Rapid Removal of Organic Pollutants from Aqueous Systems under Solar Irradiation Using ZrO2/Fe3O4 Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
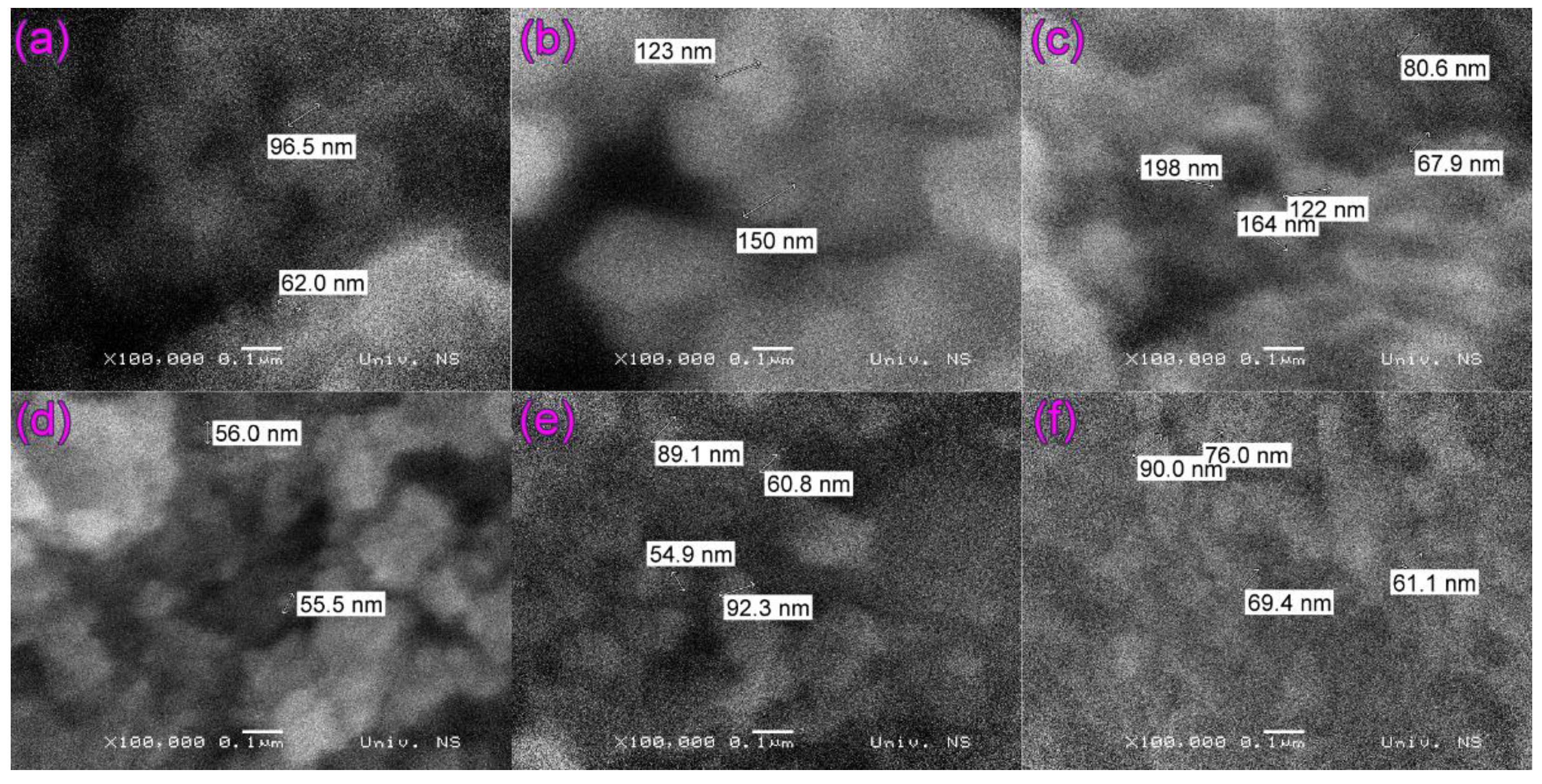
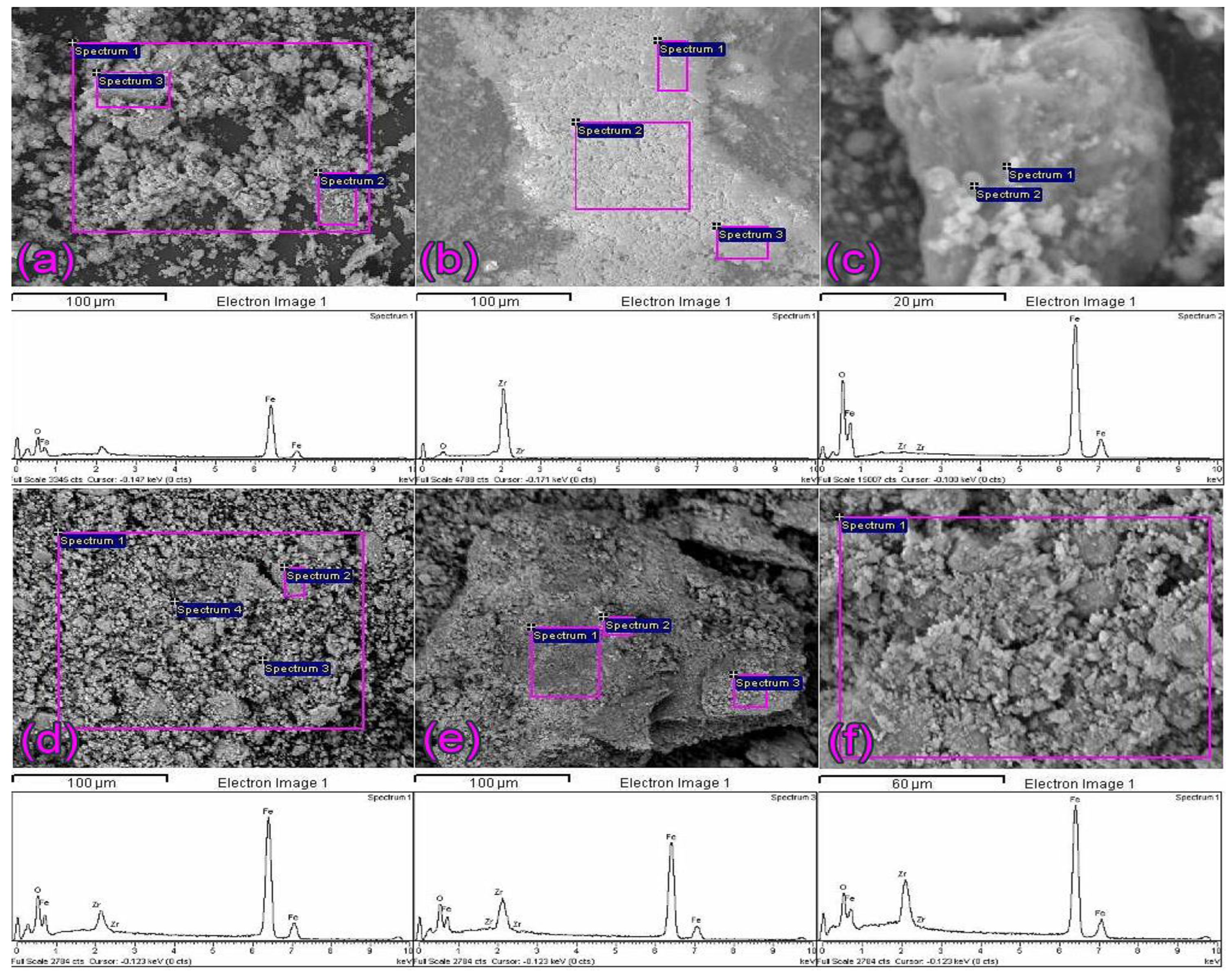
2.1. SEM and EDS Analysis of ZrO2/Fe3O4 Catalysts
2.2. XRD and Raman Measurements of ZrO2/Fe3O4 Catalysts
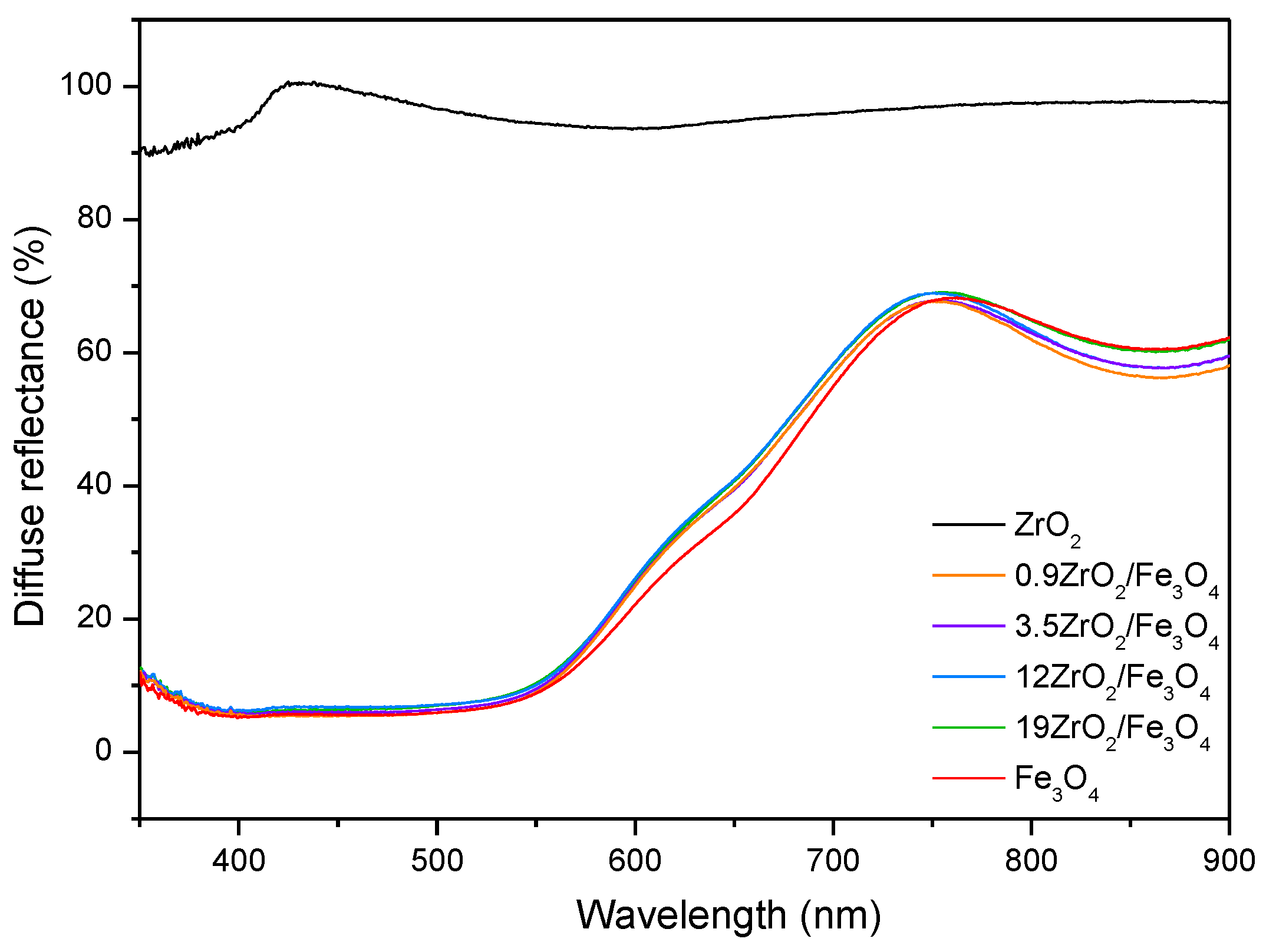
2.3. UV-Vis DRS of ZrO2/Fe3O4 Catalysts
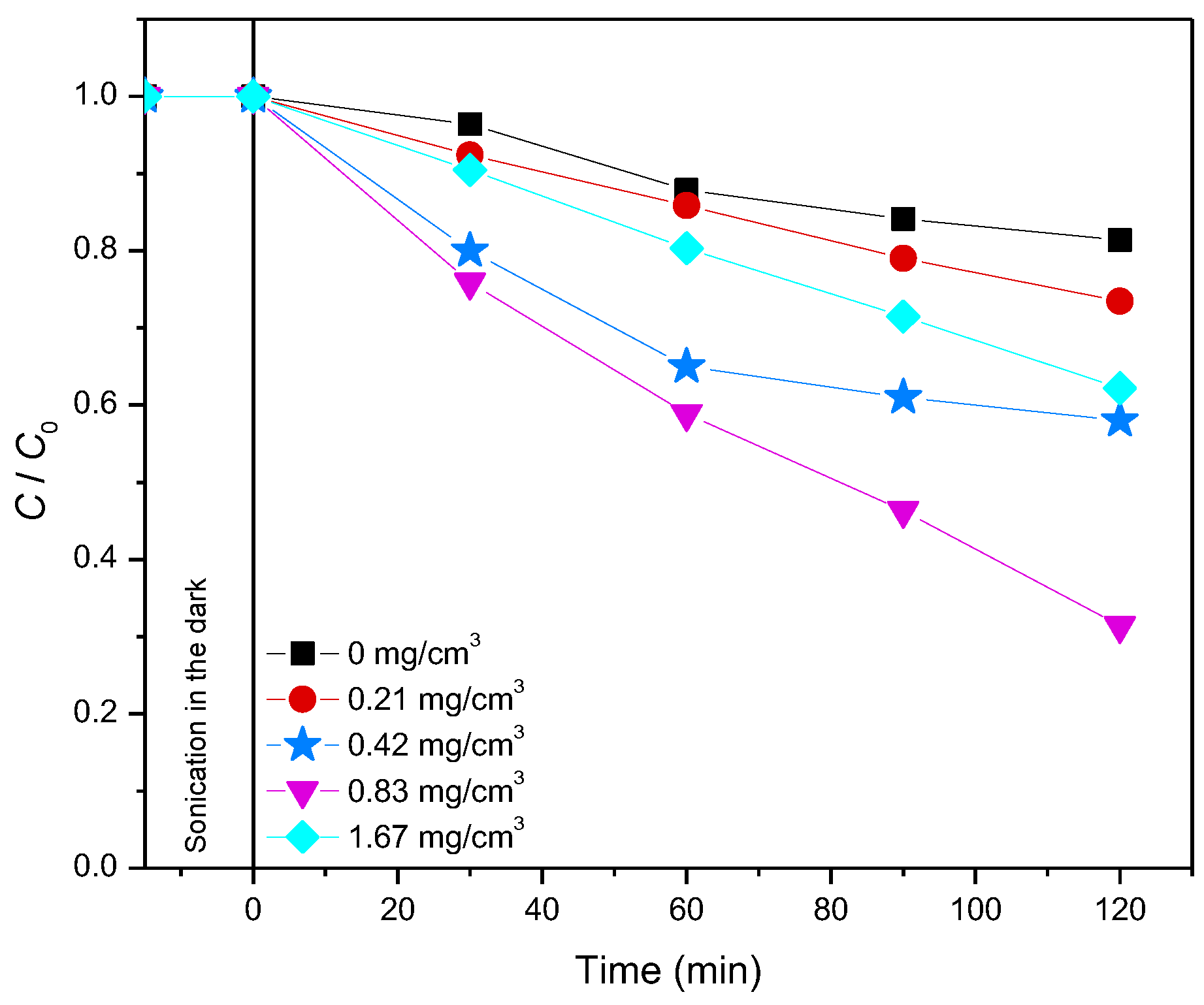
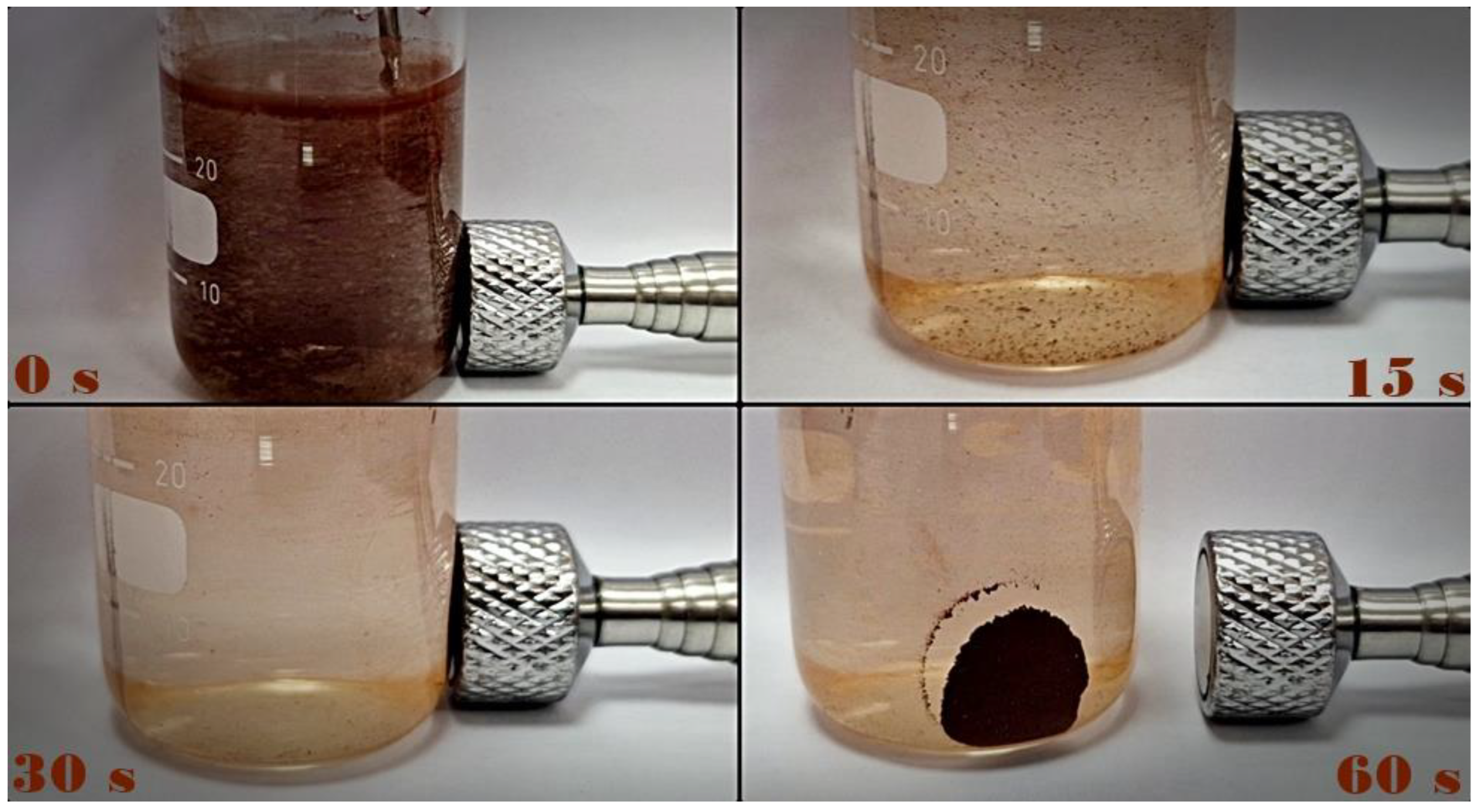
2.4. The Efficiency of Photocatalytic Degradation of the Selected Pesticides and API Using ZrO2/Fe3O4 Nanopowders
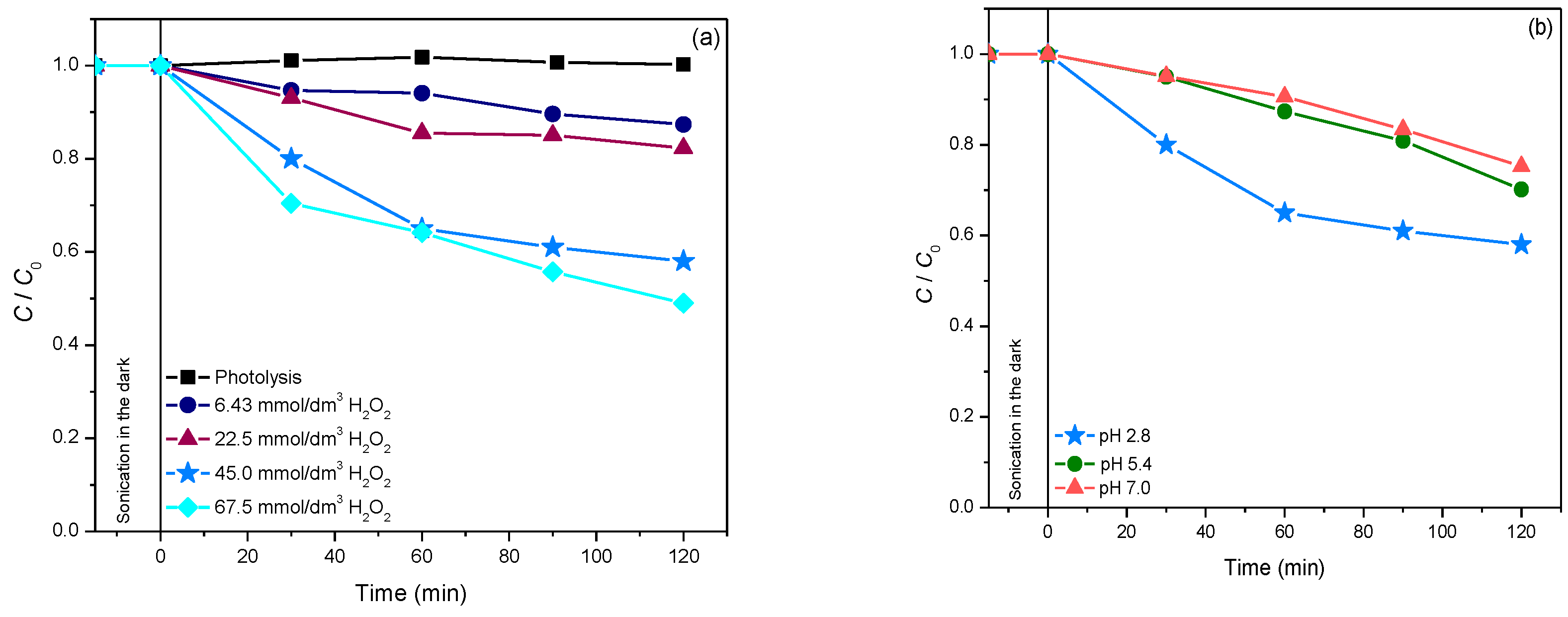
2.4.1. Photodegradation of Pesticides
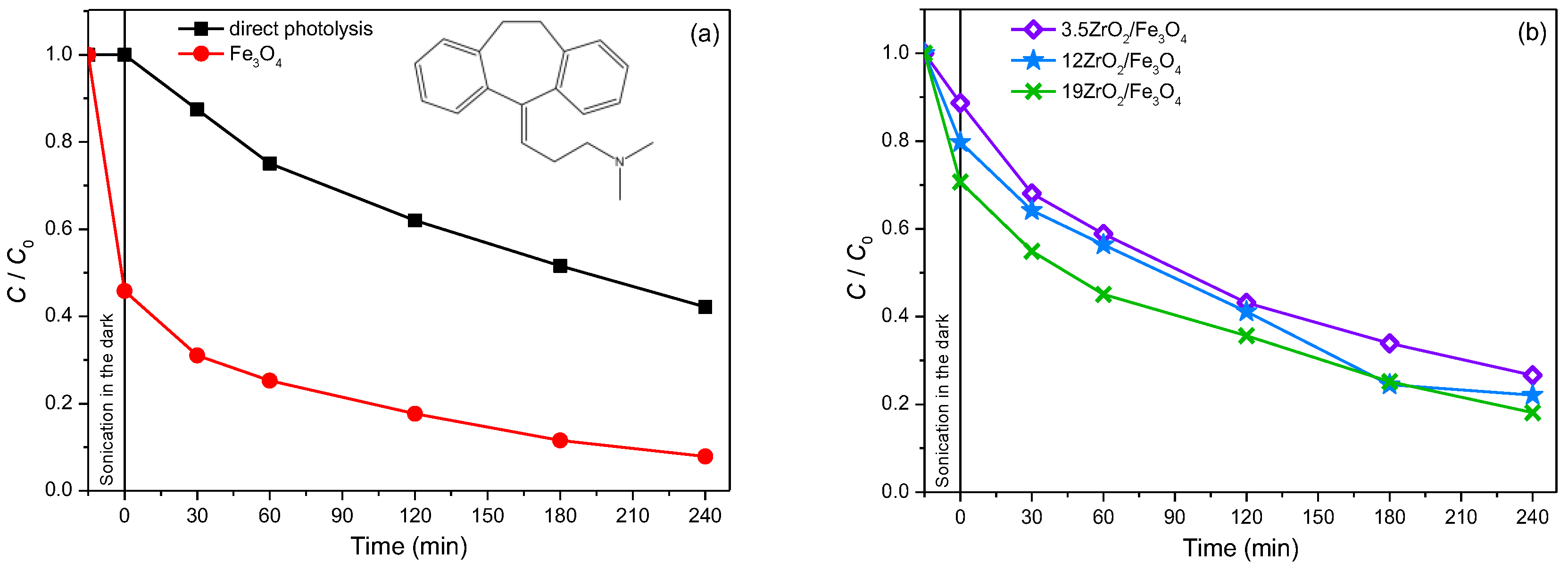
2.4.2. Photodegradation of Pharmaceutically Active Compound
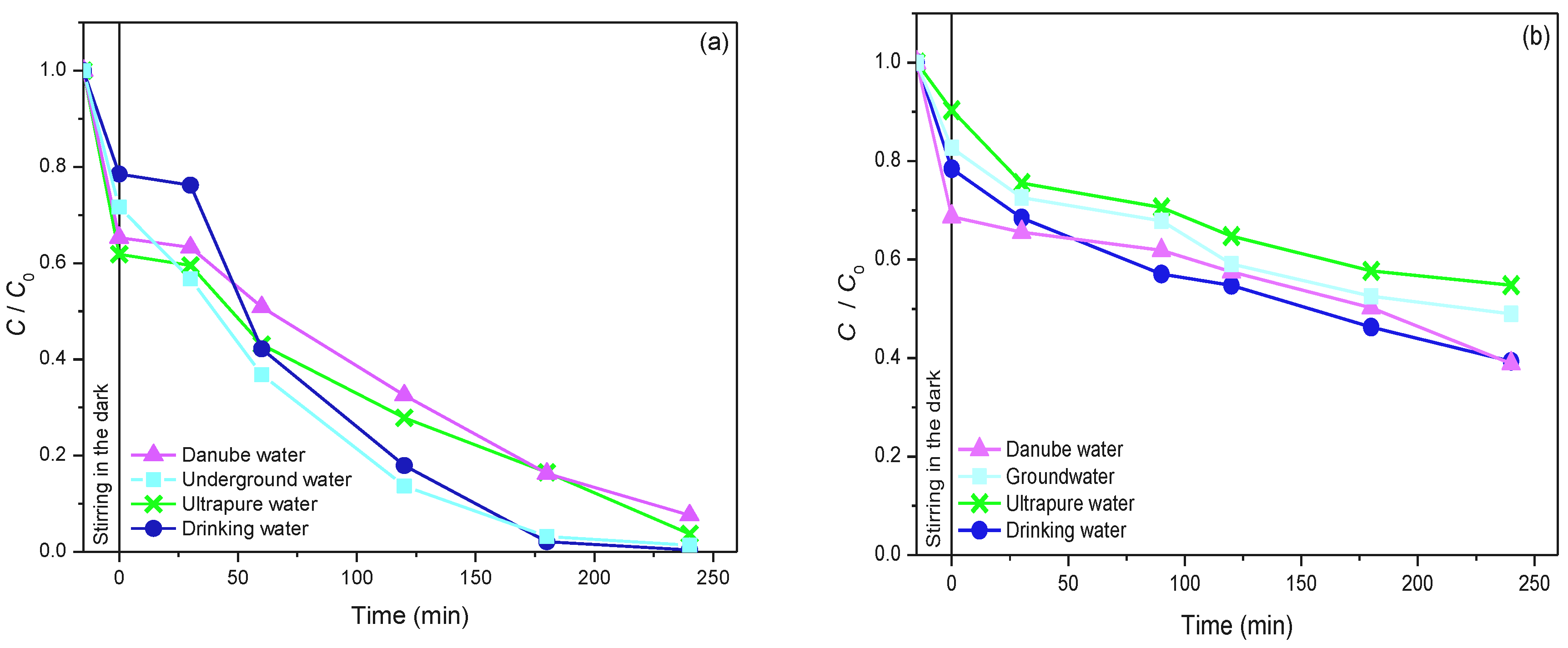
2.5. The Influence of Natural Water Matrix on the Efficiency of Photocatalytic Degradation of Selected Pesticide and API Using 19ZrO2/Fe3O4 Nanopowder
3. Materials and Methods
3.1. Chemicals and Solutions
3.2. Catalyst Synthesis
3.3. Characterization Methods
3.4. Sample Preparation and Irradiation
3.5. Analytical Procedures
4. Conclusions and Outlooks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A.U.; Rehman, M.U.; Zahoor, M.; Shah, A.B.; Zekker, I. Biodegradation of brown 706 dye by bacterial strain pseudomonas aeruginosa. Water 2021, 13, 2959. [Google Scholar] [CrossRef]
- Edwards, S.J.; Kjellerup, B.V. Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl. Microbiol. Biotechnol. 2013, 97, 9909–9921. [Google Scholar] [CrossRef]
