Fluorine-Containing Flow Modifier for BN/PPS Composites Enabled by Low Surface Energy
Abstract
1. Introduction
2. Experimental
2.1. Main Materials
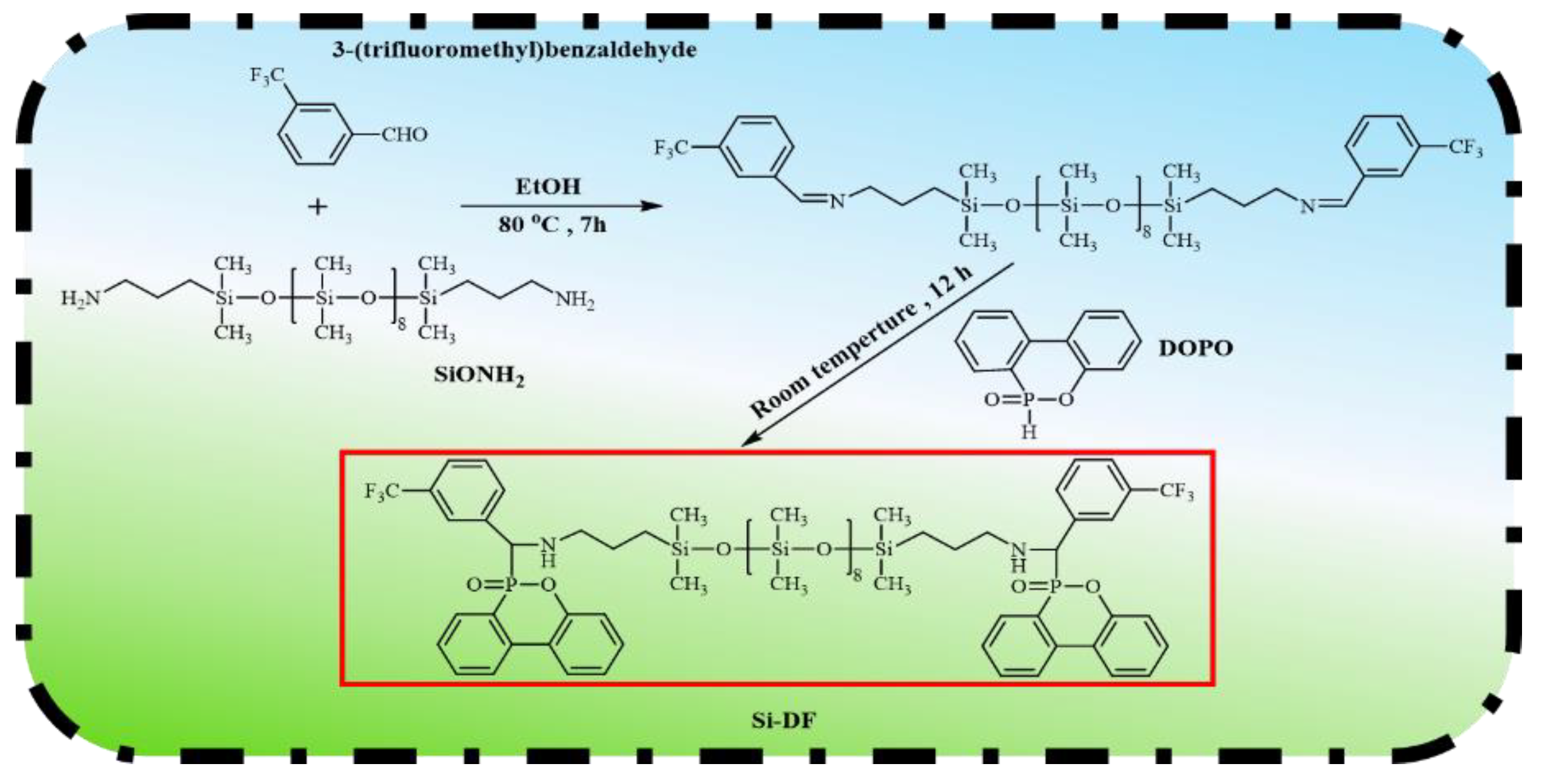
2.2. Synthesis of Si-DF
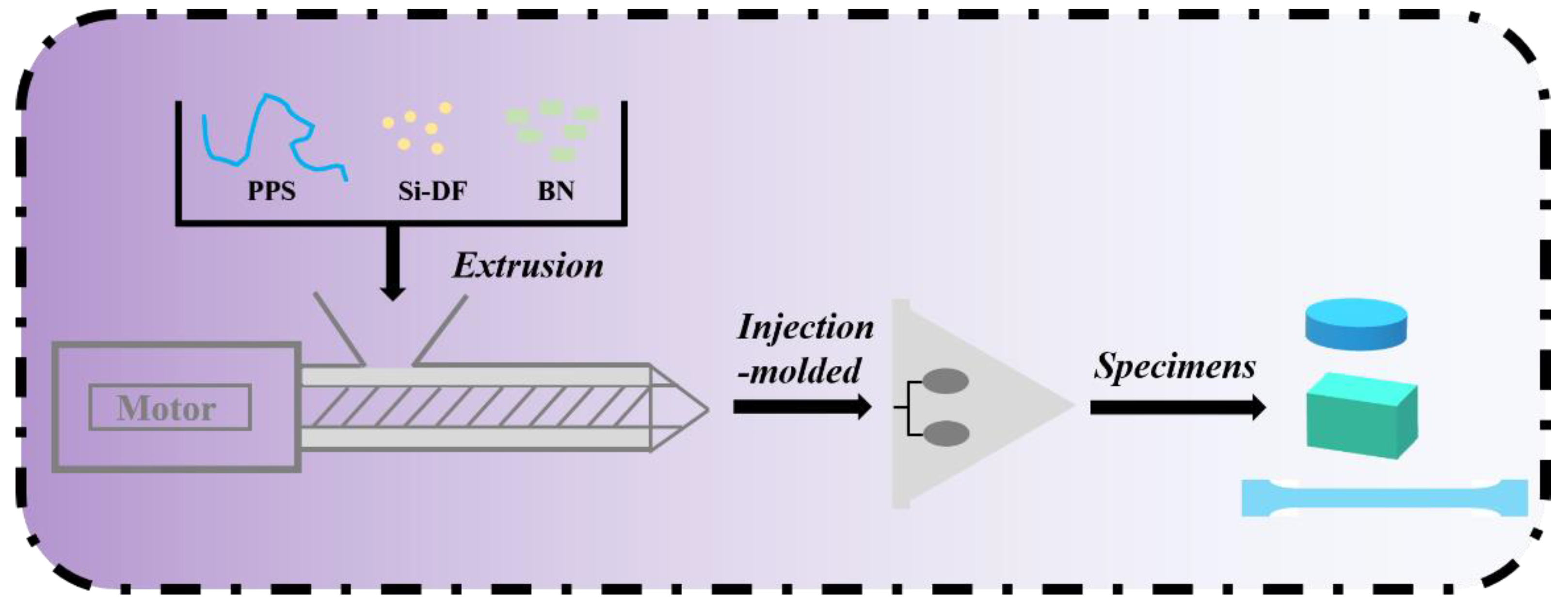
2.3. Preparation of BN/PPS Composites
2.4. Characterization
3. Results and Discussion
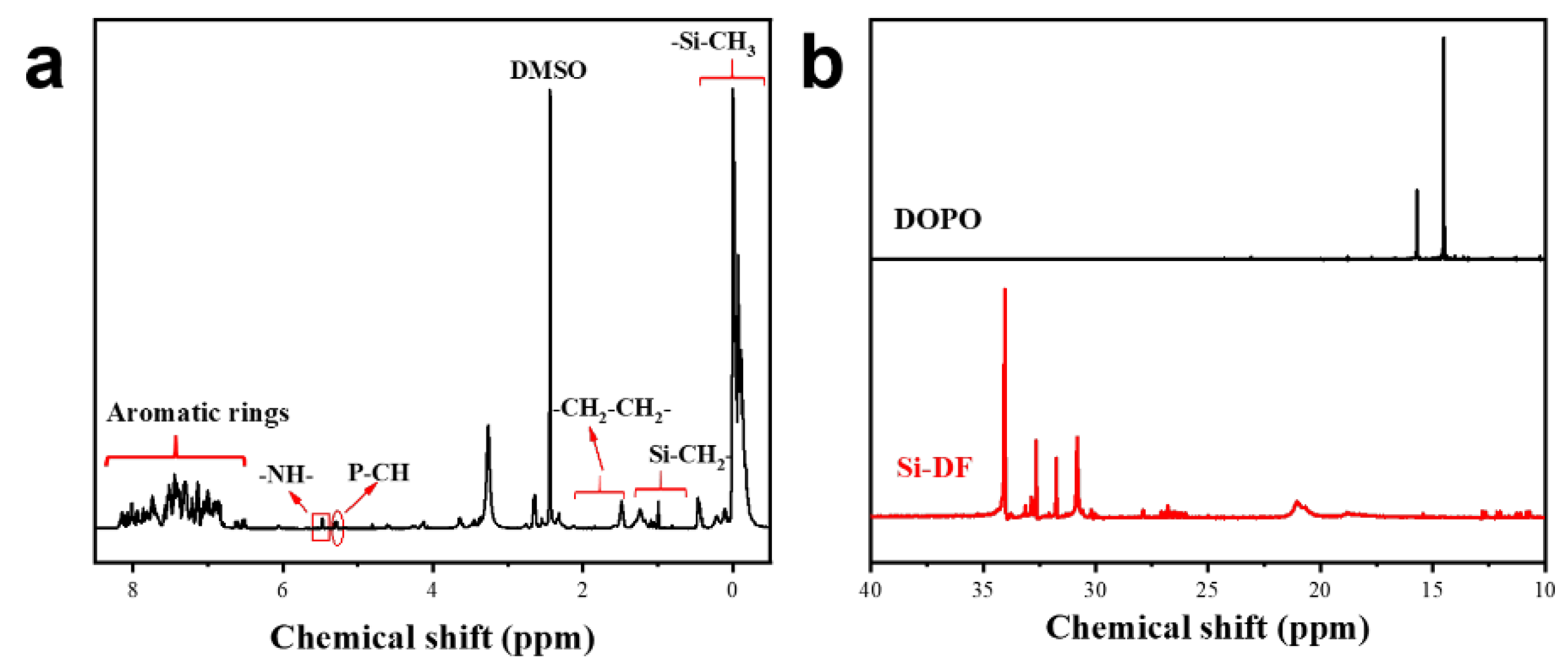
3.1. Characterization of Si-DF
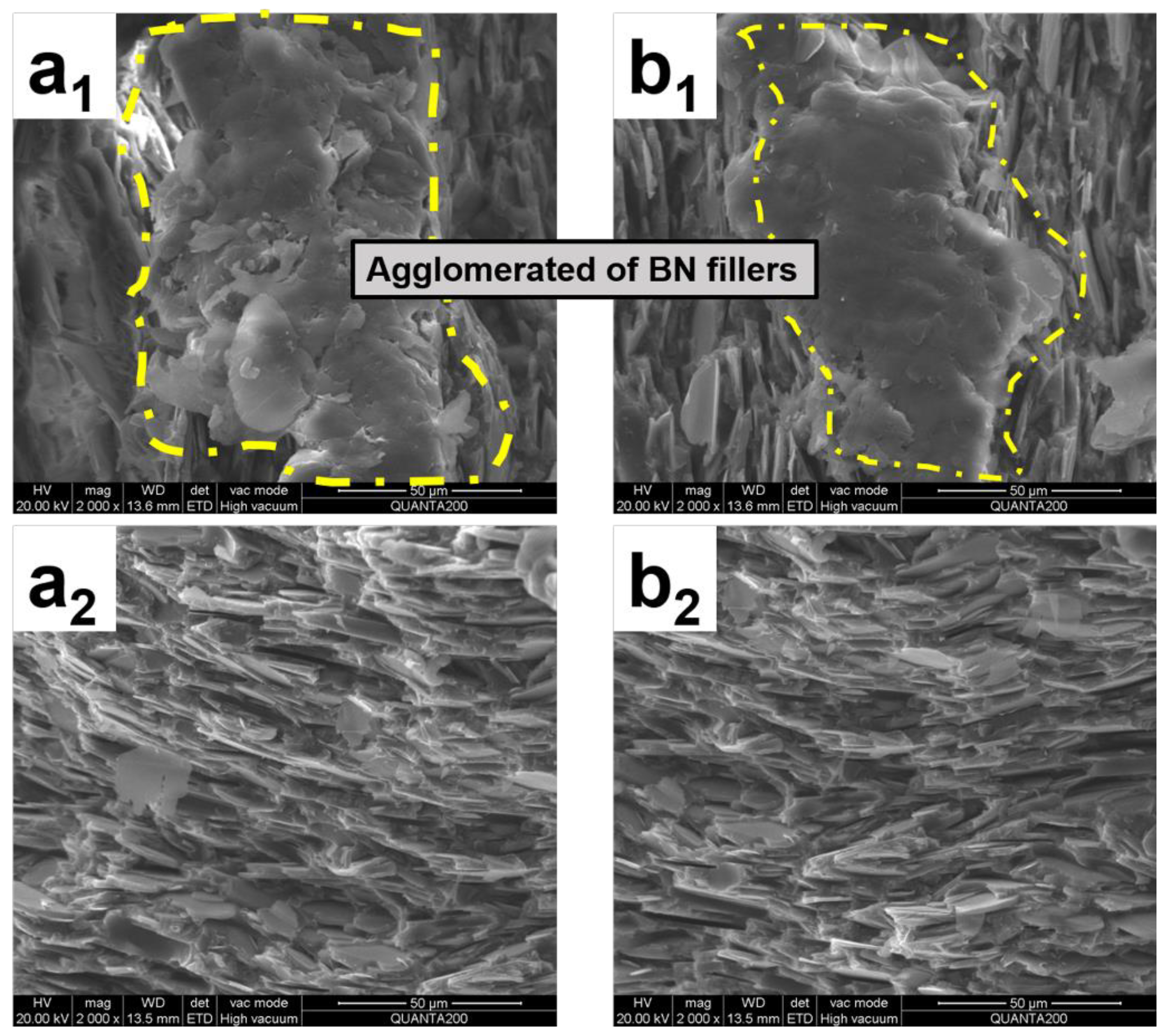
3.2. Phase Morphology
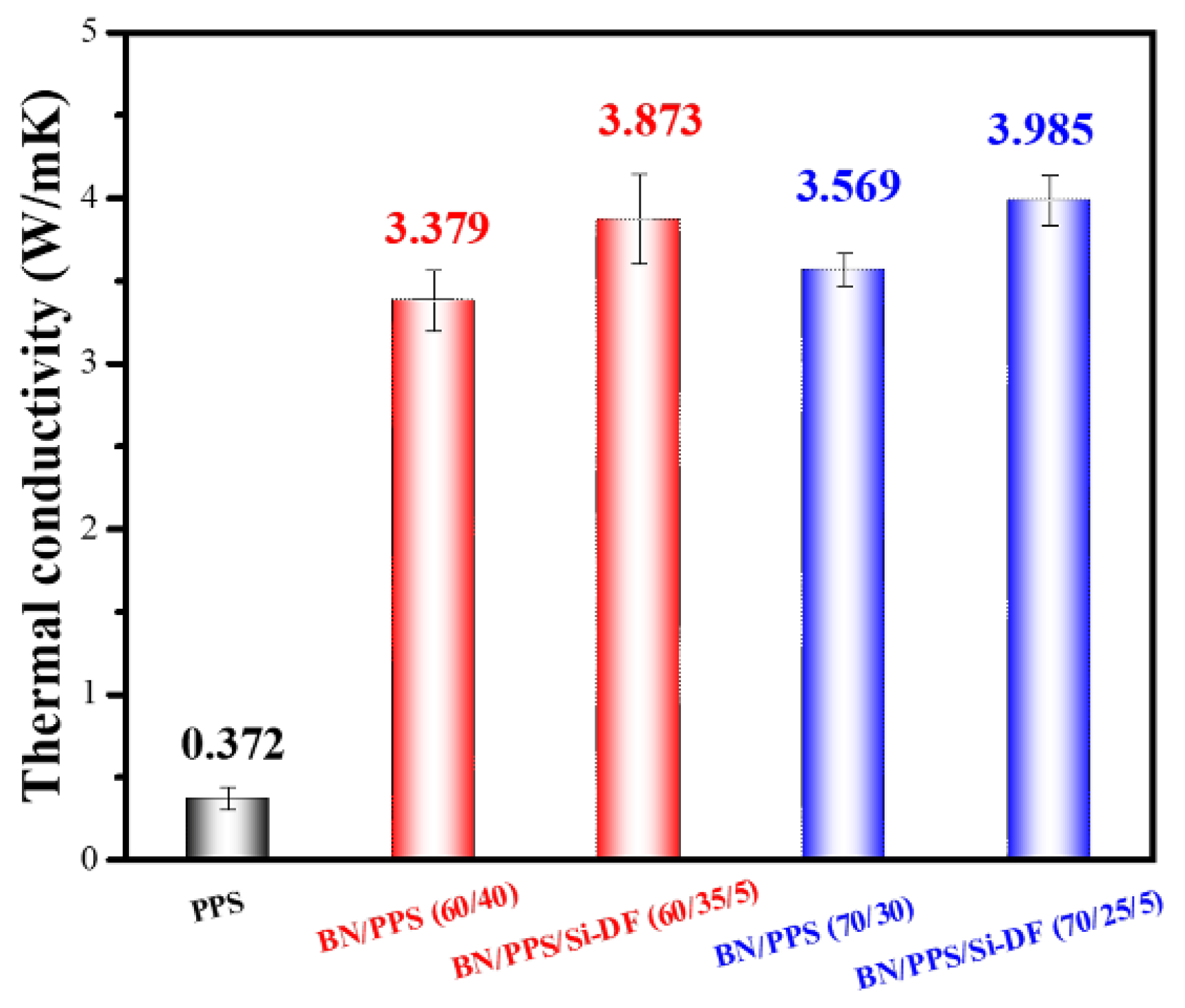
3.3. Thermal Conductivity
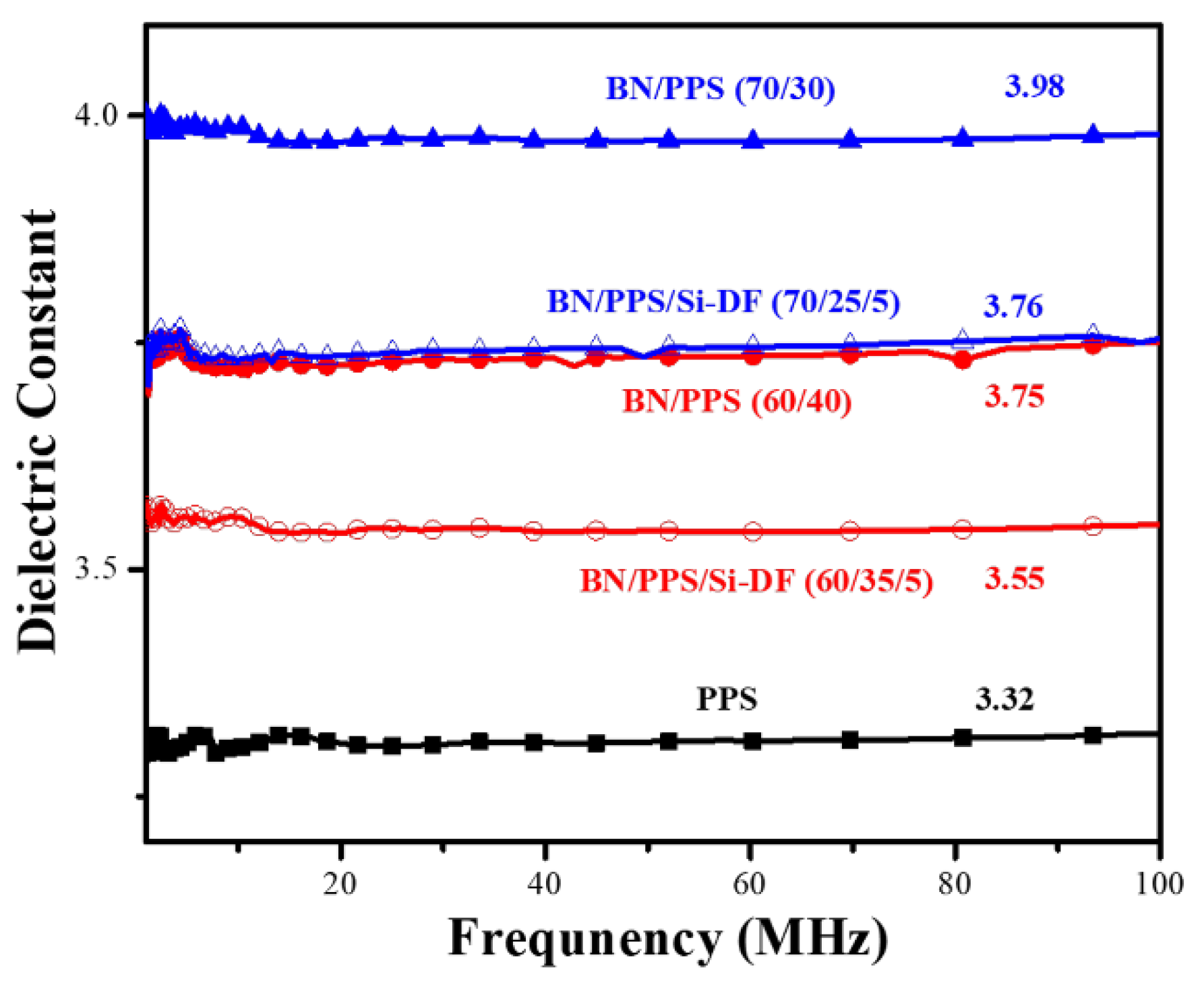
3.4. Dielectric Property
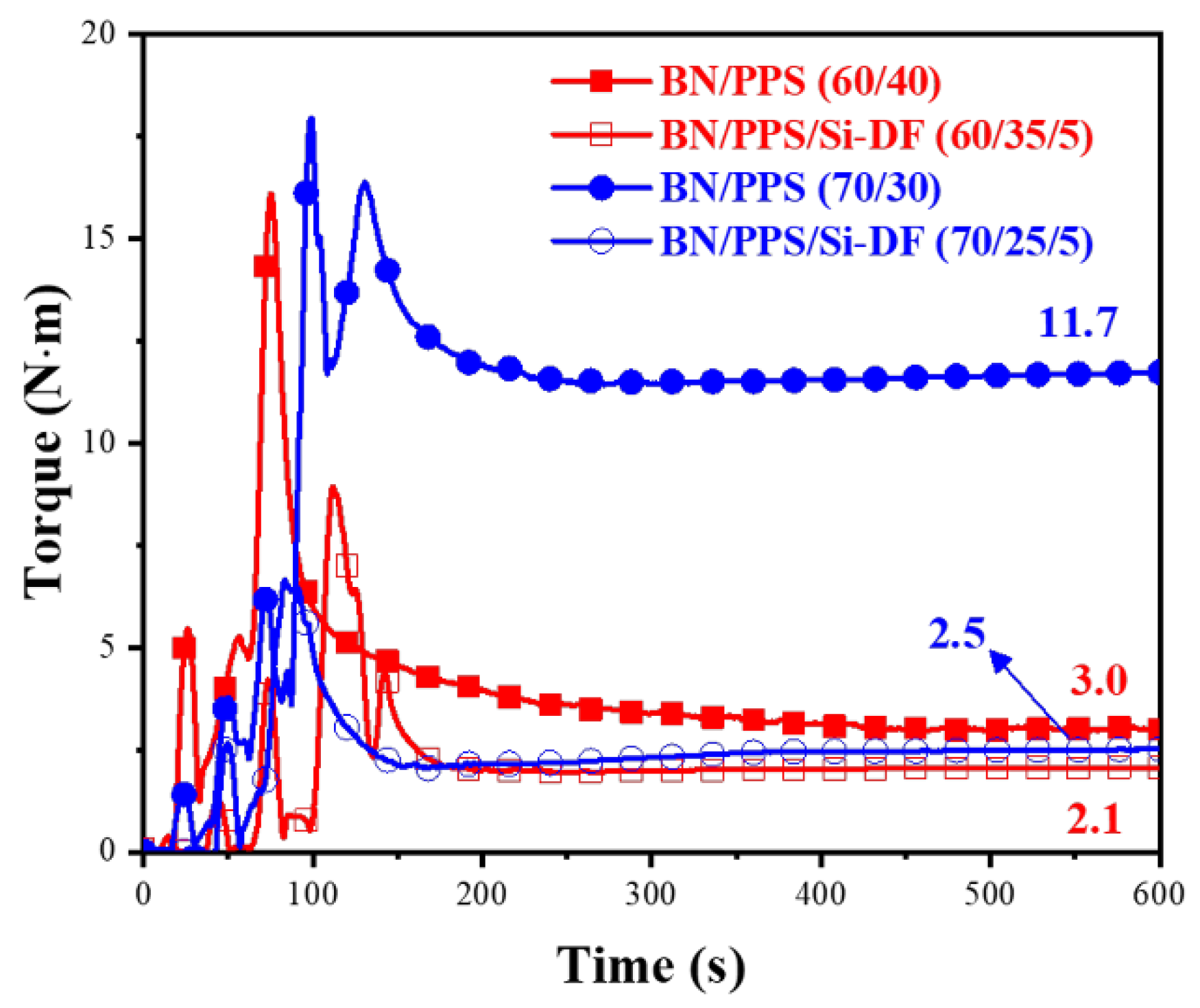
3.5. Processability
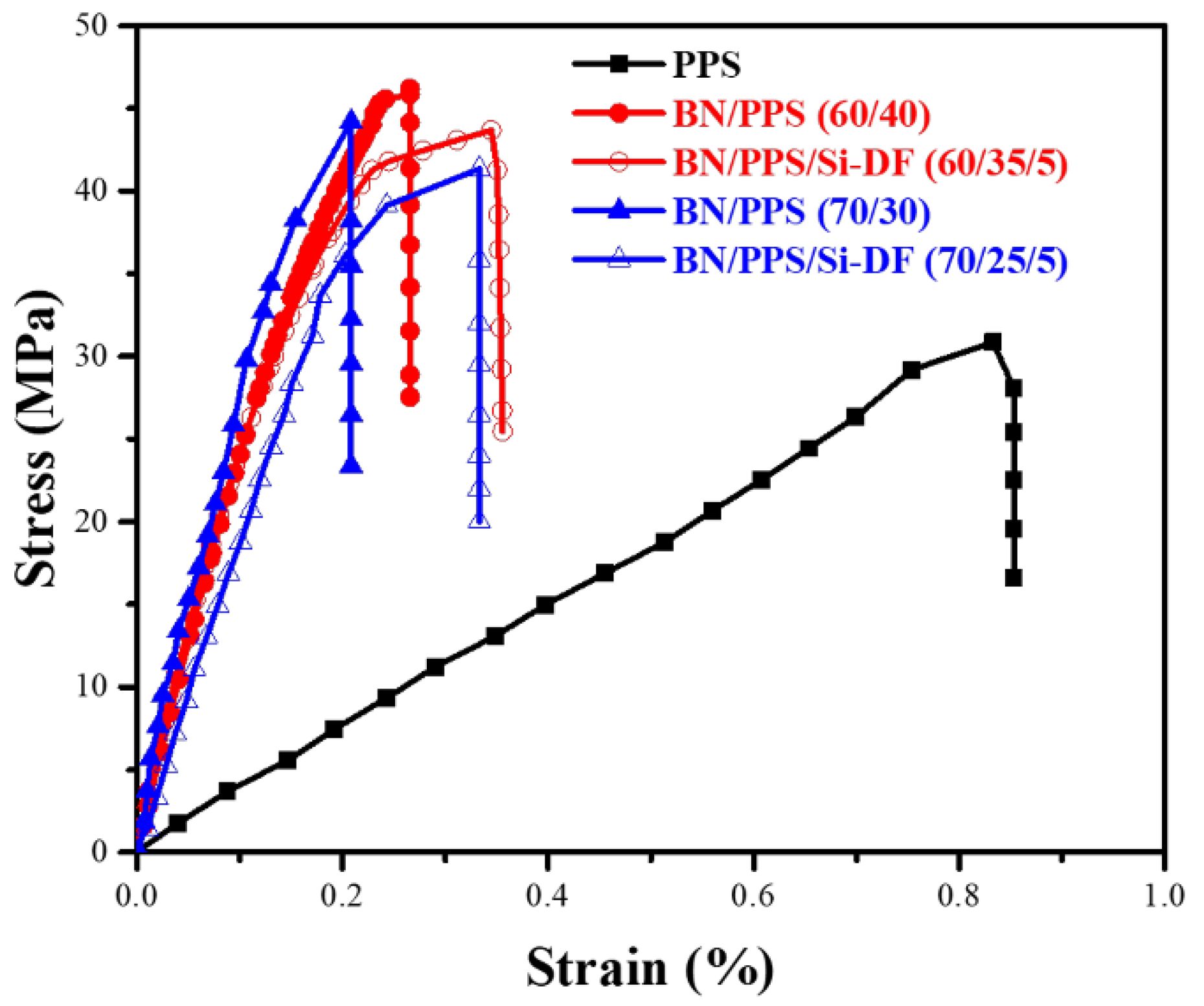
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shahil, K.M.; Balandin, A.A. Graphene-Multilayer Graphene Nanocomposites as Highly Efficient Thermal Interface Materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Lee, G.W.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced Thermal Conductivity of Polymer Composites Filled with Hybrid Filler. Compos. Part A Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.S.; Paik, K.W.; Jeon, S. Enhanced Thermal Conductivity of Epoxy-Graphene Composites by Using Non-Oxidized Graphene Flakes with Non-Covalent Functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, G.; Wang, W.; Li, L.; Fang, X. A