The Identification and Quantitative Analysis of Unusual Keto-Carotenoids in Ripe Fruits of Maclura tricuspidate and Its Potential as a Valuable Source of Cryptocapsin
Abstract
1. Introduction
2. Results and Discussion
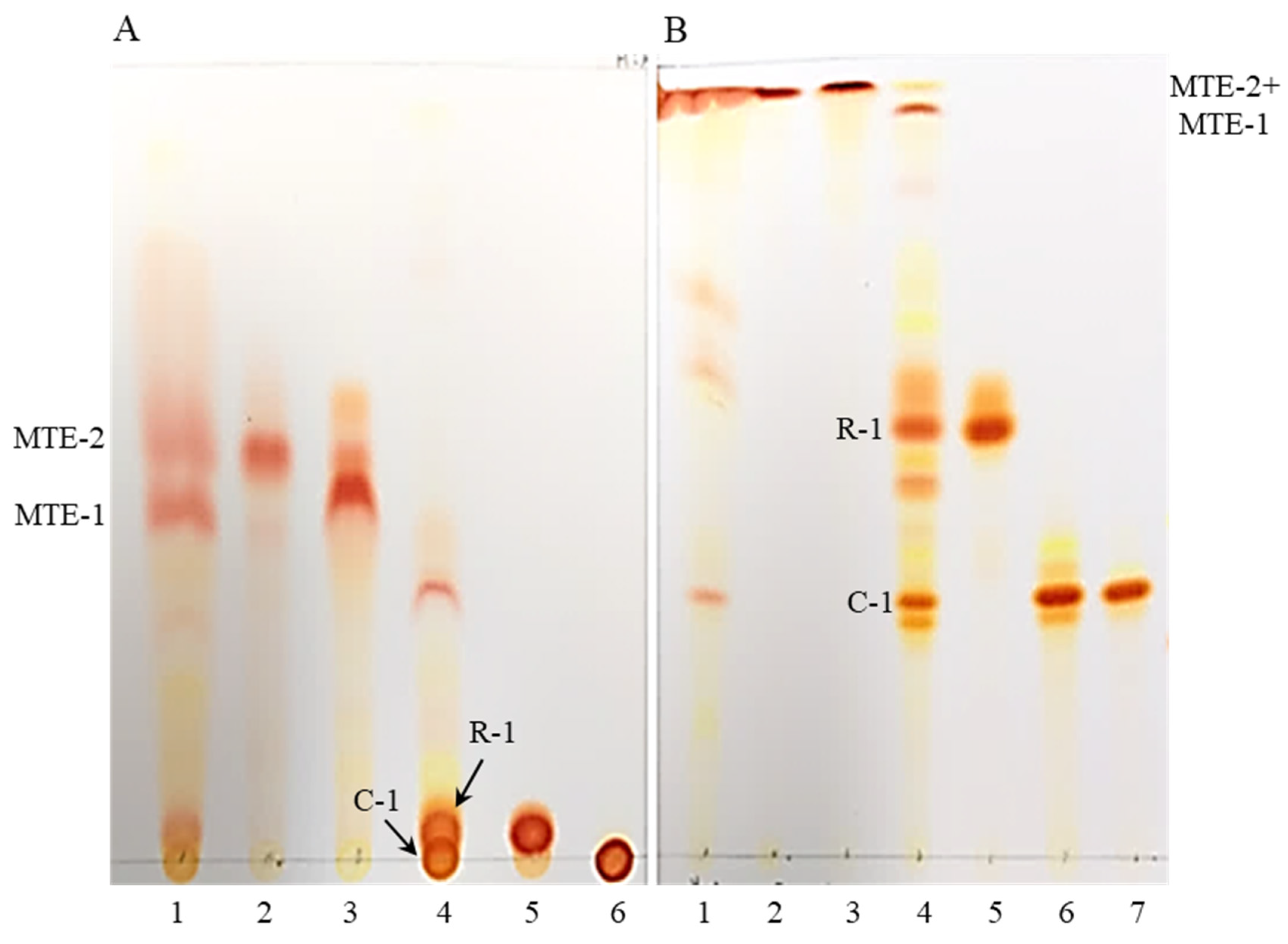
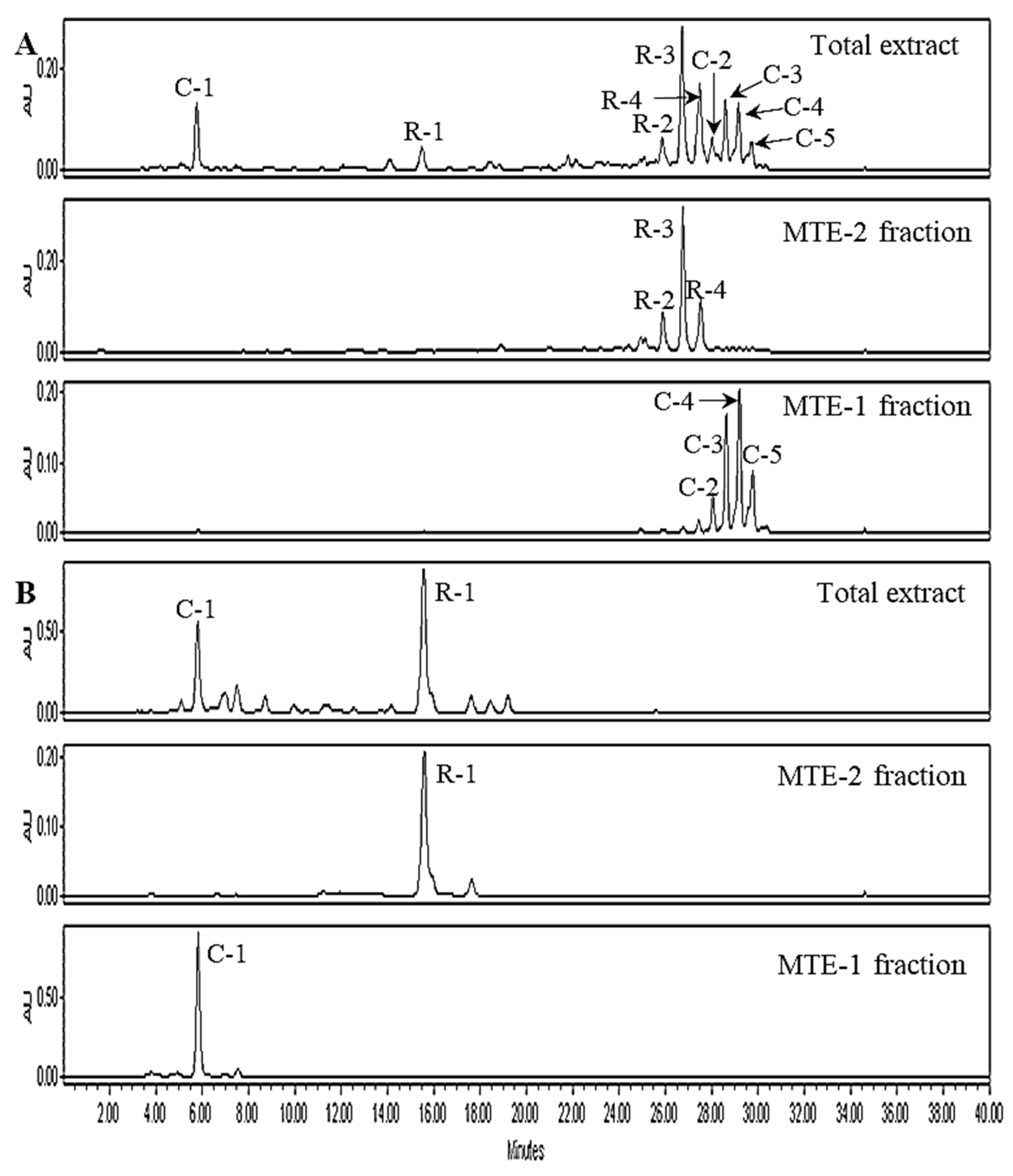
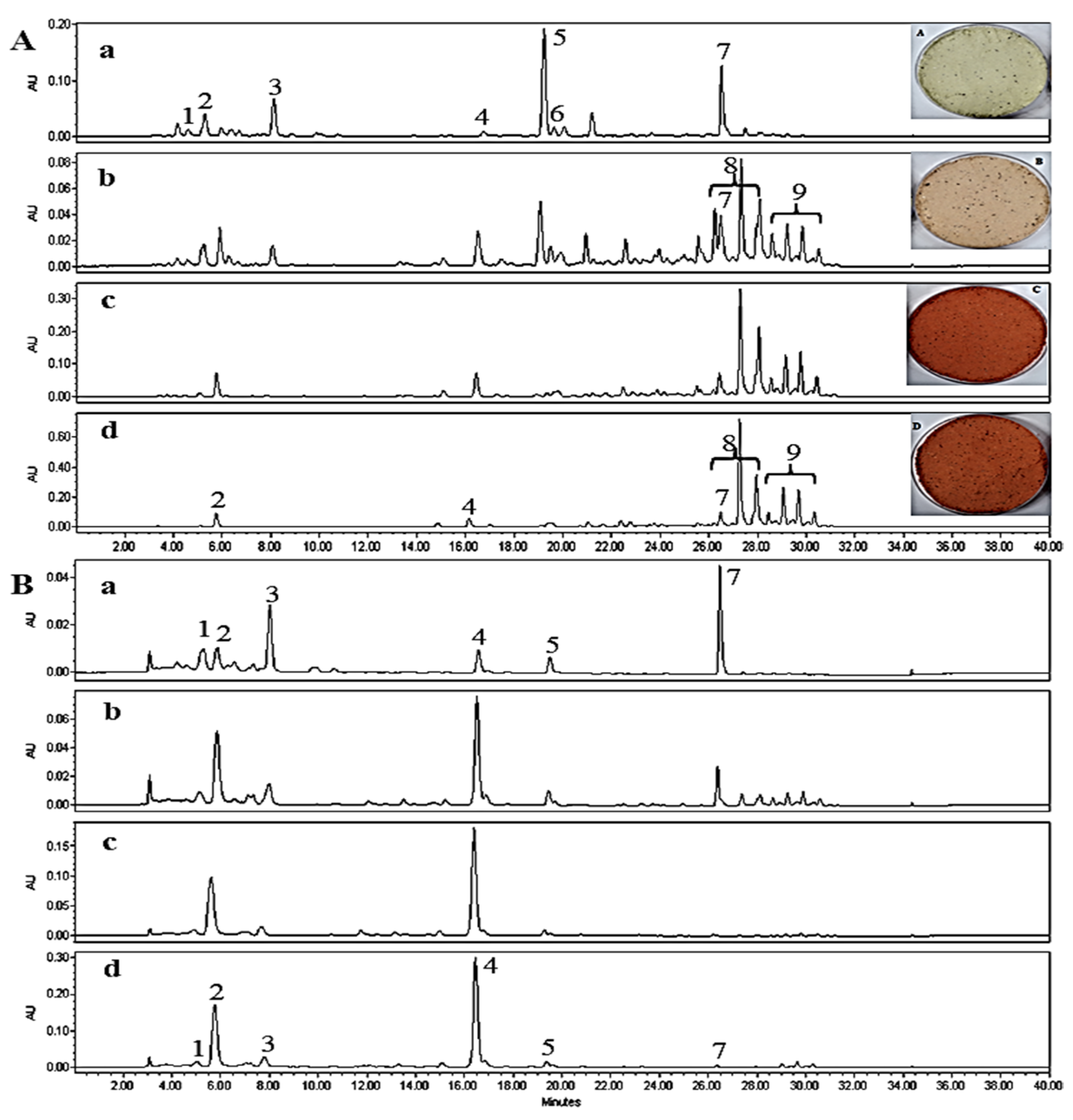
2.1. Analysis of Free and Esterified Carotenoids in MT Fruits
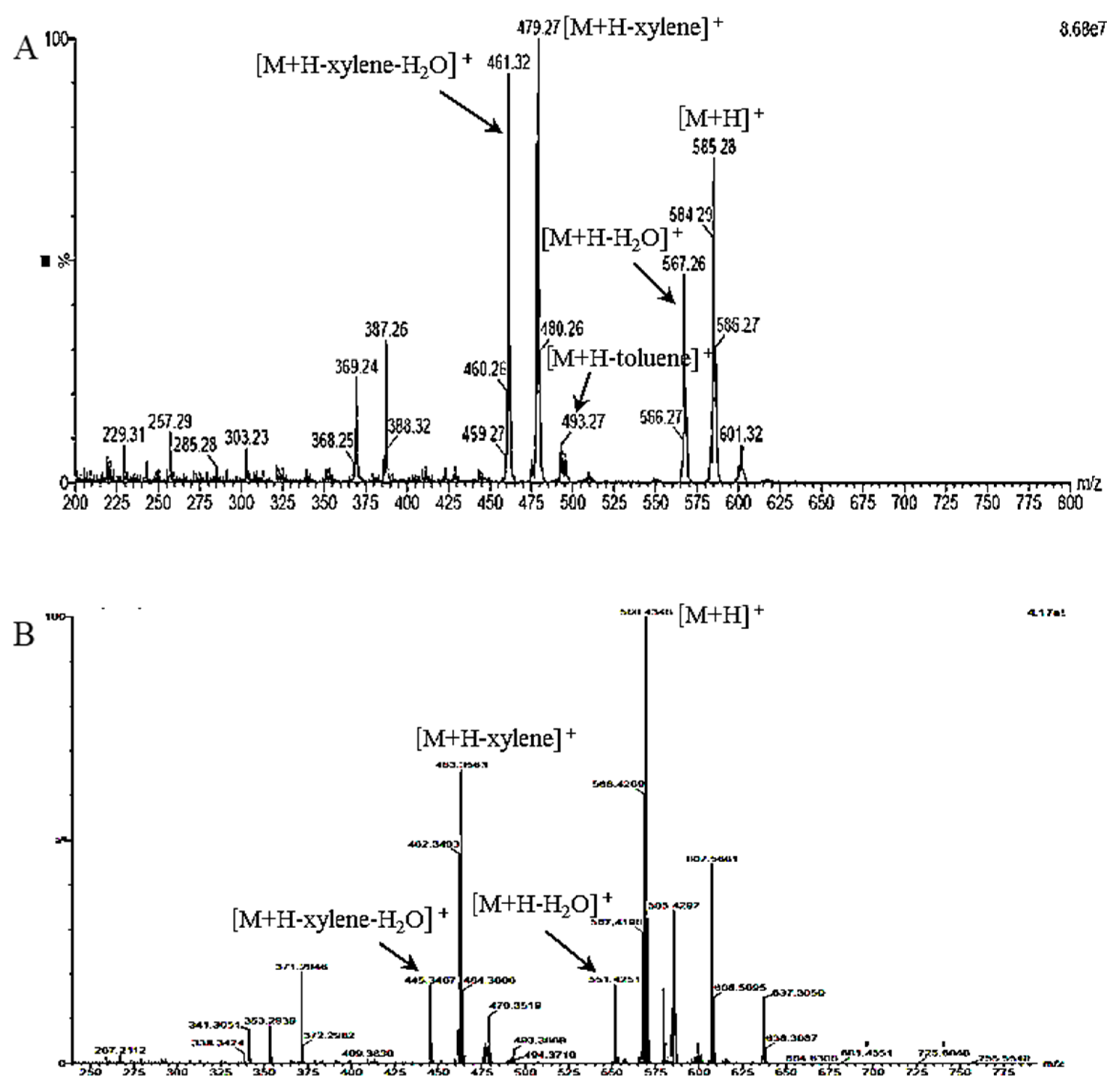
2.2. Structure Elucidation of C-1 and R-1 from Saponified Extract
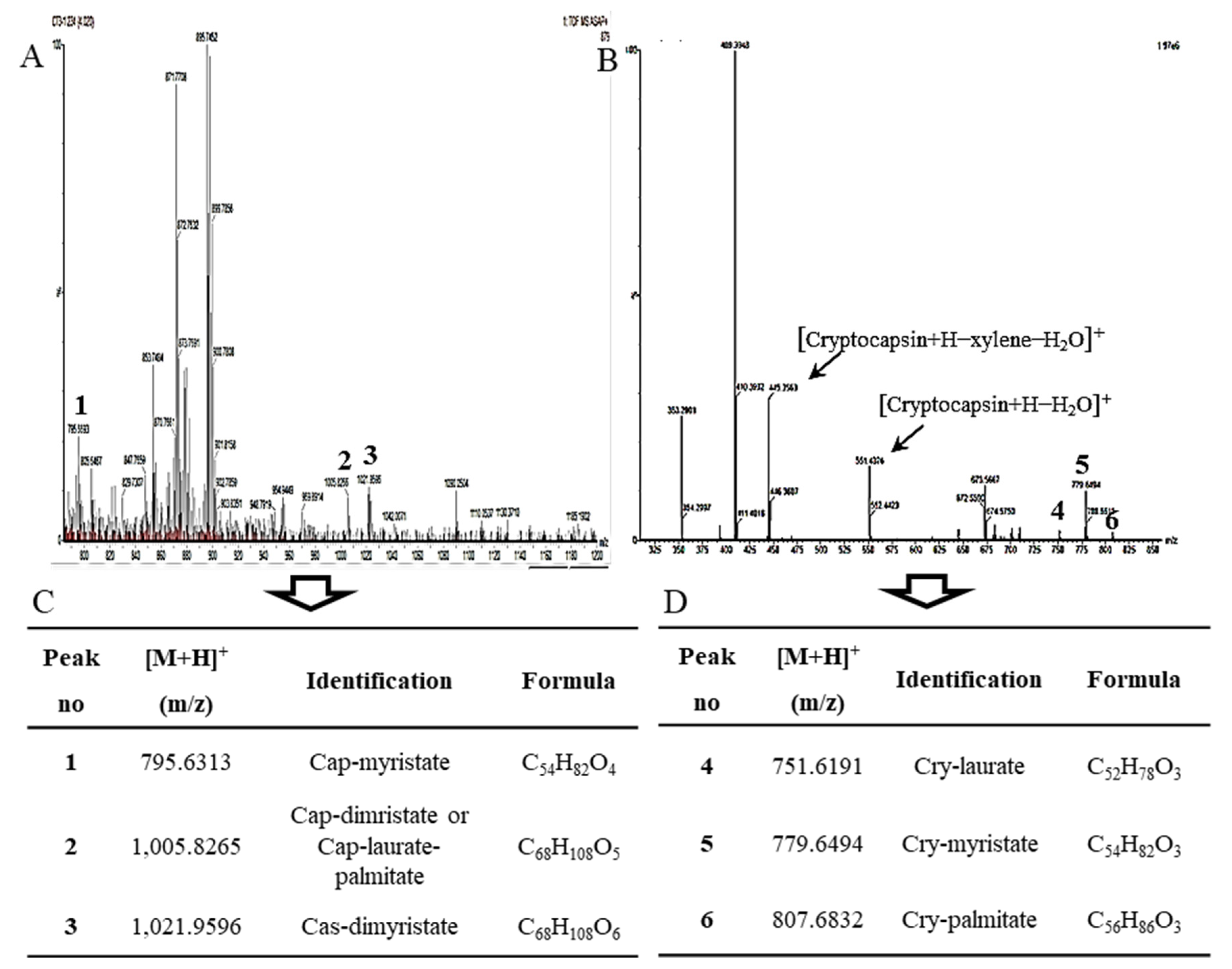
2.3. The Identification of Esterified Carotenoids from Un-Saponified MTE-1 and MTE-2 Fractions
2.4. Comparison of Keto-Carotenoid Profiles at Different Maturity Stages
3. Materials and Methods
3.1. Plant Materials
3.2. Reagents
3.3. Extraction of Carotenoids
3.4. Isolation of Esterified Carotenoid Fractions from Un-Saponified Extract
3.5. Isolation of Capsanthin and Cryptocapsin from Saponified Extract
3.6. TLC, HPLC and Q-TOF-MS Analysis
3.7. Preparation of Standard Solution
3.8. Carotenoid Contents at Different Maturity Stages
3.9. Spectroscopic Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Leong, H.Y.; Show, P.L.; Lim, M.H.; Ooi, C.W.; Ling, T.C. Natural red pigments from plants and their health benefits—A review. Food Rev. Int. 2017, 34, 463–482. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.C.; Kim, S.H.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Bunea, A.; Socaciu, C.; Pintea, A. Xanthophyll esters in fruits and vegetables. Not. Bot. Horti Agobot. 2014, 42, 310–324. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 2018, 648, 36–43. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef]
