Energy Electronegativity and Chemical Bonding
Abstract
1. Introduction
2. Electronegativity
2.1. Electronegativity of Free Atoms
2.2. Electronegativity of Atoms in Molecules
2.3. Electronegativity of Atoms in Solids
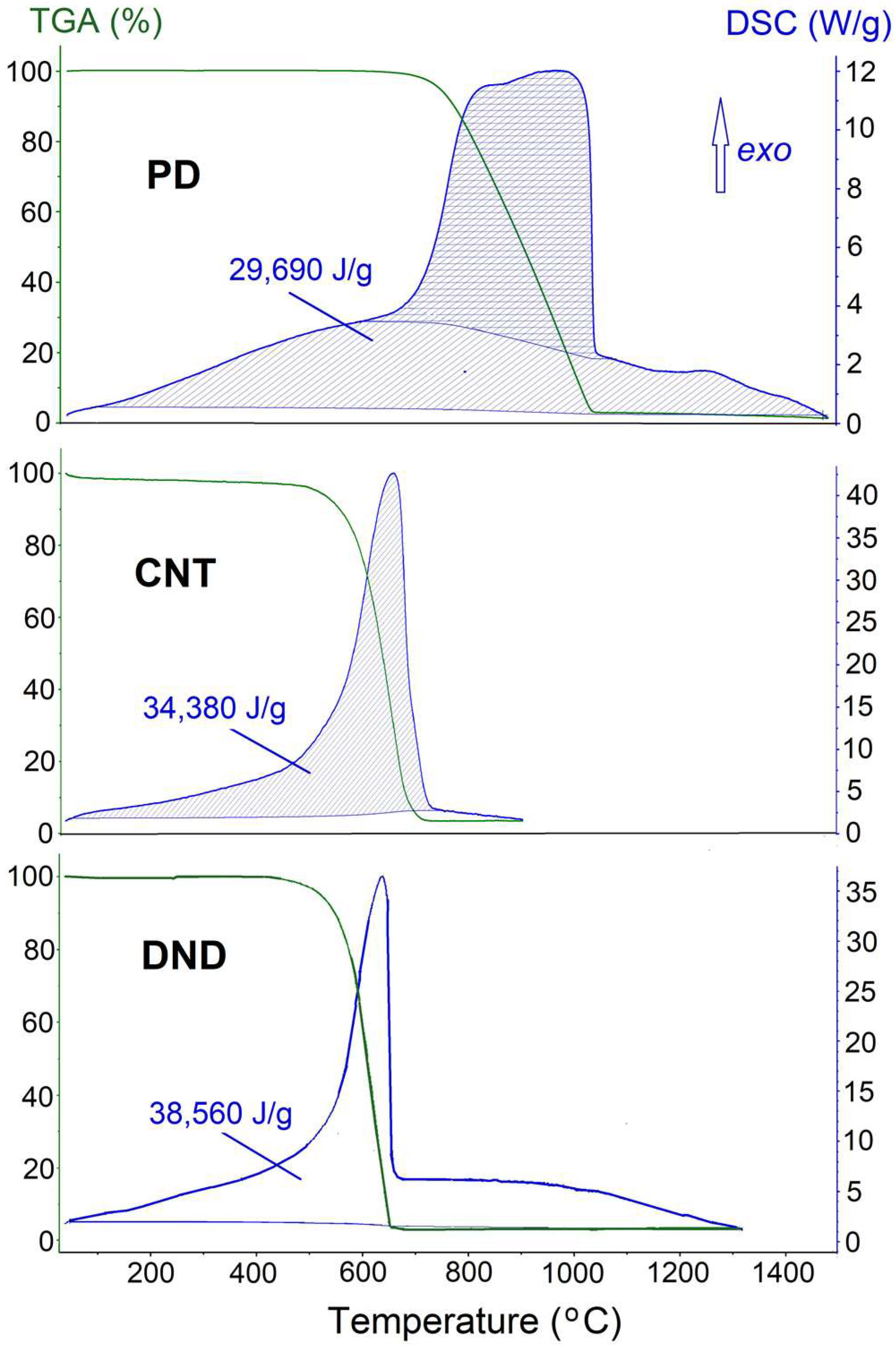
2.4. Electronegativity of Nanosized Elements
3. Effective Charges of Atoms
3.1. Bond Polarity in Molecules
3.2. Equalization of Electronegativity of Atoms in Molecules
3.3. Effective Charges of Atoms in Solid Compounds
3.4. Coordination Charges of Atoms in Chemical Compounds
3.5. Change of Chemical Bonding under Pressure
4. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, G.N. The atom and the molecule. J. Am. Chem. Soc. 1916, 38, 762–785. [Google Scholar] [CrossRef]
- Ingold, C.K. The principles of aromatic substitution, from the standpoint of the electronic theory of valency. Rec. Trav. Chim. 1929, 48, 797–812. [Google Scholar] [CrossRef]
- Jensen, W.B. Electronegativity from Avogadro to Pauling. I. Origins of the electronegativity concept. J. Chem. Educ. 1996, 73, 11–20. [Google Scholar] [CrossRef]
- Jensen, W.B. Electronegativity from Avogadro to Pauling: II. Late nineteenth- and early twentieth-century developments. J. Chem. Educ. 2003, 80, 279–287. [Google Scholar] [CrossRef]
- Sproul, G.D. Evaluation of electronegativity scales. ACS Omega 2020, 5, 11585–11594. [Google Scholar] [CrossRef]
- Fajans, K.; Berlin, T. Quantization of molecules, inter- and intra-molecular forces. Phys. Rev. 1943, 63, 309–312. [Google Scholar] [CrossRef]
- Fajans, K. Quantikel-Theorie der chemischen Bindung. Chimia 1959, 13, 349–366. [Google Scholar]
- Batsanov, S.S. On the relationship between the theory of polarization and the concept of electronegativity. Russ. J. Inorg. Chem. 1957, 2, 1482–1487. (In Russian) [Google Scholar]
- Pauling, L. The nature of the chemical bond IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 1932, 54, 3570–3582. [Google Scholar]
- Mulliken, R.S. A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 1934, 2, 782–793. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic structures of molecules. Electroaffinity, molecular orbitals and dipole moments. J. Chem. Phys. 1935, 3, 573–585. [Google Scholar] [CrossRef]
- Hinze, J.; Jaffé, H.H. Electronegativity. Orbital electronegativity of neutral atoms. J. Am. Chem. Soc. 1962, 84, 540–546. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Martynov, A.I.; Batsanov, S.S. A new approach to the determination of the electronegativity of atoms. Russ. J. Inorg. Chem. 1980, 25, 1737–1739. [Google Scholar]
- Allen, A.C. Electronegativity is the average one-electron energy of the valence-shell electrons in ground-state free atoms. J. Am. Chem.Soc. 1989, 111, 9003–9014. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Electronegativity—A perspective. J. Mol. Model. 2018, 24, 214–221. [Google Scholar] [CrossRef]
- Rahm, M.; Zeng, T.; Hoffmann, R. Electronegativity seen as the ground-state average valence electron bonding energy. J. Am. Chem. Soc. 2019, 141, 342–351. [Google Scholar] [CrossRef]
- Batsanov, S.S.; Batsanov, A.S. Introduction to Structural Chemistry; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Campero, A.; Díaz Ponce, J.A. Averaged scale in electronegativity joined to physicochemical perturbations. Consequences of periodicity. ACS Omega 2020, 5, 25520–25542. [Google Scholar] [CrossRef]
- Sproul, G. Cardinal electronegativity values correlate with physicochemical properties. Inorg. Chem. 2021, 60, 10970–10978. [Google Scholar] [CrossRef]
- Tantardini, C.; Oganov, A.R. Thermochemical electronegativities of the elements. Nat. Commun. 2021, 12, 2087. [Google Scholar] [CrossRef]
- Batsanov, S.S. Thermochemical electronegativities of metals. Russ. J. Phys. Chem. 2000, 74, 267–270. [Google Scholar]
- Stevenson, D.P. Heat of chemisorption of hydrogen in metals. J. Chem. Phys. 1955, 23, 203. [Google Scholar] [CrossRef]