Facile Assembly of Polyimide/Graphene Core–Shell Structured Nanocomposites with Both High Electrical and Thermal Conductivities. Compos. Part A Appl. Sci. Manuf. 2016, 84, 472–481. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, J.U.; Yu, J.; Kim, S.Y. Thermal Conductivity of Polymer Composites Based on the Length of Multi-Walled Carbon Nanotubes. Compos. Part B Eng. 2015, 79, 505–512. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lv, Z.; Li, N.; Liang, C.; Zhang, Q. Functionalized Graphite Nanoplatelets/Epoxy Resin Nanocomposites with High Thermal Conductivity. Int. J. Heat Mass Transf. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral Oligosilsesquioxane-Modified Boron Nitride Nanotube Based Epoxy Nanocomposites: An Ideal Dielectric Material with High Thermal Conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Han, Y.; Shi, X.; Yang, X.; Guo, Y.; Zhang, J.; Kong, J.; Gu, J. Enhanced Thermal Conductivities of Epoxy Nanocomposites Via Incorporating in-Situ Fabricated Hetero-Structured SiC-BNNS Fillers. Compos. Sci. Technol. 2020, 187, 10944. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, G.; Yang, X.; Ruan, K.; Ma, T.; Zhang, Q.; Gu, J.; Wu, Y.; Liu, H.; Guo, Z. Significantly Enhanced and Precisely Modeled Thermal Conductivity in Polyimide Nanocomposites with Chemically Modified Graphene Via in Situ Polymerization and Electrospinning-Hot Press Technology. J. Mater. Chem. C 2018, 6, 3004–3015. [Google Scholar] [CrossRef]
