Cyclo- and Polyphosphazenes for Biomedical Applications
Abstract
1. Introduction
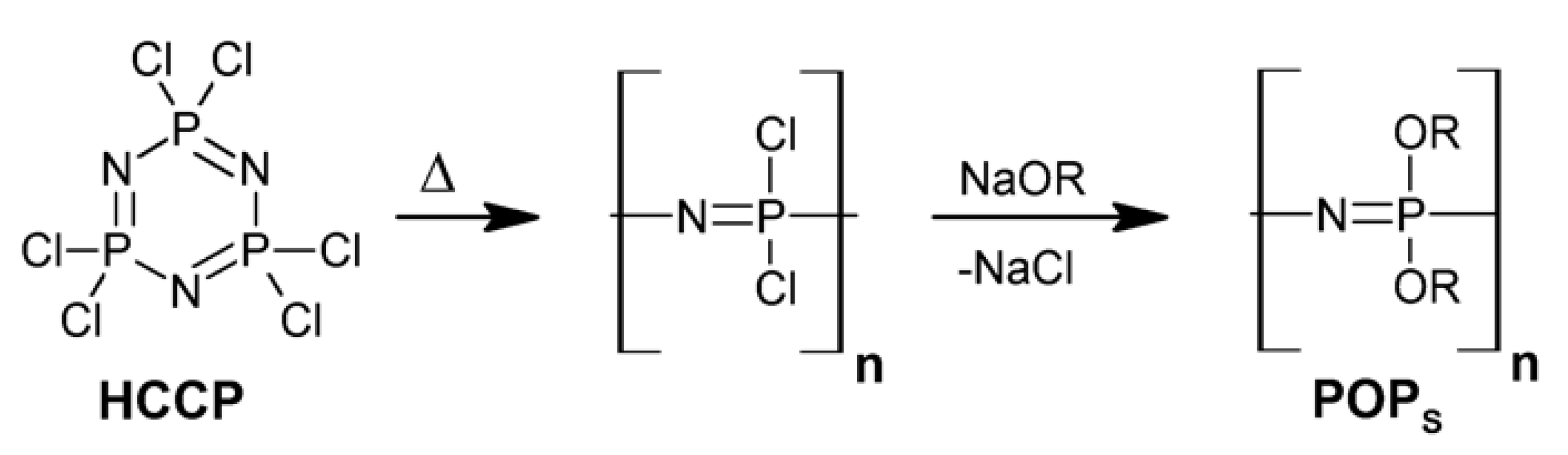
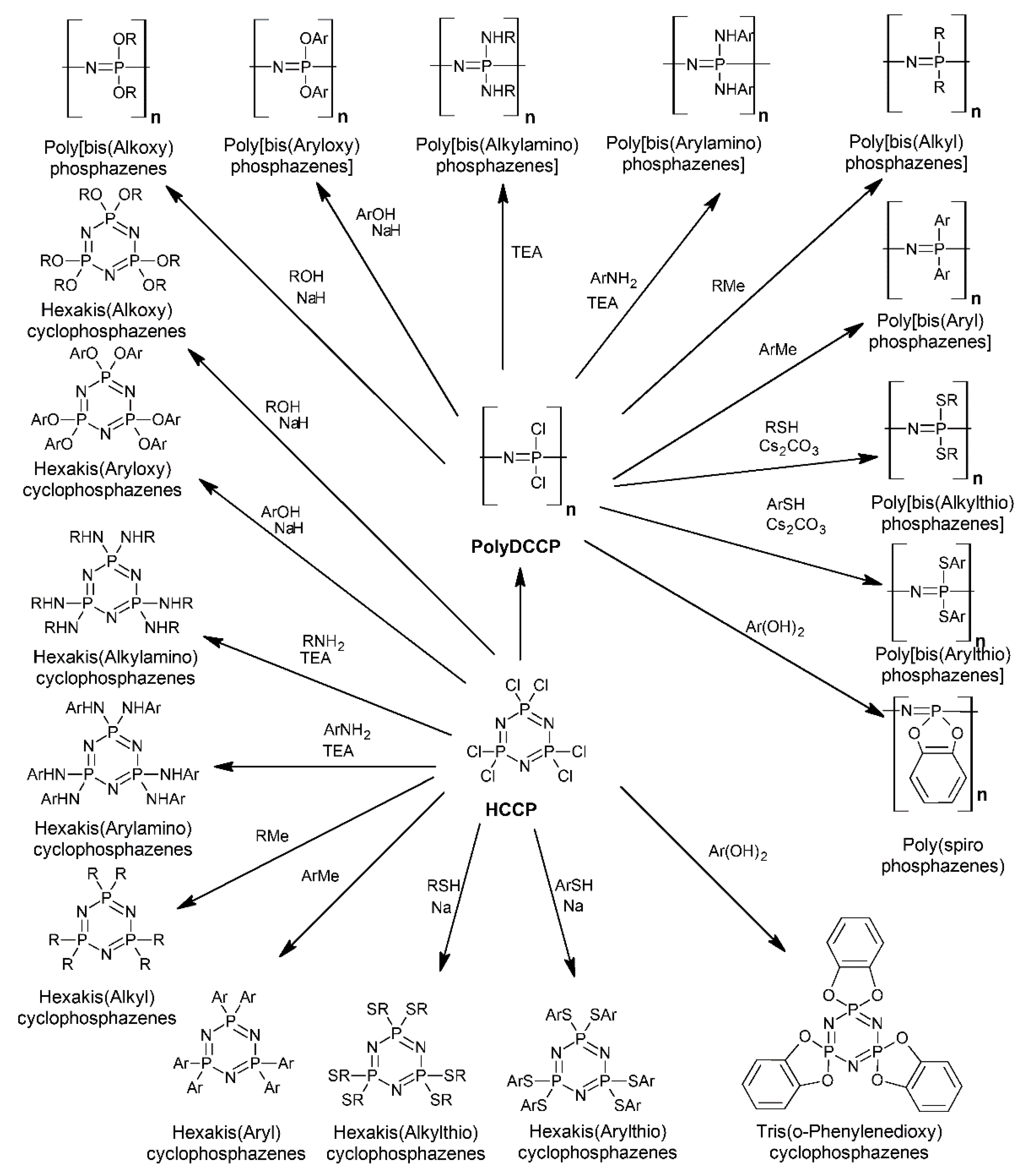
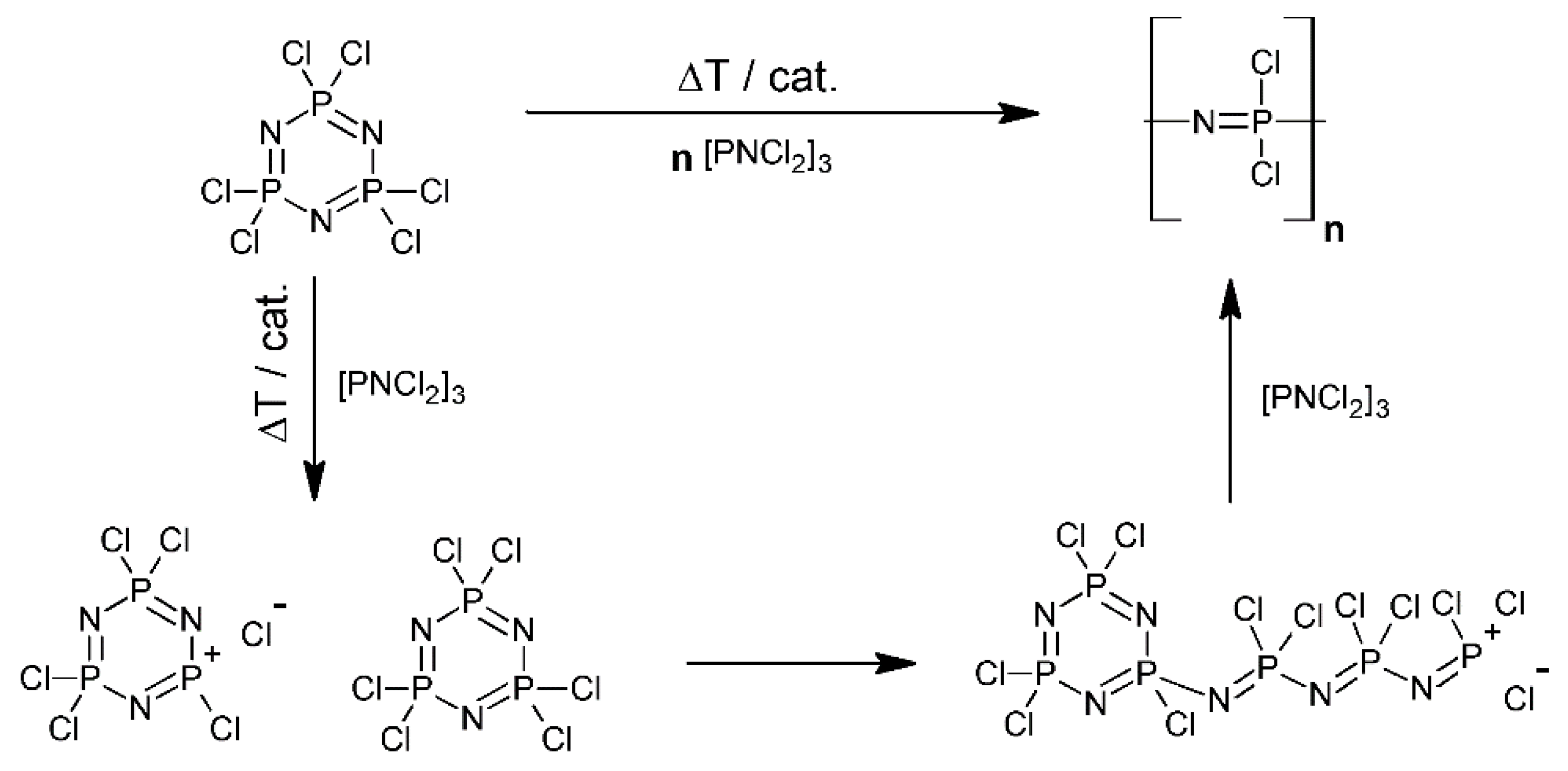
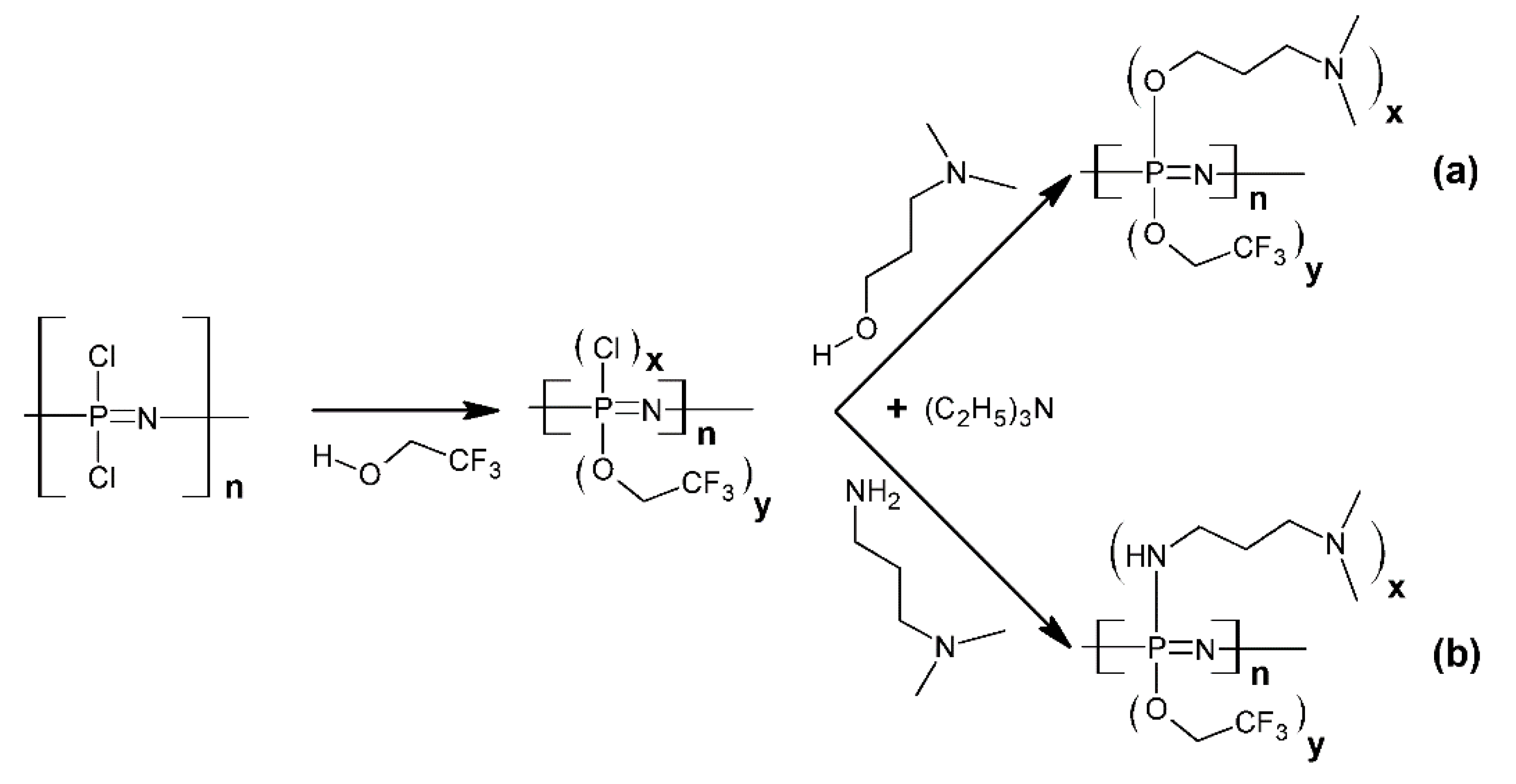
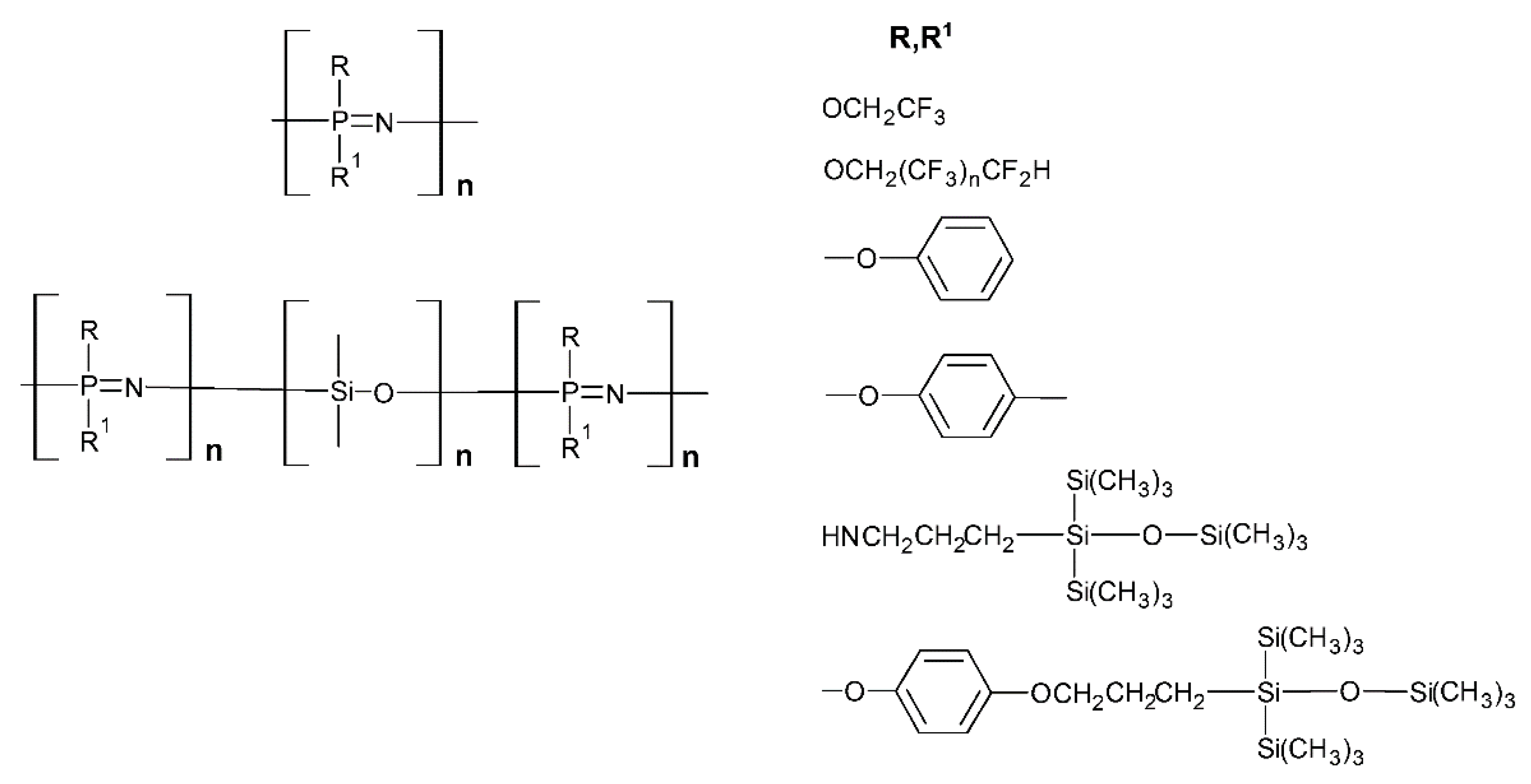
2. Synthesis and Characterizations
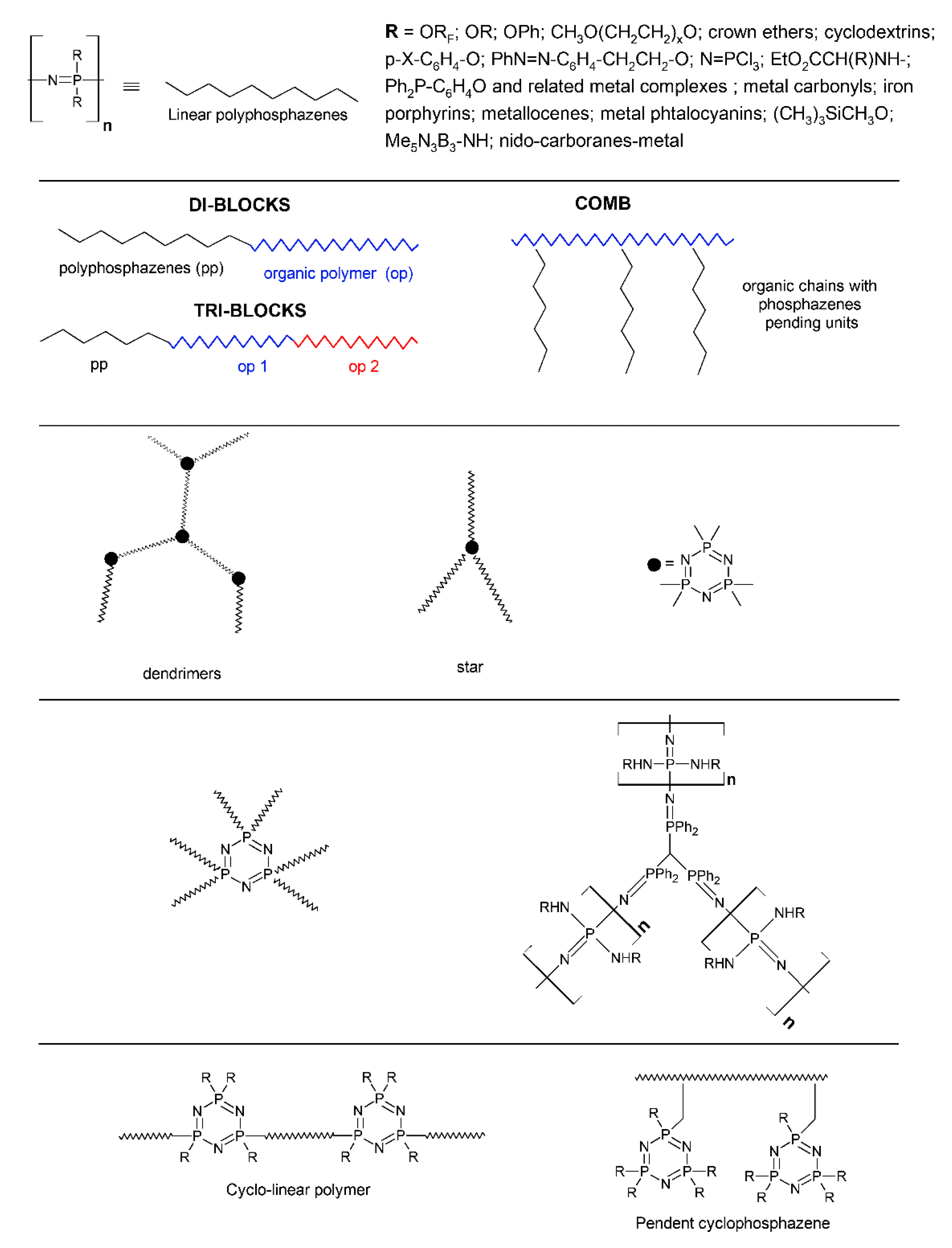
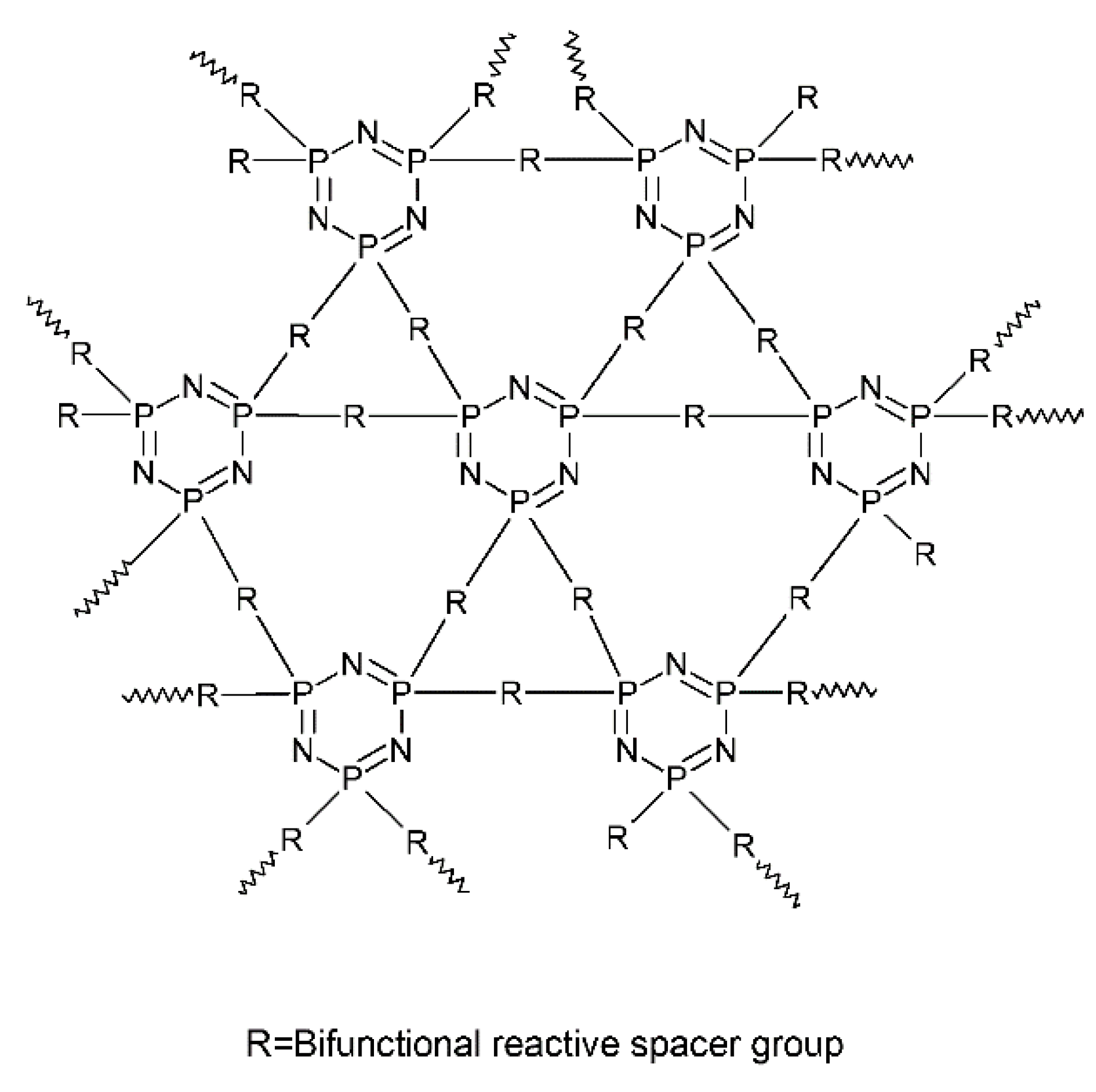
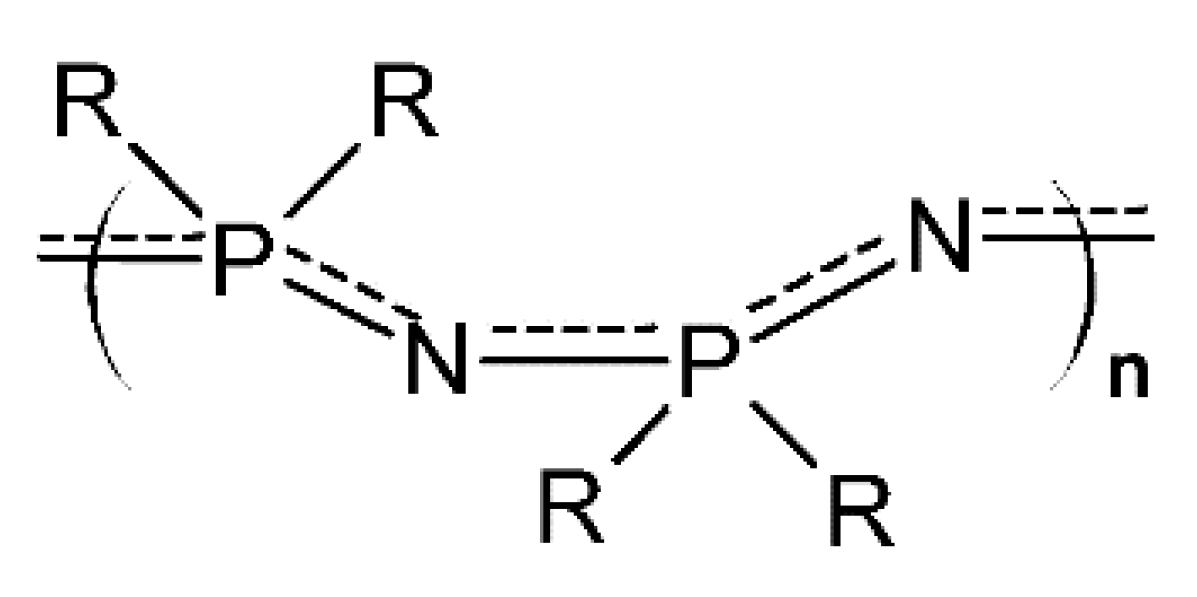
2.1. The Syntheses and the Architectures
- (i)
- (ii)
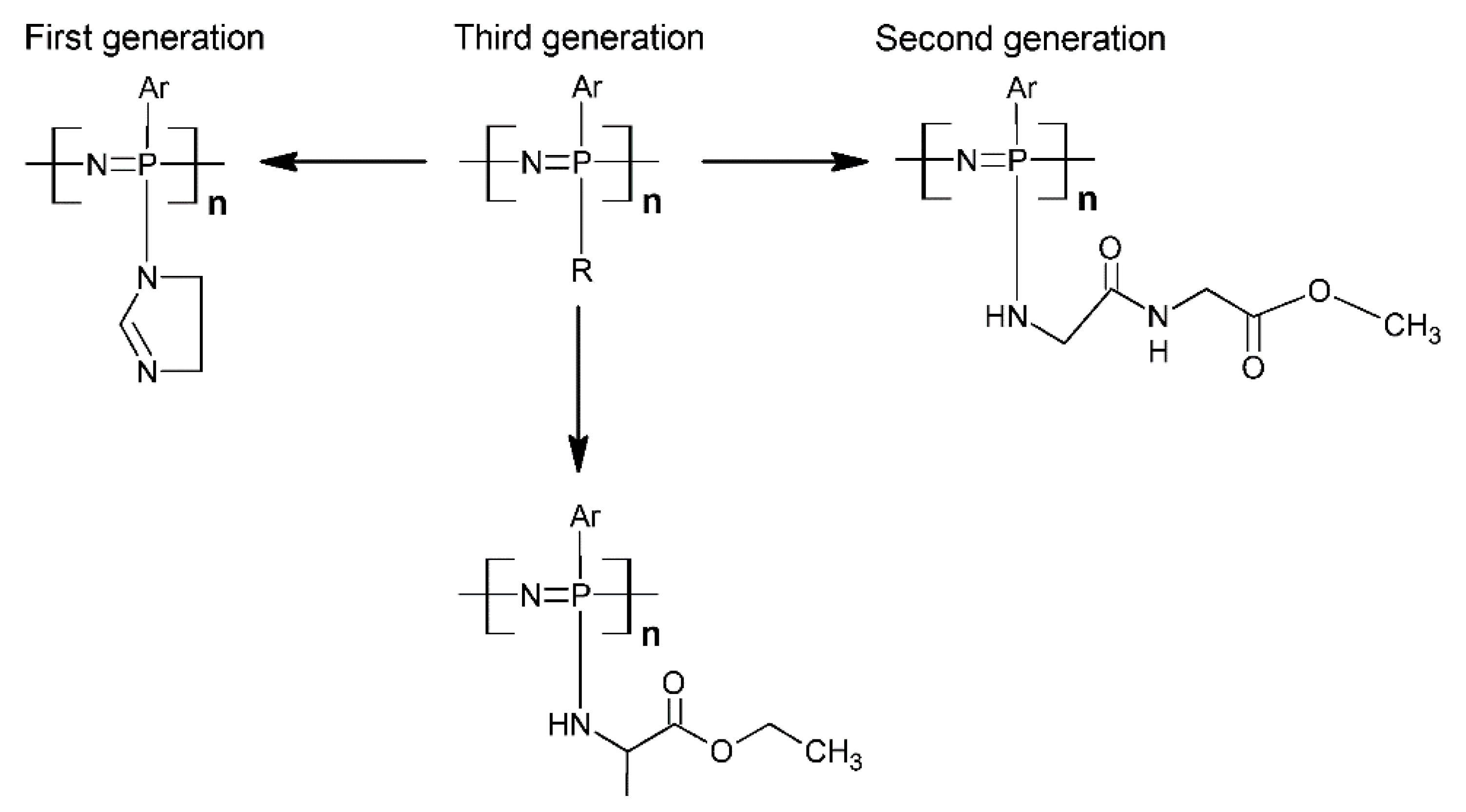
- Living cationic polymerization method by the reaction of (Cl3P=NSiMe3) with PCl5 [43]. An intriguing study concerning the mechanism of the ambient temperature PCl5-initiated living cationic chain growth polycondensation of Cl3P=NSiMe3 provided evidence that, under the usual polymerization conditions, the propagation occurs at both chain ends and identified factors to potentially control the molecular weight and broadening of the molecular weight distribution [44]. It has been observed that good control over the molecular weight and polydispersity can be achieved for short polymer chains (up to 50 units), while in the case of longer polymer chains, a lower control can be obtained. Detailed kinetic studies have been carried out to investigate the mechanism of the reactions and optimize the polymerization conditions (Scheme 4) [42,45,46]. A wide variety of reactions, from enhancing the basicity of the backbone N atoms to the electrophilic substitution on the phenyl ring or the exploitation of the relative acidity of the P-CH3 groups for the formation of carbanions, which can react with a wide variety of electrophiles, have been investigated (Scheme 5).
- (iii)
2.2. The Characterization
2.3. Computational Approaches to Phosphazenes
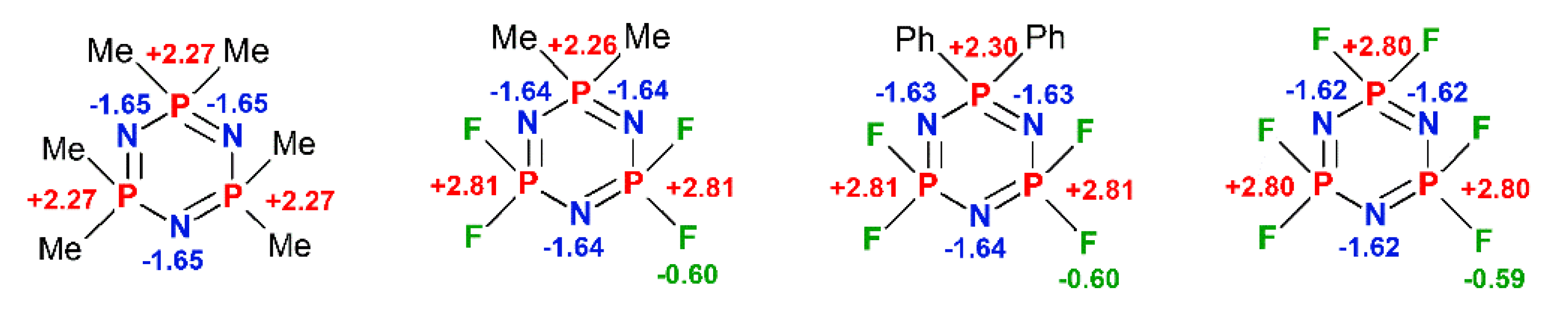
2.3.1. Quantum Chemical View of the P-N Bond in Phosphazenes
2.3.2. Molecular Dynamic Simulations of Phosphazenes
3. Biomedical Applications
- (i)
- Glass transition temperature compared with the physiological temperature: as for bone tissue engineering, a glass transition temperature higher than the physiological one to maintain structural integrity in an in vivo environment is required [105];
- (ii)
- Mechanical properties: substituents must be chosen in order to match the mechanical properties of the POPs (compressive and tensile strengths) and those of the native tissues;
