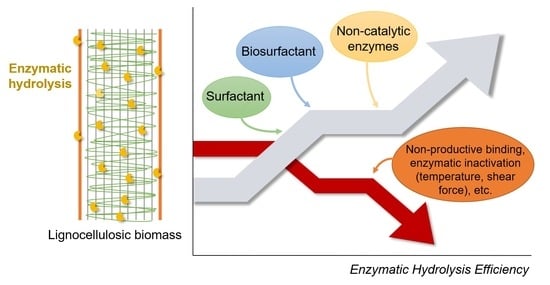
Surfactants, Biosurfactants, and Non-Catalytic Proteins as Key Molecules to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass
Abstract
1. Introduction
2. Structure, Lignin Recalcitrance, and Cellulose Crystallinity as Main Challenges to Valorize Lignocellulosic Biomass
3. Additives as Emerging Tools to Enhance Enzymatic Hydrolysis
3.1. Surfactants
Molecules with Surfactant Properties: Polymers
| Biomass | Enzyme Loading | Solids Loading (%, w/v) | Time (h) | Conversion Yield (Glucose) % | References | |
|---|---|---|---|---|---|---|
| Without Additive | With Additive | |||||
| Dilute acid-pretreated wheat straw | 15 FPU/g solid (Cellic CTec2) | 5 | 100 | ~65.0 | 80.0 Tween 20 (5.0 g/L) | [19] |
| Steam-pretreated poplar pulp | 5.625 FPU/g (Cellic CTec2) | 5 | 96 | 40.0 | 57.0 TritonX-100 (5.0%) | [28] |
| 40.0 | 41.0 Tween 80 (5.0%) | |||||
| Ground acid-pretreated sugarcane bagasse | 20 FPU/g cellulose (cellulase Imperial Jade Bio-Technology) | 10 | 72 | 27.0 | 46.0 Tween 20 (0.5%) | [34] |
| AlCl3-pretreated sugarcane bagasse | 20 FPU/g dry pretreated solids (Cellic CTec2) | 2 | 72 | 70.0 | 88.0 Tween 80 (150 mg/g biomass) | [20] |
| Dilute acid-pretreated bamboo residue | 20 FPU/g glucan (Cellic CTec2) | 5 | 72 | 29.4 | 61.6 Tween 80 (0.3 g/g lignin) | [35] |
| Alkaline-pretreated sugarcane bagasse | 40 FPU/g cellulose (cellulase) and 30 U/g hemicellulose (xylanase) | 5 | 72 | 40.7 | 57.3 Tween 60 (2.0%) | [36] |
| 40.7 | 60.0 Tween 61 (0.5%) | |||||
| Acid-pretreated wheat straw | 20 FPU/g (cellulase from Trichoderma reesei ATCC26924) | 10 | 72 | 67.4 | 72.6 Tween 80 (53.5% on dry pulp) | [37] |
| 67.4 | 74.4 PEG 6000 (50% on dry pulp) | |||||
3.2. Biosurfactants
3.3. Non-Catalytic Proteins
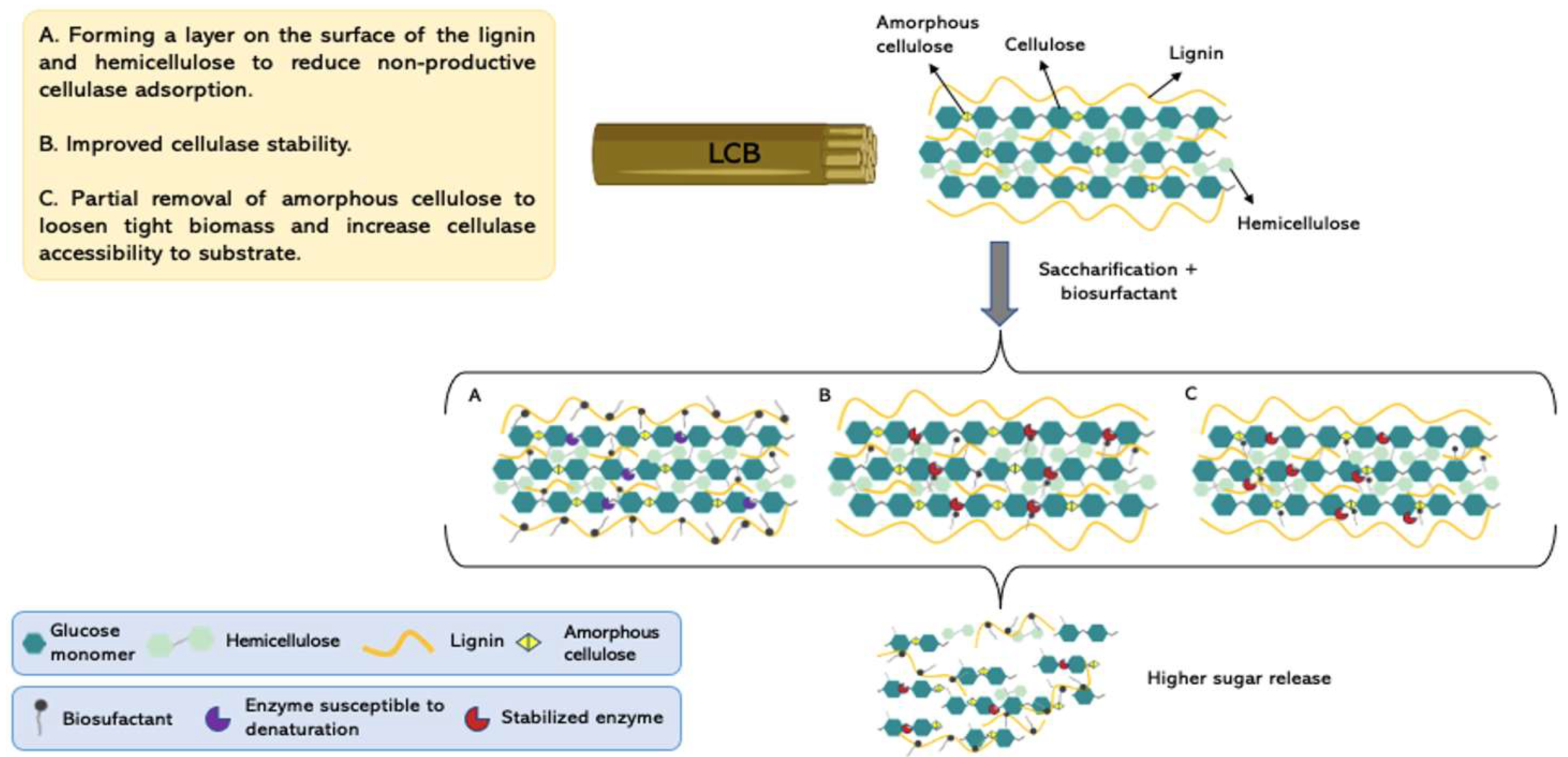
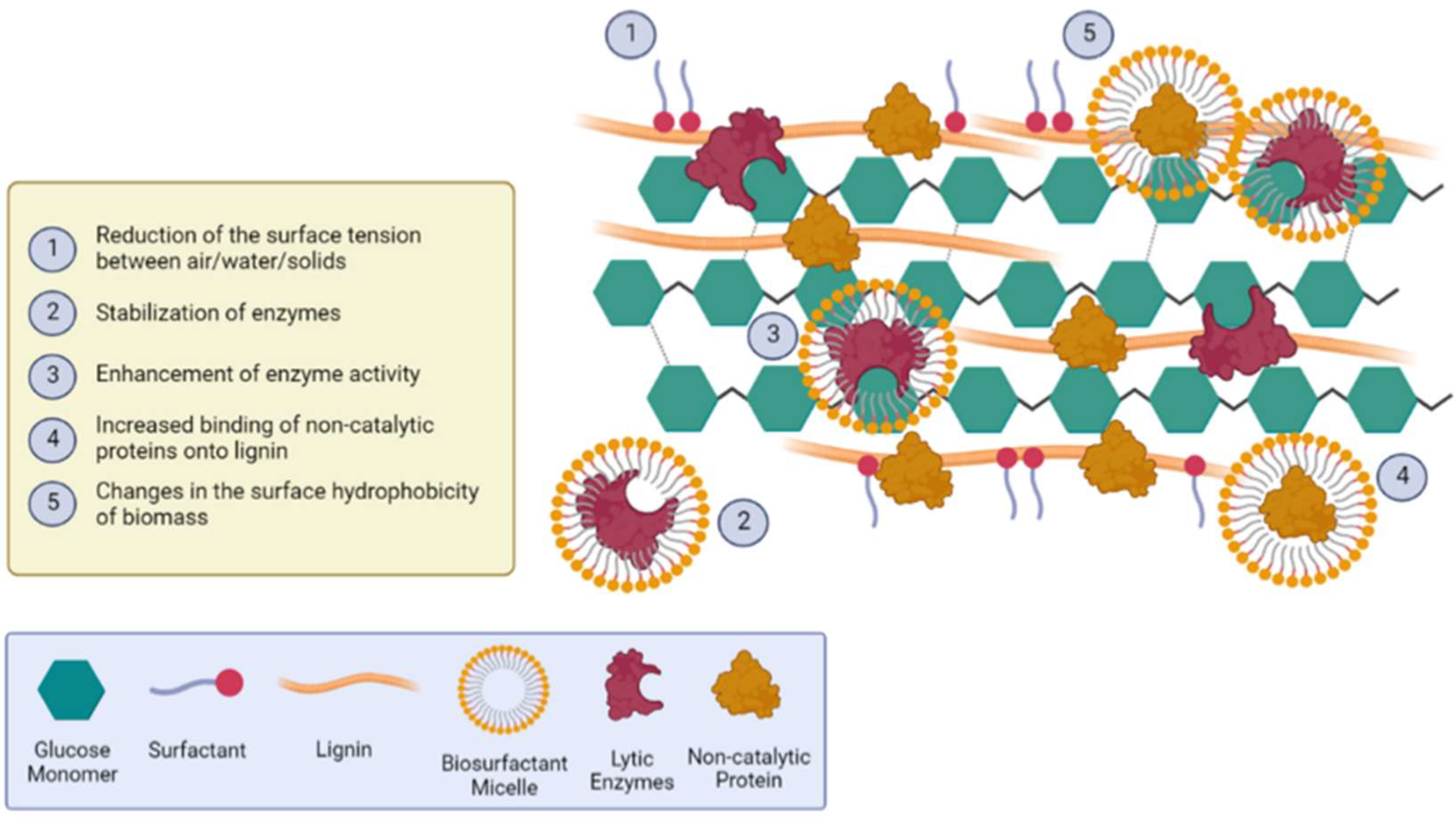
3.4. Surfactants, Biosurfactants, and Non-Catalytic Proteins Integration to Enhance the Saccharification Process
4. Potential Enzyme Cost Reduction and Future Perspectives in the Use of Additives in the Enzymatic Hydrolysis Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Alriksson, B.; Cavka, A.; Jönsson, L.J. Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in-situ detoxification with reducing agents. Bioresour. Technol. 2011, 102, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Terán-Hilares, R.; Reséndiz, A.L.; Martínez, R.T.; Silva, S.S.; Santos, J.C. Successive pretreatment and enzymatic saccharification of sugarcane bagasse in a packed bed flow-through column reactor aiming to support biorefineries. Bioresour. Technol. 2016, 203, 42–49. [Google Scholar] [CrossRef]
- Muñoz, S.S.; Balbino, T.R.; Alba, E.M.; Barbosa, F.G.; de Pier, F.T.; de Almeida, A.L.M.; Zilla, A.H.B.; Antunes, F.A.F.; Hilares, R.T.; Balagurusamy, N.; et al. Surfactants in biorefineries: Role, challenges & perspectives. Bioresour. Technol. 2022, 345, 126477. [Google Scholar]
- Mesquita, R.; Hallwass, F.; Santana, A.; Baudel, H.; Ribeiro, E. Influence of di-rhamnolipids on the enzymatic hydrolysis of steam-pretreated eucalyptus wastes. Ind. Crops Prod. 2019, 141, 111835. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, G.; Song, J.; Zheng, P.; Liu, J.; Zhu, W.; Huang, L.; Chen, L.; Luo, X.; Shuai, L. Understanding the promoting effect of non-catalytic protein on enzymatic hydrolysis efficiency of lignocelluloses. Bioresour. Bioprocess. 2021, 8, 9. [Google Scholar] [CrossRef]
