A Review of In Situ Methods—Solid Phase Adsorption Toxin Tracking (SPATT) and Polar Organic Chemical Integrative Sampler (POCIS) for the Collection and Concentration of Marine Biotoxins and Pharmaceuticals in Environmental Waters
Abstract
1. Introduction
2. Marine Biotoxins
2.1. Lipophilic Biotoxins
2.1.1. Yessotoxins (YTXs)
2.1.2. Azaspiracids (AZAs)
| Biotoxin | Limited Level |
|---|---|
| Paralytic Shellfish Poison (PSP) | <800 microgram/kg |
| Amnesic Shellfish Poison (ASP) | <20 milligrams of domoic acid/kg |
| okadaic acid (OA), dinophysistoxins and pectenotoxins together | <160 micrograms of OA equivalents/kg |
| Yessotoxins (YTXs) | <3.75 milligram of YTX equivalent/kg |
| Saxitoxin (STXs) | ≤800 µg STX.2HCL equivalent/kg |
| Azaspiracids (AZAs) | <160 micrograms of AZA equivalents/kg |
| Domoic acid (DA) | <20 mg domoic acid/kg |
| Brevetoxin | <200 mouse units or equivalent |
| Ciguatoxins | <0.1 µg/kg fish |
2.2. Hydrophilic Biotoxins
2.2.1. Domoic Acid
2.2.2. Saxitoxin (STX)
2.3. Emerging Biotoxins
2.3.1. Brevetoxins
2.3.2. Ciguatoxins
| Toxin | Formula | MW (g/mol) | Chemical Class | Syndrome Category | Solubility | Origin | Polarity | Refs. |
|---|---|---|---|---|---|---|---|---|
| Okadaic acid (OA) | C10H17N7 O4 | 804 | Polyether, spiro-keto ring assembly | DSP | Lipophilic | Halichondria okadaii | Low polarity | [30,41] |
| Yessotoxin (YTX) | C55H82O21S2 | 1143.4 | Sulfur bearing polyether | Gastrointestinal, Neurological | Lipophilic | Protoceratium reticulatum, Lingulodinium polyedrum and Gonyaulax spinifera | Highly polar | [50] |
| Azaspiracids (AZA) | C47H71NO12 | 842.1 | Polyether, second amine, 3-spiro-ring assembly | DSP | Lipophilic | A. spinosum | Low polarity | [66,114] |
| Domoic acid (DA) | C15H21NO6 | 311 | Cyclic amino acid, 3 carboxyilic acid groups | ASP | Hydrophilic | Phytoplankton | Highly polar | [115] |
| Saxitoxin (STX) | C10H17N7O4 | 299.3 | Tetrahydro-purine alkaloid | PSP | Hydrophilic | Phytoplankton | High polarity | [89] |
| Brevetoxin (PbTxs) | C49H70O13 | 867.1 | Polyether with contiguously fused rings | NSP | Lipophilic | Dinoflagellates | Polar | [99,101] |
| Ciguatoxins (CTX) | C60H86O19 | 1111.313 | Polyether | Gastrointestinal, Cardiovascular, Neurological | Lipophilic | Dinoflagellate | Polar to moderate polarity | [105] |
3. Solid Phase Absorption Toxin Tracking
3.1. SPATT Bag Construction and Adsorbent Phase Activation
3.2. SPATT Sorbents
3.2.1. Aromatic Adsorbents
3.2.2. Modified Aromatic Adsorbents
3.2.3. Methacrylic Adsorbents
3.2.4. Other SPATT Adsorbents
3.2.5. SPATT Sorbent Comparisons
3.3. SPATT Bag Preparation and Analyte Extraction
3.4. SPATT Bag Storage and Stability
- (i)
- simplicity, low cost, ease of application, transport and storage [160];
- (ii)
- allows sampling throughout the water column where no shellfish exist naturally [19];
- (iii)
- (iv)
- impervious to biotransformation with no sign of degradation when stored in −20 °C [160];
- (v)
- a sufficient pre-concentration technique to ensure adequate adsorption and analytical detection;
- (vi)
- can be used as an early warning system for bloom events when coupled with appropriate analysis (e.g., ELISA, LC-MS) [160];
- (vii)
- (viii)
- profiles the water for toxins generated by HABs prior to their biochemical transformations within shellfish tissues that leads to a variety of toxin derivatives and
- (ix)
- assesses biotoxin frequency, and the duration of algae blooms in a specific region [160].
- (i)
- high cost of instrumentation with training requirements and complex sample preparation and clean-up optimisation and validation processes;
- (ii)
- biochemical transformations within shellfish tissues leads to a variety of toxin derivatives, a more complex toxin profile than what originated from the HABs and
- (iii)
3.5. SPATT Applications
3.5.1. Application of SPATT to Marine and Freshwater Toxins
| SPATT Resins | Toxins Detected | Year | Country | Elute | Application | Deployment Condition | Adsorbent Quantity | Analyte % Recoveries | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| DIAION HP-20 (Bags) | PTX, PTX2 SA, PTX11, PT11 SA, OA, OA-ester & YTX. | 2004 | NZ | MeOH Ace MeOH > Ace | Marine | Deployed at selected depth | 12 mL = 3 g dry weight | Ave. = 62% | [116] |
| DIAION SP-207 (Bags) | OA, DTX1, PTX2, YTX, 36% less recovery than DIAION HP-20 | 2004 | NZ | MeOH Ace MeOH > Ace | Marine | Deployed at selected depth | 12 mL = 3 g dry weight | Ave. = 36% | [116] |
| DIAION HP-20 (Large scale pumping) | OA DTX-2 PTX-2 PTX-2SA | 2007 | Norway | MeOH | Marine | Seawater | 0.5 kg/column | DTX-2: 73% OA: 78% Accumulation: 2.7 mg OA, 1.3 mg DTX-2 and 1.8 mg PTX-2 during an 18-h | [161] |
| DIAION HP-20, SP850, Sepabeads1 SP825L, Amberlite1 XAD4, Dowex1 Optipore1 L-493 (Bags & Disks) | OA, DTX2, PTX2, AZA1, -2 and -3 | 2008 | Ireland | MeOH | Marine | Deployed | 3 g | OA and DTX1 were determined in positive ionisation mode | [172] |
| Membrane (Polycarbonate, polyethersulfone, polyester, nylon) and POCIS Oasis HLB | MC-RR, MC- LR | 2008 | Czech Republic | 90:10 v/v MeOH/water Acidified with 0.1% TFA | Fresh water | Exposed in a natural reservoir | Membrane exchanging area: 47.5 cm2, Oasis HLB: 2.75, 5.55, 11.1 mg/cm2 | MC-RR: 0.022 L/day, MC-LR: 0.017 L/day | [168] |
| POCIS Oasis HLB | 29 organic chemicals: Antibiotics, fungicides, herbicides, biocides | 2009 | Spain | MeOH acidified in three different levels | Marine | Fish farm | 200 mg | The detected conc’s do not have impact on aquatic organism | [179] |
| Diaion HP20 (disk) | D. acuta bloom | 2009 | Spain | MeOH | Marine | Deployed at different depth | 3 g | Plankton: PTX2 ranged from 19–73 pg/cell | [180] |
| DIAION HP-20 (Disks) | OA, PTX, YTX & AZA group | 2009 | Ireland | MeOH | Marine | Deployed at different depth | 3 g | Accumulation rate of toxins in the mussels and SPATT discs correlated | [177] |
| DIAION HP-20 (Disks) | 20-methylSPX-G, AZA-1, AZA-2, OA, DTX-1, DTX-2, PTX-2, PTX-12 & YTX. | 2009 | Norway | MeOH | Marine and Fresh water | Deployed attaching to a fixed point (1 m depth) | 3 g | PTX-2: 5–40 ng/disk 20-methylSPX-G: 706.5 ng/disk SPX-C: 164.2 ng/disk | [181] |
| SEPABEADS SP825L, SP850 & SP700 (Bags) | OA, PTX2, AZA, YTX | 2010 | NZ | MeOH | Marine | Deployed | - | OA: 61% PTX2: 22% AZA: 41% YTX: 47% | [160] |
| DIAION HP-20 (Disks) | OA, PTX, YTX and AZA group | 2010 | Ireland | MeOH | Marine (Deployed in four different depth) | Deployed Different depth | 3 g | Recovery discussed based on period and depth | [5] |
| HP20, SP700, SP207, SP207SS | Domoic acid and saxitoxin | 2010 | USA | MeOH | Coastal | Deployed | 3 g | SP700: 69–72% HP20: 99% | [170] |
| DIAON HP20 | MCY-RR and -LR, | 2010 | USA | MeOH | Freshwater and Lake water | Deployed | 3 g | 2.9 million ppb | [157] |
| Strata-X (Bags) | ATX, HTX, Dihydroanatoxin-a, Dihydrohomotoxin-a | 2011 | NZ | MeOH | Freshwater | River 1.2 m3/s | 1 g | 7% of water loading | [162] |
| PAC G-60 (Bags) | ATX, HTX, Dihydroanatoxin-a, Dihydrohomotoxin-a | 2011 | NZ | 5% FA and 70% MeOH | Freshwater | River 1.2 m3/s | 1 g | 4–12% of water loading | [162] |
| Diaion HP20 | CTX, MTX | 2011 | Spain | MeOH | Marine G. Pacificus culture | In vitro experiment | 10 g wet | CTX1B: 85.5–90.9% MTX: 66.2% | [169] |
| SP700 | PSP toxins | 2010 | Spain | MeOH | Marine | PSP and LSTs producing culture | 1 g | GTX2,3: 406.02 ± 13.30 ng/g resin STX: 219.02 ± 37.71 ng/g resin | [182] |
| DIAION HP-20 (Disks) | Spirolide C, iso-spirolide C,13-desmethylspirolide C, 20-methylspirolide G | 2011 | Norway | MeOH | Marine | Deployed | 3 g | Spirolide C: 69%, iso-spirolide C: 13%, 13-desmethylspirolide C: 22% 20-methylspirolide G: 77% 13,19-didesmethylspirolide C: 33% | [183] |
| SEPABEADS SP700 | Toxic Alexandrium okadaic acid, 13-desmethyl SPX C, 20-methyl SPX G | 2011 | Ireland | MeOH | Harbour | Deployed in water | 5 g | OA, DTX-2 and PTX: 2.5 ng/g | [184] |
| HP20 | Pinnatoxin (PnTx), analogues PnTx-E, PnTx-F, okadaic acid (OA) and its esters | 2011 | NZ | MeOH | Marine | Deployed over two summers | 4 g | OA: 14% PTXs: 50% OA-esters: 10% | [185] |
| DIAION HP20 | MC-LR, -YR, -LA, and -RR | 2011 | USA | MeOH | Freshwater | Deployed for 16 months | 3 g | MC-LR: 66.4 ng/L, 18,400 ng/g resin equal parts MC-RR, MC-YR, MC-LR | [156] |
| DIAION HP20 | Chlorophyll-a, Secchi depth, total phosphorus and total nitrogen | 2012 | NZ | MeOH | Lake | Deployed | 3 g | CYN82/91, CYN83/87/95) and the Calothrix sp. (CYN100) had low similarities (<94%) to GenBank sequences | [186] |
| HP20 and SP700 | Cyanobacterial cultures | 2013 | China | MeOH | Freshwater M. aeruginosa cultures | Deployed | 2 g | HP20 better result than SP700 | [173] |
| HP20 | OA, DTX2, PTX2) | 2013 | Spain | MeOH | Marine | Deployed at 3, 7 and 12 m depths | 2.5 g | OA/DTX2: 1.5–6.0 ng PTX2: 1.8–7.0 ng PTX2SA: 0.5–3.0 ng | [187] |
| Diaion HP20, Strata-X, Oasis HLB, BondElut C18 | SPX1, AZA1, PnTX-G, ovTX-a | 2014 | France | MeOH | Seawater Agilent reservoir | Conditioning method the same as Shea et al. 2006 | Oasis (30 mg), Strata-X (200 mg), HP20 (200 mg), Bond Elut C18 (200 mg) | SPX1: 14 ng, AZA1: 19 ng, PnTX-G: 238 ng, ovTX-a: 359 ng | [2] |
| HP20 XAD761 | DSP toxins | 2014 | Ireland | MeOH: Water 80:20 | Marine | Deployed at different depth | 5 g of each separately | HP20 XAD761 | [165] |
| DIAON HP20 | Cyclic imines (SPXs, PnTXs, GYMs) | 2014 | Spain | MeOH | Marine | Deployed | 10 g wet | DIAON HP20 | [188] |
| DIAION HP-20 resin | Microcystin-LR, RR, YR, LA | 2014 | US | MeOH | Freshwater | Deployed blow surface water | 3 g | DIAION HP-20 resin | [189] |
| HP20 | A range of lipophilic toxins | 2015 | France | MeOH | Marine | Deployed | 300 mg | HP20 | [190] |
| Amberlite XAD761, HP20 | OA, PTX2, DTX2 | 2015 | Ireland | 80:20 MeOH: Water | Marine | Deployed in different depth | 5 g dry weight | Amberlite XAD761, HP20 | [6] |
| Diaion HP20 | OA, DTX1, DTX2, PTX2 | 2016 | Spain | MeOH | Marine | Deployed in semi enclosed river at 3 m depth | 10 g | OA 17.75 pg/cell PTX2 13.2 pg/cell DTX1 trace amount | [191] |
| HP20 | PTX2, D. fortii, D. acuminate, P. rotundatum, OA, DTX1 | 2016 | China | MeOH | Marine | Deployed at 8 m depth | 3 g | D. fortii (0.28 pg/cell), D. acuminata complex (0.08 pg/cell) & P. rotundatum (D. rotundata) (0.02 pg/cell). PTX2 (nd~5.7 mg/kg), OA (nd~2.8 mg/kg) and DTX1 (nd~1.6 mg/kg) | [192] |
| HP20 | OA, DTX1, PTX2, PTX2sa, 13-desMe-C, PnTX-G | 2016 | France | MeOH | Marine | Deployed during summer | 0.3, 3, 10 g | The higher amount of resin captured more toxins | [154] |
| HP20 | DSTs, AZA | 2017 | USA | MeOH | Marine | Deployed | 3 g | Conc’s during different month are discussed. | [193] |
| SPATT HP20 | Microcystins | 2017 | USA | MeOH | Water reservoir and lake | Deployed at different sites | 3 g | MC-LR: ~88%, MC-YR: ~100%, MC-LA: ~100% | [178] |
| HP20 | Gambierdiscus toxins (CTXs) | 2018 | France | MeOH | Marine | Deployed | 20 g | 55 ng P-CTX-3C equiv./g resin | [194] |
| DIAION HP20 | DSTs | 2018 | USA | MeOH & then MeOH: ammonium acetate | Marine | Deployed | 3 g | Four toxins were identified in 37% of mussels. one toxin in 99% of mussels | [176] |
| Strata-X | Toxic Microcoleus autumnalis (Basionym Phormidium autumnale)-dominated | 2018 | NZ | MeOH acidified with FA | Stream water | Deployed in River 10, 20, 40 m | 1 g | 0.91 ng mL−1 and 95 ng g−1 of strata-x hr−1 | [195] |
| HP20 (Diaion) and XAD-2 (Amberlite) | OA, STX, DTX1, PTX2, PTX2 isomers | 2018 | USA | ACN acidified with FA | Marine | Deployed | 3 g | For both resins: OA: 53% DTX1: 20% Esterified OA: 19% Esterified DTX1: 8% PTX2: 88% PTX2 isomers: 5% PTX11: 4% secoPTX2: 3% | [196] |
| Diaion HP20 | PCTXs, MTXs | 2018 | NZ | DCM and aqueous MeOH | Marine | In vitro | 2.5–10 g | PCTX- 3C (70%) P-CTX-1B (92%). MTX3 not possible to detect | [197] |
| HP20 | Domoic acid (DA), saxitoxin (STX), okadaic acid (OA) | 2019 | USA | Extract 1 50% MeOH (v/v) and Extract 2 and Extract 3 with 1 M C2H7NO2 in 50% MeOH | Marine | Deployed | DA from 9.2 to 37 ng/g STX from 1.3 to 5.3 ng/g | [198] | |
| HP20 | Phycotoxin pectenotoxin-2 (PTX2) | 2020 | Antarctica | MeOH | Marine | Deployed in cove | 10 g | Very low background conc. | [199] |
3.5.2. SPATT Sorbents and Biotoxin Harvesting
4. Polar Organic Chemical Integrative Sampler (POCIS)
4.1. POCIS Applications in the Marine Environment
| POCIS Resins | Analyte | Year | Country | Elute | Application | Deployment Condition | Adsorbent Quantity | Analyte %Recoveries | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Modified POCIS Strata-X and Chemcatcher™ (SDB-RPS) | Caffeine, Carbamazapine, Dapsone, DEET, Hydrochlorothiazide, Troclosan | 2014 | Australia | MeOH, ACN & Ace | Pharmaceuticals | Marine and freshwater Environments (grab sampling & passive sampler) | 600 mg PES membranes (47 mm diameter) with 147 mm thickness and a pore size of 0.2 mm (used on Chemcatcher with SDB-RPS) or 140 mm thickness and a pore size of 0.45 mm | Caf: 102% CBZ: 104% Dap: 74% DEET: 77% Hydro: 99% Tro: 84 | [214] |
| POCIS Oasis HLB | Carbamazapine (CBZ), Oxacarbamazapine (Ox), and their related metabolites | 2014 | Mediterranean Sea | MeOH | Pharmaceuticals one POCIS disk was placed into glass aquaria containing 1.5 L of filtered spiked seawater at 5 μg/L | Marine environment (lab experiment) | 200 mg | CBZ: 110 ± 4 (5 ng), 95 ± 11 (10 ng) & OX: 58 ± 7 (5 ng), 69 ± 3 (10 ng) | [212] |
| POCIS HLB PES membrane | 93 pharmaceuticals | 2018 | Sweden | DCM/ACN (8/2, v/v), & DCM | Pharmaceuticals | Marine (grab sampling) | 200 mg | Conc’s ranging between 0.01 & 80 ng/L | [215] |
4.2. POCIS and Wastewater Monitoring
| POCIS Resins | Analyte | Year | Country | Elute | Application | Deployment Condition | Adsorbent Quantity | Analyte %Recoveries | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Oasis HLB | Omeprazole, fluoxetine, azithromycin, levothyroxine, methamphetamine, and methylenedioxymethamphetamine | 2004 | USA | MeOH | Pharmaceuticals | WW River water Deployed | 200 mg | Azithromycin 15–66 ng/L, methamphetamine, 2 ng/L, methylenedioxymethamphetamine 0.5 ng/L | [216] |
| POCIS Oasis HLB | 25 pharmaceuticals and personal care products | 2007 | Canada | MeOH | Pharmaceuticals Uptake rates were 0.040 to 2.462 L/d in uptake rates between 0.016 and 0.223 L/d | WW and SW | 200 mg | RS values for 13 of the 25 analytes could be determined | [220] |
| Oasis HLB PES membrane | A range of Pharmaceuticals, personal care products, endocrine disrupting | 2010 | Switzerland | MeOH | Pharmaceuticals, personal care products, endocrine disrupting | Treated WW Flow rate 2.6 and 37 cm/s | 200 mg | Different recoveries based on flow rate is reported | [221] |
| Oasis HLB (pharmaceutical & pesticide), PES membrane | A range of pharmaceuticals, hormones and pesticide are reported | 2011 | US | MeOH | Pharmaceuticals, steroid hormones, pesticides | WW | 200 mg | Recoveries as reported in the paper | [222] |
| Oasis HLB PES membrane | Beta-blockers and hormones | 2012 | France | MeOH containing 5% NH4OH. | Pharmaceuticals, hormones | WW | 200 mg | Hormones low conc’s prevented determination of reliable sampling rates. Suitable for b-blockers | [223] |
| POCIS Oasis HLB | Atenolol, Prednisolone, Methylprednisolone, Sulfamethoxazole, Ofloxacin, Ketoprofen | 2013 | France | MeOH | Pharmaceuticals Uptake profiles (25 °C, flow velocity 0.16 m/s), an automatic sampler, taking 100 mL every 15 min between 7:00 a.m. and 9:00 p.m. and 100 mL every 30 min during the night. | Hospital sewage | 200 mg | 64–95% | [224] |
| SBSE Oasis HLB | 19 moderately hydrophobic to hydrophobic pesticides | 2013 | France | MeOH then, MeOH/EtOAc (75:25, v/v) | Pesticide | Agricultural WW | 200 mg | SBSE was able to integrate a concentration peak triggered by a quick flood | [225] |