- Turcsi, E.; Murillo, E.; Kurtán, T.; Szappanos, Á.; Illyés, T.Z.; Gulyás-Fekete, G.; Agócs, A.; Nagy, V.; Deli, J. Isolation of β-cryptoxanthin-epoxides, precursors of cryptocapsin and 3′-deoxycapsanthin, from red mamey (Pouteria sapota). J. Agric. Food Chem. 2015, 63, 6059–6065. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Esquivel, P.; Jiménez, V.M.; Carle, R.; Schweiggert, R.M. Deposition form and bioaccessibility of keto-carotenoids from mamey sapote (Pouteria sapota), red bell pepper (Capsicum annuum), and sockeye salmon (Oncorhynchus nerka) filet. J. Agric. Food Chem. 2016, 64, 1989–1998. [Google Scholar] [CrossRef]
- Murillo, E.; Watts, M.; Reyna, G.; Giuffrida, D.; Durant-Archibold, A.A. Carotenoid composition of Cionosicyos macranthus fruit. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Schweiggert, R.M.; Bosy-Westphal, A.; Jimenez, V.M.; Carle, R.; Esquivel, P. Carotenoids and carotenoid esters of orange- and yellow-fleshed mamey sapote (Pouteria sapota (Jacq.) H.E. Moore & Stearn) fruit and their postprandial absorption in humans. Food Chem. 2017, 221, 673–682. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Doens, D.; Kumar, D.J.; Murillo, E.; Fernandez, P.L.; Rao, K.; Durant-Archibold, A.A. Anti-amyloid aggregation activity of novel carotenoids: Implications for Alzheimer’s drug discovery. Clin. Interv. Aging 2017, 12, 815–822. [Google Scholar] [CrossRef]
- Xin, L.T.; Yue, S.J.; Fan, Y.C.; Wu, J.S.; Yan, D.; Guan, H.S.; Wang, C.Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017, 7, 31807–31832. [Google Scholar] [CrossRef]
- Novruzov, E.N.; Agamirov, U.M. Carotenoids of Cudrania tricuspidata fruit. Chem. Nat. Compd. 2002, 38, 468–469. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef]
