Regulation of the Gut Microbiota and Inflammation by β-Caryophyllene Extracted from Cloves in a Dextran Sulfate Sodium-Induced Colitis Mouse Model
Abstract
1. Introduction
2. Results
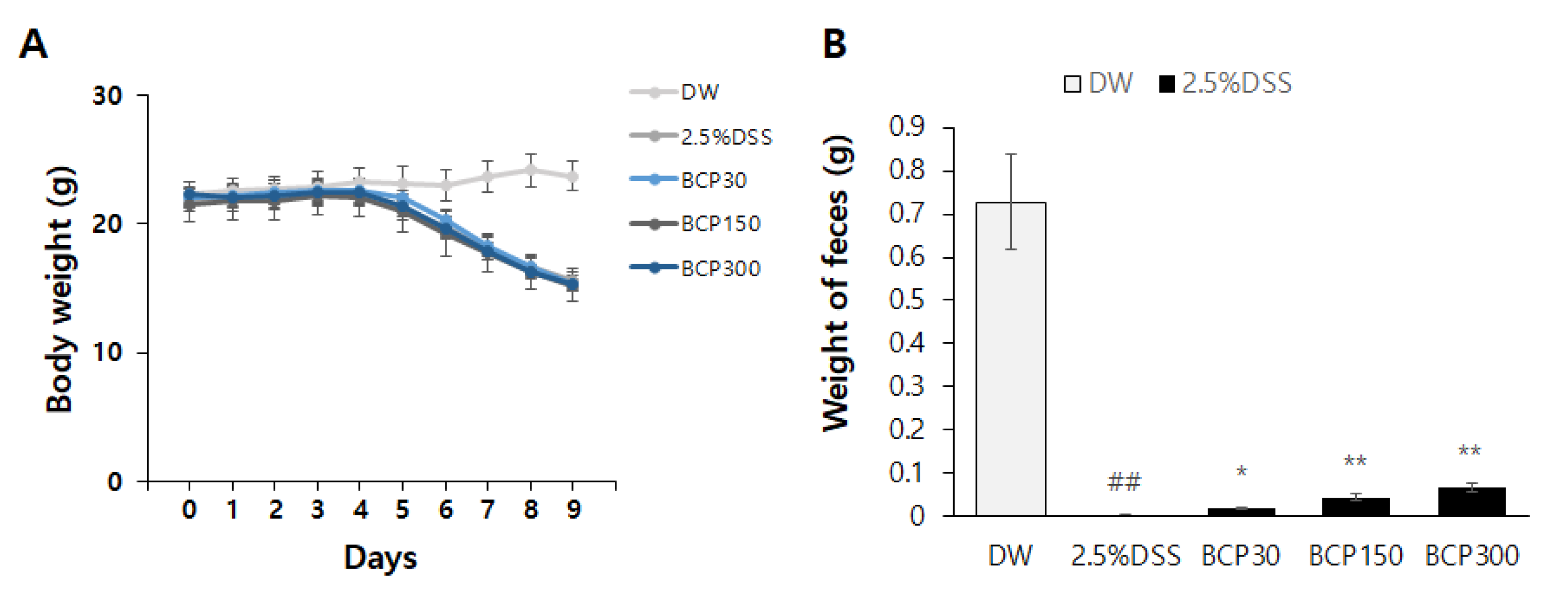
2.1. Evaluation of Changes in Stool Weight
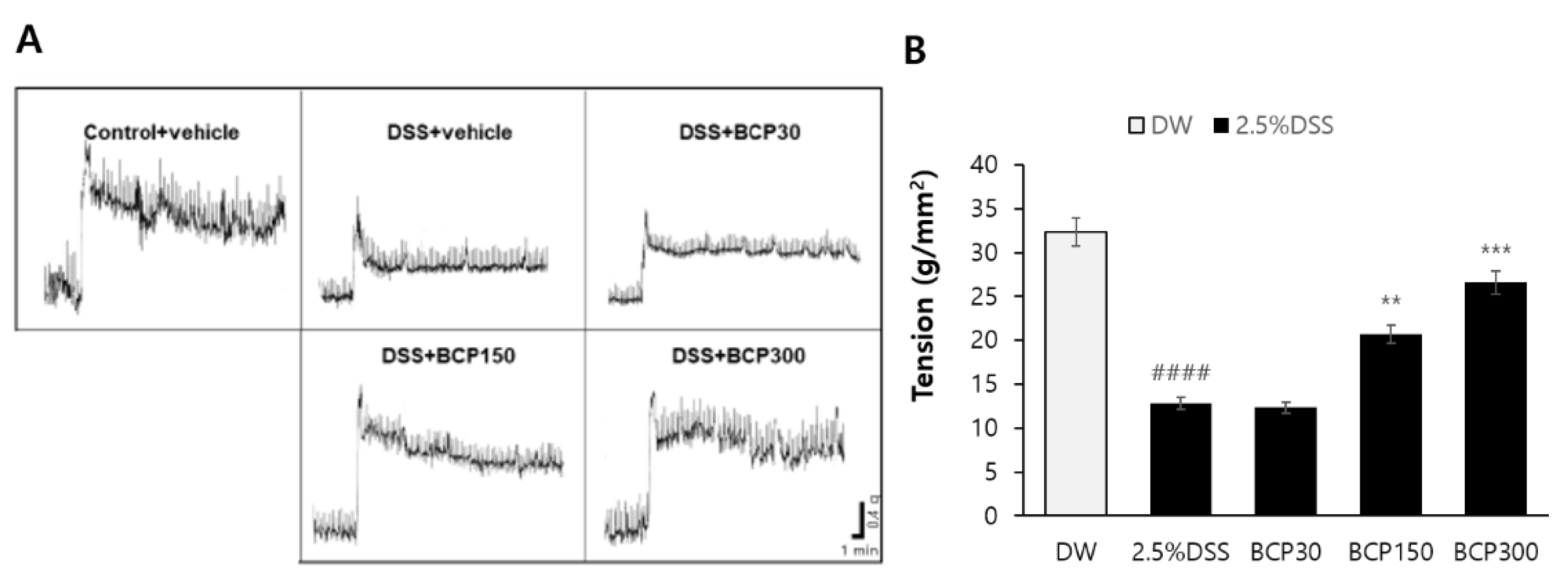
