Antimicrobial Activity of Smilax china L. Root Extracts against the Acne-Causing Bacterium, Cutibacterium acnes, and Its Active Compounds
Abstract
1. Introduction
2. Results
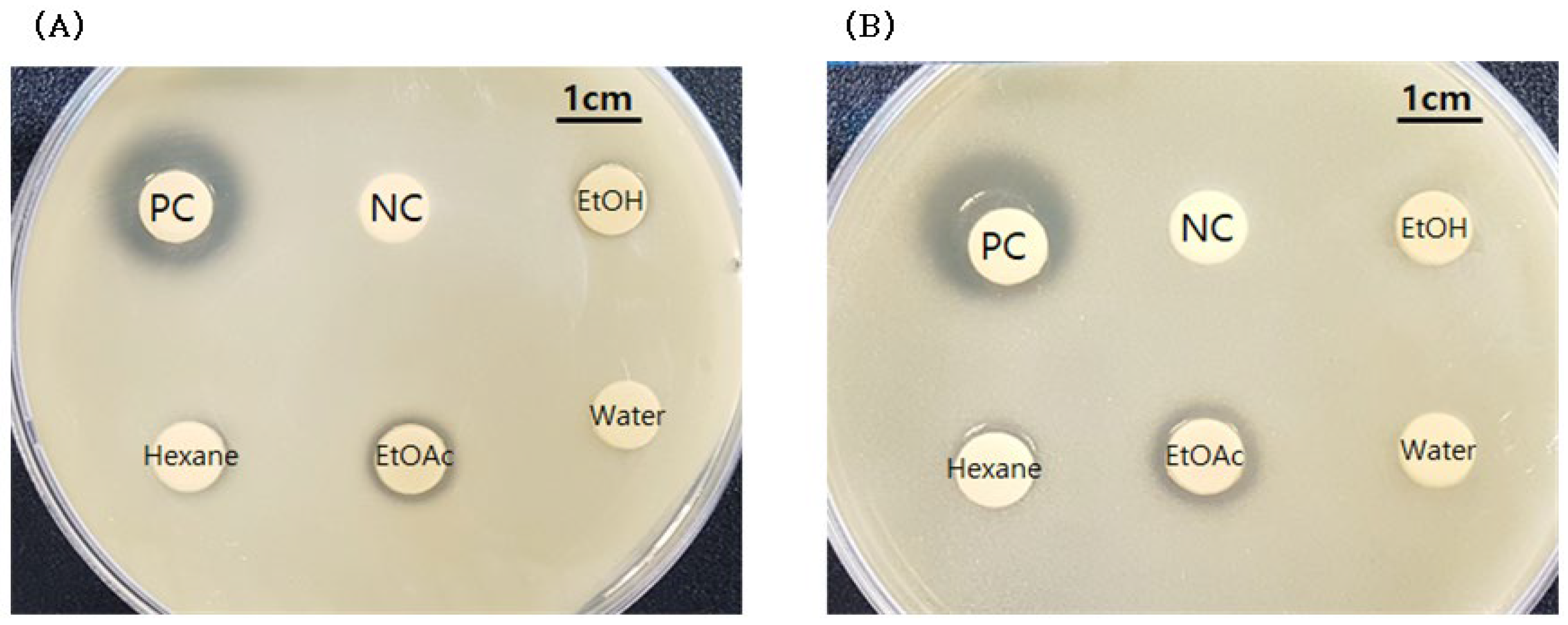
2.1. Antimicrobial Activity of Extract of Smilax china L. Root
2.2. Analysis for Chemical Compositions of the Ethyl Acetate Fraction from Smilix china L. Root Crude Extract
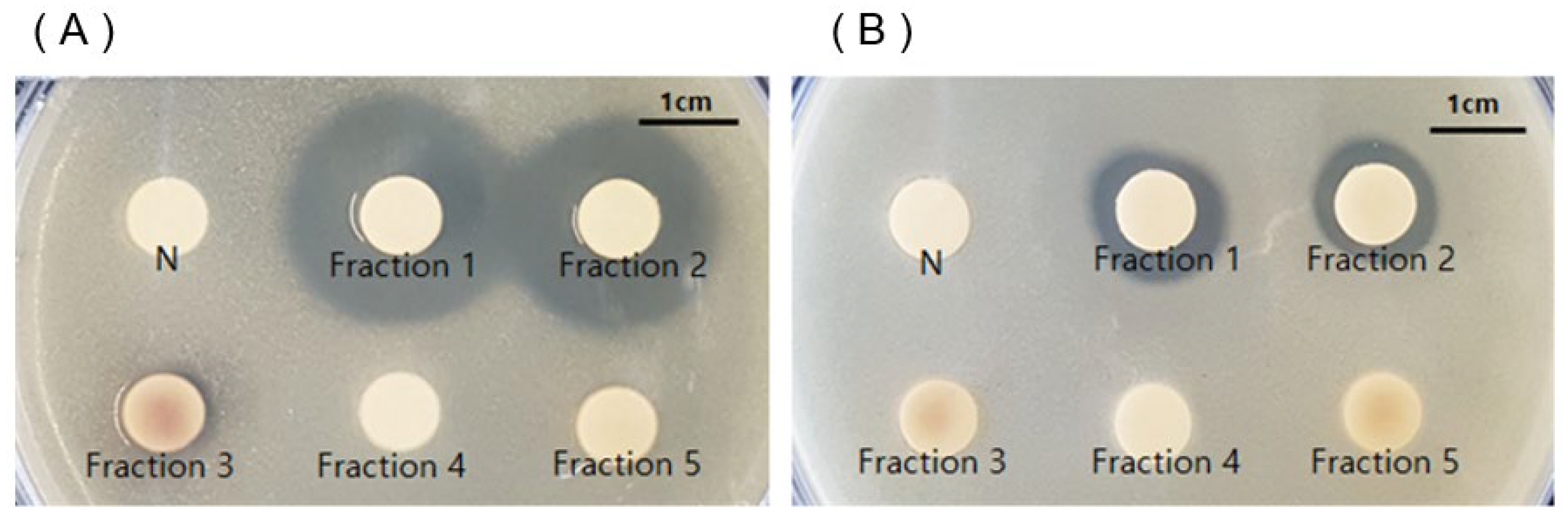
2.3. Antimicrobial Effect against Cutibacterium acnes of Fractions 1–5 Obtained from Silica Gel Column Chromatography
2.4. Antimicrobial Effect of Isolated Compounds (Quercetin, Resveratrol, and Oxyresveratrol) against Cutibacterium acnes
2.5. Cytotoxicity of Compounds 3~5, 11, 13, 17, and 30
3. Discussion
4. Materials and Methods
4.1. Materials
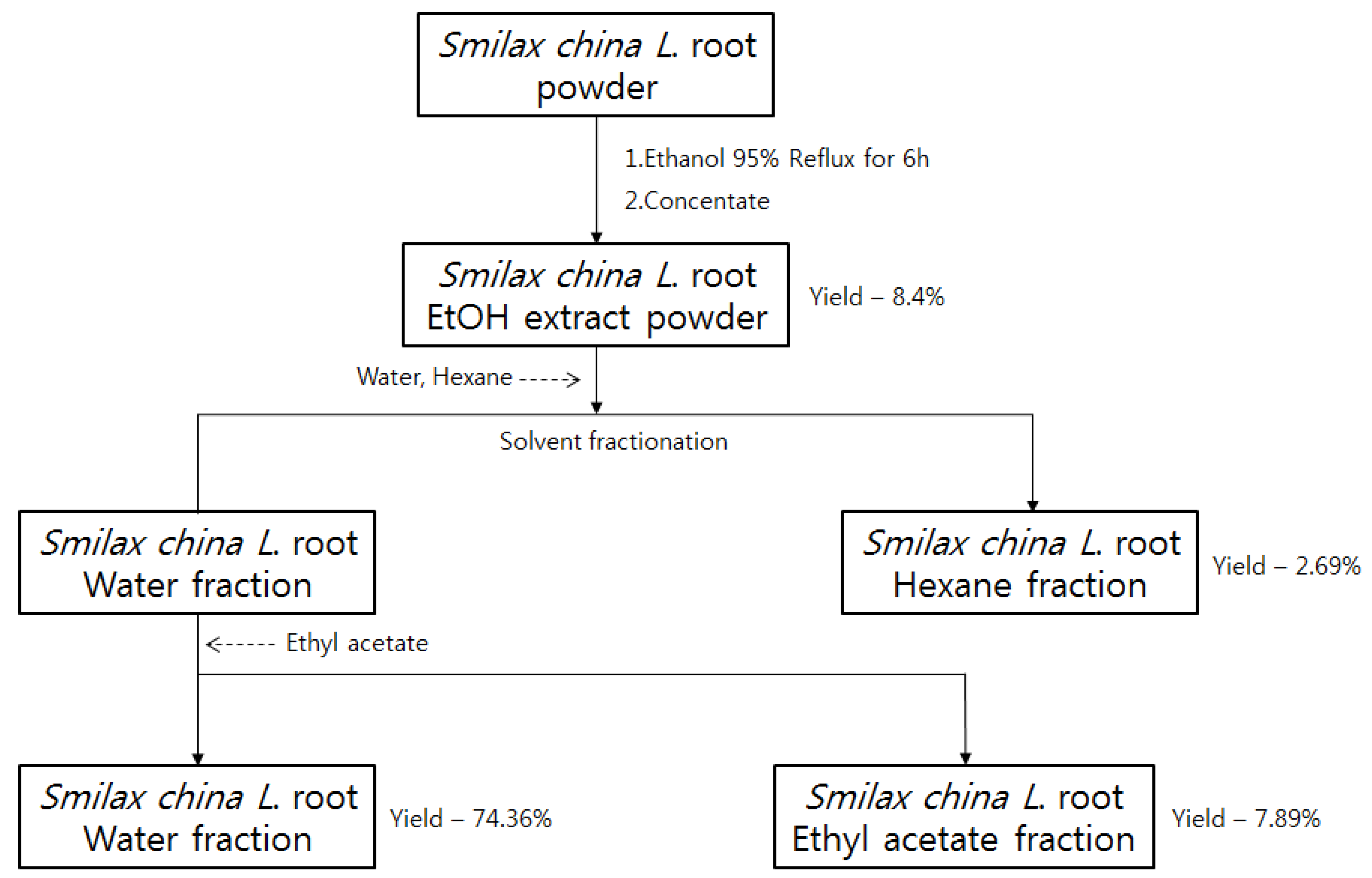
4.2. Preparation of Extract of Smilax china L. Root
4.3. High-Resolution UPLC/QTOF/MS Analysis
4.4. HPLC Analysis
4.5. Preliminary Antimicrobial Activity
4.6. Determination of Minimum Inhibitory Concentrations and Minimum Bactericidal Concentration
4.7. Cell Viability Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dreno, B.; Pecastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32 (Suppl. S2), 5–14. [Google Scholar] [CrossRef]
- Aslam, I.; Fleischer, A.; Feldman, S. Emerging drugs for the treatment of acne. Expert Opin. Emerg. Drugs 2015, 20, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R.P. Acne vulgaris: Pathogenesis, treatment, and needs assessment. Dermatol. Clin. 2012, 30, 99–106. [Google Scholar] [CrossRef]
- Ross, J.I.; Snelling, A.M.; Carnegie, E.; Coates, P.; Cunliffe, W.J.; Bettoli, V.; Tosti, G.; Katsambas, A.; Galvan Perez Del Pulgar, J.I.; Rollman, O.; et al. Antibiotic-resistant acne: Lessons from Europe. Br. J. Dermatol. 2003, 148, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Habeshian, K.A.; Cohen, B.A. Current Issues in the Treatment of Acne Vulgaris. Pediatrics 2020, 145, S225–S230. [Google Scholar] [CrossRef]