- Philip, T.; Francis, J. Isolation and chemical properties of capsanthin and derivatives. J. Food Sci. 1971, 36, 823–827. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Dong, L.; Pajkovic, N.D. Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. Int. J. Mass Spectrom. 2012, 15, 163–172. [Google Scholar] [CrossRef]
- Butnariu, M. Methods of analysis (extraction, separation, identification and quantification) of carotenoids from natural products. J. Ecosyst. Ecography 2016, 6, 1000193. [Google Scholar] [CrossRef]
- Rüttimann, A.; Englert, G.; Mayer, H.; Moss, G.P.; Weedon, B.C.L. Synthesis of optically active natural carotenoids and structurally related compounds. X. Synthesis of (3R,3′S,5′R)-capsanthin, (3S,5R,3′S,5′R)-capsorubin, (3′S,5′R)-cryptocapsin, and some related compounds. A new approach to optically active, five-membered-ring carotenoid building units by hydroboration. Helv. Chim. Acta 1983, 66, 1939–1960. [Google Scholar] [CrossRef]
- Maoka, T.; Akimoto, N.; Fujiwara, Y.; Hashimoto, K. Structure of new carotenoids with the 6-oxo-κ end group from the fruits of paprika, Capsicum annuum. J. Nat. Prod. 2004, 67, 115–117. [Google Scholar] [CrossRef]
- Hornero-Mendez, D.; Minquez-Mosquera, M.I. Xanthophyll esterification accompanying carotenoid overaccumulation in chromoplast of Capsicum annuum ripening fruits is a constitutive process and useful for ripeness index. J. Agric. Food Chem. 2000, 48, 1617–1622. [Google Scholar] [CrossRef]
- Schweiggert, U.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of carotenoids and carotenoid esters in red pepper pods (Capsicum annuum L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2617–2628. [Google Scholar] [CrossRef]
