Organocatalytic Enantioselective Michael Reaction of Aminomaleimides with Nitroolefins Catalyzed by Takemoto’s Catalyst
Abstract
1. Introduction
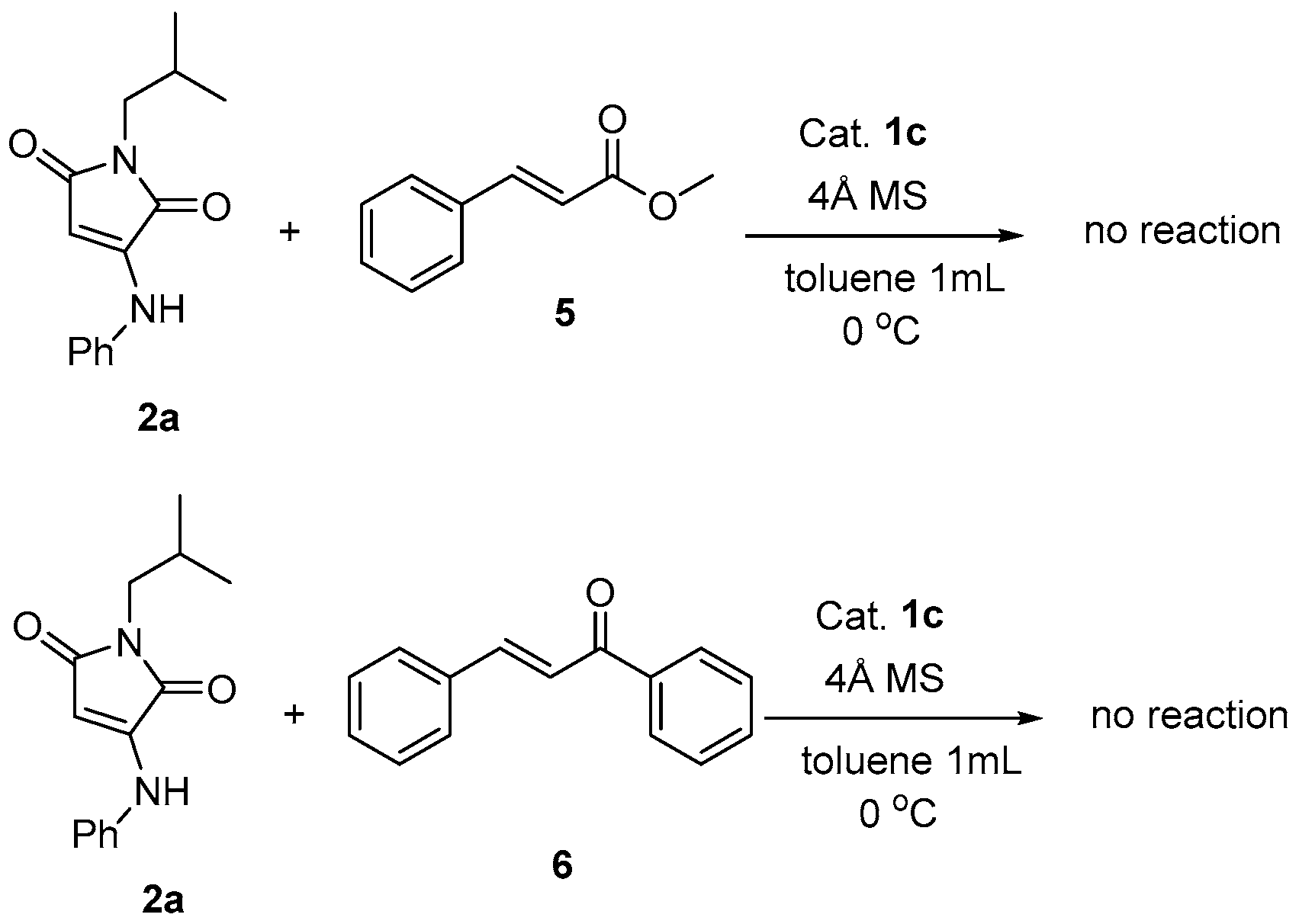
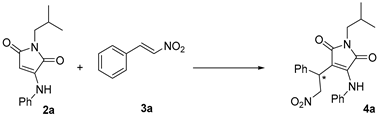
2. Results and Discussion
3. Experimental
3.1. Chemistry
3.2. General Procedure for the Enantioselective Michael Reaction of α-Aminomaleimides and β-Nitrostyrenes
3.2.1. (S)-1-Isobutyl-3-(2-nitro-1-phenylethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4a)
3.2.2. (S)-3-(1-(2-Fluorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4b)
3.2.3. (S)-3-(1-(2-Chlorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4c)
3.2.4. (S)-3-(1-(2-Bromophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5-dione(4d)
3.2.5. (S)-3-(1-(3-Bromophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4e)
3.2.6. (S)-3-(1-(3-Fluorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4f)
3.2.7. (S)-3-(1-(4-Fluorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4g)
3.2.8. (S)-3-(1-(4-Chlorophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4h)
3.2.9. (S)-3-(1-(4-Bromophenyl)-2-nitroethyl)-1-isobutyl-4-(phenylamino)-1H-pyrrole-2,5- dione (4i)
3.2.10. (S)-1-Isobutyl-3-(2-nitro-1-(p-tolyl)ethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4j)
3.2.11. (S)-1-Isobutyl-3-(1-(4-methoxyphenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4k)
3.2.12. (S)-1-Isobutyl-3-(1-(naphthalen-2-yl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole- 2,5-dione (4l)
3.2.13. (S)-1-Isobutyl-3-(2-nitro-1-(thiophen-2-yl)ethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4m)
3.2.14. (S)-3-((4-Chlorophenyl)amino)-1-isobutyl-4-(2-nitro-1-phenylethyl)-1H-pyrrole- 2,5-dione (4n)
3.2.15. (S)-3-((4-Chlorophenyl)amino)-1-isobutyl-4-(1-(2-chlorophenyl)-2-nitroethyl)-1H- pyrrole-2,5-dione (4o)
3.2.16. (S)-3-((3-Chlorophenyl)amino)-1-isobutyl-4-(2-nitro-1-phenylethyl)-1H-pyrrole- 2,5-dione (4p)
3.2.17. (S)-1-Benzyl-3-(2-nitro-1-phenylethyl)-4-(phenylamino)-1H-pyrrole-2,5-dione (4q)
3.2.18. (S)-1-Benzyl-3-(1-(2-bromophenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4r)
3.2.19. (S)-1-Benzyl-3-(1-(3-bromophenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4s)