- Phillips, J.C. Ionicity of the chemical bond in crystals. Rev. Mod. Phys. 1970, 42, 317–356. [Google Scholar] [CrossRef]
- Trasatti, S. Electronegativity, work function, and heat of adsorption of hydrogen on metals. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1972, 68, 229–236. [Google Scholar] [CrossRef]
- Frese, K.W., Jr. Simple method for estimating levels of solids. J. Vac. Sci. Technol. 1979, 16, 1042–1044. [Google Scholar] [CrossRef]
- Duffy, J.A. Variable electronegativity of oxygen in binary oxides: Possible relevance to molten fluorides. J. Chem. Phys. 1977, 67, 2930–2931. [Google Scholar] [CrossRef]
- Duffy, J.A. Chemical bonding in the oxides of the elements: A new appraisal. J. Solid State Chem. 1986, 62, 145–157. [Google Scholar] [CrossRef]
- Batsanov, S.S. System of electronegativities and effective atomic charge in crystalline compounds. Russ. J. Inorg. Chem. 1975, 20, 1437–1440. [Google Scholar]
- Pettifor, D.G. A chemical scale for crystal-structure maps. Solid State Commun. 1984, 5, 31–34. [Google Scholar] [CrossRef]
- Pettifor, D.G. Structure maps revisited. J. Phys. Condens. Matter 2003, 15, V13–V16. [Google Scholar] [CrossRef]
- Batsanov, S.S.; Batsanov, A.S. Solid-state electronegativity of atoms: New approaches. Acta Cryst. B 2021, 77, 495–505. [Google Scholar] [CrossRef]
- Wilson, R.G.; Stevie, F.A.; Magee, C.W. Secondary Ion Mass Spectrometry: A Practical Handbook for Depth Profiling and Bulk Impurity Analysis; Wiley-Interscience: New York, NY, USA, 1989. [Google Scholar]
- Yang, C.C.; Mai, Y.-W. Thermodynamics at the nanoscale: A new approach to the investigation of unique physicochemical properties of nanomaterials. Mater. Sci. Eng. R Rep. 2014, 79, 1–40. [Google Scholar]
- Shandiz, M.A.; Safaei, A.; Sanjabi, S.; Barber, Z.H. Modeling the cohesive energy and melting point of nanoparticles by their average coordination number. Solid State Commun. 2008, 145, 432–437. [Google Scholar] [CrossRef]
- Pirkkalainen, K.; Serimaa, R. Coordination number in ideal spherical nanocrystals. J. Appl. Cryst. 2009, 42, 442–447. [Google Scholar] [CrossRef]
- Batsanov, S.S.; Dan’kin, D.A. Size effect in cohesive energy of elements. Mater. Chem. Phys. 2017, 196, 245–248. [Google Scholar] [CrossRef]
- Kulakova, I.I. Surface chemistry of nanodiamonds. Phys. Solid State 2004, 46, 636–643. [Google Scholar] [CrossRef]
- Chang, S.L.Y.; Dwyer, C.; Osawa, E.; Barnard, A.S. Size dependent surface reconstruction in detonation nanodiamonds. Nanoscale Horiz. 2018, 3, 213–217. [Google Scholar] [CrossRef]
- Fang, X.W.; Mao, J.D.; Levin, E.M.; Schmidt-Rohr, K. Nonaromatic core-sell structure of nanodiamond from solid-state NMR spectroscopy. J. Am. Chem. Soc. 2009, 131, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Kadas, K.; Nabi, Z.; Kwon, S.K.; Vitos, L.; Ahuja, R.; Johansson, B. Surface relaxation and surface stress of 4d metals. Surf. Sci. 2006, 600, 395–402. [Google Scholar] [CrossRef]
- Batsanov, S.S.; Guriev, E.D.L.; Gavrilkin, S.M.; Hamilton, K.A.; Lindsey, K.; Mendis, B.G.; Riggs, H.J.; Batsanov, A.S. On the nature of fibres grown from nanodiamond colloids. Mater. Chem. Phys. 2016, 173, 325–332. [Google Scholar]
- Taylor, C.D.; Neurock, M.; Scully, J.R. First-principles investigation of the fundamental corrosion properties of a model Cu38 nanoparticle and the (111), (113) surfaces. J. Electrochem. Soc. 2008, 155, C407–C414. [Google Scholar] [CrossRef]
- Tang, L.; Han, B.; Persson, K.; Friesen, C.; He, T.; Sieradzki, K.; Ceder, G. Electrochemical stability of nanometer-scale Pt particles in acidic environments. J. Am. Chem. Soc. 2010, 132, 596–600. [Google Scholar] [CrossRef]
- Tang, I.; Li, X.; Cammarata, R.C.; Friesen, C.; Sieradzki, K. Electrochemical stability of elemental metal nanoparticles. J. Am. Chem. Soc. 2010, 132, 11722–11726. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, S.S.; Gavrilkin, S.M.; Shatalova, T.B.; Mendis, B.G.; Batsanov, A.S. Fixation of atmospheric nitrogen by nanodiamonds. New J. Chem. 2018, 42, 11160–11164. [Google Scholar] [CrossRef]