- Kim, S.; Ha, T.Y.; Hwang, I.K. Analysis, bioavailability, and potential healthy effects of capsanthin, natural red pigment from Capsicum spp. Food Rev. Int. 2009, 25, 198–213. [Google Scholar] [CrossRef]
- Murillo, E.; Nagy, V.; Agócs, A.; Deli, J. Carotenoids with κ-end group. Chapter III. In Carotenoids; Yamaguchi, M., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 49–76. [Google Scholar]
- Fisher, C.; Kocis, J.A. Separation of paprika pigments by HPLC. J. Agric. Food Chem. 1987, 35, 55–57. [Google Scholar] [CrossRef]
- Márkus, F.; Daood, H.G.; Kapitány, J.; Biacs, P.A. Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. J. Agric. Food Chem. 1999, 47, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Murillo, E.; Giuffrida, D.; Menchaca, D.; Dugo, P.; Torre, G.; Antonio, J.; Meléndez-Martinez, A.J.; Mondello, L. Native carotenoids composition of some tropical fruits. Food Chem. 2013, 140, 825–836. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Iida, K.; Madono, Y.; Yungyuen, W.; Yahata, M.; Yamawaki, K.; Kato, M. Identification and quantitative analysis of β-cryptoxanthin and β-citraurin esters in Satsuma mandarin fruit during the ripening process. Food Chem. 2017, 234, 356–364. [Google Scholar] [CrossRef]
- Breithaupt, D.F.; Bamedi, A. Carotenoid esters in vegetables and fruits: A screening with emphasis on β-cryptoxanthin esters. J. Agric. Food Chem. 2001, 49, 2064–2070. [Google Scholar] [CrossRef]
