55%, mp: 120–122 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 9.06 (s, 1H), 7.70 (d, J = 8.6 Hz, 2H), 7.25–7.19 (m, 1H), 7.02–6.97 (m, 3H), 6.93–6.85 (m, 2H), 5.83 (dd, J = 11.6 Hz, J = 4.2 Hz, 1H), 4.63 (d, J = 14.3 Hz, 1H), 4.56 (d, J =14.3 Hz, 1H), 3.79 (s, 3H), 3.78 (dd, J = 18.2 Hz, J = 11.6 Hz, 1H), 3.15 (dd, J = 18.2 Hz, J = 4.2 Hz, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.60, 164.79, 161.36, 155.35, 154.69, 130.23, 128.77, 128.71, 125.67, 124.12, 121.64, 114.65, 112.64, 66.62, 55.80, 55.04, 41.71, 22.17; HRMS (AP-ESI) m/z [M+H]+ calcd for C20H22N3O5: 384.1559, found: 384.1564.
2-(2-(1-Acetyl-3-(2-fluorophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14g)
White solid, yield: 50%, mp: 176–178 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.75 (s, 1H), 9.03 (s, 1H), 7.90–7.85 (m, 1H), 7.54–7.48 (m, 1H), 7.33–7.29 (m, 2H), 7.27–7.20 (m, 1H), 6.99–6.91 (m, 3H), 5.84 (dd, J = 11.8 Hz, J = 4.5 Hz, 1H), 4.62–4.53 (m, 2H), 3.93–3.85 (m, 1H), 3.20–3.13 (m, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.12, 164.79, 159.46, 154.73, 151.82, 132.70, 130.02, 129.53, 128.88, 125.83, 125.28, 121.66, 119.69, 117.18, 112.67, 66.51, 55.08, 43.74, 22.15; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H19FN3O4: 372.1360, found: 372.1365.
2-(2-(1-Acetyl-3-(2-chlorophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14h)
White solid, yield: 54%, mp: 166–168 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H), 9.02 (s, 1H), 7.72 (dd, J = 7.5 Hz, J = 2.1 Hz, 1H), 7.55 (dd, J = 7.7 Hz, J = 1.6 Hz, 1H), 7.48–7.40 (m, 2H), 7.26–7.22 (m, 1H), 6.99–6.91 (m, 3H), 5.84 (dd, J = 11.8 Hz, J = 4.5 Hz, 1H), 4.62–4.53 (m, 2H), 3.95 (dd, J = 18.1 Hz, J = 11.8 Hz, 1H), 3.21 (dd, J = 18.1 Hz, J = 4.5 Hz, 1H), 2.32 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.25, 164.80, 154.78, 154.25, 132.12, 131.64, 131.27, 131.17, 130.66, 129.90, 128.92, 127.90, 125.98, 121.68, 112.70, 66.53, 55.37, 44.25, 22.20; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H19ClN3O4: 388.1064, found: 388.1072.
2-(2-(1-Acetyl-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14i)
White solid, yield: 58%, mp: 174–176 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.92 (s, 1H), 9.02 (s, 1H), 8.04 (d, J = 7.8 Hz, 1H), 7.58–7.47 (m, 3H), 7.21–7.07 (m, 3H), 5.89 (dd, J = 12.0 Hz, J = 5.4 Hz, 1H), 4.64 (d, J = 13.2 Hz, 1H), 4.43 (d, J = 13.2 Hz, 1H), 3.94 (dd, J = 18.3 Hz, J = 12.0 Hz, 1H), 3.16 (dd, J = 18.3 Hz, J = 5.4 Hz, 1H), 2.31 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.05, 164.83, 156.19, 154.82, 137.70, 132.03, 130.45, 130.15, 129.88, 129.79, 128.82, 126.53, 125.79, 121.67, 112.70, 66.60, 54.37, 43.76, 23.37, 22.24; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H19IN3O4: 480.0420, found: 480.0418.