| POCIS HLB PES membrane | Naproxen, Ibuprofen, triclosan | 2014 | South Africa | ACN, then ACN: MeOH, 50:50 (v/v), | Pharmaceuticals | WW | 200 mg | Naproxen 92%, Ibuprofen 108%, triclosan 75% | [226] |
| POCIS HLB | Acetaminophen, caffeine, 1,7-dimethylxanthine, cotinine, dextroamphetamine, diethyltoluamide (DEET), diphenhydramine, ibuprofen, methamphetamine, carbamazepine, azithromycin, erthyromycin, lincomycin, monensin, sulfachloropyridazine, sulfamethazine, sulfadimethoxine, sulfamethazole, sulfamethoxazole, sulfamerazine, sulfathiazole, thiabendazole, tiamulin, tylosin, and ractopamine | 2015 | Nebraska | MeOH | Pharmaceuticals | WW effluents | 200 mg | Results available for two different locations | [227] |
| POCIS HLB, PES membrane | 17-β-estradiol, 17-α-estradiol, 17-αethinylestradiol, estrone and estriol | 2016 | Czech republic | DCM, Ace, MeOH | Hormones | WW | 200 mg | 3.4 8 to 3.66 at nominal steroid concentration in water 100 ng/L | [228] |
| POCIS HLB | A range of Pharmaceuticals, artificial sweeteners, food additives, antibiotics personal care product, fragrances, sugar substitutes, steroid hormone | 2016 | Canada | MeOH | Pharmaceuticals, artificial sweeteners, food additives, antibiotics personal care product, fragrances, sugar substitutes, steroid hormone | WW | 220 mg | Detected concentrations discussed | [217] |
| POCIS HLB | Ciprofloxacin | 2016 | France | ACN | Pharmaceutical | Hospital effluents | 200 mg | Indicate a potential ecotoxicological risk | [229] |
| POCIS HLB PES membrane | Atrazine, carbendazim, desethylatrazine, desethylterbutylazine, diuron, S-metolachlor, terbutylazine, alprazolam, atenolol, carbamazepine, diazepam, diclofenac, ibuprofen, naproxen, 17-alpha-estradiol, 17-alpha-ethinylestradiol, 17-beta-estradiol, estriol, estrone, (BDE 28, BDE 47, BDE 99, BDE 100, BDE 153, BDE 154, PFOA, PFOS, Bisphenol A, triclosan | 2016 | Germany | - | Pesticides, pharmaceuticals, hormones, fluorinated surfactants, bisphenol A, triclosan | Treated WW | 200 mg | Recovery details discussed | [204] |
| C18 & triphasic | Alkylphenols (APs), several hormones, bisphenol-A (BPA), synthetic musk fragrances and herbicides, e.g., trifluralin (Tri) & alachlor (Ala), DES hormones | 2016 | Germany | DCM/EtOAc/MeOH (4:4:2, v/v) | Herbicides, alkyphenols, hormones | WW treatment plant | 200 mg | Recovery percentages vary between 6% for DES to 96% for Tri | [219] |
| POCIS OASIS HLB | A range of Pharmaceuticals and illicit drugs | 2017 | Norway | 5% NH4OH in MeOH, and 5% HOAc in MeOH | Pharmaceuticals and illicit drugs | WW | 220 mg | Results discussed in the paper | [218] |
| POCIS HLB PES membrane | PFHxA, PFOA, PFHxS, PFDoDA, PFOS | 2017 | China | MeOH containing 5% NH4OH | Perfluorinated compounds | WW | 30 mg | Concentration shown on diagram | [136] |
| POCIS HLB | 12 pharmaceuticals | 2017 | Ukraine | MeOH | Pharmaceuticals | Surface water Hospital WW | 200 mg | Removal patterns of pharmaceuticals were discussed based on specific physical chemical properties of molecules | [230] |
| POCIS HLB MIP membrane | BTEX, chlorinated pollutants and pharmaceuticals | 2017 | Czech republic | MeOH, & MeOH/DCM (1:1, v/v), then MeOH | Pharmaceuticals | Water remediation | 200 mg | (POCIS) for the pharmaceuticals and in situ soil microcosms for microbial community analysis, was proven | [231] |
| POCIS HLB PES membrane | Carbamazepine (CBZ) and sucralose (SCR) | 2018 | Brazil | MeOH/water (1:2, v/v), | Pharmaceuticals | Sewage | 200 mg | CBZ: <LOD 3.6 ng/g, SCR: <LOD 139.9 ng/g | [232] |
| POCIS | Clarithromycin, metoprolol, propranolol, carbamazepine, sulfamethoxazole, Atenolol | 2018 | Canada | - | Pharmaceuticals | WW | - | Recoveries compared over three years | [233] |
| Modified- POCIS Strata-X PES membrane | 8 organophosphate flame retardants (OPFRs) | 2018 | China | MeOH | Pharmaceuticals and their metabolites | WW | 200 mg | Results discussed | [234] |
| Oasis HLB PES membrane | Biocides, carbamazepine, diclofenac, terbutryne, diuron, carbendazime | 2020 | Luxembourg | DCM/ACN (1:1, v/v), | Pesticides, pharmaceuticals | WW | 200 mg | Results discussed in different flooding time | [203] |
| ODGT and POCIS Oasis HLB PES membrane | Neonicotinoids, organophosphates, triazines, antibiotics, b-blockers, SSRI’s, and sodium channel blockers | 2020 | Canada | - | Pharmaceuticals | WW | 200 mg | Quantitative comparison of o-DGT, POCIS is discussed | [201] |
4.3. Application of POCIS to the Detection of Pollutants in Freshwater, Rivers, Lakes and Drinking Water Sources
| POCIS Resins | Analyte | Year | Country | Elute | Application | Deployment Condition | Adsorbent Quantity | Analyte %Recoveries | Ref |
| Oasis HLB | Azithromycin; Fluoxetine; Levothyroxine; Omeprazole | 2004 | UK | MeOH | Pharmaceuticals | River, deployment at 8 sites | 200 mg | Ranging between 86–100% | [241] |
| Oasis Tribasic admixture | Atrazine, Diazinon, Diuron, 17a-Ethynylestradiol, Isoproturon | 2004 | UK | MeOH | Pharmaceuticals | River, deployment at 8 sites | 200 mg | Ranging between 88–99% | [241] |
| Oasis HLB PES, PE membrane | Estrone, 17-estradiol, 17-ethynylestradiol, bisphenol A, propranolol, sulfamethoxazole, meberverine, thioridazine, carbamazepine, tamoxifen, indomethacine, diclofenac and meclofenamic acid in sewage effluent and river water. | 2008 | UK | MeOH | Pharmaceuticals, endocrine disrupting compounds, personal care products | River | 100 mg | Propranolol, sulfamethoxazole, carbamazepine, indomethacine & diclofenac, varied between 3.0 & 45.6 ng L−1, <LOD & 17.6 ng L−1, 16.6 and 539 ng L−1, 0.4 & 7.2 ng L−1 & 2.4 & 65.2 ng L−1, respectively; applying POCIS, conc’s were between 2.8 & 40.5 ng L−1, <LOD &18.2 ng L−1, 26.3 & 427 ng L−1, 0.5 & 11.9 ng L−1 & 4.4 & 165 ng L−1, respectively. | [242] |
| Oasis HLB, PES membrane | Diuron, (1-(3,4-dichlorophenyl) urea (DCPU), 1-(3,4-dichlorophenyl)-3-methylurea (DCPMU) | 2010 | France | MeOH, & 75% MeOH/25%, EtOAc (v/v), | Herbicides | River | 500 mg | Conc’s of diuron and its transformation products in microcosm | [269] |
| Oasis HLB, PES membrane | 20 pesticide analytes | 2010 | USA | MeOH | Pesticides, polycyclic, aromatic hydrocarbons | Ground water cave | 200 mg | Vary during different month | [270] |
| Strata-X, PES membrane | E1, E2, EE2 | 2010 | UK | Ethyl acetate, & MeOH & ultrapure water | Endocrine disrupting substances | River | 300 mg | Ranging between 0.9–2.2 ng/L | [250] |
| Strata-X, PES membrane | Prometryn | 2011 | Germany | MeOH | Prometryn | River | 300 mg | 0.01–0.07 mg/L | [251] |
| Oasis HLB, PES membrane | Desethylatrazine, Deisopropylatrazine, Simazine, Desethylterbuthylazine, Atrazine, Metolachlor, Terbuthylazine | 2011 | France | MeOH, 75% & MeOH/25% ethyl acetate (v/v) | Pesticide | River deployment | 200 mg | After 24 h terbuthylazine > atrazine > simazine > metolachlor. Daily conc. varies | [10] |
| Oasis HLB, PES membrane | Range of substances in different pH reported | 2011 | Canada | MeOH | Pharmaceuticals, personal care products, disrupting substances | River & Tap water (Water chamber in lab) | 200 mg | Recoveries in different pH are reported | [174] |
| Oasis HLB, PES membrane | A wide range of pollutants Pharmaceuticals, Polycyclic aromatic hydrocarbons, Hormones, Pesticides, Phenols | 2011 | France | DCM/MeOH (50:50 v/v), | Pharmaceuticals, polycyclic aromatic hydrocarbons, hormones, phenols | River deployment | 200 mg | Recovery tables are reported in the paper | [244] |
| Oasis HLB | 33 Pesticides | 2011 | France | MeOH & MeOH/ethyl acetate, 75/25 (v/v) | Pesticide | River | 60 mg, 150 mg, 500 mg | Dimetomorph: 14.8 ng/L, linuron: 5.1 ng/L, metazachlor: 11.3 ng/L, terbuthylazine: 4.8 ng/L | [16] |
| Oasis HLB, PES membrane | Alkylphenols, Phenolated polymer, Oestrogenic hormones, Antidepressants, Anti-inflammatory, b-Blockers, Hypolipidemic agent | 2012 | France | - | Alkylphenols, phenolated polymers, hormones, pharmaceuticals | River and wastewater treatment plant | 200 mg | Results shows the diagnostic capacity of POCIS tools | [271] |
| Oasis HLB, PES membrane | Sulfamethoxazole | 2012 | Czech Republic | MeOH:water (9:1 v/v acidified with 0.1% TFA) | Pharmaceuticals Sulphonamides in stream | River | 200 mg | 20 up to 736 ng/L | [243] |
| Oasis HLB, PES membrane | Perfluorinated alkylcarboxylates | 2012 | Australia | 0.1% (v/v) ammonia solution in MeOH, then MeOH | Perfluorinated chemicals | Harbour | 200 mg | 0.1−12 ng/L | [272] |
| Oasis HLB, PES membrane | Range of pharmaceuticals | 2012 | France | MeOH, MeOH/DCM mixture (50:50), & DCM | Pharmaceuticals | River | 200 mg | Ranging between 51–97% | [245] |
| Oasis HLB PES membrane | A range of 14 different pesticides | 2012 | France | - | Pesticide | River | 200 mg | Concs. discussed | [206] |
| Oasis HLB PES membrane | Chloro, Propic a, Propic b, Hex, Phos | 2012 | USA | MeOH | Pesticide | Synthesized river water (Effect of flow velocity was assessed) | 200 mg | Levels of organic carbon (<0.1–5 mg/L) | [273] |
| Oasis HLB PES membrane | 21 pharmaceuticals, 6 alkylphenols and 27 hydrophilic pesticides and biocides | 2012 | France | MeOH & MeOH/DCM (v/v: 50/50), & DCM | Pharmaceuticals, alkylphenols and pesticides | Surface water | 200 mg | Ranging between 2.5–33 ng/L | [274] |
| Pharma-POCIS Oasis HLB | Polar pesticides | 2013 | France | MeOH | Pesticide | Ground water Deployed in 15 m depth and drinking water | 450 mg | POCIS could be tested on groundwater sites which present temporal variations in concentrations for studying its integrative capacity | [247] |
| Strata XAW, PES membrane | Range of prefluorinated chemicals | 2013 | Australia | 0.1% (v/v) ammonia sol in MeOH & MeOH | Perfluorinated chemicals | River | 600 mg | Ranging between 71–92% | [254] |
| POCIS HLB PES membrane | Terbuthylazine, diuron and linuron | 2014 | Switzerland | MeOH | Herbicides | River | 200 mg | Terbuthylazine: 220 ng/L, diuron: 70 ng/L linuron: 50 ng/L | [275] |
| POCIS HLB | Range of pesticides | 2014 | France | MeOH | Pesticides | River | 200 mg | Ranging between 138–1080 ng/L | [276] |
| POCIS HLB, PES membrane | 23 polar pesticides and 8 metabolites | 2014 | France | MeOH, & MeOH/ethyl acetate, 75:25 (v/v) | Pesticides | River | 200 mg | Details discussed in the paper | [277] |
| POCIS HLB, PES membrane | Atrazine | 2014 | Canada | MeOH | Pesticides | River | 200 mg | Atrazine conc. in 24 streams discussed | [278] |
| POCIS HLB PES membrane | Atrazine, propazine, terbutylazine, diclofenac, ibuprofen, ketoprofen, perfluorooctanoic acid and perfluorooctanesulfonate | 2014 | Italy | Acetone | Perfluorinated chemicals, pharmaceuticals, pesticides | River (linear velocity of 2.0, 5.1, 10.2 and 15.3 cm/s) | 200 mg | Spiked conc. in different flow velocities is shown | [279] |
| POCIS HLB PES membrane | Diuron | 2014 | France | MeOH, & MeOH/DCM (v/v: 50/50), then DCM | Pesticides | Coastal water | 200 mg | Oysters were exposed to diuron integrated conc’s as low as 0.2 and 0.3 g/L | [280] |
| POCIS HLB | 39 pesticides and metabolites | 2015 | France | MeOH, & MeOH: EtOAc, 75:25 (v/v) | Pesticides and metabolites | River | 200 mg | Frequency of detection and concentration reported | [281] |
| POCIS HLB | 12 veterinary antibiotics | 2015 | USA | MeOH | Pharmaceuticals | River | 200 mg | Conc’s ranging from 0.0003 ng/L to 68 ng/L | [282] |
| POCIS HLB, PES membrane | APIs and pesticides | 2015 | Portugal | MeOH & DCM/MeOH (50:50; v/v), & DCM | Pharmaceuticals, pesticides | River | 200 mg | Caffeine: 804 ± 209 ng/L, theophylline:184 ± 44 ng/L, Carbendazim: 45 ± 18 ng/L, atrazine, diuron, Isoproturon and simazine levels were below the Environmental Quality Stds | [283] |
| POCIS-Pest and POCISPharm, PES membrane | Organ halogen herbicides, organophosphorous pesticides, carbamate, triazine, urea, pharmaceuticals, phenols, and industrial chemicals | 2016 | Greece | Hexane/DCM Additionally, DCM/EtOAc (50:50 v/v) | Pesticides, carbamate, triazine, urea, pharmaceuticals, phenols, and industrial chemicals | River | 200 mg | Most compounds showed recoveries ranged from 60 to 110%. The coefficient of variation (CV) ranged from 0.84 to 23.8%. LOD and LOQ ranged from 6.4 to 40.1 ng/L and from 21.5 to 134 ng/L, respectively. | [284] |
| POCIS sorbents, HLB and Strata X-CW, PES membrane | Benzotriazole, methylbenzotriazole, atrazine, diuron, isoproturon, linuron, metolachlor, penconazole, terbuthylazine, carbamazepine, diclofenac, metformin, sulfamethoxazole | 2016 | Switzerland | MeOH | Corrosion inhibitors, pesticides, pharmaceuticals | River | 200 mg | Rs: Benzotriazole: 0.134, methylbenzotriazole: 0.148, atrazine: 0.26, diuron: 0.15, isoproturon: 0.254, metolachlor: 0.139, terbuthylazine: 0.197, carbamazepine: 0.231, diclofenac: 0.165, sulfamethoxazole: 0.103 | [253] |
| POCIS HLB PES membrane | Metaldehyde, isoproturon, simazine, chlorotoluron, atrazine, epoxiconazole, chlorpyrifos, cypermethrin and permethrin | 2016 | UK | Ethyl acetate | Pesticides | River | 200 mg | Results compared in three different sites | [285] |
| POCIS HLB | In vitro (i.e., zf liver cell lines stably expressing zfERα, zfERβ1 and zfERβ2 subtypes) and in vivo (i.e., transgenic cyp19a1b-GFP zf embryos) | 2016 | France | MeOH, & DCM/MeOH (50:50; v/v), & DCM | Endocrine disrupting substances | River | 200 mg | Results in different sites discussed | [202] |
| POCIS HLB PES membrane | 20 parent compounds (PCs) and 11 characteristic TPs in four 11 wastewater-impacted rivers | 2016 | Sweden | - | Pharmaceuticals | River | 200 mg | Results of four different rivers discussed | [192] |
| POCIS HLB PES membrane | CBZ: carbamazepine, CAF: caffeine, BPA: bisphenol A, LIN: lincomycin, SFA: sulfamethazine, SFO: Sulfamethoxazole, ATZ: atrazine, GEM: gemfibrozil | 2016 | Singapore | - | Pharmaceutical | River | 200 mg | Sediment concentrations for carbamazepine (r = 0.79, p b 0.001), caffeine (r = 0.93, p b 0.001) but not BPA (p = 0.16) | [286] |
| POCIS HLB PES membrane | Complex mixtures of micropollutants, including emerging substances or transformation products | 2016 | France | ACN, MeOH | Rodenticide, hormones, antiparasitic, cardiovascular agent, pharmaceuticals, pesticides and their metabolites | Groundwater | 200 mg | Results of two different sites discussed | [287] |
| POCIS HLB PES membrane | Tebuconazole, Propiconazole, Carbendazim, Azoxystrobin, Myclobutanil, Iprodione Fluconazole, Ketoconazole, Climbazole, Mecoprop, Agriculture, Dicamba, 2,4-D, Irgarol 1051, Terbutryn, Estrone Natural estrogen, Androstenedione, Ibuprofen, Acetaminophen, Naproxen, Trimethoprim, Sulfamethoxazole, Gemfibrozil, Carbamazepine, Sucralose | 2016 | Canada | MeOH | Pharmaceuticals, steroid hormones, the artificial sweetener, sucralose, fungicides, herbicides, biocides | River | 200 mg | Results compared in different sites | [288] |
| Passive sampler copolymer of poly(divinylbenzene)-N-vinylpyrrolidone | 46 pesticides, 17 pharmaceuticals, 1 stimulant (caffeine) and 1 artificial sweetener (sucralose) | 2017 | France | MeOH, & MeOH/EtOAc (50/50 v/v) and EtOAc | Pharmaceuticals Average flow of river over ten years 1.0 m3/s | The Marque River because of agricultural activities | 200 mg | Atrazine 0.22 L/day, Cyprodinil 0.22 L/day, Desethylatrazine 0.09 L/day, Desisopropylatrazine 0.09 L/day, Diclofenac 0.08 L/day, Dimethenamid 0.20 L/day, Isoproturon 0.16 L/day, Metolachlor 0.17 L/day, Metalaxyl 0.19 L/day | [252] |
| POCIS HLB, PES membrane | 13 parent pharmaceuticals and 8 of their transformation products (TPs) | 2017 | China | MeOH | Pharmaceuticals and their metabolites | River | 200 mg | The max concentration: 544.0 ng/L (CBZE), and the minimum value was 0.43 ng/L1 (SDZ) | [289] |
| POCIS HLB, PES membrane | 45 pesticides | 2017 | France | MeOH | Pesticides | Surface water in vitro | 200 mg | Average concentrations discussed in the paper | [290] |
