Improved Thermoelectric Properties of SrTiO3 via (La, Dy and N) Co-Doping: DFT Approach
Abstract
1. Introduction
2. Methodology
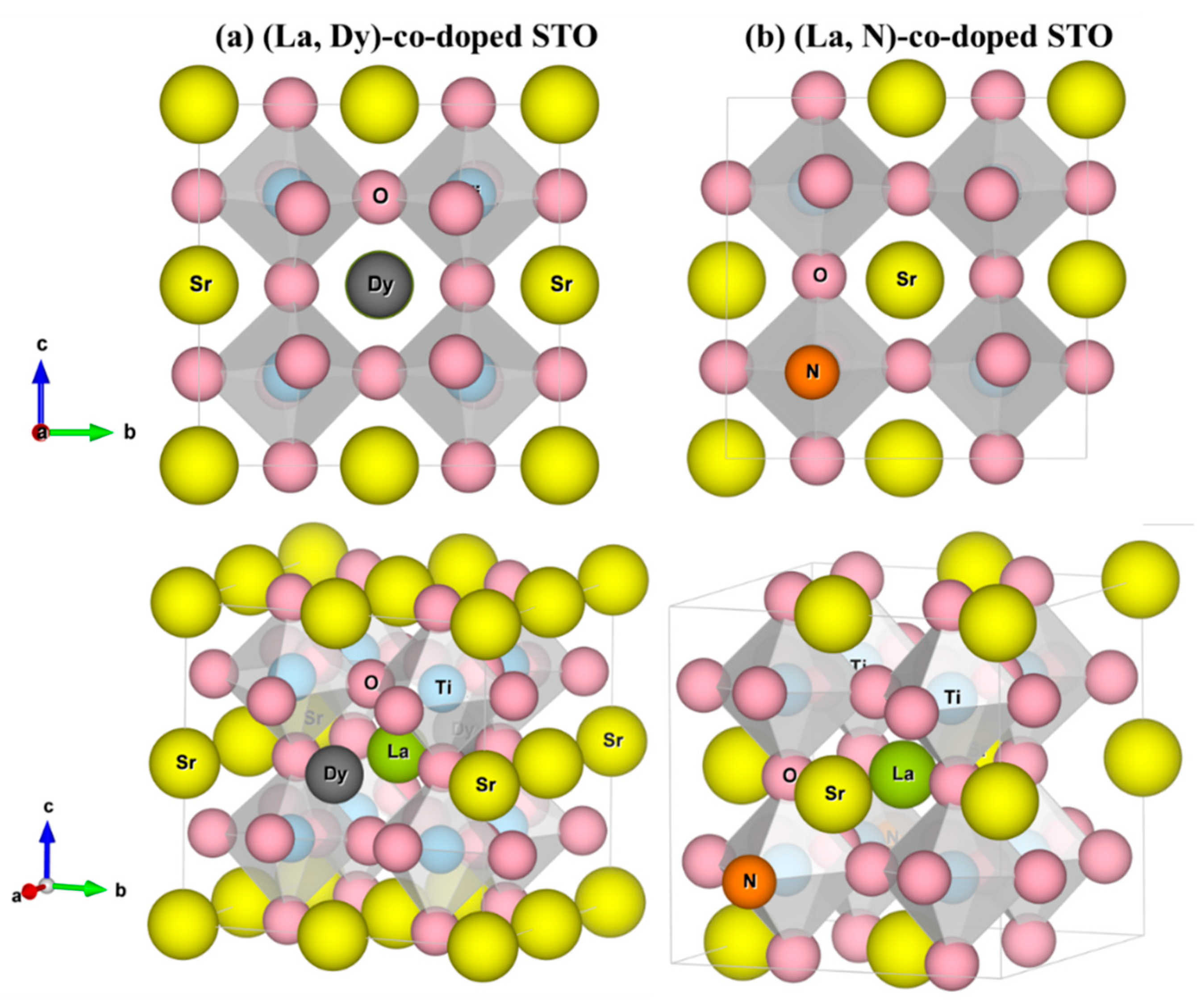
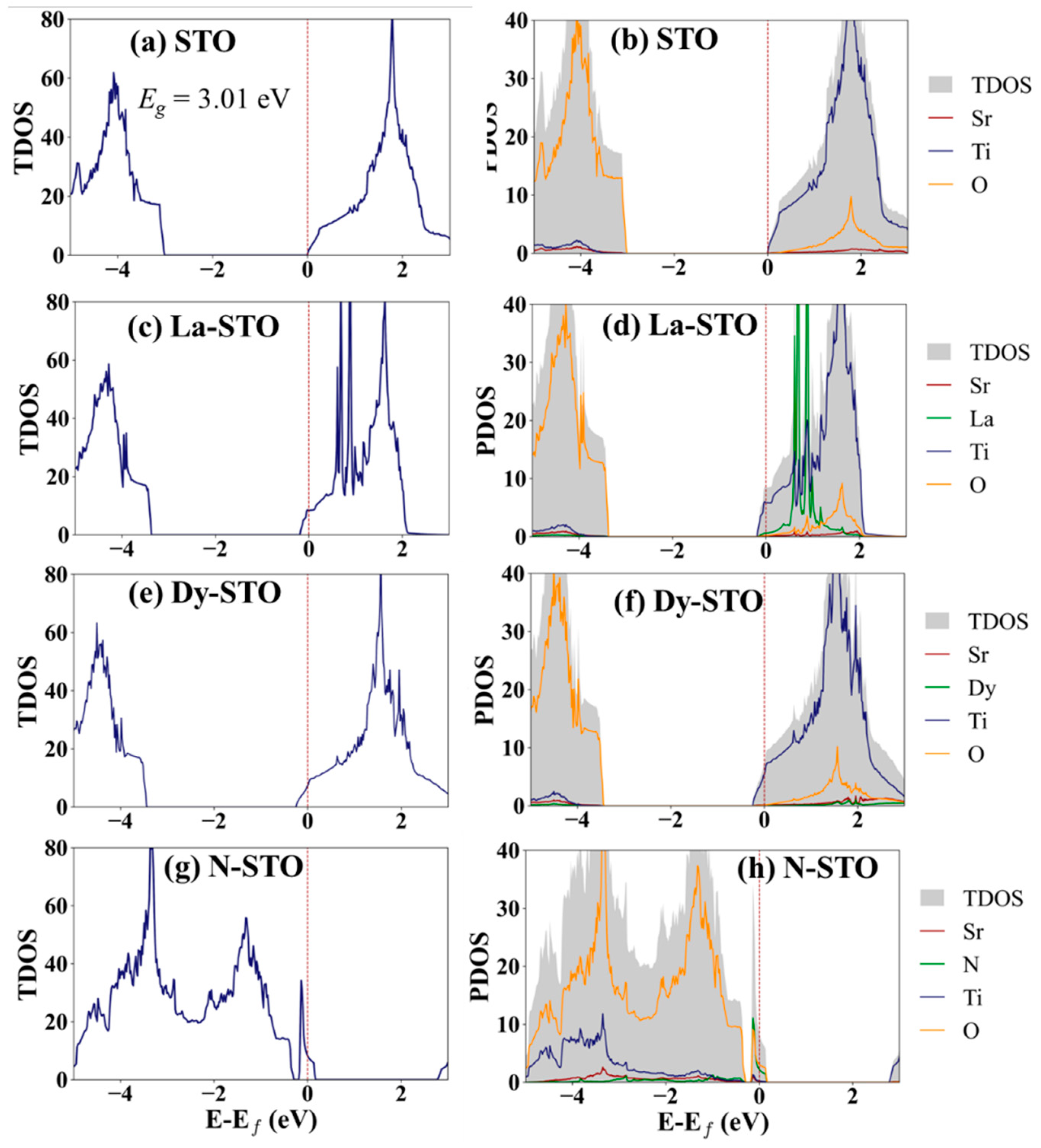
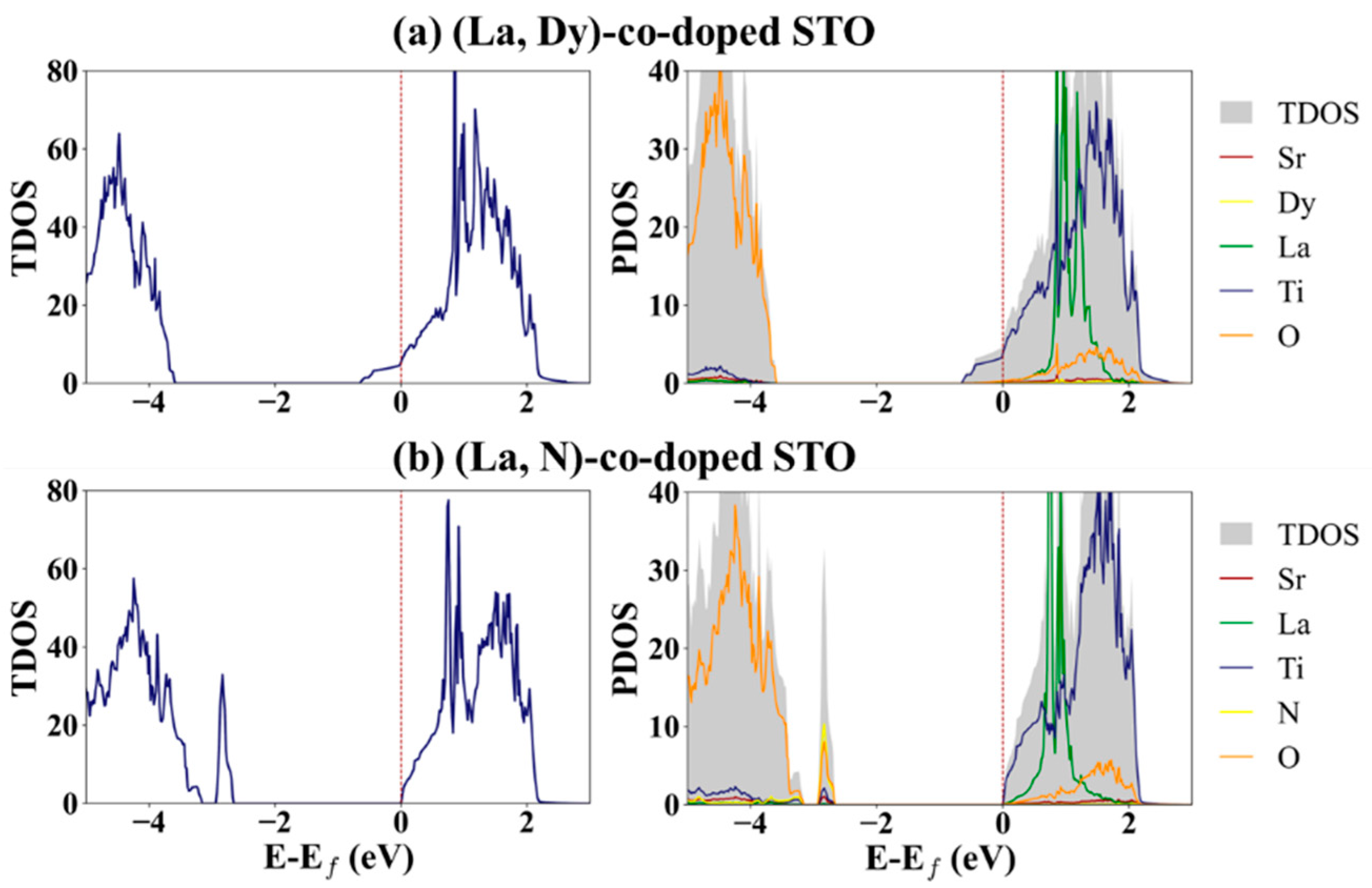
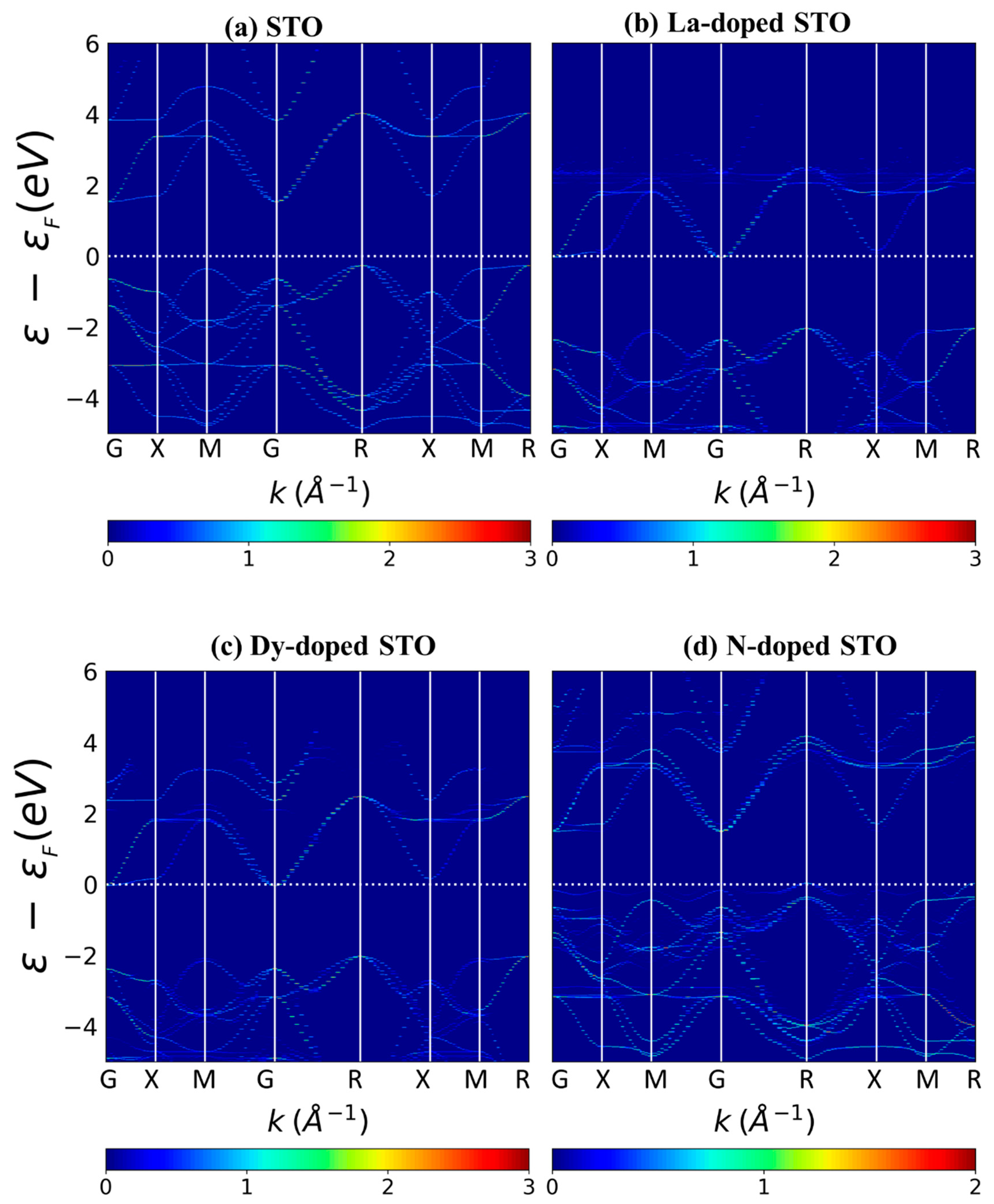
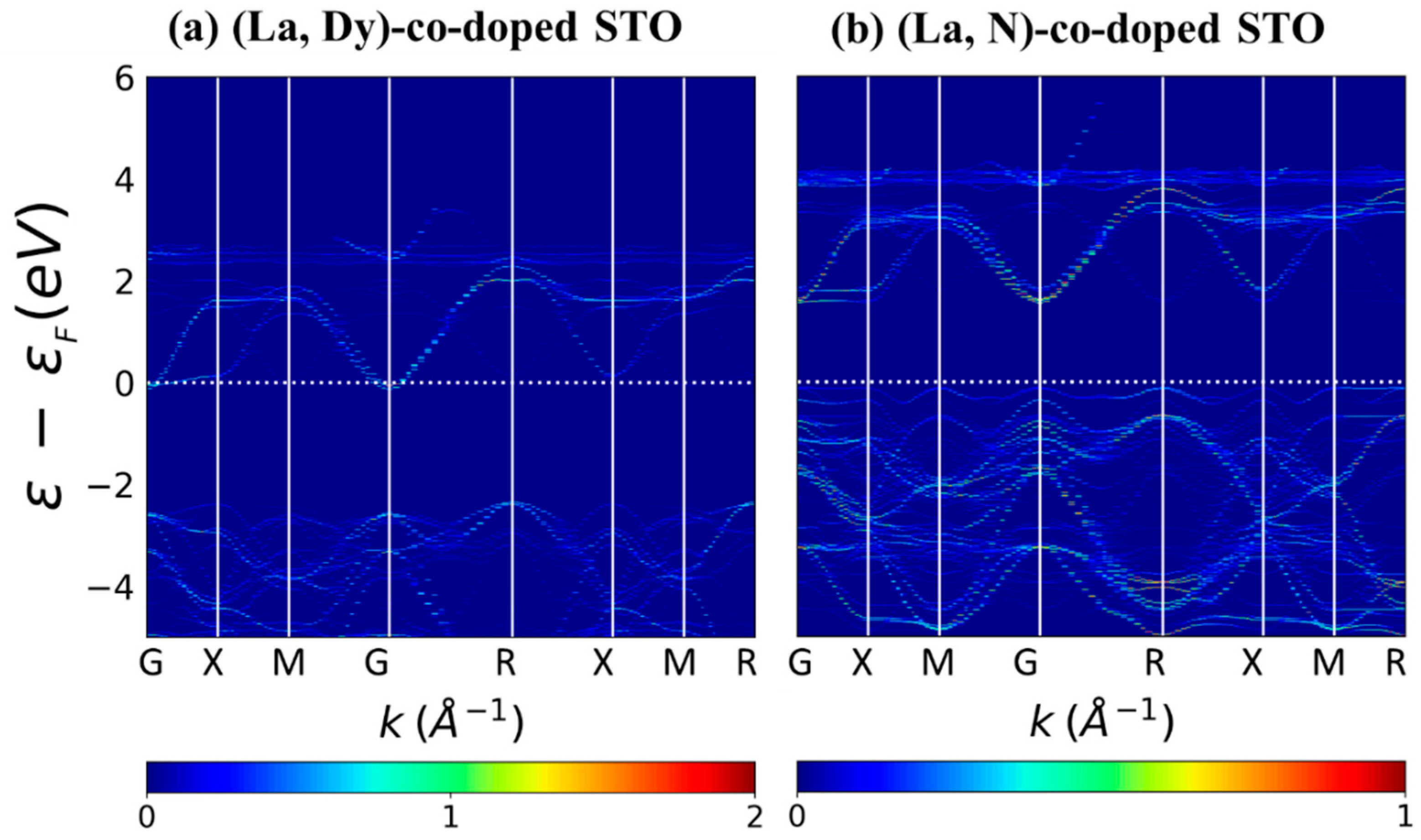
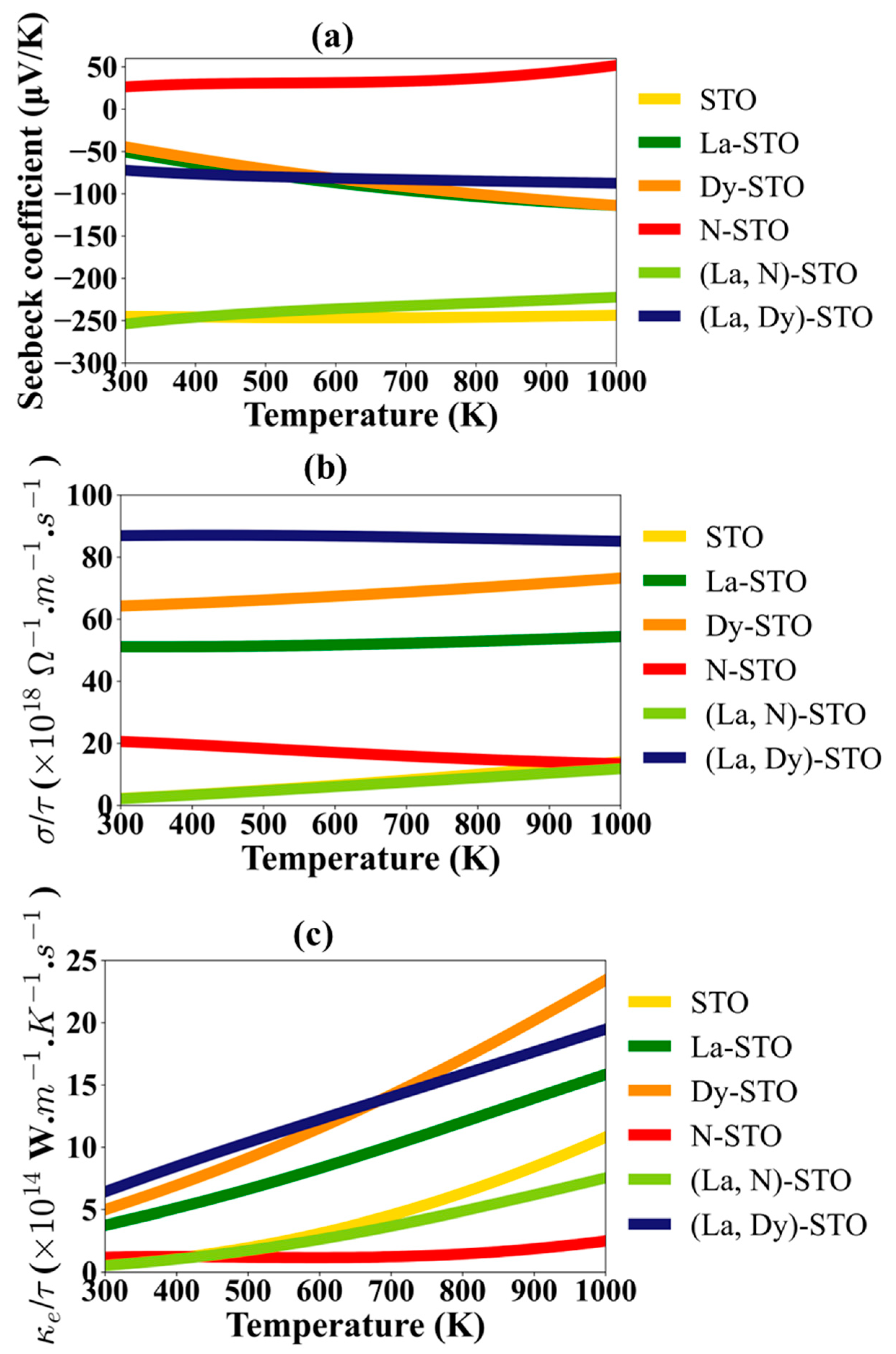
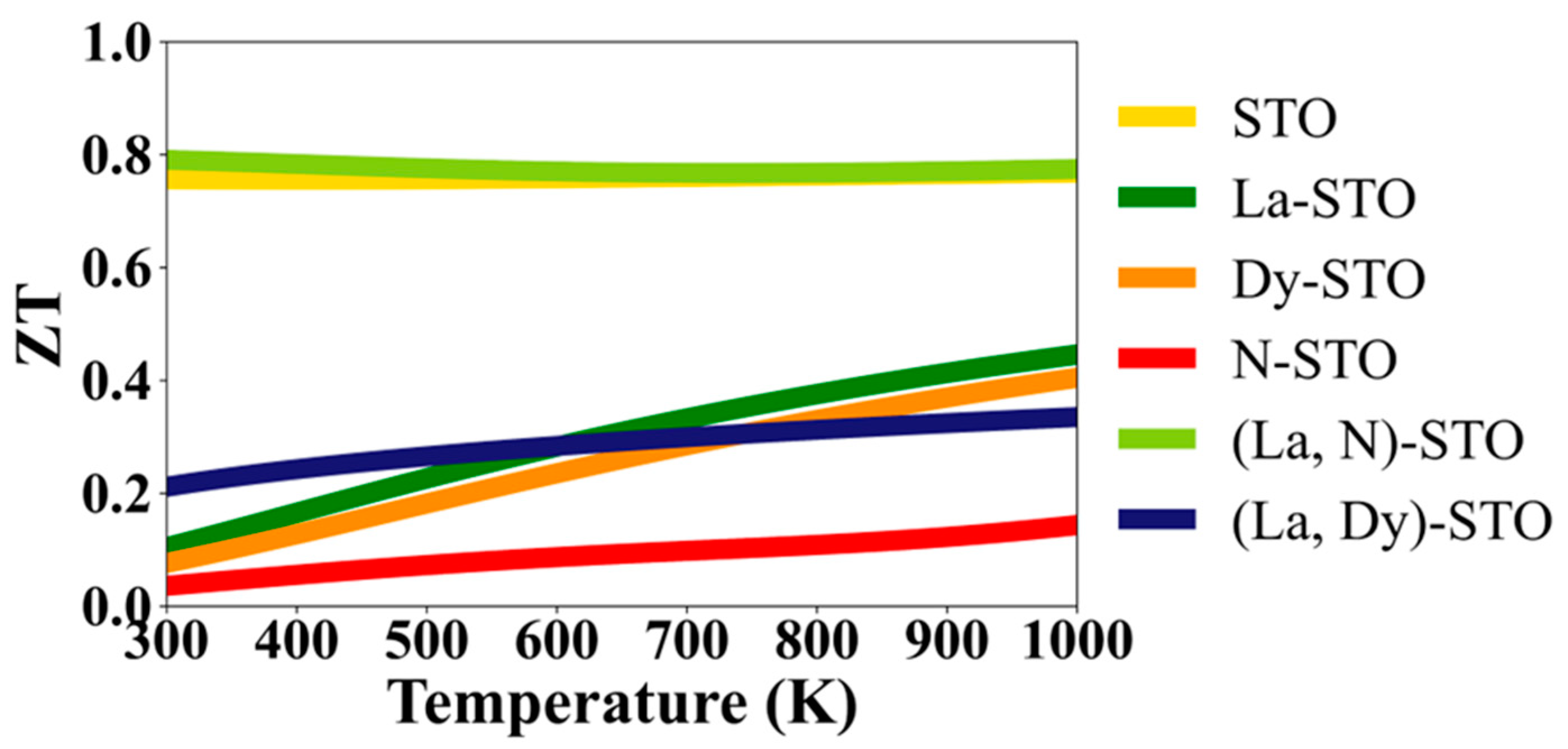
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Li, P.; Xu, D.; Luo, B.; Hong, Y.; Shi, W.; Liu, C. Hydrothermal Synthesis and Visible-Light-Driven Photocatalytic Degradation for Tetracycline of Mn-Doped SrTiO3 Nanocubes. Appl. Surf. Sci. 2015, 333, 39–47. [Google Scholar] [CrossRef]
- Jayabal, P.; Sasirekha, V.; Mayandi, J.; Jeganathan, K.; Ramakrishnan, V. A Facile Hydrothermal Synthesis of SrTiO3 for Dye Sensitized Solar Cell Application. J. Alloys Compd. 2014, 586, 456–461. [Google Scholar] [CrossRef]
- Okamoto, Y.; Fukui, R.; Fukazawa, M.; Suzuki, Y. SrTiO3/TiO2 Composite Electron Transport Layer for Perovskite Solar Cells. Mater. Lett. 2017, 187, 111–113. [Google Scholar] [CrossRef][Green Version]
- Aravinthkumar, K.; John Peter, I.; Anandha Babu, G.; Navaneethan, M.; Karazhanov, S.; Raja Mohan, C. Enhancing the Short Circuit Current of a Dye-Sensitized Solar Cell and Photocatalytic Dye Degradation Using Cr Doped SrTiO3 Interconnected Spheres. Mater. Lett. 2022, 319, 132284. [Google Scholar] [CrossRef]
