In Situ Polymerization of Linseed Oil-Based Composite Film: Enhancement of Mechanical and Water Barrier Properties by the Incorporation of Cinnamaldehyde and Organoclay
Abstract
1. Introduction
2. Results and Discussion
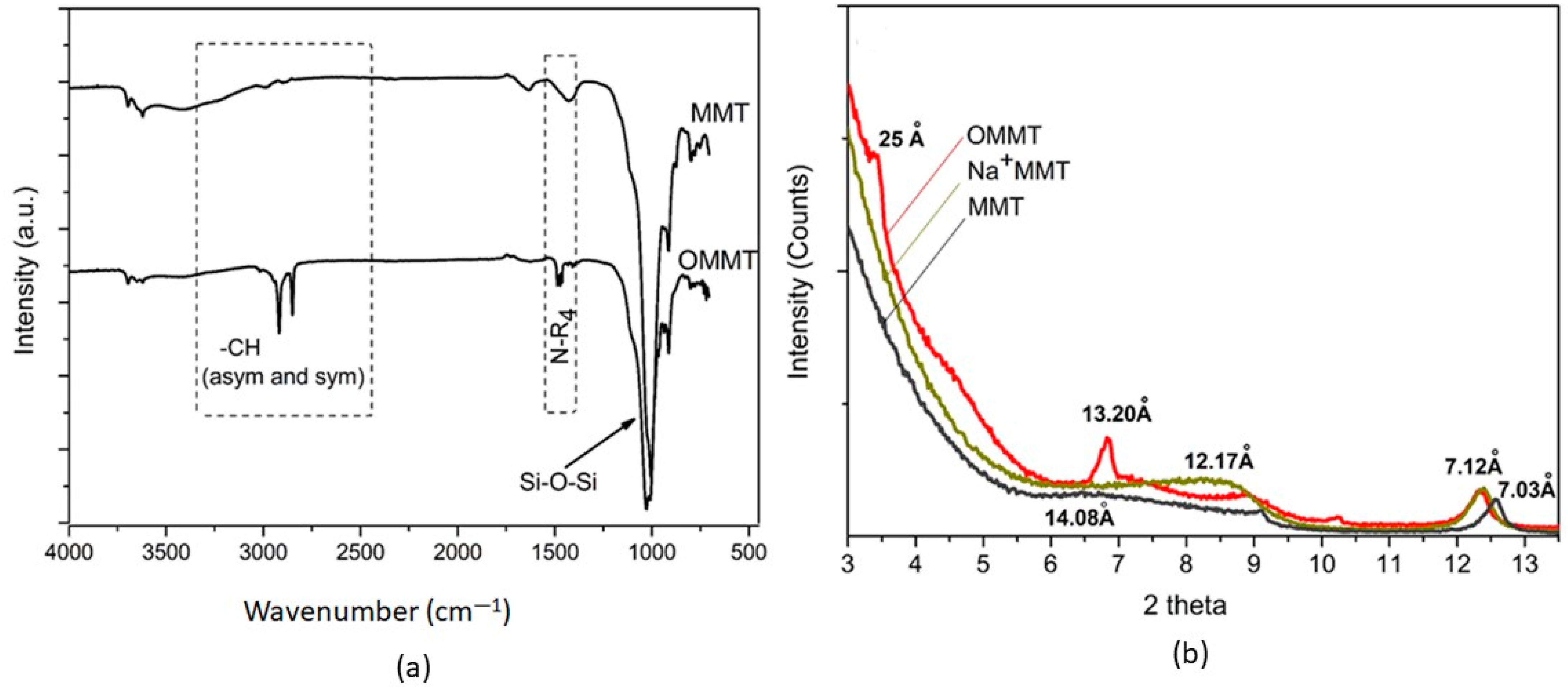
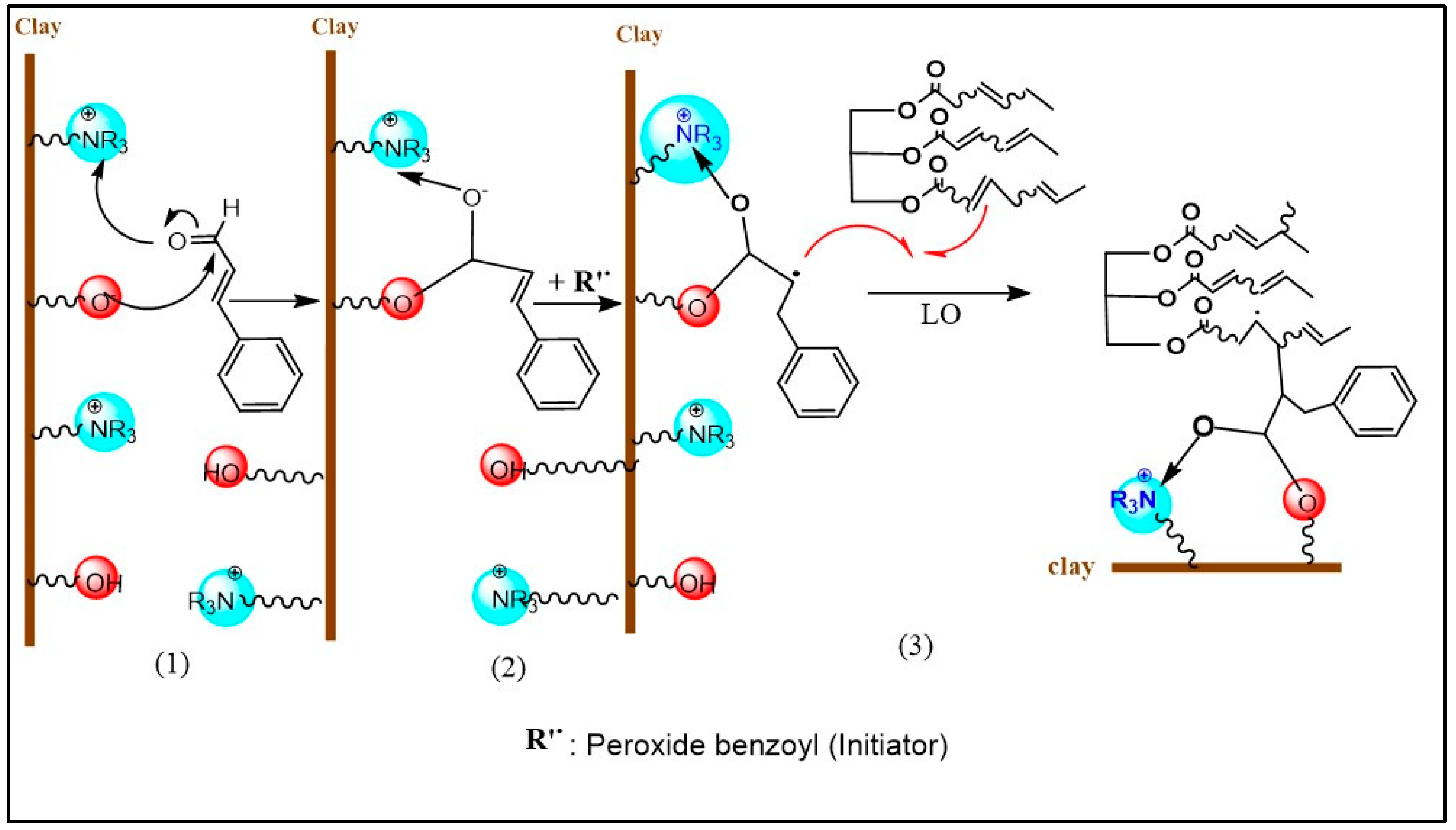
2.1. Clay Modification
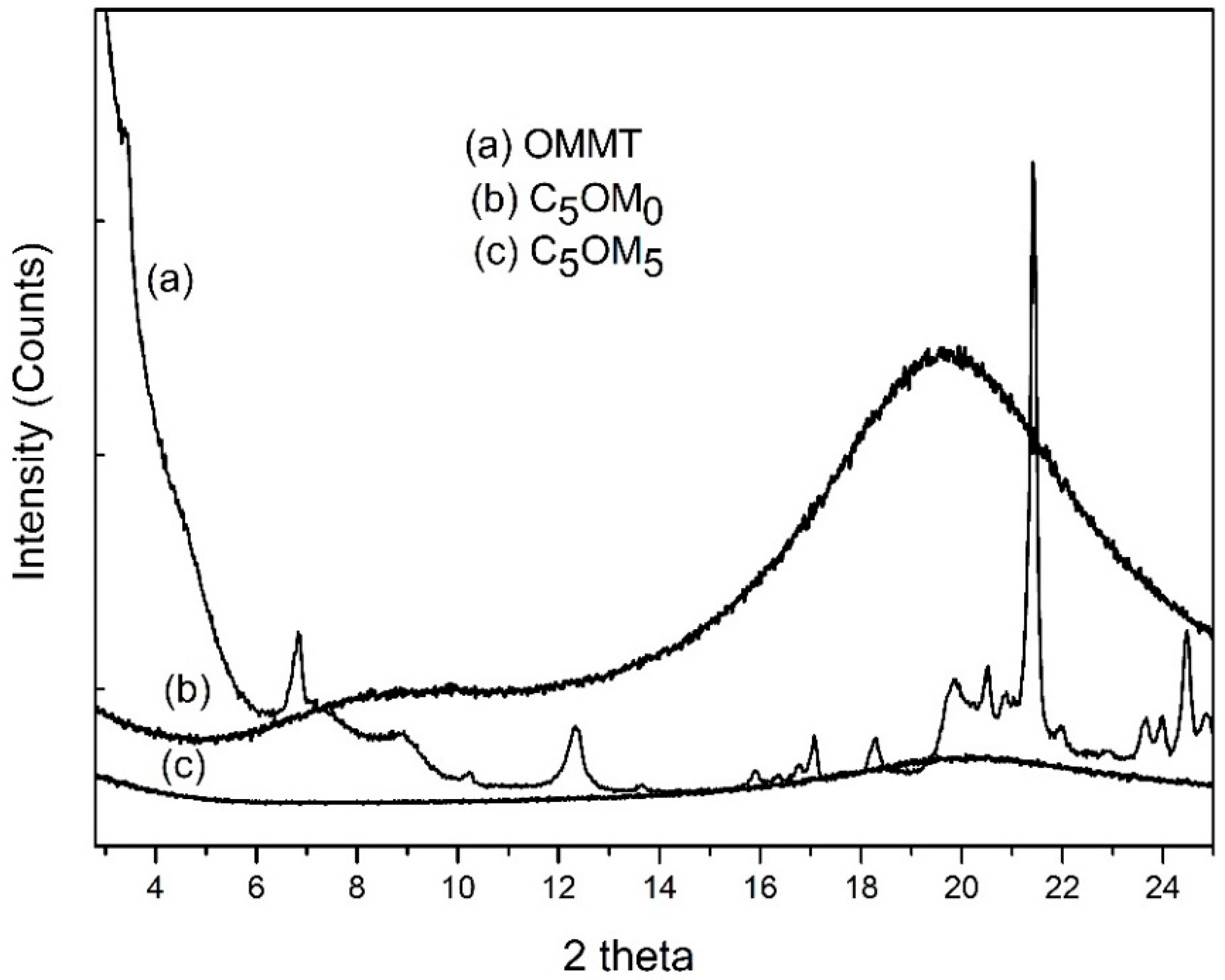
2.2. Film Composites: Morphology, Mineral Composition, Physical States, and Structural Features
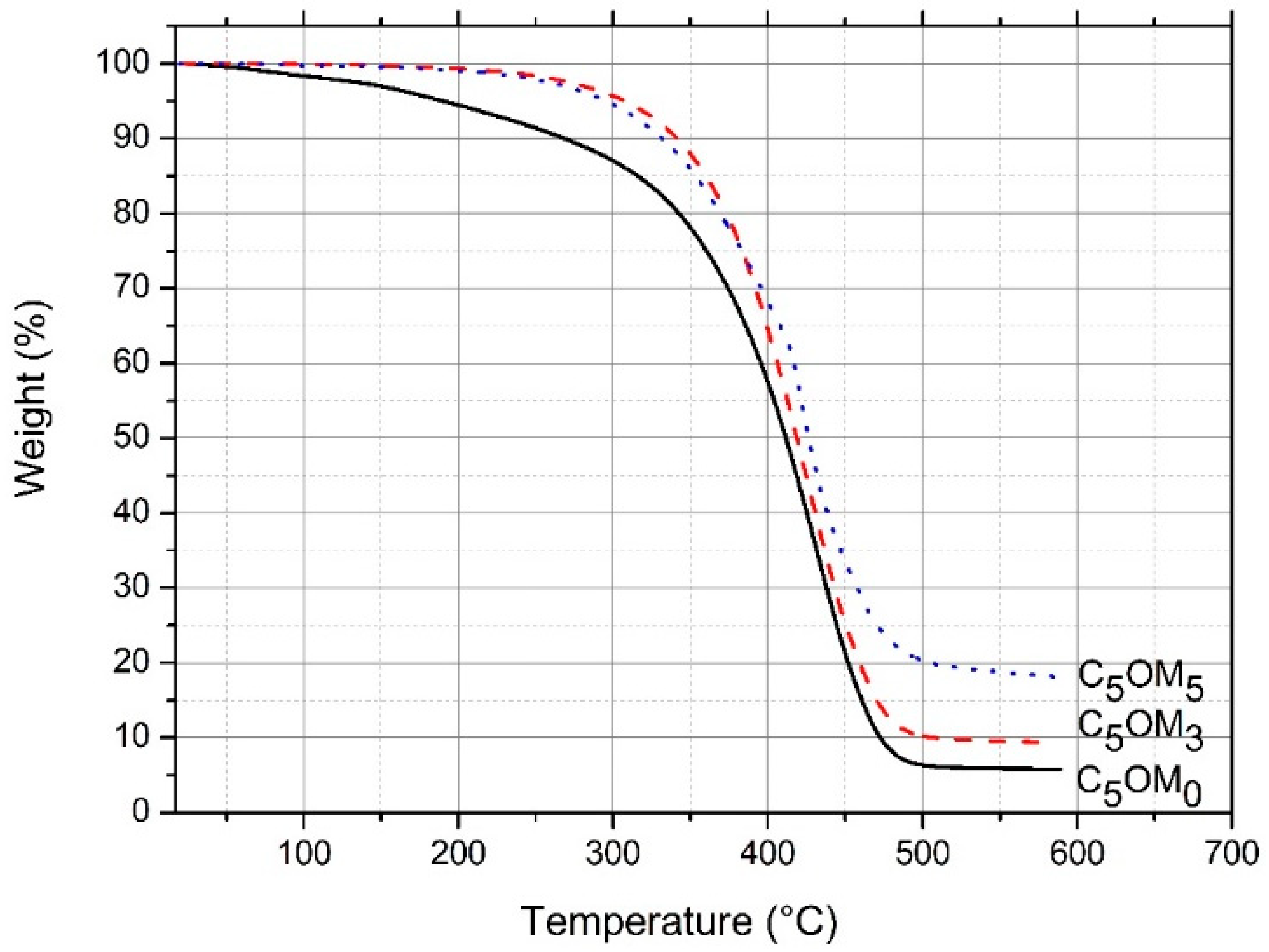
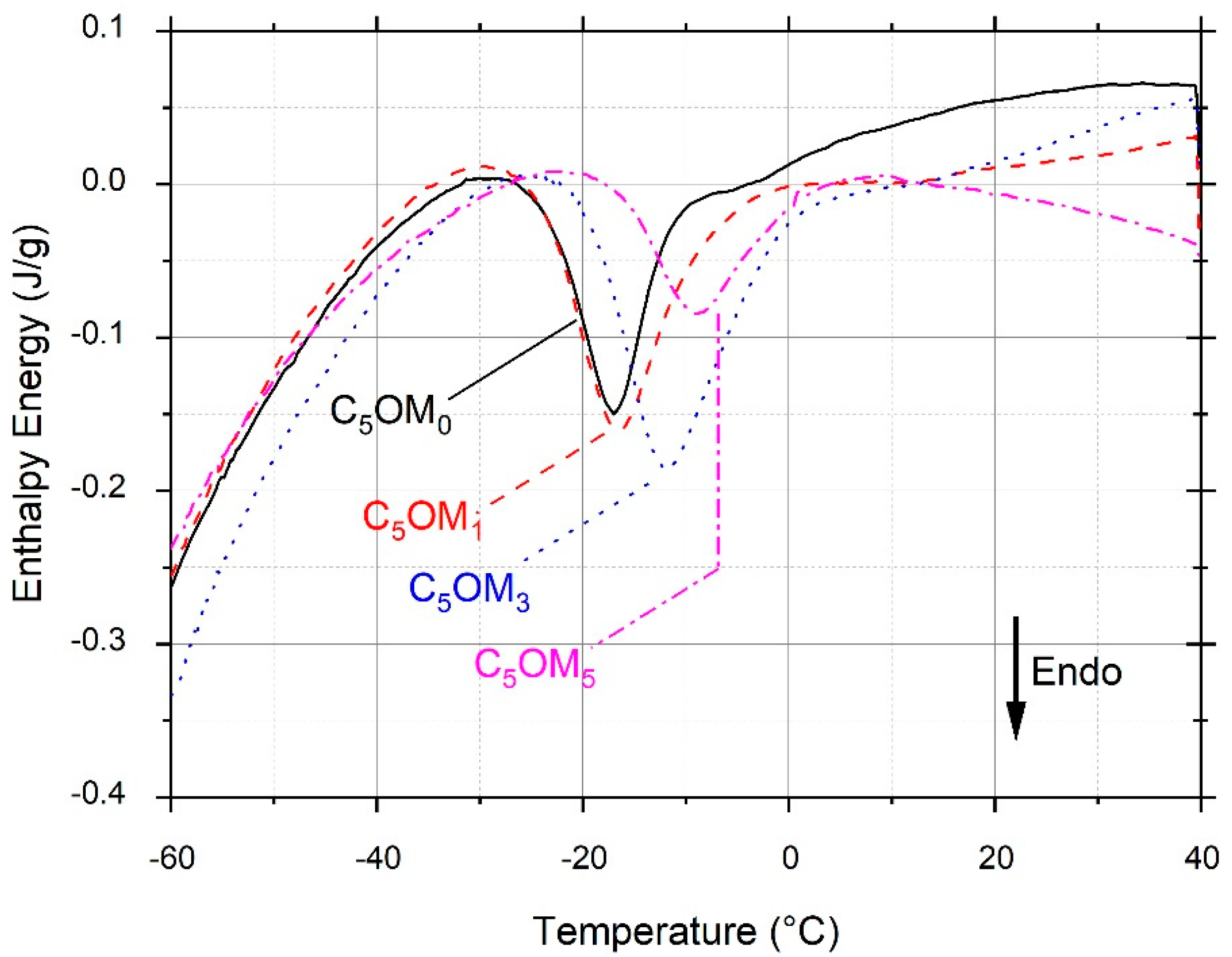
2.2.1. Thermal Analysis (TGA and DSC)
2.2.2. Structural Studies (FTIR)
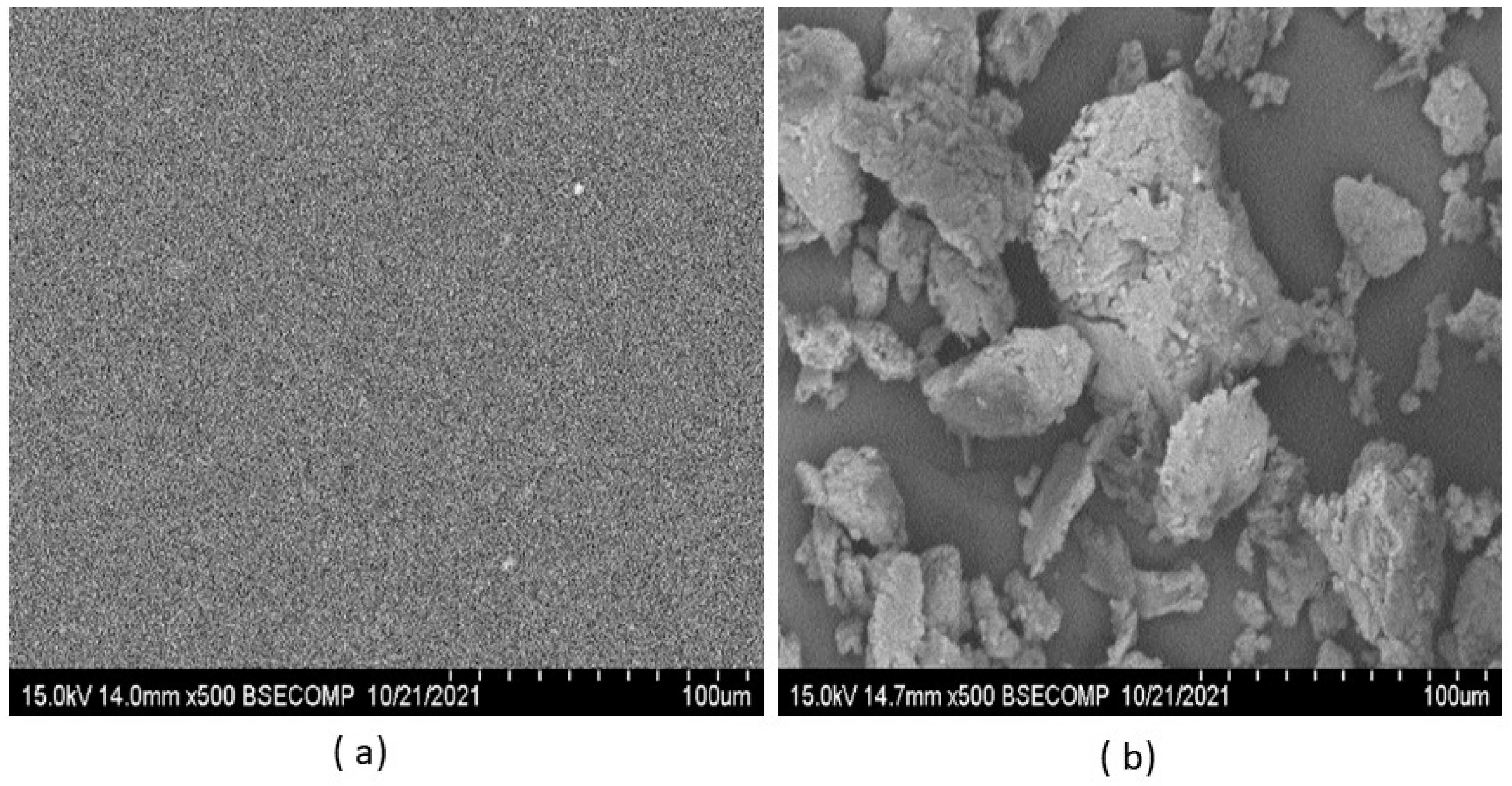
2.2.3. Physical State of the Films and Microscopy
2.3. Film Composites: Functional Properties
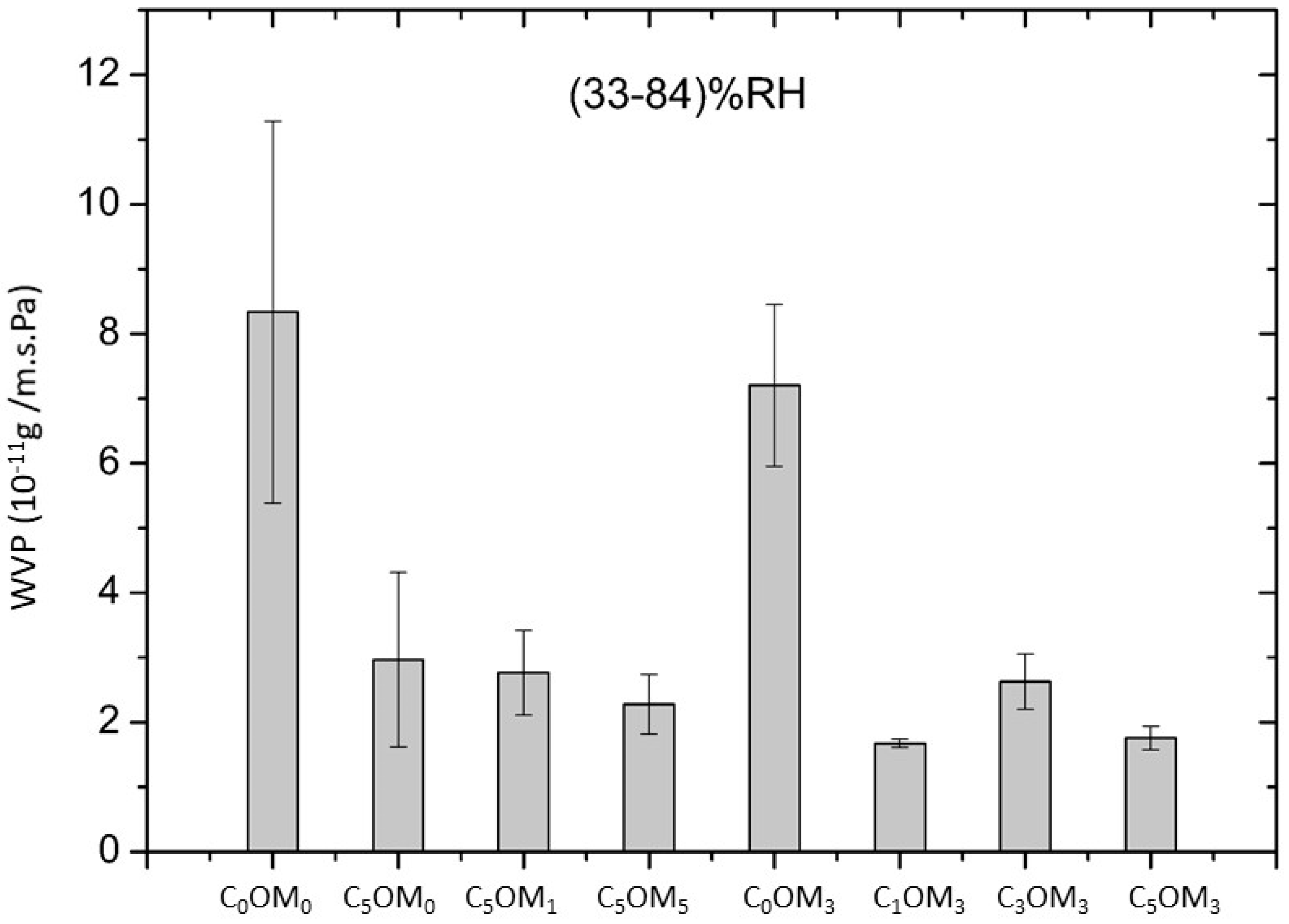
2.3.1. Water Barrier Properties
2.3.2. Mechanical Properties
2.3.3. Surface Properties
2.4. Antioxidant Activity (AA%)
3. Materials and Methods
3.1. Materials and Reagents
3.2. Methods
3.2.1. Extraction of Linseed Oil
3.2.2. Modification of the Montmorillonite (OMMT)
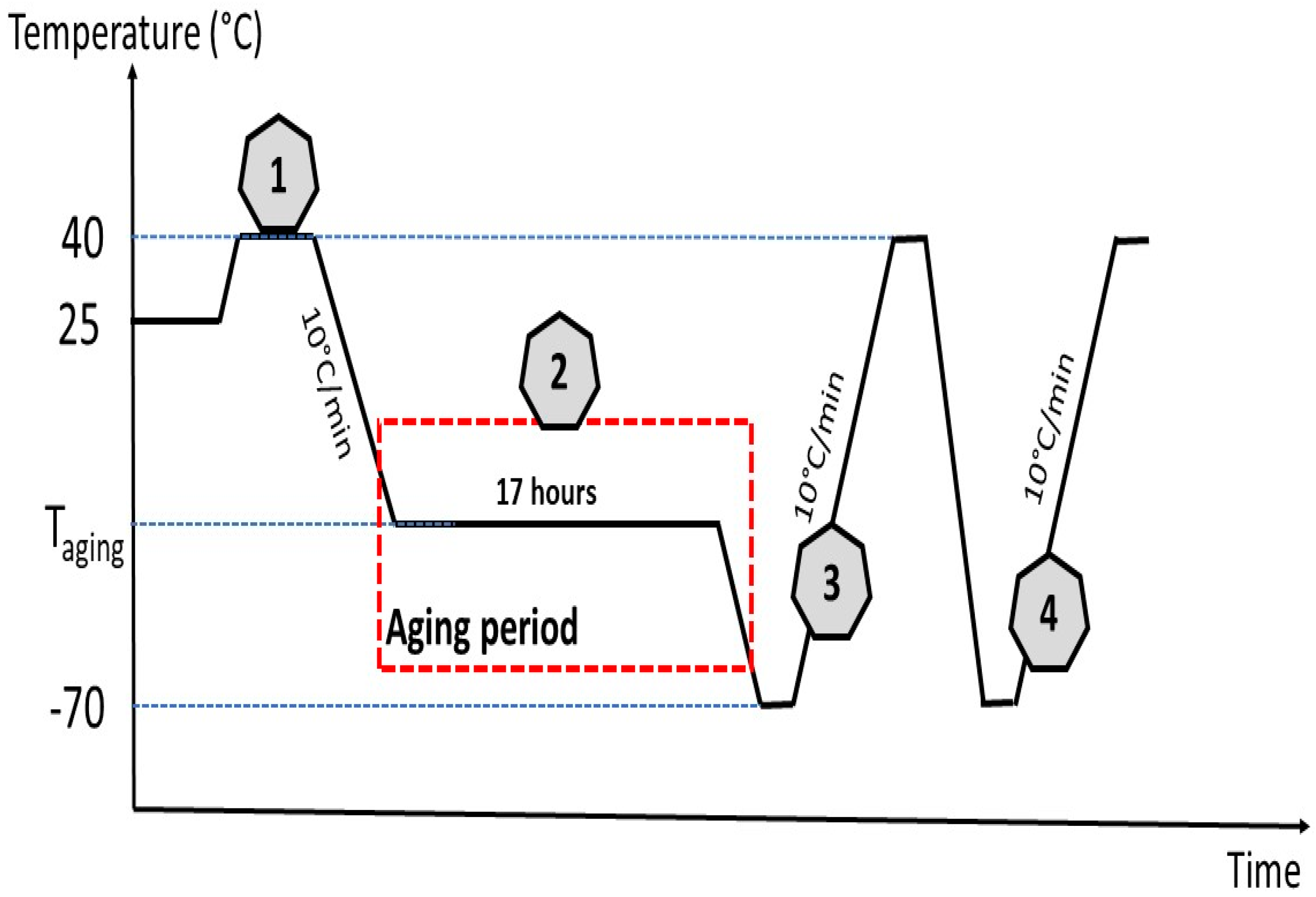
3.2.3. Film Preparation
3.3. Film Characterization
3.3.1. Thickness Measurement of Films
3.3.2. Water Vapor Permeability (WVP)
3.3.3. Characterization of Surface Properties of Films
3.3.4. Mechanical Properties
3.3.5. X-ray Diffraction (XRD)
3.3.6. Scanning Electron Microscopy (SEM)
3.3.7. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.8. Differential Scanning Calorimetry (DSC)
3.3.9. Thermogravimetric Analysis (TGA)
3.4. Antioxidant Efficacy Testing (DPPH Test)
DPPH·purple at 515 nm + ArOH → DPPH yellow +ArO· + SET
3.5. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer Films Based on Chitosan/Potato Protein/Linseed Oil/ZnO NPs to Maintain the Storage Quality of Raw Meat. Food Chem. 2020, 332, 127–375. [Google Scholar] [CrossRef] [PubMed]
