Abstract
Solenostemma argel is a desert medicinal plant indigenous to African countries. This research aims to study the pharmacological properties of Solenostemma argel plant. Aerial parts (leaves and flowers) of Solenostemma argel (Delile) Hayane were tested for antibacterial activity, antioxidant activity, anticancer, and anti-inflammatory activity. Phenolic and flavonoid contents of the plant were characterized. There was an increase in the antioxidant activity of Solenostemma argel extract from 12.16% to 94.37% by increasing concentration from10 µg/mL to 1280 µg/mL. The most sensitive organism was S. epidermidis with chloroform extract. The MTT assay revealed that methanolic extracts of Solenostemma argel showed potent cytotoxic effects on the A549, Caco-2, and MDAMB-231 cell lines, respectively. The anti-inflammatory activity increased by increasing the concentration of methanolic extract of Solenostemma argel, using indomethacin as a standard. Gallic acid was the most abundant phenolic acid, followed by synergic acid and p-coumaric acid, respectively. Catechin, quercetin, luteolin, kaempferol and rutin flavonoids were also found in the methanolic extract. GC-mass analysis showed that aerial parts of Solenostemma argel were rich in 2-(5-methyl-5 vinyl tetrahydro-2-furanyl)-2-propanol (11.63%), hexanoic acid methyl ester (10.93%), 3-dioxolane,4-methyl-2-pentadecyl (9.69%), phenol, 2-(1,1-dimethylethyl) (8.50%). It can be concluded that Solenostemma argel methanolic extract contain natural bioactive constituents with potential medicinal importance such as antioxidants, antimicrobial, anti-inflammatory, and anticancer activities.
1. Introduction
The use of medicinal plants for therapeutic purposes is an ancient form of medication, which is universal among most non-Western countries and predates modern medicine [1]. It is thought that a significant percentage (~75%) of the human population uses herbs and other forms of traditional medicines to treat diseases [2]. In addition, a substantial number of current pharmaceuticals such as aspirin, codeine, and quinine used for disease therapeutics in modern medicine are derived from historical herbal remedies and medicinal plants [2,3]. Therefore, medicinal plants are important sources of highly effective pharmaceuticals for disease treatment and sustainable human health [3].
Most common human diseases are caused by microbial infections and cellular damages caused by oxidative stress related to imbalances between the formation and neutralization of pro-oxidants [4,5]. Oxidative stress, caused by free radicals alongside lipid peroxidation, is a contributory factor to multiple diseases including cardiovascular diseases, atherosclerosis, cancer, and inflammatory diseases [6,7]. Anand et al. [8]; Marrelli, [9] showed that some medicinal plants can be used to treat diseases linked to pathogenic micro-organisms and cellular damages such as inflammatory and immune disorders. Medicinal plants contain primary or secondary metabolites such as lignans, flavonoids, terpenoids, tannins, phenolic acids, alkaloids, quinones, and coumarins, which exhibit significant antioxidant, antimicrobial, and anti-cancer activities [8,9,10].
Cancer is one of the main causes of morbidity and mortality worldwide [11]. Cancer is caused by unregulated cell proliferation, which leads to the disruption in the function and structure of surrounding tissues [12,13]. There are more than 100 forms of cancer with prostate cancer (in men) and breast cancer (in women) being the major forms of cancer in humans [14,15]. Despite the availability of treatments such as radiation, chemotherapy, and immune and hormone therapy, cancer remains one of the leading causes of death worldwide aside from cardiovascular and infectious diseases [13]. While some of these cancer treatment approaches are moderately effective, their long-term use and success rates are hampered by associated chronic toxic effects [13]. Given that free radical activities and inflammation are associated with cancer development, drug candidates that can scavenge free radicals and inhibit inflammation with limited toxic effects are better suited for use as anticancer agents.
Therefore, medicinal plants continue to be investigated as a resource to treat different cancers such as colon cancer [16], breast cancer [17], and prostate cancer [18]. Nevertheless, no silver bullet is yet to be found from medicinal plants for the complete treatment and cure of cancer despite the evaluation of many candidates. Hence, there is a need to continue the evaluation of more plants for their anti-cancer and anti-inflammatory properties that would inhibit or prevent the development of cancer. One of the promising candidates is a plant called argel.
Argel (Solenostemma argel) is a wild herbal plant widely found in different countries such as Sudan, Saudi Arabia, and Egypt. Different parts of this plants (leaves, stems, and bark) are used for pain relief and treating infections affecting the respiratory, urinary, and gastrointestinal systems [19]. These parts have also been used to treat liver, kidney, diabetes, and cardiovascular diseases in these countries [19]. Pregnane and phenolic glycosides extracts from the plant’s aerial and root parts have been shown to possess antioxidant properties in human tumor cell lines [20]. Acetone extracts of this plant have also been shown to have antioxidant and anti-inflammatory properties in laboratory rats [21]. In spite of Solenostemma argel widespread use and numerous properties, very little research has been carried out to evaluate its therapeutic properties. The purpose of this study was to assess the anticancer, antibacterial, anti-inflammatory, and antioxidant activity of different extracts from Solenostemma argel, as well as to conduct GC-mass and HPLC analyses of volatile components and phenolic acids.