- Deli, J.D.; Molnar, P.; Matus, Z.; Toth, G. Carotenoid composition in the fruits of red paprika (Capsicum annuum var. Lycopersiciforme rubrum) during ripening; Biosynthesis of carotenoids in red paprika. J. Agric. Food Chem. 2001, 49, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Murillo, E.; Turcsi, E.; Szabó, I.; Mosquera, Y.; Agócs, A.; Nagy, V.; Gulyás-Fekete, G.; Deli, J. Carotenoid composition of the fruit of red mamey (Pouteria sapota). J. Agric. Food Chem. 2016, 64, 7148–7155. [Google Scholar] [CrossRef] [PubMed]
- Murillo, E.; Mosquera, Y.; Kurtán, T.; Gulyás-Feketec, G.; Nagyc, V.; Deli, J. Isolation and characterization of novel capsorubin-like carotenoids from the red mamey (Pouteria sapota). Helv. Chim. Acta 2021, 95, 983–988. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold carotenoids: Much more than lutein esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Lee, W.J.; Gebru, Y.A.; Choi, H.S.; Yeo, S.H.; Jeong, Y.J.; Kim, S.; Kim, H.Y.; Kim, M.K. Comparison of bioactive compounds and antioxidant activities of Maclura tricuspidata fruit extracts at different maturity stages. Molecules 2019, 24, 567. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.J.; Failla, M.L.; Yahia, E.M.; Gardea-Bejar, A. Impact of the stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in ‘Ataulfo’ mango. J. Agric. Food Chem. 2008, 56, 1511–1516. [Google Scholar] [CrossRef]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DAD-APCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef]
- Lux, P.E.; Carle, R.; Zacarías, L.; Rodrigo, M.J.; Schweiggert, R.M.; Steingass, C.B. Genuine carotenoid profiles in sweet orange [Citrus sinensis (L.) Osbeck cv. Navel] peel and pulp at different maturity stages. J. Agric. Food Chem. 2019, 67, 13164–13175. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I.; Hornero-Mendéz, D. Changes in provitamin A during paprika processing. J. Food Protect. 1997, 60, 853–857. [Google Scholar] [CrossRef]
