Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction
Abstract
1. Introduction
2. Bioactive Polymers
3. Other Applications
4. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreye, O.; Toth, T.; Meier, M.A. Introducing multicomponent reactions to polymer science: Passerini reactions of renewable monomers. J. Am. Chem. Soc. 2011, 133, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.X.; Li, L.; Li, Z.L.; Lv, A.; Du, F.S.; Li, Z.C. Sequence regulated Poly(ester-amide)s based on passerini reaction. ACS Macro Lett. 2012, 1, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Solleder, S.C.; Meier, M.A.R. Sequence control in polymer chemistry through the passerini three-component reaction. Angew. Chem. Int. Ed. 2014, 53, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Sehlinger, A.; Meier, M.A.R. Passerini and Ugi multicomponent reactions in polymer science. In Multi-Component and Sequential Reactions in Polymer Synthesis; Theato, P., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2015; Volume 269, pp. 61–86. [Google Scholar]
- Zhang, J.; Zhang, M.; Du, F.S.; Li, Z.C. Synthesis of functional polycaprolactones via passerini multicomponent polymerization of 6-oxohexanoic acid and isocyanides. Macromolecules 2016, 49, 2592–2600. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.H.; Wang, J.C.; Du, F.S.; Li, Z.C. Functional Poly(ester-amide)s with tertiary ester linkages via the passerini multicomponent polymerization of a dicarboxylic acid and a diisocyanide with different electron-deficient ketones. Macromolecules 2018, 51, 5842–5851. [Google Scholar] [CrossRef]
- Oelmann, S.; Travanut, A.; Barther, D.; Romero, M.; Howdle, S.M.; Alexander, C.; Meier, M.A.R. Biocompatible unimolecular micelles obtained via the passerini reaction as versatile nanocarriers for potential medical applications. Biomacromolecules 2019, 20, 90–101. [Google Scholar] [CrossRef]
- Kreye, O.; Tueruenc, O.; Sehlinger, A.; Rackwitz, J.; Meier, M.A.R. Structurally diverse polyamides obtained from monomers derived via the Ugi multicomponent reaction. Chem. Eur. J. 2012, 18, 5767–5776. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.-Q. Ugi multicomponent reaction product: The inhibitive effect on DNA oxidation depends upon the isocyanide moiety. J. Org. Chem. 2013, 78, 8696–8704. [Google Scholar] [CrossRef]
