Lupane Triterpene Derivatives Improve Antiproliferative Effect on Leukemia Cells through Apoptosis Induction
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Produces
4.2. Plant Material
4.3. Extraction and Isolation
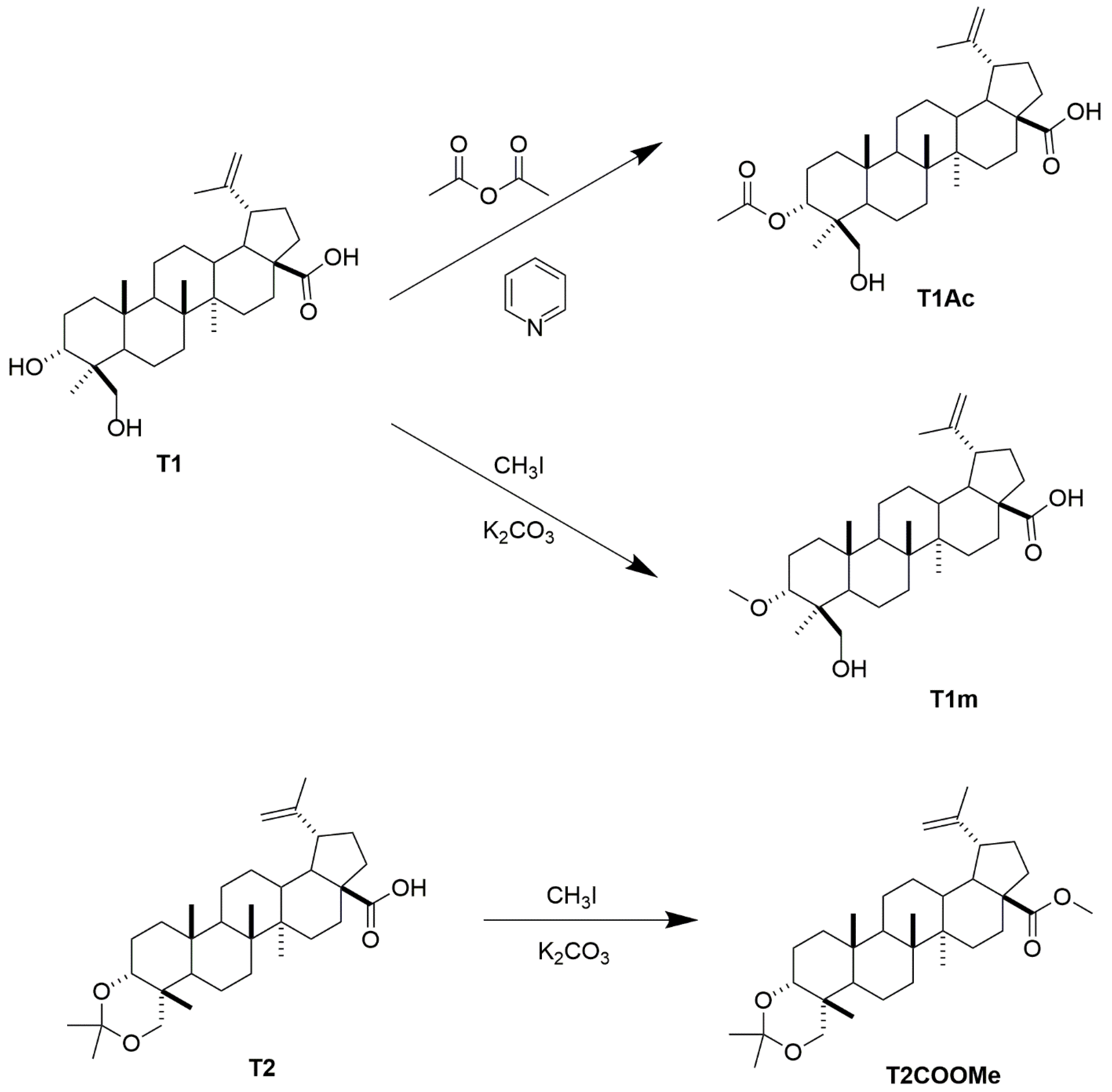