- Huang, S.-T.; Lee, W.W.; Chang, J.-L.; Huang, W.-S.; Chou, S.-Y.; Chen, C.-C. Hydrothermal Synthesis of SrTiO3 Nanocubes: Characterization, Photocatalytic Activities, and Degradation Pathway. J. Taiwan Inst. Chem. Eng. 2014, 45, 1927–1936. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, Y.; Shi, J.; Zhang, Z.; Ma, Z.; Yao, B.; Yu, X.; Wang, X. Microwave-Based Preparation of γ-Fe2O3/SrTiO3 Photocatalyst for Efficient Degradation of Organic Pollutants in Water. Mater. Chem. Phys. 2022, 288, 126357. [Google Scholar] [CrossRef]
- Valian, M.; Soofivand, F.; Yusupov, M.M.; Salavati-Niasari, M. Facile Synthesis of SrTiO3/CoAlMnO4 Nanocomposite: A Rechargeable Heterojunction Photocatalyst with Superior Hydrogen Storage Capability. Int. J. Hydrog. Energy 2022, 47, 31624–31637. [Google Scholar] [CrossRef]
- Niishiro, R.; Tanaka, S.; Kudo, A. Hydrothermal-Synthesized SrTiO3 Photocatalyst Codoped with Rhodium and Antimony with Visible-Light Response for Sacrificial H2 and O2 Evolution and Application to Overall Water Splitting. Appl. Catal. B Environ. 2014, 150, 187–196. [Google Scholar] [CrossRef]
- Sakata, Y.; Miyoshi, Y.; Maeda, T.; Ishikiriyama, K.; Yamazaki, Y.; Imamura, H.; Ham, Y.; Hisatomi, T.; Kubota, J.; Yamakata, A.; et al. Photocatalytic Property of Metal Ion Added SrTiO3 to Overall H2O Splitting. SI:Photocatalysis 2016, 521, 227–232. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, C.; Chang, Y.; Feng, Y.; Yang, Z.; Yang, T.; Lou, L.-L.; Liu, S. Novel Three-Dimensionally Ordered Macroporous SrTiO3 Photocatalysts with Remarkably Enhanced Hydrogen Production Performance. Appl. Catal. B Environ. 2017, 200, 514–520. [Google Scholar] [CrossRef]