- Kim, T.H. Pretreatment of lignocellulossic biomass. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 91–110. [Google Scholar]
- Zhang, X.; Yu, H.; Huang, H.; Liu, Y. Evaluation of biological pretreatment with white-rot fungi for the enzymatic hydrolysis of bamboo culms. Int. Biodiversity. Biodegradation 2004, 60, 159–164. [Google Scholar] [CrossRef]
- Brunow, G.; Lundquist, K.; Gellerstedt, G. Lignin. In Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Sjöström, E., Alén, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 77–124. [Google Scholar]
- Harmsen, P.F.H.; Huijgen, W.J.J.; Lopez, L.M.B.; Bakker, R.R.C. Literature Review of Physical and Chemical Pretreatment Processes for Lignocelulosic Biomass; Food and Biomass Research: Wageningen, The Netherlands, 2009. [Google Scholar]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Ingle, A.P.; Abdeshahian, P.; Ribeaux, D.R.; Prado, C.A.; Sanchez-Munos, S.; Barbosa, F.G.; Balbino, T.R.; Castro-Alonso, M.J.; Reyes-Guzman, R.; et al. Lignocellulosic Waste availability for microbial production of fuels, biochemicals and products. In Sustainable Microbial Technologies for Valorization of Agro-Industrial Wastes, 1st ed.; Sai, J.K., Singh, S., Nain, L., Eds.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Antunes, F.A.F.; Rocha, T.M.; Philippini, R.R.; Martiniano, S.E.; Prado, C.A.; Mier-Alba, E.; Hernandez-Perez, A.F.; Jofre, F.M.; Abdeshahian, P.; Ribeaux, D.R.; et al. The Potential of Vegetal Biomass for Biomolecules Production. In Comprehensive Renewable Energy; Letcher, T.M., Ed.; Elsevier: London, UK, 2022; pp. 139–164. [Google Scholar]
- Yu, H.; Hou, J.; Yu, S.; Liu, S.; Li, L.; Wu, Q.; Liu, Y.; Jiang, J.; Nie, S. Comprehensive understanding of the non-productive adsorption of cellulolytic enzymes onto lignins isolated from furfural residues. Cellulose 2019, 26, 3111–3125. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Oyegoke, T.; Tongshuwar, G.T.; Oguche, J.E. Biomass pretreatment as a key process in bioethanol productions: A review. J. Eng. Sci. 2022, 29, 130–141. [Google Scholar] [CrossRef]
- Phojaroen, J.; Jiradechakorn, T.; Kirdponpattara, S.; Sriariyanun, M.; Junthip, J.; Chuetor, S. Performance Evaluation of Combined Hydrothermal-Mechanical Pretreatment of Lignocellulosic Biomass for Enzymatic Enhancement. Polymers 2022, 14, 2313. [Google Scholar] [CrossRef]
- Petridis, L.; Smith, J.C. Molecular-level driving forces in lignocellulosic biomass deconstruction for bioenergy. Nat. Rev. Chem. 2018, 2, 382–389. [Google Scholar] [CrossRef]
- Chen, Y.A.; Zhou, Y.; Qin, Y.; Liu, D.; Zhao, X. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, W.; Han, X.; Zeng, Y.; Zhang, J.; Gao, Z.; Xie, J. Intensification of sugar production by using Tween 80 to enhance metal-salt catalyzed pretreatment and enzymatic hydrolysis of sugarcane bagasse. Bioresour. Technol. 2021, 339, 125522. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Ge, X.; Zhang, J. Effects of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnol. Biofuels 2016, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, T.; Borjesson, J.; Tjerneld, F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzym. Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Lin, W.; Chen, D.; Yong, Q.; Huang, C.; Huang, S. Improving enzymatic hydrolysis of acid-pretreated bamboo residues using amphiphilic surfactant derived from dehydroabietic acid. Bioresour. Technol. 2019, 293, 122055. [Google Scholar] [CrossRef]