4.4. Preparation of Compound T1m from 1
4.5. Preparation of Compound T1Ac from T1
4.6. Preparation of Compound T2m from T2
4.7. Cell Lines
4.8. Compounds and Controls
4.9. Bioassay of Viability in Leucemic Cell Lines
4.10. Bioassay of Viability in Vero Cell Line
4.11. Viability Test in Normal Mononuclear Cells
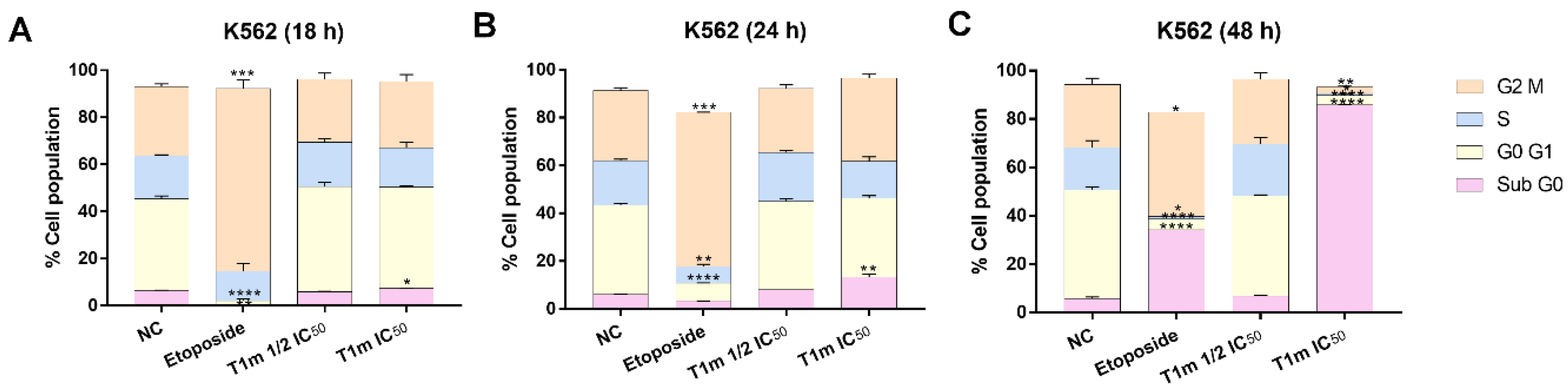
4.12. Cell Cycle Assay
4.13. Apoptosis Assay
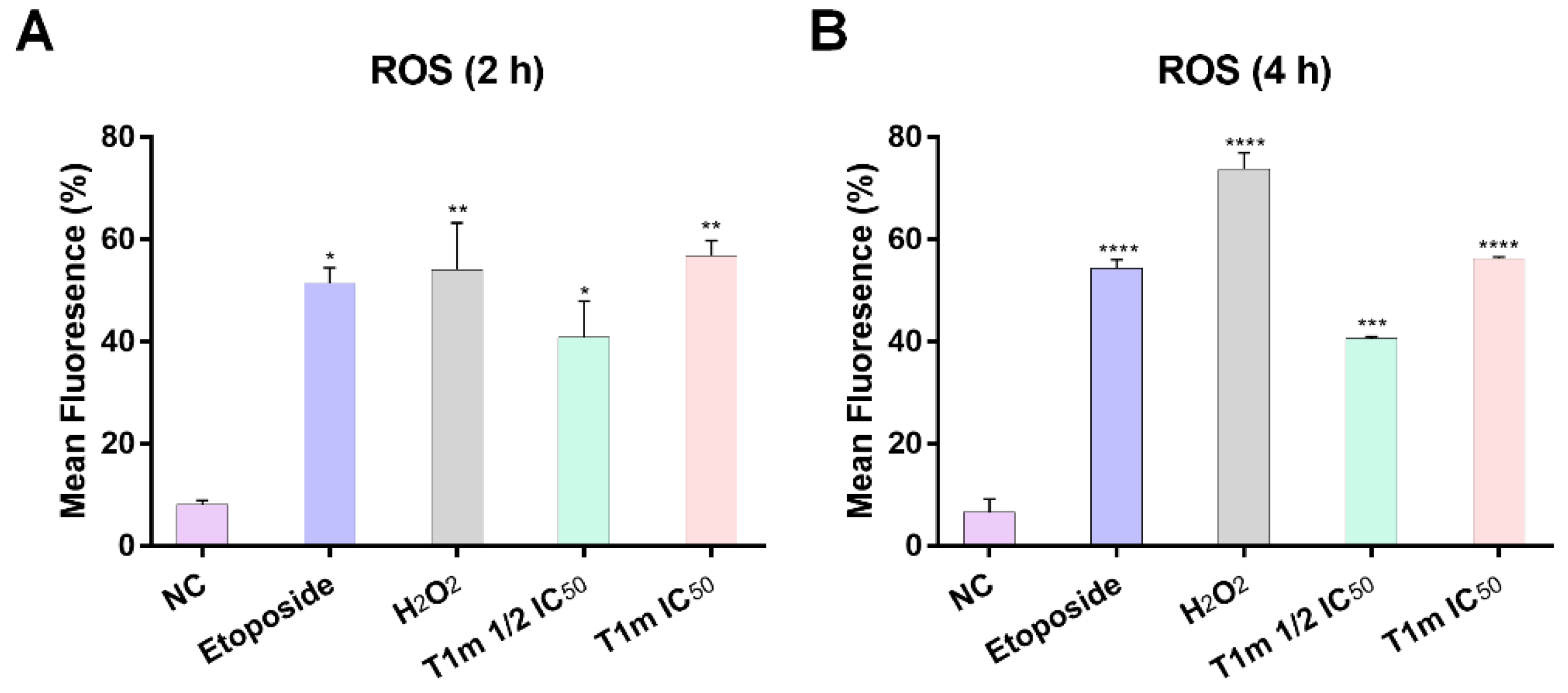
4.14. Assessment of Intracellular Reactive Oxygen Species (ROS)
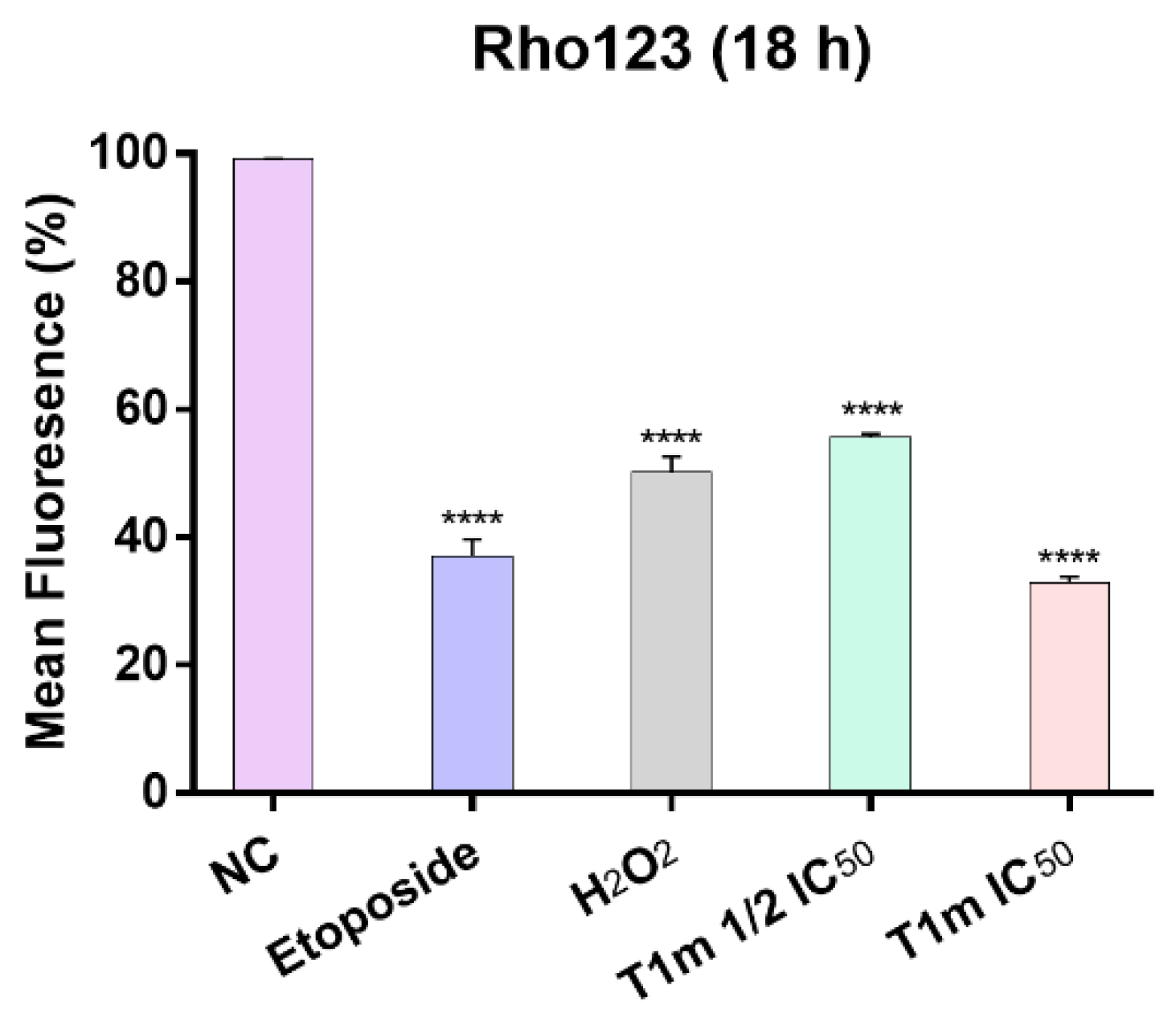