- (iii)
- Porosity and porous interconnectivity of biomaterials plays a key role either in drug delivery applications, due to their controlled degradability, or in tissue engineering, aging as materials scaffolds for cells proliferation;
- (iv)
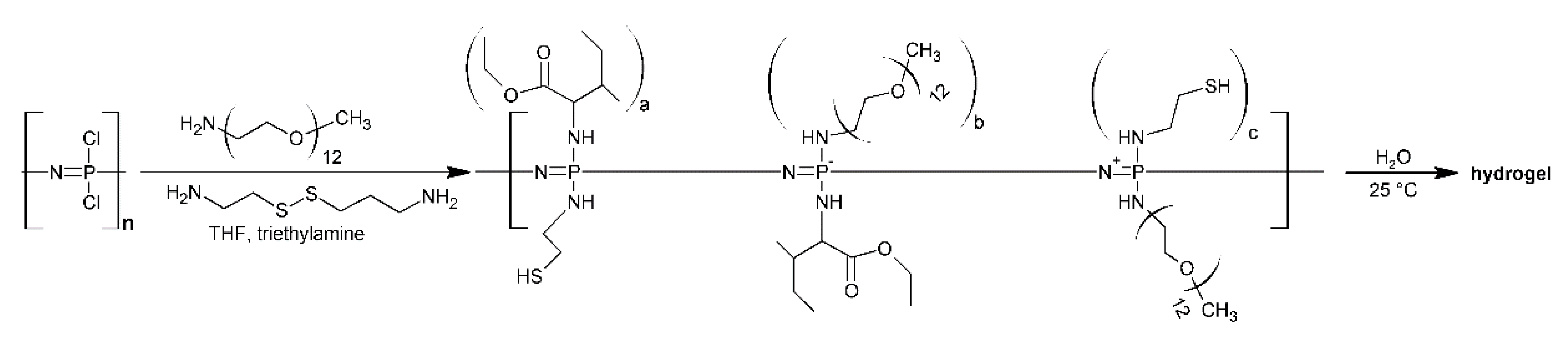
- Stimuli-responsive site behavior: temperature, ultrasound, light, pH, ionic strength, oxidative conditions, and enzyme presence are important stimuli for biomedical applications. Several stimuli-responsive materials have been prepared for tissue engineering and drug delivery due to the possibility of tuning the properties from combinations of different side groups [105,106,107,108]. The reaction of hexakis [4-(acrylamido)phenoxy]cyclotriohosphazene] with N-isopropylacrilamide and N-vinyl imidazole in the presence of ammoniumpersulfate gave crosslinked hydrogels which exhibited in vitro pH-responsive drug-release behavior [107].
3.1. Phosphazenes in Drug Delivery
3.1.1. Biological Activity of Cyclophosphazenes
3.1.2. Polyphosphazenes
- (i)
- To achieve controlled drug release systems where the role of the polymer is to extend the half-time of the drug;
- (ii)
- To achieve targeted drug delivery systems carrying drugs to the sites of action, being usually severely cytotoxic drugs, such as anticancer ones with tumor selectivity [42].
- (i)
- Improve interpolymer complexation during the formation of the mixed polyelectrolyte;
- (ii)
- Promote the release of polynucleotides from endolysosomal compartments;
- (iii)
- Reduce polycations caused by toxicity.
3.1.3. Polyphosphazenes in Gene Therapy
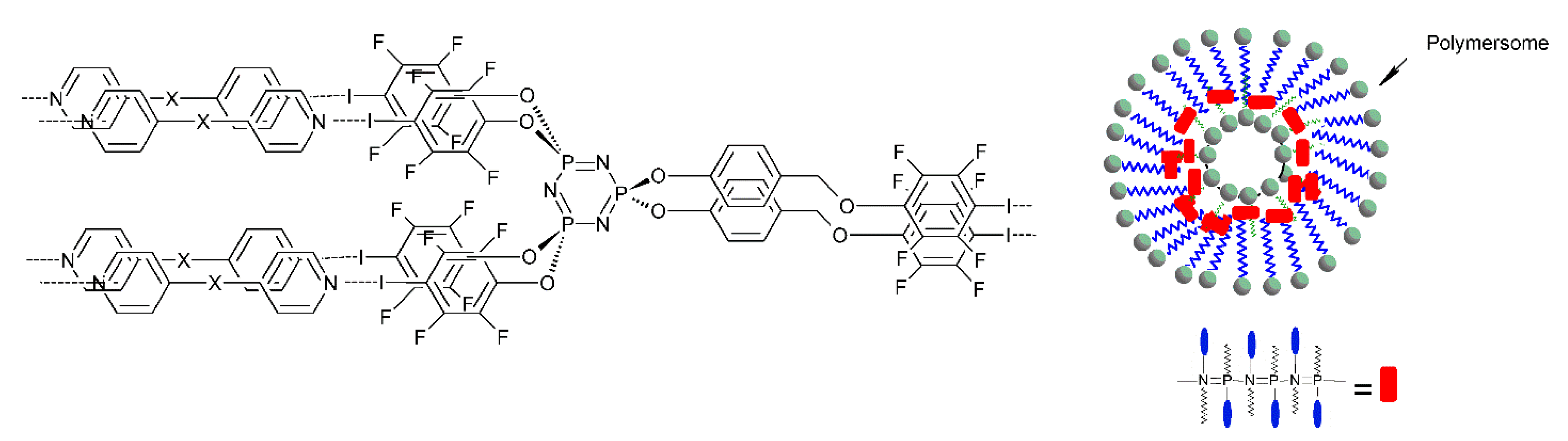
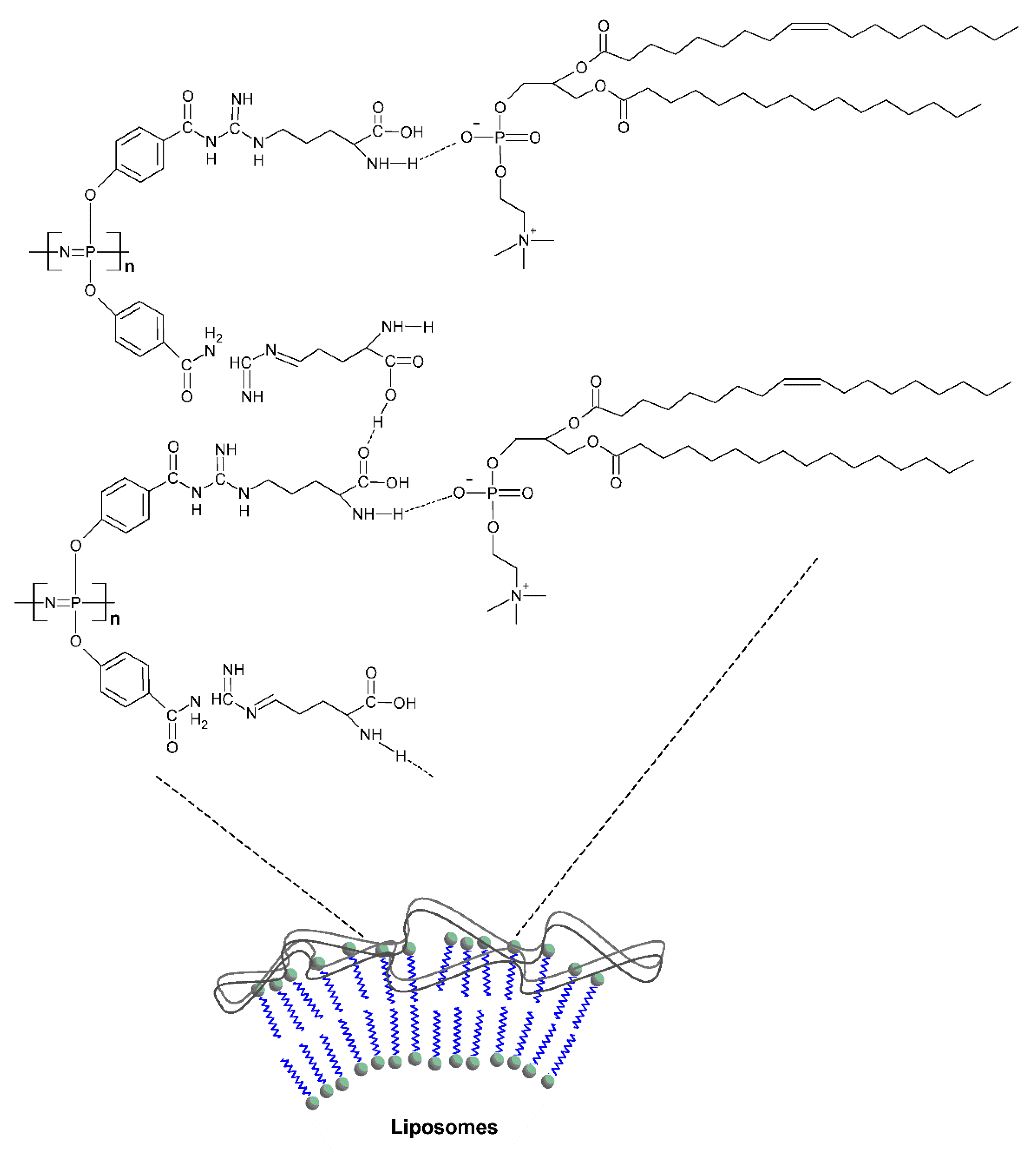
3.1.4. Micelles, Liposomes, Polymersomes
3.1.5. Nanoparticles
3.1.6. Nanofibers
3.2. Phosphazenes as Immunoadjuvants
- (i)
- (ii)
- Nanoscale constructs [188], as in the case of the spontaneous self-assembly of Resiquimod with a water-soluble poly[di(carboxylatophenoxy)phosphazene] forming an ionically paired system and a ternary one, including the Hepatitis C virus antigen. It was demonstrated that the supramolecular assembly enabled high immunostimulation in cellular assays (mouse macrophage reporter cell line) and in vitro hemocompatibility (human erythrocytes). Moreover, in vivo studies gave quite promising results (Scheme 11) [189].