- Monroe, K.; Kirk, T.; Hull, V.; Biswas, E.; Murawski, A.; Quirino, R.L. Vegetable Oil-Based Polymeric Materials: Synthesis, Properties, and Applications. Encycl. Renew. Sustain. Mater. 2020, 5, 295–302. [Google Scholar]
- De Pilli, T. Development of a Vegetable Oil and Egg Proteins Edible Film to Replace Preservatives and Primary Packaging of Sweet Baked Goods. Food Control 2020, 114, 107–273. [Google Scholar] [CrossRef]
- Yu, C.; Ke, Y.; Deng, Q.; Lu, S.; Ji, J.; Hu, X.; Zhao, Y. Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance. Appl. Sci. 2018, 8, 964. [Google Scholar] [CrossRef]
- Keleş, E.; Hazer, B. Autooxidized Polyunsaturated Oils/Oily Acids: Post-It Applications and Reactions with Fe(III) and Adhesion Properties. Macromol. Symp. 2008, 269, 154–160. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable Oil-Based Polymeric Materials: Synthesis, Properties, and Applications. Green Chem. 2010, 12, 1893. [Google Scholar] [CrossRef]
- Andjelkovic, D.D.; Valverde, M.; Henna, P.; Li, F.; Larock, R.C. Novel Thermosets Prepared by Cationic Copolymerization of Various Vegetable Oils—Synthesis and Their Structure–Property Relationships. Polymer 2005, 46, 9674–9685. [Google Scholar] [CrossRef]
- Capiel, G.; Marcovich, N.E.; Mosiewicki, M.A. Shape Memory Polymer Networks Based on Methacrylated Fatty Acids. Eur. Polym. J. 2019, 116, 321–329. [Google Scholar] [CrossRef]
- Sharma, V.; Banait, J.S.; Larock, R.C.; Kundu, P.P. Synthesis and Characterization of Styrene-Co-Divinylbenzene-Graft-Linseed Oil by Free Radical Polymerization. Express Polym. Lett. 2008, 12, 265–276. [Google Scholar] [CrossRef]
- Li, F.; Larock, R.C. Synthesis, Structure and Properties of New Tung Oil−Styrene−Divinylbenzene Copolymers Prepared by Thermal Polymerization. Biomacromolecules 2003, 4, 1018–1025. [Google Scholar] [CrossRef]