3.2.20. (S)-1-Benzyl-3-(1-(4-bromophenyl)-2-nitroethyl)-4-(phenylamino)-1H-pyrrole-2,5- dione (4t)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.J.; Choi, M.J.; Shin, J.-S.; Kim, M.J.; Choi, H.-E.; Kang, S.M.; Jin, J.H.; Lee, K.-T.; Lee, J.Y. Synthesis, biological evaluation, and docking analysis of a novel family of 1-methyl-1H-pyrrole-2,5-diones as highly potent and selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Wang, S.; Li, Y.; Zhang, F.; Song, B.; Zhao, W. Syntheses of new chlorin derivatives containing maleimide functional group and their photodynamic activity evaluation. Bioorg. Med. Chem. Lett. 2015, 25, 4078–4081. [Google Scholar] [CrossRef]
- Eloh, K.; Demurtas, M.; Mura, M.G.; Deplano, A.; Onnis, V.; Sasanelli, N.; Maxia, A.; Caboni, P. Potent Nematicidal Activity of Maleimide Derivatives on Meloidogyne incognita. J. Agric. Food Chem. 2016, 64, 4876–4881. [Google Scholar] [CrossRef]
- Eis, M.J.; Evenou, J.P.; Schuler, W.; Zenke, G.; Vangrevelinghe, E.; Wagner, J.; Matt, P. Indolyl-naphthyl-maleimides as potent and selective inhibitors of protein kinase C-α/β. Bioorg. Med. Chem. Lett. 2017, 27, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-Y.; Alam, J.; Jeyaraj, D.-A.; Wang, W.; Lin, G.-R.; Ang, S.-H.; Tan, E.-S.-W.; Lee, M.-A.; Ke, Z.; Madan, B.; et al. Scaffold Hopping and Optimization of Maleimide Based Porcupine Inhibitors. J. Med. Chem. 2017, 60, 6678–6692. [Google Scholar] [CrossRef]
- Serafim, R.A.M.; Sorrell, F.J.; Berger, B.-T.; Collins, R.J.; Vasconcelos, S.N.S.; Massirer, K.B.; Knapp, S.; Bennett, J.; Fedorov, O.; Patel, H.; et al. Discovery of a Potent Dual SLK/STK10 Inhibitor Based on a Maleimide Scaffold. J. Med. Chem. 2021, 64, 13259–13278. [Google Scholar] [CrossRef] [PubMed]
- Kjærsgaard, N.L.; Hansen, R.A.; Gothelf, K.V. Preparation of Maleimide-Modified Oligonucleotides from the Corresponding Amines Using N-Methoxycarbonyl maleimide. Bioconjug. Chem. 2022, 33, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Fernandez, S.; Martinez, A.; Zeni, M.; Reyes, L.; Rey, A.; Cerecetto, H. Glycogen Synthase Kinase-3 Maleimide Inhibitors As Potential PET-Tracers for Imaging Alzheimer’s Disease: 11C-Synthesis and In Vivo Proof of Concept. J. Med. Chem. 2022, 65, 1342–1351. [Google Scholar] [CrossRef]
- Mandal, R.; Emayavaramban, B.; Sundararaju, B. Cp*Co(III)-Catalyzed C–H Alkylation with Maleimides Using Weakly Coordinating Carbonyl Directing Groups. Org. Lett. 2018, 20, 2835–2838. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Y.; Yang, H.; Liu, D.; Sun, B.; Zhang, F.-L. Assembly of Diverse Spirocyclic Pyrrolidines via Transient Directing Group Enabled Ortho-C(sp2)–H Alkylation of Benzaldehydes. Org. Lett. 2018, 1, 146–149. [Google Scholar] [CrossRef]
