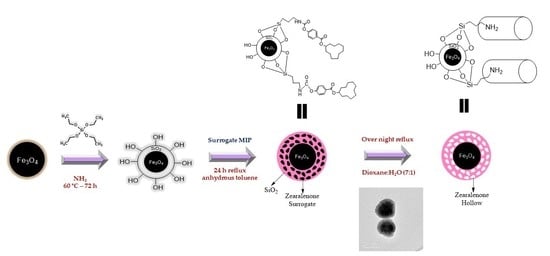
Magnetic Core-Shell Nanoparticles Using Molecularly Imprinted Polymers for Zearalenone Determination
Abstract
1. Introduction
2. Results and Discussion
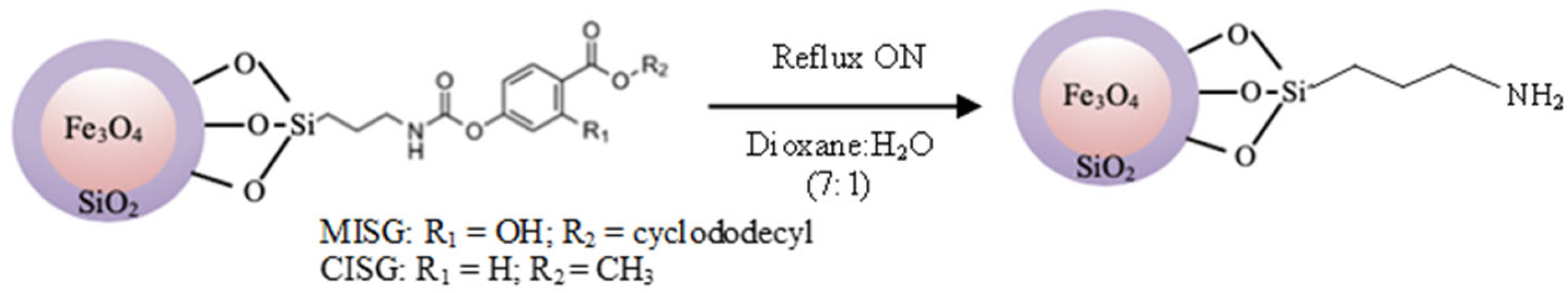
2.1. Synthesis of Fe3O4@MIP
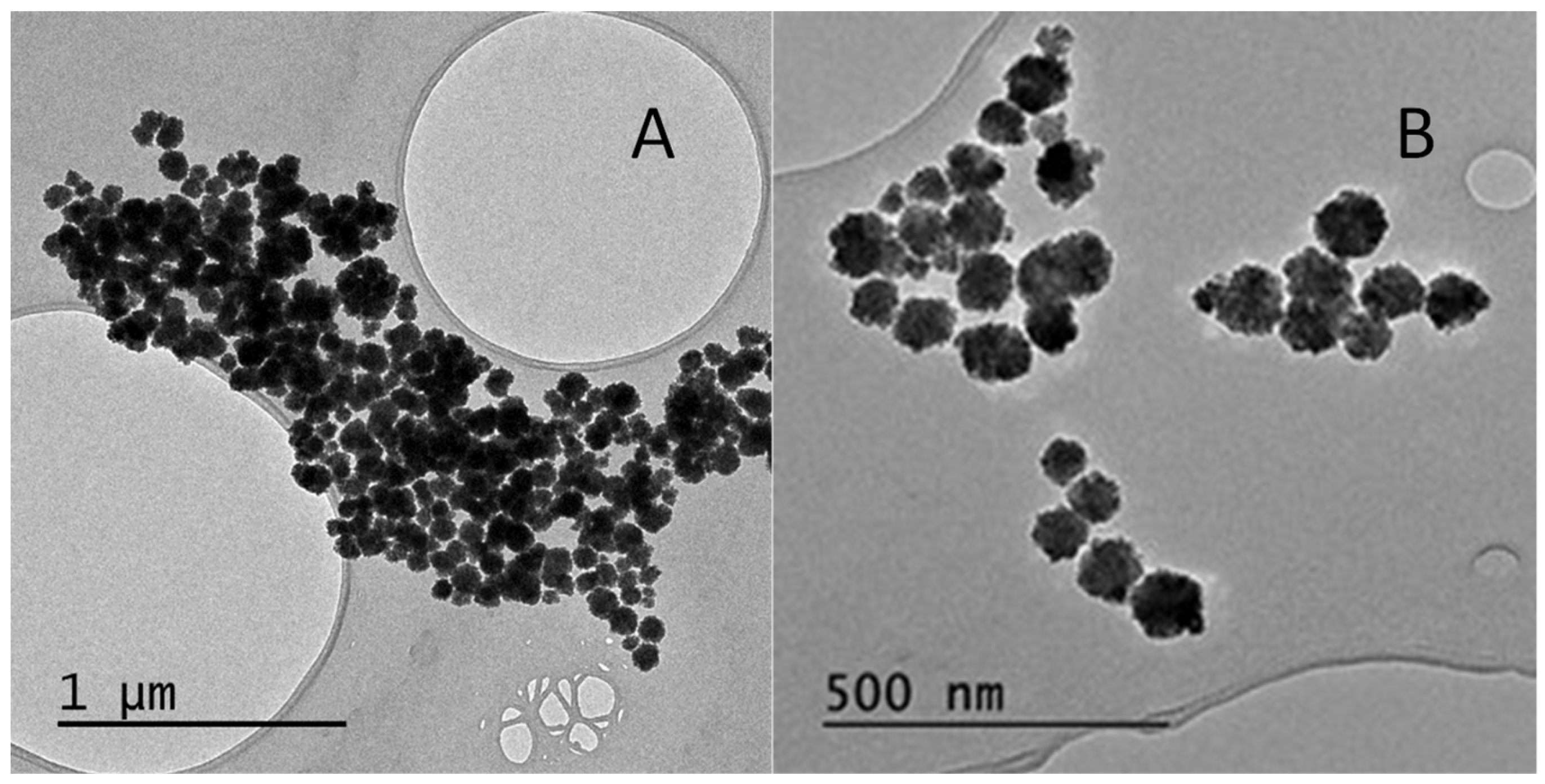
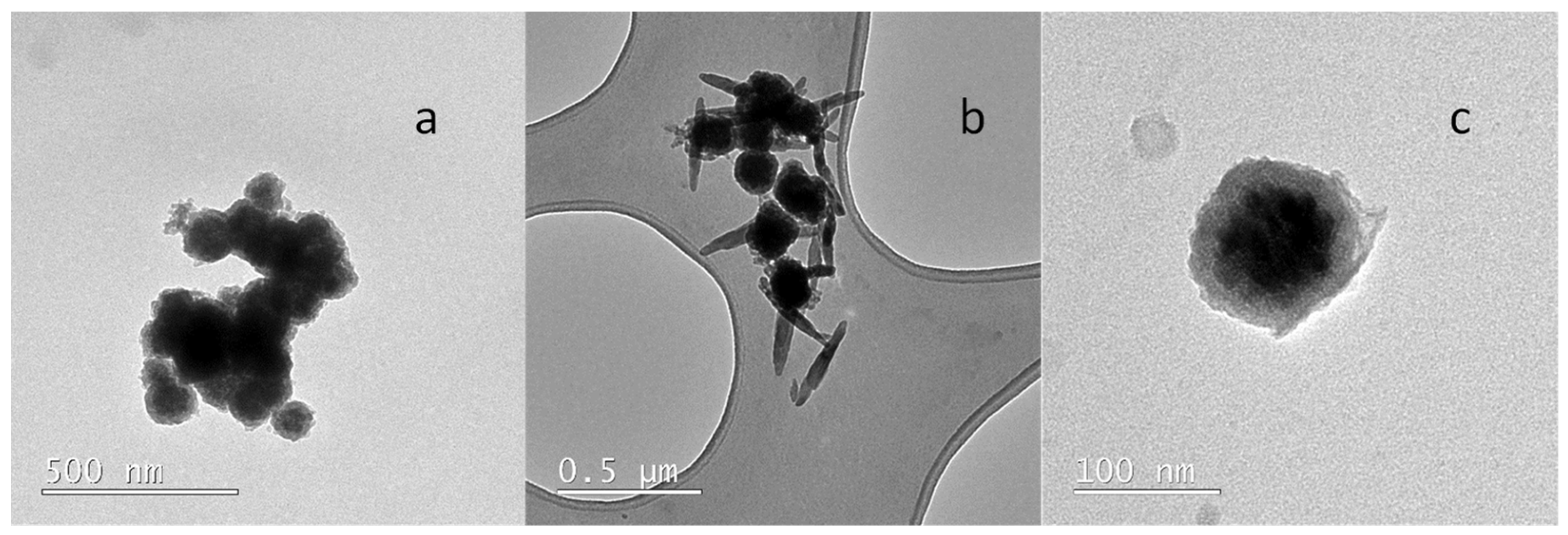
2.2. Morphology and Structure of the Core—Shell Nanostructures
2.2.1. Fourier Transform Infrared Spectroscopy (FT-IR)
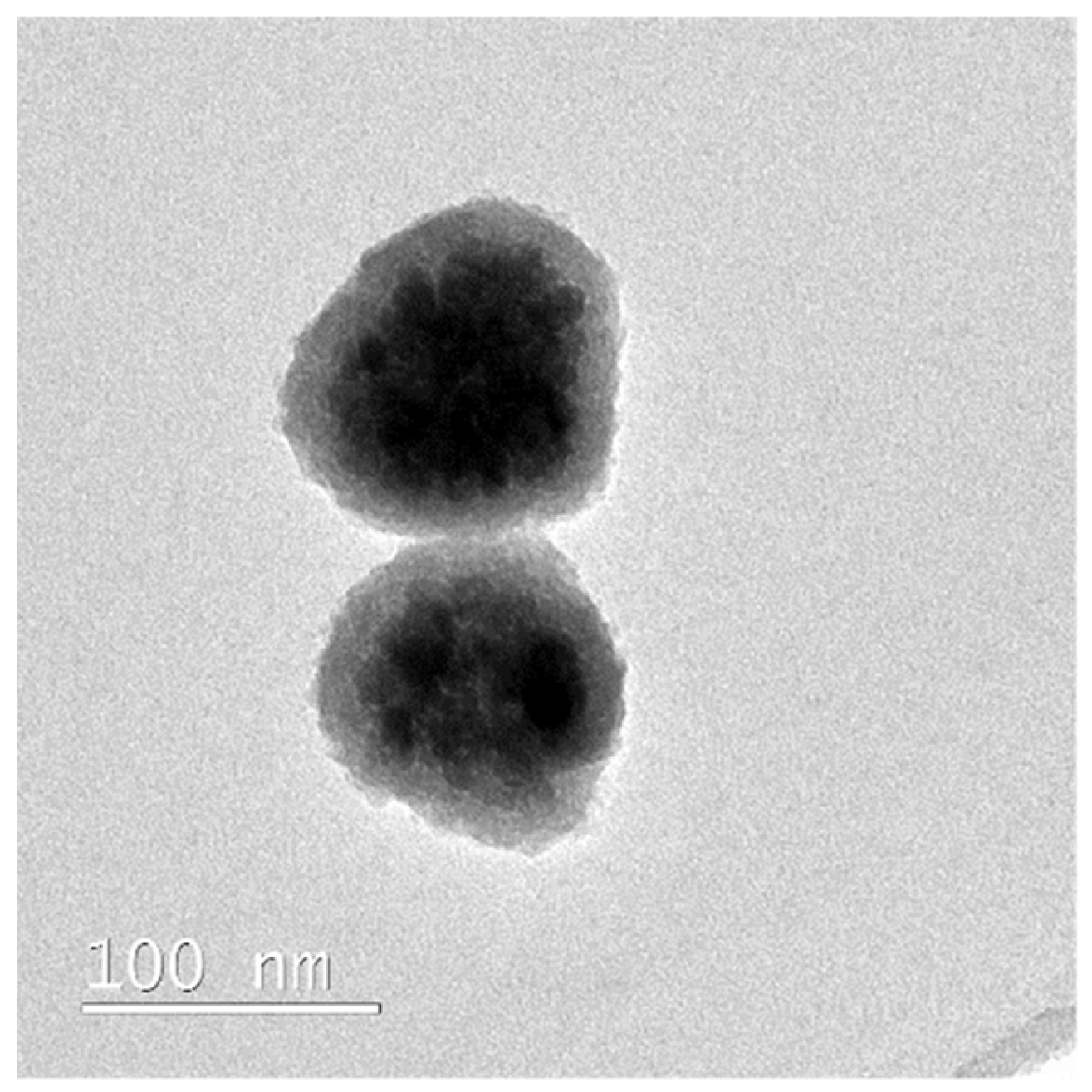
2.2.2. Transmission Electron Microscopy
2.2.3. X-ray Powder Diffraction
2.2.4. Microanalysis
2.2.5. Thermogravimetric Analysis
2.2.6. Magnetization
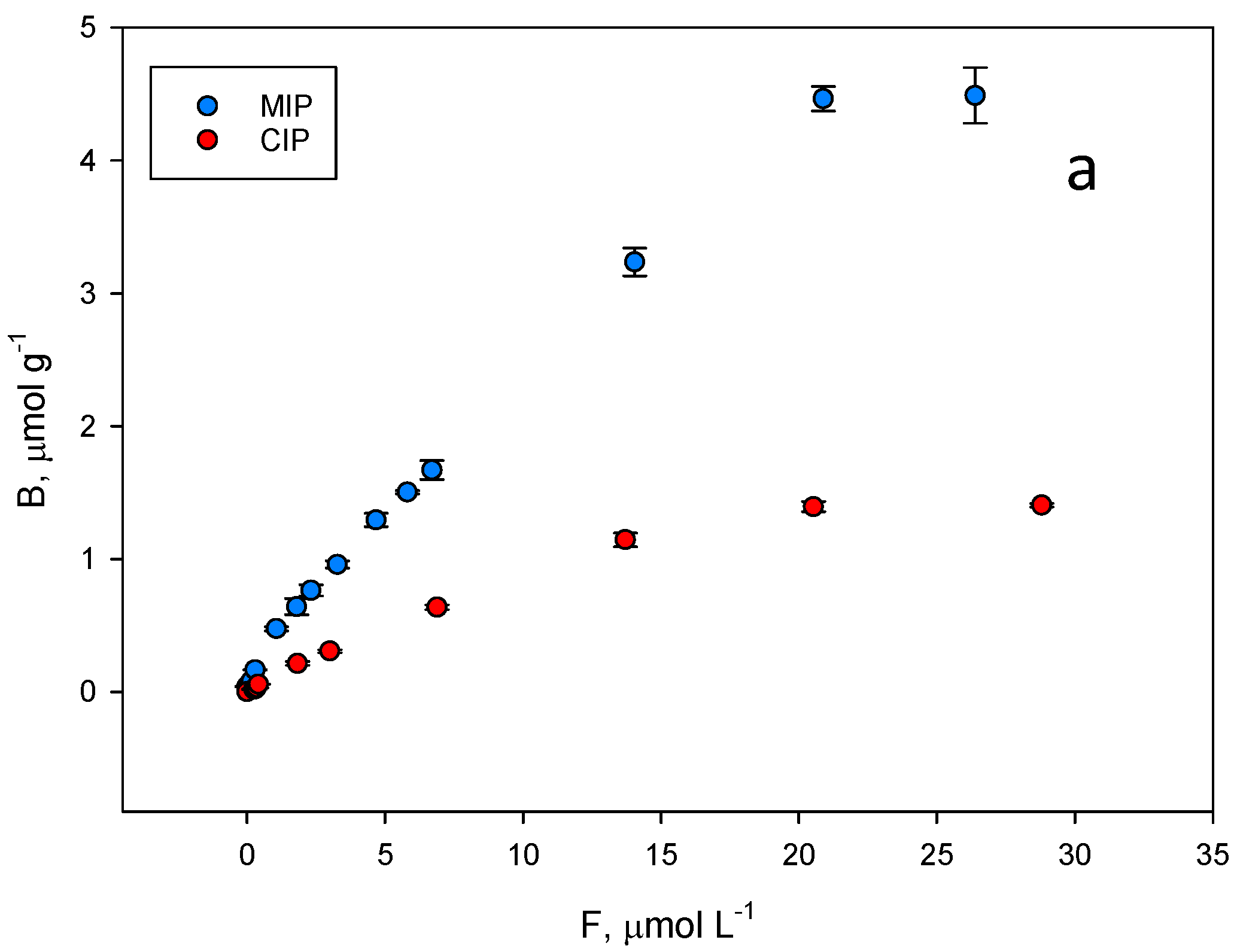
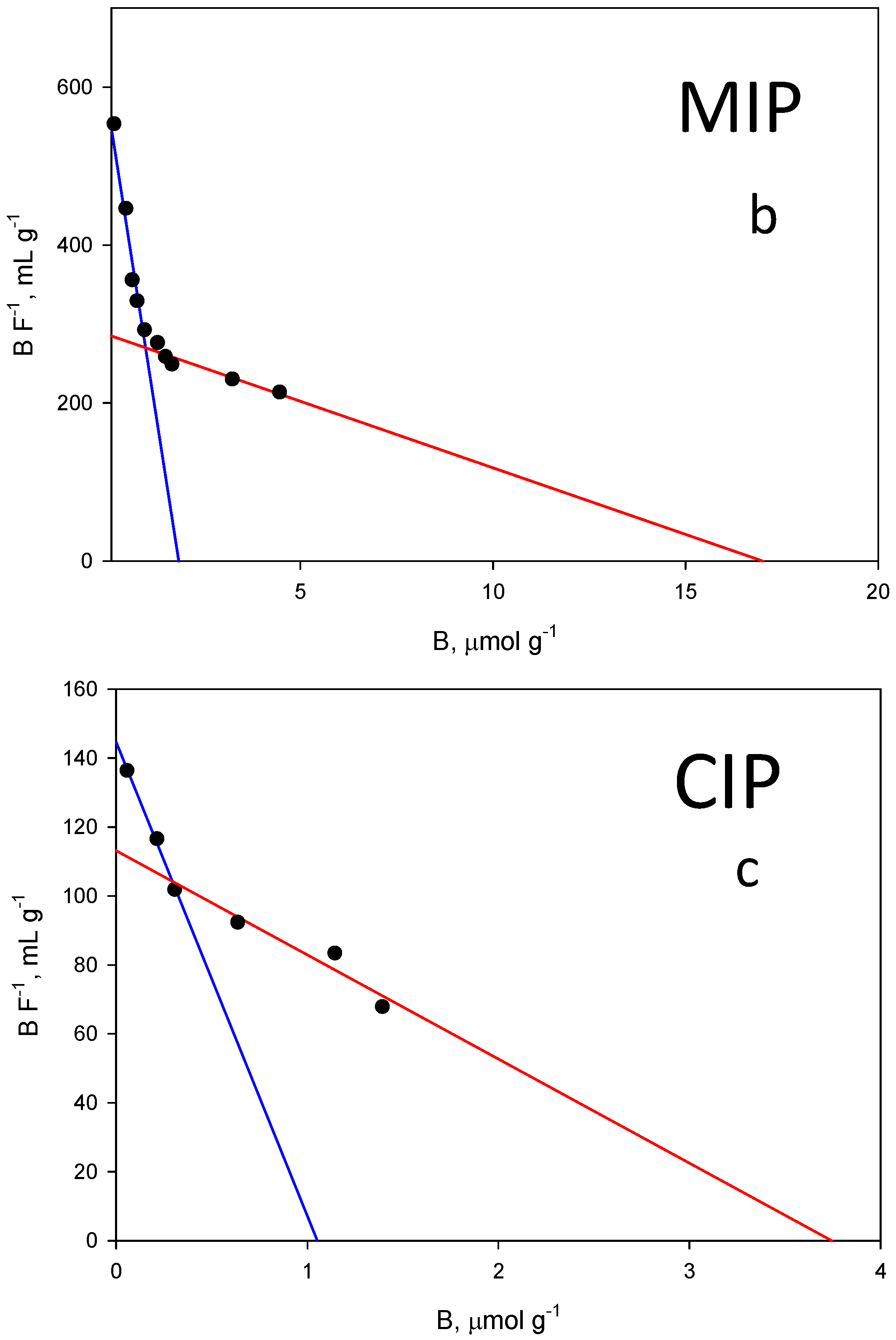
2.3. Binding Isotherm
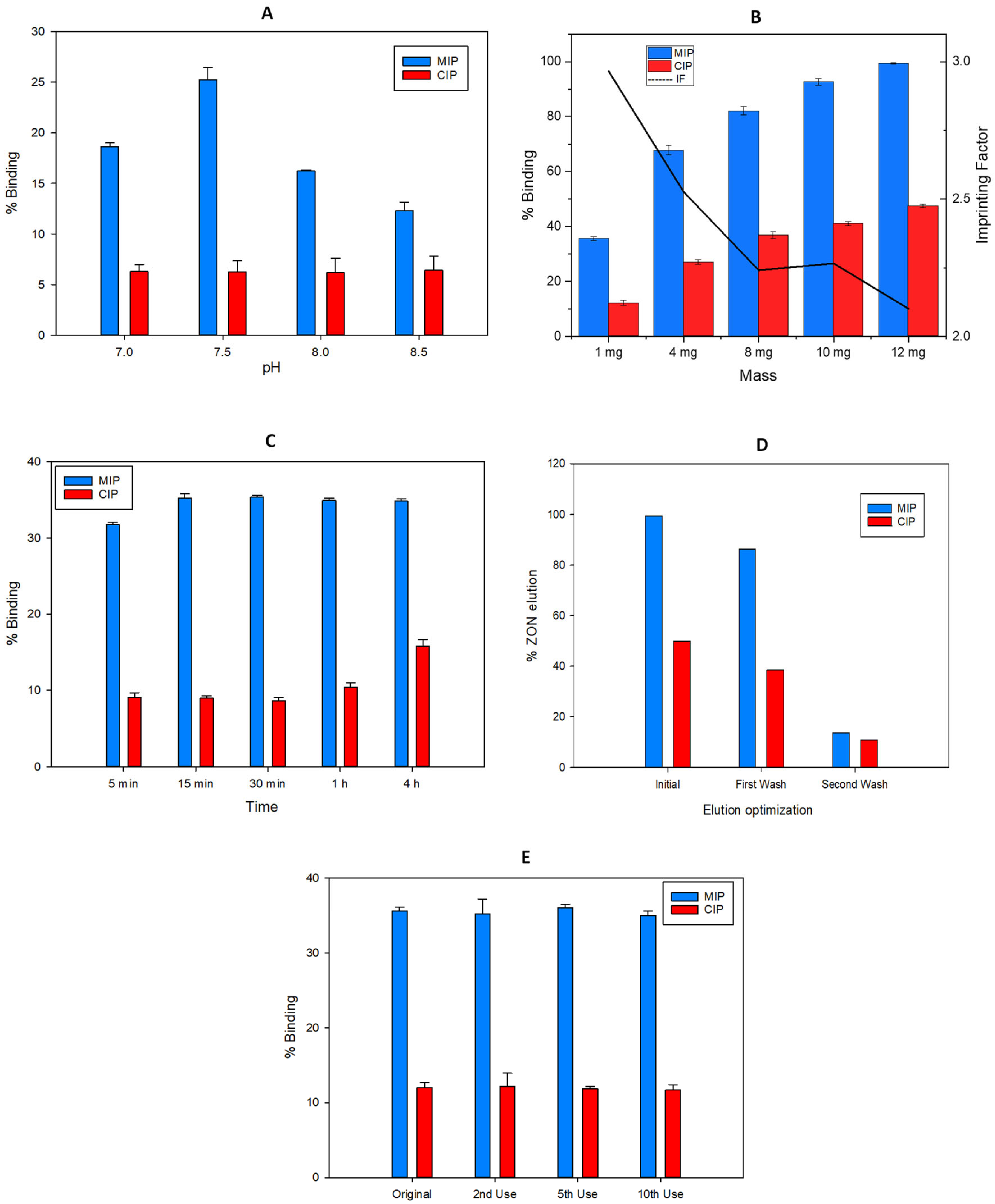
2.4. Optimization of the MISPE Process for the Extraction of ZON
2.4.1. Incubation pH
2.4.2. Mass of Fe3O4@SiO2@MIP Particles
2.4.3. Incubation Time
2.4.4. Elution Solvent Volume
2.4.5. Cross Reactivity
2.5. Sample Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Apparatus
3.3. HPLC Analysis
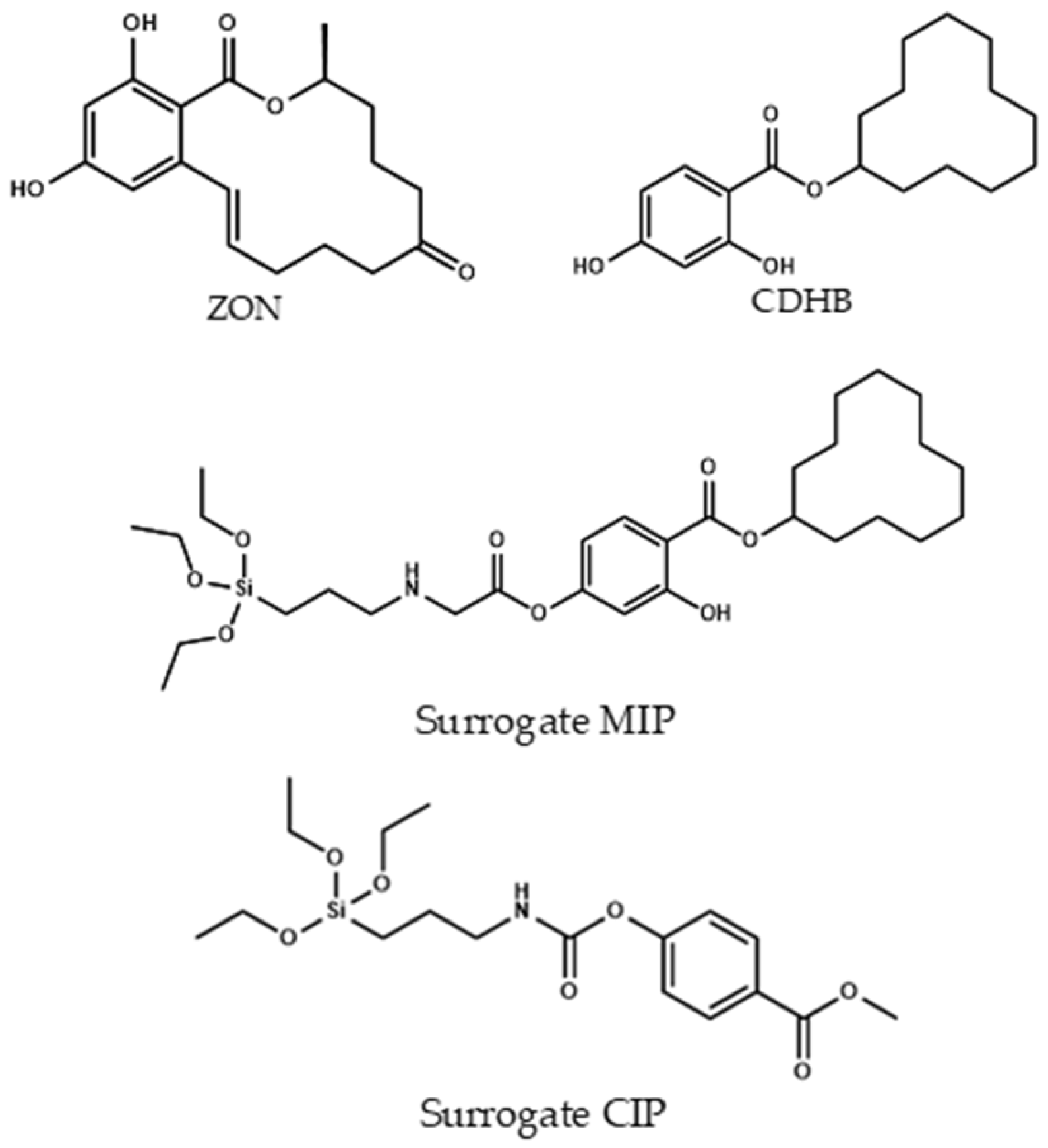
3.4. Silane Derivate Synthesis
3.4.1. Synthesis of Cyclododecyl 2,4-dihydroxybenzoate (CDHB)
3.4.2. Synthesis of Cyclododecyl 2-hydroxy-4-(3-triethoxysilylpropylcarbamoyloxy) Benzoate (Surrogate-MIP)
3.4.3. Synthesis of Methyl 4-(3-triethoxysilylpropylcarbamoyloxy) Benzoate (Surrogate-CIP)
3.5. MIPs/CIPs Fabrication
3.5.1. Fe3O4 and Fe3O4@SiO2 Magnetic Nanoparticles
3.5.2. Preparation of the Functionalized Magnetic Nanoparticles (Fe3O4@SiO2@MIP/CIP)
3.5.3. Surrogate Extraction
3.6. Evaluation of Parameters for MISPE Process
3.7. MIPs and CIPs Rebinding Performance