| POCIS HLB | A range of pesticide, fungicide, herbicide, and insecticide | 2017 | USA | MeOH | pesticides | Surface water | 200 mg | A total of 141 compounds detected at one or more of the 97 sites sampled | [291] |
| POCIS HLB, PES membrane | 37 pharmaceuticals and 3 human tracers | 2018 | France | MeOH, & MeOH/EtO Ac, 75:25 v/v | Pesticides and their Metabolites, pharmaceuticals | River | 200 mg | Frequency and concentrations in the paper | [292] |
| POCIS | Organophosphate flame retardants (OPFRs) | 2018 | China | Ethyl acetate | Endocrine disrupting substances | River | 200 mg | Six sampling locations ranged from 8.99 to 112.45 ng/L with an average concentration of 47.04 ng/L | [293] |
| POCIS HLB, PES membrane | 17 pharmaceuticals, pesticides, per- and polyfluoroalkyl substances (PFASs) | 2018 | USA | 0.1% (v/v) ammonia solution in MeOH, & MeOH | Pesticides, pharmaceuticals, and perfluorinated chemicals | River | 200 mg | Concentration shown during different month of the year | [294] |
| POCIS HLB, PES membrane | Atrazine, thiamethoxam, clothianidin, imidacloprid, 2,4-D and carbamazepine | 2018 | Canada | MeOH | Pesticides and pharmaceuticals | River | 200 mg | Recoveries compared during years | [295] |
| POCIS Oasis HLB | 37 pharmaceuticals and 3 human traces | 2018 | France | 75:25 v:v MeOH:Ethyl | Pharmaceuticals flow rate at each sampling points was calculated proportionally at the size of sampling point watershed (75, 145 and 55 km2) | agricultural rural headwater river | 200 mg | 23 compound out of 37 detected | [238] |
| POCIS HLB, PES membrane & Mixed Polymer Sampler (MPS) | Alachlor, atrazine, cybutryne, diclofenac, diuron, isoproturon, PCP, Simazine, terbutryne | 2018 | Germany | MeOH | Pharmaceuticals, pesticides | River | 220 mg | Dissolved concentration of the compounds shown | [249] |
| POCIS | S-metolachlor | 2018 | France | - | Pesticides | River | 200 mg | Concentration discussed in different sites | [296] |
| POCIS HLB, PES membrane | Malathion, diuron, carbofuran, carbendazim, trifluralin, imidacloprid, metolachlor, and acetamiprid | 2018 | Brazil | MeOH | Pesticides | River | 220 mg | Malathion 7.7%, diuron 5.1%, carbofuran 35.9%, Carbendazim 12.8%, trifluralin 5.1%, imidacloprid 5.1%, metolachlor 7.7%, acetamiprid 2.6% | [297] |
| POCIS HLB, PES membrane | Atrazine, 2,6-dichlorobenzamide, bentazone, chloridazon, isoproturon, and propiconazole | 2018 | Sweden | Ethyl acetate | pesticides | Surface water | 220 mg | Herbicides 36%–48%, fungicides 36%–21%, metabolites 11%–12%, insecticides 8%–10%, and other or mixed types 8%–10% | [298] |
| POCIS HLB | 32 selected herbicides, fungicides, and insecticides (mainly polar) | 2018 | Germany | ACN | pesticide | Surface water | 230 mg | Details discussed in the paper | [299] |
| POCIS HLB, PES membrane | A range of pesticides | 2018 | Japan | MeOH | Pesticides | River | 220 mg | Compared POCIS with grab sampling | [300] |
| Strata XAW & HLB Nylon membrane | Acetaminophen, atrazine, diuron and norfloxacin hydrochloride, amitriptyline, irbesartan, ketoprofen and progesterone | 2018 | Spain | 2.5% methanolic ammonia & MeOH | Pesticide, pharmaceuticals | Estuarine | 100 mg each | Feasibility of the simultaneous uptake of hydrophilic, acidic and basic compound | [248] |
| Oasis HLB, PES membrane | Estrone (E1), Nonylphenol (NP), 17b-estradiol (E2), ethynylestradiol (EE2), | 2019 | Germany | - | Endocrine disrupting substances | River water | 54.5 mg | NP 18 mg/L, E1 14 ng/L, E2 0.2 ng/L, EE2 0.5 ng/L | [240] |
| Oasis HLB | 20 pesticides and 32 point source chemicals | 2019 | Spain | MeOH | Pharmaceuticals, pesticides, hormones | River | 200 mg | Recovery % is reported in the paper | [301] |
| POCIS | 20 pesticides, and 32 point source chemicals, mainly pharmaceuticals | 2019 | Spain | _ | Pharmaceuticals, pesticides, hormones | River | - | High recoveries reported | [302] |
| Oasis HLB, PES membrane | Biomarkers of estrogenic endocrine disruption in smallmouth bass | 2019 | USA | DCM/MTBE 80:20 (v/v), | Endocrine disrupting substances | River | 200 mg | Ranging between 28–92% | [303] |
| Oasis HLB, PES membrane | Pharmaceuticals, endocrine disrupting substances, pesticides | 2019 | Ireland | MeOH | Pharmaceuticals, endocrine disrupting substances, pesticides | River | 230 mg | Conc’s in different years reported | [246] |
| Oasis HLB, PES membrane | 168 targeted compounds | 2019 | Slovakia | MeOH/DCM, (1:1 v/v) | Pesticides, pharmaceuticals, hormones, polycyclic aromatic hydrocarbons, polychlorinated biphenyls | River | 200 mg | Risk assessment of the detected compounds revealed | [304] |
| Oasis HLB PES membrane | A range of pesticide, herbicide, fungicide, metabolite, and insecticide | 2019 | France | MeOH, & MeOH/ethyl acetate, 75:25 v/v | pesticides | River | 200 mg Bags deployed based on the depth >100 m or <100 m | Results discussed in the paper | [305] |
| Oasis HLB Six different membrane | 25 pharmaceuticals and personal care products | 2022 | USA | Formic acid: MeOH 88:12% | Pharmaceuticals and personal care products | Vernal pools | Results compared to grab sampling in the paper | [306] |
| POCIS Resins | Analyte | Year of Study | Country | Elute | Application | Deployment Condition | Adsorbent Quantity | Analyte %Recoveries | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Oasis HLB PES memberane | Range of substances reported | 2010 | USA | MeOH | Endocrine disrupting | Lake | 200 mg | Recoveries reported | [258] |
| Oasis HLB PES membrane | Ibuprofen, Gemfibrozil, Caffeine, Carbamazepine, Trimethoprim, Venlafaxine, desmethyl-venl, Citalopram, Galaxolide, Tonalide, Triclosan | 2012 | Canada | MeOH | Pharmaceuticals, antidepressants, personal care products | Lake | 200 mg | Recoveries discussed in the paper in different seasons | [307] |
| POCIS HLB PES membrane | 35 APIs and endocrine disruption | 2014 | Singapore | - | Pharmaceuticals | Tropical Lake flow ~3–5 cm/S | 60 mg | Atorvastatin and norfluoxetine, from 52 to 196% (109 ± 32%), the 80–120% range for 26 of the compounds | [259] |
| POCIS HLB PES membrane | Pestisides, herbicides, fungicides and pharmaceuticals | 2016 | Canada | MeOH | Fungicides, herbicides, pharmaceuticals | Lake | 200 mg | Atrazine was detected at all sites, and diuron, 2,4-D, and mecoprop were frequently detected. The fungicides carbendazim and thiophanate-methyl were detected at all sites, & a hydroxy-metabolite of the fungicide chlorothalonil was also widely detected | [257] |
| POCIS HLB PES membrane | 25 pesticides | 2018 | Burkina Faso | MeOH, & MeOH/EtOAc(1:1, v/v), & EtOAc/Hx (1:4, v/v) | pesticides | Lake | 200 mg | Atrazine, azadirachtin, carbofuran, chlorpyrifos, cypermethrin, dieldrin, imidacloprid, & profenofos exceeded 0.1 μg/L | [256] |
| Oasis HLB PES membrane | microcystin-LR (MC-LR) | 2019 | Canada | MeOH | Microcystin-LR | Lake | 220 mg | The Rs 0.045 (±0.001) and 0.041 (±0.001) L per day for initial concentrations of 0.5 and 1.0 mg/L | [260] |
| Oasis HLB PES membrane | neonicotinoid insecticides NNIs, thiamethoxam, clothianidin and imidacloprid, Atrazine, 2,4-D, dicamba, carbendazim, thiophanate methyl and several azoles | 2019 | Canada | MeOH | Pesticides | Lake | 200 mg | NNIs, thiamethoxam, clothianidin and imidacloprid 0.23 μg/L, Atrazine, 2,4-D, dicamba, carbendazim, thiophanate methyl and several azole-based fungicides were also widely detected | [255] |
| Oasis HLB | Pesticides | 2019 | Tunisia | ACN | Pesticides | Lagoon watershed | The results in different sites are reported in the paper | [308] |
| POCIS Resins | Analyte | Year | Country | Elute | Application | Deployment Condition | Adsorbent Quantity | Analyte %Recoveries | Ref |
| Oasis MAX, HRX, HLB, PES membrane | Acidic herbicides | 2012 | France | MeOH, & MeOH/EtOAc 5:5 (v/v) | Pesticide | Drinking water | - | POCIS-MAX showed no influence of nitrates. MAX sorbent >82% recoveries | [265] |
| Oasis HLB, PES membrane | Eight alkylphenols, nine hormones, 11 pesticides, 27 pharmaceuticals and one UV filter | 2013 | France | MeOH, & MeOH: DCM 50:50 | Alkylphenols, hormones, pesticides, pharmaceutical, UV filters | Tap water (using external thermostat tank) | 200 mg | Details discussed in the paper | [266] |
| POCIS HLB | Carbamazepine, trimethoprim, sulfamethoxazole, ibuprofen, gemfibrozil, estrone and sucralose | 2014 | Canada | MeOH | Pharmaceuticals | Drinking water | 220 mg | After 10 days: CBZ 894.7 ± 37.2 ng/L, Ibuprofen: 262.4 ± 80.1 ng/L, Gemfibrozil: 144.5 ± 31.3 ng/L, TPM: 8.7 ± 2.2 ng/L, Sucralose: 186.1± 15.0 ng/L | [309] |
| POCIS HLB, PES membrane | Atrazine-d5, caffeine-13C3, cotinine-d3, DIA-d5, fluoranthene-d10, lindane | 2016 | USA | Acetone, DCM | Pesticides, polycyclic, aromatic hydrocarbons, personal care products | Tap water | 200 mg | 4.51 ± 0.34 g/g (atrazine-d5), 4.62 ± 0.30 g/g (caffeine-13C3), 4.01 ± 0.08 g/g (cotinine-d3), 3.87 ± 0.24 g/g (DIA-d5), 4.42 ± 0.16 g/g (fluoranthene-d10), and 4.65 ± 0.14 g/g (lindane). | [264] |
| POCIS HLB, Additionally, DOWEX, PES membrane | 34 pesticide, personal care products and hydrocarbons | 2016 | USA | Acetone, & DCM, | Pesticides, polycyclic aromatic hydrocarbons, personal care products | Tap water | 200 mg | Recoveries average: Dowex Optipore L-493: 90% (range: 66–127%), HLB: 91% (range: 66–135%), and Osorb media: 96% (range:63–127%) | [263] |
| POCIS HLB, PES membrane | 73 compounds | 2016 | Norway | MeOH | Pharmaceuticals, endocrine disrupting substances, pesticides, herbicides, drugs of abuse | Drinking water | 200 mg | Results for prediction model discussed | [262] |
| POCIS HLB, PES membrane | Diclofenac (DIC), ketoprofen (KET), mefenamic acid (MEF), naproxen (NAP), ibuprofen (IBU), ketoprofen-d3 (KET-d3), perfluorooctanoic acid (PFOA), perfluorooctanesulfonate (PFOS) and Caffeine (CAF) | 2018 | Italy | Acetone | Pharmaceuticals, perfluorinated compounds, caffeine | Drinking water in vitro | 200 mg | Caffeine: 0.07–0.93 ng/L, perfluorinated compounds: 2.93–13.42 ng/L | [267] |
| POCIS HLB, PES membrane | Imidacloprid, clothianidin, thiamethoxam, acetamiprid, thiacloprid, a hydroxy metabolite | 2018 | Canada | MeOH: Acetone 60:40 v/v | Pesticides | Drinking water | 220 mg | Clothianidin 300 µg/L imidaclopid 500 µg/L thiamethoxam 5 µg/L | [261] |
| Oasis HLB | Microcystins risk assessment | 2019 | Czech Republic | - | Microcystins | Drinking water reservoir Depth: 13, 28, 46 m, flow velocities ranging between 0.01 and 0.15 m/s | 90 mg | 20–200 pg/L after 14-d deployment and 1–12 ng/L | [268] |
| POCIS Oasis WAX, PES membrane | 26 per- and polyfluoroalkyl substances (PFASs) | 2019 | Sweden | MeOH | Per, polyfluoroalkyl substances (PFASs) | Drinking water in treatment plant | 200 mg | 64–89% | [310] |
4.4. Application of POCIS, In Vitro Laboratory Studies
| POCIS Resins | Analyte | Year | Country | Elute | Application | Deployment Condition | Adsorbent Quantity | Analyte %Recoveries | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oasis HLB, PES membrane | Caffein, Amitripthiline, Doxepine, Imipramine, Carbamazapine, Diazepam, Nordizepam, Ibuprofen, Gemfibrozile, Naproxine, Diclofenac, Ketoprofen | 2007 | France | EtOAc/Ace 50/50 v/v | Pharmaceuticals | Laboratory simulation | 200 mg | Average recoveries: Caf: 1622 ng/L, Ami: 355 ng/L, Dox: 253 ng/L, Imi: 377 ng/L, Cbz: 226 ng/L, Dzp: 435 ng/L, Ndzp: 629 ng/L, Ibu: 1128 ng/L, Gem: 1744 ng/L, Nap: 673 ng/L, Diclo: 606 ng/L, Keto: 388 ng/L | [315] | ||
| Oasis HLB, PES membrane | Range of substances in different pH reported | 2011 | Canada | MeOH | Pharmaceuticals, personal care products, disrupting substances | Laboratory scale River and Tap water (water chamber in lab) | 200 mg | Recoveries in different pH are reported | [174] | ||
| Oasis HLB, PES membrane | Atrazine, simazine, desethylatrazine (DEA), desisopropylatrazine (DIA), desethylterbuthylazine (DET), terbuthylatrazine, diuron, isoproturon, chlortoluron, linuron, propyzamide, alachlor, metolachlor, acetochlor, metalaxyl, penconazole, and azoxystrobine | 2013 | France | ACN | pesticide | Laboratory calibration experiment | 230 mg | Sampling rate: 67.9–279 mg/L | [313] | ||
| Pharma-POCIS Oasis HLB PES membrane | Polar pesticides and metabolites | 2013 | France | ACN | pesticide | Laboratory in situ sampling | 200 mg | 169 to 479 mL/g day | [311] | ||
| POCIS HLB, Nylon membrane | A wide range of pharmaceuticals and pesticides | 2014 | France | MeOH, DCM/MeOH (50:50; v/v), and DCM | Pesticides, pharmaceuticals | Laboratory water samples | 200 mg | Results discussed in the paper | [317] | ||
| Strata XAW, PES membrane | Perfluorohexanoate (PFHxA), perfluoroheptanoate (PFHpA), perfluorooctanoate (PFOA), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA) | 2014 | Australia | 0.1% (v/v) ammonia MeOH, & MeOH | Perfluorinated chemicals (PFCs) | Laboratory water sample | 600 mg | PFPeA 0.078 ± 0.02 L/d, PFHxA 0.118 ± 0.01 L/d, PFNA 0.165 ± 0.004 L/d, PFHxS 0.182 ± 0.01 L/d | [214] | ||
| POCIS HLB, PES membrane | 2,4-dichlorophenoxyacetic acid (2,4-D), acetochlor ethanesulfonic acid (ESA), acetochlor oxanilic acid, bentazon, dicamba, esotrione, and metsulfuron, atrazine, diuron, esisopropylatrazine herbicides | 2014 | France | MeOH & MeOH/EtOAc 50: 50 (v/v) | Herbicides | Laboratory water samples | 200, 600 mg | Increasing sorbent to 600 mg resulted in sampling rates (Rss) twice as high as those observed with 200 mg | [239] | ||
| POCIS (5 different types) SR, POCIS-A, POCIS-B, Chemcatcher RPS, Chemcatcher C18 | 124 pesticides | 2015 | Sweden | MeOH, & DCM/MeOH (8/2, v/v) | Pesticides | Laboratory condition | 220 mg | Results for different POCIS devices discussed | [316] | ||
| Pharma-POCIS HLB PES membrane | 20 pesticides, insecticides, herbicides | 2016 | Japan | EtOH | Pesticides | Laboratory pesticide sample water | 220 mg | Sampling rate increased at 18 °C from 0.00676 to 0.262, 24 °C 0.00603 to 0.312, 30 °C 0.00426 to 0.603. | [314] | ||
| Carbon nanotubes, PES membrane | Carbamazepine, diclofenac, β-estradiol, p-nitrophenol, 3,5-dichlorphenol, sulfapyridine, sulfamethoxazole | 2017 | Poland | ACN/MeOH/DCM, (40:40:20; v/v), | Pharmaceuticals, phenols | Laboratory water sample | 100 mg | Sulfapyridine: 79.8 ± 0.2%, Sulfamethoxazole: 41.5 ± 0.1% Carbamazepine: 96.6 ± 1.5% p-nitrophenol: 70.5 ± 0.1% 17-β-estradiol: 77.1 ± 0.5% 3,5-dichlorophenol: 103.1 ± 1.8% diclofenac: 76.3 ± 1.4% | [318] | ||
| Oasis HLB PES membrane | Atenolol, cabamazapine, Diclofenac, Fluoxetine, Ketoprofen, Metoprolol, Paroxetine, Propaonalol, Sulfamethaxazole, Trimethoprime | 2019 | France | MeOH, & MeOH/ EtOAc, 75:25 v/v | Pharmaceuticals | Ultrapure water | 200 mg | Effect of flow velocities is assessed (2 < V < 18 cm/s) | [312] | ||
| POCIS Oasis HLB | 44 pharmaceuticals | 2020 | France | MeOH & 75:25 (v/v) MeOH: EtOAc | Pharmaceuticals pump (flow rate = 13 m3/h) | laboratory-scale artificial river | 200 mg | Econazole, fenbendazole, fenofibrate, metformin, thioridazine, and triclabendazole) were not sampled by POCIS and 12 compounds are not available | [319] | ||
| POCIS HLB PES membrane | neonicotinoid pesticides | 2020 | Japan | MeOH: ACE 2:1 | neonicotinoid pesticides | Laboratory scale | 20 mg | Suitable for neonicotinoid detection in lower concentration | [320] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vrana, B.; Smedes, F.; Hilscherová, K. Passive sampling of waterborne contaminants. In In Situ Bioavailability and Toxicity of Organic Chemicals in Aquatic Systems; Springer: New York, NY, USA, 2020. [Google Scholar]
- Zendong, Z.; Herrenknecht, C.; Abadie, E.; Brissard, C.; Tixier, C.; Mondeguer, F.; Séchet, V.; Amzil, Z.; Hess, P. Extended evaluation of polymeric and lipophilic sorbents for passive sampling of marine toxins. Toxicon 2014, 91, 57–68. [Google Scholar] [CrossRef] [PubMed]
- The Occurrence of Marine Biotoxins and Risk of Exposure to Seafood Consumers in Ireland; Food Safety Authority of Ireland: Dublin, Ireland, 2016; p. 64.