5-(2-(2-(Hydroxyamino)-2-oxoethoxy)phenyl)-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14j)
White solid, yield: 51%, mp: 164–166 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.93 (s, 1H), 9.01 (s, 1H), 8.00 (d, J = 7.9 Hz, 1H), 7.57–7.52 (m, 2H), 7.50–7.46 (m, 1H), 7.23–7.11 (m, 3H), 6.48 (s, 2H), 5.77 (dd, J = 12.2 Hz, J = 6.2 Hz, 1H), 4.64 (d, J = 13.1 Hz, 1H), 4.41 (d, J = 13.1 Hz, 1H), 3.92 (dd, J = 18.1 Hz, J = 12.2 Hz, 1H), 3.12 (dd, J = 18.1 Hz, J = 6.2 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.84, 155.54, 154.59, 153.22, 141.08, 136.17, 131.32, 131.27, 130.80, 128.77, 128.72, 126.20, 121.66, 112.44, 95.70, 66.56, 55.10, 44.46; HRMS (AP-ESI) m/z [M+H]+ calcd for C18H18IN4O4: 481.0373, found: 481.0371.
2-(2-(1-Acetyl-3-(o-tolyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14k)
White solid, yield: 50%, mp: 170–172 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.75 (s, 1H), 9.03 (s, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.33 (d, J = 4.3 Hz, 2H), 7.29–7.21 (m, 2H), 6.99–6.92 (m, 3H), 5.78 (dd, J = 11.7 Hz, J = 4.4 Hz, 1H), 4.64–4.54 (m, 2H), 3.88 (dd, J = 17.8 Hz, J = 11.7 Hz, 1H), 3.22 (dd, J = 17.8 Hz, J = 4.4 Hz, 1H), 2.61 (s, 3H), 2.33 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.05, 164.83, 156.19, 154.82, 137.70, 132.03, 130.45, 130.15, 129.88, 129.79, 128.82, 126.53, 125.79, 121.67, 112.70, 66.60, 54.37, 43.76, 23.37, 22.24; HRMS (AP-ESI) m/z [M+H]+ calcd for C20H22N3O4: 368.1610, found: 368.1607.
2-(2-(1-Acetyl-3-(2,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14l)
White solid, yield: 53%, mp: 128–130 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.67 (s, 1H), 8.95 (s, 1H), 7.68–7.64 (m, 2H), 7.43 (dd, J = 8.6 Hz, J = 2.1 Hz, 1H), 7.17–7.13 (m, 1H), 6.90–6.82 (m, 3H), 5.74 (dd, J = 11.7 Hz, J = 4.6 Hz, 1H), 4.52 (d, J = 14.2 Hz, 1H), 4.45 (d, J = 14.2 Hz, 1H), 3.86 (dd, J = 18.1 Hz, J = 11.7 Hz, 1H), 3.14 (dd, J = 18.1 Hz, J = 4.6 Hz, 1H), 2.24 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.28, 164.73, 154.78, 153.21, 135.30, 133.10, 132.40, 130.77, 129.76, 129.59, 128.93, 128.11, 126.10, 121.63, 112.70, 66.48, 55.57, 43.99, 22.20; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H18Cl2N3O4: 422.0674, found: 422.0675.
2-(2-(1-Acetyl-3-(2,6-dimethoxyphenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14m)
White solid, yield: 55%, mp: 178–180 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.70 (s, 1H), 8.98 (s, 1H), 7.36 (t, J = 8.4 Hz, 1H), 7.25–7.21 (m, 1H), 7.07 (d, J = 7.6 Hz, 1H), 7.01–6.95 (m, 2H), 6.71 (d, J = 8.4 Hz, 2H), 5.84 (dd, J = 11.7 Hz, J = 4.3 Hz, 1H), 4.62–4.53 (m, 2H), 3.73 (s, 3H), 3.66 (dd, J = 18.1 Hz, J = 11.7 Hz, 1H), 3.33 (s, 2H), 2.75 (dd, J = 18.1 Hz, J = 4.3 Hz, 1H), 2.21 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.69, 164.84, 158.58, 154.43, 153.17, 131.56, 130.84, 128.78, 125.93, 121.87, 112.46, 110.02, 104.78, 66.70, 56.45, 53.69, 45.71, 22.22; HRMS (AP-ESI) m/z [M+H]+ calcd for C21H24N3O6: 414.1665, found: 414.1661.