- Xie, S.H.; Zhu, B.K.; Li, J.B.; Wei, X.Z.; Xu, Z.K. Preparation and Properties of Polyimide/Aluminum Nitride Composites. Polym. Test. 2004, 23, 797–801. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, X.; Zhu, Y.; Hui, D.; Qiu, Y. Mechanical, Electrical and Thermal Properties of Aligned Carbon Nanotube/Polyimide Composites. Compos. Part B Eng. 2014, 56, 408–412. [Google Scholar] [CrossRef]
- Guan, X.; Cao, B.; Cai, J.; Ye, Z.; Lu, X.; Huang, H.; Liu, S.; Zhao, J. Design and Synthesis of Polysiloxane Based Side Chain Liquid Crystal Polymer for Improving the Processability and Toughness of Magnesium Hydrate/Linear Low-Density Polyethylene Composites. Polymers 2020, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, O.; Cai, J.; Xie, W.; Liu, S.; Zhao, J. Silicone/Fluorine-Functionalized Flow Modifier with Low Surface Energy for Improving Interfaces in Highly Filled Composites. Compos. Sci. Technol. 2021, 214, 108984. [Google Scholar] [CrossRef]
- Gu, J.; Guo, Y.; Yang, X.; Liang, C.; Geng, W.; Tang, L.; Li, N.; Zhang, Q. Synergistic Improvement of Thermal Conductivities of Polyphenylene Sulfide Composites Filled with Boron Nitride Hybrid Fillers. Compos. Part A Appl. Sci. Manuf. 2017, 95, 267–273. [Google Scholar] [CrossRef]