2. Materials and Methods
2.1. Chemicals
All chemicals and HPLC standards used were of high analytical grade, purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. Plant Materials and Sample Preparation
The plant materials, Solenostemma argel (family Apocynaceae), used in this study were collected from Halaib and Shalatin (Gabal Elba) region in Egypt. The collected plant materials were washed using distilled water. The aerial parts were cut and air-dried in the shade. These air-dried plant materials were ground into a fine powder using Moulinex Dpa241 Choppers, Blender 1000, Ecully, France, and stored at −20 °C in screwed-in brown bottles until needed for further investigations.
2.3. Extraction Process
One hundred grams of the powdered Solenostemma argel (aerial parts) was extracted by shaking at 150 rpm for 24 h at 25 °C with 1 L of solvent (water, methanol, acetone, ethanol, chloroform, ether, ethyl acetate, and methylene chloride). The extracts were subsequently filtered using a Buchner funnel containing a Whatman No. 1 filter paper. The obtained residue was re-extracted with 500 mL of solvent, and filtered, after which the filtrates (extracts) were pooled together and concentrated using a rotary evaporator at reduced pressure (Heidolph VV 2000,Hei-VAPCore, Schwabach, Germany. The concentrated extract was dried in a desiccator under a vacuum until a consistent weight was obtained. The weight of the triplicate extracts (samples) was recorded, and each extract was re-suspended in the smallest amount of solvent possible to achieve a concentration of 10 mg/mL. The extracts were stored at −4 °C until use.
2.4. Antioxidant Capacity Assay
Antioxidant activity was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as described by McCue et al. [22]. The assay reaction mixture contained 40 μL of Solenostemma argel extract from different solvent extractions at different concentrations, ranging from 10 to 1280-μg/mL, which had been prepared by diluting the extract with the extraction solvent and 3 mL of methanolic solution of 0.1 mM DPPH radical. The mixture was vigorously agitated and incubated at 37 °C for 30 min. The absorbance values were measured at 515 nm using a UV–visible spectrophotometer (Milton Roy, Spectronic 1201, SpectraLab Scientific Inc., Markham, ON, Canada). Ascorbic acid was used as a positive control. The absorbance value of the reaction mixture was calculated using the following equation:
DPPH scavenging activity (%) = 100 × (A0 − A1)/(A0)
The absorbances of the control and sample, respectively, are A0 and A1. The results are presented as the average of three replicate analyses, with the major values as well as the standard deviation (SD) provided.
The 50% inhibitory concentration (IC50), the concentration required to 50% DPPH radical scavenging activity was estimated from graphic plots of the dose–response curve using Graphpad Prism software (San Diego, CA, USA).
2.5. Antimicrobial Activity
2.5.1. Microorganism
Eight clinical microbial isolates were used in the experiments (Gram-negative bacteria: Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, and Acinetobacter baumannii; Gram-positive bacteria: Staphylococcus aureus, Streptococcus epidermidis, and Enterococcus faecalis and yeast: Candida tropicalis. The isolates were collected and identified from clinical specimens (sputum, end tracheal tube (ETT), nasal swab, and laryngeal swab (from respiratory tract infections patients) from Giessen University Clinic, Giessen, Germany [23].
2.5.2. Paper Disc Diffusion Assay
For disc diffusion assay, 100 µL of cell suspension was uniformly spread onto Mueller Hinton agar (MHA). The 6 mm diameter filter paper discs (Whatman No. 41) were positioned to contact the surface of infected agar and were impregnated with 25 µL extract at a concentration of 10 mg/mL. The plates were incubated for 24 h at 37 °C. The diameter of the zone of inhibition (ZOI) was then measured precisely, and the means of the triplicates were determined [24].
2.5.3. Determination of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
MIC and MBC of different Solenostemma argel extracts (water, methanol, acetone, ethanol, chloroform, ether, ethyl acetate, and methylene chloride) against all isolates were determined. Briefly, the two-fold dilution method by Koo et al. [25] was used with a concentration range between 3.12 and 100 mg/mL (w/v) in Mueller Hinton broth. The tested bacterial inoculum load was 5 × 105 cfu/mL. Each microbial isolate was inoculated into all the dilutions and the inoculated tubes were incubated overnight at 37 °C. MIC is the lowest concentration of an antibacterial agent necessary to inhibit visible growth. MBC is the minimum concentration of an antibacterial agent that results in bacterial death [26].