3.3. Phosphazenes in Tissue Engineering
3.3.1. Bone Tissue Engineering
3.3.2. Polyphosphazenes in Nerve and Cardiac Tissue Engineering
3.4. Other Biomedical Applications
Funding
Acknowledgments
Conflicts of Interest
References
- Mark, J.E.; Allcock, H.R.; West, R. Inorganic Polymers, 2nd ed.; Oxford University Press: Oxford, UK, 2005; Volume 22, pp. 1–353. [Google Scholar]
- Gleria, M.; Jaeger, R. Phosphazenes: A Worldwide Insight; Science Publishing: Hauppage, NY, USA, 2004; p. 1047. [Google Scholar]
- Gleria, M.; de Jaeger, R. Applicative Aspects of Cyclophosphazenes; Science Publishing: Hauppage, NY, USA, 2004; p. 3. [Google Scholar]
- Gleria, M.; de Jaeger, R. Aspects of Phosphazene Research. J. Inorg. Organomet. Polym. 2001, 11, 1–45. [Google Scholar] [CrossRef]
- Allcock, H.R.; Kugel, R.L. Synthesis of High Polymeric Alkoxy-and Aryloxyphosphonitriles. J. Am. Chem. Soc. 1965, 87, 4216–4217. [Google Scholar] [CrossRef]
- Allcock, H.R.; Kugel, R.L.; Valan, K.J. Phosphonitrilic Compounds. VI. High Molecular Weight Poly(Alkoxy-and Aryloxyphosphazenes). Inorg. Chem. 1966, 5, 1709–1715. [Google Scholar] [CrossRef]
- Allcock, H.R.; Kugel, R.L. Phosphonitrilic Compounds. VII. High Molecular Weight Poly(Diaminophosphazenes). Inorg. Chem. 1966, 5, 1716–1718. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Yang, Y.; Jia, C.; Zhang, X.; Wu, J.; Zhu, Z.; Wang, J.; Yang, J. Flame-Retardant Effect of Hyperbranched Phosphazene-Based Microspheres in Poly(L-Lactic Acid). J. Mater. Sci. 2022, 57, 1516–1535. [Google Scholar] [CrossRef]
- Zarybnicka, L.; Machotova, J.; Kopecka, R.; Sevcik, R.; Hudakova, M.; Pokorny, J.; Sal, J. Effect of Cyclotriphosphazene-Based Curing Agents on the Flame Resistance of Epoxy Resins. Polymers 2020, 13, 8. [Google Scholar] [CrossRef]
- Gleria, M.; de Jaeger, R. Polyphosphazenes: A Review. Top. Curr. Chem. 2005, 250, 165–251. [Google Scholar] [CrossRef]
- Allcock, H.R. Chapter 7. Phosphazene High Polymers. In Phosphorus-Based Polymers: From Synthesis to Applications; RSC Polymer Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 125–150. [Google Scholar] [CrossRef]
- Khanin, D.A.; Kononevich, Y.N.; Temnikov, M.N.; Morgalyuk, V.P.; Vasil’ev, V.G.; Popov, A.Y.; Brel, V.K.; Papkov, V.S.; Muzafarov, A.M. New Hybrid Materials Based on Cyclophosphazene and Polysiloxane Precursors: Synthesis and Properties. Polymer 2020, 186, 122011. [Google Scholar] [CrossRef]
- Inoue, K.; Itaya, T. Synthesis and Functionality of Cyclophosphazene-Based Polymers. Bull. Chem. Soc. Jpn. 2001, 74, 1381–1395. [Google Scholar] [CrossRef]
- Kato, F.; Chandra, A.; Tokita, M.; Asano, H.; Shimomoto, H.; Ihara, E.; Hayakawa, T. Self-Assembly of Hierarchical Structures Using Cyclotriphosphazene-Containing Poly(Substituted Methylene) Block Copolymers. ACS Macro. Lett. 2018, 7, 37–41. [Google Scholar] [CrossRef]
- Andrianov, A.K. Polyphosphazenes for Biomedical Applications; John Wiley & Son: Hoboken, NJ, USA, 2009; p. 462. [Google Scholar]
- Belluco, U.; Bertani, R.; Michelin, R.A.; Mozzon, M.; Zingales, F.; Gleria, M. Organometallic Phosphazenes: Synthesis and Characterization of Pt(II) and Pt(0) Cinnammonitrile Cyclophosphazene Derivatives. Inorg. Chim. Acta 1995, 229, 13–15. [Google Scholar] [CrossRef]
- Chistyakov, E.; Yudaev, P.; Nelyubina, Y. Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-[4-{(N-Allylimino)Methyl}phenoxy]Cyclotriphosphazene. Nanomaterials 2022, 12, 2268. [Google Scholar] [CrossRef] [PubMed]
- De Jaeger, R.; Gleria, M. Synthesis and Characterizations of Poly (Organophosphazenes); Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2004; p. 372. [Google Scholar]
- Allcock, H.R. A Perspective of Polyphosphazene Research. J. Inorg. Organomet. Polym. Mater. 2006, 16, 277–294. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, J.; Yang, Z.; Huang, M.; Chen, Z.; Gong, Q.; Cao, S. Photorefractive Properties of Polyphosphazenes Containing Carbazole-Based Multifunctional Chromophores. Polymer 2008, 49, 2107–2114. [Google Scholar] [CrossRef]
- Burjanadze, M.; Paulsdorf, J.; Kaskhedikar, N.; Karatas, Y.; Wiemhöfer, H.D. Proton Conducting Membranes from Sulfonated Poly[Bis(Phenoxy)Phosphazenes] with an Interpenetrating Hydrophilic Network. Solid State Ion. 2006, 177, 2425–2430. [Google Scholar] [CrossRef]
- Ali, Z.; Basharat, M.; Wu, Z. A Review on the Morphologically Controlled Synthesis of Polyphosphazenes for Electrochemical Applications. ChemElectroChem 2021, 8, 759–782. [Google Scholar] [CrossRef]
- Gleria, M.; Bertani, R.; de Jaeger, R.; Lora, S. Fluorine Containing Phosphazene Polymers. J. Fluor. Chem. 2004, 125, 329–337. [Google Scholar] [CrossRef]
- Allcock, H.R.; Phelps, M.V.B.; Barrett, E.W.; Pishko, M.V.; Koh, W.G. Ultraviolet Photolithographic Development of Polyphosphazene Hydrogel Microstructures for Potential Use in Microarray Biosensors. Chem. Mater. 2006, 18, 609–613. [Google Scholar] [CrossRef]
- Wycisk, R.; Pintauro, P.N. Polyphosphazene Membranes for Fuel Cells. Adv. Polym. Sci. 2008, 216, 157–183. [Google Scholar] [CrossRef]
- Amin, A.M.; Wang, L.; Wang, J.; Yu, H.; Huo, J.; Gao, J.; Xiao, A. Recent Research Progress in the Synthesis of Polyphosphazene and Their Applications. Des. Monomers Polym. 2009, 12, 357–375. [Google Scholar] [CrossRef]
- Singler, R.E. Historical Overview of the Army Contributions to Phosphazene Chemistry. J. Inorg. Organomet. Polym. Mater. 2007, 16, 307–309. [Google Scholar] [CrossRef]
- Teasdale, I.; Brüggemann, O. Polyphosphazenes for Medical Applications; Smithers Rapra: Shropshire, UK, 2014. [Google Scholar]
- Nielsen, M.L.; Cranford, G.; Quimby, O.T. Trimeric Phosphonitrile Chloride and Tetrameric Phosphonitrile Chloride. J. Am. Chem. Soc. 2007, 6, 94–97. [Google Scholar] [CrossRef]
- Bowers, D.J.; Wright, B.D.; Scionti, V.; Schultz, A.; Panzner, M.J.; Twum, E.B.; Li, L.L.; Katzenmeyer, B.C.; Thome, B.S.; Rinaldi, P.L.; et al. Structure and Conformation of the Medium-Sized Chlorophosphazene Rings. Inorg. Chem. 2014, 53, 8874–8886. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.A.; Fitzsimmons, B.W.; Smith, B.C. The Phosphazenes (Phosphonitrilic Compounds). Chem. Rev. 1962, 62, 247–281. [Google Scholar] [CrossRef]
- Stewart, F.F. Phosphazenes. In Organophosphorus Chemistry; RSC Publishing: Cambridge, UK, 2013; Volume 42, pp. 216–262. [Google Scholar] [CrossRef]
- Stewart, F.F. Phosphazenes. In Organophosphorus Chemistry; RSC Publishing: Cambridge, UK, 2014; Volume 43, pp. 366–412. [Google Scholar] [CrossRef]
- Allcock, H.R. Recent Developments in Polyphosphazene Materials Science. Curr. Opin. Solid State Mater. Sci. 2006, 10, 231–240. [Google Scholar] [CrossRef]
- Ahmad, M.; Nawaz, T.; Hussain, I.; Chen, X.; Imran, M.; Hussain, R.; Assiri, M.A.; Ali, S.; Wu, Z. Phosphazene Cyclomatrix Network-Based Polymer: Chemistry, Synthesis, and Applications. ACS Omega 2022, 7, 28694–28707. [Google Scholar] [CrossRef] [PubMed]
- Sedláková, V.; Voráč, Z.; Jaroš, J.; Bačovská, R.; Kloučková, M.; Svoboda, M.; Streit, L.; Dumková, J.; Vašíčková, K.; Alberti, M.; et al. Enhanced Bioactivity of Electrospun PCL and PLLA Scaffolds Blended with Amino-Phosphazene. Mater. Lett. 2018, 228, 339–343. [Google Scholar] [CrossRef]
- Fu, J.; Qiu, L. Optimizing Hydrophobic Groups in Amphiphiles to Induce Gold Nanoparticle Complex Vesicles for Stability Regulation. Langmuir 2017, 33, 12291–12299. [Google Scholar] [CrossRef]
- Henke, H.; Brüggemann, O.; Teasdale, I. Branched Macromolecular Architectures for Degradable, Multifunctional Phosphorus-Based Polymers. Macromol. Rapid Commun. 2017, 38, 1600644. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, C.; Allcock, H.R. Synthesis and Assembly of Novel Poly(Organophosphazene) Structures Based on Noncovalent “Host-Guest” Inclusion Complexation. Macromolecules 2014, 47, 1065–1072. [Google Scholar] [CrossRef]
- Bertani, R.; Chaux, F.; Gleria, M.; Metrangolo, P.; Milani, R.; Pilati, T.; Resnati, G.; Sansotera, M.; Venzo, A. Supramolecular Rods via Halogen Bonding-Based Self-Assembly of Fluorinated Phosphazene Nanopillars. Inorg. Chim. Acta 2007, 360, 1191–1199. [Google Scholar] [CrossRef]
- Rothemund, S.; Teasdale, I. Preparation of Polyphosphazenes: A Tutorial Review. Chem. Soc. Rev. 2016, 45, 5200–5215. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Wang, L.; Yu, H.; Zain-ul-Abdin; Akram, M.; Wu, J.; Haroon, M.; Ullah, R.S.; Deng, Z.; Xia, X. Poly(Organo)Phosphazenes: Recent Progress in the Synthesis and Applications in Tissue Engineering and Drug Delivery. Russ. Chem. Rev. 2018, 87, 109–150. [Google Scholar] [CrossRef]
- Wang, B. Development of a One-Pot in Situ Synthesis of Poly(Dichlorophosphazene) from PCl3. Macromolecules 2005, 38, 643–645. [Google Scholar] [CrossRef]
- Blackstone, V.; Lough, A.J.; Murray, M.; Manners, I. Probing the Mechanism of the PCl5-Initiated Living Cationic Polymerization of the Phosphoranimine Cl3P=NSiMe3 Using Model Compound Chemistry. J. Am. Chem. Soc. 2009, 131, 3658–3667. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Olshavsky, M.A. A New Route to the Phosphazene Polymerization Precursors, Cl3P=NSiMe3 and (NPCl2)3. Inorg. Chem. 1999, 38, 280–283. [Google Scholar] [CrossRef]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Nelson, J.M.; Reeves, S.D.; Honeyman, C.H.; Manners, I. “Living” Cationic Polymerization of Phosphoranimines as an Ambient Temperature Route to Polyphosphazenes with Controlled Molecular Weights. Macromolecules 1996, 29, 7740–7747. [Google Scholar] [CrossRef]
- Wisian-Neilson, P.; Neilson, R.H.; Graaskamp, J.M.; Dunn, B.S. Poly (Dimethylphosphazene) and Poly (Methylphenylphosphazene): {Poly[Nitrilo (Dimethylphosphoranylidyne)] and Poly[Nitrilo (Methylphenylphosphoranylidyne)]}. Inorg. Synth. 1989, 25, 69–74. [Google Scholar] [CrossRef]
- Schwesinger, R.; Schlemper, H. Peralkylated Polyaminophosphazenes—Extremely Strong, Neutral Nitrogen Bases. Angew. Chem. Int. Ed. Engl. 1987, 26, 1167–1169. [Google Scholar] [CrossRef]
- Weitkamp, R.F.; Neumann, B.; Stammler, H.G.; Hoge, B. Phosphorus-Containing Superbases: Recent Progress in the Chemistry of Electron-Abundant Phosphines and Phosphazenes. Chem. Eur. J. 2021, 27, 10807–10825. [Google Scholar] [CrossRef]