- Yu, J.T.; Chen, R.; Jia, H.; Pan, C. Rhodium-Catalyzed Site-Selective ortho-C–H Activation: Enone Carbonyl Directed Hydroarylation of Maleimides. J. Org. Chem. 2018, 83, 12086–12093. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wang, Y.; Wu, C.; Yu, J.-T. Rhodium-catalyzed C7-alkylation of indolines with maleimides. Org. Biomol. Chem. 2018, 16, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Uno, B.-E.; Dicken, R.-D.; Redfern, L.-R.; Stern, C.-M.; Krzywicki, G.-G.; Scheidt, K.-A. Calcium(II)—Catalyzed enantioselective conjugate additions of amines. Chem. Sci. 2018, 9, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Shamsianpour, M.; Issazadeh, S.; Dorrani, M.; Hazrati, H. Palladium-catalyzed direct arylation of maleimides: A simple route to bisaryl-substituted maleimides. Tetrahedron 2017, 73, 1668–1672. [Google Scholar] [CrossRef]
- Jafarpour, F.; Shamsianpour, M. Palladium-catalyzed cross-dehydrogenative coupling of maleimides with simple arenes: A fast track to highly substituted maleimides. RSC Adv. 2016, 6, 103567–103570. [Google Scholar] [CrossRef]
- Yang, Z.-H.; An, Y.-L.; Chen, Y.; Shao, Z.-Y.; Zhao, S.-Y. Copper(I) Iodide-Catalyzed Sulfenylation of Maleimides and Related 3-Indolylmaleimides with Thiols. Adv. Synth. Catal. 2016, 358, 3869–3875. [Google Scholar] [CrossRef]
- Dana, S.; Mandal, A.; Sahoo, H.; Baidya, M. Ru(II)-Catalyzed C–H Functionalization on Maleimides with Electrophiles: A Demonstration of Umpolung Strategy. Org. Lett. 2017, 19, 1902–1905. [Google Scholar] [CrossRef]
- An, Y.-L.; Zhang, H.-H.; Yang, Z.-H.; Lin, L.; Zhao, S.-Y. Cu/Ag-Cocatalyzed Aerobic Oxidative Amination and CuCl2-Mediated Aerobic Oxidative Chloroamination of Maleimides. Eur. J. Org. Chem. 2016, 2016, 5405–5414. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Tan, H.-R.; An, Y.-L.; Zhao, Y.-W.; Lin, H.-P.; Zhao, S.-Y. Three-Component Coupling Reactions of Maleimides, Thiols, and Amines: One-Step Construction of 3,4-Heteroatom-functionalized Maleimides by Copper-Catalyzed C(sp2)–H Thioamination. Adv. Synth. Catal. 2018, 360, 173–179. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Tan, H.-R.; Zhu, J.-N.; Zheng, J.; Zhao, S.-Y. Regioselective Silver-Catalyzed Carbon-Phosphorus Difunctionalization of Maleimides: One-Step Construction of Phosphonylated Indolylmaleimides and Pyrrolylmaleimides. Adv. Synth. Catal. 2018, 360, 1523–1529. [Google Scholar] [CrossRef]