3-(2,6-Dimethoxyphenyl)-5-(2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14n)
White solid, yield: 52%, mp: 212–214 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H), 8.94 (s, 1H), 7.35 (t, J = 8.4 Hz, 1H), 7.24–7.17 (m, 2H), 7.00 (t, J = 7.5 Hz, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.70 (d, J = 8.5 Hz, 2H), 6.33 (s, 2H), 5.73 (dd, J = 11.9 Hz, J = 4.8 Hz, 1H), 4.61 (d, J = 14.4 Hz, 1H), 4.54 (d, J = 14.4 Hz, 1H), 3.73 (s, 6H), 3.63 (dd, J = 17.9 Hz, J = 11.9 Hz, 1H), 2.72 (dd, J = 17.9 Hz, J = 4.8 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.88, 158.65, 155.48, 154.25, 149.53, 132.23, 131.35, 128.59, 126.36, 121.80, 112.09, 110.34, 104.65, 66.56, 56.37, 53.38, 45.88; HRMS (AP-ESI) m/z [M+H]+ calcd for C20H23N4O6: 415.1618, found: 415.1622.
2-(2-(1-Acetyl-3-(2,6-dichlorophenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14o)
White solid, yield: 54%, mp: 174–176 °C. 1H NMR (600 MHz, DMSO-d6): δ 10.73 (s, 1H), 9.02 (s, 1H), 7.61–7.57 (m, 1H), 7.52 (t, J = 8.1 Hz, 1H), 7.29–7.20 (m, 1H), 7.04 (d, J = 7.7 Hz, 1H), 7.02–6.95 (m, 2H), 5.98 (dd, J = 11.9 Hz, J = 4.7 Hz, 1H), 4.60 (d, J = 14.2 Hz, 1H), 4.55 (d, J = 14.2 Hz, 1H), 3.82 (dd, J = 18.6 Hz, J = 11.9 Hz, 1H), 2.91 (dd, J = 18.6 Hz, J = 4.7 Hz, 1H), 2.28 (s, 3H); 13C NMR (150 MHz, DMSO-d6): δ 168.21, 164.73, 154.49, 153.23, 134.25, 132.58, 130.55, 129.99, 129.04, 128.91, 125.53, 121.70, 112.68, 66.72, 54.86, 44.88, 22.17; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H18Cl2N3O4: 422.0674, found: 422.0668.
2-(2-(1-Acetyl-3-(naphthalen-1-yl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxy acetamide (14p)
White solid, yield: 55%, mp: 198–200 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.81 (s, 1H), 9.25 (d, J = 8.7 Hz, 1H), 9.06 (s, 1H), 8.04–8.01 (m, 2H), 7.75 (d, J = 7.2 Hz, 1H), 7.72–7.68 (m, 1H), 7.63–7.60 (m, 1H), 7.55 (t, J = 7.7 Hz, 1H), 7.25 (td, J = 7.7 Hz, J = 1.8 Hz, 1H), 7.03–6.99 (m, 2H), 6.94 (t, J = 7.4 Hz, 1H), 5.86 (dd, J = 11.7 Hz, J = 4.3 Hz, 1H), 4.65 (d, J = 14.3 Hz, 1H), 4.58 (d, J = 14.3 Hz, 1H), 4.07 (dd, J = 17.8 Hz, J = 11.7 Hz, 1H), 3.42–3.38 (m, 1H), 2.45 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.05, 164.82, 155.98, 154.88, 134.09, 131.38, 130.32, 130.09, 129.41, 129.27, 128.84, 128.17, 127.90, 126.77, 125.89, 125.66, 121.66, 112.70, 66.58, 54.27, 44.11, 22.43; HRMS (AP-ESI) m/z [M+H]+ calcd for C23H22N3O4: 404.1610, found: 404.1605.