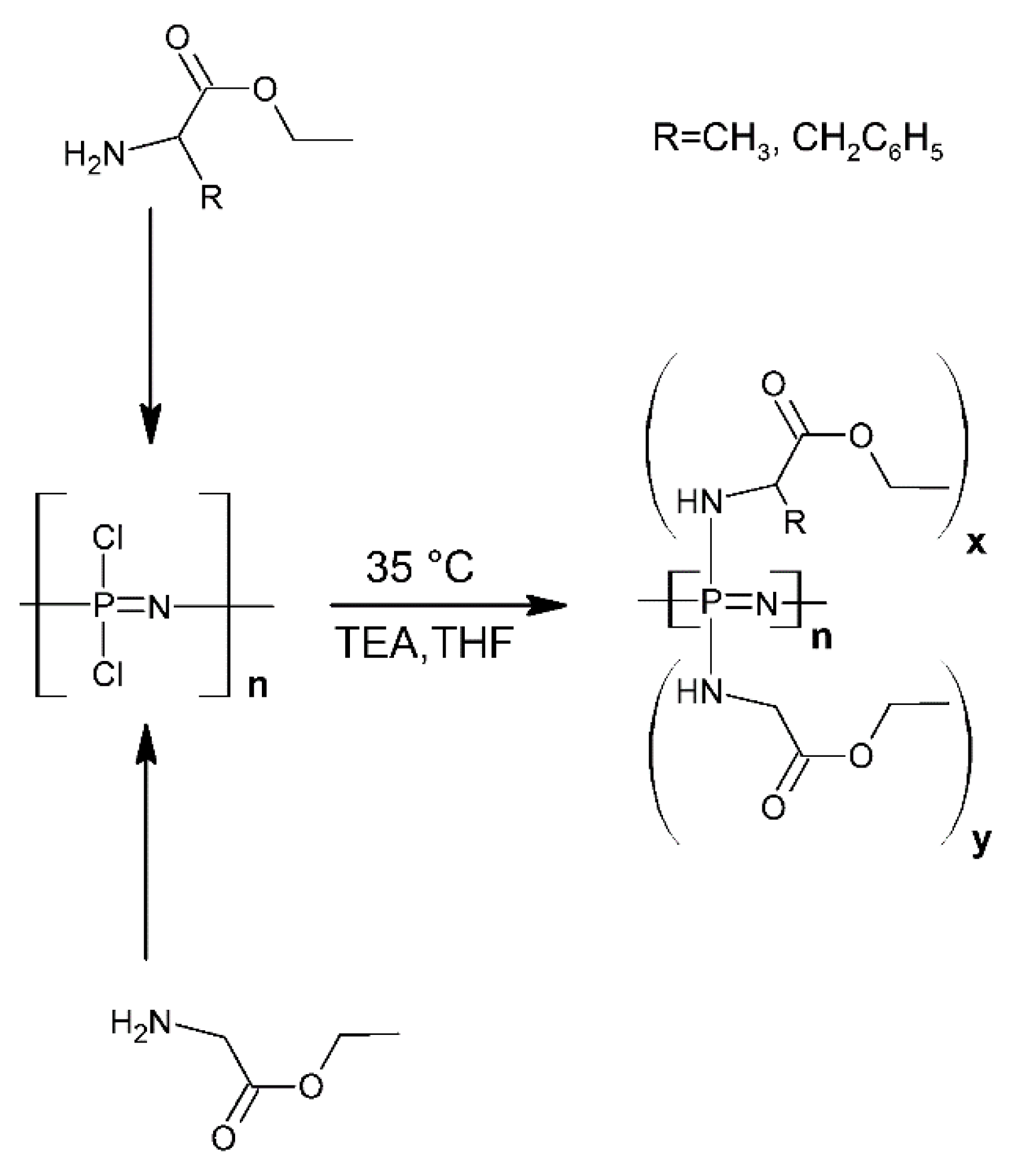
- Huang, Z.; Chen, S.; Lu, X.; Lu, Q. Water-Triggered Self-Assembly Polycondensation for the One-Pot Synthesis of Cyclomatrix Polyphosphazene Nanoparticles from Amino Acid Ester. Chem. Commun. 2015, 51, 8373–8376. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Huang, X. Cyclomatrix Polyphosphazenes Frameworks (Cyclo-POPs) and the Related Nanomaterials: Synthesis, Assembly and Functionalisation. Mater. Today Commun. 2017, 11, 38–60. [Google Scholar] [CrossRef]
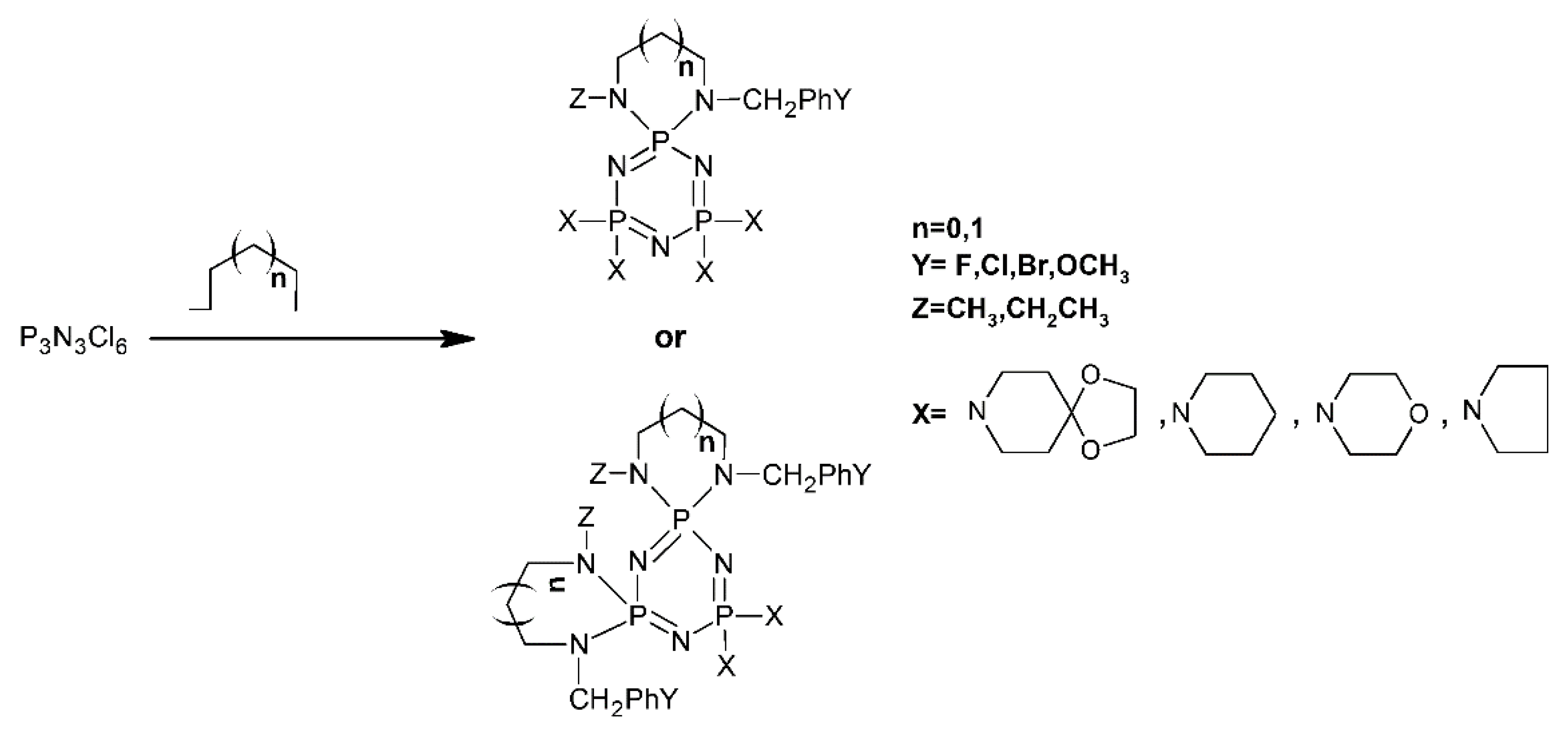
- Bartlett, S.W.; Coles, S.J.; Davies, D.B.; Hursthouse, M.B.; Ibişoǧlu, H.; Kiliç, A.; Shaw, R.A.; Ün, I. Structural Investigations of Phosphorus-Nitrogen Compounds. 7. Relationships between Physical Properties, Electron Densities, Reaction Mechanisms and Hydrogen-Bonding Motifs of N3P3Cl(6-n)(NHBut)n Derivatives. Acta Cryst. B 2006, 62, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R. Recent Advances in Phosphazene (Phosphonitrilic) Chemistry. Chem. Rev. 1972, 72, 315–356. [Google Scholar] [CrossRef]
- Ün, Ş.Ş.; Özcan, E.; Uslu, A.; Yuksel, F.; Kiliç, A. Cyclotriphosphazene Derivatives with Three Different Chiral Centres: Synthesis, Characterization and Investigation of Their Stereogenic Properties. Polyhedron 2013, 62, 250–259. [Google Scholar] [CrossRef]
- Asmafiliz, N. Syntheses of Chiral Phosphazenes with Stereogenic Centers: NMR Behavior in the Presence of a Chiral Solvating Agent. Heteroat. Chem. 2014, 25, 83–94. [Google Scholar] [CrossRef]
- Yeşilot, S.; Uslu, A. Stereochemical Aspects of Polyphosphazenes. Polym. Rev. 2017, 57, 213–247. [Google Scholar] [CrossRef]
- Uslu, A.; Yeşilot, S. Chiral Configurations in Cyclophosphazene Chemistry. Coord. Chem. Rev. 2015, 291, 28–67. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Lucken, E.A.C.; Whitehead, M.A. The Structure of the Phosphonitrilic Halides. J. Chem. Soc. 1960, 2423–2429. [Google Scholar] [CrossRef]
- Chaplin, A.B.; Harrison, J.A.; Dyson, P.J. Revisiting the Electronic Structure of Phosphazenes. Inorg. Chem. 2005, 44, 8407–8417. [Google Scholar] [CrossRef]
- Calichman, M.; Derecskei-Kovacs, A.; Allen, C.W. The Origin of Endocyclic Bond Length Variations in Disubstituted Cyclotriphosphazenes. Inorg. Chem. 2007, 46, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Guajardo Maturana, R.; Valenzuela, M.L.; Schott, E.; Rojas-Poblete, M. Bonding and Optical Properties of Spirocyclic-Phosphazene Derivatives. A DFT Approach. Phys. Chem. Chem. Phys. 2017, 19, 31479–31486. [Google Scholar] [CrossRef]
- Linares-Flores, C.; Ramirez-Tagle, R.; Rojas-Poblete, M.; Arratia-Perez, R.; Muñoz-Castro, A.; Guajardo-Maturana, R. Role of Donor-Acceptor Functional Groups in N3P3 Cyclic-Triphosphazene Backbone. Unraveling Bonding Characteristics from Natural Orbitals within an Extended Transition State-Natural Orbital for the Chemical Valence Scheme. Int. J. Quantum Chem. 2020, 120, e26057. [Google Scholar] [CrossRef]
- Jancik, V.; Cortés-Guzmán, F.; Herbst-Irmer, R.; Matínez-Otero, D. Is Hexachloro-Cyclo-Triphosphazene Aromatic? Evidence from Experimental Charge Density Analysis. Chem. Eur. J. 2017, 23, 6964–6968. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, I.I.; Tur, D.R.; Papkov, V.S.; Antipin, M.Y. X-ray Crystal Structures and DFT Calculations of Differently Charged Aminocyclophosphazenes. J. Mol. Struct. 2009, 928, 1–11. [Google Scholar] [CrossRef]
- Calichman, M.; Allen, C.W. Organophophazenes 28: The Nature of the Exocyclic Phosphorus–Carbon Bond in Organophosphazenes. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2110–2115. [Google Scholar] [CrossRef]
- Gershoni-Poranne, R.; Stanger, A. NICS—Nucleus Independent Chemical Shift in Aromaticity. Modern Computational Methods and Applications; Fernandez, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Jaeger, R.; Vancso, G.J. An Ab Initio and Force Field Study on the Conformation and Chain Flexibility of the Dichlorophosphazene Trimer. Macromol. Theory Simul. 1996, 5, 673–689. [Google Scholar] [CrossRef]
- Caminiti, R.; Gleria, M.; Lipkowitz, K.B.; Lombarde, G.M.; Pappalardo, G.C. Molecular Dynamics Simulations Combined with Large Angle X-Ray Scattering Technique for the Determination of the Structure, Conformation, and Conformational Dynamics of Polyphosphazenes in Amorphous Phase: Study of Poly[Di(4-Methylphenoxy)Phosphazene]. J. Am. Chem. Soc. 1997, 119, 2196–2204. [Google Scholar] [CrossRef]
- Laguna, M.T.R.; Saiz, E.; Tarazona, M.P. Solution Properties of Poly(Diphenoxyphosphazene) below the θ Temperature Obtained by SEC/MALLS. Polymer 2000, 41, 7993–8000. [Google Scholar] [CrossRef]
- Amato, M.E.; Grassi, A.; Lipkowitz, K.B.; Lombardo, G.M.; Pappalardo, G.C.; Sadun, C. Molecular Dynamics Simulations of Polyphosphazenes: Poly[Bis(Chloro)Phosphazene][NPCl2] n. J. Inorg. Organomet. Polym. 1996, 6, 237–253. [Google Scholar] [CrossRef]
- Caminiti, R.; Gleria, M.; Lipkowitz, K.B.; Lombardo, G.M.; Pappalardo, G.C. Molecular Modeling and Large-Angle x-Ray Scattering Studies of the Structure of Semicrystalline Poly [Bis(Phenoxy)Phosphazene]. Chem. Mater. 1999, 11, 1492–1497. [Google Scholar] [CrossRef]
- Tarazona, M.P.; Saiz, E. A Conformational Model for Poly(Dichlorophosphazene) Derived from Molecular Dynamics Simulations. Polymer 2000, 41, 3337–3347. [Google Scholar] [CrossRef]
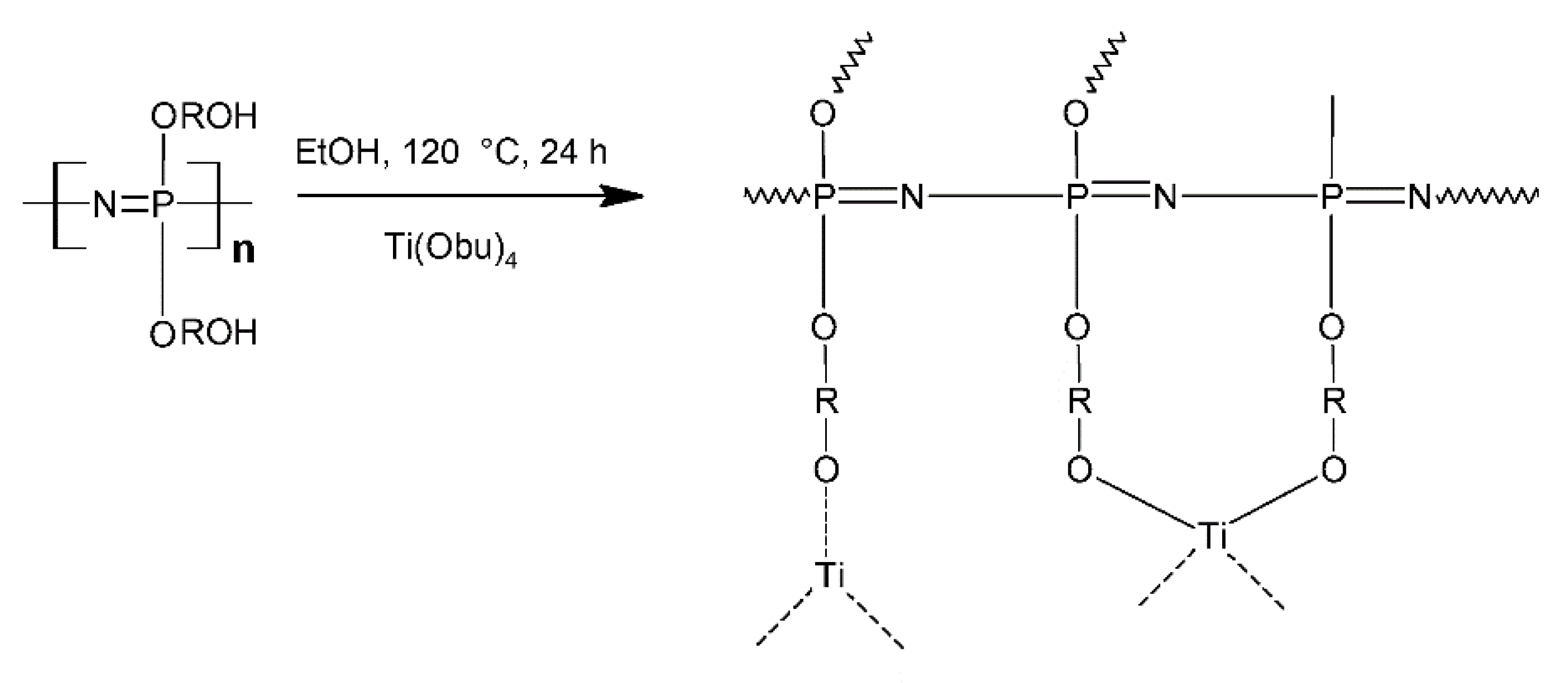
- Wang, Y.; Balbuena, P.B. Combined Ab Initio Quantum Mechanics and Classical Molecular Dynamics Studies of Polyphosphazene Polymer Electrolytes: Competitive Solvation of Li+ and LiCF3SO3. J. Phys. Chem. B 2004, 108, 15694–15702. [Google Scholar] [CrossRef]
- Sun, H. The COMPASS Force Field: Parameterization and Validation for Phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Fried, J.R.; Ren, P. Molecular Simulation of the Glass Transition of Polyphosphazenes. Comput. Theor. Polym. Sci. 1999, 9, 111–116. [Google Scholar] [CrossRef]
- Fried, J.R.; Ren, P. The Atomistic Simulation of the Gas Permeability of Poly(Organophosphazenes). Part 1. Poly(Dibutoxyphosphazenes). Comput. Theor. Polym. Sci. 2000, 10, 447–463. [Google Scholar] [CrossRef]
- Hu, N.; Fried, J.R. The Atomistic Simulation of the Gas Permeability of Poly(Organophosphazenes). Part 2. Poly[Bis(2,2,2-Trifluoroethoxy)Phosphazene]. Polymer 2005, 46, 4330–4343. [Google Scholar] [CrossRef]
- Fried, J.R. Gas Diffusion and Solubility in Poly(Organophosphazenes): Results of Molecular Simulation Studies. J. Inorg. Organomet. Polym. Mater. 2006, 16, 407–418. [Google Scholar] [CrossRef]
- Kroger, J.L.; Fried, J.R. Molecular Simulations of Polyphosphazenes for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2012, 22, 973–984. [Google Scholar] [CrossRef]
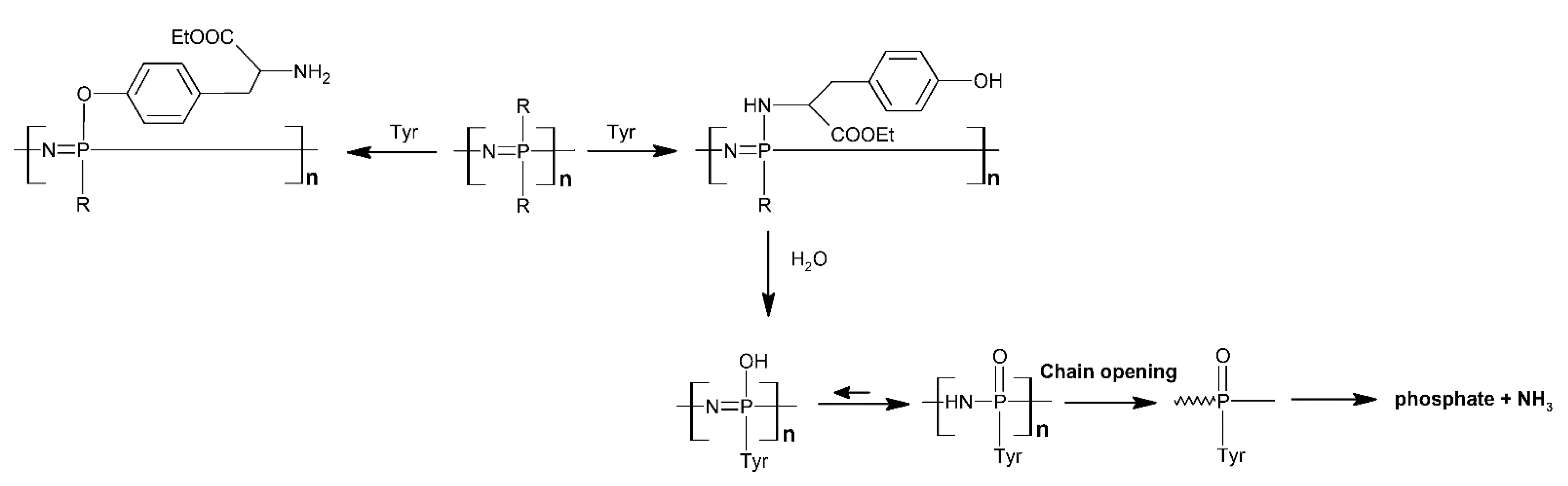
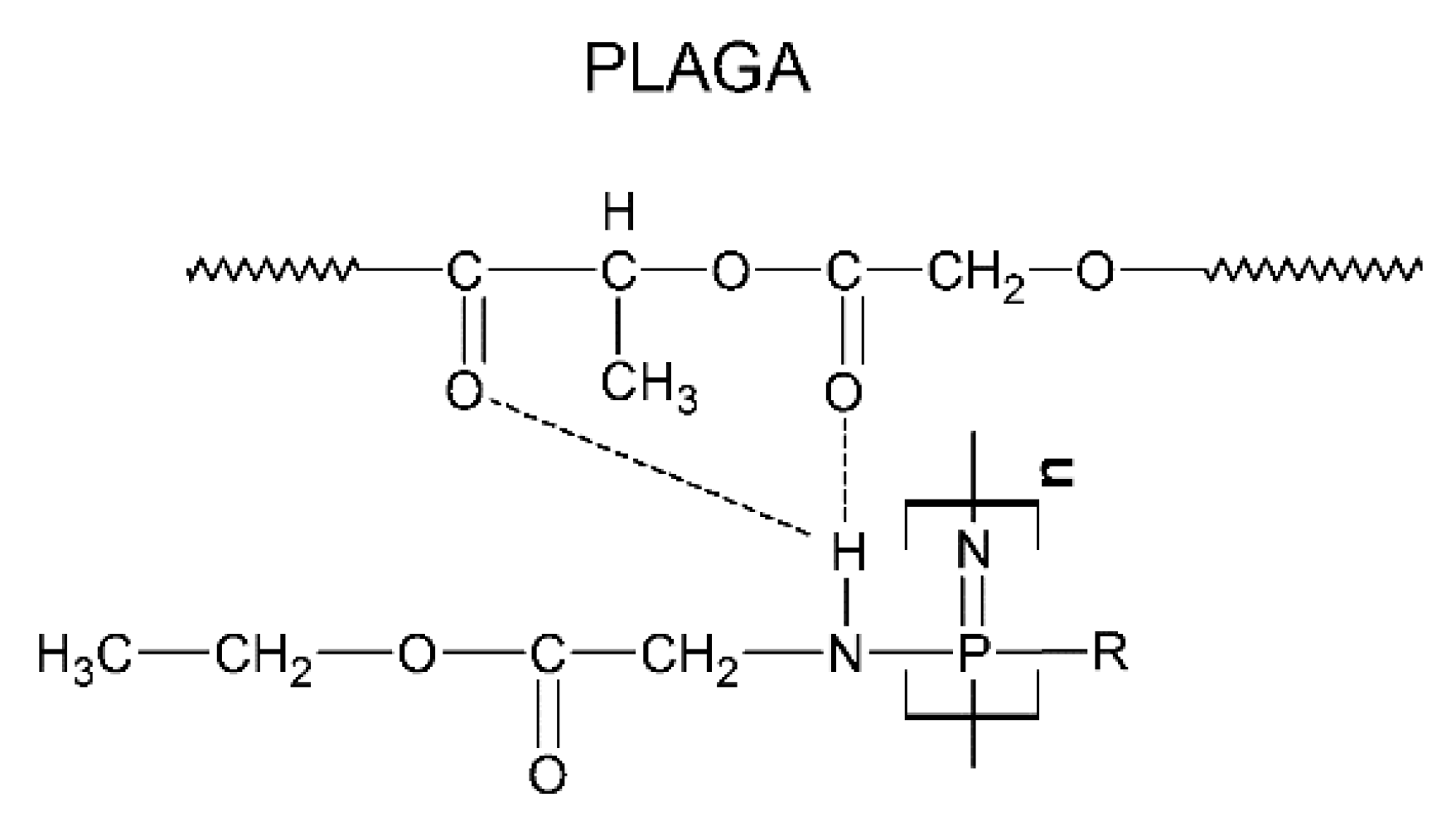
- Allcock, H.R.; Pucher, S.R.; Scopelianos, A.G. Poly[(Amino Acid Ester)Phosphazenes] as Substrates for the Controlled Release of Small Molecules. Biomaterials 1994, 15, 563–569. [Google Scholar] [CrossRef]
- Veronese, F.M.; Marsilio, F.; Lora, S.; Caliceti, P.; Passi, P.; Orsolini, P. Polyphosphazene Membranes and Microspheres in Periodontal Diseases and Implant Surgery. Biomaterials 1999, 20, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Caliceti, P.; Veronese, F.M.; Lora, S. Polyphosphazene Microspheres for Insulin Delivery. Int. J. Pharm. 2000, 211, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Basharat, M.; Wu, S.; Zhang, S.; Zhang, X.; Ma, H.; Liu, W.; Wu, D.; Wu, Z. Effect of Side Groups on Glass Transition Temperatures of Poly(Ethoxy/Phenoxy)Phosphazenes: Prediction and Synthesis. Polymer 2021, 230, 124068. [Google Scholar] [CrossRef]
- Chen, K.; Demir, B.A.; Chen, K.; Demir, B. A Computational Procedure for Atomistic Modelling of Polyphosphazenes towards Better Capturing Molecular-Level Structuring and Thermo-Mechanical Properties. Polymers 2022, 14, 1451. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Decollibus, D.P.; Andrianov, A.K. Protein Stabilization in Aqueous Solutions of Polyphosphazene Polyelectrolyte and Non-Ionic Surfactants. Biomacromolecules 2010, 11, 2268–2273. [Google Scholar] [CrossRef]
- Decollibus, D.P.; Marin, A.; Andrianov, A.K. Effect of Environmental Factors on Hydrolytic Degradation of Water-Soluble Polyphosphazene Polyelectrolyte in Aqueous Solutions. Biomacromolecules 2010, 11, 2033–2038. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Ogueri, K.S.; Ude, C.C.; Allcock, H.R.; Laurencin, C.T. Biomedical Applications of Polyphosphazenes. Med. Devices Sens. 2020, 3, e10113. [Google Scholar] [CrossRef]
- Singh, A.; Krogman, N.R.; Sethuraman, S.; Nair, L.S.; Sturgeon, J.L.; Brown, P.W.; Laurencin, C.T.; Allcock, H.R. Effect of Side Group Chemistry on the Properties of Biodegradable L-Alanine Cosubstituted Polyphosphazenes. Biomacromolecules 2006, 7, 914–918. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A. Degradation of Polyaminophosphazenes: Effects of Hydrolytic Environment and Polymer Processing. Biomacromolecules 2006, 7, 1581–1586. [Google Scholar] [CrossRef]
- Lakshmi, S.; Katti, D.S.; Laurencin, C.T. Biodegradable Polyphosphazenes for Drug Delivery Applications. Adv. Drug Deliv. Rev. 2003, 55, 467–482. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Penev, K.I.; Mequanint, K. One-Pot Substitution Approach for the Syntheses of Nonfunctional and Functional Poly[(Amino Acid Ester)Phosphazene] Biomaterials. Macromol. Mater. Eng. 2017, 302, 1600318. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Mequanint, K. Biodegradable Polyphosphazene Biomaterials for Tissue Engineering and Delivery of Therapeutics. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, I.; Brüggemann, O. Polyphosphazenes: Multifunctional, Biodegradable Vehicles for Drug and Gene Delivery. Polymers 2013, 5, 161–187. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Deng, M.; Kumbar, S.G.; Laurencin, C.T. Polyphosphazenes. Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 193–206. [Google Scholar] [CrossRef]
- Morozowich, N.L.; Mondschein, R.J.; Allcock, H.R. Comparison of the Synthesis and Bioerodible Properties of N-Linked Versus O-Linked Amino Acid Substituted Polyphosphazenes. J. Inorg. Organomet. Polym. Mater. 2014, 24, 164–172. [Google Scholar] [CrossRef]