2.6. Cell Viability and Cytotoxic Effects
2.6.1. Mammalian Cell Lines
Human lung cancer cell line (A549), human colon adenocarcinoma cell line (Caco-2), human breast cancer cell line (MDA-MB-231), and normal lung fibroblasts (HEL299) were used to assess the cytotoxic effects of the different extracts. These cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) through personal communication with Prof. Dr. Mahmoud Al-Aeser, the Regional Center for Mycology and Biotechnology (RCMB) at Al-Azhar University, Egypt.
2.6.2. Propagation of Cell Lines
Dulbecco’s modified Eagle’s medium (DMEM) was used to propagate the cells. The media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine, HEPES buffer, and 50 µg/mL gentamycin. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 and were sub-cultured two times a week.
2.6.3. Cytotoxicity Assay
The cytotoxicity assay was evaluated as described by Mosmann [27]. Briefly, the cells were seeded in a 96-well plate at a cell concentration of 1 × 104 cells per well in 100 µL of growth medium using triplicate samples. A fresh medium containing different concentrations of the methanolic extract was added 24 h after seeding. Two-fold diluents of the methanolic extract were also added to confluent cell monolayers dispensed into 96-well, flat-bottomed microtiter plates (Falcon, NJ, USA) using a multichannel pipette. The microtiter plates were incubated at 37 °C in a humidified incubator with 5% CO2 for a period of 24 h. Control cells were incubated without extract and with or without DMSO. The DMSO concentration in each well was too low (maximal 0.1%) to affect the experiment.
After the end of the incubation period, the media in the well were aspirated and crystal violet solution (1%) was added to each well for at least 30 min. The plates were then rinsed with tap water until all the excess stains had been removed. Glacial acetic acid (30%) was subsequently added to all wells, mixed thoroughly and the plates placed into the microplate reader (SunRise, TECAN, Inc., Morrisville, NC, USA) for absorbance measurements at a test wavelength of 490 nm. All the results were corrected for the background absorbance detected in wells without added stain. Treated samples were compared with the control cell in the absence of the tested compounds. The cytotoxic effects of different concentrations of the methanolic extracts on cells were calculated as described by Mosmann [27].
The viability is expressed as percentage and the IC50 was calculated by using a dose–response curve for each concentration using GraphPad Prism software (San Diego, CA, USA).
2.7. In Vitro Anti-Inflammatory Activity
2.7.1. Erythrocyte Suspension Preparation
The membrane stabilization approach was used to test the anti-inflammatory efficacy in vitro. RBCs were extracted from a healthy human volunteer who had not taken any non-steroidal anti-inflammatory medicines for two weeks before the trial. The blood was cleansed three times in a 10 mM sodium phosphate-buffered isotonic buffered solution (154 mM NaCl) (pH 7.4). The blood was centrifuged at 3000× g for 10 min.
2.7.2. Hypotonic Solution-Induced Erythrocyte Hemolysis
Membrane stabilizing activities of the methanolic extract (with high cytotoxic activity) were assessed using hypotonic solution-induced erythrocyte hemolysis. The sample used consisted of a stock erythrocyte (RBCs) suspension (0.50 mL) mixed with 5 mL of hypotonic solution (50 mM NaCl) in 10 mM sodium phosphate-buffered saline (pH 7.4) containing the methanolic extracts (7.81–1000 µg/mL) or indomethacin (as positive control). The negative control sample consisted of 0.5 mL of RBCs mixed with hypotonic-buffered saline solution alone. The mixtures were incubated in 96 well plates for 10 min at room temperature and centrifuged for 10 min at 3000× g and the absorbance of the supernatant was measured at 540 nm. The percentage inhibition of hemolysis or membrane stabilization was calculated according to the modified method described by Shinde et al. [28].
Inhibition of hemolysis (membrane stabilization %) = OD1 − OD2/OD1 × 100
OD1 = Optical density of the hypotonic-buffered saline solution alone
OD2 = Optical density of extract in the hypotonic solution
The IC50 value was defined as the concentration of the sample to inhibit 50% RBCs hemolysis under the assay conditions.
2.8. Determination of the Phenolic Acids and Flavonoids of Solenostemma argel Methanolic Extract
HPLC (Agilent 1100, Agilent Technologies, Inc., Santa Clara, CA, USA), which was used for the detection of phenolic acids and flavonoids, consisted of two LC pumps, a UV/V detector, and a C18 column (150 × 4.60 mm, 5 µm particle size). The Agilent ChemStation was used to obtain and analyze chromatograms. Phenolic acids were separated using a gradient mobile phase consisting of two solvents: solvent A (methanol) and solvent B (acetic acid in ultrapure water, 1:25). Elution from the column was achieved with the following gradients: 0 to 3 min of solvent B, followed by 50% eluent A for the next 5 min, after which the concentration of A was increased to 80% for the next 2 min and then reduced to 50% again for the following 5 min. Flavonoids were separated using a gradient mobile phase consisting of two solvents: solvent A (acetonitrile) and solvent B (0.2%, v/v aqueous formic acid) with an isocratic elution (70:30) program. The detection wavelength was set between 200 and 450 nm, with specific monitoring conducted at 220 nm. Identification of the phenolic and flavonoid compounds was performed by comparing the retention times of the analyses with reference standards according to the methods described by Mattila et al. [29].