- Muta, H.; Kurosaki, K.; Yamanaka, S. Thermoelectric Properties of Rare Earth Doped SrTiO3. J. Alloys Compd. 2003, 350, 292–295. [Google Scholar] [CrossRef]
- Fu, Q.-Q.; Gu, H.; Xing, J.-J.; Cao, Z.; Wang, J. Controlling the A-Site Deficiency and Oxygen Vacancies by Donor-Doping in Pre-Reductive-Sintered Thermoelectric SrTiO3 Ceramics. Acta Mater. 2022, 229, 117785. [Google Scholar] [CrossRef]
- Ma, Z.; Qi, Y.; Wang, J.; Bi, X.; Liu, X.; Li, X.; Li, J.; Sun, X. Effect of Annealing Temperature in Carbon Powder on Thermoelectric Properties of the SrTiO3−δ Single Crystal in the [111] Crystal Orientation. Ceram. Int. 2022, 48, 18876–18883. [Google Scholar] [CrossRef]
- Sun, J.; Singh, D.J. Thermoelectric Properties of N-Type SrTiO3. APL Mater. 2016, 4, 104803. [Google Scholar] [CrossRef]
- Zhou, E.; Raulot, J.-M.; Xu, H.; Hao, H.; Shen, Z.; Liu, H. Structural, Electronic, and Optical Properties of Rare-Earth-Doped SrTiO3 Perovskite: A First-Principles Study. Phys. B Condens. Matter 2022, 643, 414160. [Google Scholar] [CrossRef]
- Fadlallah, M.M.; Gogova, D. Theoretical Study on Electronic, Optical, Magnetic and Photocatalytic Properties of Codoped SrTiO3 for Green Energy Application. Micro Nanostructures 2022, 168, 207302. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, P.; Qin, M.; Lou, Z.; Gong, L.; Xu, J.; Kong, J.; Yan, H.; Gao, F. Effect of La3+, Ag+ and Bi3+ Doping on Thermoelectric Properties of SrTiO3: First-Principles Investigation. Ceram. Int. 2022, 48, 13803–13816. [Google Scholar] [CrossRef]