- Yoon, S.H.; Robyt, J.F. Activation and stabilization of 10 starch-degrading enzymes by Triton X-100, polyethylene glycols, and polyvinyl alcohols. Enzym. Microb. Technol. 2005, 37, 556–562. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, S.B.; Ryu, D.D.; Reese, E.T. Surface deactivation of cellulase and its prevention. Enzym. Microb. Technol. 1982, 4, 99–103. [Google Scholar] [CrossRef]
- Okino, S.; Ikeo, M.; Ueno, Y.; Taneda, D. Effects of Tween 80 on cellulase stability under agitated conditions. Bioresour. Technol. 2013, 142, 535–539. [Google Scholar] [CrossRef]
- Alhammad, A.; Adewale, P.; Kuttiraja, M.; Christopher, L.P. Enhancing enzyme-aided production of fermentable sugars from poplar pulp in the presence of non-ionic surfactants. Bioprocess Biosyst. Eng. 2018, 41, 1133–1142. [Google Scholar] [CrossRef]
- Nogueira, C.D.C.; de Padilha, C.E.A.; de Filho, P.F.S.; dos Santos, E.S. Effects of the addition of poly (ethylene glycol) and non-ionic surfactants on pretreatment, enzymatic hydrolysis, and ethanol fermentation. BioEnergy Res. 2022, 15, 889–904. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Xia, F.; Zhang, Z.; Huang, X.; Cheng, Y.; Wang, H. The hydrothermal-alkaline/oxygen two-step pretreatment combined with the addition of surfactants reduced the amount of cellulase for enzymatic hydrolysis of reed. Bioresour. Technol. 2020, 308, 123324. [Google Scholar] [CrossRef]
- Tejirian, A.; Xu, F. Inhibition of enzymatic cellulolysis by phenolic compounds. Enzym. Microb. Technol. 2011, 48, 239–247. [Google Scholar] [CrossRef]
- Chylenski, P.; Felby, C.; Haven, M.Ø.; Gama, M.; Selig, M.J. Precipitation of Trichoderma reesei commercial cellulase preparations under standard enzymatic hydrolysis conditions for lignocelluloses. Biotechnol. Lett. 2012, 34, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zeng, M.; Hu, Q.; Cai, C.; Lin, X.; Qiu, X.; Yang, D.; Pang, Y. Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour. Technol. 2018, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C.; Zahoor; Chen, X.; Yu, Q.; Wang, Z.; Zhuang, X.; Yuan, Z. Effect of a nonionic surfactant on enzymatic hydrolysis of lignocellulose based on lignocellulosic features and enzyme adsorption. ACS Omega 2020, 5, 15812–15820. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, C.; Yong, Q.; Ragauskas, A.; Meng, X. Revealing the mechanism of surfactant-promoted enzymatic hydrolysis of dilute acid pretreated bamboo. Bioresour. Technol. 2022, 360, 127524. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhuang, X.; Tan, X.; Wang, Q.; Chen, X.; Yu, Q.; Qi, W.; Wang, Z.; Yuan, Z. Dual effect of nonionic surfactants on improving the enzymatic hydrolysis of lignocellulose. Energy Fuels 2018, 32, 5951–5959. [Google Scholar] [CrossRef]