- Cao, T.L.; Song, K.B. Active Gum Karaya/Cloisite Na+ Nanocomposite Films Containing Cinnamaldehyde. Food Hydrocoll. 2019, 89, 453–460. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Trans-Cinnamaldehyde-Doped Quadripartite Biopolymeric Films: Rheological Behavior of Film-Forming Solutions and Biofunctional Performance of Films. Food Hydrocoll. 2021, 112, 106339. [Google Scholar] [CrossRef]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Study on Carvacrol and Cinnamaldehyde Polymeric Films: Mechanical Properties, Release Kinetics and Antibacterial and Antibiofilm Activities. Appl. Microbiol. Biotechnol. 2012, 96, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Satoh, K.; Kamigaito, M. Controlled Radical Copolymerization of Cinnamic Derivatives as Renewable Vinyl Monomers with both Acrylic and Styrenic Substituents: Reactivity, Regioselectivity, Properties, and Functions. Biomacromolecules 2019, 20, 192–203. [Google Scholar] [CrossRef]
- Calambas, H.L.; Fonseca, A.; Adames, D.; Aguirre-Loredo, Y.; Caicedo, C. Physical-Mechanical Behavior and Water-Barrier Properties of Biopolymers-Clay Nanocomposites. Molecules 2021, 26, 6734. [Google Scholar] [CrossRef]
- Zhang, K.; Park, B.-J.; Fang, F.F.; Choi, H.J. Sonochemical Preparation of Polymer Nanocomposites. Molecules 2009, 14, 2095–2110. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Novel Biobased Nanocomposites from Soybean Oil and Functionalized Organoclay. Biomacromolecules 2006, 7, 2692–2700. [Google Scholar] [CrossRef]
- Kumar, S.; Mohanty, S.; Nayak, S.K. Nanocomposites of Epoxidized Soybean Oil (ESO)-Based Epoxy (DGEBA) Blends and Clay Platelets: Cured with Methylhexahydrophthalic Anhydride Crosslinker. J. Macromol. Sci. Part A 2020, 57, 654–662. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Avgeropoulos, A.; Proestos, C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-Lactic Acid Antioxidant Active Packaging Films. Molecules 2022, 27, 1231. [Google Scholar] [CrossRef]
- Sharma, V.; Banait, J.S.; Kundu, P.P. Spectroscopic Characterization of Linseed Oil Based Polymer Nano-Composites. Polym. Test. 2008, 27, 916–923. [Google Scholar] [CrossRef]