- Sarkar, C.R.; Bhagawati, B.; Das, L.; Goswami, B.C. An efficient condition of saponification of lutein ester from marigold flower. Ann. Biol. Res. 2012, 3, 1461–1466. [Google Scholar]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Kiokias, S. HPLC analysis and determination of carotenoid pigments in commercially available plant extracts. Curr. Res. Nutr. Food Sci. 2016, 4, 1–4. [Google Scholar] [CrossRef]

| Carbon No | C-1 (Capsanthin) | R-1 (Cryptocapsin) | ||
|---|---|---|---|---|
| 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | |
| 1 | 37.14 | 34.28 | ||
| 2 | 48.42 | 1.48 (2H, m) | 39.63 | 1.47 (2H, overlapped) |
| 3 | 65.13 | 3.99 (1H, m) | 19.26 | 1.64 (2H, m) |
| 4 | 42.54 | 2.39 (1H, dd, 17.2, 4.8) | 33.12 | 2.02 (2H, m) |
| 5 | 126.25 | 129.50 | ||
| 6 | 137.75 | 137.89 | ||
| 7 | 125.53 | 6.12 (3H, s) | 126.96 | 6.19 (1H, overlapped) |
| 8 | 138.45 | 6.12 (3H, s) | 137.71 | 6.14 (1H, overlapped) |
| 9 | 135.91 | 136.53 | ||
| 10 | 131.66 | 6.17(1H, d, 11.4) | 131.71 | 6.71(1H, overlapped) |
| 11 | 125.87 | 6.89 (1H, overlapped) | 125.70 | 6.89 (1H, overlapped) |
| 12 | 137.61 | 6.36 (1H, d, 15.2) | 137.03 | 6.36 (1H, br. d, 14.5) |
| 13 | 132.39 | 137.71 | ||
| 14 | 132.00 | 6.26 (1H, d, 12.0) | 132.17 | 6.26 (1H, br. d, 11.5) |
| 15 | 131.24 | 6.15 (1H, overlapped) | 130.75 | 6.15 (1H, overlapped) |
| 16 | 28.74 | 1.07 (3H, s) | 28.98 | 1.03 (3H, s) |
| 17 | 30.27 | 1.26 (3H, s) | 29.71 | 1.25 (3H, s) |
| 18 | 21.63 | 1.74 (3H, s) | 21.79 | 1.72 (3H, s) |
| 19 | 12.79 | 1.99 (3H, s) | 12.74 | 1.99 (3H, s) |
| 20 | 12.96 | 1.96 (3H, s) | 12.80 | 1.96 (3H, s) |
| 1′ | 43.99 | 43.97 | ||
| 2′ | 50.84 | 2.06 (1H, m) | 50.84 | 2.00 (1H, m) 1.71 (1H, overlapped) |
