Nano-WSe2 Is Absorbable and Transformable by Rice Plants †
Abstract
1. Introduction
2. Results and Discussion
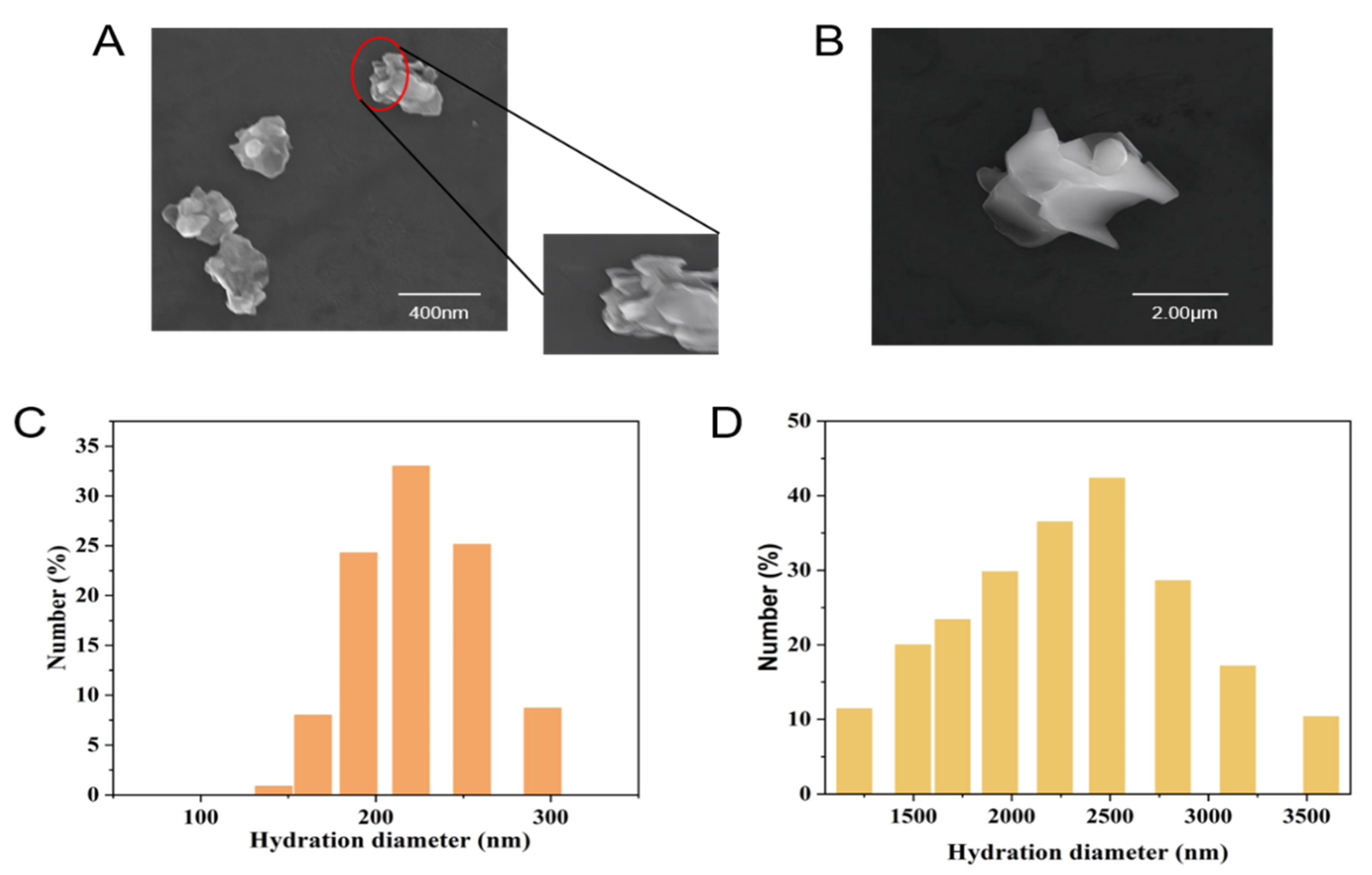
2.1. The Size of Nano-WSe2 and Micro-WSe2
2.2. The Impacts on the Germination and Growth of Rice Plants by Nano-WSe2 or Micro-WSe2
2.2.1. The Impacts on Germination Rate of Rice Seeds
2.2.2. The Impacts on the Growth of Rice Seedlings
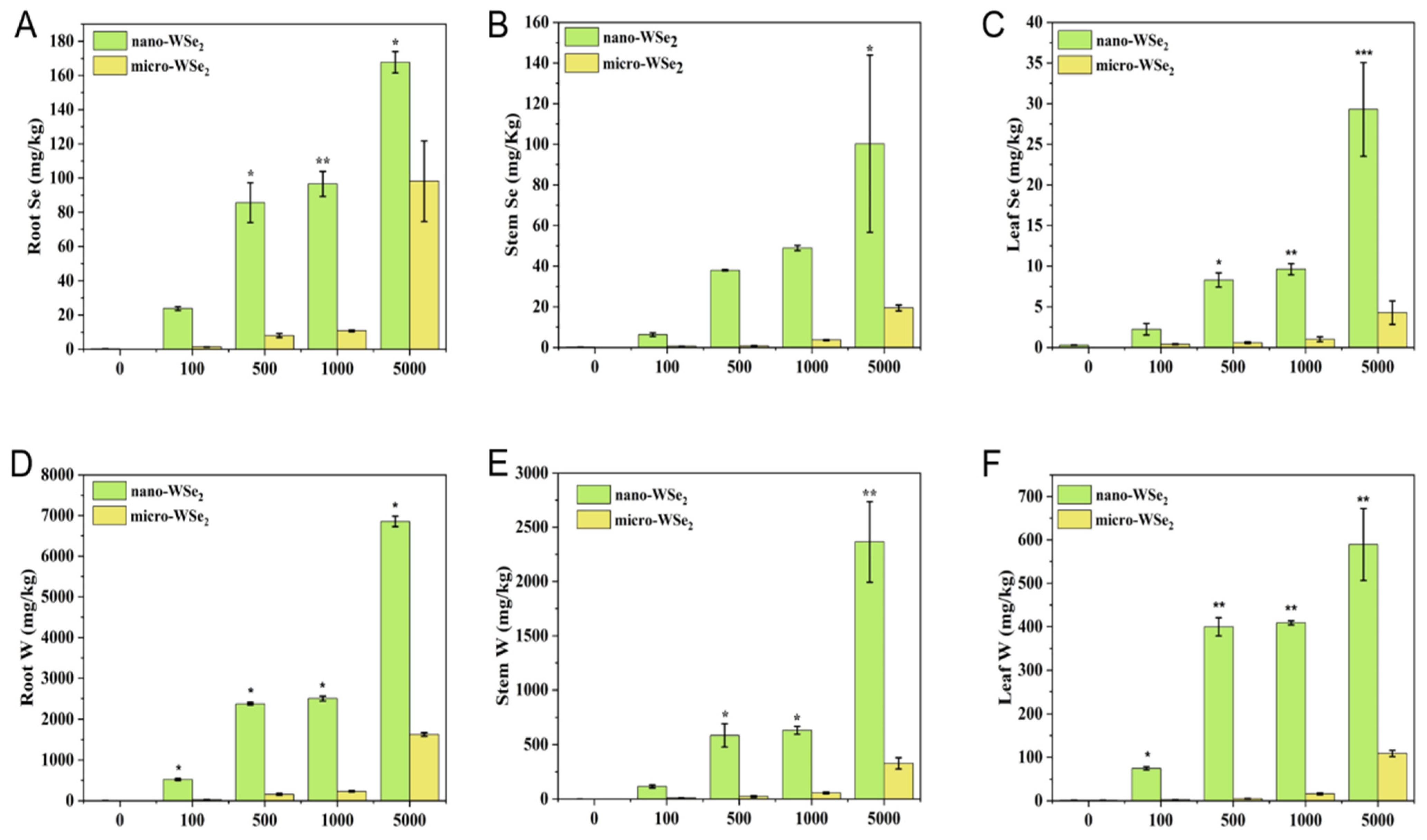
2.3. The Concentration of Se and W in Rice Plants after Exposure to Nano-WSe2 or Micro-WSe2
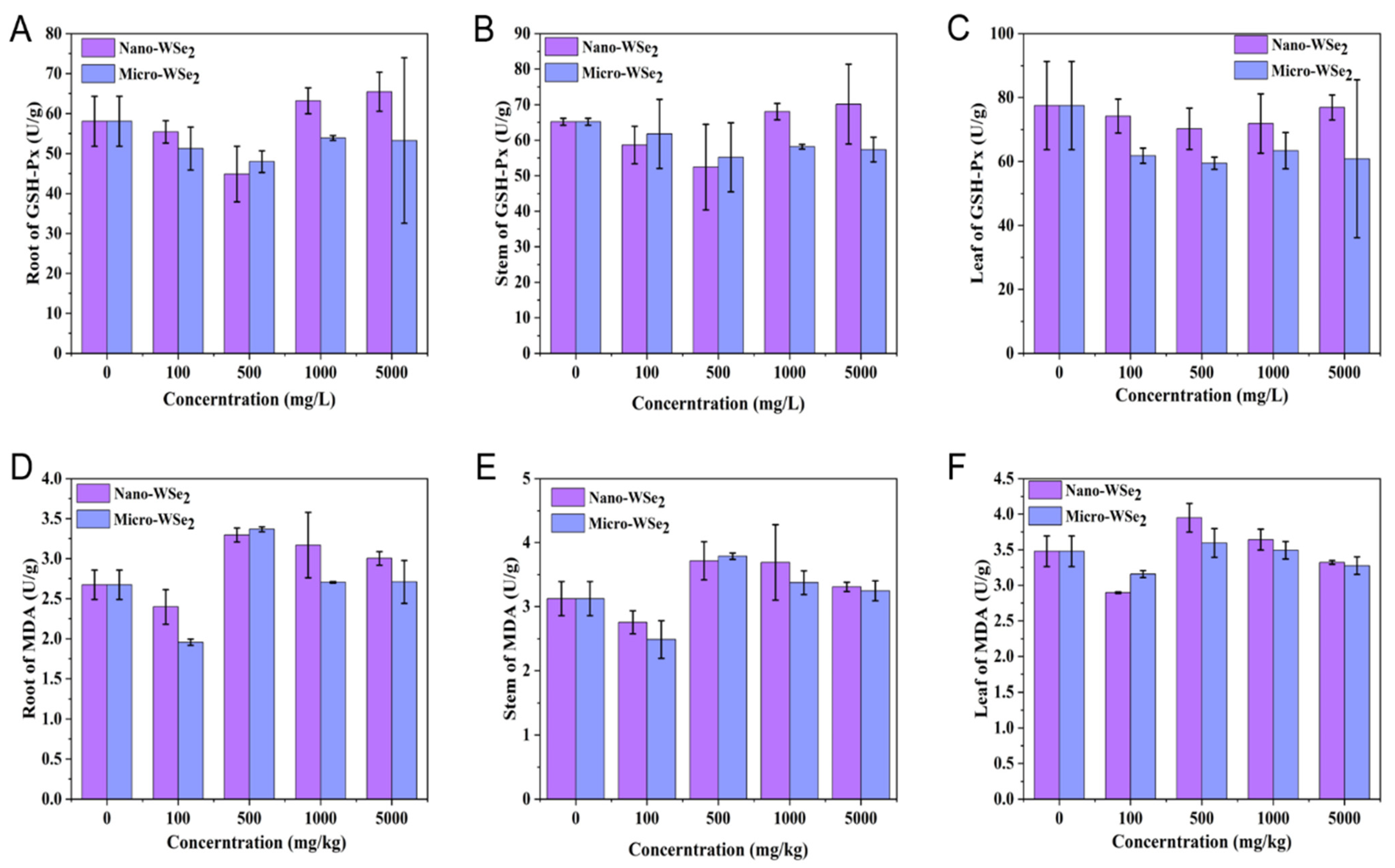
2.4. The Levels of GSH-Px an MDA in Rice Roots, Stems, and Leaves after Exposure to by Nano-WSe2 or Micro-WSe2
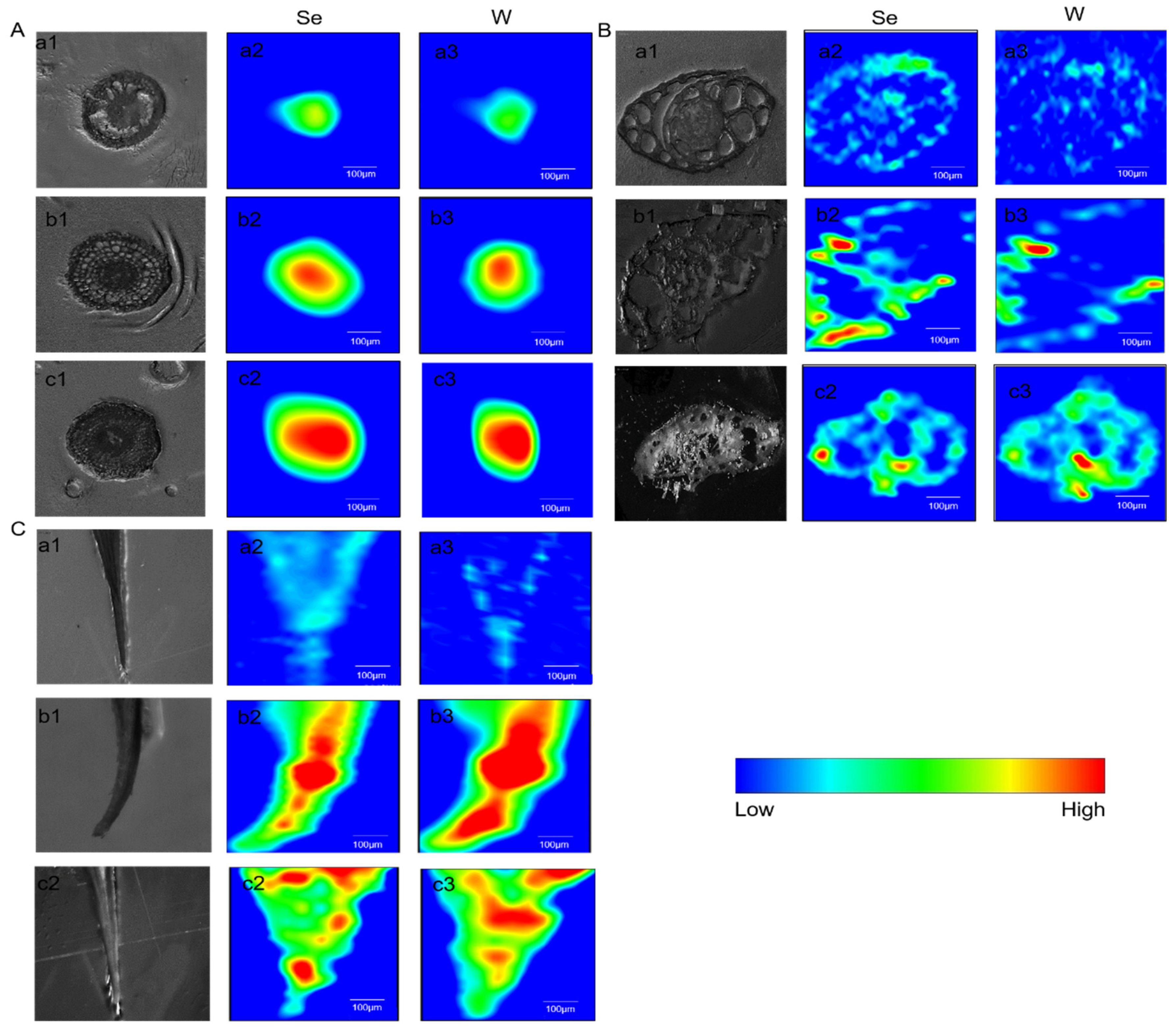
2.5. The Distribution and Transformation of Se and W in Rice Plants
2.5.1. The Distribution of Se and W in Rice Plants
2.5.2. The Transformation of Se and W in Rice Roots
3. Materials and Methods
3.1. Reagents and Materials
3.2. Characterization of the Micro-WSe2 and Nano-WSe2
3.3. Rice Germination and Hydroponic Cultivation
3.4. Analytical Method
3.4.1. Quantification of W and Se in Rice Plants
3.4.2. Measurement of GSH-Px and MDA
3.4.3. The Speciation of Se in Rice Tissues
3.4.4. The Distribution and Transformation of W and Se in Rice Tissues
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nair, N.L.; Maniv, E.; John, C.; Doyle, S.; Orenstein, J.; Analytis, J.G. Electrical switching in a magnetically intercalated transition metal dichalcogenide. Nat. Mater. 2020, 19, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-T.; Kang, W.; Xu, J.; Sun, Y.-W.; Jiang, M.; Ng, T.-W.; Xue, H.