- Salam, H.; Dong, Y.; Davies, I. Development of Biobased Polymer/Clay Nanocomposites. Fill. Reinf. Adv. Nanocomposites 2015, 10, 101–132. [Google Scholar]
- Uyama, H.; Kuwabara, M.; Tsujimoto, T.; Nakano, M.; Usuki, A.; Kobayashi, S. Green Nanocomposites from Renewable Resources: Plant Oil−Clay Hybrid Materials. Chem. Mater. 2003, 15, 2492–2494. [Google Scholar] [CrossRef]
- Miyagawa, H.; Misra, M.; Drzal, L.T.; Mohanty, A.K. Novel Biobased Nanocomposites from Functionalized Vegetable Oil and Organically-Modified Layered Silicate Clay. Polymer 2005, 46, 445–453. [Google Scholar] [CrossRef]
- Bashar, M.; Mertiny, P.; Sundararaj, U. Effect of Nanocomposite Structures on Fracture Behavior of Epoxy-Clay Nanocomposites Prepared by Different Dispersion Methods. J. Nanomater. 2014, 2014, 70. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Bio-Based Nanocomposites from Corn Oil and Functionalized Organoclay Prepared by Cationic Polymerization. Macromol. Mater. Eng. 2007, 292, 863–872. [Google Scholar] [CrossRef]
- Nejib, A.; Joelle, D.; Fadhila, A.; Sophie, G. Adsorption of Anionic Dye on Natural and Organophilic Clays: Effect of Textile Dyeing Additives. Desalination Water Treat. 2014, 54, 1754–1769. [Google Scholar] [CrossRef]
- Msadok, I.; Hamdi, N.; Gammoudi, S.; Rodríguez, M.A.; Srasra, E. Effect of Cationic Surfactant HDPy+ on the Acidity and Hydrophilicity of Tunisian Clay. Mater. Chem. Phys. 2019, 225, 279–283. [Google Scholar] [CrossRef]
- Kriaa, A.; Hamdi, N.; Srasra, E. Acid-Base Chemistry of Montmorillonitic and Beidellitic-Montmorillonitic Smectite. Russ. J. Electrochem. 2007, 43, 167–177. [Google Scholar] [CrossRef]
- Sharma, V.; Banait, J.S.; Larock, R.C.; Kundu, P.P. Synthesis and Characterization of Linseed Oil-Based Nanocomposites. Polym. Compos. 2009, 31, 630–637. [Google Scholar] [CrossRef]
- Albayrak, Ö.; Şen, S.; Çaylı, G.; Ortaç, B. Bio-Based Polymer Nanocomposites Based on Layered Silicates Having a Reactive and Renewable Intercalant. J. Appl. Polym. Sci. 2013, 130, 2031–2041. [Google Scholar] [CrossRef]