3.8. Real Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dalefield, R. Mycotoxins and Mushrooms. In Veterinary Toxicology for Australia and New Zealand; Elsevier: Masterton, New Zealand, 2017; pp. 373–419. [Google Scholar] [CrossRef]
- D’Mello, J.P.F.; Macdonald, A.M.C. Mycotoxins. Anim. Feed. Sci. Technol. 1997, 69, 155–166. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, Metabolism, and Impact of Mycotoxins on Humans and Animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I. Mycotoxins. In Foodborne Infections and Intoxications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 409–418. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the Risks for Public Health Related to the Presence of Zearalenone in Food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Vesonder, R.F.; Goliński, P.; Plattner, R.; Zietkiewicz, D.L. Mycotoxin Formation by Different Geographic Isolates of Fusarium Crookwellense. Mycopathologia 1991, 113, 11–14. [Google Scholar] [CrossRef]
- Urraca, J.L.; Benito-Peña, E.; Pérez-Conde, C.; Moreno-Bondi, M.C.; Pestka, J.J. Analysis of Zearalenone in Cereal and Swine Feed Samples Using an Automated Flow-Through Immunosensor. J. Agric. Food Chem. 2005, 53, 3338–3344. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T.; Scott, P.M.; Watanabe, H. Risk Assessment of the Mycotoxin Zearalenone. Regul. Toxicol. Pharmacol. 1987, 7, 253–306. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Risks for Animal Health Related to the Presence of Zearalenone and Its Modified Forms in Feed|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4851 (accessed on 26 September 2022).
- European Food Safety Authority. Evaluation of the Increase of Risk for Public Health Related to a Possible Temporary Derogation from the Maximum Level of Deoxynivalenol, Zearalenone and Fumonisins for Maize and Maize Products. EFSA J. 2014, 12, 3699. [Google Scholar] [CrossRef]