- Sehlinger, A.; Dannecker, P.-K.; Kreye, O.; Meier, M.A.R. Diversely substituted polyamides: Macromolecular design using the Ugi four-component reaction. Macromolecules 2014, 47, 2774–2783. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Fu, C.; Zhu, C.; Zhang, Y.; Wang, S.; Wei, Y.; Tao, L. Introducing the Ugi reaction into polymer chemistry as a green click reaction to prepare middle-functional block copolymers. Polym. Chem. 2014, 5, 2704–2708. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Wei, Y.; Fu, C.; Tao, L. The Ugi reaction in polymer chemistry: Syntheses, applications and perspectives. Polym. Chem. 2015, 6, 8233–8239. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Liu, J.; Xie, Z.; Luan, S.; Xiao, C.; Tao, Y.; Wang, X. Ugi reaction of natural amino acids: A general route toward facile synthesis of polypeptoids for bioapplications. ACS Macro Lett. 2016, 5, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Dannecker, P.-K.; Sehlinger, A.; Meier, M.A.R. Polymacrocycles derived via Ugi multi-component reactions. Macromol. Rapid Commun. 2019, 40, 1800748. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Z.; Tao, Y. Polypeptoids synthesis based on Ugi reaction: Advances and perspectives. Biopolymers 2019, 110, e23288. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial self-healing hydrogel via the Ugi reaction. ACS Appl. Polym. Mater. 2020, 2, 404–410. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, G.; Lv, T.; He, X.; Wei, Y.; Pan, R.; Yang, L.; Tao, L. Antioxidant polymers via the Ugi reaction for in vivo protection of UV-induced oxidative stress. Chem. Mater. 2022, 34, 2645–2654. [Google Scholar] [CrossRef]
- Wu, G.M.; Sun, W.L.; Shen, Z.Q. Synthesis and properties of two Poly(phenyl methacylate)s functionalized with pedent Dihydropyrimid(thi)one groups. Chin. J. Polym. Sci. 2009, 27, 293–296. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, B.; Zhao, Y.; Fu, C.; Tao, L.; Wei, Y. A new insight into the biginelli reaction: The dawn of multicomponent click chemistry? Polym. Chem. 2013, 4, 5395–5400. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, H.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Q.; Ren, X.; Fu, C.; Wei, Y.; Wang, Z.; et al. Postpolymerization modification of Poly(dihydropyrimidin-2(1H)-thione)s via the thiourea-haloalkane reaction to prepare functional polymers. ACS Macro Lett. 2015, 4, 843–847. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Y.; Zhang, Q.; Fu, C.; Wei, Y.; Tao, L. From drug to adhesive: A new application of Poly(dihydropyrimidin-2(1H)-one)s via the biginelli polycondensation. Polym. Chem. 2015, 6, 4940–4945. [Google Scholar] [CrossRef]