- Fernandez, R.; Maman, L.; Jaen, D.; Fernandez Fuentes, L.; Ocana, M.A.; Gordillo, M.M. Dinophysis Species and Diarrhetic Shellfish Toxins: 20 Years of Monitoring Program in Andalusia, South of Spain. Toxins 2019, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Fux, E.; Gonzalez-Gil, S.; Lunven, M.; Gentien, P.; Hess, P. Production of diarrhetic shellfish poisoning toxins and pectenotoxins at depths within and below the euphotic zone. Toxicon 2010, 56, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Bane, V.; García-Altares, M.; van Pelt, F.N.; Furey, A.; O’Halloran, J. Assessment of emerging biotoxins (pinnatoxin G and spirolides) at Europe’s first marine reserve: Lough Hyne. Toxicon 2015, 108, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Górecki, T.; Namieśnik, J. Passive sampling. TrAC Trends Anal. Chem. 2002, 21, 276–291. [Google Scholar] [CrossRef]
- Salim, F.; Gorecki, T. Theory and modelling approaches to passive sampling. Environ. Sci. Process. Impacts 2019, 21, 1618–1641. [Google Scholar] [CrossRef]
- Palmes, E.; Gunnison, A.F. Personal monitoring device for gaseous contaminants. Am. Ind. Hyg. Assoc. J. 1973, 34, 78–81. [Google Scholar] [CrossRef]
- Pesce, S.; Morin, S.; Lissalde, S.; Montuelle, B.; Mazzella, N. Combining polar organic chemical integrative samplers (POCIS) with toxicity testing to evaluate pesticide mixture effects on natural phototrophic biofilms. Environ. Pollut. 2011, 159, 735–741. [Google Scholar] [CrossRef]
- Grodtke, M.; Paschke, A.; Harzdorf, J.; Krauss, M.; Schuurmann, G. Calibration and field application of the Atlantic HLB Disk containing Chemcatcher(R) passive sampler—Quantitative monitoring of herbicides, other pesticides, and transformation products in German streams. J. Hazard. Mater. 2021, 410, 124538. [Google Scholar] [CrossRef]
- Network of Reference Laboratories, Research Centres and Related Organisations for Monitoring of Emerging Environmental Substances. Available online: https://www.norman-network.net/ (accessed on 27 June 2022).
- Miège, C.; Mazzella, N.; Allan, I.; Dulio, V.; Smedes, F.; Tixier, C.; Vermeirssen, E.; Brant, J.; O’Toole, S.; Budzinski, H.; et al. Position paper on passive sampling techniques for the monitoring of contaminants in the aquatic environment–achievements to date and perspectives. Trends Environ. Anal. Chem. 2015, 8, 20–26. [Google Scholar] [CrossRef]
- Vrana, B.; Allan, I.J.; Greenwood, R.; Mills, G.A.; Dominiak, E.; Svensson, K.; Knutsson, J.; Morrison, G. Passive sampling techniques for monitoring pollutants in water. TrAC Trends Anal. Chem. 2005, 24, 845–868. [Google Scholar] [CrossRef]
- Gong, X.; Li, K.; Wu, C.; Wang, L.; Sun, H. Passive sampling for monitoring polar organic pollutants in water by three typical samplers. Trends Environ. Anal. Chem. 2018, 17, 23–33. [Google Scholar] [CrossRef]
- Lissalde, S.; Mazzella, N.; Fauvelle, V.; Delmas, F.; Mazellier, P.; Legube, B. Liquid chromatography coupled with tandem mass spectrometry method for thirty-three pesticides in natural water and comparison of performance between classical solid phase extraction and passive sampling approaches. J. Chromatogr. A 2011, 1218, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- DGT, Research. DGT Research for Measurements in Waters, Soils and Sediments; DGT, Research: Lancaster, UK; Available online: https://www.dgtresearch.com/what-dgt-does/ (accessed on 27 June 2022).
- Howard, M.D.; Kudela, R.; Caron, D.; Smith, J.; Hayashi, K. Standard Operating Procedure for Solid Phase Adsorption Toxin Testing (SPATT) Assemblage and Extraction of HAB Toxins; University of California and University of Southern California: Santa Cruz, CA, USA, 2018. [Google Scholar]
- Roué, M.; Darius, H.T.; Chinain, M. Solid phase adsorption toxin tracking (SPATT) technology for the monitoring of aquatic toxins: A review. Toxins 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Reeve, J. Risk assessment of shellfish toxins. Toxins 2013, 5, 2109–2137. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.; Clarke, D. Review of DSP Toxicity in Ireland: Long-Term Trend Impacts, Biodiversity and Toxin Profiles from a Monitoring Perspective. Toxins 2019, 11, 61. [Google Scholar] [CrossRef]
- Young, N.; Robin, C.; Kwiatkowska, R.; Beck, C.; Mellon, D.; Edwards, P.; Turner, J.; Nicholls, P.; Fearby, G.; Lewis, D.; et al. Outbreak of diarrhetic shellfish poisoning associated with consumption of mussels, United Kingdom, May to June 2019. Eurosurveillance 2019, 24, 1900513. [Google Scholar] [CrossRef]
- Stoecker, D.K. Mixotrophy among Dinoflagellates 1. J. Eukaryot. Microbiol. 1999, 46, 397–401. [Google Scholar] [CrossRef]
- Ulrich Lüttge, F.M.C.; Risueño, M.-C.; Leuschner, C. Progress in Botany; Springer: Berlin, Germany, 2021; Volume 82. [Google Scholar]
- Azad, H.S.; Borchardt, J.A. Variations in phosphorus uptake by algae. Environ. Sci. Technol. 1970, 4, 737–743. [Google Scholar] [CrossRef]
- Lee, T.C.; Fong, F.L.; Ho, K.C.; Lee, F.W. The Mechanism of Diarrhetic Shellfish Poisoning Toxin Production in Prorocentrum spp.: Physiological and Molecular Perspectives. Toxins 2016, 8, 272. [Google Scholar] [CrossRef]
- Chain, E. Scientific Opinion on marine biotoxins in shellfish—Palytoxin group. EFSA J. 2009, 7, 1393. [Google Scholar]
- Council, E. Regulation (EC) No 853/2004 of the European parliament and of the council of 29 April 2004 laying down specific hygiene rules for food of animal origin. J. Eur. Union 2004, 139, 55–205. [Google Scholar]
- Manita, D.; Alves, R.N.; Braga, A.C.; Fogaca, F.H.; Marques, A.; Costa, P.R. In vitro bioaccessibility of the marine biotoxins okadaic acid, dinophysistoxin-2 and their 7-O-acyl fatty acid ester derivatives in raw and steamed shellfish. Food Chem. Toxicol. 2017, 101, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Zhao, X.Y.; Ji, L.D.; Xu, J. Okadaic acid (OA): Toxicity, detection and detoxification. Toxicon 2019, 160, 1–7. [Google Scholar] [CrossRef]
- Solter, P.F.; Beasley, V.R. Phycotoxins. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1155–1186. [Google Scholar]
- Guo, F.; An, T.; Rein, K.S. The algal hepatoxoxin okadaic acid is a substrate for human cytochromes CYP3A4 and CYP3A5. Toxicon 2010, 55, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, F.; Crain, S.; Quilliam, M.A.; Wang, X.; Rein, K.S. The structures of three metabolites of the algal hepatotoxin okadaic acid produced by oxidation with human cytochrome P450. Bioorganic Med. Chem. 2012, 20, 3742–3745. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pasaro, E.; Mendez, J.; Laffon, B. Okadaic acid: More than a diarrheic toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef]
- Suganuma, M.; Fujiki, H.; Suguri, H.; Yoshizawa, S.; Hirota, M.; Nakayasu, M.; Ojika, M.; Wakamatsu, K.; Yamada, K.; Sugimura, T. Okadaic acid: An additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc. Natl. Acad. Sci. USA 1988, 85, 1768–1771. [Google Scholar] [CrossRef]
- Kamat, P.K.; Rai, S.; Nath, C. Okadaic acid induced neurotoxicity: An emerging tool to study Alzheimer’s disease pathology. Neurotoxicology 2013, 37, 163–172. [Google Scholar] [CrossRef]
- Jiao, Y.-H.; Dou, M.; Wang, G.; Li, H.-Y.; Liu, J.-S.; Yang, X.; Yang, W.-D. Exposure of okadaic acid alters the angiogenesis in developing chick embryos. Toxicon 2017, 133, 74–81. [Google Scholar] [CrossRef]
- Jayaraj, R.; Gupta, N.; Rao, P.L. Multiple signal transduction pathways in okadaic acid induced apoptosis in HeLa cells. Toxicology 2009, 256, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Florio, C.; Luxich, E.; Vertua, R.; Yasumoto, T. Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon 1996, 34, 965–974. [Google Scholar] [CrossRef]
- Chen, L. Okadaic acid induces apoptosis through the PKR, NF-κB and caspase pathway in human osteoblastic osteosarcoma MG63 cells. Toxicol. Vitr. 2011, 25, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.G.; Norte, M.; Creus, A.H.; Fernandez, J.J.; Daranas, A.H. Self-association of okadaic acid: Structural and pharmacological significance. Mar. Drugs 2013, 11, 1866–1877. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Vale, P.; Chaveca, T.; Laires, A.; Rueff, J.; Oliveira, N.G. Naturally contaminated shellfish samples: Quantification of diarrhetic shellfish poisoning toxins in unhydrolysed and hydrolysed extracts and cytotoxicity assessment. J. Appl. Toxicol. 2010, 30, 699–707. [Google Scholar] [CrossRef]
- Huynh-Delerme, C.; Fessard, V.; Kiefer-Biasizzo, H.; Puiseux-Dao, S. Characteristics of okadaic acid—Induced cytotoxic effects in CHO K1 cells. Environ. Toxicol. Int. J. 2003, 18, 383–394. [Google Scholar] [CrossRef]
- Coates, C.J.; Lim, J.; Harman, K.; Rowley, A.F.; Griffiths, D.J.; Emery, H.; Layton, W. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biol. Toxicol. 2019, 35, 219–232. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Balazs, G.H.; Steidinger, K.A.; Baden, D.G.; Work, T.M.; Russell, D.J. The potential role of natural tumor promoters in marine turtle fibropapillomatosis. J. Aquat. Anim. Health 1999, 11, 199–210. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.Y.; Lin, L.; Gao, Y.; Hong, H.S.; Wang, D.Z. Quantitative proteomic analysis of okadaic acid treated mouse small intestines reveals differentially expressed proteins involved in diarrhetic shellfish poisoning. J. Proteom. 2012, 75, 2038–2052. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine Biotoxins: Occurrence, Toxicity, Regulatory Limits and Reference Methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef]
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Moita, M.T.; Pazos, Y.; Rocha, C.; Nolasco, R.; Oliveira, P.B. Toward predicting Dinophysis blooms off NW Iberia: A decade of events. Harmful Algae 2016, 53, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, A.; de la Rosa, L.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Yessotoxin, a novel phycotoxin, activates phosphodiesterase activity. Effect of yessotoxin on cAMP levels in human lymphocytes. Biochem. Pharmacol. 2003, 65, 193–208. [Google Scholar] [CrossRef]
- Murata, M.; Kumagai, M.; Lee, J.S.; Yasumoto, T. Isolation and structure of yessotoxin, a novel polyether compound implicated in diarrhetic shellfish poisoning. Tetrahedron Lett. 1987, 28, 5869–5872. [Google Scholar] [CrossRef]
- Alfonso, A.; Vieytes, M.R.; Botana, L.M. Yessotoxin, a Promising Therapeutic Tool. Mar. Drugs 2016, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Fattorusso, E.; Forino, M.; Magno, S.; Poletti, R.; Satake, M.; Viviani, R.; Yasumoto, T. Yessotoxin in mussels of the northern Adriatic Sea. Toxicon 1997, 35, 177–183. [Google Scholar] [CrossRef]
- Paz, B.; Riobo, P.; Ramilo, I.; Franco, J.M. Yessotoxins profile in strains of Protoceratium reticulatum from Spain and USA. Toxicon 2007, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; McNabb, P.; De Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L.; et al. Toxin levels and profiles in microalgae from the North-Western Adriatic Sea—15 years of studies on cultured species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, R.-C.; Kong, F.-Z.; Li, C.; Dai, L.; Chen, Z.-F.; Geng, H.-X.; Zhou, M.-J. Contamination status of lipophilic marine toxins in shellfish samples from the Bohai Sea, China. Environ. Pollut. 2019, 249, 171–180. [Google Scholar] [CrossRef]
- Suzuki, T.; Horie, Y.; Koike, K.; Satake, M.; Oshima, Y.; Iwataki, M.; Yoshimatsu, S. Yessotoxin analogues in several strains of Protoceratium reticulatum in Japan determined by liquid chromatography-hybrid triple quadrupole/linear ion trap mass spectrometry. J. Chromatogr. A 2007, 1142, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Kumagai, M.; Yasumoto, T. Toxicologic evaluation of yessotoxin. Nat. Toxins 1997, 5, 255–259. [Google Scholar] [CrossRef]
- Pérez-Gómez, A.; Ferrero-Gutierrez, A.; Novelli, A.; Franco, J.M.; Paz, B.; Fernández-Sánchez, M.T. Potent neurotoxic action of the shellfish biotoxin yessotoxin on cultured cerebellar neurons. Toxicol. Sci. 2006, 90, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.; Andreosso, A.; Seymour, J. Cardiovascular toxicity from marine envenomation. In Heart and Toxins; Elsevier: Amsterdam, The Netherlands, 2015; pp. 203–223. [Google Scholar]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobo, P.; Franco, J.M.; Fernandez, J.J. Yessotoxins, a group of marine polyether toxins: An overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef]
- Rubini, S.; Albonetti, S.; Menotta, S.; Cervo, A.; Callegari, E.; Cangini, M.; Dall’Ara, S.; Baldini, E.; Vertuani, S.; Manfredini, S. New Trends in the Occurrence of Yessotoxins in the Northwestern Adriatic Sea. Toxins 2021, 13, 634. [Google Scholar] [CrossRef]
- Bianchi, C.; Fato, R.; Angelin, A.; Trombetti, F.; Ventrella, V.; Borgatti, A.R.; Fattorusso, E.; Ciminiello, P.; Bernardi, P.; Lenaz, G. Yessotoxin, a shellfish biotoxin, is a potent inducer of the permeability transition in isolated mitochondria and intact cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004, 1656, 139–147. [Google Scholar] [CrossRef]
- Barroso, J.M. Commission Regulation (EU) No 786/2013.of 16 August 2013 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards the permitted limits of yessotoxins in live bivalve molluscs. Off. J. Eur. Union 2013, 14, 1. [Google Scholar]
- Twiner, M.J.; Rehmann, N.; Hess, P.; Doucette, G.J. Azaspiracid shellfish poisoning: A review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs 2008, 6, 39–72. [Google Scholar] [CrossRef]
- Furey, A.; O’Doherty, S.; O’Callaghan, K.; Lehane, M.; James, K.J. Azaspiracid poisoning (AZP) toxins in shellfish: Toxicological and health considerations. Toxicon 2010, 56, 173–190. [Google Scholar] [CrossRef]
- McGirr, S.; Clarke, D.; Kilcoyne, J.; Salas, R.; Koehler, H.; Silke, J.; Touzet, N. Insights into the discrepancy between Azadinium spp. and azaspiracid toxins near strategically important aquaculture operations in the west and southwest of Ireland. Estuar. Coast. Shelf Sci. 2021, 262, 107622. [Google Scholar] [CrossRef]
- Hess, P.; McCarron, P.; Krock, B.; Kilcoyne, J.; Miles, C.O. Azaspiracids: Chemistry, biosynthesis, metabolism, and detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection; CRC Press: Boca Raton, FL, USA, 2014; pp. 799–821. [Google Scholar]
- Satake, M.; Ofuji, K.; Naoki, H.; James, K.J.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. Azaspiracid, a new marine toxin having unique spiro ring assemblies, isolated from Irish mussels, Mytilus edulis. J. Am. Chem. Soc. 1998, 120, 9967–9968. [Google Scholar] [CrossRef]
- Wu, X.; Hou, L.; Lin, X.; Xie, Z. Application of novel nanomaterials for chemo-and biosensing of algal toxins in shellfish and water. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 353–414. [Google Scholar]
- Lehane, M.; Brana-Magdalena, A.; Moroney, C.; Furey, A.; James, K. Liquid chromatography with electrospray ion trap mass spectrometry for the determination of five azaspiracids in shellfish. J. Chromatogr. A 2002, 950, 139–147. [Google Scholar] [CrossRef]
- Diaz Sierra, M.; Furey, A.; Hamilton, B.; Lehane, M.; James, K.J. Elucidation of the fragmentation pathways of azaspiracids, using electrospray ionisation, hydrogen/deuterium exchange, and multiple-stage mass spectrometry. J. Mass Spectrom. 2003, 38, 1178–1186. [Google Scholar] [CrossRef]
- Hamilton, B.; Díaz Sierra, M.; Lehane, M.; Furey, A.; James, K.J. The fragmentation pathways of azaspiracids elucidated using positive nanospray hybrid quadrupole time-of-flight (QqTOF) mass spectrometry. Spectroscopy 2004, 18, 355–362. [Google Scholar] [CrossRef]
- Hess, P.; Grune, B.; Anderson, D.B.; Aune, T.; Botana, L.M.; Caricato, P.; van Egmond, H.P.; Halder, M.; Hall, S.; Lawrence, J.F.; et al. Three Rs Approaches in Marine Biotoxin Testing: The Report and Recommendations of a joint ECVAM/DG SANCO Workshop (ECVAM Workshop 54). Altern. Lab. Anim. 2006, 34, 193–224. [Google Scholar] [CrossRef] [PubMed]
- Abal, P.; Louzao, M.C.; Fraga, M.; Vilarino, N.; Ferreiro, S.; Vieytes, M.R.; Botana, L.M. Absorption and Effect of Azaspiracid-1 Over the Human Intestinal Barrier. Cell. Physiol. Biochem. 2017, 43, 136–146. [Google Scholar] [CrossRef]
- Ronzitti, G.; Hess, P.; Rehmann, N.; Rossini, G.P. Azaspiracid-1 alters the E-cadherin pool in epithelial cells. Toxicol. Sci. 2007, 95, 427–435. [Google Scholar] [CrossRef]
- Authority, E.F.S. Marine biotoxins in shellfish–Azaspiracid group-Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2008, 6, 723. [Google Scholar]
- Alves, R.N.; Rambla-Alegre, M.; Braga, A.C.; Maulvault, A.L.; Barbosa, V.; Campàs, M.; Reverté, L.; Flores, C.; Caixach, J.; Kilcoyne, J.; et al. Bioaccessibility of lipophilic and hydrophilic marine biotoxins in seafood: An in vitro digestion approach. Food Chem. Toxicol. 2019, 129, 153–161. [Google Scholar] [CrossRef]
- Jeffery, B.; Barlow, T.; Moizer, K.; Paul, S.; Boyle, C. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A.M. Domoic acid in Portuguese shellfish and fish. Toxicon 2001, 39, 893–904. [Google Scholar] [CrossRef]