-T.; Yu, D.Y.W.; Zhang, W.; Lee, C.-S. Hierarchical nanotubes assembled from MoS2-carbon monolayer sandwiched superstructure nanosheets for high-performance sodium ion batteries. Nano Engry 2016, 22, 27–37. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, C.; Tan, X.; Xu, H.; Zhu, K. Polyaniline-modified 3D-flower-like molybdenum disulfide composite for efficient adsorption/photocatalytic reduction of Cr(VI). J. Colloid Interface Sci. 2016, 476, 62–70. [Google Scholar] [CrossRef]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Tan, X.; Yan, X.; Zhang, W.; Huang, Y.; Ji, R.; White, J.C. Environmental implications of MoS2 nanosheets on rice and associated soil microbial communities. Chemosphere 2022, 291 Pt 1, 133004. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Cao, M.; Cai, R.; Zhao, L.; Guo, M.; Wang, L.; Wang, Y.; Zhang, L.; Wang, X.; Yao, H.; Xie, C.; et al. Molybdenum derived from nanomaterials incorporates into molybdenum enzymes and affects their activities in vivo. Nat. Nanotechnol. 2021, 16, 708–716. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Q.; Yang, D.; Cui, J. Molybdenum Sulfide Induce Growth Enhancement Effect of Rice (Oryza sativa L.) through Regulating the Synthesis of Chlorophyll and the Expression of Aquaporin Gene. J. Agric. Food Chem. 2018, 66, 4013–4021. [Google Scholar] [CrossRef]
- Wen, B.; Zhu, Y.; Yudistira, D.; Boes, A.; Zhang, L.; Yidirim, T.; Liu, B.; Yan, H.; Sun, X.; Zhou, Y.; et al. Ferroelectric-Driven Exciton and Trion Modulation in Monolayer Molybdenum and Tungsten Diselenides. ACS Nano 2019, 13, 5335–5343. [Google Scholar] [CrossRef]