2.9. Determination of the Volatile Components of Solenostemma argel Methanolic Extract
GC-MS analysis of the methanolic extract was carried out using Thermo Scientific TRACE 1310 Gas Chromatograph (Waltham, MA, USA) coupled with an ISQ LT (single quadrupole mass spectrometer). The column was DB5-MS, 30 m, 0.25 mm ID (J&W Scientific, Folsom, CA, USA). Helium at a flow rate of 1.0 mL/min was used as carrier gas. The temperature program was as follows: started at 40 °C, sample held at 40 °C for 3 min; increasing to 280 °C with 5.0 °C/min heating rate ramp and maintained at this temperature for 5 min and before an increase to 290 °C with 7.5 °C/min heating rate and samples then maintained at this temperature for 1 min. The injection and detector temperatures were 200 and 300 °C, respectively. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z 40–450. The compounds were identified by Wiley and Nist mass spectral data base.
2.10. Statistical Analysis
An analysis of variance (ANOVA) was performed on the data to examine any significant difference between the samples used in his study. The means of triplicate samples were calculated as well as their standard deviation (SD). Duncan’s multiple range tests (p ≤ 0.05) were used to examine the significance of the variable mean differences. All statistical analyses were carried out using IBM SPSS version 16 (SPSS Inc, Chicago, IL, USA).
3. Results and Discussion
3.1. DPPH Radical Scavenging Activity of Solenostemma argel Extracts
To investigate the potential health properties of Solenostemma argel, we carried out a screening of the antioxidant abilities of different extracts of Solenostemma argel. Regarding the effect of the extraction solvent on antioxidant activity, methanol extract had the highest scavenging potency at 79.36%, followed by ethanol at 66.17% and acetone at 61.77%, respectively, while chloroform showed the lowest scavenging potency at 40.34% (Table 1).

Table 1.
Radical scavenging activity of Solenostemma argel extracts at different concentrations toward DPPH.
Regarding the effect of the extract’s concentration, the scavenging potency increased with the increasing Solenostemma argel concentration. The highest scavenging percentage 94.37% was detected using the concentration of 1280 µg/mL that was followed by 89.72% at a concentration of 640 µg/mL, and 79.31% at a concentration of 320 µg/mL, respectively. On the other hand, the concentration of 10 µg/mL gave the lowest scavenging potency with an average of 12.16%. The lowest IC50 value of 16.8 μg/mL was recorded with methanolic extract, while the highest value of 129.6 μg/mL was recorded with water extract (Table 2).

Table 2.
IC50 values of Solenostemma argel extracts.
The observed results were consistent with the findings of Kebbab-Massime et al. [30], who found that the methanolic extract of Solenostemma argel showed higher radical scavenging activity than the aqueous extract at all doses evaluated using the DPPH assay. Taj et al. [31] stated that the radical scavenging activity of Solenostemma argel increased from 32% at a concentration of 250 µg /mL to 84% at a concentration of 1000 µg/mL. Al-Juhaimi et al. [32] stated that the antioxidant activity of argel extract was due to the presence of phenolic acids, flavones, glycosylated flavonoids, polyphenols, b-carotene, b-sitosterol, monoterpenes, pregnenes, and pregnan. Benmaarouf et al. [21] stated that the high content of flavonoids such as rutin, kaempferol-3-o-rutinoside, kaempferol-3-o-diglucoside-7-o-glucoside, astragalin, and kaempfero in Solenostemma argel imparted its antioxidant activity.
3.2. Antimicrobial Activity of Solenostemma argel Extracts
Acetone, chloroform, and ethyl acetate were the most active solvents on most isolates, whereas methylene chloride and methanol exhibited moderate activity. Water, ether, and ethanol extracts, on the other hand, exhibited no inhibitory impact on isolated bacteria. (Table 3). The most sensitive organism was S. epidermidis with an inhibition zone of 27 mm using chloroform extract.

Table 3.
Antimicrobial activity of different Solenostemma argel extracts against respiratory tract infection isolates measured as zone of inhibition diameter (ZOI, mm).
Chloroform was the best extract with least MIC and MBC followed by acetone and ethyl acetate. The MIC of chloroform ranged from 6.25 to 25 mg/mL, while the MBCs of the chloroform extract ranged from 12.5 to 100 mg/mL among the tested strains. The least MIC and MBC were (6.25, 12.5) mg/mL, respectively, which were recorded with S. epidermidis (Table 4).

Table 4.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for different extracts of Solenostemma argel against respiratory tract infections microbes.