| 3′ | 70.38 | 4.52 (1H, m) | 70.36 | 4.52(1H, m) |
| 4′ | 45.29 | 1.51 (1H, overlapped) 2.96 (1H, dd, 14.4, 8.4) | 45.29 | 1.49 (1H, overlapped) 2.96 (1H, dd, 14.0, 8.5) |
| 5′ | 58.96 | 58.94 | ||
| 6′ | 203.05 | 202.96 | ||
| 7′ | 120.88 | 6.44 (1H, d, 15.0) | 120.84 | 6.44 (1H, d, 15.0) |
| 8′ | 146.95 | 7.33 (1H, d, 15.0) | 146.89 | 7.33 (1H, d, 15.0) |
| 9′ | 133.64 | 133.59 | ||
| 10′ | 140.79 | 140.76 | 6.55 (1H, br, d, 10.0) | |
| 11′ | 124.10 | 6.62 (1H, overlapped) | 124.04 | 6.62 (1H, overlapped) |
| 12′ | 142.02 | 142.01 | 6.55 (1H, br. d, 10.0) | |
| 13′ | 136.13 | 135.80 | ||
| 14′ | 135.28 | 6.36 (1H, d, 14.4) | 135.31 | 6.36 (1H, br. d, 14.5) |
| 15′ | 129.71 | 6.64 (1H, overlapped) | 129.55 | 6.63 (1H, overlapped) |
| 16′ | 25.08 | 1.21 (3H, s) | 25.09 | 1.21 (3H, s) |
| 17′ | 25.88 | 0.84 (3H, s) | 25.86 | 0.84 (3H, s) |
| 18′ | 21.32 | 1.36 (3H, s) | 21.29 | 1.37 (3H, s) |
| 19′ | 12.84 | 1.96 (3H, s) | 12.85 | 1.98 (3H, s) |
| 20′ | 12.93 | 1.96 (3H, s) | 12.90 | 1.98 (3H, s) |
| Capsorubin (Peak-1) | Capsanthin (Peak-2) | Lutein (Peak-3) | Cryptocapsin (Peak-4) | β-Crypto xanthin (Peak-6) | β-Carotene (Peak-7) | |
|---|---|---|---|---|---|---|
| Unsaponified | ||||||
| Immature | 5.97 ± 0.22 b | 1.89 ± 0.08 a | 55.68 ± 0.85 b | 3.42 ± 0.15 a | 12.65 ± 0.17 a | 41.66 ± 1.48 b |
| Premature | 4.14 ± 0.03 a | 4.32 ± 0.23 b | 16.28 ± 0.57 a | 12.2 ± 0.61 b | 11.53 ± 0.07 a | 18.01 ± 0.61 a |
| Fully mature | 6.72 ± 0.02 d | 23.72 ± 0.55 c | - | 55.01 ± 1.51 d | 13.13 ± 1.18 b | - |
| Overmature | 6.33 ± 0.01 c | 30.55 ± 0.77 d | - | 15.52 ± 1.07 c | 13.12 ± 0.23 b | - |
| Saponified | ||||||
| Immature | 3.34 ± 0.05 a | 1.37 ± 0.14 a | 26.25 ± 0.95 b | 4.07 ± 0.24 a | 7.97 ± 0.13 a | 15.1 ± 0.26 d |