- Bader, R.W.F. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Meister, J.; Schwarz, W.H.E. Principal components of ionicity. J. Phys. Chem. 1994, 98, 8245–8252. [Google Scholar] [CrossRef]
- Coppens, P. X-ray Charge Densities and Chemical Bonding; Oxford University: Oxford, UK, 1997. [Google Scholar]
- Fajans, K. Deformation von Ionen und Molekeln auf Grund refraktometrischer Daten. Z. Phys. 1928, 50, 531–536. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Pauling, L. Interatomic distances and bond character in the oxygen acids and related substances. J. Phys. Chem. 1952, 56, 361–365. [Google Scholar] [CrossRef]
- Thomas, J.M.; Walker, N.R.; Cooke, S.A.; Gerry, M.C.L. Microwave spectra and structures of KrAuF, KrAgF, and KrAgBr; 83Kr nuclear quadrupole coupling and the nature of noble gas-noble metal halide bonding. J. Am. Chem. Soc. 2004, 126, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Steimle, T.C.; Cheng, L.; Stanton, J.F. The permanent electric dipole moment of gold chloride, AuCl. Mol. Phys. 2015, 113, 2073–2080. [Google Scholar] [CrossRef]
- Coulson, C.A. Valence; Clarendon Press: Oxford, UK, 1952. [Google Scholar]
- Hou, S.; Bernath, P.F. Relationship between dipole moments and harmonic vibrational frequencies in diatomic molecules. J. Phys. Chem. A 2015, 119, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Qureshi, A.H.; Wei, Z. Atomic charges in highly ionic diatomic molecules. ACS Omega 2018, 3, 17180–17187. [Google Scholar] [CrossRef] [PubMed]
- Groß, L.; Herrmann, C. Local electric dipole moments: A generalized approach. J. Comput. Chem. 2016, 37, 2260–2265. [Google Scholar] [CrossRef] [PubMed]
- Gussoni, M.; Castiglioni, C.; Zerbi, G. Physical meaning of electrooptical parameters derived from infrared intensities. J. Phys. Chem. 1984, 88, 600–604. [Google Scholar] [CrossRef]
- Gussoni, M.; Castiglioni, C.; Ramos, M.N.; Zerbi, G. Ab initio counterpart of infrared atomic charges. Chem. Phys. Lett. 1987, 142, 515–518. [Google Scholar] [CrossRef]
- Haiduke, R.L.A.; Bruns, R.E. An atomic charge-charge flux-dipole flux atom-in-molecule decomposition for molecular dipole-moment derivatives and infrared fundamental intensities. J. Phys. Chem. A 2005, 109, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Richter, W.E.; Bassi, A.B.M.S.; Bruns, R.E. Dynamic atomic contributions to infrared intensities of fundamental bands. Phys. Chem. Chem. Phys. 2015, 17, 30378–30388. [Google Scholar] [CrossRef]
- Richter, W.E.; Duarte, L.J.; Silva, A.F.; Bruns, R.E. Review of experimental GAPT and infrared atomic charges in molecules. J. Braz. Chem. Soc. 2016, 27, 979–991. [Google Scholar] [CrossRef]
- Richter, W.E.; Duarte, L.J.; Bruns, R.E. Are “GAPT charges” really just charges? J. Chem. Inf. Model. 2021, 61, 3881–3890. [Google Scholar] [CrossRef]
- Pauling, L. The modern theory of valency. J. Chem. Soc. 1948, 1461–1467. [Google Scholar] [CrossRef]
- Sanderson, R.T. An interpretation of bond lengths and a classification of bonds. Science 1951, 114, 670–672. [Google Scholar] [CrossRef]
- Sanderson, R.T. Electronegativity and bond energy. J. Am. Chem. Soc. 1983, 105, 2259–2261. [Google Scholar] [CrossRef]
- Batsanov, S.S. Simple semi-empirical method for evaluating bond polarity in molecular and crystalline halides. J. Mol. Struct. 2010, 980, 225–229. [Google Scholar] [CrossRef]
- Fuentealba, P.; Parr, R.G. Higher-order derivatives in density-functional theory, especially the hardness derivative ∂η/∂N. J. Chem. Phys. 1991, 94, 5559–5564. [Google Scholar] [CrossRef]
- Politzer, P. Anomalous properties of fluorine. J. Am. Chem. Soc. 1969, 91, 6235–6237. [Google Scholar] [CrossRef]