- Boukis, A.C.; Llevot, A.; Meier, M.A. High glass transition temperature renewable polymers via biginelli multicomponent polymerization. Macromol. Rapid Commun. 2016, 37, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhao, Y.; Wu, H.; Wang, Z.; Yang, B.; Wei, Y.; Wang, Z.; Tao, L. Multicomponent combinatorial polymerization via the biginelli reaction. J. Am. Chem. Soc. 2016, 138, 8690–8693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, H.; Wang, Z.; Wei, Y.; Wang, Z.; Tao, L. Training the old dog new tricks: The applications of the biginelli reaction in polymer chemistry. Sci. China Chem. 2016, 59, 1541–1547. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Tao, L. Polymer synthesis by mimicking nature’s strategy: The combination of ultra-fast RAFT and the biginelli reaction. Polym. Chem. 2017, 8, 5679–5687. [Google Scholar] [CrossRef]
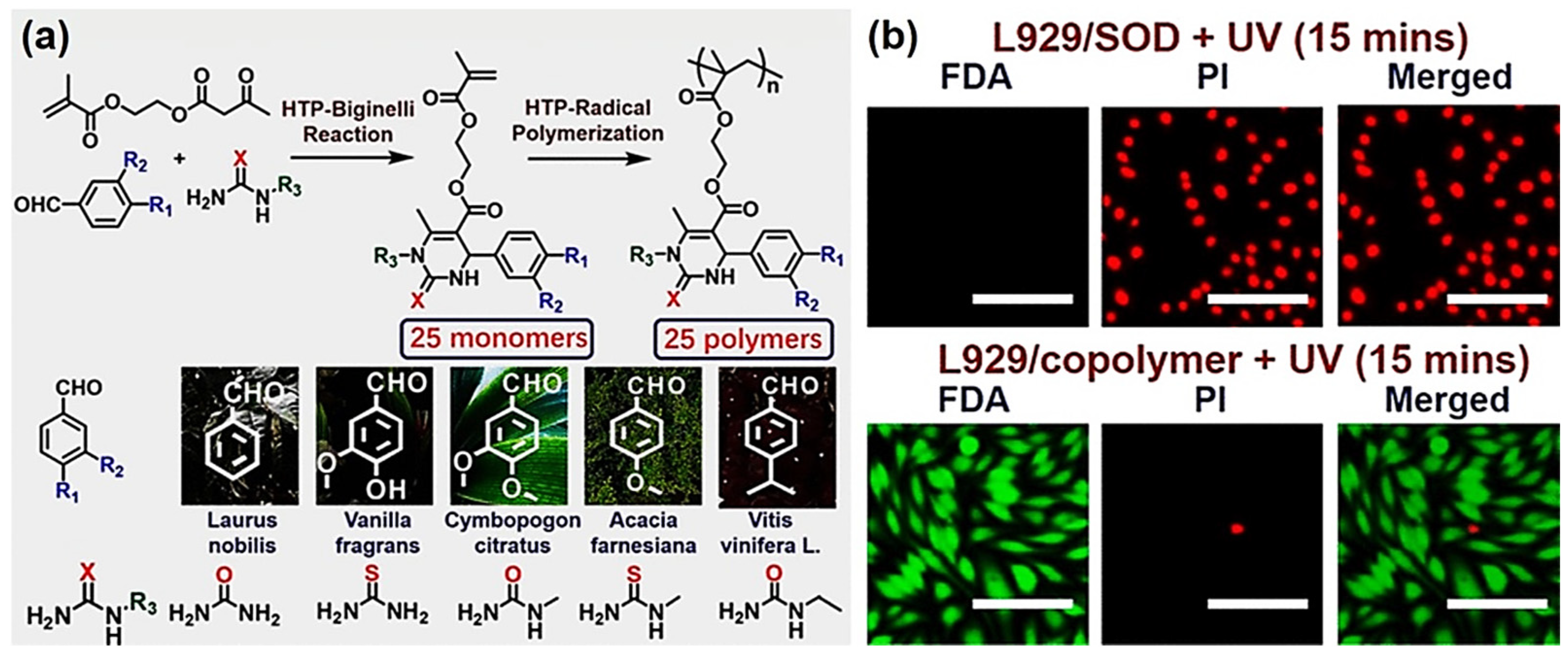
- Mao, T.; Liu, G.; Wu, H.; Wei, Y.; Gou, Y.; Wang, J.; Tao, L. High throughput preparation of UV-protective polymers from essential oil extracts via the biginelli reaction. J. Am. Chem. Soc. 2018, 140, 6865–6872. [Google Scholar] [CrossRef]
- Wu, H.; Fu, C.; Zhao, Y.; Yang, B.; Wei, Y.; Wang, Z.; Tao, L. Multicomponent copolycondensates via the simultaneous hantzsch and biginelli reactions. ACS Macro Lett. 2015, 4, 1189–1193. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Q.; Li, Y.; Wang, X.; Wu, H.; Wei, Y.; Zeng, Y.; Tao, L. High-throughput preparation of antibacterial polymers from natural product derivatives via the Hantzsch reaction. iScience 2020, 23, 100754. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, Y.; Lv, T.; Mao, T.; Wei, Y.; Jia, S.; Gou, Y.; Tao, L. High-throughput preparation of radioprotective polymers via Hantzsch’s reaction for in vivo x-ray damage determination. Nat. Commun. 2020, 11, 6214. [Google Scholar] [CrossRef]
- Liu, G.; Pan, R.; Wei, Y.; Tao, L. The Hantzsch reaction in polymer chemistry: From synthetic methods to applications. Macromol. Rapid Commun. 2021, 42, e2000459. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Z.; Dai, X.; Zeng, Y.; Wei, Y.; He, X.; Yan, L.T.; Tao, L. De novo design of entropy-driven polymers resistant to bacterial attachment via multicomponent reactions. J. Am. Chem. Soc. 2021, 143, 17250–17260. [Google Scholar] [CrossRef]
- Kakuchi, R.; Theato, P. Efficient multicomponent postpolymerization modification based on Kabachnik-fields reaction. ACS Macro Lett. 2014, 3, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Yang, B.; Zhu, C.; Wei, Y.; Tao, L. ‘One pot’ synthesis of well-defined Poly(aminophosphonate)s: Time for the Kabachnik–fields reaction on the stage of polymer chemistry. Polym. Chem. 2014, 5, 1857–1862. [Google Scholar] [CrossRef]