- Vergara, P.; Ladero, M.; Carbajo, J.M.; García-Ochoa, F.; Villar, J.C. Effect of additives on the enzymatic hydrolysis of pre-treated wheat straw. Braz. J. Chem. Eng. 2021, 38, 241–249. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.G.; Ribeaux, D.R.; Rocha, T.; Costa, R.A.; Guzmán, R.R.; Marcelino, P.R.; dos Santos, J.C.; Silva, S.S.D. Biosurfactants: Sustainable and Versatile Molecules. J. Braz. Chem. Soc. 2022, 33, 870–893. [Google Scholar] [CrossRef]
- Xu, C.; Alam, M.A.; Wang, Z.; Chen, H.; Zhang, J.; Huang, S.; Zhuang, W.; Xu, J. Mechanisms of bio-additives on boosting enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2021, 337, 125341. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, J.; Yao, J. Surfactant-promoted hydrolysis of lignocellulose for ethanol production. Fuel Process. Technol. 2021, 213, 106660. [Google Scholar] [CrossRef]
- Zhang, Q.; He, G.; Wang, J.; Cai, W.; Xu, Y. Mechanisms of the stimulatory effects of rhamnolipid biosurfactant on rice straw hydrolysis. Appl. Energy 2009, 86, S233–S237. [Google Scholar] [CrossRef]
- Oliva-Taravilla, A.; Carrasco, C.; Jönsson, L.J.; Martín, C. Effects of biosurfactants on enzymatic saccharification and fermentation of pretreated softwood. Molecules 2020, 25, 3559. [Google Scholar] [CrossRef]
- Wang, H.Y.; Fan, B.Q.; Li, C.H.; Liu, S.; Li, M. Effects of rhamnolipid on the cellulase and xylanase in hydrolysis of wheat straw. Bioresour. Technol. 2011, 102, 6515–6521. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, S.; Qiu, Z.; Han, H.; Zhang, Q. Stimulatory effect and adsorption behavior of rhamnolipids on lignocelluloses degradation system. Bioresour. Technol. 2017, 224, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Eslami, P.; Hajfarajollah, H.; Bazsefidpar, S. Recent advancements in the production of rhamnolipid biosurfactants by Pseudomonas aeruginosa. RSC Adv. 2020, 10, 34014–34032. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tan, F.; Zhong, H.; Liu, G.; Ahmad, Z.; Liang, Q. Sub-CMC solubilization of n-alkanes by rhamnolipid biosurfactant: The Influence of rhamnolipid molecular structure. Colloids Surf. B Biointerfaces 2020, 192, 111049. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, B.; Cai, Q.; Cao, Y.; Ling, J.; Lee, K.; Chen, B. A critical review on the environmental application of lipopeptide micelles. Bioresour. Technol. 2021, 339, 125602. [Google Scholar] [CrossRef]
- Menon, V.; Prakash, G.; Prabhune, A.; Rao, M. Biocatalytic approach for the utilization of hemicellulose for ethanol production from agricultural residue using thermostable xylanase and thermotolerant yeast. Bioresour. Technol. 2010, 101, 5366–5373. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, J.; Zhang, Y.; Guo, Y.; Xu, H.; Xu, J.; Wang, Z. Enhancement of high-solids enzymatic hydrolysis efficiency of alkali pretreated sugarcane bagasse at low cellulase dosage by fed-batch strategy based on optimized accessory enzymes and additives. Bioresour. Technol. 2019, 292, 121993. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; El-Sheshtawy, H.S. Effect of biosurfactant on hydrolysis of municipal waste by cellulases producing bacteria for bioethanol production. Curr. Res. Green Sustain. Chem. 2022, 5, 100294. [Google Scholar] [CrossRef]
- Madadi, M.; Song, G.; Sun, F.; Sun, C.; Xia, C.; Zhang, E.; Karimi, K.; Tu, M. Positive role of non-catalytic proteins on mitigating inhibitory effects of lignin and enhancing cellulase activity in enzymatic hydrolysis: Application, mechanism, and prospective. Environ. Res. 2022, 215, 114291. [Google Scholar] [CrossRef]
