Synthesis and Anti-Leishmanial Properties of Quinolones Derived from Zanthosimuline
Abstract
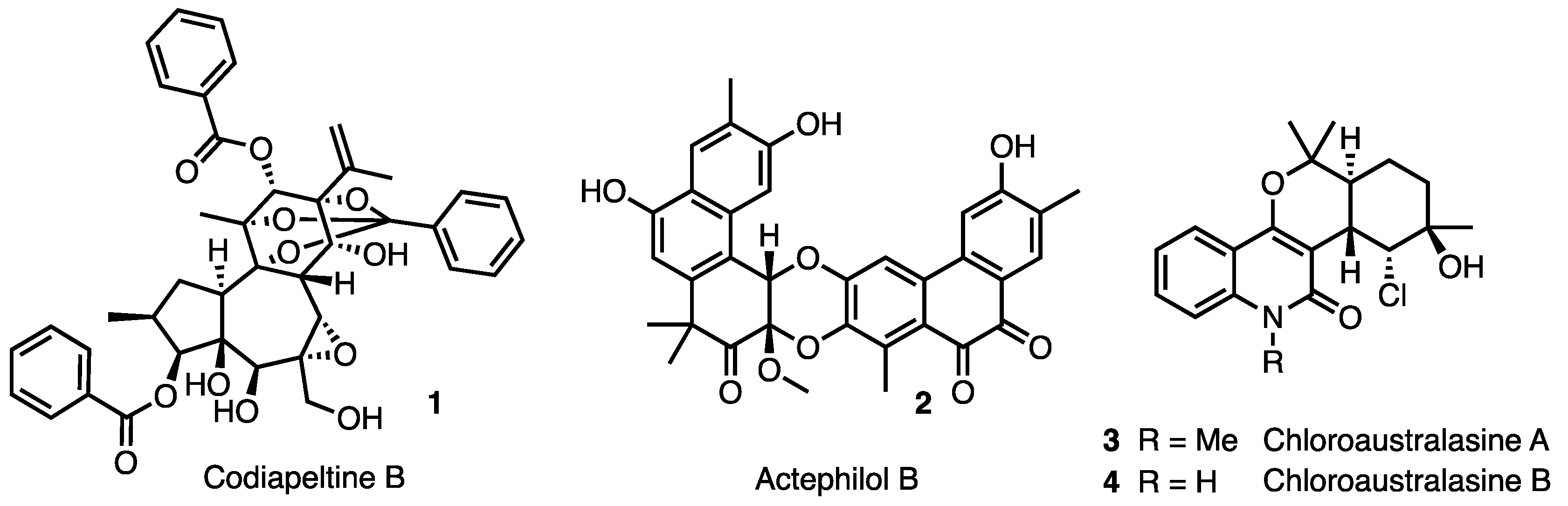
1. Introduction
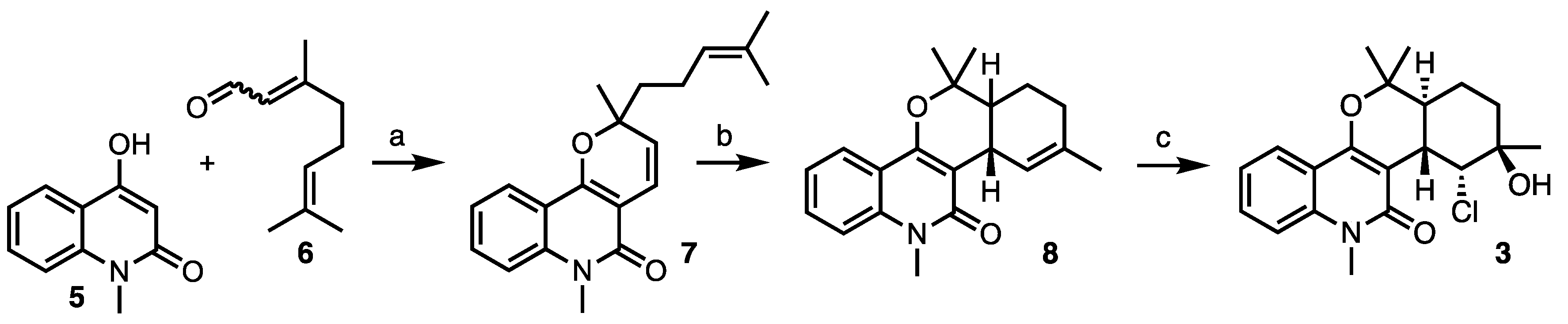
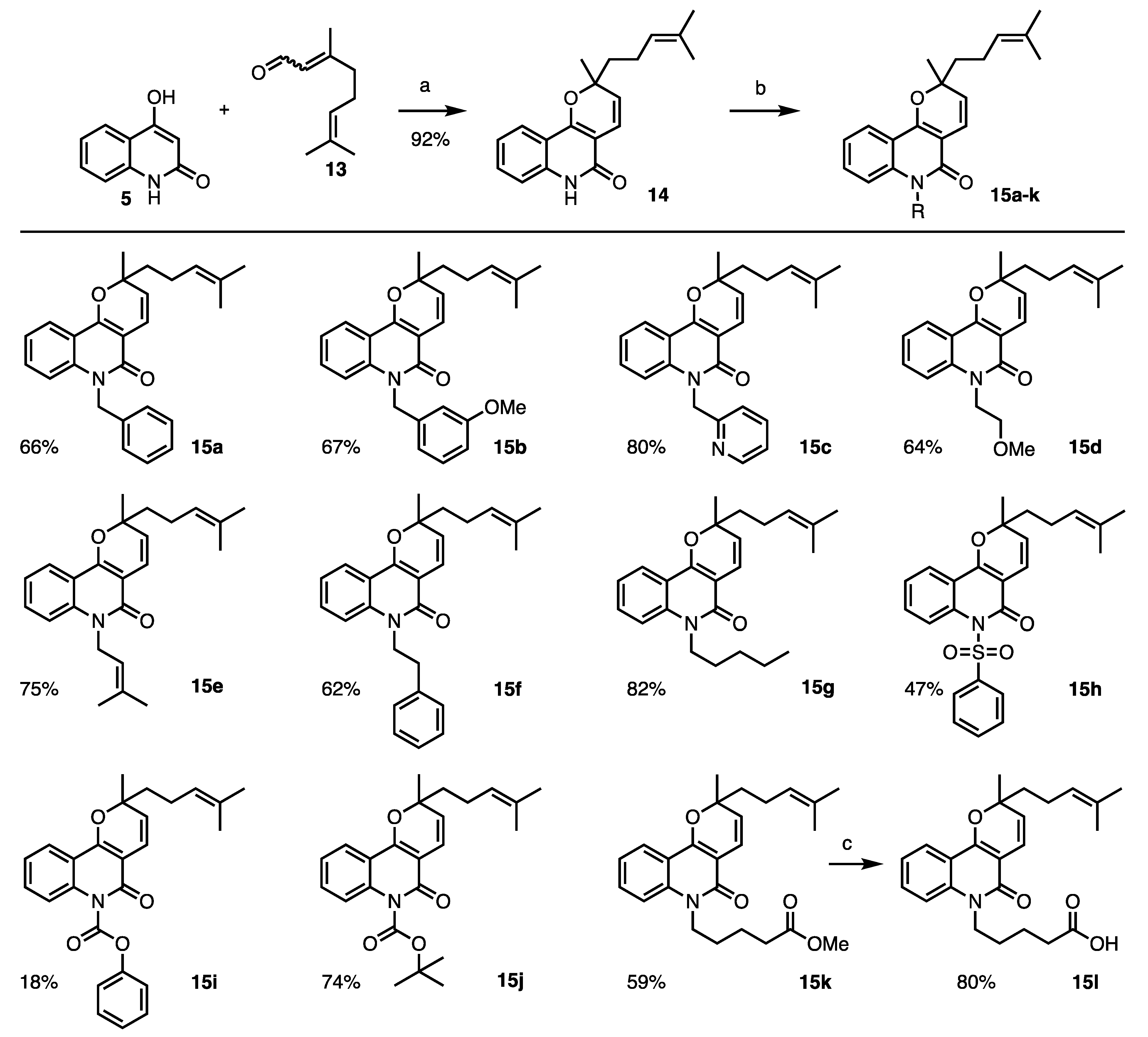