2-(2-(1-Acetyl-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazol-5-yl)-6-bromophenoxy)-N-hydroxyacetamide (14q)
White solid, yield: 50%, mp: 96–98 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.92 (s, 1H), 9.02 (s, 1H), 8.04 (d, J = 7.8 Hz, 1H), 7.58–7.47 (m, 3H), 7.21–7.07 (m, 3H), 5.89 (dd, J = 12.0 Hz, J = 5.4 Hz, 1H), 4.64 (d, J = 13.2 Hz, 1H), 4.43 (d, J = 13.2 Hz, 1H), 3.94 (dd, J = 18.3 Hz, J = 12.0 Hz, 1H), 3.16 (dd, J = 18.3 Hz, J = 5.4 Hz, 1H), 2.31 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.41, 164.17, 155.77, 151.90, 141.24, 138.41, 135.59, 132.92, 131.68, 130.96, 128.86, 127.46, 126.00, 116.95, 95.70, 70.77, 54.74, 44.92, 22.40; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H18BrIN3O4: 557.9525, found: 557.9528.
5-(3-Bromo-2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14r)
White solid, yield: 52%, mp: 140–142 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.93 (s, 1H), 9.01 (s, 1H), 8.00 (d, J = 7.9 Hz, 1H), 7.57–7.52 (m, 2H), 7.50–7.46 (m, 1H), 7.23–7.11 (m, 3H), 6.48 (s, 2H), 5.77 (dd, J = 12.2 Hz, J = 6.2 Hz, 1H), 4.64 (d, J = 13.1 Hz, 1H), 4.41 (d, J = 13.1 Hz, 1H), 3.92 (dd, J = 18.1 Hz, J = 12.2 Hz, 1H), 3.12 (dd, J = 18.1 Hz, J = 6.2 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.28, 155.45, 152.79, 151.72, 140.93, 139.58, 136.11, 132.66, 131.39, 130.94, 128.79, 127.34, 126.41, 116.93, 95.96, 70.81, 55.10, 45.27; HRMS (AP-ESI) m/z [M+H]+ calcd for C18H17BrIN4O4: 558.9478, found: 558.9482.
2-(2-(1-Acetyl-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazol-5-yl)-5-bromophenoxy)-N-hydroxyacetamide (14s)
White solid, yield: 54%, mp: 168–170 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H), 9.08 (s, 1H), 8.02 (d, J = 7.9 Hz, 1H), 7.54–7.43 (m, 2H), 7.19–7.10 (m, 3H), 6.96 (d, J = 8.2 Hz, 1H), 5.76 (dd, J = 11.8 Hz, J = 4.8 Hz, 1H), 4.68–4.58 (m, 2H), 3.88 (dd, J = 18.1 Hz, J = 11.8 Hz, 1H), 3.20 (dd, J = 18.1 Hz, J = 4.8 Hz, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.42, 164.43, 156.35, 155.72, 141.31, 135.66, 131.60, 130.81, 129.41, 128.83, 128.08, 124.45, 121.20, 116.03, 95.54, 66.71, 55.28, 43.69, 22.40; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H18BrIN3O4: 557.9525, found: 557.9520.
5-(4-Bromo-2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14t)
White solid, yield: 57%, mp: 196–198 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 9.04 (s, 1H), 7.99 (d, J = 7.9 Hz, 1H), 7.50–7.44 (m, 2H), 7.18–7.13 (m, 3H), 7.01 (d, J = 8.5 Hz, 1H), 6.49 (s, 2H), 5.68 (dd, J = 12.0 Hz, J = 5.4 Hz, 1H), 4.69–4.58 (m, 2H), 3.87 (dd, J = 18.0 Hz, J = 12.0 Hz, 1H), 3.13 (dd, J = 18.0 Hz, J = 5.4 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.48, 155.51, 155.48, 153.43, 141.00, 136.14, 131.32, 130.82, 130.79, 128.77, 128.04, 124.47, 120.96, 115.78, 95.80, 66.73, 55.06, 44.12; HRMS (AP-ESI) m/z [M+H]+ calcd for C18H17BrIN4O4: 558.9478, found: 558.9482.