- SDG Knowledge Hub. Available online: https://sdg.iisd.org/news/world-bank-finds-poor-water-quality-might-halve-economic-growth/ (accessed on 19 September 2022).
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Murray, K.E.; Thomas, S.M.; Bodour, A.A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ. Pollut. 2010, 158, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef]
- Hincapié, M.; Maldonado, M.I.; Oller, I.; Gernjak, W.; Sánchez-Pérez, J.A.; Ballesteros, M.M.; Malato, S. Solar photocatalytic degradation and detoxification of EU priority substances. Catal. Today 2005, 101, 203–210. [Google Scholar] [CrossRef]
- Godiya, C.B.; Park, B.J. Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques: A review. Environ. Chem. Lett. 2022, 20, 1–37. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron–hole recombination time at TiO2 single-crystal surfaces: Influence of surface band bending. J. Phys. Chem. Lett. 2014, 5, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Zahoor, M.; Rehman, M.U.; Shah, A.B.; Zekker, I.; Khan, F.A.; Ullah, R.; Albadrani, G.M.; Bayram, R.; Mohamed, H.R.H. Biological mineralization of methyl orange by pseudomonas aeruginosa. Water 2022, 14, 1551. [Google Scholar] [CrossRef]
- Zahoor, M.; Shah, A.B.; Naz, S.; Ullah, R.; Bari, A.; Mahmood, H.M. Isolation of quercetin from Rubus fruticosus, their concentration through NF/RO membranes, and recovery through carbon nanocomposite. A pilot plant study. Biomed. Res. Int. 2020, 2020, 8216435. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Naeem, M.; Zahoor, M.; Rahim, A.; Hanafiah, M.M.; Oyekanmi, A.A.; Shah, A.B.; Mahnashi, M.H.; Ali, A.A.; Jalai, N.A.; et al. Biodegradation of azo dye methyl red by pseudomonas aeruginosa: Optimization of process conditions. Int. J. Environ. Res. Public Health 2022, 19, 9962. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- ManiRahulan, K.; Vinitha, G.; Devaraj Stephen, L.; Kanakama, C.C. Synthesis and optical limiting effects in ZrO2 and ZrO2@SiO2 core–shell nanostructures. Ceram. Int. 2013, 39, 5281–5286. [Google Scholar] [CrossRef]
- Mansouri, M.; Mozafari, N.; Bayati, B.; Setareshenas, N. Photo-catalytic dye degradation of methyl orange using zirconia–zeolite nanoparticles. Bull. Mater. Sci. 2019, 42, 230. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Saeed, K.; Sadiq, M.; Khan, I.; Ullah, S.; Ali, N.; Khan, A. Synthesis, characterization, and photocatalytic application of Pd/ZrO2 and Pt/ZrO2. Appl. Water Sci. 2018, 8, 60. [Google Scholar] [CrossRef]
- Zare, M.H.; Mehrabani-Zeinabad, A. Photocatalytic activity of ZrO2/TiO2/Fe3O4 ternary nanocomposite for the degradation of naproxen: Characterization and optimization using response surface methodology. Sci. Rep. 2022, 12, 10388. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Alhazmi, A.; Mohammad, A.; Khan, S.; Bahadur Pal, D.; Haque, S.; Singh, R.; Mishra, P.K.; Kumar Gupta, V. Sustainable green approach to synthesize Fe3O4/α-Fe2O3 nanocomposite using waste pulp of Syzygiumcumini and its application in functional stability of microbial cellulases. Sci. Rep. 2021, 11, 24371. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Hong, S. Green synthesis of spinel magnetite iron oxide nanoparticles. Adv. Mater. Res. 2014, 1051, 39–42. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Nano magnetite decorated multiwalled carbon nanotubes: A robust nanomaterial for enhanced carbon dioxide adsorption. Energy Environ. Sci. 2011, 4, 889–895. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venaüncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Finčur, N.L.; Abramović, B.F.; Dimitrievska, M.R.; Štrbac, G.R.; Čajko, K.O.; Miljević, B.B.; Đačanin, L.j.R.; Lukić-Petrović, S.R. Environmentally friendly photoactive heterojunction zinc tin oxide nanoparticles. Ceram. Int. 2016, 42, 3575–3583. [Google Scholar] [CrossRef]
- Boi, F.S.; Ivaturi, S.; Taallah, A.; Wang, S.; Wen, J. Evidence of band gap features in Fe3O4 Bbmm filled carbon nano-onions. Mater. Res. Express 2020, 7, 055603. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Vasquez, M.I.; Kümmerer, K. Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes—Degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 2011, 85, 693–709. [Google Scholar] [CrossRef]
- Calza, P.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. Pure Appl. Chem. 2001, 73, 1839–1848. [Google Scholar] [CrossRef]
- Habibi, M.H.; Hassanzadeh, A.; Mahdavi, S. The effect of operational parameters on the photocatalytic degradation of three textile azo dyes in aqueous TiO2 suspensions. J. Photochem. Photobiol. A Chem. 2005, 172, 89–96. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Wei, M.; Chen, P.; Shan, G. Photolytic reaction mechanism and impacts of coexisting substances on photodegradation of bisphenol A by Bi2WO6 in water. Water Res. 2012, 46, 845–853. [Google Scholar] [CrossRef]