- Yang, X.; Tang, L.; Guo, Y.; Liang, C.; Zhang, Q.; Kou, K.; Gu, J. Improvement of Thermal Conductivities for Pps Dielectric Nanocomposites Via Incorporating NH2-POSS Functionalized nBN Fillers. Compos. Part A Appl. Sci. Manuf. 2017, 101, 237–242. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Jia, Q.; Zhang, Y.; Wu, G.; Ji, T. Improved Thermal Conductivity and Dielectric Properties of hBN/PTFE Composites Via Surface Treatment by Silane Coupling Agent. Compos. Part B Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Min, P.; Sui, G. BN-MWCNT/PPS core-shell Structure Particles and Their 3D Segregated Architecture Composites with High Thermal Conductivities. Compos. Sci. Technol. 2017, 144, 63–69. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, K.; Kim, J. Silane Surface Modification of Boron Nitride for High Thermal Conductivity with Polyphenylene Sulfide Via Melt Mixing Method. Polym. Adv. Technol. 2017, 28, 1489–1494. [Google Scholar] [CrossRef]
- Chao, P.; Li, Y.; Gu, X.; Han, D.; Jia, X.; Wang, M.; Zhou, T.; Wang, T. Novel Phosphorus–Nitrogen–Silicon Flame Retardants and Their Application in Cycloaliphatic Epoxy Systems. Polym. Chem. 2015, 6, 2977–2985. [Google Scholar] [CrossRef]