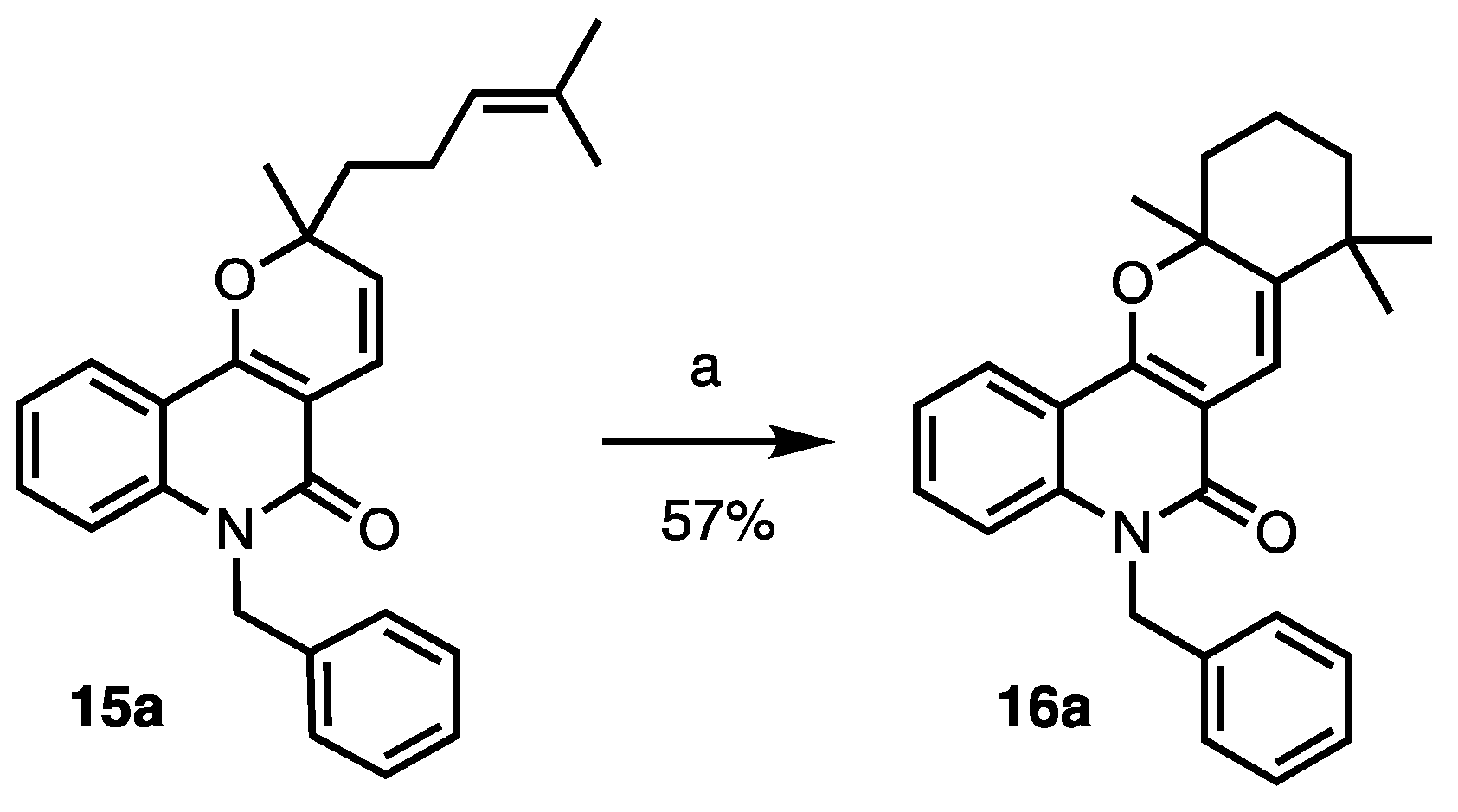
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Details
3.2. Procedures and Analytical Description of Compounds
3.3. Cell Cultures
3.4. In Vitro Cytotoxicity Evaluation of Compounds
3.5. In Vitro Antileishmanial Evaluation of Compounds on Axenic and Intramacrophage Amastigotes
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef] [PubMed]
- WHO/Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 7 November 2022).