- Guzsvány, V.; Csanádi, J.; Gaál, F. NMR study of the influence of pH on the persistence of some neonicotinoids in water. Acta Chim. Slov. 2006, 53, 52–57. [Google Scholar]
- Černigoj, U.; Štangar, U.L.; Trebše, P. Degradation of neonicotinoid insecticides by different advanced oxidation processes and studying the effect of ozone on TiO2 photocatalysis. Appl. Catal. B Environ. 2007, 75, 229–238. [Google Scholar] [CrossRef]
- Malato, S.; Fernández–Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Banić, N.D.; Abramović, B.F.; Krstić, J.B.; Šojić Merkulov, D.V.; Finčur, N.L.; Mitrić, M.N. Novel WO3/Fe3O4 magnetic photocatalysts: Preparation, characterization and thiacloprid photodegradation. J. Ind. Eng. Chem. 2019, 70, 264–275. [Google Scholar] [CrossRef]
- Abramović, B.F.; Banić, N.D.; Šojić, D.V. Degradation of thiacloprid in aqueous solution by UV and UV/H2O2 treatments. Chemosphere 2010, 81, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Banić, N.; Abramović, B.; Krstić, J.; Šojić, D.; Lončarević, D.; Cherkezova–Zheleva, Z.; Guzsvány, V. Photodegradation of thiacloprid using Fe/TiO2 as a heterogeneous photo–fenton catalyst. Appl. Catal. B Environ. 2011, 107, 363–371. [Google Scholar] [CrossRef]