- Gurunathan, T.; Chung, J.S. Physicochemical Properties of Amino–Silane-Terminated Vegetable Oil-Based Waterborne Polyurethane Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 4645–4653. [Google Scholar] [CrossRef]
- Madeleine-Perdrillat, C.; Karbowiak, T.; Debeaufort, F.; Delmotte, L.; Vaulot, C.; Champion, D. Effect of Hydration on Molecular Dynamics and Structure in Chitosan Films. Food Hydrocoll. 2016, 61, 57–65. [Google Scholar] [CrossRef]
- Shekarabi, A.S.; Oromiehie, A.R.; Vaziri, A.; Ardjmand, M.; Safekordi, A.A. Investigation of the Effect of Nanoclay on the Properties of Quince Seed Mucilage Edible Films. Food Sci. Nutr. 2014, 2, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Konwar, U.; Karak, N.; Mandal, M. Mesua ferrea L. Seed Oil Based Highly Thermostable and Biodegradable Polyester/Clay Nanocomposites. Polym. Degrad. Stab. 2009, 94, 2221–2230. [Google Scholar] [CrossRef]
- Siti Nur E’zzati1, M.A.; Siti Munirah Salimah, A.B.; Ali, F.B.; Manshor, M.R. Impregnation of Cinnamon Essential Oil into Plasticised Polylactic Acid Biocomposite Film for Active Food Packaging. J. Packag. Tech. Res. 2017, 1, 149–156. [Google Scholar]
- Han, Y.; Yu, M.; Wang, L. Physical and Antimicrobial Properties of Sodium Alginate/Carboxymethyl Cellulose Films Incorporated with Cinnamon Essential Oil. Food Package Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Srisa, A.; Harnkarnsujarit, N. Antifungal Films from Trans-Cinnamaldehyde Incorporated Poly(Lactic Acid) and Poly(Butylene Adipate-Co-Terephthalate) for Bread Packaging. Food Chem. 2020, 333, 127–537. [Google Scholar] [CrossRef]
- Pirsa, S. Biodegradable Film Based on Pectin/Nano-Clay/Methylene Blue: Structural and Physical Properties and Sensing Ability for Measurement of Vitamin C. Int. J. Biol. Macromol. 2020, 163, 666–675. [Google Scholar] [CrossRef]