- He, X.; Liu, G.; Tian, Y.; Mao, T.; Wu, H.; Wei, Y.; Tao, L. Antioxidant polymers via the Kabachnik-fields reaction to control cellular oxidative stress. Macromol. Biosci. 2020, 20, e1900419. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, Y.; Liu, G.; Tian, Y.; Wei, Y.; Zhao, L.; Yang, L.; Tao, L. Magnetic self-healing hydrogel from difunctional polymers prepared via the Kabachnik-fields reaction. ACS Macro Lett. 2022, 11, 39–45. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, L.; Liu, G.; Wei, Y.; Zeng, Y.; Tao, L. Polymer chelator prepared via the Kabachnik–fields reaction for the in vivo prevention of heavy-metal damage. Chem. Mater. 2022, 34, 9558–9568. [Google Scholar] [CrossRef]
- Hassan, S.; Müller, T.J.J. Multicomponent syntheses based upon copper-catalyzed Alkyne-Azide cycloaddition. Adv. Synth. Catal. 2015, 357, 617–666. [Google Scholar] [CrossRef]
- Kayser, L.V.; Vollmer, M.; Welnhofer, M.; Krikcziokat, H.; Meerholz, K.; Arndtsen, B.A. Metal-free, multicomponent synthesis of pyrrole-based π-conjugated polymers from imines, acid chlorides, and alkynes. J. Am. Chem. Soc. 2016, 138, 10516–10521. [Google Scholar] [CrossRef]
- Wei, B.; Li, W.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B.Z. Metal-free multicomponent tandem polymerizations of alkynes, amines, and formaldehyde toward structure- and sequence-controlled luminescent polyheterocycles. J. Am. Chem. Soc. 2017, 139, 5075–5084. [Google Scholar] [CrossRef]
- Lee, I.H.; Kim, H.; Choi, T.L. Cu-catalyzed multicomponent polymerization to synthesize a library of Poly(N-sulfonylamidines). J. Am. Chem. Soc. 2013, 135, 3760–3763. [Google Scholar] [CrossRef]
- Leitch, D.C.; Kayser, L.V.; Han, Z.Y.; Siamaki, A.R.; Keyzer, E.N.; Gefen, A.; Arndtsen, B.A. A palladium-catalysed multicomponent coupling approach to conjugated Poly(1,3-dipoles) and polyheterocycles. Nat. Commun. 2015, 6, 7411. [Google Scholar] [CrossRef]
- Kim, H.; Bang, K.T.; Choi, I.; Lee, J.K.; Choi, T.L. Diversity-oriented polymerization: One-shot synthesis of library of graft and dendronized polymers by Cu-catalyzed multicomponent polymerization. J. Am. Chem. Soc. 2016, 138, 8612–8622. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Bang, K.T.; Yang, H.S.; Choi, T.L. Recent advances in diversity-oriented polymerization using Cu-catalyzed multicomponent reactions. Macromol. Rapid Commun. 2022, 43, e2100642. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th anniversary perspective: Polymer functionalization. Macromolecules 2017, 50, 5215–5252. [Google Scholar] [CrossRef]
- Afshari, R.; Shaabani, A. Materials functionalization with multicomponent reactions: State of the art. ACS Comb. Sci. 2018, 20, 499–528. [Google Scholar] [CrossRef]
