Synthesis and Characterization of Colistin-Functionalized Silica Materials for Rapid Capture of Bacteria in Water
Abstract
1. Introduction
2. Results and Discussion
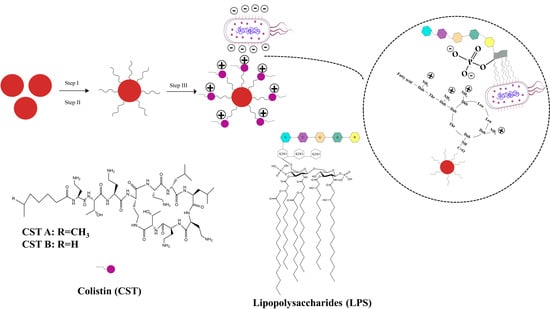
2.1. Preparation of SiO2@NH2@COOH@CST
2.1.1. Selection of Synthesis Route
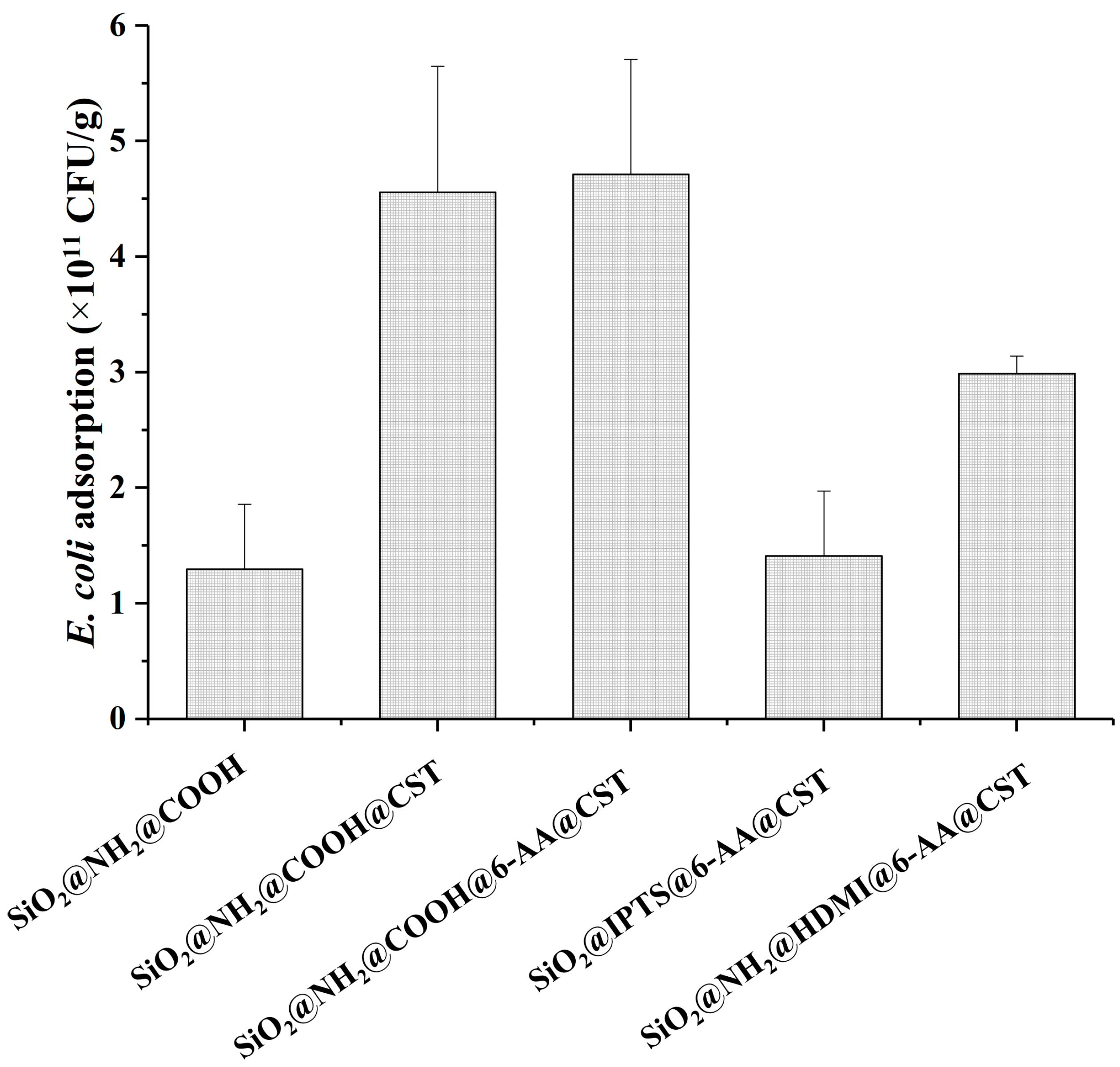
2.1.2. Optimization of Linkers
2.1.3. Selection of Buffer Systems
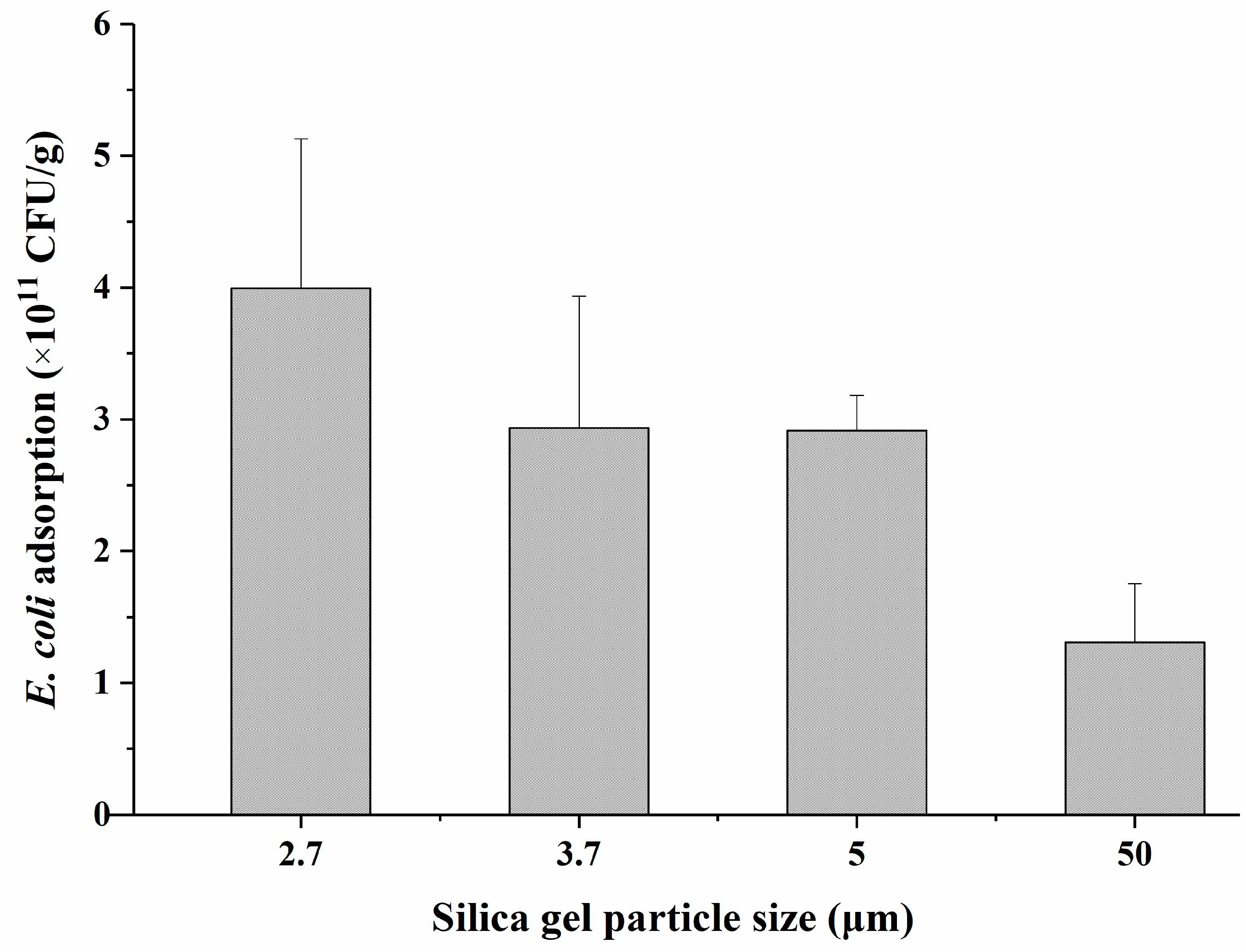
2.1.4. Optimization of Silica Particle Size
2.2. Characterization
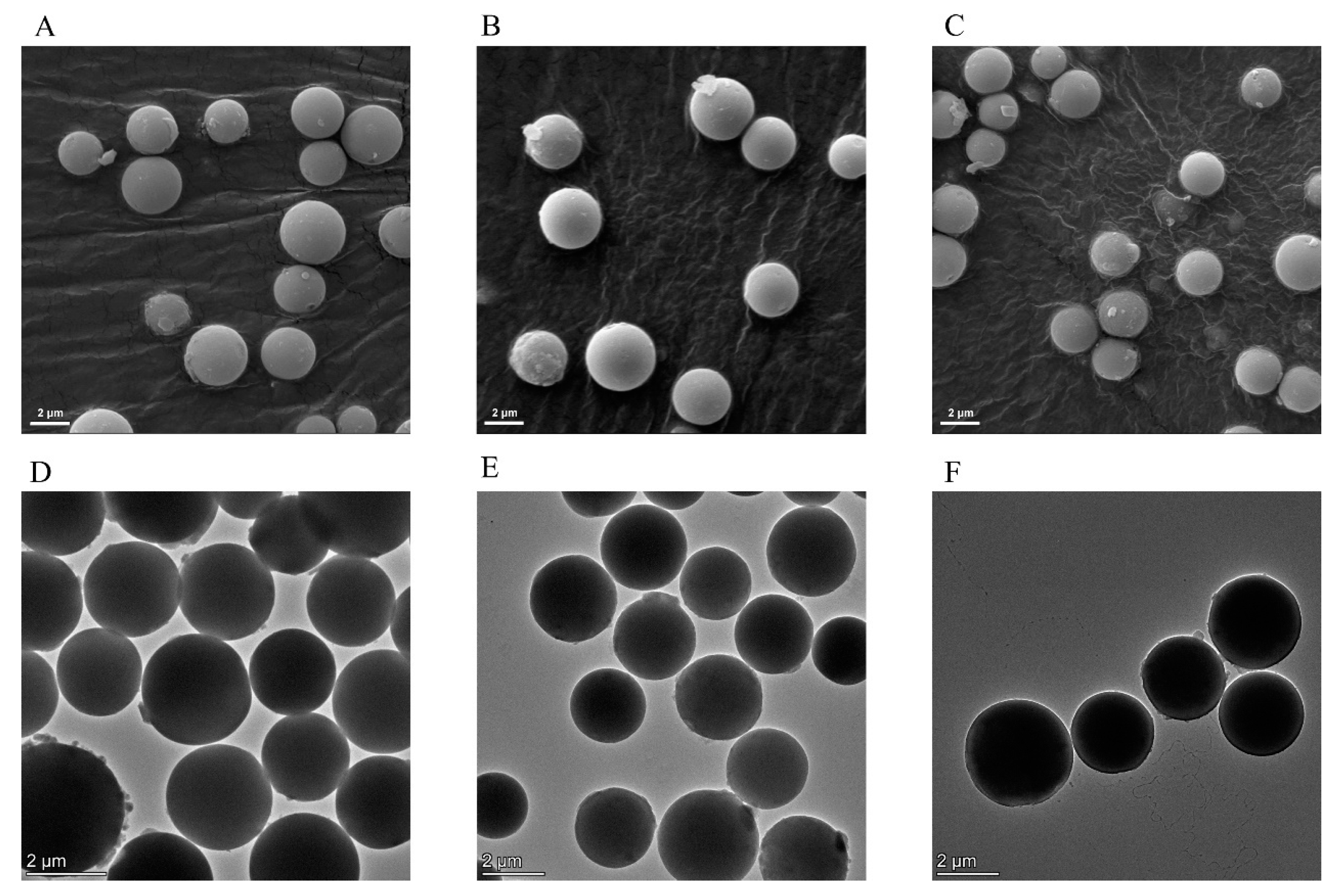
2.2.1. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) Analysis
2.2.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.2.3. Thermogravimetric Analysis
2.2.4. Zeta Potential Measurement
2.3. Adsorption Properties
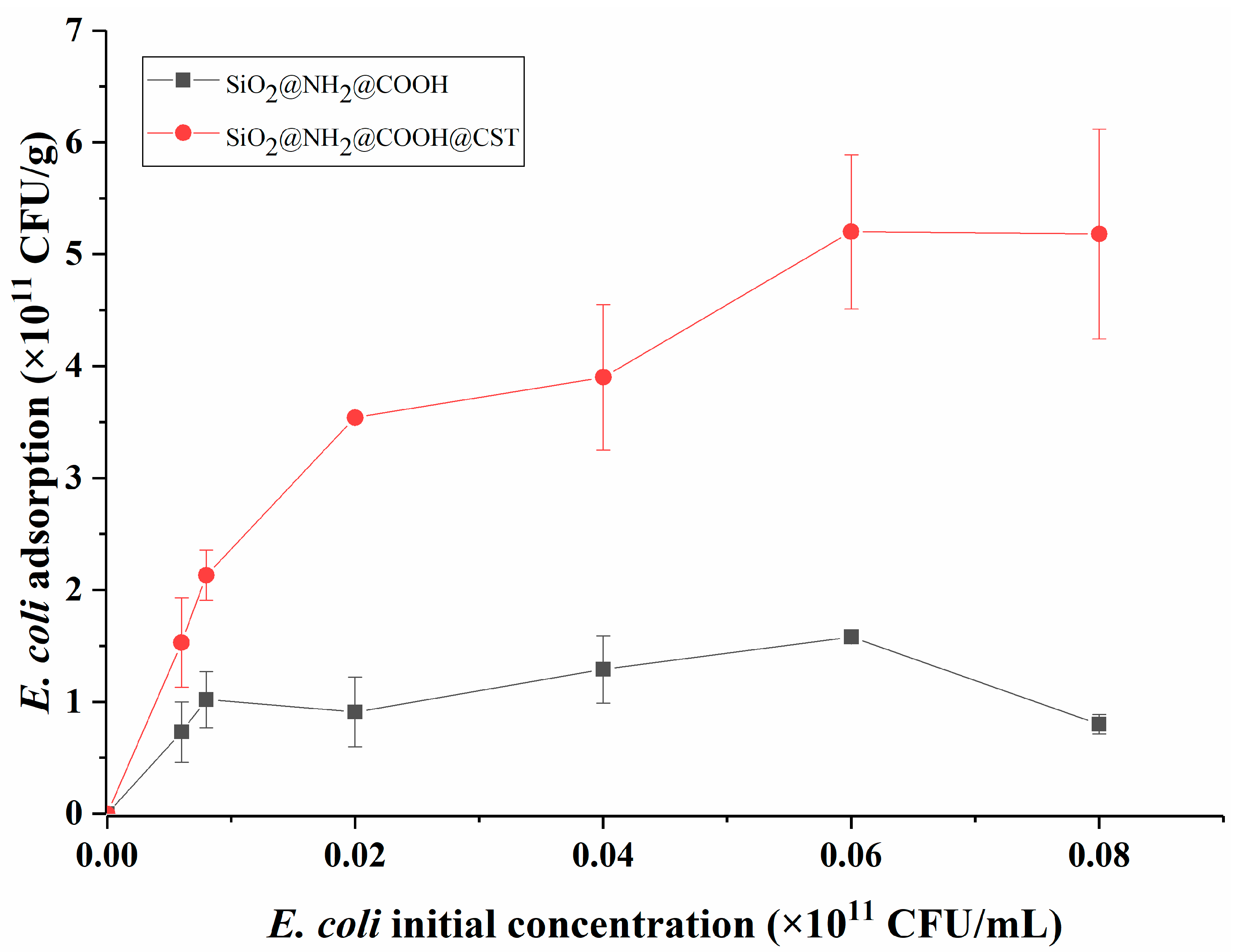
2.3.1. Adsorption Isotherm
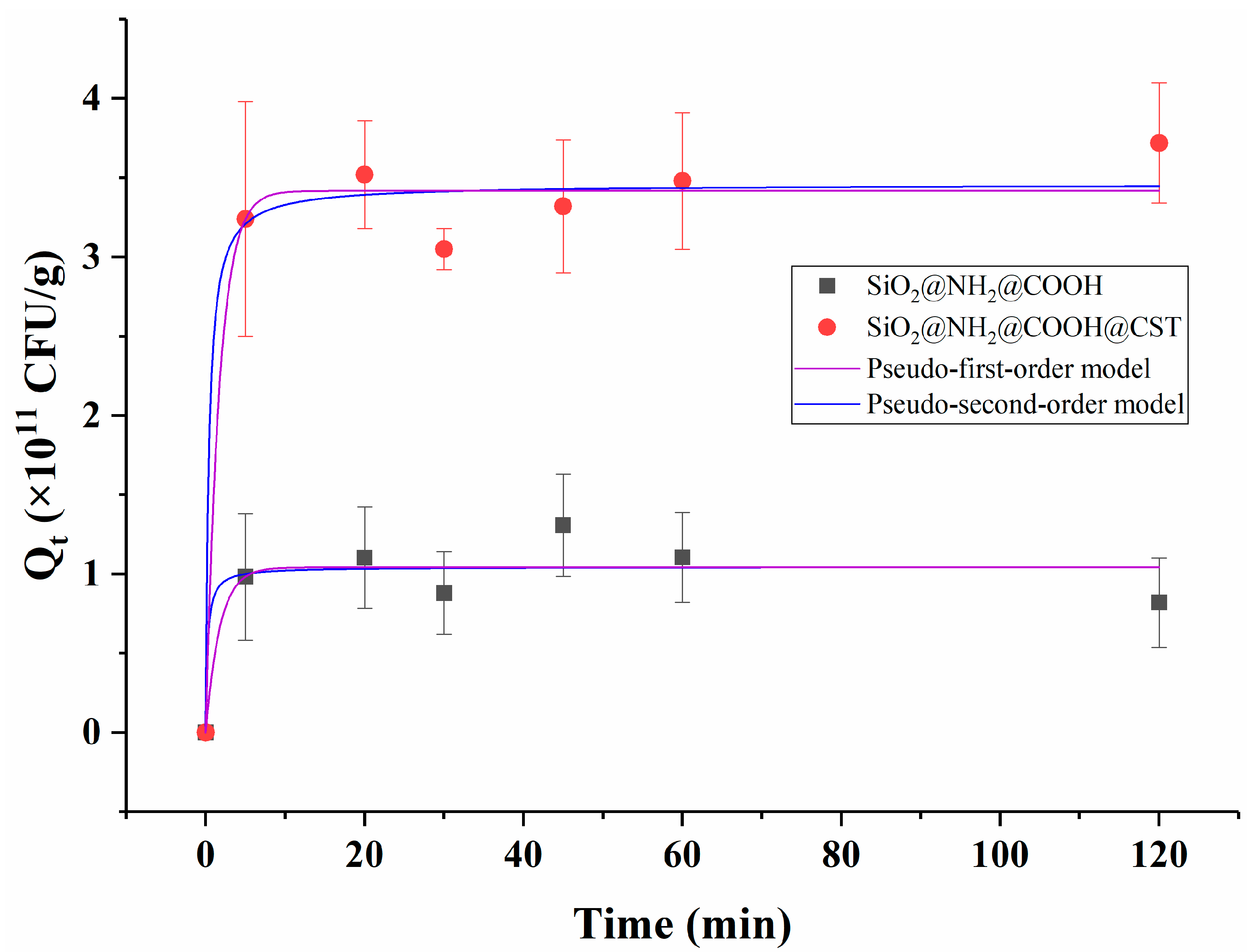
2.3.2. Adsorption Kinetics
2.4. Factors Affecting Bacterial Adsorption
2.4.1. Effect of pH on E. coli Adsorption
2.4.2. Effect of Temperature on E. coli Adsorption
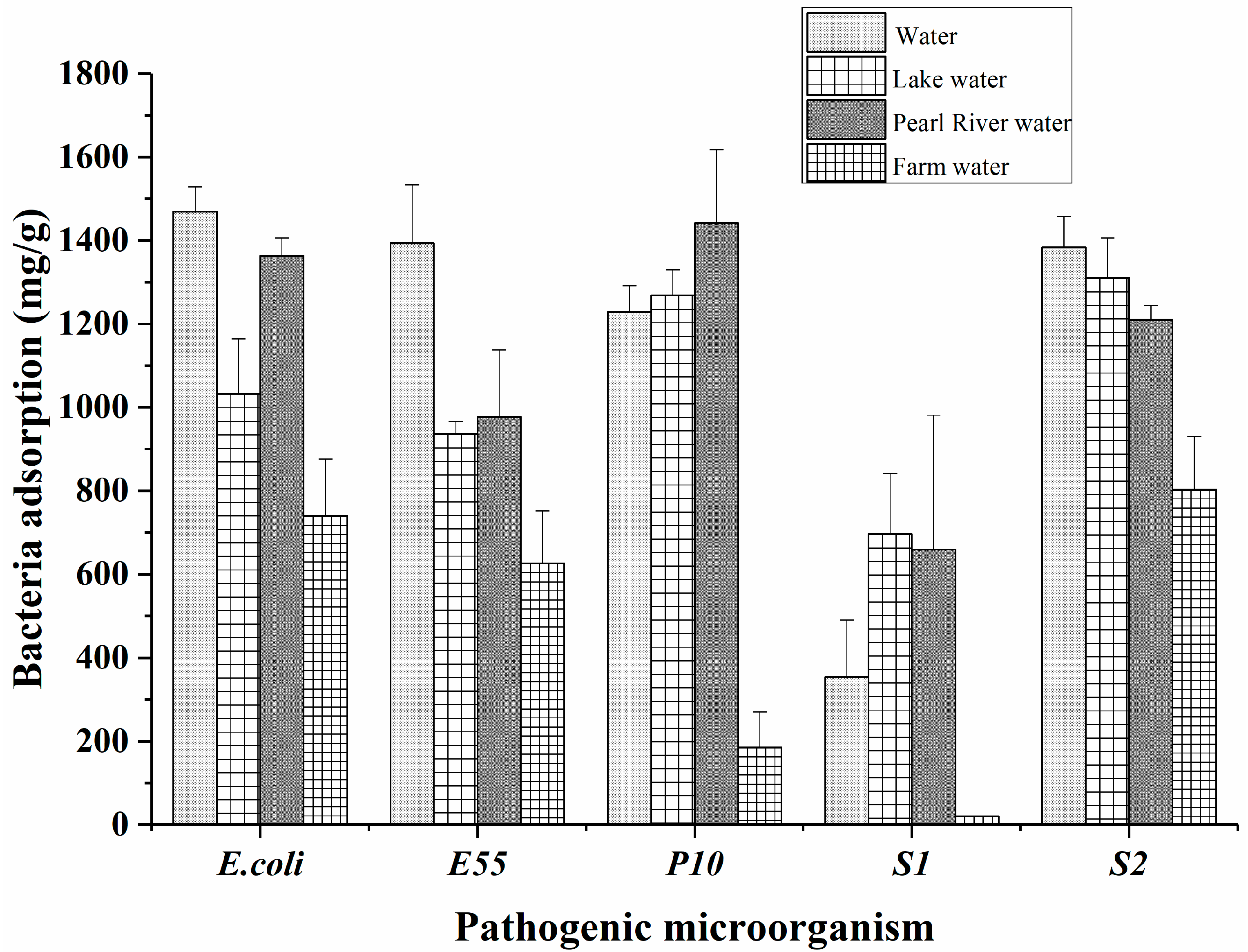
2.5. Application
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of CST-Modified Silica
3.2.1. Synthesis of Amino-Modified Silica (SiO2@NH2)
3.2.2. Carboxylation on the Surface of SiO2@NH2
3.2.3. Synthesis of SiO2@NH2@COOH@CST
3.3. Characterization of SiO2@NH2@COOH@CST
3.4. Preparation of Bacteria Suspension
3.5. Adsorption Experiments
3.5.1. Measurement of the Adsorbed Bacteria