- Naidu, D.S.; John, M.J. Effect of Clay Nanofillers on the Mechanical and Water Vapor Permeability Properties of Xylan–Alginate Films. Polymers 2020, 12, 2279. [Google Scholar] [CrossRef]
- De, S.; Pasbakhsh, P.; Goh, K.L.; Chai, S.P.; Ismail, H. Physico-Chemical Characterisation of Chitosan/Halloysite Composite Membranes. Polym. Test. 2013, 32, 265–271. [Google Scholar]
- Ortiz-Zarama, M.A.; Jiménez-Aparicio, A.R.; Solorza-Feria, J. Obtainment and Partial Characterization of Biodegradable Gelatin Films with Tannic Acid, Bentonite and Glycerol. J. Sci. Food Agric. 2016, 96, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of Halloysite Nanoclay on the Physical, Mechanical, and Antioxidant Properties of Chitosan Films Incorporated with Clove Essential Oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Rekik, S.B.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Development and Characterization of Porous Membranes Based on Kaolin/Chitosan Composite. Appl. Clay Sci. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced Physico-Mechanical, Barrier and Antifungal Properties of Soy Protein Isolate Film by Incorporating Both Plant-Sourced Cinnamaldehyde and Facile Synthesized Zinc Oxide Nanosheets. Colloids Surf. B Biointerfaces 2019, 180, 31–38. [Google Scholar] [CrossRef]
- Kanmani, P.; Jong-Whan, R. Physical, Mechanical and Antimicrobial Properties of Gelatin Based Active Nanocomposite Films Containing AgNPs and Nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver Nanoparticle-Loaded Chitosan–Starch Based Films: Fabrication and Evaluation of Tensile, Barrier and Antimicrobial Properties. Mater. Sci. Eng. C 2010, 30, 891–897. [Google Scholar] [CrossRef]
- Cyras, V.P.; Manfredi, L.B.; Ton-That, M.-T.; Vázquez, A. Physical and Mechanical Properties of Thermoplastic Starch/Montmorillonite Nanocomposite Films. Carbohydr. Polym. 2008, 1, 55–63. [Google Scholar] [CrossRef]
- Symoniuk (Popis), E.; Ratusz, K.; Krygier, K. Oxidative Stability and the Chemical Composition of Market Cold-Pressed Linseed Oil: Market Linseed Oil Quality. Eur. J. Lipid Sci. Technol. 2017, 119, 1700062. [Google Scholar]