- Ding, C.; Hu, D.; He, X.; Lai, Y.; Shao, G. Fabrication and microstructure evolution of monolithic bridged polysilsesquioxane-derived SiC ceramic aerogels. Ceram. Int. 2022, 48, 25833–25839. [Google Scholar] [CrossRef]
- Shao, G.; Shen, X.; Huang, X. Multilevel Structural Design and Heterointerface Engineering of a Host–Guest Binary Aerogel toward Multifunctional Broadband Microwave Absorption. ACS Mater. Lett. 2022, 4, 1787–1797. [Google Scholar] [CrossRef]
- Cao, B.; Zhou, Y.; Wu, Y.; Cai, J.; Guan, X.; Liu, S.; Zhao, J.; Zhang, M. Simultaneous Improvement of Processability and Toughness of Highly Filled MH/LLDPE Composites by Using Fluorine-Containing Flow Modifiers. Compos. Part A Appl. Sci. Manuf. 2020, 134, 105900. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Shan, Z.; Wang, S.; Xiao, Y. Surface Modification of Magnesium Hydroxide by Wet Process and Effect on the Thermal Stability of Silicone Rubber. Appl. Surf. Sci. 2019, 465, 740–746. [Google Scholar] [CrossRef]
- Jalali Dil, E.; Favis, B.D. Localization of Micro- and Nano-Silica Particles in Heterophase Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blends. Polymer 2015, 76, 295–306. [Google Scholar] [CrossRef]