- Florencio, C.; Badino, A.C.; Farinas, C.S. Soybean protein as a cost-effective lignin-blocking additive for the saccharification of sugarcane bagasse. Bioresour. Technol. 2016, 221, 172–180. [Google Scholar] [CrossRef]
- Brethauer, S.; Studer, M.H.; Yang, B.; Wyman, C.E. The effect of bovine serum albumin on batch and continuous enzymatic cellulose hydrolysis mixed by stirring or shaking. Bioresour. Technol. 2011, 102, 6295–6298. [Google Scholar] [CrossRef]
- Gourlay, K.; Hu, J.; Arantes, V.; Andberg, M.; Saloheimo, M.; Penttilä, M.; Saddler, J. Swollenin aids in the amorphogenesis step during the enzymatic hydrolysis of pretreated biomass. Bioresour. Technol. 2013, 142, 498–503. [Google Scholar] [CrossRef]
- Almeida, R.M.; Pimentel, W.R.; Santos-Rocha, M.S.; Buffo, M.M.; Farinas, C.S.; Ximenes, E.A.; Ladisch, M.R. Protective effects of non-catalytic proteins on endoglucanase activity at air and lignin interfaces. Biotechnol. Prog. 2021, 37, e3134. [Google Scholar] [CrossRef]
- Leamon, A.K.M. Heterologous Expression and Biophysical Characterization of Non-Catalytic Proteins Secreted by Lignocellulose-Degrading Fungi. Master’s Thesis, Department of Food and Environmental Sciences, Faculty of Agriculture and Forestry, University of Helsinki, Helsinki, Finland, 2019. [Google Scholar]
- Agrawal, R.; Verma, A.; Singhania, R.R.; Varjani, S.; Di Dong, C.; Patel, A.K. Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour. Technol. 2021, 332, 125042. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, Z.; Zhang, R.; Wang, D.; Jenkins, B. Non-ionic surfactants, and non-catalytic protein treatment on enzymatic hydrolysis of pretreated creeping wild ryegrass. In Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2007; pp. 351–368. [Google Scholar]
- Ge, X.; Sun, Z.; Xin, D.; Zhang, J. Enhanced xylanase performance in the hydrolysis of lignocellulosic materials by surfactants and non-catalytic protein. Appl. Biochem. Biotechnol. 2014, 172, 2106–2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kobayashi, S.; Mochidzuki, K. Effect of non-enzymatic proteins on enzymatic hydrolysis and simultaneous saccharification and fermentation of different lignocellulosic materials. Bioresour. Technol. 2015, 190, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Madadi, M.; Wang, Y.; Xu, C.; Liu, P.; Wang, Y.; Xia, T.; Tu, Y.; Lin, X.; Song, B.; Yang, X.; et al. Using Amaranthus green proteins as universal biosurfactant and biosorbent for effective enzymatic degradation of diverse lignocellulose residues and efficient multiple trace metals remediation of farming lands. J. Hazard. Mater. 2021, 406, 124727. [Google Scholar] [CrossRef]
- Jørgensen, H.; Vibe-Pedersen, J.; Larsen, J.; Felby, C. Liquefaction of lignocellulose at high-solids concentrations. Biotechnol. Bioeng. 2007, 96, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.B.; Karim, M.N.; Schell, D.J.; McMillan, J.D. Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2008, 99, 8940–8948. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Feng, X.; Li, X.; Zhao, J. The adsorption properties of endoglucanase to lignin and their impact on hydrolysis. Bioresour. Technol. 