- Barthet, V.; Klensporf-Pawlik, D.; Przybylski, R. Antioxidant Activity of Flaxseed Meal Components. Can. J. Plant Sci. 2014, 94, 593–602. [Google Scholar] [CrossRef]
- Gaurav, K.; Dipak, K.M. In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against strepto zotocin-induced toxicity in albino rats. Eur. J. Lipid Sci. Tech. 2012, 114, 1237–1245. [Google Scholar]
- Bardaa, S.; Turki, M.; Ben Khedir, S.; Mzid, M.; Rebai, T.; Ayadi, F.; Sahnoun, Z. The Effect of Prickly Pear, Pumpkin, and Linseed Oils on Biological Mediators of Acute Inflammation and Oxidative Stress Markers. BioMed Res. Int. 2020, 2020, 5643465. [Google Scholar] [CrossRef] [PubMed]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [PubMed]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose Acetate/AgNPs-Organoclay and/or Thymol Nano-Biocomposite Films with Combined Antimicrobial/Antioxidant Properties for Active Food Packaging Use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef] [PubMed]
- López-Mata, M.A.; Ruiz-Cruz, S.; de Jesús Ornelas-Paz, J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E.; Silva-Beltrán, N.P.; Cira-Chávez, L.A.; Burruel-Ibarra, S.E. Mechanical, Barrier and Antioxidant Properties of Chitosan Films Incorporating Cinnamaldehyde. J. Polym. Environ. 2018, 26, 452–461. [Google Scholar] [CrossRef]
- ASTM E96/E96M-10; Committee Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 1998.
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Karbowiak, T.; Debeaufort, F.; Voilley, A. Importance of Surface Tension Characterization for Food, Pharmaceutical and Packaging Products: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 391–407. [Google Scholar] [CrossRef] [PubMed]
- BS EN ISO 527-3:1995/Cor 2:2001; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. International Organization for Standardization: London, UK, 2001.