2-(2-(1-Acetyl-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazol-5-yl)-4-bromophenoxy)-N-hydroxyacetamide (14u)
White solid, yield: 51%, mp: 106–108 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 9.04 (s, 1H), 8.03 (d, J = 7.9 Hz, 1H), 7.53–7.42 (m, 3H), 7.18 (t, J = 7.5 Hz, 1H), 7.11 (d, J = 2.5 Hz, 1H), 6.97 (d, J = 8.8 Hz, 1H), 5.79 (dd, J = 11.9 Hz, J = 4.9 Hz, 1H), 4.64–4.53 (m, 2H), 3.89 (dd, J = 18.2 Hz, J = 11.9 Hz, 1H), 3.23 (dd, J = 18.2 Hz, J = 4.9 Hz, 1H), 2.35 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 168.58, 164.46, 156.34, 154.25, 141.36, 135.52, 132.41, 131.65, 131.49, 130.85, 128.87, 115.21, 113.23, 95.47, 66.72, 55.30, 43.74, 22.41; HRMS (AP-ESI) m/z [M+H]+ calcd for C19H18BrIN3O4: 557.9525, found: 557.9522.
5-(5-Bromo-2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14v)
White solid, yield: 54%, mp: 202–204 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 9.02 (s, 1H), 8.00 (d, J = 7.9 Hz, 1H), 7.51–7.41 (m, 3H), 7.18–7.14 (m, 2H), 6.96 (d, J = 8.8 Hz, 1H), 6.53 (s, 2H), 5.71 (dd, J = 12.0 Hz, J = 5.4 Hz, 1H), 4.64–4.54 (m, 2H), 3.89 (dd, J = 18.0 Hz, J = 12.0 Hz, 1H), 3.15 (dd, J = 18.0 Hz, J = 5.4 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.48, 155.53, 154.04, 153.53, 141.04, 136.04, 133.76, 131.38, 131.23, 130.82, 128.81, 128.76, 115.01, 113.24, 95.72, 66.77, 55.16, 44.18; HRMS (AP-ESI) m/z [M+H]+ calcd for C18H17BrIN4O4: 558.9478, found: 558.9472.
2-(2-(1-Acetyl-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazol-5-yl)-3-bromophenoxy)-N-hydroxyacetamide (14w)
White solid, yield: 55%, mp: 152–154 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.65 (s, 1H), 8.98 (s, 1H), 8.08–8.02 (m, 1H), 7.61–7.59 (m, 1H), 7.53–7.45 (m, 1H), 7.24–7.06 (m, 3H), 6.93–6.82 (m, 1H), 6.28–5.86 (m, 1H), 4.66–4.45 (m, 2H), 3.93–3.73 (m, 1H), 3.61–3.23 (m, 1H), 2.26–2.20 (m, 3H); HRMS (AP-ESI) m/z [M+H]+ calcd for C19H18BrIN3O4: 557.9525, found: 557.9520.
5-(2-Bromo-6-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14x)
White solid, yield: 59%, mp: 132–134 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.62 (m, 1H), 8.99 (s, 1H), 8.04–8.00 (m, 1H), 7.61–7.57 (m, 1H), 7.51–7.44 (m, 1H), 7.28–6.93 (m, 4H), 6.21–5.79 (m, 3H), 4.72–4.43 (m, 2H), 3.88–3.70 (m, 1H), 3.64–3.28 (m, 1H); HRMS (AP-ESI) m/z [M+H]+ calcd for C18H17BrIN4O4: 558.9478, found: 558.9484.
2-(2-(1-Acetyl-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazol-5-yl)-3-chlorophenoxy)-N-hydroxyacetamide (14y)
White solid, yield: 56%, mp: 168–170 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.68–10.63 (m, 1H), 9.01 (s, 1H), 8.08–8.03 (m, 1H), 7.61–7.46 (m, 2H), 7.30–7.15 (m, 2H), 7.08–6.88 (m, 2H), 6.26–5.89 (m, 1H), 4.68–4.45 (m, 2H), 3.95–3.73 (m, 1H), 3.64–3.24 (m, 1H), 2.25–2.21 (m, 3H); HRMS (AP-ESI) m/z [M+H]+ calcd for C19H18ClIN3O4: 514.0031, found: 514.0035.