4.15. Assessment of Mitochondrial Membrane Potential (ΔψM)
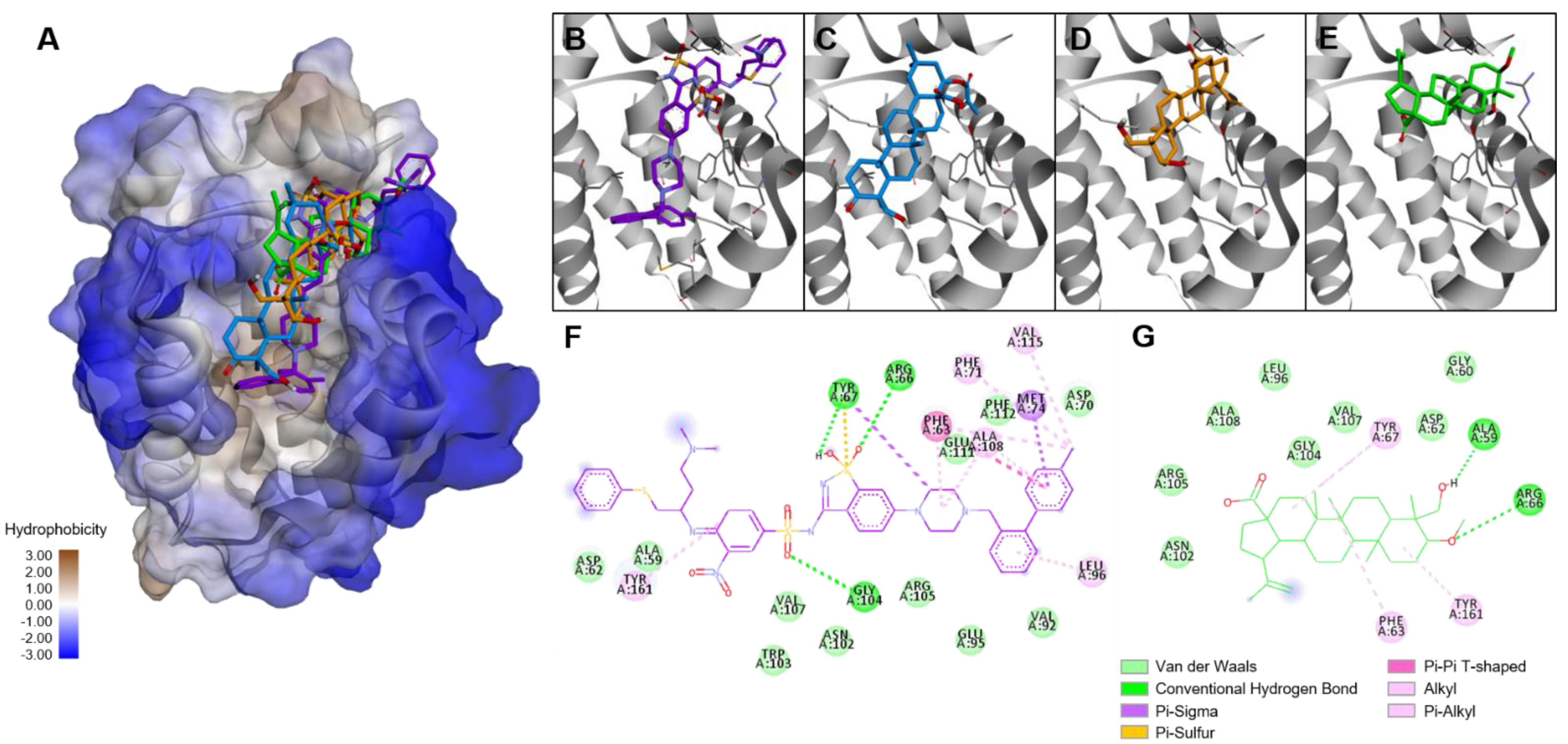
4.16. Molecular Docking
4.17. Analysis of Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistis, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr (accessed on 2 September 2022).