- Zha, X.J.; Pu, J.H.; Ma, L.F.; Li, T.; Bao, R.Y.; Bai, L.; Liu, Z.Y.; Yang, M.B.; Yang, W. A Particular Interfacial Strategy in PVDF/OBC/MWCNT Nanocomposites for High Dielectric Performance and Electromagnetic Interference Shielding. Compos. Part A Appl. Sci. Manuf. 2018, 105, 118–125. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.; Chen, H.; Yu, X.; Zhao, X. Mechanical Properties, Rheological Behaviors, and Phase Morphologies of High-Toughness PLA/PBAT Blends by in-Situ Reactive Compatibilization. Compos. Part B Eng. 2019, 173, 107028. [Google Scholar] [CrossRef]
- Yuan, C.; Duan, B.; Li, L.; Xie, B.; Huang, M.; Luo, X. Thermal Conductivity of Polymer-Based Composites with Magnetic Aligned Hexagonal Boron Nitride Platelets. ACS Appl. Mater. Interfaces 2015, 7, 13000–13006. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors Affecting Thermal Conductivities of the Polymers and Polymer Composites: A Review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Kim, J. Thermal and Mechanical Properties of Epoxy Composites with a Binary Particle Filler System Consisting of Aggregated and Whisker Type Boron Nitride Particles. Compos. Sci. Technol. 2014, 103, 72–77. [Google Scholar] [CrossRef]
- Hidalgo, J.; Jiménez-Morales, A.; Torralba, J.M. Torque Rheology of Zircon Feedstocks for Powder Injection Moulding. J. Eur. Ceram. Soc. 2012, 32, 4063–4072. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Zhu, S.; Sui, C.; Wang, C.; Kuang, Y.; Ray, U.; Liu, D.; Brozena, A.; Leiste, U.H.; et al. A Printed, Recyclable, Ultra-Strong, and Ultra-Tough Graphite Structural Material. Mater. Today 2019, 30, 17–25. [Google Scholar] [CrossRef]