- Amin, A.M.; Shahid, S.A.; Li, W.; Haojie, Y.; Ali, Z.; Rehman, H.; Ghaffar, A.; Sarfraz, M.; Waqas, M. An Efficient Synthesis, Structural Characterization and Hydrolytic Degradation Studies of Poly[Bis(3-Phenyl-1-Propoxide Amino Benzoic Acid Diethylamino)Phosphazene] as Potential Materials for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1117–1121. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Bhattacharyya, S.; Nukavarapu, S.P.; Khan, Y.M.; Nair, L.S.; Laurencin, C.T. In Vitro and in Vivo Characterization of Biodegradable Poly(Organophosphazenes) for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2006, 16, 365–385. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Chen, J. Synthesis, Properties, and Biological Activity of Poly[Di(Sodium Carboxylatoethylphenoxy)Phosphazene]. Biomacromolecules 2006, 7, 394–399. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K.; Prasad, D.N.; Bhardwaj, T.R. Synthesis and in Vitro Degradation Studies of Substituted Poly(Organophosphazenes) for Drug Delivery Applications. J. Drug Deliv. Sci. Technol. 2017, 38, 135–142. [Google Scholar] [CrossRef]
- Andrianov, A.K. Water-Soluble Polyphosphazenes for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2006, 16, 397–406. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Peterson, P. Water-Soluble Biodegradable Polyphosphazenes Containing N-Ethylpyrrolidone Groups. Macromolecules 2005, 38, 7972–7976. [Google Scholar] [CrossRef]
- Wilfert, S.; Iturmendi, A.; Schoefberger, W.; Kryeziu, K.; Heffeter, P.; Berger, W.; Brüggemann, O.; Teasdale, I. Water-Soluble, Biocompatible Polyphosphazenes with Controllable and PH-Promoted Degradation Behavior. J. Polym. Sci. A Polym. Chem. 2014, 52, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Heyde, M.; Claeyssens, M.; Schacht, E.H. Interaction between Proteins and Polyphosphazene Derivatives Having a Galactose Moiety. Biomacromolecules 2008, 9, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.F. Phosphazenes. Organophosphorus Chem. 2012, 41, 349–384. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/Nano-Hydroxyapatite Composite Microsphere Scaffolds for Bone Tissue Engineering. Biomacromolecules 2008, 9, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Iturmendi, A.; Monkowius, U.; Teasdale, I. Oxidation Responsive Polymers with a Triggered Degradation via Arylboronate Self-Immolative Motifs on a Polyphosphazene Backbone. ACS Macro Lett. 2017, 6, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Ozay, H.; Sahin, O.; Koc, O.K.; Ozay, O. The Preparation and Applications of Novel Phosphazene Crosslinked Thermo and PH Responsive Hydrogels. J. Ind. Eng. Chem. 2016, 43, 28–35. [Google Scholar] [CrossRef]
- Fu, J.; Liang, L.; Qiu, L.; Fu, J.; Liang, L.; Qiu, L. In Situ Generated Gold Nanoparticle Hybrid Polymersomes for Water-Soluble Chemotherapeutics: Inhibited Leakage and PH-Responsive Intracellular Release. Adv. Funct. Mater. 2017, 27, 1604981. [Google Scholar] [CrossRef]
- Chen, F.; Teniola, O.R.; Ogueri, K.S.; Laurencin, C.T. Recent Trends in the Development of Polyphosphazenes for Bio-Applications. Regen. Eng. Transl. Med. 2022, 1, 1–22. [Google Scholar] [CrossRef]
- Ding, G.; Wang, A.; Shi, X.; Li, J.; You, L.; Wang, S. Preparation of Multiple-Spectra Encoded Polyphosphazene Microspheres and Application for Antibody Detection. Polym. Bull. 2022, 79, 6409–6429. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, S.; Wu, Z.; Qin, S.; Li, F.; Song, T.; Cao, X.; Wang, Z.L.; Zhang, L. On the Understanding of Dielectric Elastomer and Its Application for All-Soft Artificial Heart. Sci. Bull. 2021, 66, 981–990. [Google Scholar] [CrossRef]
- Kuzey, N.G.; Özgür, M.; Cemaloğlu, R.; Asmafiliz, N.; Kılıç, Z.; Açık, L.; Aydın, B.; Hökelek, T. Mono- and Dispirocyclotriphosphazenes Containing 4-Bromobenzyl Pendant Arm(s): Synthesis, Spectroscopy, Crystallography and Biological Activity Studies. J. Mol. Struct. 2020, 1220, 128658. [Google Scholar] [CrossRef]
- Asmafiliz, N.; Berberoğlu, İ.; Özgür, M.; Kılıç, Z.; Kayalak, H.; Açık, L.; Türk, M.; Hökelek, T. Phosphorus-Nitrogen Compounds: Part 46. The Reactions of N3P3Cl6 with Bidentate and Monodentate Ligands: The Syntheses, Structural Characterizations, Antimicrobial and Cytotoxic Activities, and DNA Interactions of (N/N)Spirocyclotriphosphazenes with 4-Chlorobenzyl Pendant Arm. Inorg. Chim. Acta 2019, 495, 118949. [Google Scholar] [CrossRef]
- Güven Kuzey, N.; Cemaloğlu, R.; Yakut, M.; Asmafiliz, N.; Kılıç, Z.; Aydın, B.; Açık, L.; Hökelek, T. Phosphorus–Nitrogen Compounds Part 55. Syntheses of 4-Methoxybenzylspiro(N/N)Cyclotriphosphazenes: Chemical, Structural and Biological Properties. Res. Chem. Intermed. 2021, 47, 3933–3962. [Google Scholar] [CrossRef]
- İnci Tanrıkulu, G.; Yakut Özgür, M.; Okumuş, A.; Kılıç, Z.; Hökelek, T.; Aydın, B.; Açık, L. Phosphorus-Nitrogen Compounds Part 47: The Conventional and Microwave-Assisted Syntheses of Dispirocyclotriphosphazene Derivatives with (4-Fluoro/4-Nitrobenzyl) Pendant Arms: Structural and Stereogenic Properties and DNA Interactions. Inorg. Chim Acta 2019, 490, 179–189. [Google Scholar] [CrossRef]
- İşcan, Ö.; Cemaloğlu, R.; Asmafiliz, N.; Zeyrek, C.T.; Kılıç, Z.; Açık, L.; Aydın, B.; Türk, M.; Hökelek, T. Phosphorus–Nitrogen Compounds: Part 53—Synthesis, Characterization, Cytotoxic and Antimicrobial Activity, DNA Interaction and Molecular Docking Studies of New Mono- and Dispirocyclotriphosphazenes with Pendant Arm(s). Mol. Divers. 2022, 26, 1077–1100. [Google Scholar] [CrossRef] [PubMed]
- Okumuş, A.; Akbaş, H.; Karadağ, A.; Aydın, A.; Kılıç, Z.; Hökelek, T. Antiproliferative Effects against A549, Hep3B and FL Cell Lines of Cyclotriphosphazene-Based Novel Protic Molten Salts: Spectroscopic, Crystallographic and Thermal Results. ChemistrySelect 2017, 2, 4988–4999. [Google Scholar] [CrossRef]
- Elmas, G.; Okumuş, A.; Koç, L.Y.; Soltanzade, H.; Kílíç, Z.; Hökelek, T.; Dal, H.; Açík, L.; Üstündağ, Z.; Dündar, D.; et al. Phosphorus-Nitrogen Compounds. Part 29. Syntheses, Crystal Structures, Spectroscopic and Stereogenic Properties, Electrochemical Investigations, Antituberculosis, Antimicrobial and Cytotoxic Activities and DNA Interactions of Ansa-Spiro-Ansa Cyclotetraphosphazenes. Eur. J. Med. Chem. 2014, 87, 662–676. [Google Scholar] [CrossRef]
- Elmas, G.; Okumuş, A.; Kılıç, Z.; Çam, M.; Açık, L.; Hökelek, T. Phosphorus-Nitrogen Compounds. Part 40. The Syntheses of (4-Fluorobenzyl) Pendant Armed Cyclotetraphosphazene Derivatives: Spectroscopic, Crystallographic and Stereogenic Properties, DNA Interactions and Antimicrobial Activities. Inorg. Chim. Acta 2018, 476, 110–122. [Google Scholar] [CrossRef]
- Okumuş, A.; Elmas, G.; Cemaloǧlu, R.; Aydin, B.; Binici, A.; Şimşek, H.; Açik, L.; Türk, M.; Güzel, R.; Kiliç, Z.; et al. Phosphorus–Nitrogen Compounds. Part 35. Syntheses, Spectroscopic and Electrochemical Properties, and Antituberculosis, Antimicrobial and Cytotoxic Activities of Mono-Ferrocenyl-Spirocyclotetraphosphazenes. New J. Chem. 2016, 40, 5588–5603. [Google Scholar] [CrossRef]
- Asmafiliz, N.; Civan, M.; Özben, A.; Kılıç, Z.; Ramazanoğlu, N.; Açık, L.; Hökelek, T. Phosphorus-Nitrogen Compounds. Part 39. Syntheses and Langmuir-Blodgett Thin Films and Antimicrobial Activities of N/N and N/O Spirocyclotriphosphazenes with Monoferrocenyl Pendant Arm. Appl. Organomet. Chem. 2018, 32, e4223. [Google Scholar] [CrossRef]
- Binici, A.; Okumuş, A.; Elmas, G.; Kiliç, Z.; Ramazanoǧlu, N.; Açik, L.; Şimşek, H.; Çaǧdaş Tunali, B.; Türk, M.; Güzel, R.; et al. Phosphorus–Nitrogen Compounds. Part 42. The Comparative Syntheses of 2-Cis-4-Ansa(N/O) and Spiro(N/O) Cyclotetraphosphazene Derivatives: Spectroscopic and Crystallographic Characterization, Antituberculosis and Cytotoxic Activity Studies. New J. Chem. 2019, 43, 6856–6873. [Google Scholar] [CrossRef]
- Başterzi, N.S.; Bilge Koçak, S.; Okumuş, A.; Kiliç, Z.; Hökelek, T.; Çelik, Ö.; Türk, M.; Koç, L.Y.; Açik, L.; Aydin, B. Syntheses, Structural Characterization and Biological Activities of Spiro-Ansa-Spiro-Cyclotriphosphazenes. New J. Chem. 2015, 39, 8825–8839. [Google Scholar] [CrossRef]
- Okumuş, A.; Elmas, G.; Kılıç, Z.; Binici, A.; Ramazanoğlu, N.; Açık, L.; Çoşut, B.; Hökelek, T.; Güzel, R.; Tunalı, B.Ç.; et al. The Comparative Reactions of 2-Cis-4-Ansa and Spiro Cyclotetraphosphazenes with Difunctional Ligands: Structural and Stereogenic Properties, Electrochemical, Antimicrobial and Cytotoxic Activity Studies. Appl. Organomet. Chem. 2021, 35, e6150. [Google Scholar] [CrossRef]
- Koçak, S.B.; Koçoǧlu, S.; Okumus, A.; Kiliç, Z.; Öztürk, A.; Hökelek, T.; Öner, Y.; Açik, L. Syntheses, Spectroscopic Properties, Crystal Structures, Biological Activities, and DNA Interactions of Heterocyclic Amine Substituted Spiro-Ansa-Spiro- and Spiro-Bino-Spiro-Phosphazenes. Inorg. Chim. Acta 2013, 406, 160–170. [Google Scholar] [CrossRef]
- Asmafiliz, N.; Kiliç, Z.; Civan, M.; Avci, O.; Yasemin Gönder, L.; Açik, L.; Aydin, B.; Türk, M.; Hökelek, T. Phosphorus–Nitrogen Compounds. Part 36. Syntheses, Langmuir–Blodgett Thin Films and Biological Activities of Spiro-Bino-Spiro Trimeric Phosphazenes. New J. Chem. 2016, 40, 9609–9626. [Google Scholar] [CrossRef]
- Koran, K.; Ozkaya, A.; Ozen, F.; Cil, E.; Arslan, M. Synthesis, Characterization, and Biological Evaluation of New Oxime-Phosphazenes. Res. Chem. Intermed. 2013, 39, 1109–1124. [Google Scholar] [CrossRef]
- Gascón, E.; Maisanaba, S.; Otal, I.; Valero, E.; Repetto, G.; Jones, P.G.; Jiménez, J. (Amino)Cyclophosphazenes as Multisite Ligands for the Synthesis of Antitumoral and Antibacterial Silver(I) Complexes. Inorg. Chem. 2020, 59, 2464–2483. [Google Scholar] [CrossRef]
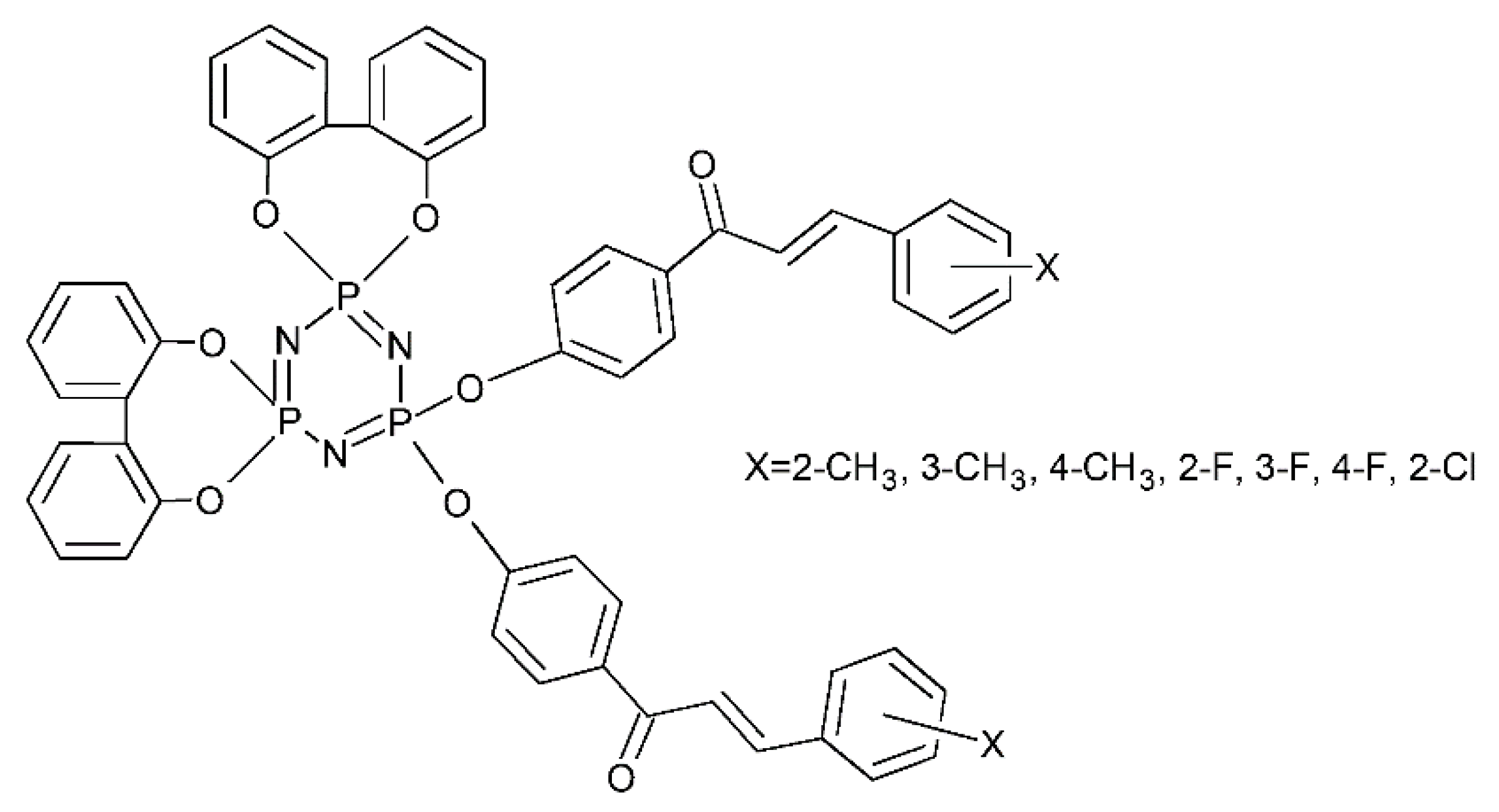
- Doğan, H.; Bahar, M.R.; Çalışkan, E.; Tekin, S.; Uslu, H.; Akman, F.; Koran, K.; Sandal, S.; Görgülü, A.O. Synthesis and Spectroscopic Characterizations of Hexakis[(1-(4′-Oxyphenyl)-3-(Substituted-Phenyl)Prop-2-En-1-One)]Cyclotriphosphazenes: Their in Vitro Cytotoxic Activity, Theoretical Analysis and Molecular Docking Studies. J. Biomol. Struct. Dyn. 2022, 40, 3258–3272. [Google Scholar] [CrossRef]