- Sevimli Dikicier, B. Topical treatment of acne vulgaris: Efficiency, side effects, and adherence rate. J. Int. Med. Res. 2019, 47, 2987–2992. [Google Scholar] [CrossRef]
- Leyden, J.J.; Wortzman, M.; Baldwin, E.K. Antibiotic-resistant Propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis 2008, 82, 417–421. [Google Scholar] [PubMed]
- Lubtikulthum, P.; Kamanamool, N.; Udompataikul, M. A comparative study on the effectiveness of herbal extracts vs 2.5% benzoyl peroxide in the treatment of mild to moderate acne vulgaris. J. Cosmet. Dermatol. 2019, 18, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Keating, G.M. Clindamycin/benzoyl peroxide gel (BenzaClin): A review of its use in the management of acne. Am. J. Clin. Dermatol. 2008, 9, 193–204. [Google Scholar] [CrossRef]
- Emrich, S.; Schuster, A.; Schnabel, T.; Oostingh, G.J. Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts. Molecules 2022, 27, 2817. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.; Yoo, S.; Hong, Y.H.; Han, S.Y.; Jeong, S.; Jeong, D.; Kim, J.H.; Cho, J.Y.; Park, J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J. Ginseng. Res. 2018, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xue, H.; Li, X.; Zhao, Y.; Lin, L.; Yang, L.; Zheng, G. Chemical composition, antibacterial properties, and mechanism of Smilax china L. polyphenols. Appl. Microbiol. Biotechnol. 2019, 103, 9013–9022. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, N.; Xiong, L.; Sun, D.; Chen, Z.; Chen, L. A new phenylpropanoid-substituted epicatechin from the rhizome of Smilax china. Nat. Prod. Res. 2022, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, T.S.; Yang, S.H.; Suh, J.W.; Shim, S.M. Microbial bioconversion and processing methods enhance the phenolic acid and flavonoids and the radical scavenging capacity of Smilax china L. leaf. J. Sci. Food Agric. 2016, 96, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Guo, H.Z.; Cui, Y.J.; Liu, A.H.; Yu, H.L.; Guo, H.; Xu, M.; Guo, D.A. Simultaneous determination of six major stilbenes and flavonoids in Smilax china by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 44, 737–742. [Google Scholar] [CrossRef]
- Sashida, Y.; Kubo, S.; Mimaki, Y.; Nikaido, T.; Ohmoto, T. Steroidal saponins from Smilax riparia and S. china. Phytochemistry 1992, 31, 2439–2443. [Google Scholar] [CrossRef]
- Shao, B.; Guo, H.; Cui, Y.; Ye, M.; Han, J.; Guo, D. Steroidal saponins from Smilax china and their anti-inflammatory activities. Phytochemistry 2007, 68, 623–630. [Google Scholar] [CrossRef]
- Tian, L.W.; Zhang, Z.; Long, H.L.; Zhang, Y.J. Steroidal Saponins from the Genus Smilax and Their Biological Activities. Nat. Prod. Bioprospect 2017, 7, 283–298. [Google Scholar] [CrossRef]
- Feng, H.; He, Y.; La, L.; Hou, C.; Song, L.; Yang, Q.; Wu, F.; Liu, W.; Hou, L.; Li, Y.; et al. The flavonoid-enriched extract from the root of Smilax china L. inhibits inflammatory responses via the TLR-4-mediated signaling pathway. J. Ethnopharmacol. 2020, 256, 112785. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Chen, G.; Lu, Z.; Wang, X.; Sha, N.; Shao, B.; Li, P.; Guo, D.A. High performance liquid chromatographic method for the determination and pharmacokinetic studies of oxyresveratrol and resveratrol in rat plasma after oral administration of Smilax china extract. Biomed. Chromatogr. 2008, 22, 421–427. [Google Scholar] [CrossRef]