Our findings support the findings of numerous studies, such as Sulieman, et al. [33], who found that Solenostemma argel extracts had potent antimicrobial activity against Aspergillus niger, Pennicilium italicum, Escerichia coli and Salmonella typhi. Tharib et al. [34] also stated that Solenostemma argel extract has potent antimicrobial activity against both Gram-positive and Gram-negative bacteria. According to Megeressa et al. [35] Gram-positive bacteria are more sensitive to plant extracts than Gram-negative bacteria, which is likely owing to the presence of phospholipid membranes in the cell wall of Gram-negative bacteria. Hamadnalla et al. [36] stated that the antibacterial activity is due to the presence of phytoconstituents such as saponin and flavonoid in different plant extracts.
3.3. Anti-Proliferative Effects on A549, Caco-2, and MDA-MB-231 Cells
IC50 of Solenostemma argel methanolic extract against the lung carcinoma cell line (A549), Caco-2 cell line and MDA-MB-231 cell line were (179, 243 and 731), µg/mL, respectively. While IC50 of Doxorubicin (reference standard) against the lung carcinoma cell line (A549), Caco-2 cell line, and MDA-MB-231 cell line were (0.95, 1.93 and 0.6), µg/mL, respectively (Table 5).

Table 5.
Anticancer activity of Solenostemma argel methanolic extract at different concentrations toward different cancer cell lines.
Abouzaid et al. [37] found that the methanolic extract of Solenostemma argel had significant cytotoxic effects on lung cancer induced by dimethylbenzanthracene (DMBA) in male Wistar rats. Hanafi and Mansour [38] investigated the anticancer activity of aqueous extract of Solenostemma argel against Ehrlich carcinoma-bearing mice. It was found that the Solenostemma argel aqueous extract activated tumor cell death, and decreased tumor volume. It also recorded a high and wide zone of apoptotic tumor cells.
3.4. In Vitro Anti-Inflammatory Activity (Membrane Stabilization %) of Solenostemma argel Methanolic Extract
At various concentrations (1000, 500, 250, 125, 62.5, 31.25, 15.63, and 7.8 g/mL), the methanolic extract of Solenostemma argel demonstrated significant stabilization of RBC membranes. It was observed that the percentage protection of methanolic extract of Solenostemma argel increased from 28.12% at a concentration of 7.8 µg/mL to 82.73% at a concentration of 1000 µg/mL. The IC50 value of methanolic extract of Solenostemma argel was 24.4 ± 0.96 µg/mL compared to 17.02 ± 0.91 µg/mL with indomethacin standard. (Table 6).

Table 6.
Anti-inflammatory activity (membrane stabilization %) of Solenostemma argel methanolic extract at different concentrations.
Innocenti et al. [39] evaluated the anti-inflammatory activity of Solenostemma argel using the Croton oil ear test in mice. The extract induced 73% oedema reduction compared to 56% reduction induced by indomethacin. Ibrahim et al. [40] anti-inflammatory activity (74.19%, 69.44% and 66.58%), respectively, by inhibiting the heat-induced albumin denaturation. Ismaiel et al. [41] reported that Solenostemma argel extract exhibited inhibition of inflammation, at a dose of 100 mg/kg body weight after 3 h (22%) of carrageenan administration. The presence of flavonoids and related polyphenols in Solenostemma argel extract may be responsible for its anti-inflammatory activity [39]. Benmaarouf et al. [21] stated that the anti-inflammatory activity of Solenostemma argel may be due to the inhibition of the release of anti-inflammatory mediators occurring during the intermediate and second phases of edema formation, such as bradykinin and prostaglandins.
3.5. Identification of Phenolic Acids and Flavonoids of Solenostemma argel Methanolic Extract
Table 7 and Figure 1A,B presented the different phenolic compounds obtained from the analyses of Solenostemma argel methanolic extract by HPLC. Data indicated the presence of a total of eleven different phenolic compounds consisting of five flavonoids (rutin, quercetin, kaempferol, luteolin, and catechin) and five phenolic acids (syringic acid, p-coumaric acid, caffeic acid, gallic acid, and ferulic acid).

Table 7.
Phenolic acids and flavonoid compositions of methanolic extract of Solenostemma argel.
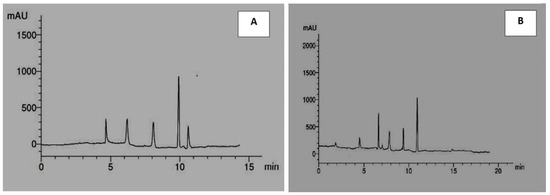
Figure 1.
HPLC chromatographic profiles of phenolic acids (A) and flavonoids (B) in methanolic extract of Solenostemma argel.
Data indicated that the most abundant phenolic compound was gallic acid at 36.49%, then synergic acid and p-coumaric acid were 18.28% and 18%, respectively. The most abundant flavonoid compound was catechin at 47.32%, then quercetin at 18.47%, then luteolin at 17.59%, then kaempferol at 14.36%, and the lowest amount of flavonoids was rutin at 2.24%.