- Bertolazzi, S.; Gobbi, M.; Zhao, Y.; Backes, C.; Samorì, P. Molecular chemistry approaches for tuning the properties of two-dimensional transition metal dichalcogenides. Chem. Soc. Rev. 2018, 47, 6845–6888. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Y.; Liu, Y.; Ye, R.; Chen, L.; Bai, G.; Xu, S. Erbium-doped tungsten selenide nanosheets with near-infrared II emission and photothermal conversion. Chem. Eng. J. 2021, 411, 128610. [Google Scholar] [CrossRef]
- Wang, W.; Gu, W.; Li, G.; Xie, H.; Wong, P.K.; An, T. Few-layered tungsten selenide as a co-catalyst for visible-light-driven photocatalytic production of hydrogen peroxide for bacterial inactivation. Environ. Sci. Nano 2020, 7, 3877–3887. [Google Scholar] [CrossRef]
- Lin, Y.T.; Zhang, X.Q.; Chen, P.H.; Chi, C.C.; Lin, E.C.; Rong, J.G.; Ouyang, C.; Chen, Y.F.; Lee, Y.H. Selective Growth of WSe2 with Graphene Contacts. Nanoscale Res. Lett. 2020, 15, 61. [Google Scholar] [CrossRef]
- Teo, W.Z.; Chng, E.L.; Sofer, Z.; Pumera, M. Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chemistry 2014, 20, 9627–9632. [Google Scholar] [CrossRef]
- Pushie, M.J.; George, G.N. Spectroscopic studies of molybdenum and tungsten enzymes. Coord. Chem. Rev. 2011, 255, 1055–1084. [Google Scholar] [CrossRef]
- Bevers, L.E.; Hagedoorn, P.-L.; Hagen, W.R. The bioinorganic chemistry of tungsten. Coord. Chem. Rev. 2009, 253, 269–290. [Google Scholar] [CrossRef]
- Seelmann, C.S.; Willistein, M.; Heider, J.; Boll, M. Tungstoenzymes: Occurrence, Catalytic Diversity and Cofactor Synthesis. Inorganics 2020, 8, 44. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Buttner, L.; de Lima Romao, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef]