- S, C.P.; Jose, R.; Vijay, A.; P, V.; K, V.S. Tuning Thermoelectric Properties of Nb and Ta Co-Doped SrTiO3 Ceramics. Int. Conf. Adv. Mater. Innov. Sustain. 2022, 64, 464–467. [Google Scholar] [CrossRef]
- Suen, C.H.; Zhang, L.; Yang, K.; He, M.Q.; Chai, Y.S.; Zhou, K.; Wang, H.; Zhou, X.Y.; Dai, J.-Y. High Thermoelectric Performance of ZrTe2/SrTiO3 Heterostructure. J. Mater. 2022, 8, 570–576. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Jiang, X.; Kang, H.; Yang, X.; Zhang, X.; Wang, T. Ultrahigh Electrical Conductivities and Low Lattice Thermal Conductivities of La, Dy, and Nb Co-Doped SrTiO3 Thermoelectric Materials with Complex Structures. J. Mater. Sci. Technol. 2020, 52, 172–179. [Google Scholar] [CrossRef]
- Shang, P.-P.; Zhang, B.-P.; Li, J.-F.; Ma, N. Effect of Sintering Temperature on Thermoelectric Properties of La-Doped SrTiO3 Ceramics Prepared by Sol–Gel Process and Spark Plasma Sintering. Solid State Sci. 2010, 12, 1341–1346. [Google Scholar] [CrossRef]
- Zhang, T.; Wan, R.; Guo, Y.; Ahmed, A.J.; Lei, Y.; Tian, G. Lanthanum-Doped SrTiO3 Theoretical Thermoelectric Properties. Ionics 2022, 28, 2021–2028. [Google Scholar] [CrossRef]