- Huston, L.P.; Pignatello, J.J. Degradation of selected pesticide active ingredients and commercial formulations in water by photo-assisted Fenton reaction. Water Res. 1999, 33, 1238–1246. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Luo, L.; Chang, Z.; Sun, X. One-pot synthesis and catalyst support application of mesoporous N-doped carbonaceous materials. J. Mater. Chem. 2012, 22, 12149–12154. [Google Scholar] [CrossRef]
- Chaabane, H.; Vulliet, E.; Joux, F.; Lantoine, F.; Conan, P.; Cooper, J.-F.; Coste, C.-M. Photodegradation of sulcotrione in various aquatic environments and toxicity of its photoproducts for some marine micro-organisms. Water Res. 2007, 41, 1781–1789. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual: A World Compendium, 15th ed.; British Crop Protection Council: Alton, UK, 2009; pp. 1007–1008. [Google Scholar]
- Zhang, S.; Qiu, C.B.; Zhou, Y.; Jin, Z.P.; Yang, H. Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonasreinhardtii. Ecotoxicology 2011, 20, 337–347. [Google Scholar] [CrossRef]
- Abbar, J.C.; Lamani, S.D.; Nandibewoor, S.T. Ruthenium (III) catalyzed oxidative degradation of amitriptyline-A tricyclic antidepressant drug by permanganate in aqueous acidic medium. J. Solut. Chem. 2011, 40, 502–520. [Google Scholar] [CrossRef]
- Li, H.; Sumarah, M.W.; Topp, E. Persistence of the tricyclic antidepressant drugs amitriptyline and nortriptyline in agriculture soils. Environ. Toxicol. Chem. 2013, 32, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Akamine, R.T.; Grossklauss, L.F.; Nozoe, K.T.; Moreira, G.A.; Oliveira, A.S.B.; Chieia, M.A.T.; Andersen, M.L.; Tufik, S. Restless leg syndrome exacerbated by amytriptiline in a patient with Duchenne Muscular Dystrophy. Sleep Sci. 