- Baker, J.-R.; Tedaldi, L.-M.; Aliev, A.-E. [2 + 2] Photocycloadditions of thiomaleimides. Chem. Commun. 2012, 48, 4725–4727. [Google Scholar] [CrossRef]
- Lin, C.; Zhen, L.; Cheng, Y.; Du, H.-J.; Zhao, H.; Wen, X.; Kong, L.-Y.; Xu, Q.-L.; Sun, H. Visible-Light Induced Isoindoles Formation To Trigger Intermolecular Diels–Alder Reactions in the Presence of Air. Org. Lett. 2015, 17, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wu, X.; Jiang, L.; Zhang, Z.; Xie, X. Reduction of Benzolactams to Isoindoles via an Alkoxide-Catalyzed Hydrosilylation. Org. Lett. 2017, 19, 6048–6051. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Lee, R.; Zhu, B.; Coote, M.-L.; Zhao, X.; Jiang, Z. Highly Enantio- and Diastereoselective [4 + 2] Cycloaddition of 5H-oxazol-4-ones with N-Maleimides. J. Org. Chem. 2016, 81, 8061–8069. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Kaur, J.; Chimni, S.S. Asymmetric Organocatalytic Addition Reactions of Maleimides: A Promising Approach Towards the Synthesis of Chiral Succinimide Derivatives. Chem. Asian J. 2013, 8, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, J.M.J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef]
- Gómez-Torres, E.; Alonso, D.A.; Gómez-Bengoa, E.; Nájera, C. Enantioselective synthesis of succinimides by Michael addition of 1,3-dicarbonyl compounds to maleimides catalyzed by a chiral bis(2-aminobenzimidazole) organocatalyst. Eur. J. Org. Chem. 2013, 2013, 1434–1440. [Google Scholar] [CrossRef]
- Yuan, F.; Duan, W.; Li, Z.; Luo, X.; Zhang, M.; Deng, H.; Song, L. One-Pot Synthesis of Trifluoromethylated Pyrazol-4-YlPyrrole-2,5-Dione Derivatives. Synthesis 2019, 51, 3345–3355. [Google Scholar] [CrossRef]
- Khan, M.M.; Shareef, S.; Khan, S.; Saigal; Sahoo, S.C. Organocatalyzed Highly Efficient Synthesis of Densely Functionalized Pyrrole-Fused 1,4-Dihydropyridine Derivatives. Synth. Commun. 2019, 49, 2884–2894. [Google Scholar] [CrossRef]
- Xie, D.-H.; Niu, C.; Du, D.-M. Enantioselective Michael/ Hemiketalization Cascade Reactions between Hydroxymaleimides and 2-Hydroxynitrostyrenes for the Construction of Chiral ChromanFused Pyrrolidinediones. Molecules 2022, 27, 5081. [Google Scholar] [CrossRef]
- Sakai, N.; Kawashima, K.; Kajitani, M.; Mori, S.; Oriyama, T. Combined Computational and Experimental Studies on the Asymmetric Michael Addition of α-Aminomaleimides to β-Nitrostyrenes Using an Organocatalyst Derived from Cinchona Alkaloid. Org. Lett. 2021, 23, 5714–5718. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Hoashi, Y.; Okino, T.; Takemoto, Y. Enantioselective Michael Addition to α, β-unsaturated Imides Catalyzed by a Bifunctional Organocatalyst. Angew. Chem. Int. Ed. Engl. 2005, 44, 4032–4035. [Google Scholar] [CrossRef] [PubMed]