- Ahmad, S.; Kifayatullah; Shah, K.A.; Hussain, H.; Haq, A.U.; Ullah, A.; Khan, A.; Rahman, N.U. Prevalence of acute and chonic forms of leukemia in various regions of Khyber Pakhtunkhawa, Pakistan: Needs much to be done! Bangladesh J. Med. Sci. 2019, 18, 222–227. [Google Scholar] [CrossRef]
- Frazer, R.; Irvine, A.E.; McMullin, M.F. Chronic myeloid leukemia in the 21st century. Ulster Med. J. 2007, 76, 8–17. [Google Scholar] [PubMed]
- Moreno-Lorenzana, D.L.; Avilés-Vazquez, S.; Sandoval-Esquivel, M.A.; Alvarado-Moreno, A.; Ortiz-Navarrete, V.; Torres-Martínez, H.; Ayala-Sánchez, M.; Mayani, H.; Chavez-González, A. CDKIs p18INK4c and p57Kip2 are involved in quiescence of CML leukemic stem cells after treatment with TKI. Cell Cycle 2016, 15, 1276–1287. [Google Scholar] [CrossRef]
- O’Reilly, E.; Alizadeh-Zeinabad, H.; Szegezdi, E. Hematopoietic versus leukemic stem cell quiescence: Challenges and therapeutic opportunities. Blood Rev. 2021, 50, 100850. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly for decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, C.; Zhou, X.; Han, Y.; He, Y.; Ouyang, J.; Zhou, W.; Wang, Z.; Wang, H.; Li, G. Anti-inflamatory activity of three triterpene from Hippophae rhamnoides L. Lipopolysaccharide-stimulated RAW264.7 cells. Int. J. Mol. Sci. 2021, 22, 12009. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical role of polyphenols and triterpenes present in the extract of fruits and leaves of Olea europea as antioxidants, anti-infectives and anticancer agents on healty growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef]
- Darshani, P.; Sarma, S.S.; Srivastava, A.K.; Baishya, R.; Kumar, D. Anti-viral triterpenes: A review. Phytochem. Rev. 2022, 21, 1761–1842. [Google Scholar] [CrossRef]
- Mokoka, T.A.; McGaw, L.J.; Mdee, L.K.; Bagla, V.P.; Iwalewa, E.O.; Eloff, J.N. Antimicrobial activity and cytotoxicy of triterpenes isolated from leaves of Maytenus undata (Celastraceae). BMC Complement. Altern. Med. 2013, 13, 111. [Google Scholar] [CrossRef]
- Lehbili, M.; Magid, A.A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Abedini, A.; Morjani, H.; Gangloff, S.C.; Kabouche, Z. Antibaterial, antioxidant and cytotoxic activities of triterpenes and flavonoids from the aerial parts of Salvia barrelieri Etl. Nat. Prod. Res. 2017, 32, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Sajid, A.R.; Javeed, A.; Aslam, M.; Ahsan, T.; Hussain, D.; Mateen, A.; Li, X.; Qin, P.; Ji, M. Antioxidant, antifungal, and aphicidal activity of the triterpenoids spinasterol and 22,23-dihydrospinasterol from leaves of Citrullus colocynthis L. Sci. Rep. 2022, 12, 4910. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic triterpenoids with nitrogen-containing heterocyclic moiety, privileged hybrids in anticancer drug discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Semenova, A.A.; Nedopekina, D.A.; Davletshin, E.V.; Spivak, A.Y.; Belosludtsev, K.N. Effect of F16-Betulin conjugate on mitochondrial membranes and its role in cell death initiation. Membranes 2021, 11, 352. [Google Scholar] [CrossRef]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef]
- Alfaro-Almaguer, J.; Mejía-Manzano, L.A.; González-Valdez, J. State-of-the-art and opportunities for bioactive pentacyclic triterpenes from native mexican plants. Plants 2022, 11, 2184. [Google Scholar] [CrossRef]
- Valencia-Chan, L.S.; García-Cámara, I.; Torres-Tapia, L.W.; Moo-Puc, R.E.; Peraza-Sánchez, S.R. Lupane-type triterpenes of Phoradendron vernicosum. J. Nat. Prod. 2017, 80, 3038–3042. [Google Scholar] [CrossRef]
- Valencia-Chan, L.S.; Moreno-Lorenzana, D.; Ceballos-Cruz, J.J.; Peraza-Sánchez, S.R.; Chávez-González, A.; Moo-Puc, R.E. Apoptotic and Cell Cycle Effects of Triterpenes Isolated from Phoradendron wattii on Leukemia Cell Lines. Molecules 2022, 27, 5616. [Google Scholar] [CrossRef]
- Urban, M.; Sarek, J.; Klinot, J.; Korinkova, G.; Hajduch, M. Synthesis of Aseco Derivatives of betulinic acid with cytotoxic activity J. Nat. Prod. 2004, 67, 1100–1105. [Google Scholar] [CrossRef]