- Hambright, K.D.; Zamor, R.M.; Easton, J.D.; Allison, B. Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1. [Google Scholar]
- Truelove, J.; Mueller, R.; Pulido, O.; Martin, L.; Fernie, S.; Iverson, F. 30-day oral toxicity study of domoic acid in Cynomolgus monkeys: Lack of overt toxicity at doses approaching the acute toxic dose. Nat. Toxins 1997, 5, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Grimmelt, B.; Nijjar, M.S.; Brown, J.; Macnair, N.; Wagner, S.; Johnson, G.R.; Amend, J.F. Relationship between domoic acid levels in the blue mussel (Mytilus edulis) and toxicity in mice. Toxicon 1990, 28, 501–508. [Google Scholar] [CrossRef]
- Alfonso, M.; Duran, R.; Arufe, M.C. Effect of excitatory amino acids on serum TSH and thyroid hormone levels in freely moving rats. Horm. Res. Paediatr. 2000, 54, 78–83. [Google Scholar] [CrossRef]
- Hampson, D.R.; Huang, X.-p.; Wells, J.W.; Walter, J.A.; Wright, J.L. Interaction of domoic acid and several derivatives with kainic acid and AMPA binding sites in rat brain. Eur. J. Pharmacol. 1992, 218, 1–8. [Google Scholar] [CrossRef]
- Burns, J.M.; Hall, S.; Ferry, J.L. The adsorption of saxitoxin to clays and sediments in fresh and saline waters. Water Res. 2009, 43, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Schantz, E.J.; Ghazarossian, V.E.; Schnoes, H.K.; Strong, F.M.; Springer, J.P.; Pezzanite, J.O.; Clardy, J. Letter: The structure of saxitoxin. J. Am. Chem. Soc. 1975, 97, 1238. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Walter, J.A.; Burton, I.W.; Stirling, D.J.; Fattorusso, E.; Quilliam, M.A. Isolation and structure elucidation of new and unusual saxitoxin analogues from mussels. J. Nat. Prod. 2008, 71, 1518–1523. [Google Scholar] [CrossRef]
- Fox, J.W. 114—Venoms and Poisons from Marine Organisms. In Goldman’s Cecil Medicine, 24th ed.; Goldman, L., Schafer, A.I., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 697–700. [Google Scholar] [CrossRef]
- Faber, S. Saxitoxin and the induction of paralytic shellfish poisoning. J. Young Investig. 2012, 23, 1–7. [Google Scholar]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R. Algal Toxins in Seafood and Drinking Water; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Rogers, R.S.; Rapoport, H. The pKa’s of saxitoxin. J. Am. Chem. Soc. 1980, 102, 7335–7339. [Google Scholar] [CrossRef]
- Harland, F.; Wood, S.A.; Broady, P.; Williamson, W.; Gaw, S. Changes in saxitoxin-production through growth phases in the metaphytic cyanobacterium Scytonema cf. crispum. Toxicon 2015, 103, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef]
- Botana, L.M.; Alfonso, A. Phycotoxins: Chemistry and Biochemistry; John Wiley & Sons: New York, NY, USA, 2015. [Google Scholar]
- Plakas, S.M.; Dickey, R.W. Advances in monitoring and toxicity assessment of brevetoxins in molluscan shellfish. Toxicon 2010, 56, 137–149. [Google Scholar] [CrossRef]
- Turchiano, R. Brief review of natural nonprotein neurotoxins. Biol. Warf. 2015, 89, 16–24. [Google Scholar]
- Abraham, A.; Plakas, S.M.; Wang, Z.; Jester, E.L.; El Said, K.R.; Granade, H.R.; Henry, M.S.; Blum, P.C.; Pierce, R.H.; Dickey, R.W. Characterization of polar brevetoxin derivatives isolated from Karenia brevis cultures and natural blooms. Toxicon 2006, 48, 104–115. [Google Scholar] [CrossRef]
- Poli, M.A. Laboratory procedures for detoxification of equipment and waste contaminated with brevetoxins PbTx-2 and PbTx-3. J. Assoc. Off. Anal. Chem. 1988, 71, 1000–1002. [Google Scholar] [CrossRef]
- Trainer, V.L.; Edwards, R.A.; Szmant, A.M.; Stuart, A.M.; Mende, T.J.; Baden, D.G. Brevetoxins: Unique Activators of Voltage-Sensitive Sodium Channels; ACS Publications: Washington, DC, USA, 1990. [Google Scholar]
- Poli, M.A.; Templeton, C.B.; Pace, J.G.; Hines, H.B. Detection, Metabolism, and Pathophysiology of Brevetoxins; ACS Publications: Washington, DC, USA, 1990. [Google Scholar]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fleming, L.E.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.-Y.; Backer, L.; Ayyar, R.; Weisman, R. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–479. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Dechraoui, M.Y.; Tiedeken, J.A.; Persad, R.; Wang, Z.; Granade, H.R.; Dickey, R.W.; Ramsdell, J.S. Use of two detection methods to discriminate ciguatoxins from brevetoxins: Application to great barracuda from Florida Keys. Toxicon 2005, 46, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, C.; Hagelstein, J.G.; Burchard, G.D.; Schmiedel, S. Outbreak of ciguatera fish poisoning on a cargo ship in the port of hamburg. J. Travel Med. 2012, 19, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Chateau-Degat, M.L.; Dewailly, E.; Cerf, N.; Nguyen, N.L.; Huin-Blondey, M.O.; Hubert, B.; Laudon, F.; Chansin, R. Temporal trends and epidemiological aspects of ciguatera in French Polynesia: A 10-year analysis. Trop. Med. Int. Health 2007, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Arena, P.; Levin, B.; Fleming, L.; Friedman, M.; Blythe, D. A pilot study of the cognitive and psychological correlates of chronic ciguatera poisoning. Harmful Algae 2004, 3, 51–60. [Google Scholar] [CrossRef]
- Friedman, M.A.; Arena, P.; Levin, B.; Fleming, L.; Fernandez, M.; Weisman, R.; Bernstein, J.; Schrank, K.; Blythe, D.; Backer, L.; et al. Neuropsychological study of ciguatera fish poisoning: A longitudinal case-control study. Arch. Clin. Neuropsychol. 2007, 22, 545–553. [Google Scholar] [CrossRef]
- Caillaud, A.; de la Iglesia, P.; Darius, H.T.; Pauillac, S.; Aligizaki, K.; Fraga, S.; Chinain, M.; Diogene, J. Update on methodologies available for ciguatoxin determination: Perspectives to confront the onset of ciguatera fish poisoning in Europe. Mar. Drugs 2010, 8, 1838–1907. [Google Scholar] [CrossRef]
- James, K.; Lehane, M.; Moroney, C.; Fernandez-Puente, P.; Satake, M.; Yasumoto, T.; Furey, A. Azaspiracid shellfish poisoning: Unusual toxin dynamics in shellfish and the increased risk of acute human intoxications. Food Addit. Contam. 2002, 19, 555–561. [Google Scholar] [CrossRef]
- La Barre, S.; Bates, S.S.; Quilliam, M.A. Domoic Acid. In Outstanding Marine Molecules; Wiley-VCH: Weinheim, Germany, 2014; pp. 189–216. [Google Scholar] [CrossRef]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef]
- McCarthy, H.P.; Crowder, L.B. An overlooked scale of global transport: Phytoplankton species richness in ships’ ballast water. Biol. Invasions 2000, 2, 321. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Transport of toxic dinoflagellates via ships ballast water: Bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar. Ecol. Prog. Ser. 1998, 168, 297–309. [Google Scholar] [CrossRef]
- van den Bergh, J.C.; Nunes, P.A.; Dotinga, H.M.; Kooistra, W.H.; Vrieling, E.G.; Peperzak, L. Exotic harmful algae in marine ecosystems: An integrated biological–economic–legal analysis of impacts and policies. Mar. Policy 2002, 26, 59–74. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. In Environmental Health; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–12. [Google Scholar]
- Callaway, R.; Shinn, A.P.; Grenfell, S.E.; Bron, J.E.; Burnell, G.; Cook, E.J.; Crumlish, M.; Culloty, S.; Davidson, K.; Ellis, R.P.; et al. Review of climate change impacts on marine aquaculture in the UK and Ireland. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 389–421. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal bloom and its economic impact. Eur. Comm. Jt. Res. Cent. Inst. Environ. Sustain. 2016. [CrossRef]
- Panda, D.; Dash, B.P.; Manickam, S.; Boczkaj, G. Recent advancements in LC-MS based analysis of biotoxins: Present and future challenges. Mass Spectrom. Rev. 2021, 41, 766–803. [Google Scholar] [CrossRef] [PubMed]
- Vilarino, N.; Louzao, M.C.; Fraga, M.; Rodriguez, L.P.; Botana, L.M. Innovative detection methods for aquatic algal toxins and their presence in the food chain. Anal. Bioanal. Chem. 2013, 405, 7719–7732. [Google Scholar] [CrossRef]
- Turrell, E.A.; Stobo, L. A comparison of the mouse bioassay with liquid chromatography-mass spectrometry for the detection of lipophilic toxins in shellfish from Scottish waters. Toxicon 2007, 50, 442–447. [Google Scholar] [CrossRef]
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.; Van den Top, H.J.; De Boer, J. Marine toxins: Chemistry, toxicity, occurrence and detection, with special reference to the Dutch situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef]
- Puente, P.F.; Sáez, M.J.F.; Hamilton, B.; Lehane, M.; Ramstad, H.; Furey, A.; James, K.J. Rapid determination of polyether marine toxins using liquid chromatography–multiple tandem mass spectrometry. J. Chromatogr. A 2004, 1056, 77–82. [Google Scholar] [CrossRef]
- Bane, V.; Brosnan, B.; Barnes, P.; Lehane, M.; Furey, A. High-resolution mass spectrometry analysis of tetrodotoxin (TTX) and its analogues in puffer fish and shellfish. Food Addit. Contam. Part A 2016, 33, 1468–1489. [Google Scholar] [CrossRef]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.P. Functional assays in marine biotoxin detection. Toxicology 2005, 207, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Jellett, J.F.; Marks, L.J.; Stewart, J.E.; Dorey, M.L.; Watson-Wright, W.; Lawrence, J.F. Paralytic shellfish poison (saxitoxin family) bioassays: Automated endpoint determination and standardization of the in vitro tissue culture bioassay, and comparison with the standard mouse bioassay. Toxicon 1992, 30, 1143–1156. [Google Scholar] [CrossRef]
- Nicholson, R.A.; Li, G.H.; Buenaventura, E.; Graham, D. A rapid and sensitive assay for paralytic shellfish poison (PSP) toxins using mouse brain synaptoneurosomes. Toxicon 2002, 40, 831–838. [Google Scholar] [CrossRef]
- Vieytes, M.; Fontal, O.; Leira, F.; de Sousa, J.B.; Botana, L. A fluorescent microplate assay for diarrheic shellfish toxins. Anal. Biochem. 1997, 248, 258–264. [Google Scholar] [CrossRef]
- Louhimies, S. Directive 86/609/EEC on the protection of animals used for experimental and other scientific purposes. Altern. Lab. Anim. 2002, 30, 217–219. [Google Scholar] [CrossRef]
- Garthwaite, I.; Ross, K.M.; Miles, C.O.; Hansen, R.P.; Foster, D.; Wilkins, A.L.; Towers, N.R. Polyclonal antibodies to domoic acid, and their use in immunoassays for domoic acid in sea water and shellfish. Nat. Toxins 1998, 6, 93–104. [Google Scholar] [CrossRef]
- Wang, L.; Gong, X.; Wang, R.; Gan, Z.; Lu, Y.; Sun, H. Application of an immobilized ionic liquid for the passive sampling of perfluorinated substances in water. J. Chromatogr. A 2017, 1515, 45–53. [Google Scholar] [CrossRef]
- Taniyama, S.; Arakawa, O.; Terada, M.; Nishio, S.; Takatani, T.; Mahmud, Y.; Noguchi, T. Ostreopsis sp., a possible origin of palytoxin (PTX) in parrotfish Scarus ovifrons. Toxicon 2003, 42, 29–33. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Wang, L.; Du, X.; Wei, D. Production of a soluble single-chain variable fragment antibody against okadaic acid and exploration of its specific binding. Anal. Biochem. 2016, 503, 21–27. [Google Scholar] [CrossRef]
- Bodero, M.; Gerssen, A.; Portier, L.; Klijnstra, M.D.; Hoogenboom, R.; Guzman, L.; Hendriksen, P.J.M.; Bovee, T.F.H. A Strategy to Replace the Mouse Bioassay for Detecting and Identifying Lipophilic Marine Biotoxins by Combining the Neuro-2a Bioassay and LC-MS/MS Analysis. Mar. Drugs 2018, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Charbonneau, C.F.; Ménard, C. Liquid chromatographic determination of domoic acid in mussels, using AOAC paralytic shellfish poison extraction procedure: Collaborative study. J. Assoc. Off. Anal. Chem. 1991, 74, 68–72. [Google Scholar] [CrossRef]
- Hess, P.; McGovern, E.; McMahon, T.; Morris, S.; Stobo, L.A.; Brown, N.A.; Gallacher, S.; McEvoy, J.D.G.; Kennedy, G.; Young, P.B.; et al. LC-UV and LC-MS methods for the determination of domoic acid. TrAC Trends Anal. Chem. 2005, 24, 358–367. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Hendrix, A.; Halaska, B.; Duignan, P.; Shum, S.; Isoherranen, N.; Marcinek, D.J.; Gulland, F.M. Domoic acid in California sea lion fetal fluids indicates continuous exposure to a neuroteratogen poses risks to mammals. Harmful Algae 2018, 79, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Bogan, Y. Factors Affecting the Concentration of Domoic Acid in Scallop, Pecten maximus. Ph.D. Thesis, Letterkenny Institute of Technology, Letterkenny, Ireland, 2006. [Google Scholar]
- López-Rivera, A.; Pinto, M.; Insinilla, A.; Isla, B.S.; Uribe, E.; Alvarez, G.; Lehane, M.; Furey, A.; James, K.J. The occurrence of domoic acid linked to a toxic diatom bloom in a new potential vector: The tunicate Pyura chilensis (piure). Toxicon 2009, 54, 754–762. [Google Scholar] [CrossRef]
- Furey, A.; Lehane, M.; Gillman, M.; Fernandez-Puente, P.; James, K.J. Determination of domoic acid in shellfish by liquid chromatography with electrospray ionization and multiple tandem mass spectrometry. J. Chromatogr. A 2001, 938, 167–174. [Google Scholar] [CrossRef]
- Lee, J.S.; Yanagi, T.; Kenma, R.; Yasumoto, T. Fluorometric determination of diarrhetic shellfish toxins by high-performance liquid chromatography. Agric. Biol. Chem. 1987, 51, 877–881. [Google Scholar]
- Dorne, J.; Bordajandi, L.; Amzal, B.; Ferrari, P.; Verger, P. Combining analytical techniques, exposure assessment and biological effects for risk assessment of chemicals in food. TrAC Trends Anal. Chem. 2009, 28, 695–707. [Google Scholar] [CrossRef]
- Kilcoyne, J.; Fux, E. Strategies for the elimination of matrix effects in the liquid chromatography tandem mass spectrometry analysis of the lipophilic toxins okadaic acid and azaspiracid-1 in molluscan shellfish. J. Chromatogr. A 2010, 1217, 7123–7130. [Google Scholar] [CrossRef]
- Holland, P.; McNabb, P.; Selwood, A.; Neil, T.; Slattery, D.; Van de Riet, J.; Van Egmond, H.; Van den Topp, H.; Yasumoto, T. A multiresidue LC-MS method for algal toxins in shellfish: Inter-laboratory study. In Proceedings of the HABTech03 Workshop, Nelson, New Zealand, November 2003. [Google Scholar]
- Otero, P.; Miguéns, N.; Rodríguez, I.; Botana, L.M. LC–MS/MS analysis of the emerging toxin pinnatoxin-G and high levels of esterified OA group toxins in Galician commercial mussels. Toxins 2019, 11, 394. [Google Scholar] [CrossRef]
- Harju, K.; Rapinoja, M.-L.; Avondet, M.-A.; Arnold, W.; Schär, M.; Burrell, S.; Luginbühl, W.; Vanninen, P. Optimization of sample preparation for the identification and quantification of saxitoxin in proficiency test mussel sample using liquid chromatography-tandem mass spectrometry. Toxins 2015, 7, 4868–4880. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: Collaborative study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Takizawa, A. Fluorometric measurement of yessotoxins in shellfish by high-pressure liquid chromatography. Biosci. Biotechnol. Biochem. 1997, 61, 1775–1777. [Google Scholar] [CrossRef]
- Zendong, Z.; Bertrand, S.; Herrenknecht, C.; Abadie, E.; Jauzein, C.; Lemée, R.; Gouriou, J.; Amzil, Z.; Hess, P. Passive Sampling and High Resolution Mass Spectrometry for Chemical Profiling of French Coastal Areas with a Focus on Marine Biotoxins. Environ. Sci. Technol. 2016, 50, 8522–8529. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.; Metcalf, J.; Spáčil, Z.; Downing, T.; Downing, S.; Long, A.; Nunn, P.B.; Cox, P.A. Distinguishing the cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) from other diamino acids. Toxicon 2011, 57, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Kudela, R.M. Characterization and deployment of Solid Phase Adsorption Toxin Tracking (SPATT) resin for monitoring of microcystins in fresh and saltwater. Harmful Algae 2011, 11, 117–125. [Google Scholar] [CrossRef]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef]
- Mackenzie, L.; White, D.; Oshima, Y.; Kapa, J. The resting cyst and toxicity of Alexandrium ostenfeldii (Dinophyceae) in New Zealand. Phycologia 1996, 35, 148–155. [Google Scholar] [CrossRef]
- Kudela, R.M. Passive sampling for freshwater and marine algal toxins. Compr. Anal. Chem. 2017, 78, 379–409. [Google Scholar]
- MacKenzie, L.A. In situ passive solid-phase adsorption of micro-algal biotoxins as a monitoring tool. Curr. Opin. Biotechnol. 2010, 21, 326–331. [Google Scholar] [CrossRef]
- Rundberget, T.; Sandvik, M.; Larsen, K.; Pizarro, G.M.; Reguera, B.; Castberg, T.; Gustad, E.; Loader, J.I.; Rise, F.; Wilkins, A.L.; et al. Extraction of microalgal toxins by large-scale pumping of seawater in Spain and Norway, and isolation of okadaic acid and dinophysistoxin-2. Toxicon 2007, 50, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Holland, P.T.; MacKenzie, L. Development of solid phase adsorption toxin tracking (SPATT) for monitoring anatoxin-a and homoanatoxin-a in river water. Chemosphere 2011, 82, 888–894. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, J.-H. Isotherm, kinetic and thermodynamic characteristics of adsorption of paclitaxel onto Diaion HP-20. Process Biochem. 2016, 51, 917–924. [Google Scholar] [CrossRef]
- Mitsubishi Chemical Corporation. In Diaion HP20 Product Data Sheet; Mitsubishi Chemical Corporation: Tokyo, Japan.