2.2. Evaluation of Digestive Tract Motility Recovery
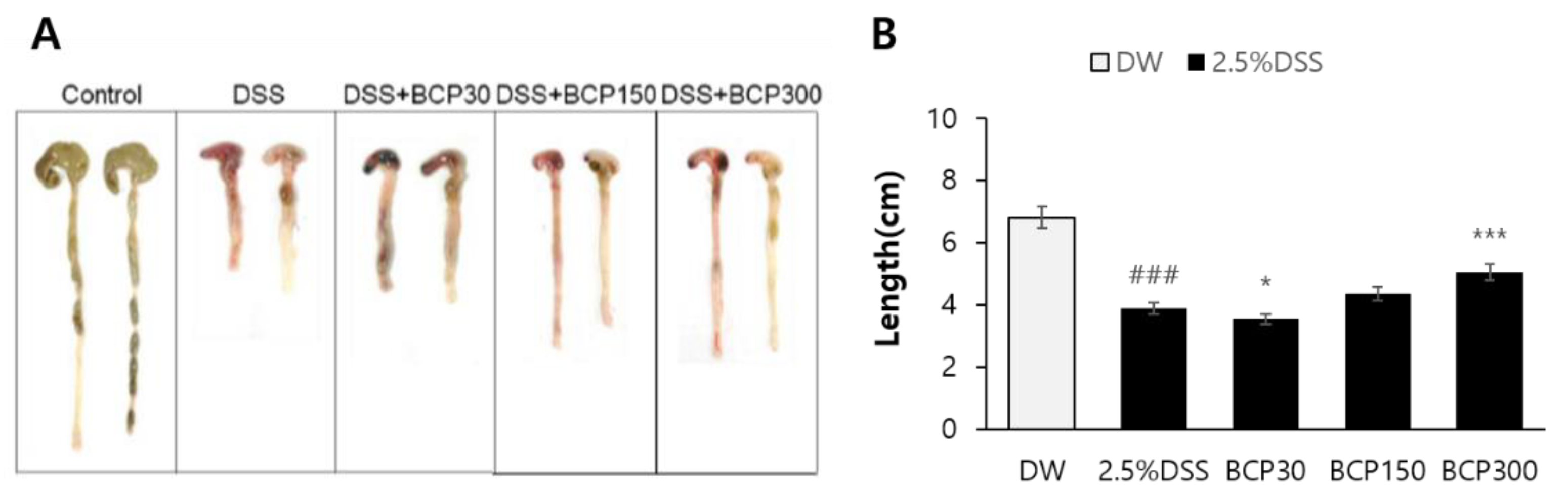
2.3. Evaluation of Colitis Relief after Autopsy