- Gillani, S.S.A.; Ahmad, R.; Islah-u-din; Rizwan, M.; Shakil, M.; Rafique, M.; Murtaza, G.; Jin, H.B. First-Principles Investigation of Structural, Electronic, Optical and Thermal Properties of Zinc Doped SrTiO3. Optik 2020, 201, 163481. [Google Scholar] [CrossRef]
- Gillani, S.S.A.; Jawad, A.; Zeba, I.; Shakil, M.; Tahir, M.B.; Ahmad, R. Effect of Li, K and Be Doping on Phase Stability, Band Structure and Optoelectronic Response of SrTiO3 Perovskite for Semiconductor Devices: A Computational Insight. Optik 2021, 227, 166044. [Google Scholar] [CrossRef]
- Wang, H.C.; Wang, C.L.; Su, W.B.; Liu, J.; Zhao, Y.; Peng, H.; Zhang, J.L.; Zhao, M.L.; Li, J.C.; Yin, N.; et al. Enhancement of Thermoelectric Figure of Merit by Doping Dy in La0.1Sr0.9TiO3 Ceramic. Mater. Res. Bull. 2010, 45, 809–812. [Google Scholar] [CrossRef]
- Ito, M.; Matsuda, T. Thermoelectric Properties of Non-Doped and Y-Doped SrTiO3 Polycrystals Synthesized by Polymerized Complex Process and Hot Pressing. J. Alloys Compd. 2009, 477, 473–477. [Google Scholar] [CrossRef]
- Dadgostar, S.; Pura Ruiz, J.L.; Serrano Gutierrez, J.; Lepine, B.; Schieffer, P.; Jimenez, J. Luminescence in Undoped and Nb-Doped SrTiO3 Crystals: Bulk and Surface Emission. Mater. Sci. Eng. B 2022, 283, 115830. [Google Scholar] [CrossRef]
- Blennow, P.; Hagen, A.; Hansen, K.K.; Wallenberg, L.R.; Mogensen, M. Defect and Electrical Transport Properties of Nb-Doped SrTiO3. Solid State Ion. 2008, 179, 2047–2058. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okinaka, N.; Akiyama, T. A Large Thermoelectric Figure of Merit of La-Doped SrTiO3 Prepared by Combustion Synthesis with Post-Spark Plasma Sintering. Scr. Mater. 