- Jiang, S.; Wei, Q.; Ye, X.; Luo, D.; Zhang, X.; Li, Z.; You, P.; Huang, X.; Liu, Y. The Anti-Inflammatory Effect of Smilax china L. Extract on LPS-Stimulated THP-1 via Downregulation of MAPK and NF-kappaB Signaling Pathway. Evid. Based Complement Altern. Med. 2021, 2021, 9958808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Song, X.Y.; Zhang, D.D.; Li, Z.L.; Yang, Y.Y.; Luo, X.Y.; Ye, X.C. Spectrum-effect relationship between UPLC fingerprint of Smilax china and anti-pelvic inflammation in rats. Zhongguo Zhong Yao Za Zhi 2019, 44, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, W.; Liu, S.; Zhong, Z.; Zheng, G. Quercetin, Engelitin and Caffeic Acid of Smilax china L. Polyphenols, Stimulate 3T3-L1 Adipocytes to Brown-like Adipocytes Via beta3-AR/AMPK Signaling Pathway. Plant Foods Hum. Nutr. 2022, 77, 529–537. [Google Scholar] [CrossRef]
- Lee, H.E.; Kim, J.A.; Whang, W.K. Chemical Constituents of Smilax china L. Stems and Their Inhibitory Activities against Glycation, Aldose Reductase, alpha-Glucosidase, and Lipase. Molecules 2017, 22, 451. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.S.; Gao, Z.H.; Yang, X.L. Supercritical fluid extraction of sapogenins from tubers of Smilax china. Fitoterapia 2004, 75, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Hsu, Y.W.; Liaw, C.C.; Lee, J.K.; Huang, H.C.; Kuo, L.M. Cytotoxic phenylpropanoid glycosides from the stems of Smilax china. J. Nat. Prod. 2005, 68, 1475–1478. [Google Scholar] [CrossRef]
- Fan, E.; Zhang, K.; Jiang, S.; Yan, C.; Bai, Y. Analysis of trans-resveratrol in grapes by micro-high performance liquid chromatography. Anal. Sci. 2008, 24, 1019–1023. [Google Scholar] [CrossRef][Green Version]