- Batsanov, S.S. Structure and properties of fluorine, oxygen, and nitrogen atoms in covalent bonds. Russ. Chem. Bull. 1989, 38, 410–412. [Google Scholar] [CrossRef]
- Murray, J.S.; Seybold, P.G.; Politzer, P. The many faces of fluorine: Some noncovalent interactions of fluorine compounds. J. Chem. Thermodyn. 2021, 156, 106382. [Google Scholar] [CrossRef]
- Manz, T.A. Seven confluence principles: A case study of standardized statistical analysis for 26 methods that assign net atomic charges in molecules. RSC Adv. 2020, 10, 44121–44148. [Google Scholar] [CrossRef]
- Born, M. Über elektrostatische Gitterpotentiale. Z. Physik 1921, 7, 124–140. [Google Scholar] [CrossRef]
- Szigeti, B. Polarisability and dielectric constant of ionic crystals. Trans. Faraday Soc. 1949, 45, 155–166. [Google Scholar] [CrossRef]
- Szigeti, B. Compressibility and absorption frequency of ionic crystals. Proc. R. Soc. Lond. Ser. A 1950, 204, 51–62. [Google Scholar]
- Szigeti, B. Higher -order terms in the dielectric constant of ionic crystals. Proc. R. Soc. Lond. Ser. A 1959, 252, 217–235. [Google Scholar]
- Szigeti, B. The magnitude of the anharmonic contribution to the static dielectric constant. Proc. R. Soc. Lond. Ser. A 1961, 261, 274–280. [Google Scholar]
- Batsanov, S.S. Dielectric methods of studying the chemical bond and the concept of electronegativity. Russ. Chem. Rev. 1982, 51, 684–697. [Google Scholar] [CrossRef]
- Berkowitz, J.; Batson, C.; Goodsman, G. Photoelectron spectroscopy of AgCl, AgBr, and AgI vapors. J. Chem. Phys. 1980, 72, 5829–5837. [Google Scholar] [CrossRef]
- Tossel, J.A.; Vaughan, D.J. Relationships between valence orbital binding energies and crystal structures in compounds of copper, silver, gold, zinc, cadmium, and mercury. Inorg. Chem. 1981, 20, 3333–3340. [Google Scholar] [CrossRef]
- Batsanov, S.S. The concept of electronegativity. Conclusions and prospects. Russ. Chem. Rev. 1968, 37, 332–351. [Google Scholar] [CrossRef]
- Suchet, J.P. Chemical Physics of Semiconductors; Van Nostrand: London, UK, 1965. [Google Scholar]
- Catlow, C.R.A.; Stoneham, A.M. Ionicity in solids. J. Phys. C Solid State Phys. 1983, 16, 4321–4338. [Google Scholar] [CrossRef]
- Barinskii, R.L. Determination of effective charges of atoms in complexes from the X-ray absorption spectra. J. Struct. Chem. 1960, 1, 183–190. [Google Scholar] [CrossRef]
- Vainshtein, E.E.; Kopelev, Y.F. X-ray spectral analysis of aromatic complexes of the transition elements. J. Struct. Chem. 1962, 3, 433–441. [Google Scholar] [CrossRef]
- Barinskii, R.L. On the paper by E.E. Vainstein and Yu. F. Kopelev “X-ray spectral analysis of aromatic complexes of the transition elements”. J. Struct. Chem. 1962, 3, 442–445. [Google Scholar] [CrossRef]
- Batsanov, S.S.; Ovsyannikova, I.A. Atomic charges in nickelecene and nickelecinium determined by means of X-ray spectra. Dokl. Akad. Nauk SSSR 1965, 165, 855–856. (In Russian) [Google Scholar]
- Iwata, M. X-ray determination of the electron distribution in crystals of [Co(NH3)6] [Cr(CN)6] at 80 K. Acta Crystallogr. Sect. B 1977, 33, 59–69. [Google Scholar] [CrossRef]
- Batsanov, S.S.; Ruchkin, E.D. Mixed halides of tetravalent platinum. Russ. J. Inorg. Chem. 1959, 4, 779–783. [Google Scholar]
- Batsanov, S.S.; Ruchkin, E.D. About isomers in mixed platinum halides. Russ. J. Inorg. Chem. 1965, 10, 1415–1418. [Google Scholar]
- Batsanov, S.S.; Podberezskaya, N.V.; Khripin, L.A. Mercury salts with mixed anions. Synthesis and properties of mixed halides. Russ. Chem. Bull. 1965, 14, 199–203. [Google Scholar] [CrossRef]
- Batsanov, S.S.; Rigin, V.I. Chalcogenohalides of thalium. Dokl. Akad. Nauk SSSR 1964, 158, 1355–1357. (In Russian) [Google Scholar]
- Batsanov, S.S.; Rigin, V.I. Isomerism of thallium selenobromides. Dokl. Akad. Nauk SSSR 1966, 167, 89–90. (In Russiasn) [Google Scholar]
- Batsanov, S.S.; Zalivina, E.N.; Derbeneva, S.S.; Borodaevsky, V.E. Synthesis and properties of copper bromo- and iodo-chlorides. Dokl. Akad. Nauk SSSR 1968, 181, 599–602. (In Russian) [Google Scholar]