2010, 63, 407–410. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, Y.Z.; Jing, Y.; Yao, Y.; Ma, J.; Sun, J.H. Effects of N Concentration on Electronic Structure and Optical Absorption Properties of N-Doped SrTiO3 from First Principles. Mater. Sci. Forum 2013, 749, 561–568. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Madsen, G.K.H.; Singh, D.J. BoltzTraP. A Code for Calculating Band-Structure Dependent Quantities. Comput. Phys. Commun. 2006, 175, 67–71. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W. VASPKIT: A Pre- and Post-Processing Program for VASP Code. arXiv 2019, arXiv:1908.0826. [Google Scholar]
- Okuda, T.; Nakanishi, K.; Miyasaka, S.; Tokura, Y. Large Thermoelectric Response of Metallic Perovskites: Sr1−xLaxTiO3 (0<~ x<~0.1). Phys. Rev. B 2001, 63, 113104. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Guimarães, L.G.; dos Santos, C.A.M.; de Lima, B.S.; da Luz, M.S. Electrical and Thermodynamic Study of SrTiO3 Reduction Using the van Der Pauw Method. Mater. Chem. Phys. 2021, 263, 124428. [Google Scholar] [CrossRef]
- Sikam, P.; Moontragoon, P.; Sararat, C.; Karaphun, A.; Swatsitang, E.; Pinitsoontorn, S.; Thongbai, P. DFT Calculation and Experimental Study on Structural, Optical and Magnetic Properties of Co-Doped SrTiO3. Appl. Surf. Sci. 2018, 446, 92–113. [Google Scholar] [CrossRef]