- Tian, T.; Hu, R.; Tang, B.Z. Room temperature one-step conversion from elemental sulfur to functional polythioureas through catalyst-free multicomponent polymerizations. J. Am. Chem. Soc. 2018, 140, 6156–6163. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Multicomponent reactions-based modified/functionalized materials in the biomedical platforms. ACS Appl. Bio Mater. 2020, 3, 156–174. [Google Scholar] [CrossRef]
- Pan, R.; Liu, G.; Zeng, Y.; He, X.; Ma, Z.; Wei, Y.; Chen, S.; Yang, L.; Tao, L. A multi-responsive self-healing hydrogel for controlled release of curcumin. Polym. Chem. 2021, 12, 2457–2463. [Google Scholar] [CrossRef]
- Zhang, J.; Zang, Q.; Yang, F.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Sulfur conversion to multifunctional Poly(O-thiocarbamate)s through multicomponent polymerizations of sulfur, diols, and diisocyanides. J. Am. Chem. Soc. 2021, 143, 3944–3950. [Google Scholar] [CrossRef]
- Peng, F.; Liu, H.; Hu, S.; Yue, F.; Xiao, D.; Guo, L.; Qi, H. High throughput preparation of antioxidant polysaccharide-based polymers with UV-resistant and antibacterial performance. Food Hydrocoll. 2022, 133, 107936. [Google Scholar] [CrossRef]
- Biginelli, P. Ueber Aldehyduramide des Acetessigäthers. Ber. Dtsch. Chem. Ges. 1891, 24, 1317–1319. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.H.S.; Masson, F.T.; Simeoni, L.A.; Homem-de-Mello, M. Biological Activity of Dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, B.; Zhao, Y.; Zhang, X.; Wang, X.; Wei, Y.; Tao, L. One-pot polymer conjugation on carbon nanotubes through simultaneous π–π stacking and the biginelli reaction. Polymer 2015, 64, 210–215. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Y.; Li, Y.; Yang, L.; Zhao, Y.; Liu, G.; Wei, Y.; Wang, X.; Tao, L. Post-polymerization modification via the biginelli reaction to prepare water-soluble polymer adhesives. Polym. Chem. 2017, 8, 5490–5495. [Google Scholar] [CrossRef]
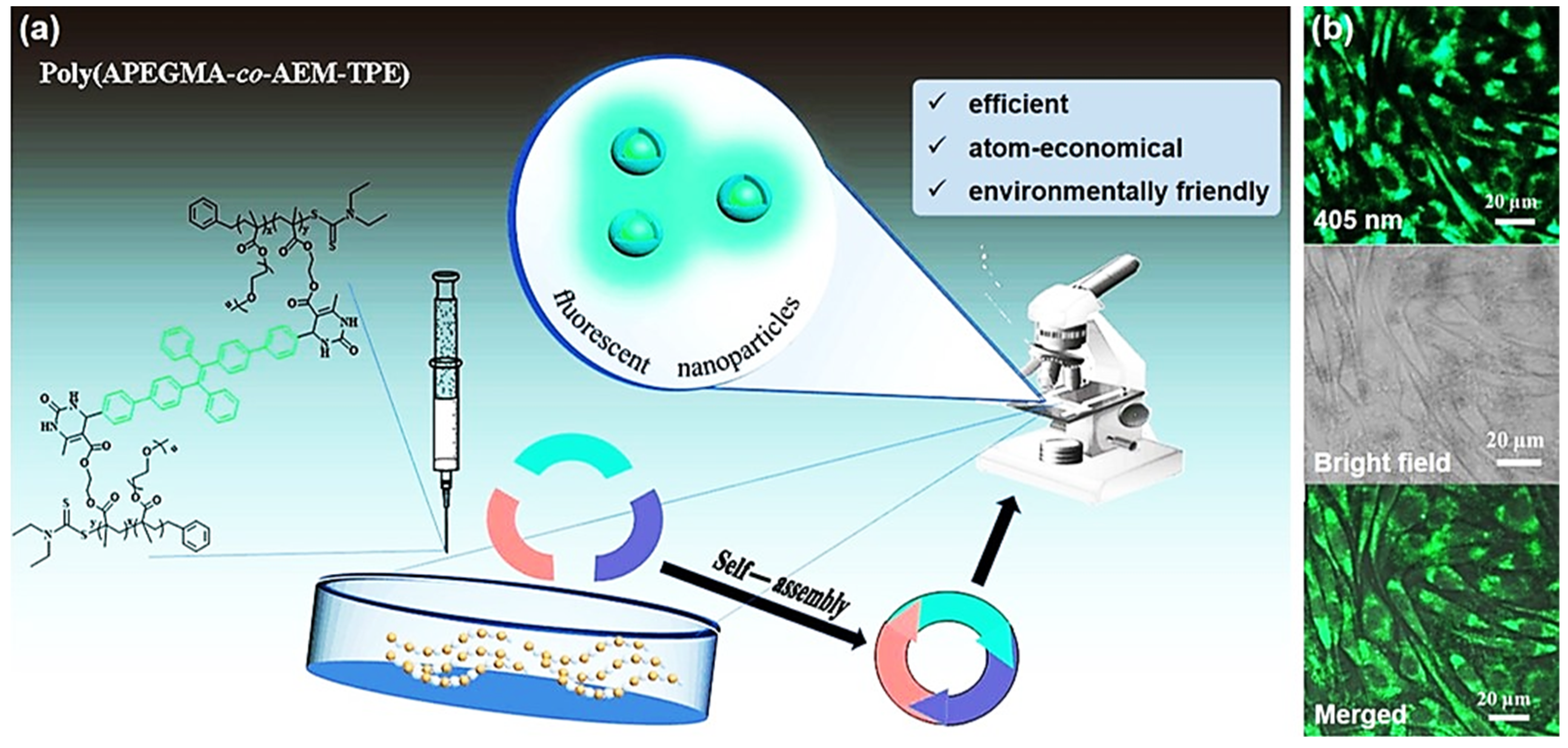
- Dong, J.; Liu, M.; Jiang, R.; Huang, H.; Wan, Q.; Wen, Y.; Tian, J.; Dai, Y.; Zhang, X.; Wei, Y. Synthesis and biological imaging of cross-linked fluorescent polymeric nanoparticles with aggregation-induced emission characteristics based on the combination of RAFT polymerization and the biginelli reaction. J. Colloid. Interface Sci. 2018, 528, 192–199. [Google Scholar] [CrossRef]
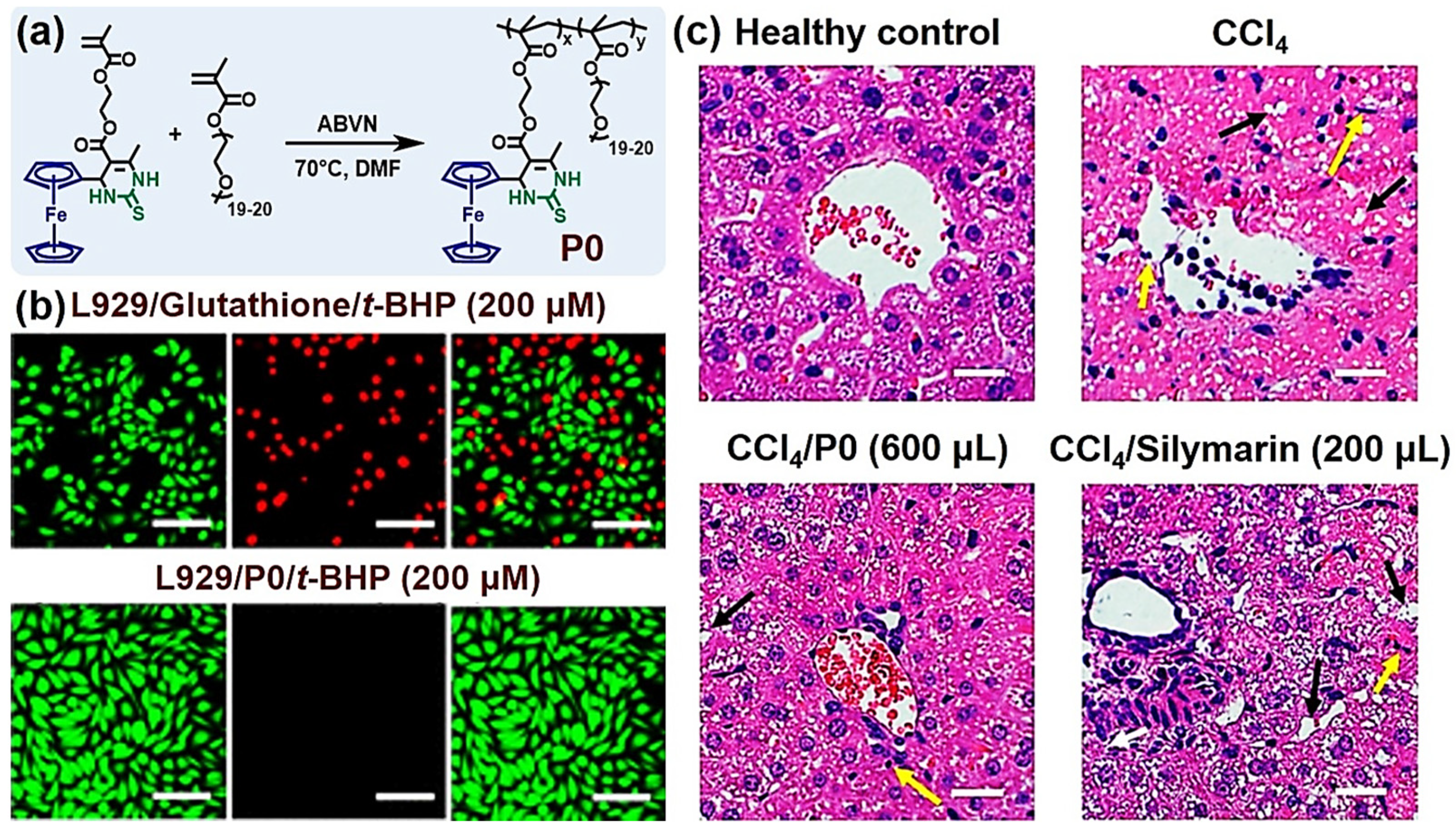
- Mao, T.; Yang, L.; Liu, G.; Wei, Y.; Gou, Y.; Wang, J.; Tao, L. Ferrocene-containing polymer via the biginelli reaction for in vivo treatment of oxidative stress damage. ACS Macro Lett. 2019, 8, 639–645. [Google Scholar] [CrossRef]