5-(2-Chloro-6-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-3-(2-iodophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14z)
White solid, yield: 57%, mp: 190–192 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.62 (s, 1H), 8.97 (s, 1H), 8.04–8.00 (m, 1H), 7.59–7.56 (m, 1H), 7.51–7.44 (m, 1H), 7.28–7.00 (m, 4H), 6.19–5.83 (m, 3H), 4.73–4.44 (m, 2H), 3.90–3.70 (m, 1H), 3.67–3.28 (m, 1H); HRMS (AP-ESI) m/z [M+H]+ calcd for C18H17ClIN4O4: 514.9983, found: 514.9987.
2-(2-(1-Acetyl-3-(2-(benzyloxy)phenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14aa)
White solid, yield: 55%, mp: 162–164 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H), 9.02 (s, 1H), 7.77 (d, J = 7.8 Hz, 1H), 7.44–7.39 (m, 3H), 7.36–7.28 (m, 3H), 7.24–7.20 (m, 2H), 7.02 (t, J = 7.5 Hz, 1H), 6.95 (d, J = 8.3 Hz, 1H), 6.92–6.86 (m, 2H), 5.79 (dd, J = 11.6 Hz, J = 4.2 Hz, 1H), 5.15 (s, 2H), 4.57 (d, J = 14.2 Hz, 1H), 4.47 (d, J = 14.2 Hz, 1H), 3.82 (dd, J = 18.5 Hz, J = 11.6 Hz, 1H), 3.18 (dd, J = 18.5 Hz, J = 4.2 Hz, 1H), 2.29 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.88, 164.76, 157.33, 154.92, 154.61, 136.97, 132.05, 130.28, 129.54, 128.90, 128.76, 128.34, 128.14, 125.58, 121.66, 121.32, 121.01, 114.02, 112.57, 70.37, 66.53, 54.82, 44.91, 22.19; HRMS (AP-ESI) m/z [M+H]+ calcd for C26H26N3O5: 460.1872, found: 460.1868.
3-(2-(Benzyloxy)phenyl)-5-(2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14bb)
White solid, yield: 52%, mp: 176–178 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.75 (s, 1H), 8.99 (s, 1H), 7.88 (d, J = 7.7 Hz, 1H), 7.40–7.28 (m, 6H), 7.22–7.17 (m, 2H), 7.02–6.88 (m, 4H), 6.47 (s, 2H), 5.70 (dd, J = 11.9 Hz, J = 4.8 Hz, 1H), 5.17–5.10 (m, 2H), 4.58 (d, J = 14.4 Hz, 1H), 4.47 (d, J = 14.4 Hz, 1H), 3.80 (dd, J = 18.2 Hz, J = 11.9 Hz, 1H), 3.15 (dd, J = 18.2 Hz, J = 4.8 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.79, 157.09, 155.48, 154.40, 151.32, 137.04, 131.75, 131.55, 129.58, 128.92, 128.55, 128.35, 128.15, 125.74, 121.64, 121.34, 121.23, 113.93, 112.29, 70.34, 66.48, 54.54, 45.22; HRMS (AP-ESI) m/z [M+H]+ calcd for C25H25N4O5: 461.1825, found: 461.1830.