- Gémes, B.; Takács, E.; Gádoros, P.; Barócsi, A.; Kocsányi, L.; Lenk, S.; Csákányi, A.; Kautny, S.; Domján, L.; Szarvas, G.; et al. Development of an Immunofluorescence Assay Module for Determination of the Mycotoxin Zearalenone in Water. Toxins 2021, 13, 182. [Google Scholar] [CrossRef]
- Arranz, I.; Mischke, C.; Stroka, J.; Sizoo, E.; van Egmond, H.; Neugebauer, M. Liquid Chromatographic Method for the Quantification of Zearalenone in Baby Food and Animal Feed: Interlaboratory Study. J. AOAC Int. 2007, 90, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H.; Mañes, J. A Survey of Trichothecenes, Zearalenone and Patulin in Milled Grain-Based Products Using GC–MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; D’Ascenzo, G.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Determination of Type B Trichothecenes and Macrocyclic Lactone Mycotoxins in Field Contaminated Maize. Food Chem. 2005, 92, 559–568. [Google Scholar] [CrossRef]
- Josephs, R.D.; Schuhmacher, R.; Krska, R. International Interlaboratory Study for the Determination of the Fusarium Mycotoxins Zearalenone and Deoxynivalenol in Agricultural Commodities. Food Addit. Contam. 2001, 18, 417–430. [Google Scholar] [CrossRef]
- Santos Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent Mycotoxins in Animal Feed: Occurrence and Analytical Methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, S.; Bai, Y.; Prates, L.L.; Lei, Y.; Yu, P. Mycotoxin Contamination of Food and Feed in China: Occurrence, Detection Techniques, Toxicological Effects and Advances in Mitigation Technologies. Food Control 2018, 91, 202–215. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Multiclass Mycotoxin Analysis in Silybum Marianum by Ultra High Performance Liquid Chromatography–Tandem Mass Spectrometry Using a Procedure Based on QuEChERS and Dispersive Liquid–Liquid Microextraction. J. Chromatogr. A 2013, 1282, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, J.N.; Zhou, L.; Wang, Y.; Zhao, Y.; Xing, F.; Dai, X.; Liu, Y. Mycotoxin Detection—Recent Trends at Global Level. J. Integr. Agric. 2015, 14, 2265–2281. [Google Scholar] [CrossRef]
- Mateo, J.J.; Mateo, R.; Hinojo, M.J.; Llorens, A.; Jiménez, M. Liquid Chromatographic Determination of Toxigenic Secondary Metabolites Produced by Fusarium Strains. J. Chromatogr. A 2002, 955, 245–256. [Google Scholar] [CrossRef]
- Shi, L.; Yu, T.; Luo, M.; Wang, H. Preparation Monoclonal β-Type Anti-Idiotype Antibody of Zearalenone and Development of Green ELISA Quantitative Detecting Technique. Prep. Biochem. Biotechnol. 2020, 50, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, L.; Zhou, B.; Zhu, L.; Zhang, Y.; Huang, B. Simultaneous Detection of Deoxynivalenol and Zearalenone by Dual-Label Time-Resolved Fluorescence Immunoassay: Detection of Deoxynivalenol and Zearalenone by Immunoassay. J. Sci. Food Agric. 2011, 91, 193–197. [Google Scholar] [CrossRef]
- Gagkaeva, T.; Gavrilova, O.; Orina, A.; Lebedin, Y.; Shanin, I.; Petukhov, P.; Eremin, S. Analysis of Toxigenic Fusarium Species Associated with Wheat Grain from Three Regions of Russia: Volga, Ural, and West Siberia. Toxins 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hany, R.A.G.; Urraca, J.L.; Descalzo, A.B.; Gómez-Arribas, L.N.; Moreno-Bondi, M.C.; Orellana, G. Tailoring Molecularly Imprinted Polymer Beads for Alternariol Recognition and Analysis by a Screening with Mycotoxin Surrogates. J. Chromatogr. A 2015, 1425, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, G. Preparation of Magnetic Molecularly Imprinted Polymer for Melamine and Its Application in Milk Sample Analysis by HPLC. J. Biomed. Sci. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Mehdinia, A.; Dadkhah, S.; Baradaran Kayyal, T.; Jabbari, A. Design of a Surface-Immobilized 4-Nitrophenol Molecularly Imprinted Polymer via Pre-Grafting Amino Functional Materials on Magnetic Nanoparticles. J. Chromatogr. A 2014, 1364, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Appell, M.; Mueller, A. Mycotoxin Analysis Using Imprinted Materials Technology: Recent Developments. J. AOAC Int. 2016, 99, 861–864. [Google Scholar] [CrossRef]
- Caro, E.; Masqué, N.; Marcé, R.M.; Borrull, F.; Cormack, P.A.G.; Sherrington, D.C. Non-Covalent and Semi-Covalent Molecularly Imprinted Polymers for Selective on-Line Solid-Phase Extraction of 4-Nitrophenol from Water Samples. J. Chromatogr. A 2002, 963, 169–178. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, H. A Novel Fluorescence and SPE Adsorption Nanomaterials of Molecularly Imprinted Polymers Based on Quantum Dot-Grafted Covalent Organic Frameworks for the High Selectivity and Sensitivity Detection of Ferulic Acid. Nanomaterials 2019, 9, 305. [Google Scholar] [CrossRef]
- Bakhtiar, S.; Bhawani, S.A.; Shafqat, S.R. Synthesis and Characterization of Molecularly Imprinted Polymer for TheRemoval/Extraction of Thymol from Spiked Blood Serum and River Water. Asian J. Chem. 2019, 31, 2479–2484. [Google Scholar] [CrossRef]
- Cáceres, C.; Bravo, C.; Rivas, B.; Moczko, E.; Sáez, P.; García, Y.; Pereira, E. Molecularly Imprinted Polymers for the Selective Extraction of Bisphenol A and Progesterone from Aqueous Media. Polymers 2018, 10, 679. [Google Scholar] [CrossRef]