- Pradhan, S.; Schwartz, R.A.; Patil, A.; Grabbe, S.; Goldust, M. Treatment options for leishmaniasis. Clin. Exp. Dermatol. 2022, 47, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Olivon, F.; Remy, S.; Grelier, G.; Apel, C.; Eydoux, C.; Guillemot, J.-C.; Neyts, J.; Delang, L.; Touboul, D.; Roussi, F.; et al. Antiviral compounds from Codiaeum peltatum targeted by multi-informative network approach. J. Nat. Prod. 2019, 82, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Olivon, F.; Apel, C.; Retailleau, P.; Allard, P.M.; Wolfender, J.L.; Touboul, T.; Litaudon, M.; Desrat, S. Searching for original natural products by molecular networking: Detection, isolation and total synthesis of chloroaustralasines. Org. Chem. Front. 2018, 5, 2171–2178. [Google Scholar] [CrossRef]
- Riveira, M.J.; La-Venia, A.; Mischne, M.P. Pericyclic cascade toward isochromenes: Application to the synthesis of alkaloid benzosimuline. J. Org. Chem. 2016, 81, 7977–7983. [Google Scholar] [CrossRef]
- Dib, M.; Ouchetto, H.; Ouchetto, K.; Hafid, A.; Khouili, M. Recent developments of quinoline derivatives and their potential biological activities. Curr. Org. Synth. 2021, 18, 248–269. [Google Scholar] [CrossRef]
- Pérez-Pertejo, Y.; Reguera, R.M.; Carbajo-Andrés, R.; Balana-Fouce, R.; Alonso, C.; Martin-Encinas, E.; Selas, A.; Rubiales, G.; Palacios, F. Antileishmanial activity of new hybrid tetrahydroquinoline and quinoline derivatives with phosphorous substituents. Eur. J. Med. Chem. 2019, 162, 18–31. [Google Scholar]
- Barak Almandil, N.; Taha, M.; Rahim, F.; Wadood, A.; Imran, S.; Alqahtani, M.A.; Bamarouf, Y.A.; Ibrahim, M.; Mosaddik, A.; Gollapalli, M. Synthesis of novel quinoline-based thiadazole, evaluation of their antileishmanial potential and molecular docking studies. Biorg. Chem. 2019, 85, 109–116. [Google Scholar] [CrossRef]
- Carlos Coa, J.; Cardona-Galeano, W.; Restrepo, A. Fe3+ chelating quinoline-hydrazone hybrids with proven cytotoxicity, leishmanicidal, and trypanocidal activities. Phys. Chem. Chem. Phys. 2018, 20, 20382–20390. [Google Scholar]
- Sharma, M.G.; Vala, R.M.; Patel, H.M. Pyridine-2-carboxylic acid as an effectual catalyst for rapid multi-component synthesis of pyrazolo[3,4-b]quinolinones. RSC Adv. 2020, 10, 35499–35504. [Google Scholar] [CrossRef]