2-(2-(1-Acetyl-3-(2-((2-bromobenzyl)oxy)phenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14cc)
White solid, yield: 53%, mp: 82–84 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.68 (s, 1H), 9.04 (s, 1H), 7.79 (d, J = 7.7 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 7.6 Hz, 1H), 7.47–7.36 (m, 2H), 7.30 (t, J = 7.6 Hz, 1H), 7.23–7.19 (m, 2H), 7.06 (t, J = 7.5 Hz, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.90–6.84 (m, 2H), 5.79 (dd, J = 11.7 Hz, J = 4.2 Hz, 1H), 5.20–5.12 (m, 2H), 4.56 (d, J = 14.3 Hz, 1H), 4.45 (d, J = 14.3 Hz, 1H), 3.78 (dd, J = 18.5 Hz, J = 11.7 Hz, 1H), 3.16 (dd, J = 18.5 Hz, J = 4.2 Hz, 1H), 2.29 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.82, 164.65, 157.14, 154.73, 154.54, 135.84, 133.12, 132.10, 130.92, 130.70, 130.18, 129.63, 128.70, 128.34, 125.51, 123.33, 121.59, 121.00, 113.91, 112.49, 70.31, 66.51, 54.73, 44.75, 22.15; HRMS (AP-ESI) m/z [M+H]+ calcd for C26H25BrN3O5: 538.0978, found: 538.0982.
3-(2-((2-Bromobenzyl)oxy)phenyl)-5-(2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14dd)
White solid, yield: 56%, mp: 92–94 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.67 (s, 1H), 9.03 (s, 1H), 7.90 (d, J = 7.8 Hz, 1H), 7.64 (d, J = 7.8 Hz, 1H), 7.55 (d, J = 7.5 Hz, 1H), 7.43–7.27 (m, 3H), 7.22–7.17 (m, 2H), 7.04 (t, J = 7.5 Hz, 1H), 6.94–6.87 (m, 3H), 6.49 (s, 2H), 5.70 (dd, J = 11.8 Hz, J = 4.8 Hz, 1H), 5.19–5.11 (m, 2H), 4.57 (d, J = 14.4 Hz, 1H), 4.45 (d, J = 14.4 Hz, 1H), 3.75 (dd, J = 18.4 Hz, J = 11.8 Hz, 1H), 3.13 (dd, J = 18.4 Hz, J = 4.8 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.68, 156.92, 155.41, 154.33, 151.15, 135.87, 133.14, 131.64, 131.60, 130.94, 130.72, 129.63, 128.51, 128.36, 125.69, 123.38, 121.57, 121.48, 121.34, 113.79, 112.19, 70.28, 66.44, 54.46, 45.04; HRMS (AP-ESI) m/z [M+H]+ calcd for C25H24BrN4O5: 539.0930, found: 539.0934.
2-(2-(1-Acetyl-3-(2-((3-bromobenzyl)oxy)phenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14ee)
White solid, yield: 57%, mp: 164–166 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.73 (s, 1H), 9.01 (s, 1H), 7.74 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.66 (d, J = 2.2 Hz, 1H), 7.51 (d, J = 8.6 Hz, 1H), 7.43 (t, J = 7.4 Hz, 2H), 7.31 (t, J = 7.8 Hz, 1H), 7.23–7.18 (m, 2H), 7.03 (t, J = 7.5 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 6.89 (d, J = 7.4 Hz, 1H), 5.79 (dd, J = 11.6 Hz, J = 4.2 Hz, 1H), 5.17 (s, 2H), 4.58 (d, J = 14.4 Hz, 1H), 4.50 (d, J = 14.4 Hz, 1H), 3.85 (dd, J = 18.4 Hz, J = 11.6 Hz, 1H), 3.17 (dd, J = 18.4 Hz, J = 4.2 Hz, 1H), 2.29 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.87, 164.78, 157.01, 154.69, 154.63, 139.89, 132.01, 131.18, 131.14, 130.75, 130.24, 129.69, 128.77, 127.07, 125.61, 122.11, 121.65, 121.49, 121.06, 114.03, 112.57, 69.40, 66.51, 54.74, 44.81, 22.20; HRMS (AP-ESI) m/z [M+H]+ calcd for C26H25BrN3O5: 538.0978, found: 538.0986.