- Batsanov, S.S.; Sokolova, M.N.; Ruchkin, E.D. Mixed halides of gold. Russ. Chem. Bull. 1971, 20, 1757–1759. [Google Scholar] [CrossRef]
- Pantelouris, A.; Kueper, G.; Hormes, J.; Feldmann, C.; Jansen, M. Anionic gold in Cs3AuO and Rb3AuO established by X-ray absorption spectroscopy. J. Am. Chem. Soc. 1995, 117, 11749–11753. [Google Scholar] [CrossRef]
- Feldmann, C.; Jansen, M. Zur Kristallchemischen Ahnlichkeit von aurid- und halogenid-ionen. Z. Anorg. Allgem. Chem. 1995, 621, 1907–1912. [Google Scholar] [CrossRef]
- Mudring, A.-V.; Jansen, M. Base-induced disproportionation of elemental gold. Angew. Chem. Int. Ed. 2000, 39, 3066–3067. [Google Scholar] [CrossRef]
- Karpov, A.; Nuss, J.; Wedig, U.; Jansen, M. Cs2Pt: A platinide(-II) exhibiting complete charge separation. Angew. Chem. Int. Ed. 2003, 42, 4818–4821. [Google Scholar] [CrossRef]
- Saltykov, V.; Nuss, J.; Konuma, M.; Jansen, M. Investigation of the quasi-binary system BaAu–BaPt. Z. Allgem. Anorg. Chem. 2009, 635, 70–75. [Google Scholar] [CrossRef]
- Smetana, V.; Mudring, A.-V. Cesium platinide hydride 4Cs2Pt·CsH: An intermetallic double salt featuring metal anions. Angew. Chem. Int. Ed. 2016, 55, 14838–14841. [Google Scholar] [CrossRef] [PubMed]
- Agnarelli, L.; Prots, Y.; Burkhardt, U.; Schmidt, M.; Koželj, P.; Leithe-Jasper, A.; Grin, Y. Mg3Pt2: Anionic chains in a Eu3Ga2-type structure. Inorg. Chem. 2021, 60, 13681–13690. [Google Scholar] [CrossRef]
- Batsanov, S.S. Pressure effect on the heat of formation of condensed substances. Russ. J. Phys. Chem. 1999, 73, 1–6. [Google Scholar]
- Batsanov, S.S. Chemical bonding evolution on compression of crystals. J. Struct. Chem. 2005, 46, 306–314. [Google Scholar] [CrossRef]
- Ghandehari, K.; Luo, H.; Ruoff, A.L.; Trail, S.S.; Disalvo, F.J. Crystal structure and band gap of rubidium hydride to 120 GPa. Mod. Phys. Lett. B 1995, 9, 1133–1140. [Google Scholar] [CrossRef]
- Ghandehari, K.; Luo, H.; Ruoff, A.L.; Trail, S.S.; Disalvo, F.J. New high pressure crystal structure and equation of state of cesium hydride to 253 GPa. Phys. Rev. Lett. 1995, 74, 2264–2267. [Google Scholar] [CrossRef]
- Fajans, K. General chemistry. By Linus Pauling. J. Phys. Chem. 1951, 55, 1107–1108. [Google Scholar] [CrossRef]
- Hückel, W. Die chemische Bindung. Kritische Betrachtung der Systematik, der Ausdrucksweisen und der formelmäßigen Darstellung. J. Prakt. Chem. 1957, 5, 105–174. [Google Scholar] [CrossRef]
- Batsanov, S.S. Comments on Hückel’s book. Russ. J. Phys. Chem. 1960, 34, 444–445. [Google Scholar]
- Iczkowski, R.P.; Margrave, J.L. Electronegativity. J. Am. Chem. Soc. 1961, 83, 3547–3551. [Google Scholar] [CrossRef]
- Syrkin, Y.K. Effective charges and electronegativity. Russ. Chem. Rev. 1962, 31, 197–207. [Google Scholar] [CrossRef]
- Batsanov, S.S. Comment on the article “Effective charges and electronegativites” of Syrkin. Russ. J. Phys. Chem. 1963, 37, 761-174. [Google Scholar]
- Spiridonov, V.P.; Tatevskii, V.M. On concept of electronegativity of atoms. Russ. J. Phys. Chem. 1963, 37, 522–526, 661–664, 848–849, 1070–1072, 1177–1179. [Google Scholar]
- Batsanov, S.S. Comment on articles of Spiridonov and Tatevskii criticizing concept of electronegativity. Russ. J. Phys. Chem. 1967, 41, 1298–1301. [Google Scholar]
- Zvolinskii, V.P. A conference on the concept of electronegativity. J. Struct. Chem. 1962, 3, 478–483. [Google Scholar] [CrossRef]
- Accorinti, H.L. Incompatible models in chemistry: The case of electronegativity. Found. Chem. 2019, 21, 71–81. [Google Scholar] [CrossRef]
- Tandon, H.; Chakraborty, T.; Suhag, V. A scale of atomic electronegativity in terms of atomic nucleophilicity index. Found. Chem. 2020, 22, 335–346. [Google Scholar] [CrossRef]