- Shah, D.; Maiti, P.; Jiang, D.D.; Batt, C.A.; Giannelis, E.P. Effect of Nanoparticle Mobility on Toughness of Polymer Nanocomposites. Adv. Mater. 2005, 17, 525–528. [Google Scholar] [CrossRef]

| Samples | BN (g) | PPS (g) | Si-DF (g) |
|---|---|---|---|
| BN/PPS (60/40) | 60 | 40 | - |
| BN/PPS/Si-DF (60/35/5) | 60 | 35 | 5 |
| BN/PPS (70/30) | 70 | 30 | - |
| BN/PPS/Si-DF (70/25/5) | 70 | 25 | 5 |
| Samples | Contact Angle (°) | γ (mJ/m2) | γd mJ/m2) | γp (mJ/m2) | |
|---|---|---|---|---|---|
| Water | Diiodomethane | ||||
| PPS | 90.37 | 24.76 | 46.99 | 46.63 | 0.36 |
| BN | 89.12 | 64.89 | 26.8 | 22.5 | 4.3 |
| Si-DF | 98.45 | 51.20 | 34.08 | 33.78 | 0.3 |
| Interfacial Energy | Based on Geometric-Mean Equation (mJ/m2) | Based on Harmonic-Mean Equation (mJ/m2) | ωG | ωH | Flow Modifiers Distribution |
|---|---|---|---|---|---|
| γBN/PPS | 6.52 | 11.75 | −0.37 | −0.31 | BN/PPS interface |
| γSi-DF/PPS | 1.04 | 2.06 | |||
| γSi-DF/BN | 3.47 | 5.74 |
| Samples | Filler Loading [wt%] | Thermally Conductive Coefficient [W/m·K] | Thermal Conductivity Enhancement [%] | Strategy | Year [Ref] |
|---|---|---|---|---|---|