- Kumar, A.; Aery, N.C. Effect of tungsten on growth, biochemical constituents, molybdenum and tungsten contents in wheat. Plant Soil Environ. 2011, 57, 519–525. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Azooz, M.M. Insights into the oxidative status and antioxidative responses of germinating broccoli (Brassica oleracea var. italica L.) seeds in tungstate contaminated water. Chemosphere 2020, 261, 127585. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Dimkovikj, A.; Fisher, B.; Hutchison, K.; Van Hoewyk, D. Stuck between a ROS and a hard place: Analysis of the ubiquitin proteasome pathway in selenocysteine treated Brassica napus reveals different toxicities during selenium assimilation. J. Plant Physiol. 2015, 181, 50–54. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Lu, J.; Zhao, G.; Sun, J. Metabonomics analysis reveals the protective effect of nanoselenium against heat stress of rainbow trout (Oncorhynchus mykiss). J. Proteom. 2022, 259, 104545. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Celi, P.; Cottrell, J.J.; Chauhan, S.S.; Leury, B.J.; Dunshea, F.R. Effects of a short-term supranutritional selenium supplementation on redox balance, physiology and insulin-related metabolism in heat-stressed pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Yuanan, H.; He, K.; Sun, Z.; Chen, G.; Cheng, H. Quantitative source apportionment of heavy metal(loid)s in the agricultural soils of an industrializing region and associated model uncertainty. J. Hazard. Mater. 2020, 391, 122244. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Hu, H.; Shaheen, S.M.; Zhong, H.; Tack, F.M.G.; Wu, M.; Li, Y.F.; Gao, Y.; Rinklebe, J.; et al. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ. Int. 2021, 156, 106749. [Google Scholar] [CrossRef]