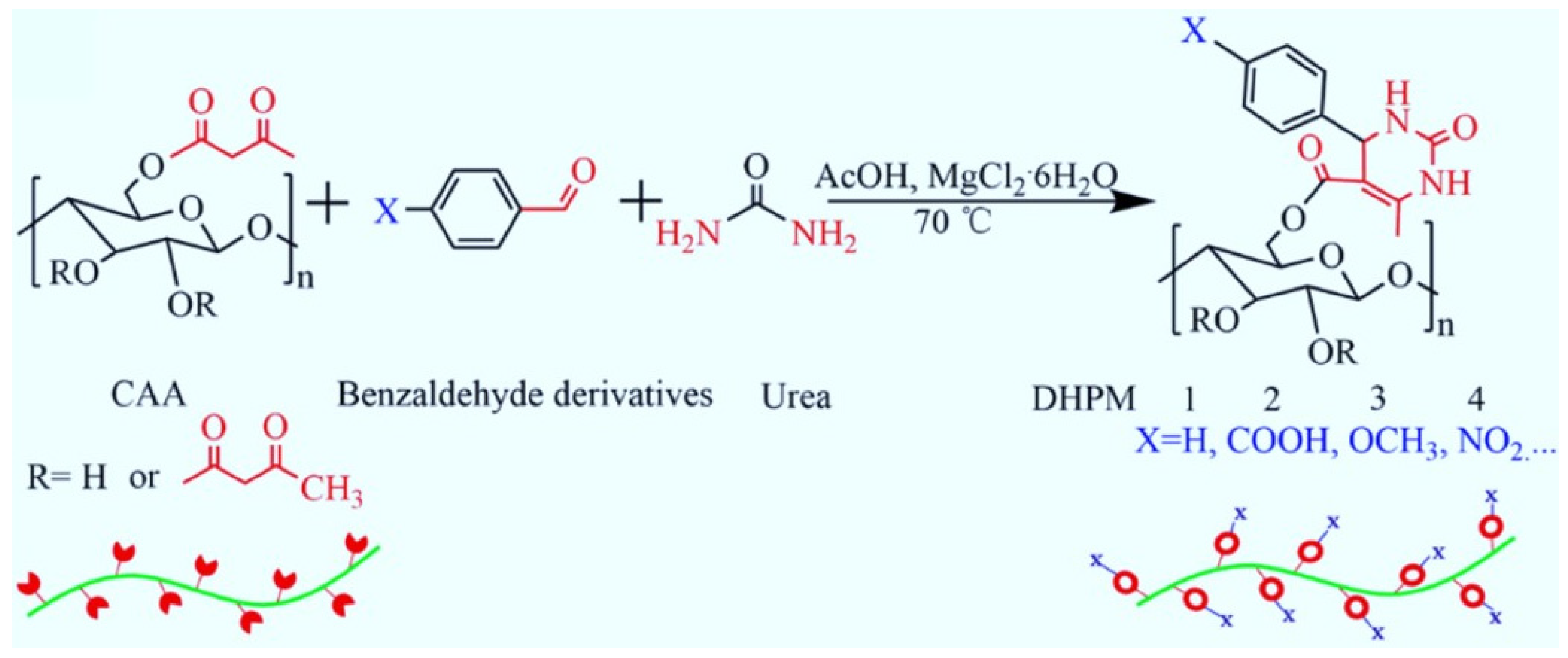
- Rong, L.; Zeng, M.; Liu, H.; Wang, B.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Yuan, J.; Sui, X. Biginelli reaction on cellulose acetoacetate: A new approach for versatile cellulose derivatives. Carbohydr. Polym. 2019, 209, 223–229. [Google Scholar] [CrossRef]
- Esen, E.; Meier, M.A.R. Modification of starch via the biginelli multicomponent reaction. Macromol. Rapid Commun. 2020, 41, e1900375. [Google Scholar] [CrossRef]
- Li, Y.; Tan, T.; Zhao, Y.; Wei, Y.; Wang, D.; Chen, R.; Tao, L. Anticancer polymers via the biginelli reaction. ACS Macro Lett. 2020, 9, 1249–1254. [Google Scholar] [CrossRef]
- Windbiel, J.T.; Meier, M.A.R. Synthesis of new biginelli polycondensates: Renewable materials with tunable high glass transition temperatures. Polym. Int. 2020, 70, 506–513. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, Y.; Wu, H.; Zhou, C.; Tao, L. An antioxidant self-healing hydrogel for 3D cell cultures. J. Mater. Chem. B 2020, 8, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; He, X.; Liu, G.; Wei, Y.; Gou, Y.; Zhou, X.; Tao, L. Fluorescent polymers via post-polymerization modification of biginelli-type polymers for cellular protection against UV damage. Polym. Chem. 2021, 12, 852–857. [Google Scholar] [CrossRef]
- Windbiel, J.T.; Meier, M.A.R. RAFT polymerization of a renewable ricinoleic acid-derived monomer and subsequent post-polymerization modification via the biginelli-3-component reaction. Macromol. Chem. Phys. 2021, 223, 2100360. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, C.; Tao, L. Stimuli-responsive multifunctional phenylboronic acid polymers via multicomponent reactions: From synthesis to application. Macromol. Rapid Commun. 2021, 42, e2100022. [Google Scholar] [CrossRef]
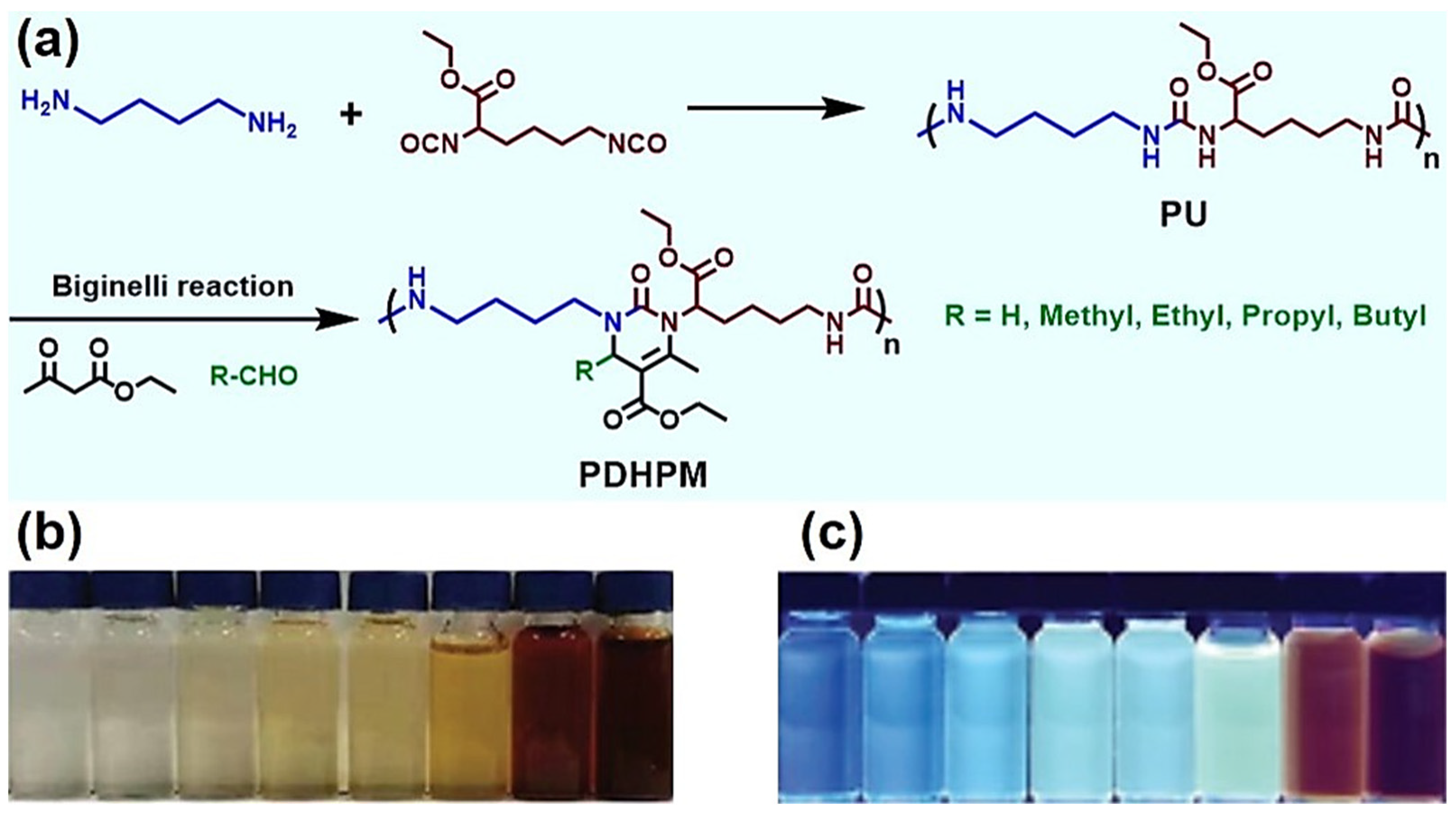
- Zhang, D.; Zheng, J.; Zhang, P.; Zhao, R.; Chen, Z.; Wang, M.; Deng, K. Polyurea modified with 4-dihydropyrimidone-2-ketone rings by biginelli reaction and its boostered AIE characteristic. Macromol. Chem. Phys. 2021, 222, 2100284. [Google Scholar] [CrossRef]
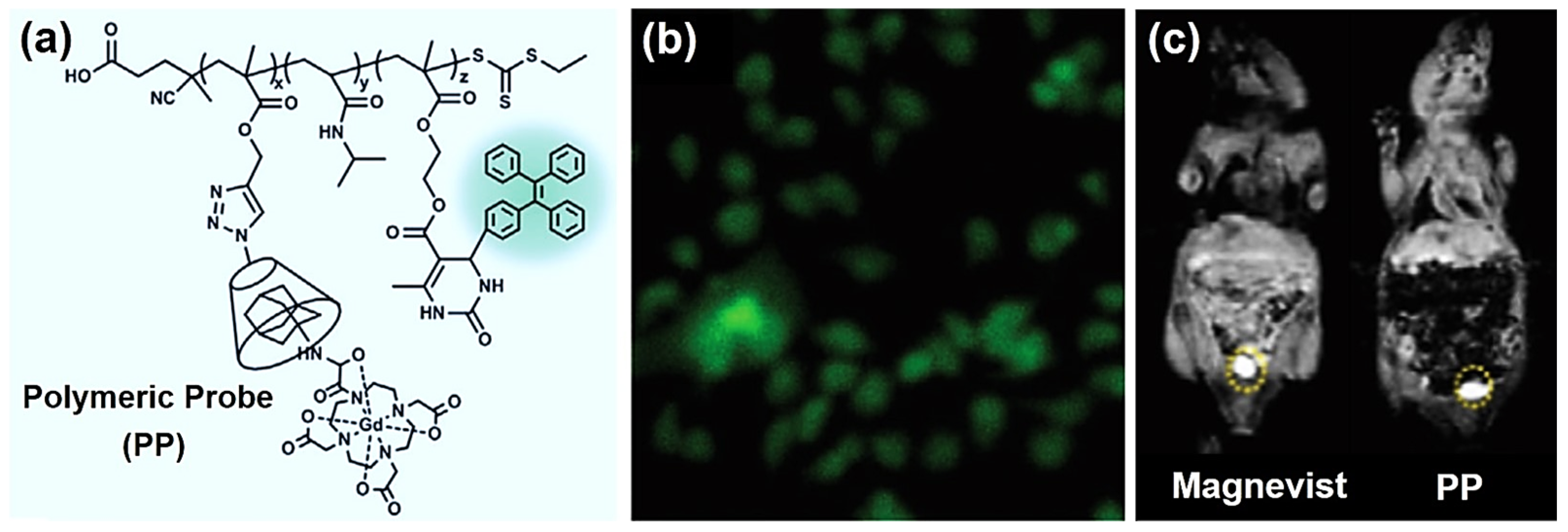
- Zhou, M.; Li, L.; Xie, W.; He, Z.; Li, J. Synthesis of a thermal-responsive dual-modal supramolecular probe for magnetic resonance imaging and fluorescence imaging. Macromol. Rapid Commun. 2021, 42, e2100248. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Zhang, X.; Lin, L. ROS-Scavenging glyco-nanoplatform for synergistic antibacterial and wound-healing therapy of bacterial keratitis. J. Mater. Chem. B 2022, 10, 4575–4587. [Google Scholar] [CrossRef]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.X.; Sun, Y.; Deng, K.; Mei, J.Y.; Chermansky, C.J.; Damaser, M.S. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat. Rev. Urol. 2022, 19, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.D.; Cyrino, L.A.R.; Ferreira, G.K.; Magro, D.D.D.; Calegari, C.R.; Cabral, H.; Cavichioli, N.; Ramos, S.A.; Ullmann, O.M.; Mayer, Y.; et al. Neuroinflammation and neuroprogression produced by oxidative stress in euthymic bipolar patients with different onset disease times. Sci. Rep. 2022, 12, 16742. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Slatore, C.G.; Littman, A.J.; Au, D.H.; Satia, J.A.; White, E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am. J. Respir. Crit. Care. Med. 2008, 177, 524–530. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, A.; Xu, P.; Pindrus, M.A.; Blasioli, D.J.; Kaplan, D.L. Expansion and osteogenic differentiation of bone marrow-derived mesenchymal stem cells on a vitamin C functionalized polymer. Biomaterials 2006, 27, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Wattamwar, P.P.; Mo, Y.; Wan, R.; Palli, R.; Zhang, Q.; Dziubla, T.D. Antioxidant activity of degradable polymer Poly(trolox ester) to suppress oxidative stress injury in the cells. Adv. Funct. Mater. 2010, 20, 147–154. [Google Scholar] [CrossRef]
- Hlushko, R.; Hlushko, H.; Sukhishvili, S.A. A family of linear phenolic polymers with controlled hydrophobicity, adsorption and antioxidant properties. Polym. Chem. 2018, 9, 506–516. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant activity of synthetic polymers of phenolic compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating antioxidant functionality into polymer materials: Fundamentals, strategies, and applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent advances in antioxidant polymers: From sustainable and natural monomers to synthesis and applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef]
- Stefani, H.A.; Oliveira, C.B.; Almeida, R.B.; Pereira, C.M.; Braga, R.C.; Cella, R.; Borges, V.C.; Savegnago, L.; Nogueira, C.W. Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: A new class of potential antioxidant agents. Eur. J. Med. Chem. 2006, 41, 513–518. [Google Scholar] [CrossRef]
- Jager, T.L.d.; Cockrell, A.E.; Plessis, S.S.D. Ultraviolet light induced generation of reactive oxygen species. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 996, pp. 15–23. [Google Scholar]
- Liu, Z.Q. Enhancing antioxidant effect against peroxyl radical-induced oxidation of DNA: Linking with ferrocene moiety! Chem. Rec. 2019, 19, 2385–2397. [Google Scholar] [CrossRef]