- Gescher, A.J. Resveratrol from red grapes—Pedestrian polyphenol or useful anticancer agent? Planta Med. 2008, 74, 1651–1655. [Google Scholar] [CrossRef]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The Potential of Systems Biology to Discover Antibacterial Mechanisms of Plant Phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Yang, J.M.; Moon, G.S. Partial Characterization of an Anti-Listerial Bacteriocin from Enterococcus faecium CJNU. Food Sci. Anim. Res. 2021, 41, 164–171. [Google Scholar] [CrossRef]
- Teh, C.H.; Nazni, W.A.; Nurulhusna, A.H.; Norazah, A.; Lee, H.L. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 2017, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.A.; Mohammed, S.A.A.; Khan, O.; Ali, H.M.; El-Readi, M.Z.; Mohammed, H.A. Cinnamaldehyde-Based Self-Nanoemulsion (CA-SNEDDS) Accelerates Wound Healing and Exerts Antimicrobial, Antioxidant, and Anti-Inflammatory Effects in Rats’ Skin Burn Model. Molecules 2022, 27, 5225. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Al-Omar, M.S.; Khan, R.A.; Mohammed, S.A.A.; Qureshi, K.A.; Abbas, M.M.; Al Rugaie, O.; Abd-Elmoniem, E.; Ahmad, A.M.; Kandil, Y.I. Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert. Plants 2021, 10, 1811. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Ali, H.M.; Qureshi, K.A.; Alsharidah, M.; Kandil, Y.I.; Said, R.; Mohammed, S.A.A.; Al-Omar, M.S.; Rugaie, O.A.; Abdellatif, A.A.H.; et al. Comparative Phytochemical Profile and Biological Activity of Four Major Medicinal Halophytes from Qassim Flora. Plants 2021, 10, 2208. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.J.; Jung, K.H.; Moon, G.S.; Moon, S.K.; Lee, H.Y. A facile and efficient method for the synthesis of crystalline tetrahydro-beta-carbolines via the Pictet-Spengler reaction in water. Sci. Rep. 2020, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.C.; Belov, L.; Davey, M.W.; Davey, R.A.; Kidman, A.D. The MTT cell viability assay for cytotoxicity testing in multidrug-resistant human leukemic cells. Leuk. Res. 1992, 16, 1165–1173. [Google Scholar] [CrossRef]