3-(2-((3-Bromobenzyl)oxy)phenyl)-5-(2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14ff)
White solid, yield: 59%, mp: 120–122 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H), 8.98 (s, 1H), 7.87 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.62 (t, J = 1.8 Hz, 1H), 7.51–7.49 (m, 1H), 7.41–7.36 (m, 2H), 7.30 (t, J = 7.8 Hz, 1H), 7.22–7.14 (m, 2H), 7.03–6.88 (m, 4H), 6.46 (s, 2H), 5.70 (dd, J = 11.9 Hz, J = 4.8 Hz, 1H), 5.16 (s, 2H), 4.59 (d, J = 14.4 Hz, 1H), 4.50 (d, J = 14.4 Hz, 1H), 3.81 (dd, J = 18.3 Hz, J = 11.9 Hz, 1H), 3.14 (dd, J = 18.3 Hz, J = 4.8 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.79, 156.81, 155.46, 154.40, 151.18, 139.92, 131.72, 131.54, 131.18, 130.80, 129.64, 128.56, 127.10, 125.77, 122.10, 121.64, 121.41, 113.97, 112.28, 69.36, 66.45, 54.53, 45.19; HRMS (AP-ESI) m/z [M+H]+ calcd for C25H24BrN4O5: 539.0930, found: 539.0936.
2-(2-(1-Acetyl-3-(2-((4-bromobenzyl)oxy)phenyl)-4,5-dihydro-1H-pyrazol-5-yl)phenoxy)-N-hydroxyacetamide (14gg)
White solid, yield: 53%, mp: 96–98 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.75 (s, 1H), 9.02 (s, 1H), 7.74 (dd, J = 7.7 Hz, J = 1.8 Hz, 1H), 7.55–7.52 (m, 2H), 7.44–7.36 (m, 3H), 7.24–7.17 (m, 2H), 7.03 (t, J = 7.5 Hz, 1H), 6.95 (d, J = 8.3 Hz, 1H), 6.91–6.85 (m, 2H), 5.78 (dd, J = 11.6 Hz, J = 4.2 Hz, 1H), 5.13 (d, J = 1.8 Hz, 2H), 4.58 (d, J = 14.2 Hz, 1H), 4.50 (d, J = 14.2 Hz, 1H), 3.82 (dd, J = 18.4 Hz, J = 11.6 Hz, 1H), 3.18 (dd, J = 18.4 Hz, J = 4.2 Hz, 1H), 2.29 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 167.86, 164.78, 157.11, 154.95, 154.64, 136.45, 132.03, 131.84, 130.37, 130.23, 129.62, 128.74, 125.63, 121.64, 121.54, 121.44, 121.10, 114.01, 112.54, 69.57, 66.48, 54.85, 44.81, 22.19; HRMS (AP-ESI) m/z [M+H]+ calcd for C26H25BrN3O5: 538.0978, found: 538.0980.
3-(2-((4-Bromobenzyl)oxy)phenyl)-5-(2-(2-(hydroxyamino)-2-oxoethoxy)phenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (14hh)
White solid, yield: 55%, mp: 96–98 °C. 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H), 8.99 (s, 1H), 7.85 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.53 (d, J = 8.3 Hz, 2H), 7.40–7.35 (m, 3H), 7.23–7.14 (m, 2H), 7.02–6.88 (m, 4H), 6.47 (s, 2H), 5.69 (dd, J = 11.9 Hz, J = 4.7 Hz, 1H), 5.16–5.09 (m, 2H), 4.59 (d, J = 14.4 Hz, 1H), 4.50 (d, J = 14.4 Hz, 1H), 3.80 (dd, J = 18.3 Hz, J = 11.9 Hz, 1H), 3.14 (dd, J = 18.3 Hz, J = 4.7 Hz, 1H); 13C NMR (100 MHz, DMSO-d6): δ 164.80, 156.86, 155.44, 154.42, 151.32, 136.53, 131.85, 131.68, 131.52, 130.36, 129.63, 128.54, 125.76, 121.61, 121.53, 121.44, 121.34, 113.95, 112.25, 69.51, 66.43, 54.58, 45.12; HRMS (AP-ESI) m/z [M+H]+ calcd for C25H24BrN4O5: 539.0930, found: 539.0938.