- Urraca, J.L.; Carbajo, M.C.; Torralvo, M.J.; González-Vázquez, J.; Orellana, G.; Moreno-Bondi, M.C. Effect of the Template and Functional Monomer on the Textural Properties of Molecularly Imprinted Polymers. Biosens. Bioelectron. 2008, 24, 155–161. [Google Scholar] [CrossRef]
- Tang, Y.; Lan, J.; Gao, X.; Liu, X.; Zhang, D.; Wei, L.; Gao, Z.; Li, J. Determination of Clenbuterol in Pork and Potable Water Samples by Molecularly Imprinted Polymer through the Use of Covalent Imprinting Method. Food Chem. 2016, 190, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Leung, B.; Danylec, B.; Harris, S.; Boysen, R.; Hearn, M. Phytosterol Recognition via Rationally Designed Molecularly Imprinted Polymers. C 2018, 4, 13. [Google Scholar] [CrossRef]
- Dąbrowski, M.; Zimińska, A.; Kalecki, J.; Cieplak, M.; Lisowski, W.; Maksym, R.; Shao, S.; D’Souza, F.; Kuhn, A.; Sharma, P.S. Facile Fabrication of Surface-Imprinted Macroporous Films for Chemosensing of Human Chorionic Gonadotropin Hormone. ACS Appl. Mater. Interfaces 2019, 11, 9265–9276. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef] [PubMed]
- Urraca, J.L.; Huertas-Pérez, J.F.; Cazorla, G.A.; Gracia-Mora, J.; García-Campaña, A.M.; Moreno-Bondi, M.C. Development of Magnetic Molecularly Imprinted Polymers for Selective Extraction: Determination of Citrinin in Rice Samples by Liquid Chromatography with UV Diode Array Detection. Anal. Bioanal. Chem. 2016, 408, 3033–3042. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Zamani, R. Synthesis of Fe3O4@SiO2 Magnetic Nanoparticle, Functionalized with 2,6-Pyridine Dicarboxylic Acid. Inorg. Nano-Met. Chem. 2019, 49, 127–131. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, T.; Gao, L.; Xing, L.; Zhu, Y.; Li, S. A Convenient Separation Method for Di(2-Ethylhexyl)Phthalate by Novel Superparamagnetic Molecularly Imprinted Polymers. RSC Adv. 2018, 8, 36191–36199. [Google Scholar] [CrossRef]