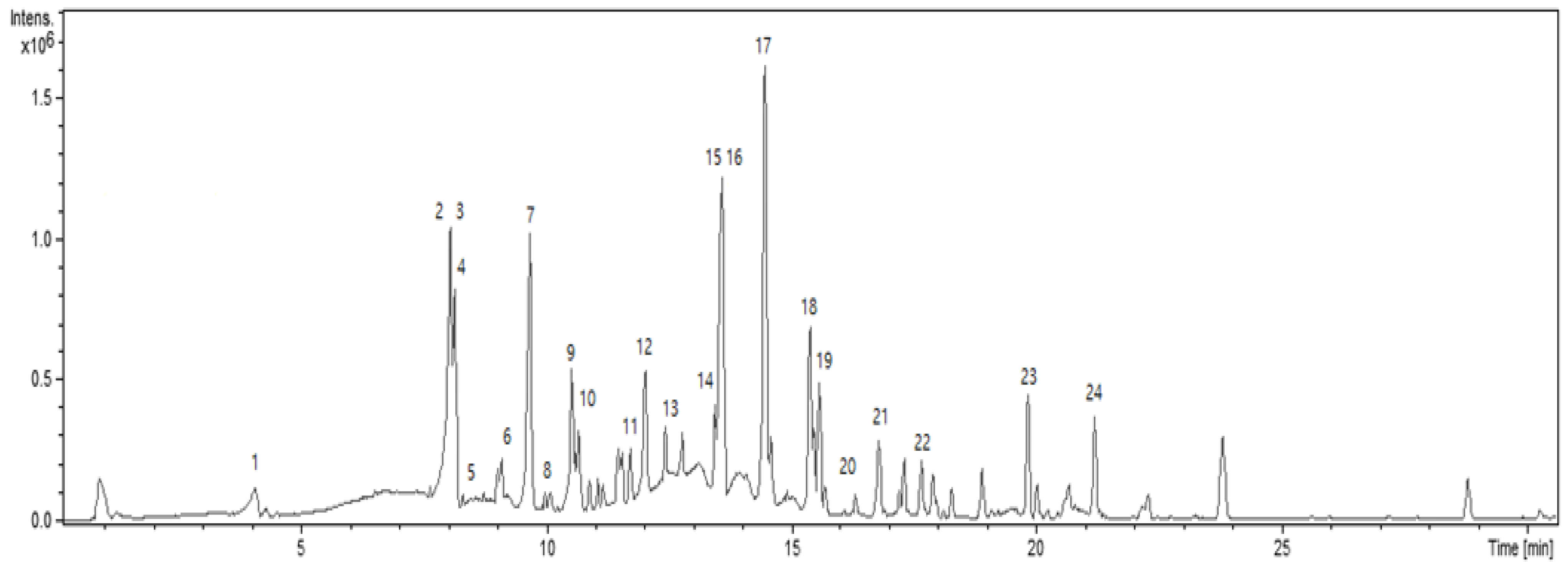


| No. | RT (min) | Compound Name | Formula | Detected Mass(M-H) * | Theoretical Exact Mass(M-H) |
|---|---|---|---|---|---|
| Phenols | |||||
| 1 | 4.3 | 1,2-Benzenediol | C6H6O2 | 109.0294 | 109.0290 |
| Acids | |||||
| 2 | 8.1 | Chlorogenic acid | C16H18O9 | 353.0878 | 353.0873 |
| 4 | 8.2 | Quinic acid | C7H12O6 | 191.0560 | 191.0556 |
| 5 | 8.3 | Caffeic acid | C9H8O4 | 179.0352 | 179.0350 |
| Flavonoids | |||||
| 3 | 8.1 | Catechin | C15H14O6 | 289.0719 | 289.0712 |
| 6 | 9.5 | Afzelechin | C15H14O5 | 273.0768 | 273.0763 |
| 7 | 9.7 | Leucopelargonidin | C15H14O6 | 289.0714 | 289.0712 |
| 8 | 9.9 | Astilbin | C21H22O11 | 449.1078 | 449.1084 |
| 10 | 10.7 | Procyanidin B3 | C30H26O12 | 577.1359 | 577.1346 |
| 14 | 13.4 | Kaempferol 7-O-β-D-glucopyranoside | C21H20O11 | 447.0938 | 447.0927 |
| 12, 15, 18 | 12.0, 13.6, 15.4 | Cinchonain Ia~c | C24H20O9 | 451.1040 | 451.1029 |
| 16 | 13.7 | Engeletin | C21H22O10 | 433.1129 | 433.1135 |
| 20 | 16.2 | Quercetin | C15H10O7 | 301.0365 | 301.0354 |
| Stillbenoids | |||||
| 11 | 11.7 | Polydatin | C20H22O8 | 389.1248 | 389.1236 |
| 13 | 12.4 | Oxyresveratrol | C14H12O4 | 243.0665 | 243.0657 |
| 17 | 14.5 | Resveratrol | C14H12O3 | 227.0715 | 227.0708 |
| 21 | 16.8 | Scirpusin A | C28H22O7 | 469.1281 | 469.1287 |
| Phenylpropanoids | |||||
| 9 | 10.5 | Cinchonain IIa | C39H32O15 | 739.1670 | 739.1663 |
| 19 | 15.6 | Helonioside A | C32H38O17 | 693.2051 | 693.2031 |
| 22 | 17.7 | Smilaside A | C36H42O19 | 777.2268 | 777.2242 |
| 23 | 19.8 | Smilaside C | C41H44O19 | 839.2405 | 839.2399 |