2014, 7, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of basic antidepressants and their N-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography—tandem mass spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Finčur, N.; Šojić Merkulov, D.; Putnik, P.; Despotović, V.; Banić, N.; Lazarević, M.; Četojević-Simin, D.; Agbaba, J.; Abramović, B. Environmental photocatalytic degradation of antidepressants with solar radiation: Kinetics, mineralization, and toxicity. Nanomaterials 2021, 11, 632. [Google Scholar] [CrossRef]
- Qiu, X.; Wan, Z.; Pu, M.; Xu, X.; Ye, Y.; Hu, C. Synthesis and photocatalytic activityof Pt-deposited TiO2 nanotubes (TNT) for Rhodamine B degradation. Front. Chem. 2022, 10, 922701. [Google Scholar] [CrossRef] [PubMed]


| Sample | O (wt.%) | Fe (wt.%) | Zr (wt.%) | Total (wt.%) |
|---|---|---|---|---|
| Fe3O4 | 24.24 | 75.76 | - | 100.00 |
| ZrO2 | 28.43 | - | 71.57 | 100.00 |
| 0.9ZrO2/Fe3O4 | 25.24 | 74.15 | 0.61 | 100.00 |
| 3.5ZrO2/Fe3O4 | 22.22 | 74.16 | 3.62 | 100.00 |
| 12ZrO2/Fe3O4 | 32.24 | 58.90 | 8.86 | 100.00 |
| 19ZrO2/Fe3O4 | 22.96 | 64.16 | 12.88 | 100.00 |
| Sample | Crystallite Size (nm) | Hematite/Magnetite Ratio | |
|---|---|---|---|
| Hematite | Magnetite | ||
| Fe3O4 | 4.6 | 13.0 | 0.45 |
| 0.9ZrO2/Fe3O4 | 7.5 | 14.7 | 0.59 |
| 3.5ZrO2/Fe3O4 | 5.1 | 14.3 | 0.77 |
| 12ZrO2/Fe3O4 | 6.0 | 12.3 | 0.75 |
| 19ZrO2/Fe3O4 | 8.2 | 16.2 | 0.31 |
| Compound | Mobile Phase Composition ACN:0.1% Water Solution H3PO4 (v/v) and pH of Mobile Phase | Injected Volume (μL) | Wavelength (nm) 1 | Flow Rate (cm3/min) | Retention Time (min) |