- Yang, J.-c.; Zhang, H.; Zhang, J.-h. Root Morphology and Physiology in Relation to the Yield Formation of Rice. J. Integr. Agric. 2012, 11, 920–926. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.D.A.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2015, 14, 123–147. [Google Scholar] [CrossRef]
- Strigul, N.; Koutsospyros, A.; Arienti, P.; Christodoulatos, C.; Dermatas, D.; Braida, W. Effects of tungsten on environmental systems. Chemosphere 2005, 61, 248–258. [Google Scholar] [CrossRef]
- Niwa, Y.; Sasaki, Y. Plant self-defense mechanisms against oxidative injury and protection of the forest by planting trees of triploids and tetraploids. Ecotoxicol. Environ. Saf. 2003, 55, 70–81. [Google Scholar] [CrossRef]
- Liu, S.; Waqas, M.A.; Wang, S.H.; Xiong, X.Y.; Wan, Y.F. Effects of increased levels of atmospheric CO2 and high temperatures on rice growth and quality. PLoS ONE 2017, 12, e0187724. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; Qin, Y.; Xu, J.; Shohag, M.J.I.; Wei, Y.; Gu, M. Effect of Different Forms of Selenium on the Physiological Response and the Cadmium Uptake by Rice under Cadmium Stress. Int. J. Environ. Res. Public Health 2020, 17, 6991. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Johnson, D.R.; Seiter, J.M.; Lindsay, J.H.; Boyd, R.E.; Bednar, A.J.; Allison, P.G. Tungsten toxicity, bioaccumulation, and compartmentalization into organisms representing two trophic levels. Environ. Sci. Technol. 