3.5.2. Adsorption Isotherm and Kinetic Adsorption
3.6. Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.J.; Liu, F.; Shan, C.; Tong, M.P.; Hou, Y.L. Efficient bacterial capture with amino acid modified magnetic nanoparticles. Water Res. 2014, 50, 124–134. [Google Scholar] [CrossRef]
- Zhan, S.H.; Zhu, D.D.; Ma, S.L.; Yu, W.C.; Jia, Y.N.; Li, Y.; Yu, H.B.; Shen, Z.Q. Highly efficient removal of pathogenic bacteria with magnetic graphene composite. ACS Appl. Mater. Interfaces 2015, 7, 4290–4298. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Huang, Q.W.; Zhang, Y.X.; Zhang, M.Y.; Xie, J.M.; He, L.M. Rapid multiresidue analysis of authorized/banned cyclopolypeptide antibiotics in feed by liquid chromatography-tandem mass spectrometry based on dispersive solid-phase extraction. J. Pharmaceut. Biomed. 2019, 170, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Wang, J.P.; Li, J. Teaching ‘old’ polymyxins new tricks: New-generation lipopeptides targeting Gram-negative ‘superbugs’. ACS Chem. Biol. 2014, 9, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, T.T.; Debowski, A.W.; Tang, H.; Nilsson, H.O.; Stubbs, K.A.; Marshall, B.J.; Benghezal, M. Lipopolysaccharide structure and biosynthesis in helicobacter pylori. Helicobacter 2016, 21, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Yin, J.H.; Meng, Q.; Cheng, D.; Fu, J.N.; Yu, Z.L. Mechanisms of bactericidal action and resistance of polymyxins for Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Kodama, M.; Tani, T.; Hanasawa, K. Treatment of sepsis by extracorporeal elimination of endotoxin using polymyxin. Am. J. Surg. 1994, 167, 412–417. [Google Scholar] [CrossRef]