- von Szentpály, L. Theorems and rules connecting bond energy and bond order with electronegativity equalization and hardness maximization. Theor. Chem. Acc. 2020, 139, 54. [Google Scholar] [CrossRef]
- Nordholm, S. From electronegativity towards reactivity—Searching for a measure of atomic reactivity. Molecules 2021, 26, 3680. [Google Scholar] [CrossRef] [PubMed]
- Racioppi, S.; Rahm, M. In-situ electronegativity and the bridging of chemical bonding concepts. Chem. Eur. J. 2021, 27, 18156–18167. [Google Scholar] [CrossRef] [PubMed]
- Cherkasov, A.R.; Galkin, V.I.; Zueva, E.M.; Cherkasov, R.A. The concept of electronegativity: The current state of the problem. Russ. Chem. Rev. 1998, 67, 375–392. [Google Scholar] [CrossRef]

| Li | Be | B | C | N | O | F | |||
| 0.90 | 1.45 | 1.90 | 2.37 | 2.85 | 3.32 | 3.78 | |||
| Na | Mg | Al | Si | P | S | Cl | |||
| 0.89 | 1.31 | 1.64 | 1.98 | 2.32 | 2.65 | 2.98 | |||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
| 0.81 | 1.17 | 1.50 | 1.25 b | 1.60 c | 1.33 b | 1.32 b | 1.35 b | 1.38 b | 1.40 b |
| 1.86 | 1.92 d | 1.66 c | 1.70 c | 1.67 c | 1.72 c | 1.76 c | |||
| 2.22 | 1.98 d | 2.03 d | |||||||
| Cu | Zn | Ga | Ge | As | Se | Br | |||
| 1.48 | 1.64 | 1.83 | 2.09 | 1.70 c | 2.61 | 2.88 | |||
| 1.66 b | 2.27 | ||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| 0.80 | 1.13 | 1.41 | 1.23 b | 1.53 c | 1.92 d | 1.88 d | 1.35 b | 1.39 b | 1. 45 b |
| 1.71 | 2.02 | 2.33 | 1.93 d | 1.94 d | 2.01 d | ||||
| Ag | Cd | In | Sn | Sb | Te | I | |||
| 1.57 | 1.65 | 1.80 | 1.29 b | 1.60 c | 2.45 | 2.68 | |||
| 2.00 | 2.24 | ||||||||
| Cs | Ba | La | Hf | Ta | W | Re | Os | Ir | Pt |
| 0.77 | 1.08 | 1.35 | 1.28 b | 1.54 c | 1.83 d | 1.85 d | 1.39 b | 1.40 b | 1.45 b |
| 1.71 | 1.99 | 2.28 | 2.52 | 1.86 d | 1.89 d | 1.95 d | |||
| Au | Hg | Tl | Pb | Bi | Po | Th | U | ||
| 1.78 | 1.79 | 0.96 a | 1.31 b | 1.58 c | 2.29 | 1.59 d | 1.68 d | ||
| 1.93 c | 1.89 | 2.07 | 2.13 | ||||||
| M (I) | χ | M (II) | χ | M (II) | χ | M (III) | χ | M (IV) | χ |
|---|---|---|---|---|---|---|---|---|---|
| Li | 0.98 | Cu | 2.04 | Hg | 1.95 | Sc | 1.34 | Ti | 1.77 |
| Na | 0.93 | Be | 1.43 | Sn | 1.42 | Y | 1.25 | Zr | 1.63 |
| K | 0.72 | Mg | 1.38 | Pb | 1.55 | La | 1.12 | Hf | 1.56 |
| Rb | 0.67 | Ca | 1.00 | Cr | 1.53 | B | 1.96 | C | 2.60 |
| Cs | 0.53 | Sr | 0.95 | Mn | 1.35 | Al | 1.64 | Si | 2.05 |
| Cu | 1.46 | Ba | 0.82 | Fe | 1.58 | In | 1.73 | Ge | 2.15 |
| Ag | 1.61 | Zn | 1.60 | Co | 1.64 | Ga | 1.73 | Sn | 2.06 |
| Au | 1.84 | Cd | 1.70 | Ni | 1.71 | V | 1.80 | Pb | 2.17 |
| Tl | 1.06 | Cr | 1.92 | W | 2.19 |
| Li | Be | B | C | ||||||
| 0.47 | 0.65 | 1.17 | 1.29 | ||||||
| Na | Mg | Al | Si | ||||||
| 0.41 | 0.62 | 1.03 | 1.18 | ||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni |
| 0.38 | 0. 52 | 0.70 | 0.98 | 1.03 | 1.03 | 0.89 | 0.98 | 0.97 | 1.16 |
| Cu | Zn | Ga | Ge | As | Se | ||||
| 0.93 | 0.64 | 1.14 | 1.28 | 1.18 | 1.46 | ||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd |
| 0.35 | 0.50 | 0.65 | 0.97 | 1.05 | 1.17 | 1.01 | 1.32 | 1.34 | 1.30 |
| Ag | Cd | In | Sn | Sb | Te | I | |||
| 1.02 | 0.76 | 1.11 | 1.01 | 0.98 | 1.34 | 1.78 | |||
| Cs | Ba | La | Hf | Ta | W | Re | Os | Ir | Pt |
| 0.28 | 0.46 | 0.66 | 0.80 | 0.83 | 1.14 | 1.22 | 1.20 | 1.28 | 1.32 |
| Au | Hg | Tl | Pb | Bi | Th | U | |||
| 1.23 | 0.82 | 0.78 | 1.07 | 1.14 | 0.89 | 0.97 | |||
| M (I) | M (II) | M (II) | M (III) | M (IV) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Li | 0.55 | Cu | 1.33 | Sn | 1.09 | Sc | 0.69 | Ti | 0.68 |
| Na | 0.48 | Be | 0.98 | Pb | 1.04 | Y | 0.72 | Zr | 0.77 |
| K | 0.37 | Mg | 0.68 | Cr | 0.96 | La | 0.58 | Hf | 0.75 |
| Rb | 0.35 | Ca | 0.44 | Mn | 0.73 | Al | 1.00 | C | 2.60 |
| Cs | 0.32 | Sr | 0.41 | Fe | 1.18 | In | 1.73 | Si | 2.05 |
| Cu | 1.25 | Ba | 0.42 | Co | 1.07 | Ga | 1.19 | Ge | 2.15 |
| Ag | 1.30 | Zn | 0.85 | Ni | 1.24 | V | 0.88 | Sn | 0.81 |
| Au | 2.01 | Cd | 0.91 | Pd | 1.55 | Nb | 0.66 | Pb | 1.53 |
| Tl | 0.85 | Hg | 1.44 | Pt | 1.64 | Sb | 1.27 | Th | 0.82 |