2018, 267, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, F.; Alam, M.A.; Chen, H.; Zhang, Y.; Liang, C.; Xu, H.; Huang, S.; Xu, J.; Wang, Z. Comparative study on the properties of lignin isolated from different pretreated sugarcane bagasse and its inhibitory effects on enzymatic hydrolysis. Int. J. Biol. Macromol. 2020, 146, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Lavenson, D.M.; Tozzi, E.J.; McCarthy, M.J.; Jeoh, T. The effects of water interactions in cellulose suspensions on mass transfer and saccharification efficiency at high solids loadings. Cellulose 2011, 18, 759–773. [Google Scholar] [CrossRef]
- Da Silva, A.S.A.; Espinheira, R.P.; Teixeira, R.S.S.; de Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P. Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: A critical review. Biotechnol. Biofuels 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Mesquita, J.F.; Ferraz, A.; Aguiar, A. Alkaline-sulfite pretreatment and use of surfactants during enzymatic hydrolysis to enhance ethanol production from sugarcane bagasse. Bioprocess Biosyst. Eng. 2016, 39, 441–448. [Google Scholar] [CrossRef]
- Agrawal, R.; Satlewal, A.; Kapoor, M.; Mondal, S.; Basu, B. Investigating the enzyme-lignin binding with surfactants for improved saccharification of pilot scale pretreated wheat straw. Bioresour. Technol. 2017, 224, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Xiao, W.; Yang, Y.; Hu, P.; Dai, Y.; Jiang, Z. Dissecting the effect of polyethylene glycol on the enzymatic hydrolysis of diverse lignocellulose. Int. J. Biol. Macromol. 2019, 131, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Bhagia, S.; Dhir, R.; Kumar, R.; Wyman, C.E. Deactivation of cellulase at the air-liquid interface is the main cause of incomplete cellulose conversion at low enzyme loadings. Sci. Rep. 2018, 8, 1350. [Google Scholar] [CrossRef]
- Matsumiya, K.; Suzuki, Y.A.; Hirata, Y.; Nambu, Y.; Matsumura, Y. Protein–surfactant interactions between bovine lactoferrin and sophorolipids under neutral and acidic conditions. Biochem. Cell Biol. 2017, 95, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The impact of biosurfactants on microbial cell properties leading to hydrocarbon bioavailability increase. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Chen, Y.; Nai, X.; Li, M.; Kong, J.; Hao, S.; Yan, H.; Liu, M.; Zhang, Q.; Liu, J. A comprehensive research on Lactone Sophorolipid (LSL) and Soy Protein Isolate (SPI) interacting mixture. J. Mol. Liq. 2021, 339, 117239. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zhong, H.; Zeng, G.; Liu, G.; Yu, M.; Liu, Y.; Yang, X.; Li, Z.; Fang, Z.; et al. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review. Microbiol. Res. 2017, 200, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, J.; Dick, R.P.; Li, H.; Shen, D.; Gao, Y.; Waigi, M.G.; Ling, W. Rhamnolipid influences biosorption and biodegradation of phenanthrene by phenanthrene-degrading strain Pseudomonas sp. Ph6. Environ. Pollut. 2018, 240, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Liu, S.; Tong, L.; Zhang, Y.; Yang, J.; Liu, W.; Guo, C.; Xie, Y.; Lu, G.; Dang, Z. Effects of rhamnolipids on the cell surface characteristics of Sphingomonas sp. GY2B and the biodegradation of phenanthrene. RSC Adv. 2017, 7, 24321–24330. [Google Scholar] [CrossRef]