- Patel, S.G.; Vala, R.M.; Patel, P.J.; Upadhyay, D.B.; Ramkumar, V.; Gardas, R.L.; Patel, H.M. Synthesis, crystal structure and in silico studies of novel 2,4-dimethoxy-tetrahydropyrimido[4,5-b]quinoline-6(7H)-ones. RSC Adv. 2022, 12, 18806–18820. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Quinoline as privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, P.M.; Balaraman, K.; Barratt, G.; Pomel, S.; Durand, R.; Frézard, F.; Figadère, B. The potential of 2-substituted quinolines as antileishmanial drug candidates. Molecules 2022, 27, 2313. [Google Scholar] [CrossRef] [PubMed]
- Billo, M.; Fournet, A.; Cabalion, P.; Waikedre, J.; Bories, C.; Loiseau, P.; Prina, E.; Rojas de Arias, A.; Yaluff, G.; Fourneau, C.; et al. Screening of New Caledonian and Vanuatu medicinal plants for antiprotozoal activity. J. Etnopharmacol. 2005, 96, 569–575. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kweon, H.I.; Koh, W.S.; Min, K.R.; Kim, Y.; Lee, S.H. One-pot preparation of pyranoquinolinones by ytterbium(III) trifluoromethanesulfonate-catalyzed reactions: Efficient synthesis of flindersine, N-methylflindersine, and zanthosimuline natural products. Synthesis 2001, 12, 1851–1855. [Google Scholar] [CrossRef]
- Wang, X.; Lee, Y.R. Efficient synthesis of substituted pyranoquinolinones from 2,4-dihydroxy quinoline: Total synthesis of zanthosimuline, cis-3′,4′-dihydroxy-3′,4′-di hydroflindersine, and orixalone D. Synthesis 2007, 19, 3044–3050. [Google Scholar]
- Wu, S.-J.; Chen, I.-S. Alkaloids from Zanthoxylum Simulans. Phytochemistry 1993, 34, 1659–1661. [Google Scholar]
- Caprioglio, D.; Mattoteia, D.; Minassi, A.; Pollastro, F.; Lopatriello, A.; Munoz, E.; Taglialatela-Scafati, O.; Appendino, G. One-pot total synthesis of cannabinol via iodine-mediated deconstructive annulation. Org. Lett. 2019, 21, 6122–6125. [Google Scholar] [CrossRef]
- Burchill, L.; Day, A.J.; Yahiaoui, O.; George, J.H. Biomimetic total synthesis of the rubiginosin meroterpenoids. Org. Lett. 2021, 23, 578–582. [Google Scholar] [CrossRef]
- Lin, S.; Ischay, M.A.; Fry, C.G.; Yoon, T.P. Radical cation Diels-Alder cycloaaditions by visible light photocatalysis. J. Am. Chem. Soc. 2011, 133, 19350–19353. [Google Scholar] [CrossRef]
- Luo, G.-Y.; Wu, H.; Li, H.; Yeom, H.-S.; Yang, K.; Hsung, R.P. A Total synthesis of (±)-rhododaurichromanic acid A via an oxa-[3+3] annulation of resorcinols. Synthesis 2015, 47, 2713–2720. [Google Scholar]
- Zahid, M.S.H.; Johnson, M.M.; Tokarski, R.J., 2nd; Satoskar, A.R.; Fuchs, J.R.; Bachelder, E.M.; Ainslie, K.M. Evaluation of synergy between host and pathogen-directed therapies against intracellular Leishmania donovani. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 125–132. [Google Scholar] [CrossRef] [PubMed]