- Benbettaïeb, N.; Tanner, C.; Cayot, P.; Karbowiak, T.; Debeaufort, F. Impact of Functional Properties and Release Kinetics on Antioxidant Activity of Biopolymer Active Films and Coatings. Food Chem. 2018, 242, 369–377. [Google Scholar]
- Ryszard, A.; Ronald, B.P. Functional Food Ingredients from Plants. Adv. Food Nut. Res. 2019, 90, 465–468. [Google Scholar]

| Sample Designation | Elongation at Break (%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Contact Angle (θ. deg) | Surface Energy (mN/m) | Polar Component (mN/m) | Dispersive Component (mN/m) |
|---|---|---|---|---|---|---|---|
| C0OM0 | 21.2 ± 2.4 a | 1.3 ± 0.2 a | 8.6 ± 1.5 a | 86.9 ± 4.3 a | 21.2 ± 1.7 a | 11.7 ± 1.2 a | 9.6 ± 0.5 a |
| C5OM0 | 26.2 ± 3.6 a | 0.8 ± 0.2 a | 4.0 ± 0.7 b | 88.3 ± 3.6 a | 22.9 ± 1.9 a | 8.7 ± 1.1 b | 14.1 ± 0.7 b |
| C5OM1 | 12.8 ± 2.2 b | 6.6 ± 1.4 b | 156.0 ± 3.0 c | 86.7 ± 4.3 a | 23.9 ± 3.5 a,d | 9.5 ± 2.3 a,b | 14.4 ± 1.2 b |
| C5OM5 | 1.1 ± 0.1 c | 9.8 ± 1.6 b,c | 735.1 ± 81.7 d | 74.0 ± 2.0 b,d | 31.2 ± 2.9 b | 21.0 ± 2.3 c | 10.3 ± 0.7 a |
| C0OM3 | 5.3 ± 1.1 d | 12.4 ± 2.9 c | 421.9 ± 124.1 e | 65.5 ± 3.0 c | 37.45 ± 1.7 c | 29.4 ± 1.4 d | 8.0 ± 0.3 c |
| C3OM3 | 8.3 ± 4.2 d | 10.5 ± 0.9 c | 288.9 ± 22.9 f | 70.6 ± 2.9 d | 35.5 ± 5.65 c, b | 26.8 ± 4.6 d,c | 8.7 ± 1.1 c |
| C5OM3 | 6.4 ± 1.7 d | 9.8 ± 1.6 c | 256.2 ± 27.7 f | 77.5 ± 3.0 d | 27.8 ± 0.5 d | 16.2 ± 0.36 e | 11.7 ± 0.1 d |
| Sample Designation | AA (%) | AA (%) |
|---|---|---|
| After 2 h | After 24 h | |
| C0OM0 | 52.6 ± 2.8 a | 94.6 ± 1.3 a |
| C5OM0 | 58.0 ± 2.6 b | 90.5 ± 0.8 b |
| C5OM1 | 28.7 ± 0.3 c | 70.7 ± 0.5 c |
| C5OM3 | 47.7 ± 0.1 d | 77.4 ± 2.0 d |
| C5OM5 | 49.1 ± 1.1 d,e | 82.1 ± 0.5 e |
| C0OM3 | 49.6 ± 0.0 d,e | 81.5 ± 0.0 e |
| C1OM3 | 49.2 ± 1.7 d,e | 81.5 ± 0.4 e |
| C3OM3 | 50.2 ± 0.2 e | 82.3 ± 0.1 e |
| 1% OMMT | 1.51 ± 0.08 f | 2.60 ± 0.18 f |
| 3% OMMT | 2.2 ± 0.49 f | 10.33 ± 0.56 g |
| 5% OMMT | 6.67 ± 0.18 g | 13.46 ± 0.58 h |
| 1% Cin | 1.36 ±0.45 f | 2.00 ± 0.71 f |
| 3% Cin | 1.34 ± 1.24 f | 3.11 ± 0.59 f |
| 5% Cin | 1.61 ± 1.70 f | 3.22 ± 0.51 f |
| Samples | % Cinnamaldehyde (w/w) | % Linseed Oil | % Peroxide Benzoyl (w/w) | % OMMT (w/w) | Time (h) | Image |
|---|---|---|---|---|---|---|
| C0OM0 | 0 | 99.8 | 0.2 | 0 | 72 |  |
| C5OM0 | 5 | 94.8 | 0.2 | 0 | 48 |  |
| C5OM1 | 5 | 93.8 | 0.2 | 1 | 48 |  |
| C5OM3 | 5 | 91.8 | 0.2 | 3 | 24 |  |
| C5OM5 | 5 | 89.8 | 0.2 | 5 | 24 |  |
| C0OM3 | 0 | 96.8 | 0.2 | 3 | 24 |  |
| C1OM3 | 1 | 95,8 | 0.2 | 3 | 24 |  |
| C3OM3 | 3 | 93.8 | 0.2 | 3 | 24 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guesmi, R.; Benbettaieb, N.; Ben Romdhane, M.R.; Barhoumi-Slimi, T.; Assifaoui, A. In Situ Polymerization of Linseed Oil-Based Composite Film: Enhancement of Mechanical and Water Barrier Properties by the Incorporation of Cinnamaldehyde and Organoclay. Molecules 2022, 27, 8089. https://doi.org/10.3390/molecules27228089
Guesmi R, Benbettaieb N, Ben Romdhane MR, Barhoumi-Slimi T, Assifaoui A. In Situ Polymerization of Linseed Oil-Based Composite Film: Enhancement of Mechanical and Water Barrier Properties by the Incorporation of Cinnamaldehyde and Organoclay. Molecules. 2022; 27(22):8089. https://doi.org/10.3390/molecules27228089
Chicago/Turabian StyleGuesmi, Rim, Nasreddine Benbettaieb, Mohamed Ramzi Ben Romdhane, Thouraya Barhoumi-Slimi, and Ali Assifaoui. 2022. "In Situ Polymerization of Linseed Oil-Based Composite Film: Enhancement of Mechanical and Water Barrier Properties by the Incorporation of Cinnamaldehyde and Organoclay" Molecules 27, no. 22: 8089. https://doi.org/10.3390/molecules27228089
APA StyleGuesmi, R., Benbettaieb, N., Ben Romdhane, M. R., Barhoumi-Slimi, T., & Assifaoui, A. (2022). In Situ Polymerization of Linseed Oil-Based Composite Film: Enhancement of Mechanical and Water Barrier Properties by the Incorporation of Cinnamaldehyde and Organoclay. Molecules, 27(22), 8089. https://doi.org/10.3390/molecules27228089