- Cao, X.D.; Zhu, B.Y.; Zhang, X.F.; Dong, H. Polymyxin B immobilized on cross-linked cellulose microspheres for endotoxin adsorption. Carbohydr Polym. 2016, 136, 12–18. [Google Scholar] [CrossRef]
- Thompson, M.; Blaszykowski, C.; Sheikh, S.; Romaschin, A. A true theranostic approach to medicine: Towards tandem sensor detection and removal of endotoxin in blood. Biosens. Bioelectron. 2015, 67, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cherak, Z.; Loucif, L.; Moussi, A.; Rolain, J.M. Carbapenemase producing Gram-negative bacteria in aquatic environments: A review. J. Glob. Antimicrob. Resist. 2021, 25, 287–309. [Google Scholar] [CrossRef]
- Eibagia, H.; Faghihia, K.; Komijanib, M. Synthesis of new environmentally friendly poly(urethane-imide)s as an adsorbent including β-cyclodextrin cavities and attached to iron nanoparticles for removal of gram-positive and Gram-negative bacteria from water samples. Polym. Test. 2020, 90, 106734. [Google Scholar] [CrossRef]
- Diene, S.M.; Rolain, J.M. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 2014, 20, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.L.; Li, N.; Zhang, Z.M.; Li, G.K. Dummy template based molecularly imprinted solid-phase microextraction coating for analysis of trace disinfection by-product of 2,6-dichloro-1,4-benzoquinone using high-performance liquid chromatography. Talanta 2022, 239, 123065. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.H.; Yang, Y.; Shen, Z.Q.; Shan, J.J.; Li, Y.; Yang, S.S.; Zhu, D.D. Efficient removal of pathogenic bacteria and viruses by multifunctional amine-modified magnetic nanoparticles. J. Hazard. Mater. 2014, 274, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.W.; Gao, T.; Zheng, Y.; Li, L.W.; Xu, D.K.; Zhang, X.F.; Hou, Y.L.; Yan, M. Fe3O4@silica nanoparticles for reliable identification and magnetic separation of listeria monocytogenes based on molecular-scale physiochemical interactions. J. Mater. Sci. Technol. 2021, 84, 116–123. [Google Scholar] [CrossRef]
- Feng, X.Y.; Meng, X.Y.; Xiao, F.B.; Aguilar, Z.P.; Xu, H.Y. Vancomycin-dendrimer based multivalent magnetic separation nanoplatforms combined with multiplex quantitative PCR assay for detecting pathogenic bacteria in human blood. Talanta 2020, 225, 121953. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, X.K.; Cai, R.; Yang, K.; Gao, Z.P.; Yuan, Y.H.; Yue, T.L.; Wang, Z.L. Aptamer modified magnetic nanoparticles coupled with fluorescent quantum dots for efficient separation and detection of Alicyclobacillus acidoterrestris in fruit juices. Food Control 2021, 126, 108060. [Google Scholar] [CrossRef]