| BN/PPS | 60 | 2.638 | 822.3 | Hot-compression | 2017 [15] |
| BN/PPS | 60 | 1.122 | 292.3 | Surface-modification, Hot-compression | 2017 [16] |
| BN/PPS | 40 (vol) | 2.45 | 880 | Hot-compression | 2017 [18] |
| BN/PPS | 60 | 3.1 | 785.8 | Surface-modification | 2017 [19] |
| BN/PPS | 60 | 2.7 | 610.5 | Melt blending | 2017 [19] |
| BN/PPS | 60 | 3.873 | 941.1 | Melt blending | This work |
| BN/PPS | 70 | 3.985 | 971.2 | Melt blending | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, B.; Huang, X.; Zhang, W.; Wu, P. Fluorine-Containing Flow Modifier for BN/PPS Composites Enabled by Low Surface Energy. Molecules 2022, 27, 8066. https://doi.org/10.3390/molecules27228066
Cao B, Huang X, Zhang W, Wu P. Fluorine-Containing Flow Modifier for BN/PPS Composites Enabled by Low Surface Energy. Molecules. 2022; 27(22):8066. https://doi.org/10.3390/molecules27228066
Chicago/Turabian StyleCao, Bo, Xiaodan Huang, Wenxiang Zhang, and Peng Wu. 2022. "Fluorine-Containing Flow Modifier for BN/PPS Composites Enabled by Low Surface Energy" Molecules 27, no. 22: 8066. https://doi.org/10.3390/molecules27228066
APA StyleCao, B., Huang, X., Zhang, W., & Wu, P. (2022). Fluorine-Containing Flow Modifier for BN/PPS Composites Enabled by Low Surface Energy. Molecules, 27(22), 8066. https://doi.org/10.3390/molecules27228066