| Bi | 1.22 | U | 0.95 | ||||||
| Cr | 1.01 | W | 1.14 |
| M | Nb | Eb | Ep | M | Nb | Eb | Ep | ||
|---|---|---|---|---|---|---|---|---|---|
| Ac | 12 | 9.828 | 406 | 332 | Mn | 12 | 10.42 | 283 | 246 |
| Ag | 12 | 10.33 | 285 | 245 | Mo | 8 | 6.863 | 659 | 565 |
| Al | 12 | 10.34 | 331 | 285 | Na | 8 | 6.450 | 107 | 86.7 |
| Au | 12 | 10.33 | 368 | 317 | Nb | 8 | 6.849 | 733 | 627 |
| Ba | 8 | 6.186 | 179 | 138 | Ni | 12 | 10.56 | 430 | 378 |
| C | 4 | 3.629 | 717 | 650 | Pb | 8 | 9.976 | 195 | 162 |
| Ca | 12 | 9.717 | 178 | 144 | Pd | 12 | 10.41 | 377 | 327 |
| Co | 12 | 10.55 | 427 | 375 | Pt | 12 | 10.40 | 566 | 490 |
| Cr | 8 | 6.958 | 397 | 346 | Ra | 8 | 6.140 | 159 | 122 |
| Cs | 8 | 5.781 | 76.5 | 55.3 | Rb | 8 | 5.939 | 80.9 | 60.0 |
| Cu | 12 | 10.69 | 337 | 301 | Rh | 12 | 10.44 | 556 | 434 |
| Fe | 8 | 6.964 | 415 | 362 | Si | 4 | 3.435 | 450 | 386 |
| Ge | 4 | 3.412 | 372 | 317 | Sr | 12 | 6.205 | 164 | 127 |
| Ir | 12 | 10.43 | 609 | 529 | Ta | 8 | 6.807 | 782 | 671 |
| K | 8 | 6.077 | 89.0 | 67.6 | Th | 12 | 9.921 | 602 | 498 |
| La | 12 | 9.839 | 431 | 353 | V | 8 | 6.907 | 515 | 445 |
| Li | 8 | 6.732 | 159 | 134 | W | 8 | 6.856 | 851 | 729 |
| M | Ns | Es | Eb/Io | Is | χnano | M | Ns | Es | Eb/Io | Is | χnano |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ac | 7.181 | 243 | 0.3652 | 6.896 | 1.02 | MnII | 7.181 | 169 | 0.2545 | 6.902 | 1.02 |
| Ag | 7.181 | 170 | 0.3897 | 4.534 | 0.83 | MoIV | 4.523 | 372 | 0.3012 | 12.82 | 1.40 |
| Al | 7.181 | 198 | 0.1931 | 10.63 | 1.27 | Na | 4.523 | 60. 8 | 0.2168 | 2.905 | 0.66 |
| Au | 7.181 | 220 | 0.4136 | 5.520 | 0.92 | NbIII | 4.523 | 414 | 0.4941 | 8.692 | 1.15 |
| Ba | 4.523 | 101 | 0.2440 | 4.298 | 0.81 | NiIII | 7.181 | 257 | 0.2192 | 112.2 | 1.36 |
| C | 2 | 358 | 0.2007 | 18.50 | 1.68 | PbII | 4.523 | 110 | 0.1802 | 6.349 | 0.98 |
| Ca | 7.181 | 106 | 0.2049 | 5.382 | 0.90 | PdII | 7.181 | 225 | 0.2811 | 8.310 | 1.12 |
| CoIII | 7.181 | 255 | 0.2269 | 11.66 | 1.33 | PtII | 7.181 | 338 | 0.4261 | 8.233 | 1.12 |
| CrIII | 4.523 | 225 | 0.2280 | 10.21 | 1.25 | Ra | 4.523 | 89.9 | 0.2136 | 4.361 | 0.81 |
| Cs | 4.523 | 43.2 | 0.2036 | 2.201 | 0.58 | Rb | 4.523 | 45.7 | 0.2007 | 2.362 | 0.60 |
| Cu | 7.181 | 202 | 0.4526 | 4.623 | 0.84 | RhIII | 7.181 | 333 | 0.3054 | 11.29 | 1.31 |
| FeIII | 4.523 | 235 | 0.2359 | 10.32 | 1.25 | Si | 2 | 225 | 0.1809 | 12.89 | 1.40 |
| Ge | 2 | 186 | 0.1586 | 12.15 | 1.36 | Sr | 7.181 | 98.1 | 0.2032 | 5.003 | 0.87 |
| IrIII | 7.181 | 364 | 0.3510 | 10.76 | 1.28 | TaIII | 4.523 | 442 | 0.5192 | 8.825 | 1.16 |
| K | 4.523 | 50.3 | 0.2125 | 2.454 | 0.61 | ThIV | 7.181 | 360 | 0.3818 | 9.777 | 1.22 |
| La | 7.181 | 258 | 0.3729 | 7.168 | 1.04 | VIII | 4.523 | 291 | 0.3162 | 9.551 | 1.20 |
| Li | 4.523 | 90.1 | 0.3062 | 3.048 | 0.68 | WIV | 4.523 | 481 | 0.4864 | 10.25 | 1.25 |
| M | F | Cl | Br | I | ||||
|---|---|---|---|---|---|---|---|---|
| μ | p | μ | p | μ | p | μ | p | |
| H | 1.826 | 0.41 | 1.108 | 0.18 | 0.827 | 0.12 | 0.448 | 0.06 |
| Li | 6.327 | 0.84 | 7.129 | 0.73 | 7.226 | 0.69 | 7.428 | 0.65 |
| Na | 8.156 | 0.88 | 9.002 | 0.79 | 9.118 | 0.76 | 9.236 | 0.71 |
| K | 8.592 | 0.82 | 10.27 | 0.80 | 10.63 | 0.78 | 10.82 | 0.74 |
| Rb | 8.546 | 0.78 | 10.51 | 0.78 | 10.86 | 0.77 | 11.48 | 0.75 |
| Cs | 7.884 | 0.70 | 10.39 | 0.73 | 10.82 | 0.73 | 11.69 | 0.73 |
| Cu | 5.77 | 0.69 | 5.2 a | 0.53 | ||||
| Ag | 6.22 | 0.65 | 6.08 | 0.55 | 5.62 | 0.49 | 4.55 | 0.37 |
| Au | 4.32 | 0.47 | 3.69 b | 0.35 | ||||
| Tl | 4.228 | 0.42 | 4.543 | 0.38 | 4.49 | 0.36 | 4.61 | 0.34 |
| Molecule | i | Molecule | i | Molecule | i | Molecule | i | Molecule | i |
|---|---|---|---|---|---|---|---|---|---|
| HF | 0.382 | OH2 | 0.236 | CH4 | 0.028 | BF3 | 0.506 | LiF | 0.861 |
| HCl | 0.184 | NH3 | 0.034 | SiH4 | 0.226 | BCl3 | 0.249 | LiCl | 0.760 |
| HBr | 0.114 | NF3 | 0.385 | GeH4 | 0.216 | CF4 | 0.512 | NaF | 0.889 |