The results obtained were consistent with those reported by Al-Juhaimi et al. [32] who found that gallic acid, caffeic acid, ferulic acid, and syringic acid are the primary phenolic acids and kaempferol, quercetin, and catechin are the common flavonoids present in methanolic extract of Solenostemma argel. Benmaarouf et al. [21] demonstrated the presence of salicylic acid, rutin, kaempferol-3-O-rutinoside, kampeferol 3-O-diglucoside-7-O glucoside in acetone extract of Solenostemma argel. Ibrahim et al. [40] reported the presence of other phenolic compounds such as pyrogallol, benzoic acid, resveratrol, and other flavonoids such as hesperetin, apigenin, and hesperidin in the methanolic extract of Solenostemma argel.
3.6. Determination of the Volatile Components of Solenostemma argel Methanolic Extract
Table 8 and Figure 2 demonstrated the volatile components of a methanolic extract of Solenostemma argel using GC-MS. Data in Table 8 and Figure 2 indicated that nineteen bioactive substances were identified and categorized based on their chemical structures using GC-MS analysis. cis-2,6-dimethyl-2,6-octadiene (8.95%), 2,6-octadiene, 2,6 dimethyl (4.19%), cyclohexene, 1-methyl-4-(1 methylethenyl)-,(S) (2.70%), 1,3-dioxolane,4-methyl-2-pentadecyl (9.69%), bicyclo [3.1.0] hexane, 4-methylene-1-(1-methylethyl) (1.64%), N,N′-bis (3-Aminopropyl) ethylenediamine (3.33%), 2-furanmethanol, 5-ethenyltetrahydro-À,À,5-trimethyl-, cis (5.01%) 2-furanmethanol,5-ethenyltetrahydro-À,À,5-trimethyl-, cis (4.41%), linalool (2.87%) nonanoic acid, 9-oxo-, methyl ester (3.33%), methyl 3-methylbutanoate (7.34%), hexanoic acid, methyl ester (10.93%), cis-3-hexenyllactate (2.97%), phenol, 2-(1,1-dimethylethyl) (8.50%), 2-(5-methyl-5 vinyltetrah ydro-2-furanyl)-2-propanol (11.63%), 1,3,5-triazine-2,4-diamine,6-chloro-n-ethyl (3.12%), hexadecanoic acid, methyl ester (4.80%), 9,12-octadecadienoic acid (z,z)-, methyl ester (1.85%), 10-octadecenoic acid, methyl ester (2.76%), and 2-(5-methyl-5 vinyl tetrahydro-2-furanyl)-2-propanol (11.63%) were the main volatile components.

Table 8.
Volatiles composition of methanolic extract of Solenostemma argel.
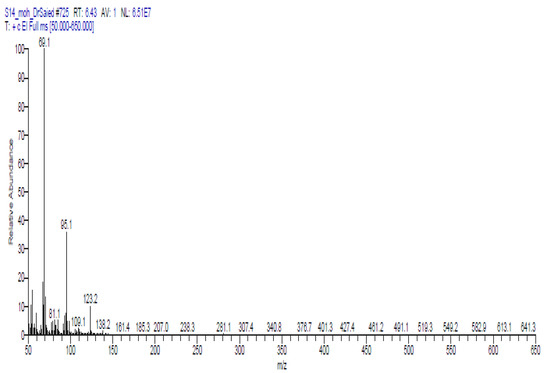
Figure 2.
GC-MS chromatographic for identification of compounds in methanolic extract of Solenostemma argel.
El-Sonbat et al. [42] recorded twenty-two volatile organic compounds within the methanolic extract of argel, which are xylitol (35.65%), 4- methylcatechol (23.89%), sinapic acid (8.13%), linalool acetate (4.28%), α-Copaene (3.21%), 2- (but-2’-enyl) phenol (2.85%), menthol,1’-(butyn3-one-1-yl)-, (1S,2S,5R)- (2.85%), α-Elemene (2.14%), and junipene (2.14%), were found to be the major constituents, while 7-methoxy-3-(4- methoxyphenyl)-2,3-dihydro-4H-chromen-4-one (2.14%), β-Guaiene (1.6%); β-Asarone (1.6%), phenol, 4-(3-hydroxy-1-propeenyl)-2-methoxy- (1.5%), isoferulic acid (1.43%), γ-Himachalene (1.25%), L-aspartic acid (1.07%), resorcinol (0.71%), valproic acid (0.71%), 6- monohydroxyflavone (0.53%), tricosanoic acid (0.53%), apigenin-7-glucoside (0.53%), 3,7,3’,4’- tetra-O-methyl-5-O-(trimethylsilyl) quercetin (0.36%), phenol, 2-methoxy-5-(1-propenyl)- (0.36%), α-Terpineol (0.36%), and 6- hydroxyflavone (0.18%) were the minor constituents.