- De Muylder, G.; Vanhollebeke, B.; Caljon, G.; Wolfe, A.R.; McKerrow, J.; Dujardin, J.C. Naloxonazine, an amastigote-specific compound, affects Leishmania parasites through modulation of host-encoded functions. PLoS Negl. Trop. Dis. 2016, 10, e0005234. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Noël, R.; Goudet, A.; Hinsinger, K.; Michau, A.; Pons, V.; Abdelkafi, H.; Secher, T.; Shima, A.; Shtanko, O.; et al. Inhibitors of retrograde trafficking active against ricin and Shiga toxins also protect cells from several viruses, Leishmania and Chlamydiales. Chem. Biol. Interact. 2017, 267, 96–103. [Google Scholar] [CrossRef]
- Levaique, H.; Pamlard, O.; Apel, C.; Bignon, J.; Arriola, M.; Kuhner, R.; Awang, K.; Loiseau, P.M.; Litaudon, M.; Pomel, S. Alkyl-resorcinol derivatives as inhibitors of GDP-mannose pyrophosphorylase with antileishmanial activities. Molecules 2021, 26, 1551. [Google Scholar] [CrossRef]
- Coleman, M.A.; Burchil, L.; Sumby, C.J.; George, J.H. Biomimetic synthesis enables the structure revision of furoerioaustralasine. Org. Lett. 2019, 21, 8776–8778. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Saar, Y.; Ransford, A.; Waldman, E.; Mazareb, S.; Amin-Spector, S.; Plumblee, J.; Turco, S.J.; Zilberstein, D. Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol. Biochem. Parasitol. 1998, 95, 9–20. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| Entry | Reaction Conditions | Conversion * | Results (Isolated Yield) |
| 1 | Silica, neat, 150 °C, 1 h | 100% | Complex mixture, 8: 26% |
| 2 | MeNO2, 150 °C, 1 h | 100% | 8: 44% |
| 3 | H2SO4 (0.01 eq.), CH2Cl2, RT, 1 h | 100% | Complex mixture ** |
| 4 | PTSA.H2O (2 eq.), CH2Cl2, RT, 24 h | 20% | Complex mixture ** |
| 5 | Montmorillonite K10, CH2Cl2, RT, 18 h | 5% | 8: 2%, 10: 2% |
| 6 | TMSCl (2 eq.), CH2Cl2, RT, 1 h | 20% | Complex mixture ** |
| 7 | BF3.OEt2 (2 eq.), CH2Cl2, RT, 18 h | 100% | 10: 30%, 11: 28% |
| 8 | TiCl4 (2 eq.), CH2Cl2, RT, 1 h | 100% | Complex mixture, 10: 10%, 11: 36% |
| 9 | SnCl4 (2 eq.), CH2Cl2, RT, 1 h | 100% | 10: 95% |
| 10 | ZnCl2 (2 eq.), CH2Cl2, RT, 1 h | <5% | − |
| 11 | FeCl3 (2 eq.), CH2Cl2, RT, 24 h | <5% | − |
| 12 | AlCl3 (2 eq.), CH2Cl2, RT, 1 h | 100% | Complex mixture ** |
| 13 | AlMe3 (2 eq.), CH2Cl2, RT, 24 h | 80% | 8: 55%, 9: 10% |
| 14 | Me2AlCl (2 eq.), CH2Cl2, RT, 18 h | 100% | 8: 95% |
| 15 | Ru(bpy)3(PF6)2 (0.05 eq.), MeNO2, RT, 24 h | 100% | 12: 86% |
| Entry | Compound | L. infantum Axenic Amastigotes IC50 (µM) ± S.D. | L. infantum Intramacrophagic Amastigotes IC50 (µM) ± S.D. | Cytotoxicity on RAW 264.7 CC50 (µM) ± S.D. | Selectivity Index * |
|---|---|---|---|---|---|