- Fo, Y.; Wang, M.; Ma, Y.; Dong, H.; Zhou, X. Origin of Highly Efficient Photocatalyst NiO/SrTiO3 for Overall Water Splitting: Insights from Density Functional Theory Calculations. J. Solid State Chem. 2020, 292, 121683. [Google Scholar] [CrossRef]
- Sikam, P.; Sararat, C.; Moontragoon, P.; Kaewmaraya, T.; Maensiri, S. Enhanced Thermoelectric Properties of N-Doped ZnO and SrTiO3: A First-Principles Study. Appl. Surf. Sci. 2018, 446, 47–58. [Google Scholar] [CrossRef]
- Atkinson, I.; Parvulescu, V.; Pandele Cusu, J.; Anghel, E.M.; Voicescu, M.; Culita, D.; Somacescu, S.; Munteanu, C.; Šćepanović, M.; Popovic, Z.V.; et al. Influence of Preparation Method and Nitrogen (N) Doping on Properties and Photo-Catalytic Activity of Mesoporous SrTiO3. J. Photochem. Photobiol. Chem. 2019, 368, 41–51. [Google Scholar] [CrossRef]
- Bilotti, E.; Fenwick, O.; Schroeder, B.; Baxendale, M.; Taroni-Junior, P.; Degousée, T.; Liu, Z. 6.14 Organic Thermoelectric Composites Materials. In Comprehensive Composite Materials II; Beaumont, P.W.R., Zweben, C.H., Eds.; Elsevier: Oxford, UK, 2018; pp. 408–430. [Google Scholar] [CrossRef]
- Lv, X.; Chen, G.; Wei, K.; Dai, R.; Wang, M.; Yu, K.; Geng, S. Influence of La Doping Concentration and A-Site Deficiency on Electrical Conductivity of La Substituted SrTiO3 and Its Chemical Compatibility with ScSZ. Ceram. Int. 2022, 48, 27527–27535. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Lei, W.; Sinclair, D.C.; Reaney, I.M. High-Figure-of-Merit Thermoelectric La-Doped A-Site-Deficient SrTiO3 Ceramics. Chem. Mater. 2016, 28, 925–935. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, L.M.; Ganz, E. Electrocatalytic reduction of CO2 by two-dimensional transition metal porphyrin sheets. J. Mater. Chem. A 2019, 7, 11944–11952. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, H.; Cheng, X. Electronic and magnetic properties of all 3d transition-metal-doped ZnO monolayers. Int. J. Quantum Chem. 2013, 113, 2243–2250. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikam, P.; Thirayatorn, R.; Kaewmaraya, T.; Thongbai, P.; Moontragoon, P.; Ikonic, Z. Improved Thermoelectric Properties of SrTiO3 via (La, Dy and N) Co-Doping: DFT Approach. Molecules 2022, 27, 7923. https://doi.org/10.3390/molecules27227923
Sikam P, Thirayatorn R, Kaewmaraya T, Thongbai P, Moontragoon P, Ikonic Z. Improved Thermoelectric Properties of SrTiO3 via (La, Dy and N) Co-Doping: DFT Approach. Molecules. 2022; 27(22):7923. https://doi.org/10.3390/molecules27227923
Chicago/Turabian StyleSikam, Pornsawan, Ruhan Thirayatorn, Thanayut Kaewmaraya, Prasit Thongbai, Pairot Moontragoon, and Zoran Ikonic. 2022. "Improved Thermoelectric Properties of SrTiO3 via (La, Dy and N) Co-Doping: DFT Approach" Molecules 27, no. 22: 7923. https://doi.org/10.3390/molecules27227923
APA StyleSikam, P., Thirayatorn, R., Kaewmaraya, T., Thongbai, P., Moontragoon, P., & Ikonic, Z. (2022). Improved Thermoelectric Properties of SrTiO3 via (La, Dy and N) Co-Doping: DFT Approach. Molecules, 27(22), 7923. https://doi.org/10.3390/molecules27227923