- Luo, K.; Jeong, K.B.; You, S.M.; Lee, D.H.; Jung, J.Y.; Kim, Y.R. Engineering, surface-engineered starch magnetic microparticles for highly effective separation of a broad range of bacteria. ACS Sustain. Chem. Eng. 2018, 6, 13524–13531. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C.; Simonet, B.M.; Valcárcel, M. Colistin-functionalised CdSe/ZnS quantum dots as fluorescent probe for the rapid detection of Escherichia coli. Biosens. Bioelectron. 2011, 26, 4368–4374. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.S.; Mejías, R.; Miller, S.E.; Greer, J.M.; McClain, M.S.; Cover, T.L.; Giorgio, T.D. Magnetic extraction of Acinetobacter baumannii using colistin functionalized gamma-Fe2O3/Au core/shell composite nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 26719–26730. [Google Scholar] [CrossRef]
- Zurier, H.S.; Duong, M.M.; Goddard, J.M.; Nugen, S.R. Engineering biorthogonal phage-based nanobots for ultrasensitive, in situ bacteria detection. ACS Appl. Bio. Mater. 2020, 3, 5824–5831. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Liu, N.; Zheng, L.Y.; Cai, G.Z.; Lin, J.H. A lab-on-chip device for sample-in-result-out detection of viable Salmonella using loop-mediated isothermal amplification and real-time turbidity monitoring. Lab. Chip. 2020, 20, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Peng, J.X.; Shi, Y.L.; Ouyang, H.; Xu, Z.Q.; Fu, Z.F. Quantification of live Gram-positive bacteria via employing artificial antibacterial peptide-coated magnetic spheres as isolation carriers. Microchem. J. 2020, 154, 104643. [Google Scholar] [CrossRef]