- Ázar, R.I.L.; Morgan, T.; Barbosa, M.H.; Guimarães, V.M.; Ximenes, E.; Ladisch, M. Impact of protein blocking on enzymatic saccharification of bagasse from sugarcane clones. Biotechnol. Bioeng. 2019, 116, 1584–1593. [Google Scholar] [CrossRef]
- Ding, D.; Li, P.; Zhang, X.; Ramaswamy, S.; Xu, F. Synergy of hemicelluloses removal and bovine serum albumin blocking of lignin for enhanced enzymatic hydrolysis. Bioresour. Technol. 2019, 273, 231–236. [Google Scholar] [CrossRef]
- Luo, X.; Liu, J.; Zheng, P.; Li, M.; Zhou, Y.; Huang, L.; Chen, L.; Shuai, L. Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnol. Biofuels 2019, 12, 51. [Google Scholar] [CrossRef]
- Wang, H.; Mochidzuki, K.; Kobayashi, S.; Hiraide, H.; Wang, X.; Cui, Z. Effect of bovine serum albumin (BSA) on enzymatic cellulose hydrolysis. Appl. Biochem. Biotechnol. 2013, 170, 541–551. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, S.; Balbino, T.R.; Terán-Hilares, R.; Mier-Alba, E.; Barbosa, F.G.; Balagurusamy, N.; Santos, J.; da Silva, S.S. Non-ionic surfactant formulation sequentially enhances the enzymatic hydrolysis of cellulignin from sugarcane bagasse and the production of Monascus ruber biopigments. Bioresour. Technol. 2022, 362, 127781. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, J.; Kuittinen, S.; Vepsäläinen, J.; Soininen, P.; Keinänen, M.; Pappinen, A. Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone–butanol–ethanol fermentation. Bioresour. Technol. 2015, 189, 131–137. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Gomes, U.V.R.; Silva, R.O.; Sarubbo, L.A.; Ribeiro, E. The potential application of biosurfactant produced by Pseudomonas aeruginosa TGC01 using crude glycerol on the enzymatic hydrolysis of lignocellulosic material. Biodegradation 2019, 30, 351–361. [Google Scholar] [CrossRef]
- Lai, C.; Jia, Y.; Yang, C.; Chen, L.; Shi, H.; Yong, Q. Incorporating lignin into polyethylene glycol enhanced its performance for promoting enzymatic hydrolysis of hardwood. ACS Sustain. Chem. Eng. 2020, 8, 1797–1804. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Martinez-Bernal, C.; Pérez-Cobas, Y.; Reyes-Sosa, F.M.; García, B.D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. [Google Scholar] [CrossRef]
- Brondi, M.G.; Vasconcellos, V.M.; Giordano, R.C.; Farinas, C.S. Alternative Low-Cost Additives to Improve the Saccharification of Lignocellulosic Biomass. Appl. Biochem. Biotechnol. 2019, 187, 461–473. [Google Scholar] [CrossRef]
- Tu, M.; Saddler, J.N. Potential Enzyme Cost Reduction with the Addition of Surfactant during the Hydrolysis of Pretreated Softwood. Appl. Biochem. Biotechnol. 2010, 161, 274–287. [Google Scholar] [CrossRef]
| Non-Catalytic Protein | Enzymatic Hydrolysis Conditions | Substrate (Pretreatment) | Increase in Conversion Yield | Reference |
|---|---|---|---|---|
| BSA 0.1 g/g dry solid | * Non-catalytic protein pretreatment All experiments were carried out for 1 h at 50 °C using 50 mM citrate buffer (pH 4.8); Enzymatic hydrolysis was carried out for 72 h at 50 °C. Enzyme formulation was composed of β-glucosidase (15 CBU/g glucan) and cellulase (15 FPU/g glucan) and a TS loading of 8% (w/w). | Pretreated creeping wild ryegrass (Acid-H2SO4) | 10.6% | [59] |