- McCarthy, M.; van Pelt, F.N.A.M.; Bane, V.; O’Halloran, J.; Furey, A. Application of passive (SPATT) and active sampling methods in the profiling and monitoring of marine biotoxins. Toxicon 2014, 89, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mitsubishi Chemical Corporation. DIAION Technical Manual; Mitsubishi Chemical Corporation: Tokyo, Japan.
- Mitsubishi Chemical Corporation. SEPABEADS SP700 Product Data Sheet; Mitsubishi Chemical Corporation: Tokyo, Japan.
- Kohoutek, J.; Babica, P.; Bláha, L.; Maršálek, B. A novel approach for monitoring of cyanobacterial toxins: Development and evaluation of the passive sampler for microcystins. Anal. Bioanal. Chem. 2008, 390, 1167–1172. [Google Scholar] [CrossRef]
- Caillaud, A.; de la Iglesia, P.; Barber, E.; Eixarch, H.; Mohammad-Noor, N.; Yasumoto, T.; Diogene, J. Monitoring of dissolved ciguatoxin and maitotoxin using solid-phase adsorption toxin tracking devices: Application to Gambierdiscus pacificus in culture. Harmful Algae 2011, 10, 433–446. [Google Scholar] [CrossRef]
- Lane, J.Q.; Roddam, C.M.; Langlois, G.W.; Kudela, R.M. Application of Solid Phase Adsorption Toxin Tracking (SPATT) for field detection of the hydrophilic phycotoxins domoic acid and saxitoxin in coastal California. Limnol. Oceanogr. Methods 2010, 8, 645–660. [Google Scholar] [CrossRef]
- Turrell, E.; Stobo, L.; Lacaze, J.-P.; Bresnan, E.; Gowland, D. Development of anearly warning system’for harmful algal blooms using solid-phase adsorption toxin tracking (SPATT). In Proceedings of the Oceans 2007-Europe, Aberdeen, UK, 18–21 June 2007; pp. 1–6. [Google Scholar]
- Fux, E.; Marcaillou, C.; Mondeguer, F.; Bire, R.; Hess, P. Field and mesocosm trials on passive sampling for the study of adsorption and desorption behaviour of lipophilic toxins with a focus on OA and DTX1. Harmful Algae 2008, 7, 574–583. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, J.; Fan, H.; Li, A. Mechanism and application of solid phase adsorption toxin tracking for monitoring microcystins. J. Chromatogr. A 2013, 1300, 159–164. [Google Scholar] [CrossRef]
- Li, A.; Ma, F.; Song, X.; Yu, R. Dynamic adsorption of diarrhetic shellfish poisoning (DSP) toxins in passive sampling relates to pore size distribution of aromatic adsorbent. J. Chromatogr. A 2011, 1218, 1437–1442. [Google Scholar] [CrossRef]
- Tesser, R.; Di Serio, M.; Casale, L.; Carotenuto, G.; Santacesaria, E. Absorption of water/methanol binary system on ion-exchange resins. Can. J. Chem. Eng. 2010, 88, 1044–1053. [Google Scholar] [CrossRef]
- Peacock, M.B.; Gibble, C.M.; Senn, D.B.; Cloern, J.E.; Kudela, R.M. Blurred lines: Multiple freshwater and marine algal toxins at the land-sea interface of San Francisco Bay, California. Harmful Algae 2018, 73, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Fux, E.; Bire, R.; Hess, P. Comparative accumulation and composition of lipophilic marine biotoxins in passive samplers and in mussels (M. edulis) on the West Coast of Ireland. Harmful Algae 2009, 8, 523–537. [Google Scholar] [CrossRef]
- Howard, M.D.; Nagoda, C.; Kudela, R.M.; Hayashi, K.; Tatters, A.; Caron, D.A.; Busse, L.; Brown, J.; Sutula, M.; Stein, E.D. Microcystin prevalence throughout lentic waterbodies in coastal Southern California. Toxins 2017, 9, 231. [Google Scholar] [CrossRef]
- Bueno, M.J.M.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Application of passive sampling devices for screening of micro-pollutants in marine aquaculture using LC–MS/MS. Talanta 2009, 77, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, G.; Paz, B.; González-Gil, S.; Franco, J.M.; Reguera, B. Seasonal variability of lipophilic toxins during a Dinophysis acuta bloom in Western Iberia: Differences between picked cells and plankton concentrates. Harmful Algae 2009, 8, 926–937. [Google Scholar] [CrossRef]
- Rundberget, T.; Gustad, E.; Samdal, I.A.; Sandvik, M.; Miles, C.O. A convenient and cost-effective method for monitoring marine algal toxins with passive samplers. Toxicon 2009, 53, 543–550. [Google Scholar] [CrossRef]
- Rodríguez, P.; Alfonso, A.; Turrell, E.; Lacaze, J.-P.; Botana, L.M. Study of solid phase adsorption of paralytic shellfish poisoning toxins (PSP) onto different resins. Harmful Algae 2011, 10, 447–455. [Google Scholar] [CrossRef]
- Rundberget, T.; Aasen, J.A.; Selwood, A.I.; Miles, C.O. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 2011, 58, 700–711. [Google Scholar] [CrossRef]
- Touzet, N.; Lacaze, J.; Maher, M.; Turrell, E.; Raine, R. Summer dynamics of Alexandrium ostenfeldii (Dinophyceae) and spirolide toxins in Cork Harbour, Ireland. Mar. Ecol. Prog. Ser. 2011, 425, 21–33. [Google Scholar] [CrossRef]
- MacKenzie, L.A.; Selwood, A.I.; McNabb, P.; Rhodes, L. Benthic dinoflagellate toxins in two warm-temperate estuaries: Rangaunu and Parengarenga Harbours, Northland, New Zealand. Harmful Algae 2011, 10, 559–566. [Google Scholar] [CrossRef]
- Wood, S.A.; Kuhajek, J.M.; de Winton, M.; Phillips, N.R. Species composition and cyanotoxin production in periphyton mats from three lakes of varying trophic status. FEMS Microbiol. Ecol. 2012, 79, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, G.; Moroño, Á.; Paz, B.; Franco, J.M.; Pazos, Y.; Reguera, B. Evaluation of passive samplers as a monitoring tool for early warning of Dinophysis toxins in shellfish. Mar. Drugs 2013, 11, 3823–3845. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Altares, M.; Casanova, A.; Bane, V.; Diogene, J.; Furey, A.; de la Iglesia, P. Confirmation of pinnatoxins and spirolides in shellfish and passive samplers from Catalonia (Spain) by liquid chromatography coupled with triple quadrupole and high-resolution hybrid tandem mass spectrometry. Mar. Drugs 2014, 12, 3706–3732. [Google Scholar] [CrossRef] [PubMed]
- Gibble, C.M.; Kudela, R.M. Detection of persistent microcystin toxins at the land–sea interface in Monterey Bay, California. Harmful Algae 2014, 39, 146–153. [Google Scholar] [CrossRef]
- Zendong, Z.; McCarron, P.; Herrenknecht, C.; Sibat, M.; Amzil, Z.; Cole, R.B.; Hess, P. High resolution mass spectrometry for quantitative analysis and untargeted screening of algal toxins in mussels and passive samplers. J. Chromatogr. A 2015, 1416, 10–21. [Google Scholar] [CrossRef]
- García-Altares, M.; Casanova, A.; Fernández-Tejedor, M.; Diogène, J.; De La Iglesia, P. Bloom of Dinophysis spp. dominated by D. sacculus and its related diarrhetic shellfish poisoning (DSP) outbreak in Alfacs Bay (Catalonia, NW Mediterranean Sea): Identification of DSP toxins in phytoplankton, shellfish and passive samplers. Reg. Stud. Mar. Sci. 2016, 6, 19–28. [Google Scholar] [CrossRef]
- Li, Z.; Sobek, A.; Radke, M. Fate of Pharmaceuticals and Their Transformation Products in Four Small European Rivers Receiving Treated Wastewater. Environ. Sci. Technol. 2016, 50, 5614–5621. [Google Scholar] [CrossRef]
- Kim, J.-H.; Tillmann, U.; Adams, N.G.; Krock, B.; Stutts, W.L.; Deeds, J.R.; Han, M.-S.; Trainer, V.L. Identification of Azadinium species and a new azaspiracid from Azadinium poporum in Puget Sound, Washington State, USA. Harmful Algae 2017, 68, 152–167. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Viallon, J.; Ung, A.; Gatti, C.; Harwood, D.T.; Chinain, M. Application of solid phase adsorption toxin tracking (SPATT) devices for the field detection of Gambierdiscus toxins. Harmful Algae 2018, 71, 40–49. [Google Scholar] [CrossRef]
- Wood, S.A.; Biessy, L.; Puddick, J. Anatoxins are consistently released into the water of streams with Microcoleus autumnalis-dominated (cyanobacteria) proliferations. Harmful Algae 2018, 80, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hattenrath-Lehmann, T.K.; Lusty, M.W.; Wallace, R.B.; Haynes, B.; Wang, Z.; Broadwater, M.; Deeds, J.R.; Morton, S.L.; Hastback, W.; Porter, L.; et al. Evaluation of Rapid, Early Warning Approaches to Track Shellfish Toxins Associated with Dinophysis and Alexandrium Blooms. Mar. Drugs 2018, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Boundy, M.J.; Selwood, A.I.; Harwood, D.T. Development of an LC–MS/MS method to simultaneously monitor maitotoxins and selected ciguatoxins in algal cultures and P-CTX-1B in fish. Harmful Algae 2018, 80, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Lie, A.A.Y.; Seubert, E.L.; Crowley, N.; Robertson, G.; Caron, D.A. Co-occurring dissolved algal toxins observed at multiple coastal sites in southern California via solid phase adsorption toxin tracking. Toxicon 2019, 171, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.; Schloss, I.R.; Trefault, N.; Tillmann, U.; Hernando, M.; Deregibus, D.; Antoni, J.; Almandoz, G.O.; Hoppenrath, M. Detection of the phycotoxin pectenotoxin-2 in waters around King George Island, Antarctica. Polar Biol. 2020, 43, 263–277. [Google Scholar] [CrossRef]
- Mathon, B.; Ferreol, M.; Togola, A.; Lardy-Fontan, S.; Dabrin, A.; Allan, I.J.; Staub, P.F.; Mazzella, N.; Miège, C. Polar organic chemical integrative samplers as an effective tool for chemical monitoring of surface waters—Results from one-year monitoring in France. Sci. Total Environ. 2022, 824, 153549. [Google Scholar] [CrossRef]
- Challis, J.K.; Almirall, X.O.; Helm, P.A.; Wong, C.S. Performance of the organic-diffusive gradients in thin-films passive sampler for measurement of target and suspect wastewater contaminants. Environ. Pollut. 2020, 261, 114092. [Google Scholar] [CrossRef]
- Sonavane, M.; Creusot, N.; Maillot-Marechal, E.; Pery, A.; Brion, F.; Aїt-Aїssa, S. Zebrafish-based reporter gene assays reveal different estrogenic activities in river waters compared to a conventional human-derived assay. Sci. Total Environ. 2016, 550, 934–939. [Google Scholar] [CrossRef]
- Galle, T.; Bayerle, M.; Pittois, D.; Huck, V. Allocating biocide sources and flow paths to surface waters using passive samplers and flood wave chemographs. Water Res. 2020, 173, 115533. [Google Scholar] [CrossRef]
- Vrana, B.; Smedes, F.; Prokeš, R.; Loos, R.; Mazzella, N.; Miege, C.; Budzinski, H.; Vermeirssen, E.; Ocelka, T.; Gravell, A.; et al. An interlaboratory study on passive sampling of emerging water pollutants. TrAC Trends Anal. Chem. 2016, 76, 153–165. [Google Scholar] [CrossRef]
- Harman, C.; Allan, I.J.; Vermeirssen, E.L. Calibration and use of the polar organic chemical integrative sampler--a critical review. Environ. Toxicol. Chem. 2012, 31, 2724–2738. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Pesce, S.; Kim-Tiam, S.; Libert, X.; Coquery, M.; Mazzella, N. Use of polar organic chemical integrative samplers to assess the effects of chronic pesticide exposure on biofilms. Ecotoxicology 2012, 21, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Stepnowski, P.; Paszkiewicz, M. Pollutant analysis using passive samplers: Principles, sorbents, calibration and applications. A review. Environ. Chem. Lett. 2021, 19, 465–520. [Google Scholar] [CrossRef]
- Alvarez, D.A.; Huckins, J.N.; Petty, J.D.; Jones-Lepp, T.; Stuer-Lauridsen, F.; Getting, D.T.; Goddard, J.P.; Gravell, A. Tool for monitoring hydrophilic contaminants in water: Polar organic chemical integrative sampler (POCIS). Compr. Anal. Chem. 2007, 48, 171–197. [Google Scholar]
- Booij, K.; Sleiderink, H.M.; Smedes, F. Calibrating the uptake kinetics of semipermeable membrane devices using exposure standards. Environ. Toxicol. Chem. Int. J. 1998, 17, 1236–1245. [Google Scholar] [CrossRef]
- Godlewska, K.; Stepnowski, P.; Paszkiewicz, M. Application of the Polar Organic Chemical Integrative Sampler for Isolation of Environmental Micropollutants—A Review. Crit. Rev. Anal. Chem. 2020, 50, 1–28. [Google Scholar] [CrossRef]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the marine environment: What are the present challenges in their monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef]
- Martínez Bueno, M.; Herrera, S.; Munaron, D.; Boillot, C.; Fenet, H.; Chiron, S.; Gómez, E. POCIS passive samplers as a monitoring tool for pharmaceutical residues and their transformation products in marine environment. Environ. Sci. Pollut. Res. 2016, 23, 5019–5029. [Google Scholar] [CrossRef]
- Vrana, B.; Urik, J.; Fedorova, G.; Svecova, H.; Grabicova, K.; Golovko, O.; Randak, T.; Grabic, R. In situ calibration of polar organic chemical integrative sampler (POCIS) for monitoring of pharmaceuticals in surface waters. Environ. Pollut. 2021, 269, 116121. [Google Scholar] [CrossRef]
- Kaserzon, S.; Hawker, D.; Kennedy, K.; Bartkow, M.; Carter, S.; Booij, K.; Mueller, J. Characterisation and comparison of the uptake of ionizable and polar pesticides, pharmaceuticals and personal care products by POCIS and Chemcatchers. Environ. Sci. Process. Impacts 2014, 16, 2517–2526. [Google Scholar] [CrossRef]
- Björlenius, B.; Ripszám, M.; Haglund, P.; Lindberg, R.H.; Tysklind, M.; Fick, J. Pharmaceutical residues are widespread in Baltic Sea coastal and offshore waters–Screening for pharmaceuticals and modelling of environmental concentrations of carbamazepine. Sci. Total Environ. 2018, 633, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Jones-Lepp, T.; Alvarez, D.; Petty, J.; Huckins, J. Polar organic chemical integrative sampling and liquid chromatography–electrospray/ion-trap mass spectrometry for assessing selected prescription and illicit drugs in treated sewage effluents. Arch. Environ. Contam. Toxicol. 2004, 47, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Sultana, T.; Murray, C.; Ehsanul Hoque, M.; Metcalfe, C.D. Monitoring contaminants of emerging concern from tertiary wastewater treatment plants using passive sampling modelled with performance reference compounds. Environ. Monit. Assess. 2016, 189, 1. [Google Scholar] [CrossRef]
- Baz-Lomba, J.A.; Harman, C.; Reid, M.; Thomas, K.V. Passive sampling of wastewater as a tool for the long-term monitoring of community exposure: Illicit and prescription drug trends as a proof of concept. Water Res. 2017, 121, 221–230. [Google Scholar] [CrossRef]
- Iparraguirre, A.; Prieto, A.; Vallejo, A.; Moeder, M.; Zuloaga, O.; Etxebarria, N.; Paschke, A. Tetraphasic polar organic chemical integrative sampler for the determination of a wide polarity range organic pollutants in water. The use of performance reference compounds and in-situ calibration. Talanta 2017, 164, 314–322. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, S.L.; McClure, E.L.; Wong, C.S. Laboratory calibration and field deployment of the polar organic chemical integrative sampler for pharmaceuticals and personal care products in wastewater and surface water. Environ. Toxicol. Chem. Int. J. 2007, 26, 2517–2529. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, G.; Qiu, J.; Li, A. Occurrence and variation of lipophilic shellfish toxins in phytoplankton, shellfish and seawater samples from the aquaculture zone in the Yellow Sea, China. Toxicon 2017, 127, 1–10. [Google Scholar] [CrossRef]