|---|---|---|---|---|---|
| Thiacloprid | 30:70, pH 2.25 | 20 | 242 | 0.8 | 8.5 |
| Sulcotrione | 50:50, pH 2.54 | 20 | 231 | 1.0 | 4.8 |
| Fluroxypyr | 50:50, pH 2.54 | 10 | 212 | 1.0 | 3.5 |
| Amitriptyline | 40:60, pH 2.50 | 10 | 206 | 0.8 | 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banić, N.; Šojić Merkulov, D.; Despotović, V.; Finčur, N.; Ivetić, T.; Bognár, S.; Jovanović, D.; Abramović, B. Rapid Removal of Organic Pollutants from Aqueous Systems under Solar Irradiation Using ZrO2/Fe3O4 Nanoparticles. Molecules 2022, 27, 8060. https://doi.org/10.3390/molecules27228060
Banić N, Šojić Merkulov D, Despotović V, Finčur N, Ivetić T, Bognár S, Jovanović D, Abramović B. Rapid Removal of Organic Pollutants from Aqueous Systems under Solar Irradiation Using ZrO2/Fe3O4 Nanoparticles. Molecules. 2022; 27(22):8060. https://doi.org/10.3390/molecules27228060
Chicago/Turabian StyleBanić, Nemanja, Daniela Šojić Merkulov, Vesna Despotović, Nina Finčur, Tamara Ivetić, Szabolcs Bognár, Dušica Jovanović, and Biljana Abramović. 2022. "Rapid Removal of Organic Pollutants from Aqueous Systems under Solar Irradiation Using ZrO2/Fe3O4 Nanoparticles" Molecules 27, no. 22: 8060. https://doi.org/10.3390/molecules27228060
APA StyleBanić, N., Šojić Merkulov, D., Despotović, V., Finčur, N., Ivetić, T., Bognár, S., Jovanović, D., & Abramović, B. (2022). Rapid Removal of Organic Pollutants from Aqueous Systems under Solar Irradiation Using ZrO2/Fe3O4 Nanoparticles. Molecules, 27(22), 8060. https://doi.org/10.3390/molecules27228060