- Kell, A.J.; Stewart, G.; Ryan, S.; Peytavi, R.; Boissinot, M.; Huletsky, A.; Bergeron, M.G.; Simard, B. Vancomycin-modified nanoparticles for efficient targeting and preconcentration of Gram-positive and Gram-negative Bacteria. ACS Nano 2008, 2, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Gilles, M.A.; Hudson, A.Q.; Borders, C.L. Stability of water-soluble carbodiimides in aqueous solution. Anal. Biochem. 1990, 184, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.B.; Yang, D.; Wagner, P.; Maclaughlin, S.; Higgins, M.J.; Molino, P.J. Zwitterion functionalized silica nanoparticle coatings: The effect of particle size on protein, bacteria, and fungal spore adhesion. Langmuir 2019, 35, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Guber, A.K.; Pachepsky, Y.A.; Shelton, D.R.; Yu, O. Effect of bovine manure on fecal coliform attachment to soil and soil particles of different sizes. Appl. Environ. Microbiol. 2007, 73, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Jiang, D.H.; Cai, P.; Rong, X.M.; Dai, K.; Liang, W.; Huang, Q.Y. Adsorption of Pseudomonas putida on soil particle size fractions: Effects of solution chemistry and organic matter. J. Soils Sediments 2012, 12, 143–149. [Google Scholar] [CrossRef]
- Liao, C.; Liang, X.; Soupir, M.L.; Jarboe, L.R. Cellular, particle and environmental parameters influencing attachment in surface waters: A review. J. Appl. Microbiol. 2015, 119, 315–330. [Google Scholar] [CrossRef]