- Ghanbari, M.; Moradi, S.; Setoodehkhah, M. Fe3O4@SiO2@ADMPT/H6P2W18O62: A Novel Wells–Dawson Heteropolyacid-Based Magnetic Inorganic–Organic Nanohybrid Material as Potent Lewis Acid Catalyst for the Efficient Synthesis of 1,4-Dihydopyridines. Green Chem. Lett. Rev. 2018, 11, 111–124. [Google Scholar] [CrossRef]
- Cregg, P.J.; Murphy, K.; Mardinoglu, A. Inclusion of Interactions in Mathematical Modelling of Implant Assisted Magnetic Drug Targeting. Appl. Math. Model. 2012, 36, 1–34. [Google Scholar] [CrossRef]
- Campos, A.F.C.; Ferreira, M.A.; Marinho, E.P.; Tourinho, F.A.; Depeyrot, J. Synthesis and Design of Functionalized Magnetic Nanocolloids for Water Pollution Remediation. Phys. Procedia 2010, 9, 45–48. [Google Scholar] [CrossRef]
- Urraca, J.L.; Marazuela, M.D.; Merino, E.R.; Orellana, G.; Moreno-Bondi, M.C. Molecularly Imprinted Polymers with a Streamlined Mimic for Zearalenone Analysis. J. Chromatogr. A 2006, 1116, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Urraca, J.L.; Marazuela, M.D.; Moreno-Bondi, M.C. Molecularly Imprinted Polymers Applied to the Clean-up of Zearalenone and α-Zearalenol from Cereal and Swine Feed Sample Extracts. Anal. Bioanal. Chem. 2006, 385, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing Company, Inc.: Reading, MA, USA; Notre Dame, IN, USA, 1956. [Google Scholar]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Li, Q.; Yang, Q.; Li, Y.; Du, J. Colorimetric Magnetic Microspheres as Chemosensor for Cu2+ Prepared from Adamantane-Modified Rhodamine and β-Cyclodextrin-Modified Fe3O4@SiO2 via Host–Guest Interaction. Talanta 2015, 141, 33–40. [Google Scholar] [CrossRef]