- Liu, Z.Q. Multicomponent reactions for integrating multiple functional groups into an antioxidant. Chem. Rec. 2020, 20, 1516–1529. [Google Scholar] [CrossRef]
- Staveren, D.R.v.; Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5985. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.-Q. Ferrocene as a functional group enhances the inhibitive effect of dihydropyrimidine on radical-induced oxidation of DNA. Org. Chem. Front. 2014, 1, 792–797. [Google Scholar] [CrossRef]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial kratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Hilliam, Y.; Kaye, S.; Winstanley, C. Pseudomonas aeruginosa and microbial keratitis. J. Med. Microbiol. 2020, 69, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 186, 971–974. [Google Scholar] [CrossRef]
- Guido, B.C.; Ramos, L.M.; Nolasco, D.O.; Nobrega, C.C.; Andrade, B.Y.; Pic-Taylor, A.; Neto, B.A.; Correa, J.R. Impact of kinesin Eg5 Inhibition by 3,4-Dihydropyrimidin-2(1H)-one derivatives on various breast cancer cell features. BMC Cancer 2015, 15, 283. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Dhfyan, A.; Al-Omar, M.A. Targeting cancer stem cells with novel 4-(4-Substituted phenyl)-5-(3,4,5-trimethoxy/3,4-dimethoxy)-benzoyl-3,4-dihydropyrimidine-2(1H)-one/thiones. Molecules 2016, 21, 1746. [Google Scholar] [CrossRef]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Gondru, R.; Peddi, S.R.; Manga, V.; Khanapur, M.; Gali, R.; Sirassu, N.; Bavantula, R. One-pot synthesis, biological evaluation and molecular docking studies of fused Thiazolo[2,3-b]pyrimidinone-pyrazolylcoumarin hybrids. Mol. Divers. 2018, 22, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zeng, Y.; He, X.; Pan, S.; Wei, Y.; Wang, B.; Tao, L. Introducing the aza-michael addition reaction between acrylate and Dihydropyrimidin-2(1H)-thione into polymer chemistry. Polym. Chem. 2022. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wang, B.; Tao, L. Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction. Molecules 2022, 27, 7886. https://doi.org/10.3390/molecules27227886
Ma Z, Wang B, Tao L. Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction. Molecules. 2022; 27(22):7886. https://doi.org/10.3390/molecules27227886
Chicago/Turabian StyleMa, Zeyu, Bo Wang, and Lei Tao. 2022. "Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction" Molecules 27, no. 22: 7886. https://doi.org/10.3390/molecules27227886
APA StyleMa, Z., Wang, B., & Tao, L. (2022). Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction. Molecules, 27(22), 7886. https://doi.org/10.3390/molecules27227886