- Zea, A.; Valero, G.; Alba, A.R.; Moyano, A.; Rios, R. Development of Diphenylamine-linked Bis(imidazoline) Ligands and Their Application in Asymmetric Friedel-Crafts Alkylation of Indole Derivatives with Nitroalkenes. Adv. Synth. Catal. 2010, 352, 1102–1106. [Google Scholar] [CrossRef]
- Raimondi, W.; Baslé, O.; Constantieux, T.; Bonne, D.; Rodriguez, J. Activation of 1, 2-Ketoesters with Takemoto’s Catalyst toward Michael Addition to Nitroalkenes. Adv. Synth. Catal. 2012, 354, 563–568. [Google Scholar] [CrossRef]
- Ansari, S.; Raabe, G.; Enders, D. Asymmetric Michael Addition of 1, 3-Bis(phenylthio)propan-2-one to Nitroalkenes Employing Takemoto’s Thiourea Catalyst. Monatsh. Chem. 2013, 144, 641–646. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, M.; Zhu, K.; Zheng, C.; Zhang, H.; Wang, W.; Shao, Z. Asymmetric Synthesis of Syn-propargylamines and Unsaturated β-amino Acids under Brønsted Base Catalysis. Nat. Commun. 2015, 6, 8544–8582. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, H.-F.; Zhao, J.-Z.; Du, Z.-H.; Da, C.-S. Organocatalytic Enantioselective Cross-aldol Reaction of o-Hydroxyarylketones and Trifluoromethyl Ketones. Org. Lett. 2017, 19, 2634–2637. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.-M.; Zhang, J.-W.; Jin, Y. Urea Derivative Catalyzed Enantioselective Hydroxyalkylation of Hydroxyindoles with Isatins. Molecules 2019, 24, 3944. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, T.-Y.; Sun, Y.-H.; Wang, L.-M.; Jin, Y. Organocatalytic enantioselective aza-Friedel–Crafts alkylation of β-naphthols and isatin-derived ketimines via a Takemoto-type catalyst. New J. Chem. 2021, 45, 10481–10487. [Google Scholar] [CrossRef]


 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Yield (%) b | %ee c | Configuration d ([α]D25) e |
| 1 | 1a | 78 | 90 | R |
| 2 | 1b | 76 | 89 | R |
| 3 | 1c | 80 | 91 | S (−6.70) |
| 4 | 1d | 73 | 90 | S |
| 5 | 1e | 74 | 87 | S |
| 6 | 1f | 69 | 76 | S |
| 7 | 1g | 72 | 82 | S |
| 8 | 1h | 70 | 74 | S |
| 9 | qunine | 80 | 53 | S (−3.82) |
| Entry | Solvent | Temperature | Catalyst Amount (% mmol) | Yield (%) b | %ee c |
|---|---|---|---|---|---|
| 1 | CH2Cl2 | rt | 10 | 82 | 90 |
| 2 | CHCl3 | rt | 10 | 80 | 91 |