4. Conclusions
The present study concludes that Solenostemma argel contains considerable quantities of phenolic compounds, flavonoids, and volatile compounds. S. argel is a very promising source of novel non-toxic, anti-inflammatory, and antioxidant compounds. It can be also used in the food industry as an antimicrobial agent. These results also provide a scientific basis for its use in cancer treatment. However, further studies are necessary to find active components in Solenostemma argel extract and to confirm its mechanism of action.
Author Contributions
R.M.E. established the study design and provided the research strategy, wrote the draft, and reviewed; Y.A.K. established the study design and provided the research strategy; M.S.M.S. and G.O.O.H. assessed the experiments and provided the data analysis; resources, M.A. and A.R.A.H.; tables and figures, statistical analysis, writing and editing, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deanship of Scientific Research, Qassim University, Kingdom of Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research, Qassim University, the Kingdom of Saudi Arabia for funding the publication of this project.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Madhuri, S. Some medicinal plants as natural anticancer agents. Pharmacogn. Rev. 2009, 3, 259. [Google Scholar]
- Akinyemi, O.; Oyewole, S.O.; Jimoh, K.A. Medicinal plants and sustainable human health: A review. Hortic. Int. J. 2018, 2, 194–195. [Google Scholar]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- Fan, C.; Lei, X.; Guo, L.; Zhang, A. Predicting the associations between microbes and diseases by integrating multiple data sources and path based HeteSim scores. Neurocomputing 2019, 323, 76–85. [Google Scholar] [CrossRef]
- Lobo, V.; Paitl, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Lushchak, V.L. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Marrelli, M. Medicinal Plants. Plants 2021, 10, 1355. [Google Scholar] [CrossRef]
- Kaur, R.; Kapoor, K.; Kaur, H. Plants as a source of anticancer agents. J. Nat. Prod. Plant Resour. 2011, 1, 119–124. [Google Scholar]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Gennari, C.; Castoldi, D.; Sharon, O. Product with taxol-like tumor activity: Approaches to eleutherobin and dicytostatin. Pure Appl. Chem. 2007, 79, 173–180. [Google Scholar] [CrossRef]
- Gezici, S.; Şekeroğlu, N. Current perspectives in the application of medicinal plants against cancer: Novel Therapeutic Agents. Anti-Cancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J. Perspectives on cancer chemoprevention research and drug development. Adv. Cancer Res. 2008, 78, 199–334. [Google Scholar]
- Soliman, A.S.; Samad, S.; Banerjee, M.; Chamberlain, R.M.; Robert, M.; Aziz, Z. Brief Continuing Medical Education (CME) Module Raises Knowledge of Developing Country Physicians International Electronic. J. Health Educ. 2006, 9, 31–41. [Google Scholar]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal plants in the prevention and treatment of colon cancer. Oxidative Med. Cell. Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef]
- Kamble, S.S.; Gacche, R.N. Evaluation of anti-breast cancer, anti-angiogenic, and antioxidant properties of selected medicinal plants. Eur. J. Integr. Med. 2019, 25, 13–19. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Lorigooini, Z.; Shirzad, H. A screening of growth inhibitory activity of Iranian medicinal plants on prostate cancer cell lines. BioMedicine 2018, 8, 8. [Google Scholar] [CrossRef]
- Awaad, A.S.; Al-Jaber, N.A.; Moses, J.E.; El-Meligy, R.M.; Zain, M.E. Antiulcerogenic activities of the extracts and isolated flavonoids of Euphorbia cuneata Vahl. Phytother. Res. 2013, 27, 126–130. [Google Scholar] [CrossRef]
- Ounaissia, K.; Pertuit, D.; Mitaine-Offer, A.C.; Miyamoto, T.; Tanaka, C.; Delemasure, S.; Dutartre, P.; Smati, D.; Lacaille-Dubois, M.A. New pregnane and phenolic glycosides from Solenostemma argel. Fitoterapia 2016, 114, 98–104. [Google Scholar] [CrossRef]
- Benmaarouf, D.K.; Pinto, D.C.G.A.; China, B.; Zenia, S.; Bendesari, K.B. Chemical analysis, antioxidant, anti-inflammatory and antinociceptive effects of acetone extract of Algerian Solenostemma argel (Delile) Hayne leaves. Int. J. Curr. Pharm. Res. 2020, 12, 72–81. [Google Scholar] [CrossRef]