- Bartelt-Hunt, S.L.; Snow, D.D.; Damon-Powell, T.; Brown, D.L.; Prasai, G.; Schwarz, M.; Kolok, A.S. Quantitative evaluation of laboratory uptake rates for pesticides, pharmaceuticals, and steroid hormones using POCIS. Environ. Toxicol. Chem. 2011, 30, 1412–1420. [Google Scholar] [CrossRef]
- Jacquet, R.; Miège, C.; Bados, P.; Schiavone, S.; Coquery, M. Evaluating the polar organic chemical integrative sampler for the monitoring of beta-blockers and hormones in wastewater treatment plant effluents and receiving surface waters. Environ. Toxicol. Chem. 2012, 31, 279–288. [Google Scholar] [CrossRef]
- Bailly, E.; Levi, Y.; Karolak, S. Calibration and field evaluation of Polar Organic Chemical Integrative Sampler (POCIS) for monitoring pharmaceuticals in hospital wastewater. Environ. Pollut. 2013, 174, 100–105. [Google Scholar] [CrossRef]
- Assoumani, A.; Lissalde, S.; Margoum, C.; Mazzella, N.; Coquery, M. In situ application of stir bar sorptive extraction as a passive sampling technique for the monitoring of agricultural pesticides in surface waters. Sci. Total Environ. 2013, 463, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Amdany, R.; Chimuka, L.; Cukrowska, E. Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): A laboratory calibration and field application. Water SA 2014, 40, 407–414. [Google Scholar] [CrossRef]
- Brown, D.; Snow, D.; Hunt, G.A.; Bartelt-Hunt, S.L. Persistence of pharmaceuticals in effluent-dominated surface waters. J. Environ. Qual. 2015, 44, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Skodova, A.; Prokes, R.; Simek, Z.; Vrana, B. In situ calibration of three passive samplers for the monitoring of steroid hormones in wastewater. Talanta 2016, 161, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ory, J.; Bricheux, G.; Togola, A.; Bonnet, J.L.; Donnadieu-Bernard, F.; Nakusi, L.; Forestier, C.; Traore, O. Ciprofloxacin residue and antibiotic-resistant biofilm bacteria in hospital effluent. Environ. Pollut. 2016, 214, 635–645. [Google Scholar] [CrossRef]
- Vystavna, Y.; Frkova, Z.; Marchand, L.; Vergeles, Y.; Stolberg, F. Removal efficiency of pharmaceuticals in a full scale constructed wetland in East Ukraine. Ecol. Eng. 2017, 108, 50–58. [Google Scholar] [CrossRef]
- Lhotský, O.; Krákorová, E.; Linhartová, L.; Křesinová, Z.; Steinová, J.; Dvořák, L.; Rodsand, T.; Filipová, A.; Kroupová, K.; Wimmerová, L. Assessment of biodegradation potential at a site contaminated by a mixture of BTEX, chlorinated pollutants and pharmaceuticals using passive sampling methods–case study. Sci. Total Environ. 2017, 607, 1451–1465. [Google Scholar] [CrossRef]
- Camotti Bastos, M.; Rheinheimer dos Santos, D.; Monteiro de Castro Lima, J.A.; Le Guet, T.; Santanna dos Santos, M.A.; Zanella, R.; Aubertheau, E.; Mondamert, L.; Caner, L.; Labanowski, J. Presence of anthropogenic markers in water: A case study of the Guaporé River watershed, Brazil. Clean–Soil Air Water 2018, 46, 1700019. [Google Scholar] [CrossRef]
- Chaves-Barquero, L.G.; Luong, K.H.; Rudy, M.D.; Frank, R.A.; Hanson, M.L.; Wong, C.S. Attenuation of pharmaceuticals, nutrients and toxicity in a rural sewage lagoon system integrated with a subsurface filtration technology. Chemosphere 2018, 209, 767–775. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zha, D.; Wang, L.; Lu, G.; Sun, Q.; Wu, D. In situ calibration of polar organic chemical integrative samplers to monitor organophosphate flame retardants in river water using polyethersulfone membranes with performance reference compounds. Sci. Total Environ. 2018, 610–611, 1356–1363. [Google Scholar] [CrossRef]
- Kim, H.; Homan, M. Evaluation of pharmaceuticals and personal care products (PPCPs) in drinking water originating from Lake Erie. J. Great Lakes Res. 2020, 46, 1321–1330. [Google Scholar] [CrossRef]
- Li, H.; Helm, P.A.; Metcalfe, C.D. Sampling in the Great Lakes for pharmaceuticals, personal care products, and endocrine-disrupting substances using the passive polar organic chemical integrative sampler. Environ. Toxicol. Chem. Int. J. 2010, 29, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Hassan, S.M.F.; Mushtaque, M.; Noor, R.; Ghayas, S.; Muhamma, I.N.; Hassan, F. Comparative analysis of biopharmaceutic classification system (BCS) based biowaiver protocols to validate equivalence of a multisource product. Afr. J. Pharm. Pharmacol. 2020, 14, 212–220. [Google Scholar]
- Guibal, R.; Lissalde, S.; Brizard, Y.; Guibaud, G. Semi-continuous pharmaceutical and human tracer monitoring by POCIS sampling at the watershed-scale in an agricultural rural headwater river. J. Hazard. Mater. 2018, 360, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Fauvelle, V.; Mazzella, N.; Belles, A.; Moreira, A.; Allan, I.J.; Budzinski, H. Optimization of the polar organic chemical integrative sampler for the sampling of acidic and polar herbicides. Anal. Bioanal. Chem. 2014, 406, 3191–3199. [Google Scholar] [CrossRef]
- Müller, A.-K.; Leser, K.; Kämpfer, D.; Riegraf, C.; Crawford, S.E.; Smith, K.; Vermeirssen, E.L.; Buchinger, S.; Hollert, H. Bioavailability of estrogenic compounds from sediment in the context of flood events evaluated by passive sampling. Water Res. 2019, 161, 540–548. [Google Scholar] [CrossRef]
- Alvarez, D.A.; Petty, J.D.; Huckins, J.N.; Jones-Lepp, T.L.; Getting, D.T.; Goddard, J.P.; Manahan, S.E. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ. Toxicol. Chem. 2004, 23, 1640–1648. [Google Scholar] [CrossRef]
- Zhang, Z.; Hibberd, A.; Zhou, J.L. Analysis of emerging contaminants in sewage effluent and river water: Comparison between spot and passive sampling. Anal. Chim. Acta 2008, 607, 37–44. [Google Scholar] [CrossRef]
- Cernoch, I.; Franek, M.; Diblikova, I.; Hilscherova, K.; Randak, T.; Ocelka, T.; Blaha, L. POCIS sampling in combination with ELISA: Screening of sulfonamide residues in surface and waste waters. J. Environ. Monit. 2012, 14, 250–257. [Google Scholar] [CrossRef]
- Tapie, N.; Devier, M.H.; Soulier, C.; Creusot, N.; Le Menach, K.; Ait-Aissa, S.; Vrana, B.; Budzinski, H. Passive samplers for chemical substance monitoring and associated toxicity assessment in water. Water Sci. Technol. 2011, 63, 2418–2426. [Google Scholar] [CrossRef]
- Vystavna, Y.; Huneau, F.; Grynenko, V.; Vergeles, Y.; Celle-Jeanton, H.; Tapie, N.; Budzinski, H.; Le Coustumer, P. Pharmaceuticals in rivers of two regions with contrasted socio-economic conditions: Occurrence, accumulation, and comparison for Ukraine and France. Water Air Soil Pollut. 2012, 223, 2111–2124. [Google Scholar] [CrossRef]
- Jones, L.; Ronan, J.; McHugh, B.; Regan, F. Passive sampling of polar emerging contaminants in Irish catchments. Water Sci. Technol. 2019, 79, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Berho, C.; Togola, A.; Coureau, C.; Ghestem, J.P.; Amalric, L. Applicability of polar organic compound integrative samplers for monitoring pesticides in groundwater. Environ. Sci. Pollut. Res. Int. 2013, 20, 5220–5228. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, L.; Ziarrusta, H.; Prieto, A.; Zugazua, O.; Zuloaga, O.; Olivares, M.; Usobiaga, A.; Paschke, A.; Etxebarria, N. Evaluation of polar organic chemical integrative and hollow fibre samplers for the determination of a wide variety of organic polar compounds in seawater. Talanta 2018, 185, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Schäffer, A.; Smith, K. A comparison of equilibrium and kinetic passive sampling for the monitoring of aquatic organic contaminants in German rivers. Water Res. 2018, 145, 248–258. [Google Scholar] [CrossRef]
- Balaam, J.L.; Grover, D.; Johnson, A.C.; Jürgens, M.; Readman, J.; Smith, A.J.; White, S.; Williams, R.; Zhou, J.L. The use of modelling to predict levels of estrogens in a river catchment: How does modelled data compare with chemical analysis and in vitro yeast assay results? Sci. Total Environ. 2010, 408, 4826–4832. [Google Scholar] [CrossRef]
- Rotter, S.; Sans-Piché, F.; Streck, G.; Altenburger, R.; Schmitt-Jansen, M. Active bio-monitoring of contamination in aquatic systems—An in situ translocation experiment applying the PICT concept. Aquat. Toxicol. 2011, 101, 228–236. [Google Scholar] [CrossRef]
- Criquet, J.; Dumoulin, D.; Howsam, M.; Mondamert, L.; Goossens, J.F.; Prygiel, J.; Billon, G. Comparison of POCIS passive samplers vs. composite water sampling: A case study. Sci. Total Environ. 2017, 609, 982–991. [Google Scholar] [CrossRef]
- Carpinteiro, I.; Schopfer, A.; Estoppey, N.; Fong, C.; Grandjean, D.; de Alencastro, L.F. Evaluation of performance reference compounds (PRCs) to monitor emerging polar contaminants by polar organic chemical integrative samplers (POCIS) in rivers. Anal. Bioanal. Chem. 2016, 408, 1067–1078. [Google Scholar] [CrossRef]
- Kaserzon, S.L.; Vermeirssen, E.L.; Hawker, D.W.; Kennedy, K.; Bentley, C.; Thompson, J.; Booij, K.; Mueller, J.F. Passive sampling of perfluorinated chemicals in water: Flow rate effects on chemical uptake. Environ. Pollut. 2013, 177, 58–63. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Helm, P.; Paterson, G.; Kaltenecker, G.; Murray, C.; Nowierski, M.; Sultana, T. Pesticides related to land use in watersheds of the Great Lakes basin. Sci. Total Environ. 2019, 648, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.; Fargues, M.; Nfon Dibié, J.-J.; Konaté, Y.; de Alencastro, L.F. Assessment of water resource contamination by pesticides in vegetable-producing areas in Burkina Faso. Environ. Sci. Pollut. Res. 2018, 25, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Sultana, T.; Li, H.; Helm, P.A. Current-use pesticides in urban watersheds and receiving waters of western Lake Ontario measured using polar organic chemical integrative samplers (POCIS). J. Great Lakes Res. 2016, 42, 1432–1442. [Google Scholar] [CrossRef]
- Writer, J.H.; Barber, L.B.; Brown, G.K.; Taylor, H.E.; Kiesling, R.L.; Ferrey, M.L.; Jahns, N.D.; Bartell, S.E.; Schoenfuss, H.L. Anthropogenic tracers, endocrine disrupting chemicals, and endocrine disruption in Minnesota lakes. Sci. Total Environ. 2010, 409, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S.; Segovia, E.; Loh, L.L.; Burger, D.F.; Eikaas, H.S.; Kelly, B.C. Application of Polar Organic Chemical Integrative Sampler (POCIS) to monitor emerging contaminants in tropical waters. Sci. Total Environ. 2014, 482–483, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Brophy, M.J.; Mackie, A.L.; Park, Y.; Gagnon, G.A. Exploring the detection of microcystin-LR using polar organic chemical integrative samplers (POCIS). Environ. Sci. Process. Impacts 2019, 21, 659–666. [Google Scholar] [CrossRef]
- Sultana, T.; Murray, C.; Kleywegt, S.; Metcalfe, C.D. Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere 2018, 202, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.H.; Baz-Lomba, J.A.; Harman, C.; Reid, M.J.; Owen, S.F.; Bury, N.R.; Thomas, K.V.; Barron, L.P. The first attempt at non-linear in silico prediction of sampling rates for polar organic chemical integrative samplers (POCIS). Environ. Sci. Technol. 2016, 50, 7973–7981. [Google Scholar] [CrossRef]
- Morrison, S.A.; Belden, J.B. Calibration of nylon organic chemical integrative samplers and sentinel samplers for quantitative measurement of pulsed aquatic exposures. J. Chromatogr. A 2016, 1449, 109–117. [Google Scholar] [CrossRef]
- Morrison, S.A.; Belden, J.B. Characterization of performance reference compound kinetics and analyte sampling rate corrections under three flow regimes using nylon organic chemical integrative samplers. J. Chromatogr. A 2016, 1466, 1–11. [Google Scholar] [CrossRef]
- Fauvelle, V.; Mazzella, N.; Delmas, F.; Madarassou, K.; Eon, M.; Budzinski, H. Use of mixed-mode ion exchange sorbent for the passive sampling of organic acids by polar organic chemical integrative sampler (POCIS). Environ. Sci. Technol. 2012, 46, 13344–13353. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Camilleri, J.; Cren-Olivé, C.; Coquery, M.; Miège, C. Determination of uptake kinetics and sampling rates for 56 organic micropollutants using “pharmaceutical” POCIS. Talanta 2013, 109, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Magi, E.; Di Carro, M.; Mirasole, C.; Benedetti, B. Combining passive sampling and tandem mass spectrometry for the determination of pharmaceuticals and other emerging pollutants in drinking water. Microchem. J. 2018, 136, 56–60. [Google Scholar] [CrossRef]
- Jaša, L.; Sadílek, J.; Kohoutek, J.; Straková, L.; Maršálek, B.; Babica, P. Application of passive sampling for sensitive time-integrative monitoring of cyanobacterial toxins microcystins in drinking water treatment plants. Water Res. 2019, 153, 108–120. [Google Scholar] [CrossRef]
- Vercraene-Eairmal, M.; Lauga, B.; Saint Laurent, S.; Mazzella, N.; Boutry, S.; Simon, M.; Karama, S.; Delmas, F.; Duran, R. Diuron biotransformation and its effects on biofilm bacterial community structure. Chemosphere 2010, 81, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Adams, G.; Sharum, M.; Steelman, K.L. Passive sampling of bioavailable organic chemicals in Perry County, Missouri cave streams. Environ. Sci. Technol. 2010, 44, 8835–8841. [Google Scholar] [CrossRef]
- Miège, C.; Budzinski, H.; Jacquet, R.; Soulier, C.; Pelte, T.; Coquery, M. Polar organic chemical integrative sampler (POCIS): Application for monitoring organic micropollutants in wastewater effluent and surface water. J. Environ. Monit. 2012, 14, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Kaserzon, S.L.; Kennedy, K.; Hawker, D.W.; Thompson, J.; Carter, S.; Roach, A.C.; Booij, K.; Mueller, J.F. Development and calibration of a passive sampler for perfluorinated alkyl carboxylates and sulfonates in water. Environ. Sci. Technol. 2012, 46, 4985–4993. [Google Scholar] [CrossRef]
- Charlestra, L.; Amirbahman, A.; Courtemanch, D.L.; Alvarez, D.A.; Patterson, H. Estimating pesticide sampling rates by the polar organic chemical integrative sampler (POCIS) in the presence of natural organic matter and varying hydrodynamic conditions. Environ. Pollut. 2012, 169, 98–104. [Google Scholar] [CrossRef]
- Munaron, D.; Tapie, N.; Budzinski, H.; Andral, B.; Gonzalez, J.-L. Pharmaceuticals, alkylphenols and pesticides in Mediterranean coastal waters: Results from a pilot survey using passive samplers. Estuar. Coast. Shelf Sci. 2012, 114, 82–92. [Google Scholar] [CrossRef]