2012, 46, 9646–9652. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, N.; Chen, C.; Luo, Y.; Li, Y.; Zhang, Z.; Zhao, Y.; Zhao, B.; Iida, A.; Chai, Z. Mapping technique for biodistribution of elements in a model organism, Caenorhabditis elegans, after exposure to copper nanoparticles with microbeam synchrotron radiation X-ray fluorescence. J. Anal. At. Spectrom. 2008, 23, 1121–1124. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Azooz, M.M. Concentration-dependent effects of tungstate on germination, growth, lignification-related enzymes, antioxidants, and reactive oxygen species in broccoli (Brassica oleracea var. italica L.). Environ. Sci. Pollut. Res. Int. 2019, 26, 36441–36457. [Google Scholar] [CrossRef]
- Shi, N.; Bai, T.; Wang, X.; Tang, Y.; Wang, C.; Zhao, L. Toxicological effects of WS2 nanomaterials on rice plants and associated soil microbes. Sci. Total Environ. 2022, 832, 154987. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Li, Y.; Gao, Y.; Li, B.; Hu, Y.; Zhao, Y.; Chai, Z. Selenium modulates mercury uptake and distribution in rice (Oryza sativa L.), in correlation with mercury species and exposure level. Metallomics 2014, 6, 1951–1957. [Google Scholar] [CrossRef]
- Pan, M.Y.; Zang, Y.; Zhou, X.R.; Lu, Y.L.; Xiong, J.P.; Li, H.M.; Feng, L.X. Inductively coupled plasma mass spectrometry for metrometallomics: The study of quantitative metalloproteins. At. Spectrosc. 2021, 42, 262–270. [Google Scholar] [CrossRef]
- Li, H. Clinimetallomics: Arsenic Speciation in Urine from Patients with Arsenism by HPLC-ICP-MS. At. Spectrosc. 2021, 42, 278–281. [Google Scholar] [CrossRef]