| 1 | 3 | 8.1 ± 1.4 | >50 | 80.8 ± 8.0 | <1.6 |
| 2 | 4 | 18.6 ± 2.5 | >6.25 | 8.3 ± 3.2 | <1.3 |
| 3 | 7 | 29.1 ± 1.0 | >100 | 75.9 ± 1.8 | <0.8 |
| 4 | 11 | >100 | 14.7 ± 1.6 | >100 | >6.8 |
| 5 | 12 | 42.6 ± 2.1 | >50 | 63.2 ± 2.5 | <1.3 |
| 6 | Miltefosine ** | 1.0 ± 0.3 | 6.7 ± 1.7 | 54.2 ± 5.8 | 8.1 |
| Entry | Compound | L. infantum Axenic Amastigotes IC50 (µM) ± S.D. | L. infantum Intramacrophagic Amastigotes IC50 (µM) ± S.D. | Cytotoxicity on RAW 264.7 CC50 (µM) ± S.D. | Selectivity Index * |
|---|---|---|---|---|---|
| 1 | 14 | 15.6 ± 1.8 | >100 | 75.9 ± 1.8 | <1.1 |
| 2 | 15a | 16.8 ± 1.8 | 23.7 ± 6.5 | >100 | >4.2 |
| 3 | 15b | 15.0 ± 1.7 | >25 | 42.7 ± 6.6 | <1.7 |
| 4 | 15c | 14.2 ± 4.3 | >25 | 31.1 ± 10.6 | <1.2 |
| 5 | 15d | 17.8 ± 2.3 | >25 | 47.8 ± 13.8 | <1.9 |
| 6 | 15e | 21.9 ± 0.6 | >25 | 44.8 ± 2.3 | <1.8 |
| 7 | 15f | 9.8 ± 2.9 | >50 | 52.3 ± 4.7 | <1.1 |
| 8 | 15g | 82.7 ± 6.5 | 29.5 ± 9.9 | >100 | <3.4 |
| 9 | 15h | >100 | >100 | >100 | − |
| 10 | 15i | 12.9 ± 3.2 | >15 | 18.1 ± 2.6 | <1.2 |
| 11 | 15j | 9.1 ± 0.4 | >25 | 27.8 ± 1.6 | <1.1 |
| 12 | 15k | 14.6 ± 2.1 | 19.0 ± 2.2 | 34.4 ± 4.0 | 1.8 |
| 13 | 15l | 16.4 ± 1.7 | >100 | >100 | |
| 14 | 16a | 7.0 ± 2.1 | >25 | 44.0 ± 10.0 | <1.8 |
| 15 | 7 | 29.1 ± 1.0 | >100 | 75.9 ± 1.8 | <0.8 |
| 16 | Miltefosine ** | 1.0 ± 0.3 | 6.7 ± 1.7 | 54.2 ± 5.8 | 8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jézéquel, G.; Cardoso, L.N.d.F.; Olivon, F.; Dennemont, I.; Apel, C.; Litaudon, M.; Roussi, F.; Pomel, S.; Desrat, S. Synthesis and Anti-Leishmanial Properties of Quinolones Derived from Zanthosimuline. Molecules 2022, 27, 7892. https://doi.org/10.3390/molecules27227892
Jézéquel G, Cardoso LNdF, Olivon F, Dennemont I, Apel C, Litaudon M, Roussi F, Pomel S, Desrat S. Synthesis and Anti-Leishmanial Properties of Quinolones Derived from Zanthosimuline. Molecules. 2022; 27(22):7892. https://doi.org/10.3390/molecules27227892
Chicago/Turabian StyleJézéquel, Gwenaëlle, Laura Nogueira de Faria Cardoso, Florent Olivon, Indira Dennemont, Cécile Apel, Marc Litaudon, Fanny Roussi, Sébastien Pomel, and Sandy Desrat. 2022. "Synthesis and Anti-Leishmanial Properties of Quinolones Derived from Zanthosimuline" Molecules 27, no. 22: 7892. https://doi.org/10.3390/molecules27227892
APA StyleJézéquel, G., Cardoso, L. N. d. F., Olivon, F., Dennemont, I., Apel, C., Litaudon, M., Roussi, F., Pomel, S., & Desrat, S. (2022). Synthesis and Anti-Leishmanial Properties of Quinolones Derived from Zanthosimuline. Molecules, 27(22), 7892. https://doi.org/10.3390/molecules27227892