| HI | 0.040 | PH3 | 0.119 | SnH4 | 0.254 | CCl4 | 0.261 | NaCl | 0.809 |
| LiH | 0.654 | PF3 | 0.580 | CO2 | 0.268 | KCl | 0.830 |
| MX | iB | iP | iS | MX | iB | iP | iS |
|---|---|---|---|---|---|---|---|
| LiF | 0.67 | 0.78 | 0.75 | CuF | 0.61 | 0.66 | 0.37 |
| LiCl | 0.63 | 0.55 | 0.67 | CuCl | 0.56 | 0.38 | 0.28 |
| LiBr | 0.62 | 0.48 | 0.62 | CuBr | 0.55 | 0.31 | 0.24 |
| LiI | 0.60 | 0.38 | 0.54 | CuI | 0.53 | 0.21 | 0.15 |
| NaF | 0.68 | 0.80 | 0.80 | AgF | 0.61 | 0.61 | 0.41 |
| NaCl | 0.64 | 0.57 | 0.71 | AgCl | 0.57 | 0.33 | 0.33 |
| NaBr | 0.63 | 0.50 | 0.67 | AgBr | 0.55 | 0.26 | 0.28 |
| NaI | 0.61 | 0.40 | 0.58 | AgI | 0.54 | 0.16 | 0.20 |
| KF | 0.71 | 0.84 | 0.85 | AuF | 0.57 | 0.53 | |
| KCl | 0.67 | 0.64 | 0.76 | AuCl | 0.53 | 0.25 | |
| KBr | 0.66 | 0.57 | 0.72 | AuBr | 0.52 | 0.18 | |
| KI | 0.64 | 0.47 | 0.64 | AuI | 0.50 | 0.13 | |
| CsF | 0.73 | 0.87 | 0.97 | TlF | 0.65 | 0.76 | 0.64 |
| CsCl | 0.69 | 0.69 | 0.89 | TlCl | 0.61 | 0.53 | 0.55 |
| CsBr | 0.68 | 0.64 | 0.84 | TlBr | 0.59 | 0.46 | 0.51 |
| CsI | 0.66 | 0.54 | 0.76 | TlI | 0.56 | 0.35 | 0.43 |
| M | F | Cl | Br | I | ||||
|---|---|---|---|---|---|---|---|---|
| is | e* | is | e* | is | e* | is | e* | |
| Li | 0.81 | 0.81 | 0.78 | 0.77 | 0.76 | 0.74 | 0.73 | 0.54 |
| Na | 0.81 | 0.83 | 0.78 | 0.78 | 0.76 | 0.75 | 0.74 | 0.74 |
| K | 0.82 | 0.92 | 0.79 | 0.81 | 0.77 | 0.77 | 0.75 | 0.75 |
| Rb | 0.82 | 0.97 | 0.80 | 0.84 | 0.77 | 0.80 | 0.75 | 0.77 |
| Cs | 0.84 | 0.96 | 0.82 | 0.85 | 0.80 | 0.82 | 0.78 | 0.78 |
| Cu | 0.71 | 0.68 | 0.98 | 0.65 | 0.96 | 0.62 | 0.91 | |
| Ag | 0.71 | 0.89 | 0.68 | 0.71 | 0.65 | 0.67 | 0.57 | 0.61 |
| Tl | 0.74 | 0.71 | 0.88 | 0.68 | 0.84 | 0.65 | 0.83 | |
| Metal | Compounds | qc (exp) | qc (cal) | Metal | Compounds | qc (exp) | qc (cal) |
|---|---|---|---|---|---|---|---|
| Cr | CrSO4·7H2O | 1.9 | 1.8 | Co | Co(NO3)3 | 1.2 | 0.6 |
| Cr(NO3)3 | 1.2 | 1.3 | Co(C5H5)2 | 0.4 | 0.7 | ||
| K2CrO4 | 0.1 | 0.5 | Co(C5H5)2Cl | 1.0 | 0.9 | ||
| Cr(C6H6)2 | 1.3 | 1.4 | Ni | Ni(C5H5)2 | 0.7 | 0.6 | |
| Mn | Mn(NO3)2·4H2O | 1.8 | 1.8 | Ni(C5H5)2Cl | 1.0 | 0.8 | |
| K3Mn(CN)6 | 0.9 | 0.6 | Os | OsO2 | 0.8 | 0.7 | |
| Mn(C5H5)2 | 1.5 | 1.3 | K2OsCl6 | 0.8 | 0.5 | ||
| Fe | (NH4)2Fe(SO4)2·6H2O | 1.9 | 1.7 | K2OsO4 | 0.8 | 0.7 | |
| K3Fe(CN)6 | 1.0 | 0.4 | K2OsNCl5 | 0.7 | 0.8 | ||
| Fe(C5H5)2 | 0.6 | 0.7 | KOsO3N | 1.0 | 0.9 |
| MI | F | Cl | Br | I | MII | O | S | Se | Te |
|---|---|---|---|---|---|---|---|---|---|
| Li | 5.2 | 1.5 | 0 | 1.9 | Be | 0 | −1.6 | 1.6 | 3.7 |
| Na | 7.0 | 2.4 | 1.5 | 4.0 | Mg | 0.9 | 0 | 0 | 5.6 |
| K | 11.7 | 5.9 | 5.4 | 4.1 | Ca | 3.7 | 2.2 | 3.3 | 7.5 |
| Rb | 10.6 | 3.9 | 2.4 | 1.7 | Sr | 7.0 | 5.9 | 7.2 | 9.6 |
| Cs | 10.4 | 6.7 | 8.3 | 7.6 | Ba | 16.5 | 16.5 | 18.0 | 24.6 |
| Cu | −8.4 | −4.7 | 6.9 | Zn | 0 | −2.2 | 0 | 4.4 | |
| Ag | −3.7 | −5.6 | −3.4 | −6.6 | Cd | 0 | −2.8 | 0 | 5.9 |
| Tl | −12.6 | −12.5 | −11.6 | −10.9 | Hg | −5.9 | −2.8 | 0.6 | 10.0 |
| MIII | N | P | As | Sb | Sn | −12.2 | −2.8 | 0 | 0 |
| B | 0.6 | 9.7 | 4.7 | Pb | −7.8 | −3.7 | 0 | 4.7 | |
| Al | 0.6 | 5.3 | 0.6 | 0 | Mn | −0.4 | −4.0 | 1.6 | |
| Ga | 2.2 | 9.4 | 2.5 | 2.8 | |||||
| In | 6.9 | 10.0 | 1.9 | 1.9 | |||||
| La | 15.3 | 14.7 | 14.4 | ||||||
| Th | 2.5 | 7.5 | |||||||
| U | 0 | 3.4 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batsanov, S.S. Energy Electronegativity and Chemical Bonding. Molecules 2022, 27, 8215. https://doi.org/10.3390/molecules27238215
Batsanov SS. Energy Electronegativity and Chemical Bonding. Molecules. 2022; 27(23):8215. https://doi.org/10.3390/molecules27238215
Chicago/Turabian StyleBatsanov, Stepan S. 2022. "Energy Electronegativity and Chemical Bonding" Molecules 27, no. 23: 8215. https://doi.org/10.3390/molecules27238215
APA StyleBatsanov, S. S. (2022). Energy Electronegativity and Chemical Bonding. Molecules, 27(23), 8215. https://doi.org/10.3390/molecules27238215