- Hu, L.; Dong, Z.; Huang, X.; Li, Y.F.; Li, B.; Qu, L.; Wang, G.; Gao, Y.; Chen, C. Determination of selenium chemical species in serum after selenium intervention in people with long-term mercury exposure by anion exchange chromatography inductively coupled plasma mass spectrometry. Chin. J. Anal. Chem. 2011, 39, 466–470. [Google Scholar] [CrossRef]
- He, L.; Lu, Y.; Li, C.; Xie, H.; Zhao, J.; Wang, Y.; Wang, L.; Wang, X.; Wang, W.; Chen, D.; et al. Non-targeted metallomics through synchrotron radiation X-ray fluorescence with machine learning for cancer screening using blood samples. Talanta 2022, 245, 123486. [Google Scholar] [CrossRef]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Li, Z.J.; Huang, Z.W.; Guo, W.L.; Wang, L.; Zheng, L.R.; Chai, Z.F.; Shi, W.Q. Enhanced Photocatalytic Removal of Uranium(VI) from Aqueous Solution by Magnetic TiO2/Fe3O4 and Its Graphene Composite. Environ. Sci. Technol. 2017, 51, 5666–5674. [Google Scholar] [CrossRef]

| Concentration (mg/L) | Germination Rate (%) |
|---|---|
| 0 | 90.66 ± 0.01 |
| (nano) 100 | 81.33 ± 0.01 |
| (micro) 100 | 82.00 ± 0.07 |
| (nano) 500 | 85.33 ± 0.02 |
| (micro) 500 | 87.86 ± 0.07 |
| (nano) 1000 | 81.33 ± 0.06 |
| (micro) 1000 | 87.33 ± 0.03 |
| (nano) 5000 | 86.67 ± 0.05 |
| (micro) 5000 | 83.33 ± 0.03 |
| Concentration (mg/L) | The Length of Root (cm) | The Length of Stem (cm) | The Length of Leaf (cm) |
|---|---|---|---|
| 0 | 6.69 ± 0.22 | 4.46 ± 1.22 | 8.59 ± 0.25 |
| (nano) 100 | 6.22 ± 0.28 | 4.13 ± 0.16 | 8.62 ± 0.71 |
| (micro) 100 | 6.30 ± 0.01 | 3.52 ± 0.28 | 8.21 ± 0.20 |