- Miller, S.E.; Bell, C.S.; Mejías, R.; McClain, M.S.; Cover, T.L.; Giorgio, T.D. Colistin-functionalized nanoparticles for the rapid capture of Acinetobacter baumannii. J. Biomed. Nanotechnol. 2016, 12, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zhuang, S.T.; Chen, R.; Liu, Y.; Wang, J.L. Magnetic COFs for the adsorptive removal of diclofenac and sulfamethazine from aqueous solution: Adsorption kinetics, isotherms study and DFT calculation. J. Hazard. Mater. 2020, 385, 121596. [Google Scholar] [CrossRef]
- Pan, J.Y.; Bai, X.T.; Li, Y.Y.; Yang, B.H.; Yang, P.Y.; Yu, F. HKUST-1 derived carbon adsorbents for tetracycline removal with excellent adsorption performance. J. Ma Environ. Res. 2022, 205, 112425. [Google Scholar] [CrossRef]
- Lyu, F.; Niu, S.L.; Wang, L.; Liu, R.Q.; Sun, W.; He, D.D. Efficient removal of Pb(II) ions from aqueous solution by modified red mud. J. Hazard. Mater. 2021, 406, 124678. [Google Scholar] [CrossRef] [PubMed]
- Pethica, B. Microbial Adhesion to Surfaces; Berkeley, R.C.W., Lynch, J.M., Melling, J., Rutter, P.R., Vincent, B., Eds.; London Society of Chemical Industry: London, UK, 1980; p. 19. [Google Scholar]
- Rivett, M.O.; Tremblay-Levesque, L.C.; Carter, R.; Thetard, R.C.H.; Tengatenga, M.; Phoya, A.; Mbalame, E.; Mchilikizo, E.; Kumwenda, K.; Mleta, P.; et al. Acute health risks to community hand-pumped groundwater supplies following Cyclone Idai flooding. Sci. Total Environ. 