- Schopfer, A.; Estoppey, N.; Omlin, J.; Udrisard, R.; Esseiva, P.; de Alencastro, L.F. The Use of Passive Samplers to Reveal Industrial and Agricultural Pollution Trends in Swiss Rivers. Chimia 2014, 68, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Tiam, S.K.; Morin, S.; Pesce, S.; Feurtet-Mazel, A.; Moreira, A.; Gonzalez, P.; Mazzella, N. Environmental effects of realistic pesticide mixtures on natural biofilm communities with different exposure histories. Sci. Total Environ. 2014, 473, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Lissalde, S.; Mazzella, N.; Mazellier, P. Polar organic chemical integrative samplers for pesticides monitoring: Impacts of field exposure conditions. Sci. Total Environ. 2014, 488, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R.L.; Pick, F.R.; Boutin, C.; Saleem, A. Atrazine contamination at the watershed scale and environmental factors affecting sampling rates of the polar organic chemical integrative sampler (POCIS). Environ. Pollut. 2014, 189, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Di Carro, M.; Bono, L.; Magi, E. A simple recirculating flow system for the calibration of polar organic chemical integrative samplers (POCIS): Effect of flow rate on different water pollutants. Talanta 2014, 120, 30–33. [Google Scholar] [CrossRef]
- Barranger, A.; Akcha, F.; Rouxel, J.; Brizard, R.; Maurouard, E.; Pallud, M.; Menard, D.; Tapie, N.; Budzinski, H.; Burgeot, T.; et al. Study of genetic damage in the Japanese oyster induced by an environmentally-relevant exposure to diuron: Evidence of vertical transmission of DNA damage. Aquat. Toxicol. 2014, 146, 93–104. [Google Scholar] [CrossRef]
- Poulier, G.; Lissalde, S.; Charriau, A.; Buzier, R.; Cleries, K.; Delmas, F.; Mazzella, N.; Guibaud, G. Estimates of pesticide concentrations and fluxes in two rivers of an extensive French multi-agricultural watershed: Application of the passive sampling strategy. Environ. Sci. Pollut. Res. 2015, 22, 8044–8057. [Google Scholar] [CrossRef]
- Jaimes-Correa, J.C.; Snow, D.D.; Bartelt-Hunt, S.L. Seasonal occurrence of antibiotics and a beta agonist in an agriculturally-intensive watershed. Environ. Pollut. 2015, 205, 87–96. [Google Scholar] [CrossRef]
- Gonzalez-Rey, M.; Tapie, N.; Le Menach, K.; Devier, M.H.; Budzinski, H.; Bebianno, M.J. Occurrence of pharmaceutical compounds and pesticides in aquatic systems. Mar. Pollut. Bull. 2015, 96, 384–400. [Google Scholar] [CrossRef]
- Terzopoulou, E.; Voutsa, D. Active and passive sampling for the assessment of hydrophilic organic contaminants in a river basin-ecotoxicological risk assessment. Environ. Sci. Pollut. Res. Int. 2016, 23, 5577–5591. [Google Scholar] [CrossRef]
- Zhang, Z.; Troldborg, M.; Yates, K.; Osprey, M.; Kerr, C.; Hallett, P.D.; Baggaley, N.; Rhind, S.M.; Dawson, J.J.C.; Hough, R.L. Evaluation of spot and passive sampling for monitoring, flux estimation and risk assessment of pesticides within the constraints of a typical regulatory monitoring scheme. Sci. Total Environ. 2016, 569–570, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S.; Estrada, E.S.; Juhel, G.; Kit, L.W.; Kelly, B.C. Pharmaceutically active compounds and endocrine disrupting chemicals in water, sediments and mollusks in mangrove ecosystems from Singapore. Mar. Pollut. Bull. 2016, 109, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Soulier, C.; Coureau, C.; Togola, A. Environmental forensics in groundwater coupling passive sampling and high resolution mass spectrometry for screening. Sci. Total Environ. 2016, 563–564, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.R.; Sultana, T.; Servos, M.R.; Metcalfe, C.D. Biological responses to contaminants in darters (Etheostoma spp.) collected from rural and urban regions of the Grand River, ON, Canada. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zha, D.; Li, Y.; Wang, L.; Yang, C.; Lu, G. Occurrence and attenuation of pharmaceuticals and their transformation products in rivers impacted by sewage treatment plants. RSC Adv. 2017, 7, 40905–40913. [Google Scholar] [CrossRef]
- Aisha, A.A.; Hneine, W.; Mokh, S.; Devier, M.-H.; Budzinski, H.; Jaber, F. Monitoring of 45 pesticides in Lebanese surface water using polar organic chemical integrative sampler (POCIS). Ocean Sci. J. 2017, 52, 455–466. [Google Scholar] [CrossRef]
- Van Metre, P.C.; Alvarez, D.A.; Mahler, B.J.; Nowell, L.; Sandstrom, M.; Moran, P. Complex mixtures of Pesticides in Midwest U.S. streams indicated by POCIS time-integrating samplers. Environ. Pollut. 2017, 220, 431–440. [Google Scholar] [CrossRef]
- Guibal, R.; Lissalde, S.; Leblanc, J.; Cleries, K.; Charriau, A.; Poulier, G.; Mazzella, N.; Rebillard, J.-P.; Brizard, Y.; Guibaud, G. Two sampling strategies for an overview of pesticide contamination in an agriculture-extensive headwater stream. Environ. Sci. Pollut. Res. 2018, 25, 14280–14293. [Google Scholar] [CrossRef]
- Zha, D.; Li, Y.; Yang, C.; Yao, C. Assessment of organophosphate flame retardants in surface water and sediment from a freshwater environment (Yangtze River, China). Environ. Monit. Assess. 2018, 190, 222. [Google Scholar] [CrossRef]
- Challis, J.K.; Cuscito, L.D.; Joudan, S.; Luong, K.H.; Knapp, C.W.; Hanson, M.L.; Wong, C.S. Inputs, source apportionment, and transboundary transport of pesticides and other polar organic contaminants along the lower Red River, Manitoba, Canada. Sci. Total Environ. 2018, 635, 803–816. [Google Scholar] [CrossRef]
- Challis, J.K.; Stroski, K.M.; Luong, K.H.; Hanson, M.L.; Wong, C.S. Field evaluation and in situ stress testing of the organic-diffusive gradients in thin-films passive sampler. Environ. Sci. Technol. 2018, 52, 12573–12582. [Google Scholar] [CrossRef] [PubMed]
- Fauvelle, V.; Belles, A.; Budzinski, H.; Mazzella, N.; Plus, M. Simulated conservative tracer as a proxy for S-metolachlor concentration predictions compared to POCIS measurements in Arcachon Bay. Mar. Pollut. Bull. 2018, 133, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Berton, A.; Brugnera, M.F.; Dores, E. Grab and passive sampling applied to pesticide analysis in the Sao Lourenco river headwater in Campo Verde—MT, Brazil. J. Environ. Sci. Health B 2018, 53, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Daneshvar, A.; Lau, A.E.; Kreuger, J. Concentrations, fluxes and field calibration of passive water samplers for pesticides and hazard-based risk assessment. Sci. Total Environ. 2018, 637–638, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Branchet, P.; Cadot, E.; Fenet, H.; Sebag, D.; Ngatcha, B.N.; Borrell-Estupina, V.; Ngoupayou, J.R.N.; Kengne, I.; Braun, J.J.; Gonzalez, C. Polar pesticide contamination of an urban and peri-urban tropical watershed affected by agricultural activities (Yaounde, Center Region, Cameroon). Environ. Sci. Pollut. Res. Int. 2018, 25, 17690–17715. [Google Scholar] [CrossRef]
- Yabuki, Y.; Ono, J.; Nagai, T.; Inao, K.; Tanimori, S. Determining the suitability of a polar organic chemical integrated sampler (POCIS) for the detection of pesticide residue in the Ishikawa River and its tributary in Osaka, Japan. J. Pestic. Sci. 2018, 43, 18–23. [Google Scholar] [CrossRef]
- Rico, A.; Arenas-Sánchez, A.; Alonso-Alonso, C.; López-Heras, I.; Nozal, L.; Rivas-Tabares, D.; Vighi, M. Identification of contaminants of concern in the upper Tagus river basin (central Spain). Part 1: Screening, quantitative analysis and comparison of sampling methods. Sci. Total Environ. 2019, 666, 1058–1070. [Google Scholar] [CrossRef]
- Arenas-Sanchez, A.; Rico, A.; Rivas-Tabares, D.; Blanco, A.; Garcia-Doncel, P.; Romero-Salas, A.; Nozal, L.; Vighi, M. Identification of contaminants of concern in the upper Tagus river basin (central Spain). Part 2: Spatio-temporal analysis and ecological risk assessment. Sci. Total Environ. 2019, 667, 222–233. [Google Scholar] [CrossRef]
- Iwanowicz, L.R.; Pinkney, A.; Guy, C.; Major, A.; Munney, K.; Blazer, V.S.; Alvarez, D.; Walsh, H.L.; Sperry, A.; Braham, R.; et al. Temporal evaluation of estrogenic endocrine disruption markers in smallmouth bass (Micropterus dolomieu) reveals seasonal variability in intersex. Sci. Total Environ. 2019, 646, 245–256. [Google Scholar] [CrossRef]
- Tousova, Z.; Vrana, B.; Smutna, M.; Novak, J.; Klucarova, V.; Grabic, R.; Slobodnik, J.; Giesy, J.P.; Hilscherova, K. Analytical and bioanalytical assessments of organic micropollutants in the Bosna River using a combination of passive sampling, bioassays and multi-residue analysis. Sci. Total Environ. 2019, 650, 1599–1612. [Google Scholar] [CrossRef]
- Bernard, M.; Boutry, S.; Lissalde, S.; Guibaud, G.; Saut, M.; Rebillard, J.P.; Mazzella, N. Combination of passive and grab sampling strategies improves the assessment of pesticide occurrence and contamination levels in a large-scale watershed. Sci. Total Environ. 2019, 651, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Hayden, K.R.; Preisendanz, H.E.; Elkin, K.R.; Saleh, L.B.; Weikel, J.; Veith, T.L.; Elliott, H.A.; Watson, J.E. Comparison of POCIS and grab sampling techniques for monitoring PPCPs in vernal pools in central Pennsylvania. Sci. Total Environ. 2022, 806, 150607. [Google Scholar] [CrossRef] [PubMed]
- Helm, P.A.; Howell, E.T.; Li, H.L.; Metcalfe, T.M.; Chomicki, K.D.; Metcalfe, C. Influence of nearshore dynamics on the distribution of organic wastewater-associated chemicals in Lake Ontario determined using passive samplers. J. Great Lakes Res. 2012, 38, 105–115. [Google Scholar] [CrossRef]
- Mhadhbi, T.; Pringault, O.; Nouri, H.; Spinelli, S.; Beyrem, H.; Gonzalez, C. Evaluating polar pesticide pollution with a combined approach: A survey of agricultural practices and POCIS passive samplers in a Tunisian lagoon watershed. Environ. Sci. Pollut. Res. 2019, 26, 342–361. [Google Scholar] [CrossRef]
- Metcalfe, C.; Hoque, M.E.; Sultana, T.; Murray, C.; Helm, P.; Kleywegt, S. Monitoring for contaminants of emerging concern in drinking water using POCIS passive samplers. Environ. Sci. Process. Impacts 2014, 16, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Gobelius, L.; Persson, C.; Wiberg, K.; Ahrens, L. Calibration and application of passive sampling for per- and polyfluoroalkyl substances in a drinking water treatment plant. J. Hazard. Mater. 2019, 362, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Togola, A.; Gonzalez, C. In-situ calibration of POCIS for the sampling of polar pesticides and metabolites in surface water. Talanta 2013, 116, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Buzier, R.; Guibal, R.; Lissalde, S.; Guibaud, G. Limitation of flow effect on passive sampling accuracy using POCIS with the PRC approach or o-DGT: A pilot-scale evaluation for pharmaceutical compounds. Chemosphere 2019, 222, 628–636. [Google Scholar] [CrossRef]
- Ibrahim, I.; Togola, A.; Gonzalez, C. Polar organic chemical integrative sampler (POCIS) uptake rates for 17 polar pesticides and degradation products: Laboratory calibration. Environ. Sci. Pollut. Res. 2013, 20, 3679–3687. [Google Scholar] [CrossRef]
- Yabuki, Y.; Nagai, T.; Inao, K.; Ono, J.; Aiko, N.; Ohtsuka, N.; Tanaka, H.; Tanimori, S. Temperature dependence on the pesticide sampling rate of polar organic chemical integrative samplers (POCIS). Biosci. Biotechnol. Biochem. 2016, 80, 2069–2075. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Development of polar organic integrative samplers for analysis of pharmaceuticals in aquatic systems. Anal. Chem. 2007, 79, 6734–6741. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Daneshvar, A.; Lau, A.E.; Kreuger, J. Characterization of five passive sampling devices for monitoring of pesticides in water. J. Chromatogr. A 2015, 1405, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belles, A.; Pardon, P.; Budzinski, H. Development of an adapted version of polar organic chemical integrative samplers (POCIS-Nylon). Anal. Bioanal. Chem. 2014, 406, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Jakubus, A.; Tyma, M.; Stepnowski, P.; Paszkiewicz, M. Application of passive sampling devices based on multi-walled carbon nanotubes for the isolation of selected pharmaceuticals and phenolic compounds in water samples–possibilities and limitations. Talanta 2017, 164, 700–707. [Google Scholar] [CrossRef]
- Guibal, R.; Lissalde, S.; Guibaud, G. Experimental Estimation of 44 Pharmaceutical Polar Organic Chemical Integrative Sampler Sampling Rates in an Artificial River under Various Flow Conditions. Environ. Toxicol. Chem. 2020, 39, 1186–1195. [Google Scholar] [CrossRef]
- Noro, K.; Endo, S.; Shikano, Y.; Banno, A.; Yabuki, Y. Development and Calibration of the Polar Organic Chemical Integrative Sampler (POCIS) for Neonicotinoid Pesticides. Environ. Toxicol. Chem. 2020, 39, 1325–1333. [Google Scholar] [CrossRef]
- Environmental Sampling Technologies Inc. Available online: https://www.est-lab.com/ (accessed on 27 June 2022).
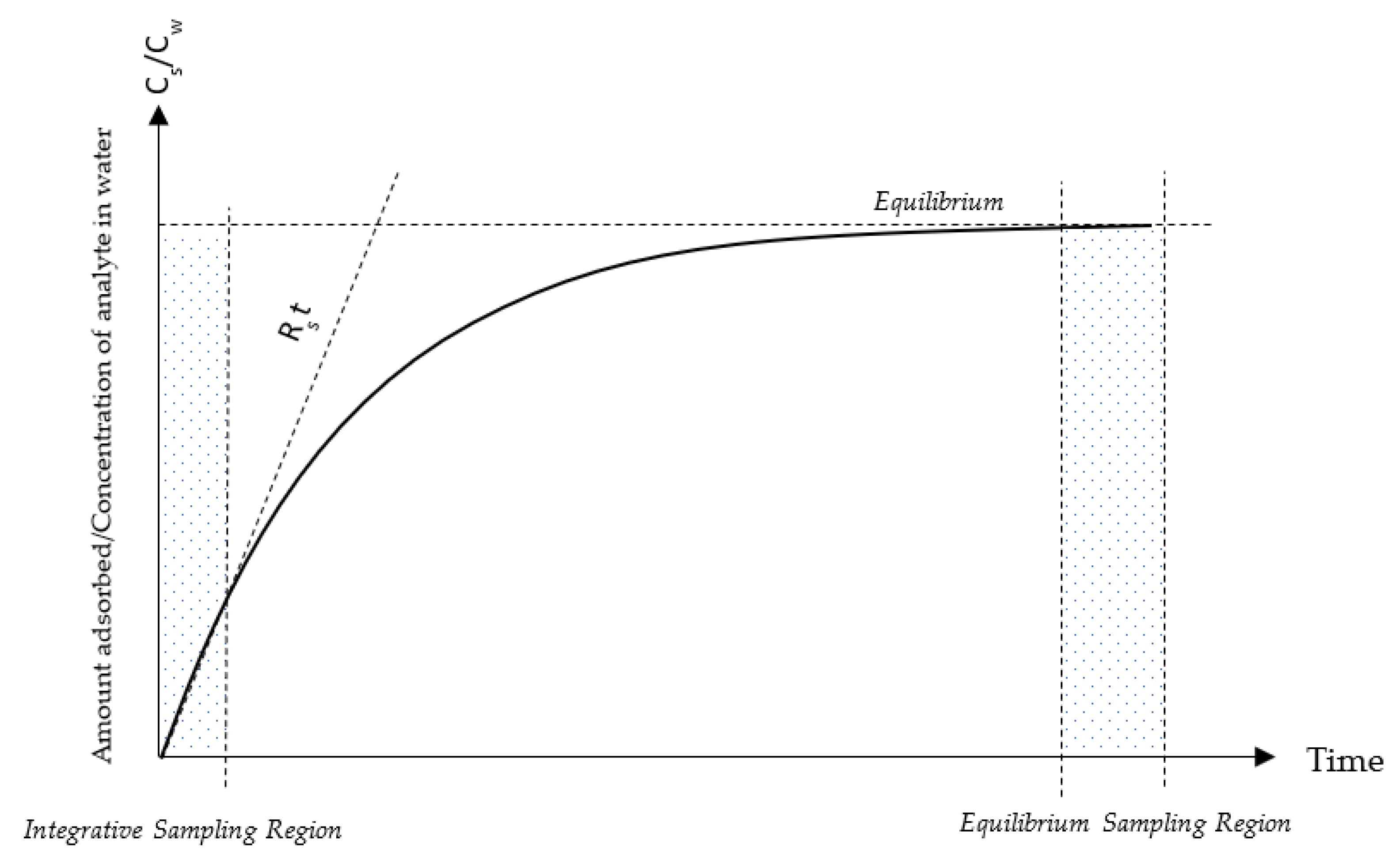
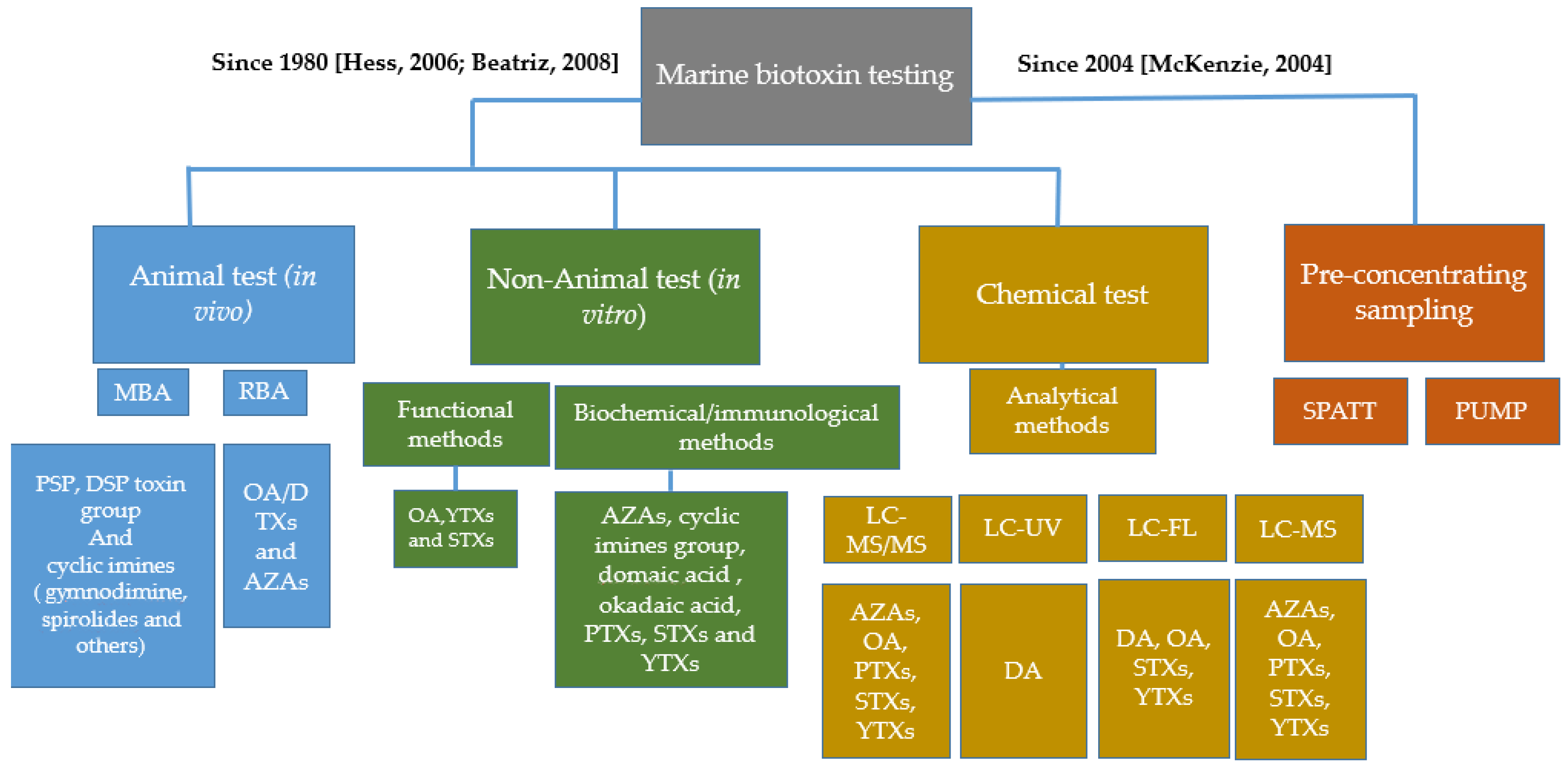
| SPATT Resin | Toxin Groups | Elute | References (Refs.) |
|---|---|---|---|
| DIAION HP20, SEPABEADS SP825L, SP850 and SEPABEADS SP700 | Microcystins | 50% MeOH | [6,165] |
| DIAION HP20, SP 207, HP2MG | PTX, PTX 2 SA, PTX 11, PT11 SA, OA, OA-ester, YTX | MeOH | [5,156] |
| DIAION HP20 | DA, CTXs | MeOH | [5,171,177] |
| Ammonium acetate in 50% MeOH | |||
| Ammonium acetate in 50% MeOH | |||
| DIAION HP20 | Cyanotoxins, Okadaic acid, Saxitoxin and related PSTs | MeOH | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamali, N.; Abbas, F.; Lehane, M.; Griew, M.; Furey, A. A Review of In Situ Methods—Solid Phase Adsorption Toxin Tracking (SPATT) and Polar Organic Chemical Integrative Sampler (POCIS) for the Collection and Concentration of Marine Biotoxins and Pharmaceuticals in Environmental Waters. Molecules 2022, 27, 7898. https://doi.org/10.3390/molecules27227898
Kamali N, Abbas F, Lehane M, Griew M, Furey A. A Review of In Situ Methods—Solid Phase Adsorption Toxin Tracking (SPATT) and Polar Organic Chemical Integrative Sampler (POCIS) for the Collection and Concentration of Marine Biotoxins and Pharmaceuticals in Environmental Waters. Molecules. 2022; 27(22):7898. https://doi.org/10.3390/molecules27227898
Chicago/Turabian StyleKamali, Naghmeh, Feras Abbas, Mary Lehane, Michael Griew, and Ambrose Furey. 2022. "A Review of In Situ Methods—Solid Phase Adsorption Toxin Tracking (SPATT) and Polar Organic Chemical Integrative Sampler (POCIS) for the Collection and Concentration of Marine Biotoxins and Pharmaceuticals in Environmental Waters" Molecules 27, no. 22: 7898. https://doi.org/10.3390/molecules27227898
APA StyleKamali, N., Abbas, F., Lehane, M., Griew, M., & Furey, A. (2022). A Review of In Situ Methods—Solid Phase Adsorption Toxin Tracking (SPATT) and Polar Organic Chemical Integrative Sampler (POCIS) for the Collection and Concentration of Marine Biotoxins and Pharmaceuticals in Environmental Waters. Molecules, 27(22), 7898. https://doi.org/10.3390/molecules27227898