| (nano) 500 | 5.79 ± 0.52 | 3.60 ± 0.31 | 9.45 ± 0.21 |
| (micro) 500 | 7.17 ± 1.06 | 3.39 ± 0.04 | 7.78 ± 0.11 |
| (nano) 1000 | 4.62 ± 0.22 * | 4.09 ± 0.32 | 8.47 ± 0.59 |
| (micro) 1000 | 6.93 ± 0.16 | 3.34 ± 0.21 | 7.77 ± 0.55 |
| (nano) 5000 | 2.23 ± 0.49 ** | 3.54 ± 0.17 | 8.39 ± 0.31 |
| (micro) 5000 | 5.97 ± 0.20 | 3.42 ± 0.02 | 6.76 ± 0.11 |
| Concentration (mg/kg) | Root | Stem | Leaf |
|---|---|---|---|
| 0 | 0.14 | 0.21 | 0.80 |
| (nano) 100 | 0.11 | 0.13 | 0.07 |
| (micro) 100 | 0.11 | 0.14 | 0.40 |
| (nano) 500 | 0.08 | 0.14 | 0.05 |
| (micro) 500 | 0.12 | 0.10 | 0.18 |
| (nano) 1000 | 0.06 | 0.07 | 0.05 |
| (micro) 1000 | 0.11 | 0.14 | 0.15 |
| (nano) 5000 | 0.06 | 0.11 | 0.09 |
| (micro) 5000 | 0.14 | 0.14 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Xie, H.; Li, J.; Cui, L.; Yu, Y.-L.; Li, B.; Li, Y.-F. Nano-WSe2 Is Absorbable and Transformable by Rice Plants. Molecules 2022, 27, 7826. https://doi.org/10.3390/molecules27227826
Tian X, Xie H, Li J, Cui L, Yu Y-L, Li B, Li Y-F. Nano-WSe2 Is Absorbable and Transformable by Rice Plants. Molecules. 2022; 27(22):7826. https://doi.org/10.3390/molecules27227826
Chicago/Turabian StyleTian, Xue, Hongxin Xie, Jincheng Li, Liwei Cui, Yong-Liang Yu, Bai Li, and Yu-Feng Li. 2022. "Nano-WSe2 Is Absorbable and Transformable by Rice Plants" Molecules 27, no. 22: 7826. https://doi.org/10.3390/molecules27227826
APA StyleTian, X., Xie, H., Li, J., Cui, L., Yu, Y.-L., Li, B., & Li, Y.-F. (2022). Nano-WSe2 Is Absorbable and Transformable by Rice Plants. Molecules, 27(22), 7826. https://doi.org/10.3390/molecules27227826