| 24 | 21.2 | Smilaside D or E | C43H46O20 | 881.2525 | 881.2504 |
| No. | Compounds | MIC (μg/mL) |
|---|---|---|
| 13 | Oxyresveratrol | 250 |
| 17 | Resveratrol | 125 |
| 20 | Quercetin | 31.25 |
| Clindamycin 1 | 0.0625 |
| No. | Compounds | MBC (μg/mL) |
|---|---|---|
| 13 | Oxyresveratrol | 250 |
| 17 | Resveratrol | 125 |
| 20 | Quercetin | 31.25 |
| Clindamycin 1 | 0.0625 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, J.-H.; Han, M.-H.; Kim, J.-I.; Kim, J.-E.; Jung, K.-H.; Oh, H.S.; Chung, Y.S.; An, H.J.; Lee, J.D.; Moon, G.-S.; et al. Antimicrobial Activity of Smilax china L. Root Extracts against the Acne-Causing Bacterium, Cutibacterium acnes, and Its Active Compounds. Molecules 2022, 27, 8331. https://doi.org/10.3390/molecules27238331
Joo J-H, Han M-H, Kim J-I, Kim J-E, Jung K-H, Oh HS, Chung YS, An HJ, Lee JD, Moon G-S, et al. Antimicrobial Activity of Smilax china L. Root Extracts against the Acne-Causing Bacterium, Cutibacterium acnes, and Its Active Compounds. Molecules. 2022; 27(23):8331. https://doi.org/10.3390/molecules27238331
Chicago/Turabian StyleJoo, Ji-Hae, Min-Hui Han, Ja-I Kim, Jong-Eun Kim, Kyung-Hwan Jung, Han Sun Oh, Young Soo Chung, Hyun Jin An, Jae Duk Lee, Gi-Seong Moon, and et al. 2022. "Antimicrobial Activity of Smilax china L. Root Extracts against the Acne-Causing Bacterium, Cutibacterium acnes, and Its Active Compounds" Molecules 27, no. 23: 8331. https://doi.org/10.3390/molecules27238331
APA StyleJoo, J.-H., Han, M.-H., Kim, J.-I., Kim, J.-E., Jung, K.-H., Oh, H. S., Chung, Y. S., An, H. J., Lee, J. D., Moon, G.-S., & Lee, H.-Y. (2022). Antimicrobial Activity of Smilax china L. Root Extracts against the Acne-Causing Bacterium, Cutibacterium acnes, and Its Active Compounds. Molecules, 27(23), 8331. https://doi.org/10.3390/molecules27238331