- McCue, P.; Horii, A.; Shetty, K. Mobilization of phenolic antioxidants from defatted soybean powders by Lentinus edodes during solid-state bioprocessing1 is associated with enhanced production of laccase. Innov. Food Sci. Emerg. Technol. 2004, 5, 385–392. [Google Scholar] [CrossRef]
- Hassan, G.O.; Karamova, N.S.; Abu Mraheil, M.; Mohamed, W.; Chakraborty, T.; Ilinskaya, O.A. Comparative evaluation of antimicrobial effect of Thymus capitatus ethanolic extract on the different respiratory tract infections isolates. BioNanoScience 2017, 7, 644–647. [Google Scholar] [CrossRef]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries: Part 2; Cambridge University Press: Cambridge, UK, 2005; pp. 105–194. [Google Scholar]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef]
- Aamer, A.A.; Abdul-Hafeez, M.M.; Sayed, S.M. Minimum Inhibitory and Bactericidal Concentrations (MIC and MBC) of Honey and Bee Propolis against Multi-Drug Resistant (MDR) Staphylococcus sp. Isolated from Bovine Clinical Mastitis. Altern. Integr. Med. 2014, 3, 171. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Shinde, U.; Phadke, A.S.; Nair, A.; Mungantiwar, A.; Dikshit, V.; Saraf, M. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Mattila, P.; Astola, J.; Kumpulainen, J. Determination of flavonoids in plant material by HPLC with diode-array and electroarray detections. J. Agric. Food Chem. 2000, 48, 5834–5841. [Google Scholar] [CrossRef]
- Kebbab-Massime, R.; Labed, B.; Boutamine-Sahki, R. Evaluation of Antimicrobial and Antioxidant Activities of Methanolic Extracts of Flavonoids Obtained from the Leaves of Solenostemma argel Plant Collected in the Region of Tamanrasset, Algeria. J. Plant Biochem. Physiol. 2017, 5, 1–5. [Google Scholar]
- Al-Deen, A.T.; Al-Naqeb, G. Hypoglycemic effect and in vitro antioxidant activity of methanolic extract from Argel (Solenostemma argel) plant. Int. J. Herb. Med. 2014, 2, 128–131. [Google Scholar]
- Al-Juhaimi, F.Y.; Ahmed, I.A.A.; Adiamo, O.Q.; Adisa, A.R.; Ghafoor, K.; Ozcan, M.M. Effect of Argel (Solenstemma argel) leaf powder on the quality attributes of camel patties during cold storage. J. Food Process. Preserv. 2017, 42, e13496. [Google Scholar]
- Sulieman, A.E.; Elzobair, W.M.; Abdelrahim, A.M. Antimicrobial activity of the extract of Solenostemma argel (harjal) plant. J. Sci. Technol. 2009, 10, 120–134. [Google Scholar]
- Tharib, S.M.; El Migirab, S.; Veitch, G.B. A Preliminary Investigation of the Potential Antimicrobial Activity of Solenostemma argel. Int. J. Crude Drug Res. 2008, 24, 101–104. [Google Scholar] [CrossRef]
- Megeressa, M.; Bisrat, D.; Mazumder, A.; Asres, K. Structural elucidation of some antimicrobial constituents from the leaf latex of Aloe trigonantha L.C. Leach. BMC Complement. Altern. Med. 2015, 15, 270. [Google Scholar] [CrossRef] [PubMed]
- Hamadnalla, H.M.Y.; El Jack, M.M. Phytochemical Screening and Antibacterial Activity of Solenostemma argel: A Medicinal Plant. Open Access J. Environ. Soil Sci. 2019, 2, 2–4. [Google Scholar]
- Abouzaid, O.A.R.; Mansour, S.Z.; Sabbah, F. Evaluation of the antitumor activity of Solenostemma argel in the treatment of lung carcinoma induced in rats. Benha Vet. Med. J. 2018, 35, 178–189. [Google Scholar] [CrossRef]
- Hanafi, N.; Mansour, S.Z. Antitumor efficacy of Solenostemma argel and or γ irradiation against Ehrlich carcinoma. J. Biol. Sci. 2010, 10, 468–479. [Google Scholar] [CrossRef]
- Innocenti, G.S.; Acqua, S.D.; Sosa, S.; Altinier, G.; Loggia, R.D. Topical anti-inflammatory activity of Solenostemma argel leaves. J. Ethnopharmacol. 2005, 10, 307–310. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Gaafar, A.A.; Salama, Z.A.; El Baz, F.K. Anti-inflammatory and Antioxidant Activity of Solenostemma argel extract. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 635–664. [Google Scholar]
- Ismaiel, S.M.O.; Mahmoud, E.N.; Mahmoud, A.M. Anti-Inflammatory Activity of Some Indigenous Sudanese Plants. Res. J. Pharmacogn. Phytochem. 2014, 6, 30–32. [Google Scholar]
- El-Sonbaty, S.M.; Mansour, S.Z.; Mahdy, E.M.E.; El-Mezayen, H.A.; Salem, F.A.M. Gas Chromatography/Mass Spectrography (GC/MS) Analysis and Biological Properties of Probiotic Fermented Solenostemma argel. Eur. J. Med. Plants 2016, 16, EJMP.27662. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).