- Hata, K.; Ogawa, S.; Makino, M.; Mukaiyama, T.; Hori, K.; Iida, T.; Fujimoto, Y. Lupane triterpenes with a carbonyl group at C-20 induce cancer cell apoptosis. J. Nat. Med. 2008, 62, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Hori, K.; Ogasawara, H.; Takihashi, S. Anti-leukemia activities of lup-28-al-20(29)-en-3-one, a lupane triterpene. Toxicol. Lett. 2003, 143, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Hori, K.; Takahashi, S. Differentiation-and Apoptosis-Inducing activities by pentacyclic triterpenes on a mouse melanoma cell line. J. Nat. Prod. 2002, 65, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Maanen, J.M.S.; Retél, J.; de Vries, J.; Pinedo, H.M. Mechanism of action of antitumor drug etoposide: A review. J. Natl. Cancer Inst. 1988, 80, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Basso, I.N.; Kim, D.D.H. Targets spectrum of the BCR-ABL tyrosine kinase inhinitors in chronic myeloid leukemia. Int. J. Hematol. 2021, 113, 632–641. [Google Scholar] [CrossRef]
- Boni, C.; Sori, C. Current views on the interplay between tyrosine kinases and phosphatases in Chonic myeloid leukemia. Cancers 2021, 13, 2311. [Google Scholar] [CrossRef]
- Okabe, S.; Tauchi, T.; Tanaka, Y.; Ohyashiki, K. Therapeutic targeting of aurora A kinase in Philadelphia chromosome-positive ABL tyrosine kinase inhibitor-resistant cells. Oncotarget 2018, 9, 32496–32506. [Google Scholar] [CrossRef][Green Version]
- Pizzatti, L.; Sá, L.A.; de Souza, J.M.; Bisch, P.M.; Abdelhay, E. Altered protein in chronic myeloid leukemia chronic phase identified by a comparative proteomic study. Biochim. Biophys. Acta 2006, 1764, 926–942. [Google Scholar] [CrossRef]
- He, R.; Liu, B.; Yang, C.R.; Han, Z.C. Inhibition of K562 leukemia angiogenesis and growth by expression of antisense vascular endothelial growth factor (VEGF) sequence. Cancer Gene Ther. 2003, 10, 879–886. [Google Scholar] [CrossRef]
- Song, G.; Li, Y.; Jaing, G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets (review). Oncol. Rep. 2012, 28, 1935–1944. [Google Scholar] [CrossRef]
- Jaiswal, S.; Traver, D.; Miyamoto, T.; Akashi, K.; Lagasse, E.; Weissman, I.L. Expression of BCR/ABL and BCL-2 in myeloid progenitors lead to myeloid leukemias. PNASM 2003, 100, 10002–10007. [Google Scholar] [CrossRef] [PubMed]
- Litaudon, M.; Jolly, C.; le Callonec, C.; din Cuong, D.; Retailleau, P.; Nosjoean, O.; van Nuguyen, H.; Pfeiffer, B.; Boutin, J.A.; Guéritte, F. Cytotoxic pentacyclic triterpenoids from Combretum sundaicum and Lantana camara as inhibitors of Bcl-xL/BakBH3 domain pepetide interation. J. Nat. Prod. 2009, 72, 1314–1320. [Google Scholar] [CrossRef]
- Elhady, S.S.; Ibrahim, E.A.; Goda, M.S.; Nafie, M.S.; Samir, H.; Diri, R.M.; Alahdal, A.M.; Thomford, A.K.; Gindy, A.E.; Hadad, G.M.; et al. GC-MS/MS Quantification of EGFR inhibitors, β-sitosterol, betulinic acid, (+) eriodictyol, (+) epipinoresinol, and secoisolariciresinol, in crude extract and ethyl acetate fraction of Thonnongia sanguinea. Molecules 2022, 27, 4109. [Google Scholar] [CrossRef]
- Roba, K. The role of terpene (secondary metabolite). Nat. Prod. Chem. Res. 2021, 9, 411. [Google Scholar]
- Ghante, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemopreventivion and anticancer treatment: An overview on targets and underling mechanism. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 359–424. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.-H.; Moreno-Jiménez, M.R.; Gónzalez-Laredo, R.F.; Gallegos-Infante, J.A.; Rocha-Gúzman, N.E. Lupane-type triperpenes and their anti-cancer activities against most common malignant tumors: A Review. EXCLI J. 2016, 15, 758–771. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Changwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor oh human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Nakagawa-Goto, K.; Yamada, K.; Taniguchi, M.; Tokuda, H.; Lee, K.H. Cancer preventive agents 9. Betulinic acid derivatives as potent cancer chemopreventive agents. Bioorg. Med. Chem. Lett. 2009, 19, 3378–3381. [Google Scholar] [CrossRef]