- Beytur, A.; Tekin, Ç.; Çalışkan, E.; Tekin, S.; Koran, K.; Orhan Görgülü, A.; Sandal, S. Hexa-Substituted Cyclotriphosphazene Derivatives Containing Hetero-Ring Chalcones: Synthesis, in Vitro Cytotoxic Activity and Their DNA Damage Determination. Bioorg. Chem. 2022, 127, 105997. [Google Scholar] [CrossRef]
- Görgülü, A.O.; Koran, K.; Özen, F.; Tekin, S.; Sandal, S. Synthesis, Structural Characterization and Anti-Carcinogenic Activity of New Cyclotriphosphazenes Containing Dioxybiphenyl and Chalcone Groups. J. Mol. Struct. 2015, 1087, 1–10. [Google Scholar] [CrossRef]
- Kim, J.K.; Toti, U.S.; Song, R.; Youn, S.S. A Macromolecular Prodrug of Doxorubicin Conjugated to a Biodegradable Cyclotriphosphazene Bearing a Tetrapeptide. Bioorg. Med. Chem. Lett. 2005, 15, 3576–3579. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Song, S.C.; Jin, J.I.; Sohn, Y.S. Synthesis and Antitumor Activity of Polyphosphazene/Methoxy-Poly(Ethylene Glycol)/(Diamine)Platinum(II) Conjugates. Polym. J. 1999, 31, 1247–1252. [Google Scholar] [CrossRef]
- Jun, Y.J.; Kim, J.I.; Jun, M.J.; Sohn, Y.S. Selective Tumor Targeting by Enhanced Permeability and Retention Effect. Synthesis and Antitumor Activity of Polyphosphazene–Platinum (II) Conjugates. J. Inorg. Biochem. 2005, 99, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.B.; Jun, Y.J.; Song, J.H.; Park, M.K.; Oh, J.H.; Chae, S.W.; Kim, I.-S.; Choi, S.-J.; Lee, H.J.; Sohn, Y.S. A Novel Micelle-Encapsulated Palitnuim(II) Anticancer Agent. J. Control. Release 2010, 147, 144–150. [Google Scholar] [CrossRef]
- Henke, H.; Kryeziu, K.; Banfić, J.; Theiner, S.; Körner, W.; Brüggemann, O.; Berger, W.; Keppler, B.K.; Heffeter, P.; Teasdale, I. Macromolecular Pt(IV) Prodrugs from Poly(Organo)Phosphazenes. Macromol. Biosci. 2016, 16, 1239–1249. [Google Scholar] [CrossRef]
- Hackl, C.M.; Schoenhacker-Alte, B.; Klose, M.H.M.; Henke, H.; Legina, M.S.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Brüggemann, O.; Teasdale, I.; et al. Synthesis and in Vivo Anticancer Evaluation of Poly(Organo)Phosphazene-Based Metallodrug Conjugates. Dalton Trans. 2017, 46, 12114–12124. [Google Scholar] [CrossRef]
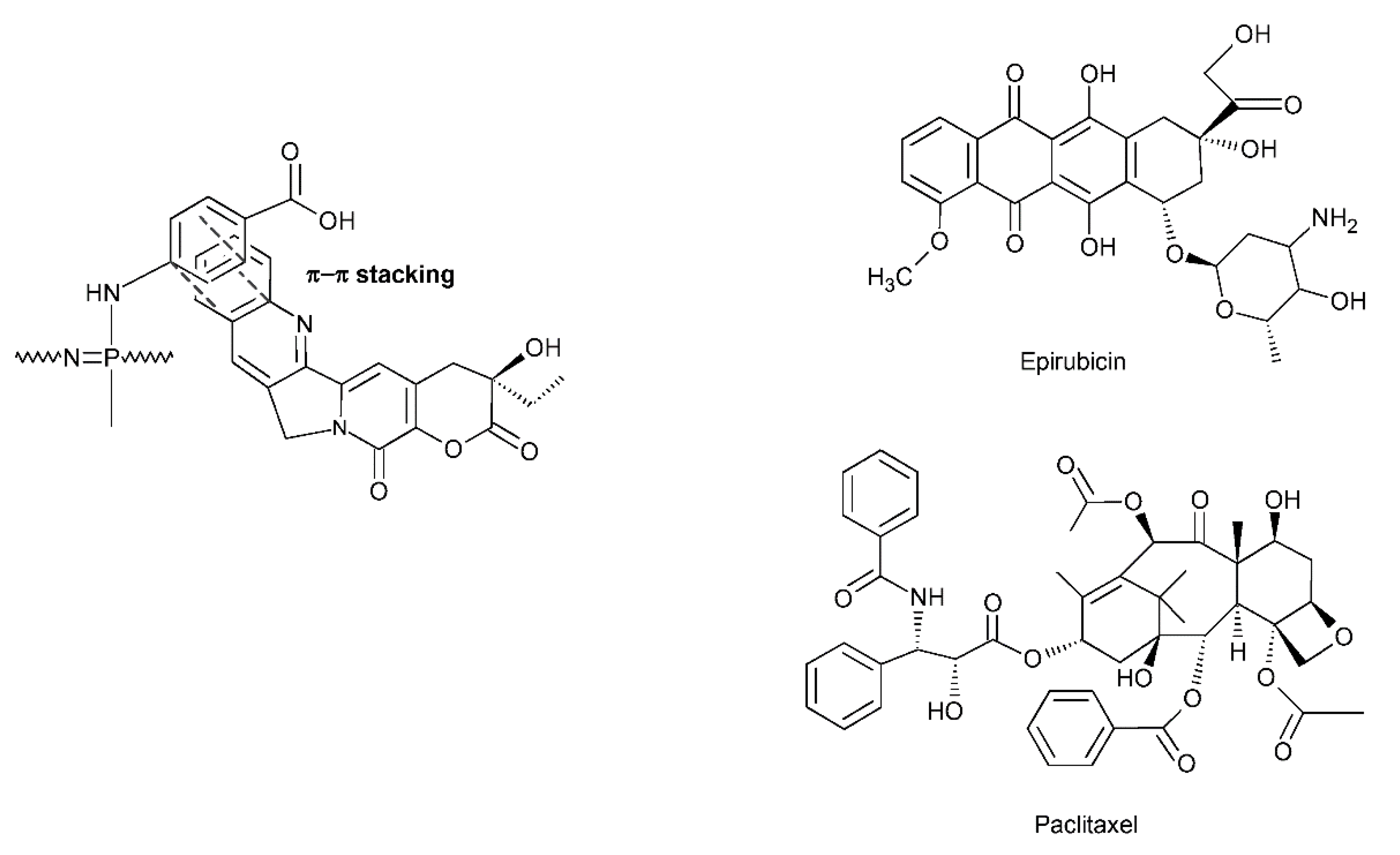
- Quiñones, J.P.; Roschger, C.; Iturmendi, A.; Henke, H.; Zierer, A.; Peniche-Covas, C.; Brüggemann, O. Polyphosphazene-Based Nanocarriers for the Release of Camptothecin and Epirubicin. Pharmaceutics 2022, 14, 169. [Google Scholar] [CrossRef]
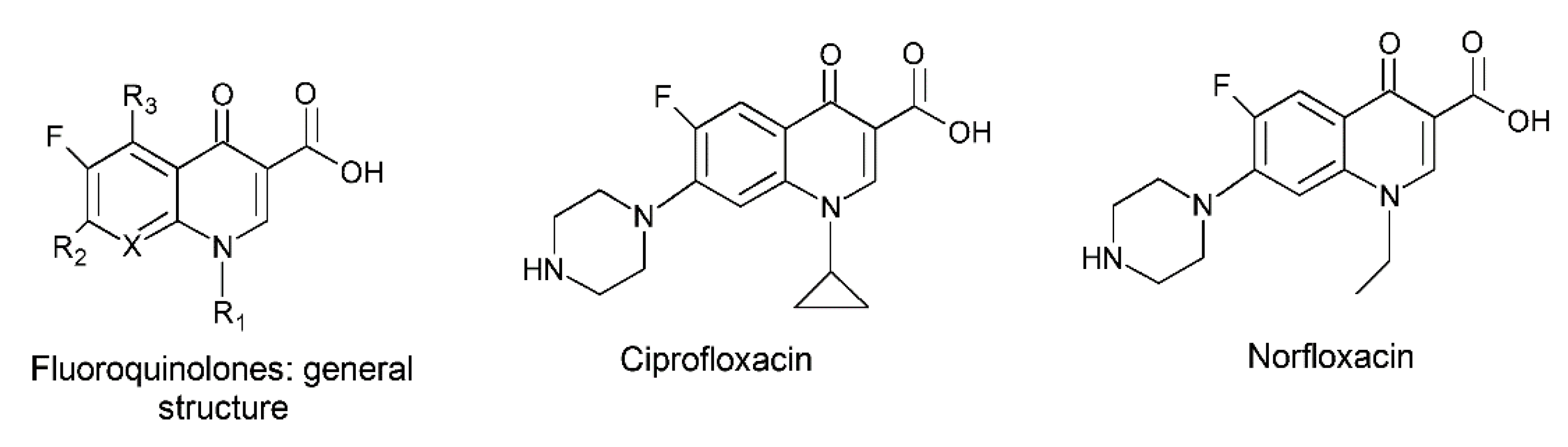
- Tian, Z.; Zhang, Y.; Liu, X.; Chen, C.; Guiltinan, M.J.; Allcock, H.R. Biodegradable Polyphosphazenes Containing Antibiotics: Synthesis, Characterization, and Hydrolytic Release Behavior. Polym. Chem. 2013, 4, 1826–1835. [Google Scholar] [CrossRef]
- Teasdale, I.; Wilfert, S.; Nischang, I.; Brüggemann, O. Multifunctional and Biodegradable Polyphosphazenes for Use as Macromolecular Anti-Cancer Drug Carriers. Polym. Chem. 2011, 2, 828–834. [Google Scholar] [CrossRef]
- Potta, T.; Chun, C.J.; Song, S.C. Injectable, Dual Cross-Linkable Polyphosphazene Blend Hydrogels. Biomaterials 2010, 31, 8107–8120. [Google Scholar] [CrossRef]
- Kang, G.D.; Cheon, S.H.; Song, S.C. Controlled Release of Doxorubicin from Thermosensitive Poly(Organophosphazene) Hydrogels. Int. J. Pharm. 2006, 319, 29–36. [Google Scholar] [CrossRef]
- Al-Abd, A.M.; Hong, K.Y.; Song, S.C.; Kuh, H.J. Pharmacokinetics of Doxorubicin after Intratumoral Injection Using a Thermosensitive Hydrogel in Tumor-Bearing Mice. J. Control. Release 2010, 142, 101–107. [Google Scholar] [CrossRef]
- Qian, Y.C.; Chen, P.C.; He, G.J.; Huang, X.J.; Xu, Z.K. Preparation of Polyphosphazene Hydrogels for Enzyme Immobilization. Molecules 2014, 19, 9850–9863. [Google Scholar] [CrossRef]
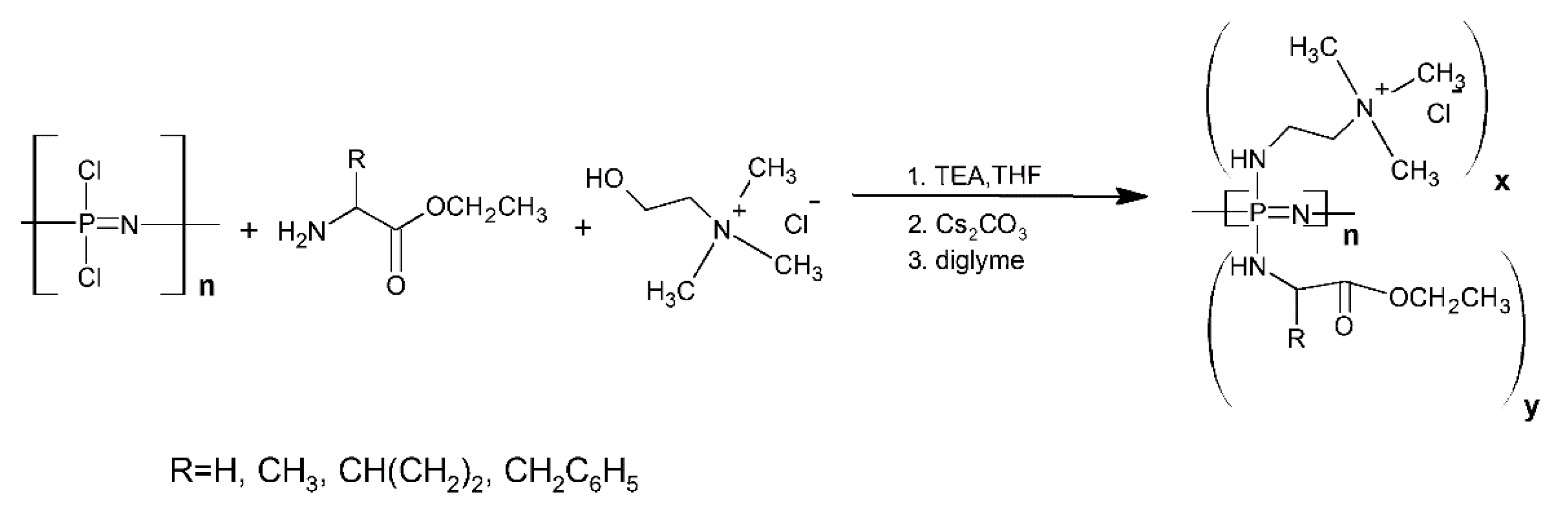
- Luten, J.; van Steenis, J.H.; van Someren, R.; Kemmink, J.; Schuurmans-Nieuwenbroek, N.M.E.; Koning, G.A.; Crommelin, D.J.A.; van Nostrum, C.F.; Hennink, W.E. Water-Soluble Biodegradable Cationic Polyphosphazenes for Gene Delivery. J. Control. Release 2003, 89, 483–497. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Jiang, J.; Gao, Y.; Gu, W.; Chen, L.; Tang, X.; Li, Y. Poly(Imidazole/DMAEA)Phosphazene/DNA Self-Assembled Nanoparticles for Gene Delivery: Synthesis and in Vitro Transfection. J. Control. Release 2008, 127, 273–279. [Google Scholar] [CrossRef]
- Luten, J.; van Steenbergen, M.J.; Lok, M.C.; de Graaff, A.M.; van Nostrum, C.F.; Talsma, H.; Hennink, W.E. Degradable PEG-Folate Coated Poly(DMAEA-Co-BA)Phosphazene-Based Polyplexes Exhibit Receptor-Specific Gene Expression. Eur. J. Pharm. Sci. 2008, 33, 241–251. [Google Scholar] [CrossRef]
- Baigude, H.; Su, J.; McCarroll, J.; Rana, T.M. In Vivo Delivery of RNAi by Reducible Interfering Nanoparticles (INOPs). ACS Med. Chem. Lett. 2013, 4, 720–723. [Google Scholar] [CrossRef]
- Hsu, W.H.; Sánchez-Gómez, P.; Gomez-Ibarlucea, E.; Ivanov, D.P.; Rahman, R.; Grabowska, A.M.; Csaba, N.; Alexander, C.; Garcia-Fuentes, M. Structure-Optimized Interpolymer Polyphosphazene Complexes for Effective Gene Delivery against Glioblastoma. Adv. Ther. 2018, 2, 1800126. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, X.; Du, C.; Zhao, B.; He, C.; Li, C.; Qiao, R. Water-Soluble Cationic Polyphosphazenes Grafted with Cyclic Polyamine and Imidazole as an Effective Gene Delivery Vector. Bioconjug. Chem. 2016, 27, 1005–1012. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Urocanic Acid Improves Transfection Efficiency of Polyphosphazene with Primary Amino Groups for Gene Delivery. Bioconjug. Chem. 2010, 21, 419–426. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Galactosylated Poly(2-(2-Aminoethyoxy)Ethoxy)Phosphazene/DNA Complex Nanoparticles: In Vitro and in Vivo Evaluation for Gene Delivery. Biomacromolecules 2010, 11, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, M.R.; Song, S.C. Injectable Polyplex Hydrogel for Localized and Long-Term Delivery of SiRNA. ACS Nano 2012, 6, 5757–5766. [Google Scholar] [CrossRef]
- Couffin-Hoarau, A.C.; Leroux, J.C. Report on the Use of Poly(Organophosphazenes) for the Design of Stimuli-Responsive Vesicles. Biomacromolecules 2004, 5, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Yu, H.; Wang, L.; Teng, L.; Zain-ul-Abdin; Nazir, A.; Fahad, S.; Elshaarani, T.; Haq, F.; Shen, D. Synthesis of Amino-Cosubstituted Polyorganophosphazenes and Fabrication of Their Nanoparticles for Anticancer Drug Delivery. J. Appl. Polym. Sci. 2020, 137, 49424. [Google Scholar] [CrossRef]
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular Permeability Enhancement in Solid Tumor: Various Factors, Mechanisms Involved and Its Implications. Int. Immunopharmacol. 2003, 3, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Metinoğlu Örüm, S.; Süzen Demircioğlu, Y. One-Pot Synthesis and Characterization of Crosslinked Polyphosphazene Dopamine Microspheres for Controlled Drug Delivery Applications. J. Macromol. Sci. Part A 2019, 56, 854–859. [Google Scholar] [CrossRef]
- Jun, Y.J.; Park, M.K.; Jadhav, V.B.; Song, J.H.; Chae, S.W.; Lee, H.J.; Park, K.S.; Jeong, B.; Choy, J.H.; Sohn, Y.S. Tripodal Amphiphiles Tunable for Self-Assembly to Polymersomes. J. Control. Release 2010, 142, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fu, J.; Zhu, L.; Tang, X.; Huang, X. Preparation of Novel Hybrid Inorganic–Organic Hollow Microspheres via a Self-Template Approach. Polym. Int. 2008, 57, 449–453. [Google Scholar] [CrossRef]
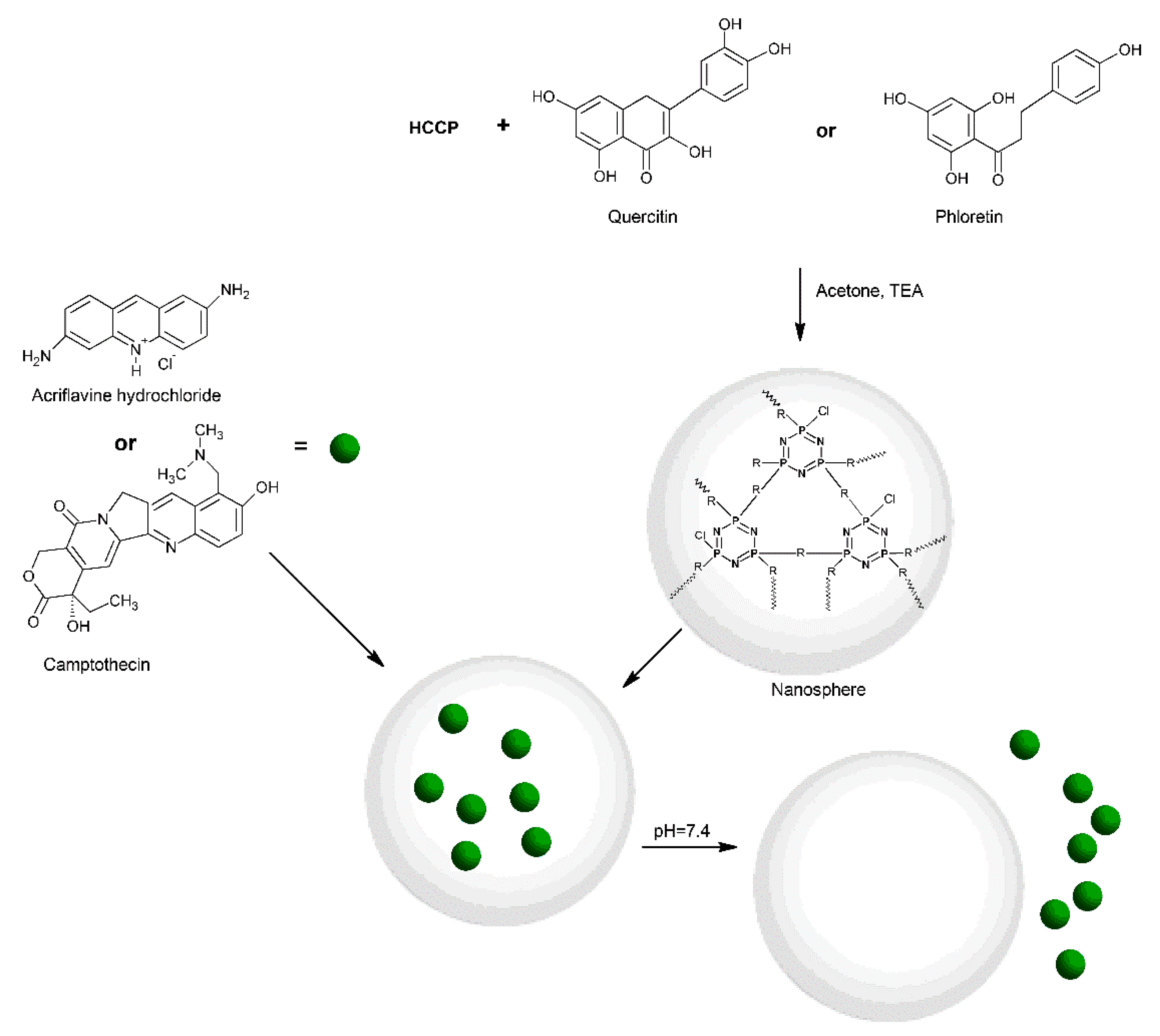
- Örüm, S.M.; Demircioğlu, Y.S. Crosslinked Polyphosphazene Nanospheres with Anticancer Quercetin: Synthesis, Spectroscopic, Thermal Properties, and Controlled Drug Release. Macromol. Res. 2018, 26, 671–679. [Google Scholar] [CrossRef]
- Mehmood, S.; Yu, H.; Wang, L.; Uddin, M.A.; Amin, B.U.; Haq, F.; Fahad, S.; Haroon, M. Cross-Linked Poly(Cyclotriphosphazene-Co-Phloretin) Microspheres and Their Application for Controlled Drug Delivery. Macromol. Res. 2022, 30, 623–630. [Google Scholar] [CrossRef]
- Onder, A.; Ozay, H. Synthesis and Characterization of Biodegradable and Antioxidant Phosphazene-Tannic Acid Nanospheres and Their Utilization as Drug Carrier Material. Mater. Sci. Eng. C 2021, 120, 111723. [Google Scholar] [CrossRef]
- Khan, R.U.; Yu, H.; Wang, L.; Zhang, Q.; Xiong, W.; Zain-ul-Abdin; Nazir, A.; Fahad, S.; Chen, X.; Elsharaarani, T. Synthesis of Polyorganophosphazenes and Preparation of Their Polymersomes for Reductive/Acidic Dual-Responsive Anticancer Drugs Release. J. Mater. Sci. 2020, 55, 8264–8284. [Google Scholar] [CrossRef]
- Mehnath, S.; Arjama, M.; Rajan, M.; Jeyaraj, M. Development of Cholate Conjugated Hybrid Polymeric Micelles for FXR Receptor Mediated Effective Site-Specific Delivery of Paclitaxel. New J. Chem. 2018, 42, 17021–17032. [Google Scholar] [CrossRef]
- Mehnath, S.; Rajan, M.; Sathishkumar, G.; Amarnath Praphakar, R.; Jeyaraj, M. Thermoresponsive and PH Triggered Drug Release of Cholate Functionalized Poly(Organophosphazene)—Polylactic Acid Co-Polymeric Nanostructure Integrated with ICG. Polymers 2017, 133, 119–128. [Google Scholar] [CrossRef]
- Simões, S.; Nuno Moreira, J.; Fonseca, C.; Düzgüneş, N.; De Lima, M.C.P. On the Formulation of PH-Sensitive Liposomes with Long Circulation Times. Adv. Drug Deliv. Rev. 2004, 56, 947–965. [Google Scholar] [CrossRef]
- Jun, Y.J.; Toti, U.S.; Kim, H.Y.; Yu, J.Y.; Jeong, B.; Jun, M.J.; Sohn, Y.S. Thermoresponsive Micelles from Oligopeptide-Grafted Cyclotriphosphazenes. Angew. Chem. Int. Ed. 2006, 45, 6173–6176. [Google Scholar] [CrossRef]
- Yu, J.Y.; Jun, Y.J.; Jang, S.H.; Lee, H.J.; Sohn, Y.S. Nanoparticulate Platinum(II) Anticancer Drug: Synthesis and Characterization of Amphiphilic Cyclotriphosphazene–Platinum(II) Conjugates. J. Inorg. Biochem. 2007, 101, 1931–1936. [Google Scholar] [CrossRef]
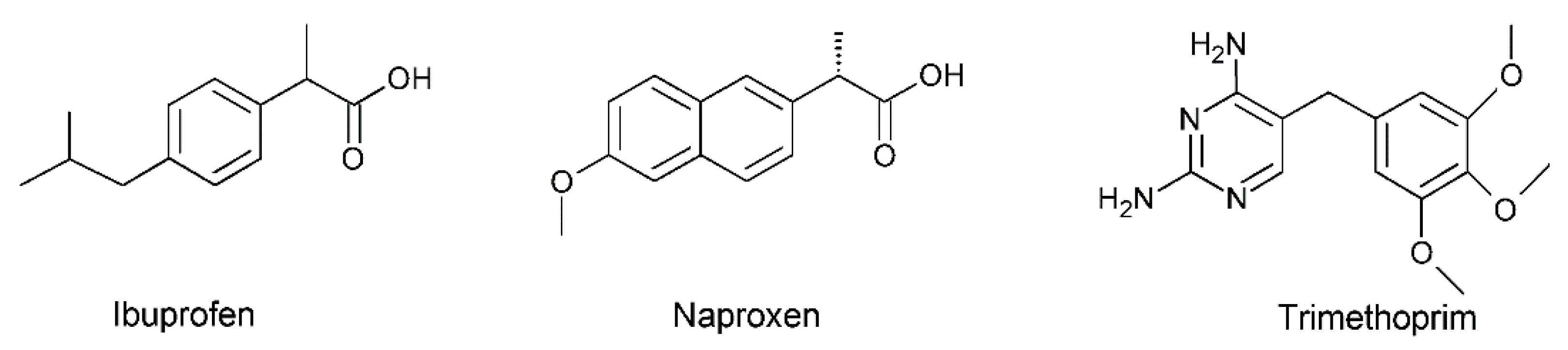
- Yurtdaş-Kırımlıoğlu, G.; Süzen-Demircioğlu, Y.; Berkman, M.S.; Metinoğlu-Örüm, S.; Altun, E. Synthesis, Spectroscopic, Thermal Properties, in Vitro Release, and Stability Studies of Ibuprofen-Loaded Microspheres Cross-Linked with Hexachlorocyclotriphosphazene/Octachlorocyclotetraphosphazene. Polym. Bull. 2021, 78, 6221–6250. [Google Scholar] [CrossRef]