| 3 | THF | rt | 10 | 76 | 88 |
| 4 | Et2O | rt | 10 | 78 | 91 |
| 5 | MTBE | rt | 10 | 80 | 90 |
| 6 | 1,4-dioxane | rt | 10 | 82 | 85 |
| 7 | PhMe | rt | 10 | 84 | 92 |
| 8 | xylene | rt | 10 | 76 | 91 |
| 9 | MeOH | rt | 10 | 70 | 23 |
| 10 | PhMe | 0 | 10 | 83 | 93 |
| 11 | PhMe | −10 | 10 | 68 | 94 |
| 12 | PhMe | −20 | 10 | 55 | 94 |
| 13 | PhMe | 0 | 5 | 74 | 93 |
| 14 | PhMe | 0 | 20 | 86 | 93 |
| 15 d | PhMe | 0 | 10 | 56 | 91 |
| 16 e | PhMe | 0 | 10 | 85 | 94 |
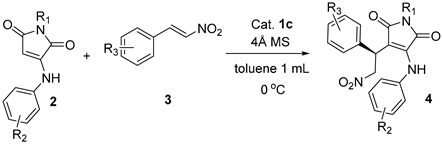 | ||||
|---|---|---|---|---|
| Entry | R 1, R 2, R 3 | Product | Yield (%) b | %ee c |
| 1 | iBu, H, H | 4a | 85 | 94 (90) d |
| 2 | iBu, H, 2-F | 4b | 83 | 92 |
| 3 | iBu, H, 2-Cl | 4c | 86 | 88 |
| 4 | iBu, H, 2-Br | 4d | 86 | 86 (88) d |
| 5 | iBu, H, 3-Br | 4e | 81 | 92 (92) d |
| 6 | iBu, H, 3-F | 4f | 82 | 93 |
| 7 | iBu, H, 4-F | 4g | 85 | 93 |
| 8 | iBu, H, 4-Cl | 4h | 84 | 93 |
| 9 | iBu, H, 4-Br | 4i | 86 | 90 (90) d |
| 10 | iBu, H, 4-Me | 4j | 78 | 93 (90) d |
| 11 | iBu, H, 4-OMe | 4k | 76 | 93 (90) d |
| 12 | iBu, H, 1-Nap | 4l | 78 | 81 (91) d |
| 13 | iBu, H, 2-thienyl | 4m | 83 | 92 (89) d |
| 14 | iBu, 4-Cl, H | 4n | 81 | 92 (92) d |
| 15 | iBu, 4-Cl, 2-Cl | 4o | 80 | 89 |
| 16 | iBu, 3-Cl, H | 4p | 85 | 93 |
| 17 | Bn, H, H | 4q | 83 | 90 (80) d |
| 18 | Bn, H, 2-Br | 4r | 80 | 84 |
| 19 | Bn, H, 3-Br | 4s | 82 | 90 |
| 20 | Bn, H, 4-Br | 4t | 84 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, H.; Jin, Y.; Zhao, R.; Wang, L.; Jin, Y. Organocatalytic Enantioselective Michael Reaction of Aminomaleimides with Nitroolefins Catalyzed by Takemoto’s Catalyst. Molecules 2022, 27, 7787. https://doi.org/10.3390/molecules27227787
Mu H, Jin Y, Zhao R, Wang L, Jin Y. Organocatalytic Enantioselective Michael Reaction of Aminomaleimides with Nitroolefins Catalyzed by Takemoto’s Catalyst. Molecules. 2022; 27(22):7787. https://doi.org/10.3390/molecules27227787
Chicago/Turabian StyleMu, Hongwen, Yan Jin, Rongrong Zhao, Liming Wang, and Ying Jin. 2022. "Organocatalytic Enantioselective Michael Reaction of Aminomaleimides with Nitroolefins Catalyzed by Takemoto’s Catalyst" Molecules 27, no. 22: 7787. https://doi.org/10.3390/molecules27227787
APA StyleMu, H., Jin, Y., Zhao, R., Wang, L., & Jin, Y. (2022). Organocatalytic Enantioselective Michael Reaction of Aminomaleimides with Nitroolefins Catalyzed by Takemoto’s Catalyst. Molecules, 27(22), 7787. https://doi.org/10.3390/molecules27227787