2022, 806, 150598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Walker, S.L.; Huang, Q.Y.; Cai, P. Adhesion of bacterial pathogens to soil colloidal particles: Influences of cell type, natural organic matter, and solution chemistry. Water Res. 2014, 53, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Li, J.Y.; Jiang, J.; Hong, Z.N.; Xu, R.K. Adhesion of Escherichia coli to nano-Fe/Al oxides and its effect on the surface chemical properties of Fe/Al oxides. Colloids Surf. B Biointerfaces 2013, 110, 289–295. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Li, J.; Du, X.; Zhou, T.; Xie, B.; He, L. Synthesis and Characterization of Colistin-Functionalized Silica Materials for Rapid Capture of Bacteria in Water. Molecules 2022, 27, 8292. https://doi.org/10.3390/molecules27238292
Qiu J, Li J, Du X, Zhou T, Xie B, He L. Synthesis and Characterization of Colistin-Functionalized Silica Materials for Rapid Capture of Bacteria in Water. Molecules. 2022; 27(23):8292. https://doi.org/10.3390/molecules27238292
Chicago/Turabian StyleQiu, Jingli, Jianli Li, Xiaoxi Du, Tong Zhou, Bingbing Xie, and Limin He. 2022. "Synthesis and Characterization of Colistin-Functionalized Silica Materials for Rapid Capture of Bacteria in Water" Molecules 27, no. 23: 8292. https://doi.org/10.3390/molecules27238292
APA StyleQiu, J., Li, J., Du, X., Zhou, T., Xie, B., & He, L. (2022). Synthesis and Characterization of Colistin-Functionalized Silica Materials for Rapid Capture of Bacteria in Water. Molecules, 27(23), 8292. https://doi.org/10.3390/molecules27238292