| BSA 50 mg/g of dry solid | Enzymatic hydrolysis was carried out for 48 h at 50 °C. Enzyme formulation was composed of cellulase (10 FPU/g of DM) and β-glucosidase (500 nkat/g DM). Xylanase was dosed at 0.18 mg/g DM. The TS loading was 1% (w/v). | Pretreated corn stover (ammonia) | 31.37% | [60] |
| BSA, CSL, YE, and P 1.0 mg/mL (total protein) | * Non-catalytic protein pretreatment All experiments were carried out for 12 h at 50 °C using 50 mM citrate buffer (pH 4.8); Enzymatic hydrolysis was carried out for 72 h at 50 °C using an enzyme dosage of 15 FPU/g of biomass and a TS loading of 2% (w/v). | Pretreated rice straw (alkaline-NaOH) | BSA-19.7% CSL-12.7% YE-13.5% P-13.7% | [61] |
| Soybean protein 4% (w/w) | 1:1 cocktail from A. niger and T. reesei. All experiments were carried out for 24 h at 50 °C using 50 mM citrate buffer (pH 4.8) and a TS loading of 5% (w/v). | Pretreated sugarcane bagasse (Steam-explosion) | 54% | [53] |
| Peanut protein 2.5 g/L (total protein) | * Non-catalytic protein pretreatment All experiments were carried out for 2 h at 50 °C using 50 mM acetate buffer (pH 5); Enzymatic hydrolysis was carried out for 72 h at 50 °C. Enzyme formulation was composed of β-glucosidase (10 CBU/g glucan) and cellulase (5 FPU/g glucan) and a TS loading of 2% (w/v). | Pretreated bamboo (phenylsulfonic acid) | 147% | [6] |
| Amaranth proteins 8% (w/w) | All experiments were carried out using 0.2 M Na-acetate buffer (pH 4.8) for 48 h at 50 °C. Enzyme formulation was composed of cellulases at 10.60 FPU/g biomass and xylanase at 6.72 U/g biomass, and a TS loading of 5% (w/v). | Pretreated Amaranth straw (liquid hot water) | 12% | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Muñoz, S.; Balbino, T.R.; de Oliveira, F.; Rocha, T.M.; Barbosa, F.G.; Vélez-Mercado, M.I.; Marcelino, P.R.F.; Antunes, F.A.F.; Moraes, E.J.C.; dos Santos, J.C.; et al. Surfactants, Biosurfactants, and Non-Catalytic Proteins as Key Molecules to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass. Molecules 2022, 27, 8180. https://doi.org/10.3390/molecules27238180
Sánchez-Muñoz S, Balbino TR, de Oliveira F, Rocha TM, Barbosa FG, Vélez-Mercado MI, Marcelino PRF, Antunes FAF, Moraes EJC, dos Santos JC, et al. Surfactants, Biosurfactants, and Non-Catalytic Proteins as Key Molecules to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass. Molecules. 2022; 27(23):8180. https://doi.org/10.3390/molecules27238180
Chicago/Turabian StyleSánchez-Muñoz, Salvador, Thércia R. Balbino, Fernanda de Oliveira, Thiago M. Rocha, Fernanda G. Barbosa, Martha I. Vélez-Mercado, Paulo R. F. Marcelino, Felipe A. F. Antunes, Elisangela J. C. Moraes, Julio C. dos Santos, and et al. 2022. "Surfactants, Biosurfactants, and Non-Catalytic Proteins as Key Molecules to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass" Molecules 27, no. 23: 8180. https://doi.org/10.3390/molecules27238180
APA StyleSánchez-Muñoz, S., Balbino, T. R., de Oliveira, F., Rocha, T. M., Barbosa, F. G., Vélez-Mercado, M. I., Marcelino, P. R. F., Antunes, F. A. F., Moraes, E. J. C., dos Santos, J. C., & da Silva, S. S. (2022). Surfactants, Biosurfactants, and Non-Catalytic Proteins as Key Molecules to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass. Molecules, 27(23), 8180. https://doi.org/10.3390/molecules27238180