- Ozay, H.; Ozay, O. Synthesis and Characterization of Drug Microspheres Containing Phosphazene for Biomedical Applications. Colloids Surf. A Phys. Eng. Asp. 2014, 450, 99–105. [Google Scholar] [CrossRef]
- Mehnath, S.; Arjama, M.; Rajan, M.; Arokia Vijayaanand, M.; Murugaraj, J. Polyorganophosphazene Stabilized Gold Nanoparticles for Intracellular Drug Delivery in Breast Carcinoma Cells. Process Biochem. 2018, 72, 152–161. [Google Scholar] [CrossRef]
- Mehnath, S.; Arjama, M.; Rajan, M.; Annamalai, G.; Jeyaraj, M. Co-Encapsulation of Dual Drug Loaded in MLNPs: Implication on Sustained Drug Release and Effectively Inducing Apoptosis in Oral Carcinoma Cells. Biomed. Pharmacother. 2018, 104, 661–671. [Google Scholar] [CrossRef]
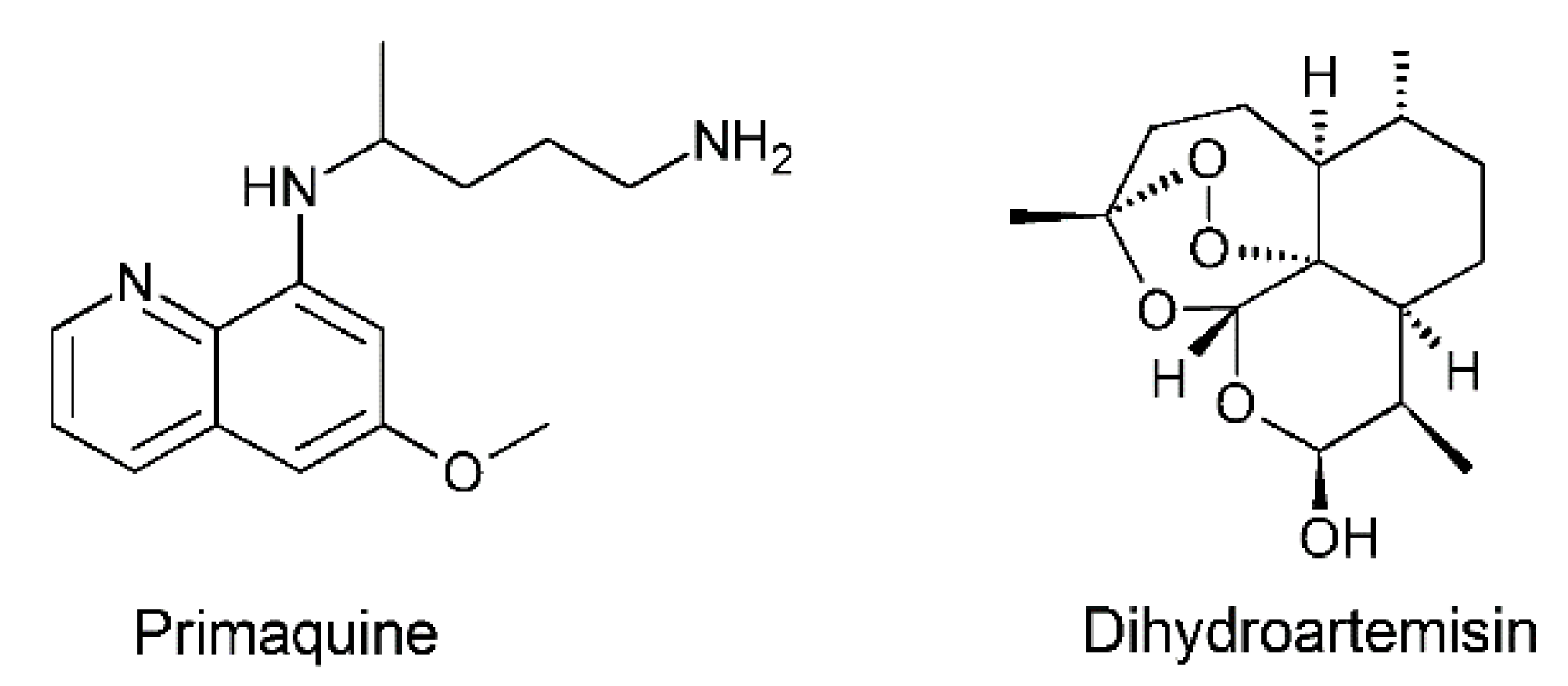
- Kumar, S.; Singh, R.K.; Murthy, R.S.R.; Bhardwaj, T.R. Synthesis and Evaluation of Substituted Poly(Organophosphazenes) as a Novel Nanocarrier System for Combined Antimalarial Therapy of Primaquine and Dihydroartemisinin. Pharm. Res. 2015, 32, 2736–2752. [Google Scholar] [CrossRef]
- Lin, Y.J.; Cai, Q.; Li, Q.F.; Xue, L.W.; Jin, R.G.; Yang, X.P. Effect of Solvent on Surface Wettability of Electrospun Polyphosphazene Nanofibers. J. Appl. Polym. Sci. 2010, 115, 3393–3400. [Google Scholar] [CrossRef]
- Wang, S.G.; Jiang, X.; Chen, P.C.; Yu, A.G.; Huang, X.J. Preparation of Coaxial-Electrospun Poly[Bis(p-Methylphenoxy)]Phosphazene Nanofiber Membrane for Enzyme Immobilization. Int. J. Mol. Sci. 2012, 13, 14136–14148. [Google Scholar] [CrossRef]
- Lin, Y.J.; Cai, Q.; Li, L.; Li, Q.F.; Yang, X.P.; Jin, R.G. Co-Electrospun Composite Nanofibers of Blends of Poly[(Amino Acid Ester)Phosphazene] and Gelatin. Polym. Int. 2010, 59, 610–616. [Google Scholar] [CrossRef]
- Nykänen, V.P.S.; Puska, M.A.; Nykänen, A.; Ruokolainen, J. Synthesis and Biomimetic Mineralization of L-Proline Substituted Polyphosphazenes as Bulk and Nanofiber. J. Polym. Sci. B Polym. Phys. 2013, 51, 1318–1327. [Google Scholar] [CrossRef]
- Peach, M.S.; James, R.; Toti, U.S.; Deng, M.; Morozowich, N.L.; Allcock, H.R.; Laurencin, C.T.; Kumbar, S.G. Polyphosphazene Functionalized Polyester Fiber Matrices for Tendon Tissue Engineering: In Vitro Evaluation with Human Mesenchymal Stem Cells. Biomed. Mater. 2012, 7, 045016. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V. Polymer Genomics: An Insight into Pharmacology and Toxicology of Nanomedicines. Adv. Drug Deliv. Rev. 2006, 58, 1597–1621. [Google Scholar] [CrossRef]
- Mutwiri, G.; Benjamin, P.; Soita, H.; Townsend, H.; Yost, R.; Roberts, B.; Andrianov, A.K.; Babiuk, L.A. Poly[Di(Sodium Carboxylatoethylphenoxy)Phosphazene] (PCEP) is a Potent Enhancer of Mixed Th1/Th2 Immune Responses in Mice Immunized with Influenza Virus Antigens. Vaccine 2007, 25, 1204–1213. [Google Scholar] [CrossRef]
- Awate, S.; Wilson, H.L.; Singh, B.; Babiuk, L.A.; Mutwiri, G. The Adjuvant PCEP Induces Recruitment of Myeloid and Lymphoid Cells at the Injection Site and Draining Lymph Node. Vaccine 2014, 32, 2420–2427. [Google Scholar] [CrossRef]
- Awate, S.; Wilson, H.L.; Lai, K.; Babiuk, L.A.; Mutwiri, G. Activation of Adjuvant Core Response Genes by the Novel Adjuvant PCEP. Mol. Immunol. 2012, 51, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Ninković, J.; Prokopowicz, Z.M.; Mancuso, C.J.; Marin, A.; Andrianov, A.K.; Dowling, D.J.; Levy, O. The Effect of Stable Macromolecular Complexes of Ionic Polyphosphazene on HIV Gag Antigen and on Activation of Human Dendritic Cells and Presentation to T-Cells. Biomaterials 2014, 35, 8876–8886. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Decollibus, D.P.; Marin, A.; Webb, A.; Griffin, Y.; Webby, R.J. PCPP-Formulated H5N1 Influenza Vaccine Displays Improved Stability and Dose-Sparing Effect in Lethal Challenge Studies. J. Pharm. Sci. 2011, 100, 1436–1443. [Google Scholar] [CrossRef]
- Le Cam, N.N.B.; Ronco, J.; Francon, A.; Blondeau, C.; Fanget, B. Adjuvants for Influenza Vaccine. Res. Immunol. 1998, 149, 19–23. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Chiu, Y.L.; Allen, M.; Lawrence, D.N.; Chapdu, C.; Israel, H.; Holman, D.; Keefer, M.C.; Wolff, M.; Frey, S.E. Long-Term Safety Analysis of Preventive HIV-1 Vaccines Evaluated in AIDS Vaccine Evaluation Group NIAID-Sponsored Phase I and II Clinical Trials. Vaccine 2003, 21, 2933–2947. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coating Formulations for Microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A.; Fuerst, T.R. Self-Assembly of Polyphosphazene Immunoadjuvant with Poly(Ethylene Oxide) Enables Advanced Nanoscale Delivery Modalities and Regulated PH-Dependent Cellular Membrane Activity. Heliyon 2016, 2, e00102. [Google Scholar] [CrossRef]
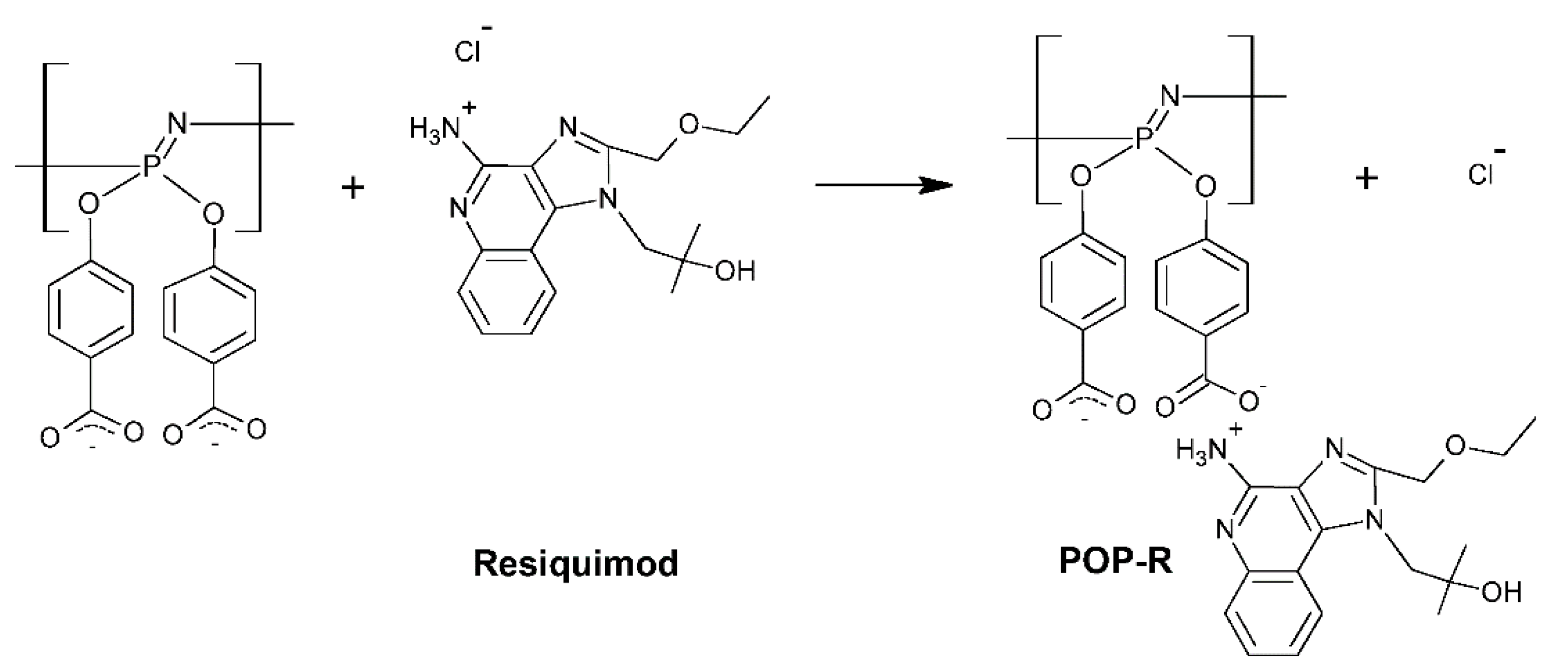
- Andrianov, A.K.; Marin, A.; Wang, R.; Karauzum, H.; Chowdhury, A.; Agnihotri, P.; Yunus, A.S.; Mariuzza, R.A.; Fuerst, T.R. Supramolecular Assembly of Toll-like Receptor 7/8 Agonist into Multimeric Water-Soluble Constructs Enables Superior Immune Stimulation in Vitro and in Vivo. ACS Appl. Bio Mater. 2020, 3, 3187–3195. [Google Scholar] [CrossRef]
- Sadat, S.M.A.; Snider, M.; Garg, R.; Brownlie, R.; van Drunen Littel-van den Hurk, S. Local Innate Responses and Protective Immunity after Intradermal Immunization with Bovine Viral Diarrhea Virus E2 Protein Formulated with a Combination Adjuvant in Cattle. Vaccine 2017, 35, 3466–3473. [Google Scholar] [CrossRef]
- Magiri, R.; Lai, K.; Chaffey, A.; Zhou, Y.; Pyo, H.M.; Gerdts, V.; Wilson, H.L.; Mutwiri, G. Intradermal Immunization with Inactivated Swine Influenza Virus and Adjuvant Polydi(Sodium Carboxylatoethylphenoxy)Phosphazene (PCEP) Induced Humoral and Cell-Mediated Immunity and Reduced Lung Viral Titres in Pigs. Vaccine 2018, 36, 1606–1613. [Google Scholar] [CrossRef]
- Magiri, R.; Lai, K.; Huang, Y.; Mutwiri, G.; Wilson, H.L. Innate Immune Response Profiles in Pigs Injected with Vaccine Adjuvants Polydi(Sodium Carboxylatoethylphenoxy)Phosphazene (PCEP) and Emulsigen. Vet. Immunol. Immunopathol. 2019, 209, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Azzam, T.; Rouiller, I.; Eisenberg, A. “Breathing” Vesicles. J. Am. Chem. Soc. 2009, 131, 10557–10566. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Feijen, J. Polymersomes for Drug Delivery: Design, Formation and Characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef]
- Ranary, S.; Hoffman, A.S.; Stayton, P.S. Antigen Delivery with Poly(Propylacrylic Acid) Conjugation Enhances MHC-1 Presentation and T-Cell Activation. Bioconjug. Chem. 2009, 20, 241–248. [Google Scholar] [CrossRef]
- Gao, M.; Peng, Y.; Jiang, L.; Qiu, L. Effective Intracellular Delivery and Th1 Immune Response Induced by Ovalbumin Loaded in PH-Responsive Polyphosphazene Polymersomes. Nanomedicine 2018, 14, 1609–1618. [Google Scholar] [CrossRef]
- Garg, R.; Brownlie, R.; Latimer, L.; Gerdts, V.; Potter, A.; van Drunen Littel-van den Hurk, S. A Chimeric Glycoprotein Formulated with a Combination Adjuvant Induces Protective Immunity against Both Human Respiratory Syncytial Virus and Parainfluenza Virus Type 3. Antivir. Res. 2018, 158, 78–87. [Google Scholar] [CrossRef]
- Valencia, S.M.; Zacharia, A.; Marin, A.; Matthews, R.L.; Wu, C.K.; Myers, B.; Sanders, C.; Difilippantonio, S.; Kirnbauer, R.; Roden, R.B.; et al. Improvement of RG1-VLP Vaccine Performance in BALB/c Mice by Substitution of Alhydrogel with the next Generation Polyphosphazene Adjuvant PCEP. Hum. Vaccin. Immunother. 2021, 17, 2748–2761. [Google Scholar] [CrossRef]
- Marin, A.; Chowdhury, A.; Valencia, S.M.; Zacharia, A.; Kirnbauer, R.; Roden, R.B.S.; Pinto, L.A.; Shoemaker, R.H.; Marshall, J.D.; Andrianov, A.K. Next Generation Polyphosphazene Immunoadjuvant: Synthesis, Self-Assembly and in Vivo Potency with Human Papillomavirus VLPs-Based Vaccine. Nanomedicine 2021, 33, 102359. [Google Scholar] [CrossRef]
- Mehnath, S.; Rajan, M.; Jeyaraj, M. Immunomodulating Polyorganophosphazene-Arginine Layered Liposome Antibiotic Delivery Vehicle against Pulmonary Tuberculosis. J. Drug Deliv. Sci. Technol. 2021, 66, 102856. [Google Scholar] [CrossRef]
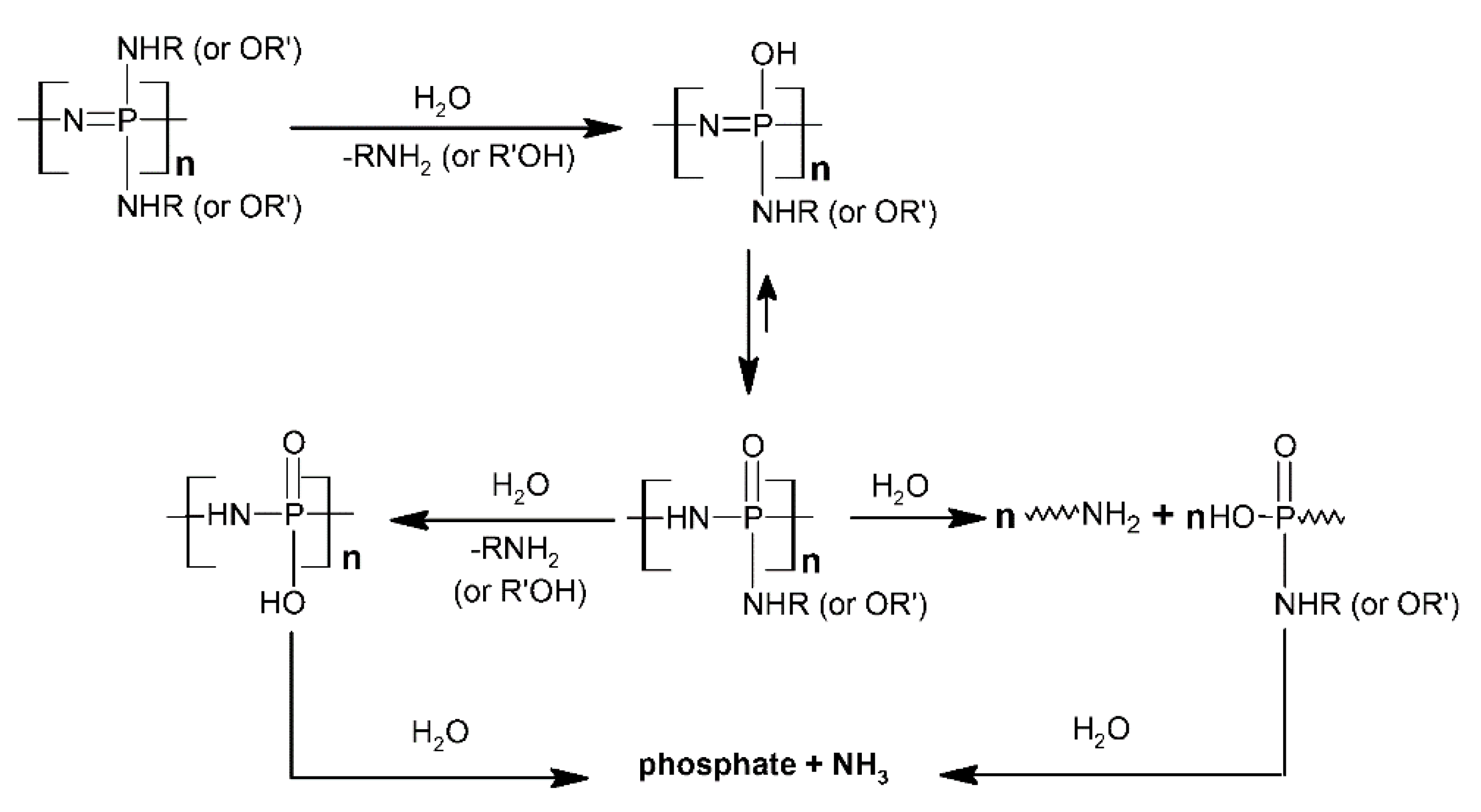
- Allcock, H.R.; Fuller, T.J.; Matsumura, K. Hydrolysis Pathways for Aminophosphazenes. Inorg. Chem. 1982, 21, 515–521. [Google Scholar] [CrossRef]
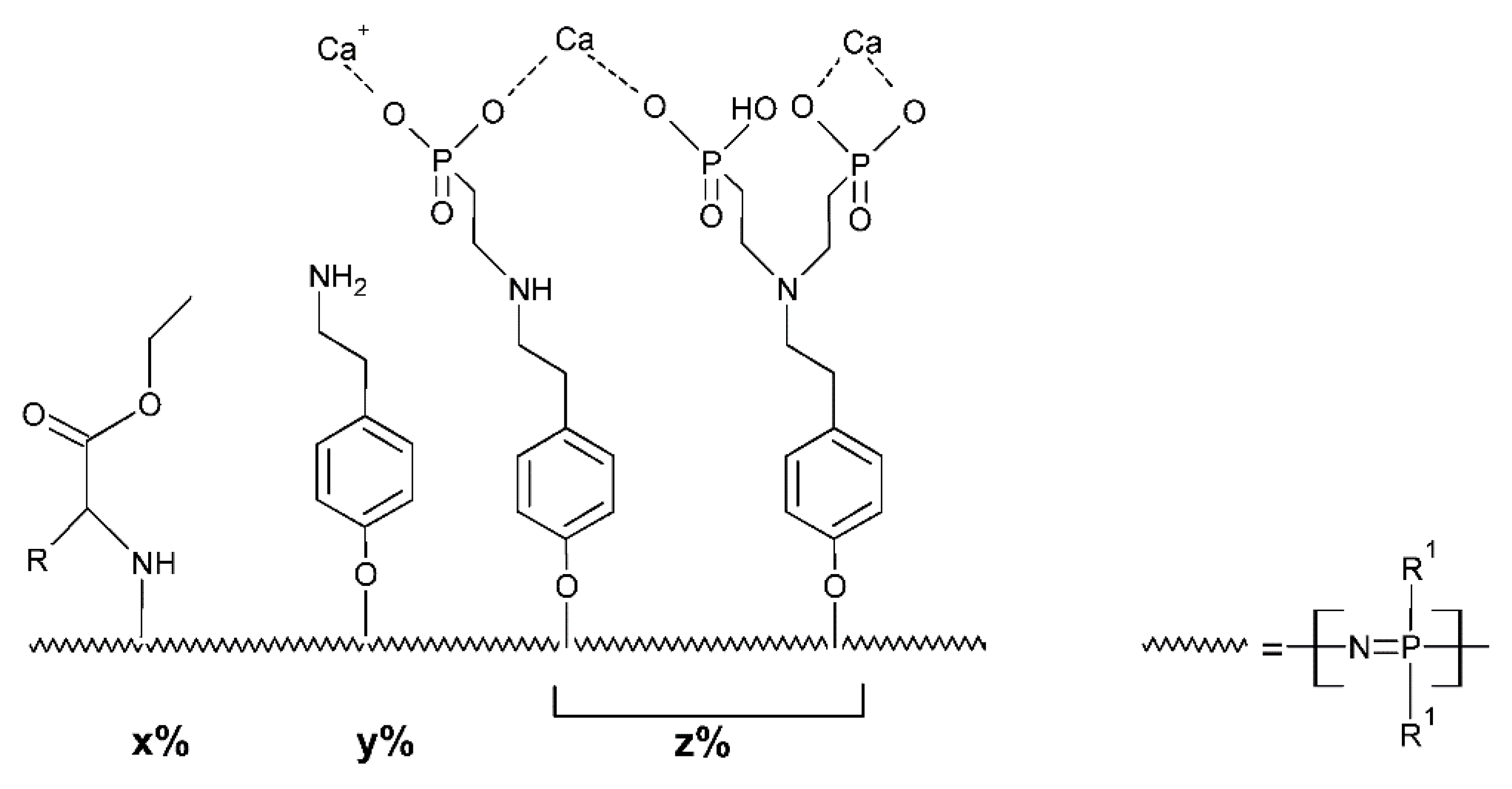
- Deng, M.; Nair, L.S.; Nukavarapu, S.P.; Kumbar, S.G.; Jiang, T.; Krogman, N.R.; Singh, A.; Allcock, H.R.; Laurencin, C.T. Miscibility and in Vitro Osteocompatibility of Biodegradable Blends of Poly[(Ethyl Alanato) (p-Phenyl Phenoxy) Phosphazene] and Poly(Lactic Acid-Glycolic Acid). Biomaterials 2008, 29, 337–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greish, Y.E.; Sturgeon, J.L.; Singh, A.; Krogman, N.R.; Touny, A.H.; Sethuraman, S.; Nair, L.S.; Laurencin, C.T.; Allcock, H.R.; Brown, P.W. Formation and Properties of Composites Comprised of Calcium-Deficient Hydroxyapatites and Ethyl Alanate Polyphosphazenes. J. Mater. Sci. Mater. Med. 2008, 19, 3153–3160. [Google Scholar] [CrossRef] [PubMed]
- Greish, Y.E.; Bender, J.D.; Lakshmi, S.; Brown, P.W.; Allcock, H.R.; Laurencin, C.T. Composite Formation from Hydroxyapatite with Sodium and Potassium Salts of Polyphosphazene. J. Mater. Sci. Mater. Med. 2005, 16, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Greish, Y.E.; Bender, J.D.; Lakshmi, S.; Brown, P.W.; Allcock, H.R.; Laurencin, C.T. Low Temperature Formation of Hydroxyapatite-Poly(Alkyl Oxybenzoate)Phosphazene Composites for Biomedical Applications. Biomaterials 2005, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Escobar Ivirico, J.L.; Li, Z.; Blumenfield, R.H.; Allcock, H.R.; Laurencin, C.T. Synthesis, Physicochemical Analysis, and Side Group Optimization of Degradable Dipeptide-Based Polyphosphazenes as Potential Regenerative Biomaterials. ACS Appl. Polym. Mater. 2019, 1, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- El-Amin, S.F.; Kwon, M.S.; Starnes, T.; Allcock, H.R.; Laurencin, C.T. The Biocompatibility of Biodegradable Glycine Containing Polyphosphazenes: A Comparative Study in Bone. J. Inorg. Organomet. Polym. Mater. 2006, 16, 387–396. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef]