- Bass, J.D.; Anderson, S.L.; Katz, A. The Effect of Outer-Sphere Acidity on Chemical Reactivity in a Synthetic Heterogeneous Base Catalyst. Angew. Chem. Int. Ed. 2003, 42, 5219–5222. [Google Scholar] [CrossRef]
- Lee, K.; Ki, C.D.; Kim, H.; Chang, J.Y. Selectivity Control by Chemical Modification of the Recognition Sites in Two-Point Binding Molecularly Imprinted Polymer. Macromolecules 2004, 37, 5544–5549. [Google Scholar] [CrossRef]
| Spiked Conc. (µg kg−1) | RA in Mineral Water (µg kg−1) | RA in Tap Water (µg kg−1) | RA in River Water (µg kg−1) |
|---|---|---|---|
| 12.5 | 12 ± 1 | 11 ± 2 | 10 ± 3 |
| 25 | 16 ± 11 | 17 ± 9 | 14 ± 9 |
| 50 | 32 ± 12 | 30 ± 4 | 31 ± 4 |
| LOD | 2.3 | 2.4 | 2.4 |
| LOQ | 7.7 | 8.0 | 8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calahorra-Rio, L.; Guadaño-Sánchez, M.; Moya-Cavas, T.; Urraca, J.L. Magnetic Core-Shell Nanoparticles Using Molecularly Imprinted Polymers for Zearalenone Determination. Molecules 2022, 27, 8166. https://doi.org/10.3390/molecules27238166
Calahorra-Rio L, Guadaño-Sánchez M, Moya-Cavas T, Urraca JL. Magnetic Core-Shell Nanoparticles Using Molecularly Imprinted Polymers for Zearalenone Determination. Molecules. 2022; 27(23):8166. https://doi.org/10.3390/molecules27238166
Chicago/Turabian StyleCalahorra-Rio, Luis, Miriam Guadaño-Sánchez, Tamara Moya-Cavas, and Javier Lucas Urraca. 2022. "Magnetic Core-Shell Nanoparticles Using Molecularly Imprinted Polymers for Zearalenone Determination" Molecules 27, no. 23: 8166. https://doi.org/10.3390/molecules27238166
APA StyleCalahorra-Rio, L., Guadaño-Sánchez, M., Moya-Cavas, T., & Urraca, J. L. (2022). Magnetic Core-Shell Nanoparticles Using Molecularly Imprinted Polymers for Zearalenone Determination. Molecules, 27(23), 8166. https://doi.org/10.3390/molecules27238166