| Premature | 3.27 ± 0.01 a | 14.69 ± 0.67 b | 18.85 ± 0.5 a | 34.68 ± 1.78 b | 10.23 ± 0.51 c | 9.9 ± 0.42 c |
| Fully mature | 6.42 ± 0.04 c | 57.65 ± 1.97 c | - | 171.66 ± 4.85 c | 9.38 ± 0.02 b | 4.55 ± 0.12 a |
| Overmature | 6.82 ± 0.04 d | 94.48 ± 1.59 d | - | 291.63 ± 13.4 d | 11.54 ± 0.22 d | 5.53 ± 0.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-K.; Kim, D.-W.; Gebru, Y.A.; Choi, H.-S.; Kim, Y.-H.; Kim, M.-K. The Identification and Quantitative Analysis of Unusual Keto-Carotenoids in Ripe Fruits of Maclura tricuspidate and Its Potential as a Valuable Source of Cryptocapsin. Molecules 2022, 27, 8317. https://doi.org/10.3390/molecules27238317
Kim J-K, Kim D-W, Gebru YA, Choi H-S, Kim Y-H, Kim M-K. The Identification and Quantitative Analysis of Unusual Keto-Carotenoids in Ripe Fruits of Maclura tricuspidate and Its Potential as a Valuable Source of Cryptocapsin. Molecules. 2022; 27(23):8317. https://doi.org/10.3390/molecules27238317
Chicago/Turabian StyleKim, Jong-Kuk, Dae-Woon Kim, Yoseph Asmelash Gebru, Han-Seok Choi, Young-Hoi Kim, and Myung-Kon Kim. 2022. "The Identification and Quantitative Analysis of Unusual Keto-Carotenoids in Ripe Fruits of Maclura tricuspidate and Its Potential as a Valuable Source of Cryptocapsin" Molecules 27, no. 23: 8317. https://doi.org/10.3390/molecules27238317
APA StyleKim, J.-K., Kim, D.-W., Gebru, Y. A., Choi, H.-S., Kim, Y.-H., & Kim, M.-K. (2022). The Identification and Quantitative Analysis of Unusual Keto-Carotenoids in Ripe Fruits of Maclura tricuspidate and Its Potential as a Valuable Source of Cryptocapsin. Molecules, 27(23), 8317. https://doi.org/10.3390/molecules27238317