- Brandes, B.; Hoenke, S.; Starke, N.; Serbian, I.; Deigner, H.P.; Al-Harrasi, A.; Csuk, R. Synthesis and cytotoxicity of apoptosis-inducing N-heterocyclic triterpene amides. Eur. J. Med. Chem. 2022, 6, 100085. [Google Scholar] [CrossRef]
- Ikeda, T.; Sporn, M.; Honda, T.; Gribble, G.W.; Kufe, D. The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res. 2003, 63, 5551–5558. [Google Scholar] [PubMed]
- Dubini, M.V.; Semenova, A.A.; Ilzorkiva, A.I.; Mikheeva, I.B.; Yashin, V.A.; Penkov, N.V.; Vydrina, V.A.; Ishmuratov, G.Y.; Sharapov, V.A.; Khoroshavina, E.I.; et al. Effect of betulin and betulonic acid on isolated rat liver mitochondria and liposomes. Biochim. Biophys. Acta Biomembr. 2020, 10, 183383. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, G.G.; Simoes, M.; Olivera, R.R.; Kaplan, M.A.C.; Gattass, C.R. 3β-acetyl tormentic acid induces apoptosis of resistant leukemia cells independently of P-gp/ABCB1 activity or expression. Investig. New Drugs 2012, 30, 105–113. [Google Scholar] [CrossRef]
- Debatin, K.M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004, 53, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.S. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Boyd, L.; Rivas, F. Triterpenoids as reactive oxygen species modulators of cell fate. Chem. Res. Toxicol. 2022, 35, 569–584. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Zhao, M.; Wang, Y.; Cheng, X.; Wang, D.; Xu, Y.; Du, Z.; Yu, X. Celastrol Targets Mitochondrial Respiratory Chain Complex I to Induce Reactive Oxygen Species-Dependent Cytotoxicity in Tumor Cells. BMC Cancer 2011, 11, 170. [Google Scholar] [CrossRef]
- Serafim, T.L.; Carvalho, F.S.; Bernardo, T.C.; Pereira, G.C.; Perkins, E.; Holy, J.; Krasutsky, D.A.; Kolomistsyna, O.N.; Krasutskym, P.A.; Olivera, P.J. New derivatives of lupane triterpenoids disturb breast cancer mitochondria and induce cell death. Bioorg. Med. Chem. 2014, 22, 6270–6287. [Google Scholar] [CrossRef]
- Leanza, L.; Checchetto, V.; Biasutto, L.; Rossa, A.; Costa, R.; Bachmann, M.; Zoratti, M.; Szabo, I. Pharmacological modulation of mitochondrial ion channels. Br. J. Pharmacol. 2019, 176, 4258–4283. [Google Scholar] [CrossRef]
- Elmore, S. Apopotosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Alam, S.; Shamsi, A.; Adnan, M.; Elasbali, A.M.; Al-Soud, W.A.; Alreshidi, M.; Hawsawi, Y.M.; Tippana, A.; Pasupuleti, V.R.; et al. Bax/Bcl-2 cascade is regulated by the EGFR Pathway: Therapeutic targeting of non-small cell lung cancer. Front. Oncol. 2022, 12, 869672. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Ito, F. EGF receptor in relation to tumor development: Molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010, 277, 316–326. [Google Scholar] [CrossRef]
- Deng, J.; Shimamura, T.; Perera, S.; Carlson, N.E.; Cai, D.; Shapiro, G.I.; Wong, K.K.; Letai, A. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007, 64, 11867–11875. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Kroemer, G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013, 23, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Meng, X.W.; Flatten, K.S.; Loegering, D.A.; Kaufmann, S.H. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ. 2013, 20, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Engeland, M.; Nieland, L.J.W.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, P.M. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, druglikeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Soymaya, T.; Lakshmipriya, T.; Klika, K.D.; Jayasree, P.R.; Kumar, P.R.M. Anticancer potential of rhizome extract and a labdane diterpenoid from Curcuma mutabilis plant endemic to Western Ghats of India. Sci. Rep. 2021, 11, 552. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Bautista, C.M.; Torres-Tapia, L.-W.; Lagunas-Martínez, A.; Contreras-Ochoa, C.O.; Peraza-Sánchez, S.R.; Moo-Puc, R.E. Secundiflorol G isolated from Aeschynomene fascicularis, a Mayan medicinal plant, induces apoptosis in cervical cancer cells. Nat. Prod. Res. 2019, 35, 826–828. [Google Scholar] [CrossRef] [PubMed]
- RCSB PDB. Protein Data Bank. Available online: https://www.rcsb.org (accessed on 3 August 2022).