- Duan, S.; Yang, X.; Mao, J.; Qi, B.; Cai, Q.; Shen, H.; Yang, F.; Deng, X.; Wang, S. Osteocompatibility Evaluation of Poly(Glycine Ethyl Ester-Co-Alanine Ethyl Ester)Phosphazene with Honeycomb-Patterned Surface Topography. J. Biomed. Mater. Res. A 2013, 101A, 307–317. [Google Scholar] [CrossRef]
- Sethuraman, S.; Nair, L.S.; El-Amin, S.; Nguyen, M.T.; Singh, A.; Krogman, N.; Greish, Y.E.; Allcock, H.R.; Brown, P.W.; Laurencin, C.T. Mechanical Properties and Osteocompatibility of Novel Biodegradable Alanine Based Polyphosphazenes: Side Group Effects. Acta Biomater. 2010, 6, 1931–1937. [Google Scholar] [CrossRef]
- Deng, M.; Nair, L.S.; Nukavarapu, S.P.; Jiang, T.; Kanner, W.A.; Li, X.; Kumbar, S.G.; Weikel, A.L.; Krogman, N.R.; Allcock, H.R.; et al. Dipeptide-Based Polyphosphazene and Polyester Blends for Bone Tissue Engineering. Biomaterials 2010, 31, 4898–4908. [Google Scholar] [CrossRef]
- Weikel, A.L.; Owens, S.G.; Morozowich, N.L.; Deng, M.; Nair, L.S.; Laurencin, C.T.; Allcock, H.R. Miscibility of Choline-Substituted Polyphosphazenes with PLGA and Osteoblast Activity on Resulting Blends. Biomaterials 2010, 31, 8507–8515. [Google Scholar] [CrossRef]
- Deng, M.; Nair, L.S.; Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Allcock, H.R.; Laurencin, C.T. Biomimetic, Bioactive Etheric Polyphosphazene-Poly(Lactide-Co-Glycolide) Blends for Bone Tissue Engineering. J. Biomed. Mater. Res. A 2010, 92A, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.B.; Koh, J.T.; Song, S.C. Tuning Physical Properties and BMP-2 Release Rates of Injectable Hydrogel Systems for an Optimal Bone Regeneration Effect. Biomaterials 2017, 122, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.B.; Chang, H.I.; Choi, H.; Koh, J.T.; Yun, K.D.; Lee, J.Y.; Song, S.C. New Approach for Vertical Bone Regeneration Using in Situ Gelling and Sustained BMP-2 Releasing Poly(Phosphazene) Hydrogel System on Peri-Implant Site with Critical Defect in a Canine Model. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, L.; Hu, X.; Huang, Y.; Cai, Q.; Ao, Y.; Yang Huang, X.Z.; Yang, L.; Huang, Y.; Cai, Q.; et al. Molecular Mechanism Study on Effect of Biodegradable Amino Acid Ester–Substituted Polyphosphazenes in Stimulating Osteogenic Differentiation. Macromol. Biosci. 2019, 19, 1800464. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jing, W.; Hu, X.; Huang, Z.; Cai, Q.; Ao, Y.; Yang, X. Time-Dependent Effect of Electrical Stimulation on Osteogenic Differentiation of Bone Mesenchymal Stromal Cells Cultured on Conductive Nanofibers. J. Biomed. Mater. Res. A 2017, 105, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yan, L.; Xie, C.; Li, P.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Wang, K.; Wang, Y.; et al. A Mussel-Inspired Persistent ROS-Scavenging, Electroactive, and Osteoinductive Scaffold Based on Electrochemical-Driven In Situ Nanoassembly. Small 2019, 15, 1805440. [Google Scholar] [CrossRef]
- Nichol, J.L.; Morozowich, N.L.; Allcock, H.R. Biodegradable Alanine and Phenylalanine Alkyl Ester Polyphosphazenes as Potential Ligament and Tendon Tissue Scaffolds. Polym. Chem. 2013, 4, 600–606. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef]
- Huang, Y.; Jing, W.; Li, Y.; Cai, Q.; Yang, X. Composites Made of Polyorganophosphazene and Carbon Nanotube Up-Regulating Osteogenic Activity of BMSCs under Electrical Stimulation. Colloids Surf. B Biointerfaces 2021, 204, 111785. [Google Scholar] [CrossRef]
- Gholivand, K.; Alavinasab Ardebili, S.A.; Mohammadpour, M.; Eshaghi Malekshah, R.; Hasannia, S.; Onagh, B. Preparation and Examination of a Scaffold Based on Hydroxylated Polyphosphazene for Tissue Engineering: In Vitro and in Vivo Studies. J. Appl. Polym. Sci. 2022, 139, 52179. [Google Scholar] [CrossRef]
- Sobhani, A.; Rafienia, M.; Ahmadian, M.; Naimi-Jamal, M.R. Fabrication and Characterization of Polyphosphazene/Calcium Phosphate Scaffolds Containing Chitosan Microspheres for Sustained Release of Bone Morphogenetic Protein 2 in Bone Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Duan, S.; Shan, D.; Wu, Z.; Cai, Q.; Yang, X. Electrospun Biodegradable Polyorganophosphazene Fibrous Matrix with Poly(Dopamine) Coating for Bone Regeneration. J. Biomed. Mater. Res. A 2014, 102, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Ogueri, K.S.; McClinton, A.; Kan, H.M.; Ude, C.C.; Barajaa, M.A.; Allcock, H.R.; Laurencin, C.T. In Vivo Evaluation of the Regenerative Capability of Glycylglycine Ethyl Ester-Substituted Polyphosphazene and Poly(Lactic-Co-Glycolic Acid) Blends: A Rabbit Critical-Sized Bone Defect Model. ACS Biomater. Sci. Eng. 2021, 7, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Du, Z.; Zheng, T.; Jing, W.; Liu, H.; Liu, X.; Mao, J.; Zhang, X.; Cai, Q.; Chen, D.; et al. Antibacterial, Conductive, and Osteocompatible Polyorganophosphazene Microscaffolds for the Repair of Infectious Calvarial Defect. J. Biomed. Mater. Res. A 2021, 109, 2580–2596. [Google Scholar] [CrossRef] [PubMed]
- Heyde, M.; Moens, M.; van Vaeck, L.; Shakesheff, K.M.; Davies, M.C.; Schacht, E.H. Synthesis and Characterization of Novel Poly[(Organo)Phosphazenes] with Cell-Adhesive Side Groups. Biomacromolecules 2007, 8, 1436–1445. [Google Scholar] [CrossRef]
- Carampin, P.; Conconi, M.T.; Lora, S.; Menti, A.M.; Baiguera, S.; Bellini, S.; Grandi, C.; Pamigotto, P.P. Electrospun Polyphosphazene Nanofibers for in Vitro Rat Endothelial Cells Proliferation. J. Biomed. Mater. Res. A 2007, 80, 661–668. [Google Scholar] [CrossRef]
- Rothemund, S.; Aigner, T.B.; Iturmendi, A.; Rigau, M.; Husár, B.; Hildner, F.; Oberbauer, E.; Prambauer, M.; Olawale, G.; Forstner, R.; et al. Degradable Glycine-Based Photo-Polymerizable Polyphosphazenes for Use as Scaffolds for Tissue Regeneration. Macromol. Biosci. 2015, 15, 351–363. [Google Scholar] [CrossRef]
- Peach, M.S.; Kumbar, S.G.; James, R.; Toti, U.S.; Balasubramaniam, D.; Deng, M.; Ulery, B.; Mazzocca, A.D.; McCarthy, M.B.; Morozowich, N.L.; et al. Design and Optimization of Polyphosphazene Functionalized Fiber Matrices for Soft Tissue Regeneration. J. Biomed. Nanotechnol. 2012, 8, 107–124. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Morris, C.D.; Pierre-Jacques, H.; Schwartz, E.R.; Keaton, A.R.; Zou, L. Osteoblast Culture on Bioerodible Polymers: Studies of Initial Cell Adhesion and Spread. Polym. Adv. Technol. 1992, 3, 359–364. [Google Scholar] [CrossRef]
- Aldini, N.N.; Fini, M.; Rocca, M.; Martini, L.; Giardino, R.; Caliceti, P.; Veronese, F.M.; Lora, S.; Maltarello, M.C. Peripheral Nerve Reconstruction with Bioabsorbable Polyphosphazene Conduits. J. Bioact. Compat. Polym. 2016, 12, 3–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y.; Li, S.; Feng, T. The Synthesis and Characterization of a Novel Biodegradable and Electroactive Polyphosphazene for Nerve Regeneration. Mater. Sci. Eng. C 2010, 30, 160–166. [Google Scholar] [CrossRef]
- Conconi, M.T.; Lora, S.; Baiguera, S.; Boscolo, E.; Polin, M.; Scienza, R.; Rebuffat, P.; Parnigotto, P.P.; Nussdorfer, G.G. In Vitro Culture of Rat Neuromicrovascular Endothelial Cells on Polymeric Scaffolds. J. Biomed. Mater. Res. A 2004, 71, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Steely, L.; Singh, A.; Hindenlang, M. Hydrophobic and Superhydrophobic Polyphosphazenes. J. Adhes. Sci. Technol. 2012, 23, 435–445. [Google Scholar] [CrossRef]
- Tang, M.; Chen, C.; Zhu, J.; Allcock, H.R.; Siedlecki, C.A.; Xu, L.C. Inhibition of Bacterial Adhesion and Biofilm Formation by a Textured Fluorinated Alkoxyphosphazene Surface. Bioact. Mater. 2021, 6, 447–459. [Google Scholar] [CrossRef]
- Xu, L.C.; Chen, C.; Zhu, J.; Tang, M.; Chen, A.; Allcock, H.R.; Siedlecki, C.A. New Cross-Linkable Poly[Bis(Octafluoropentoxy) Phosphazene] Biomaterials: Synthesis, Surface Characterization, Bacterial Adhesion, and Plasma Coagulation Responses. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3250–3260. [Google Scholar] [CrossRef]
- Marin, A.; Brito, J.; Sukhishvili, S.A.; Andrianov, A.K. Cationic Fluoropolyphosphazenes: Synthesis and Assembly with Heparin as a Pathway to Hemocompatible Nanocoatings. ACS Appl. Bio Mater. 2022, 5, 313–321. [Google Scholar] [CrossRef]
- Xu, L.C.; Li, Z.; Tian, Z.; Chen, C.; Allcock, H.R.; Siedlecki, C.A. A New Textured Polyphosphazene Biomaterial with Improved Blood Coagulation and Microbial Infection Responses. Acta Biomater. 2018, 67, 87–98. [Google Scholar] [CrossRef]
- Henn, C.; Satzl, S.; Christoph, P.; Kurz, P.; Radeleff, B.; Stampfl, U.; Stampfl, S.; Berger, I.; Richter, G.M. Efficacy of a Polyphosphazene Nanocoat in Reducing Thrombogenicity, in-Stent Stenosis, and Inflammatory Response in Porcine Renal and Iliac Artery Stents. J. Vasc. Interv. Radiol. 2008, 19, 427–437. [Google Scholar] [CrossRef]
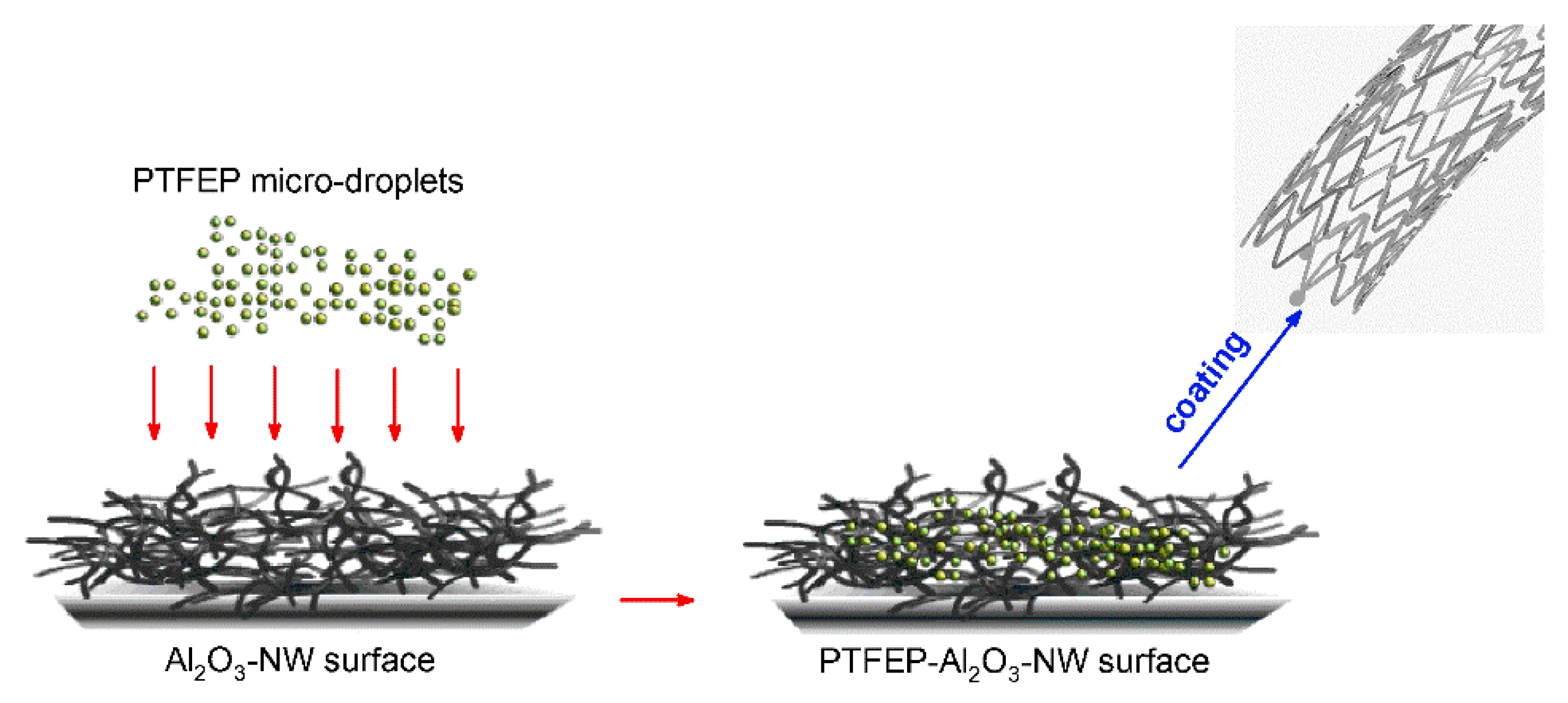
- Haidar, A.; Ali, A.A.; Veziroglu, S.; Fiutowski, J.; Eichler, H.; Müller, I.; Kiefer, K.; Faupel, F.; Bischoff, M.; Veith, M.; et al. PTFEP–Al2O3 Hybrid Nanowires Reducing Thrombosis and Biofouling. Nanoscale Adv. 2019, 1, 4659–4664. [Google Scholar] [CrossRef]
- Allcock, H.R.; Steely, L.B.; Singh, A. Hydrophobic and Superhydrophobic Surfaces from Polyphosphazenes. Polym. Int. 2006, 55, 621–625. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casella, G.; Carlotto, S.; Lanero, F.; Mozzon, M.; Sgarbossa, P.; Bertani, R. Cyclo- and Polyphosphazenes for Biomedical Applications. Molecules 2022, 27, 8117. https://doi.org/10.3390/molecules27238117
Casella G, Carlotto S, Lanero F, Mozzon M, Sgarbossa P, Bertani R. Cyclo- and Polyphosphazenes for Biomedical Applications. Molecules. 2022; 27(23):8117. https://doi.org/10.3390/molecules27238117
Chicago/Turabian StyleCasella, Girolamo, Silvia Carlotto, Francesco Lanero, Mirto Mozzon, Paolo Sgarbossa, and Roberta Bertani. 2022. "Cyclo- and Polyphosphazenes for Biomedical Applications" Molecules 27, no. 23: 8117. https://doi.org/10.3390/molecules27238117
APA StyleCasella, G., Carlotto, S., Lanero, F., Mozzon, M., Sgarbossa, P., & Bertani, R. (2022). Cyclo- and Polyphosphazenes for Biomedical Applications. Molecules, 27(23), 8117. https://doi.org/10.3390/molecules27238117