| Compound | IC50 (μg/mL) | |||
|---|---|---|---|---|
| K562 | HL60 | VERO | MNC | |
| T1 | 100 ± 1.0 (IS 1.37) | >100 | 137.2 ± 1.1 | — |
| T1Ac | >100 | >100 | >200 | — |
| T1m | 39.49 ± 1.1 (IS 1.69, 2.19) | 68.56 ± 1.2 (IS 0.97, 1.26) | 66.76 ± 1.0 | 86.49 ±1.2 |
| T2 | >100 | >100 | >200 | — |
| T2COOMe | >100 | >100 | >200 | — |
| Etoposide | 14.7 ± 1.2 (IS 2.00, 6.80) | 1.30 ± 0.3 (IS 22.63, 76.92) | 29.42 ± 1.1 | >100 |
| Compound | Score (kcal/mol) | |
|---|---|---|
| BCL-2 | EGFR (TK Domain) | |
| T1 | −4.59 | −4.78 |
| T1m | −5.27 | −4.91 |
| Icterogenin | −5.59 | — |
| Betulinic acid | — | −4.75 |
| co-crystalized inhibitor | −10.13 | −7.19 |
| PDB Entry | Search Volume | |||||
|---|---|---|---|---|---|---|
| Minimum (Å) | Maximun (Å) | |||||
| x | y | z | x | y | z | |
| 4IEH | −0.30965 | 18.84145 | 0.74902 | 22.77645 | 32.09515 | 19.18412 |
| 1M17 | 12.28565 | −3.87359 | 42.20015 | 31.74615 | 11.64701 | 57.52825 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Chan, L.S.; Estrada-Alfaro, N.; Ceballos-Cruz, J.J.; Torres-Tapia, L.W.; Peraza-Sánchez, S.R.; Moo-Puc, R.E. Lupane Triterpene Derivatives Improve Antiproliferative Effect on Leukemia Cells through Apoptosis Induction. Molecules 2022, 27, 8263. https://doi.org/10.3390/molecules27238263
Valencia-Chan LS, Estrada-Alfaro N, Ceballos-Cruz JJ, Torres-Tapia LW, Peraza-Sánchez SR, Moo-Puc RE. Lupane Triterpene Derivatives Improve Antiproliferative Effect on Leukemia Cells through Apoptosis Induction. Molecules. 2022; 27(23):8263. https://doi.org/10.3390/molecules27238263
Chicago/Turabian StyleValencia-Chan, Lía S., Neptis Estrada-Alfaro, Jimmy Josué Ceballos-Cruz, Luis W. Torres-Tapia, Sergio R. Peraza-Sánchez, and Rosa E. Moo-Puc. 2022. "Lupane Triterpene Derivatives Improve Antiproliferative Effect on Leukemia Cells through Apoptosis Induction" Molecules 27, no. 23: 8263. https://doi.org/10.3390/molecules27238263
APA StyleValencia-Chan, L. S., Estrada-Alfaro, N., Ceballos-Cruz, J. J., Torres-Tapia, L. W., Peraza-Sánchez, S. R., & Moo-Puc, R. E. (2022). Lupane Triterpene Derivatives Improve Antiproliferative Effect on Leukemia Cells through Apoptosis Induction. Molecules, 27(23), 8263. https://doi.org/10.3390/molecules27238263