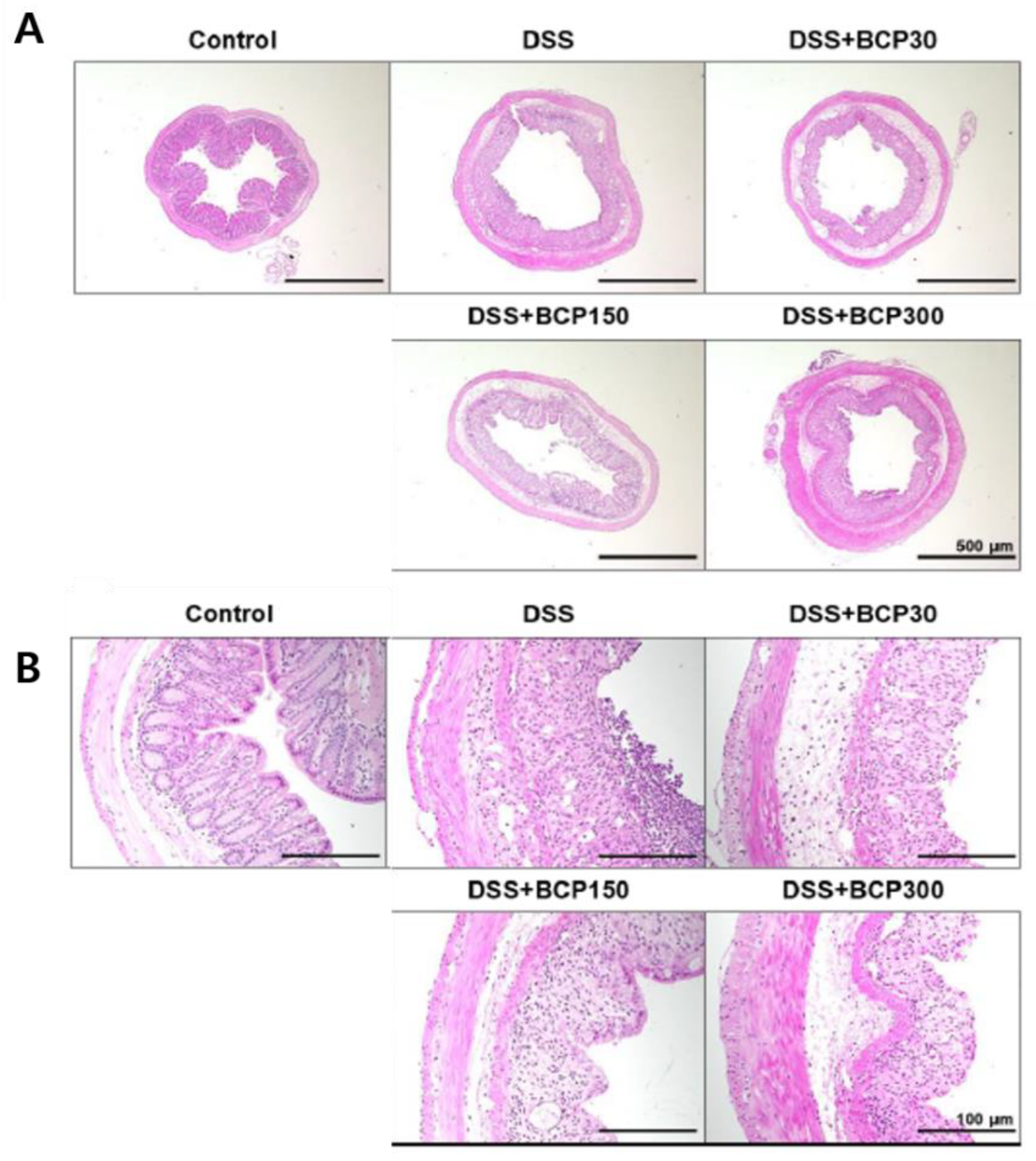
2.4. Observation of Histological Changes
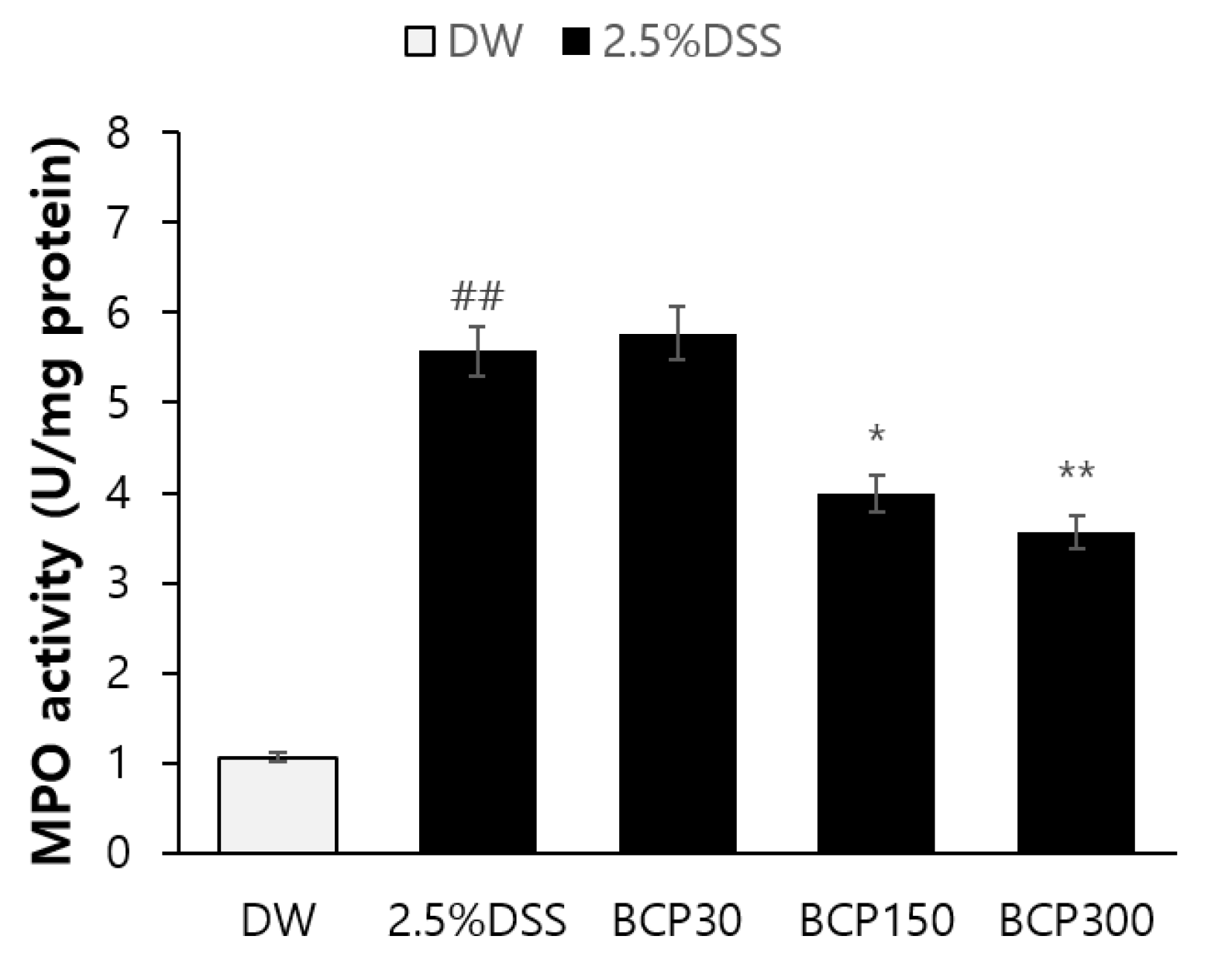
2.5. Observation of Immunological Changes
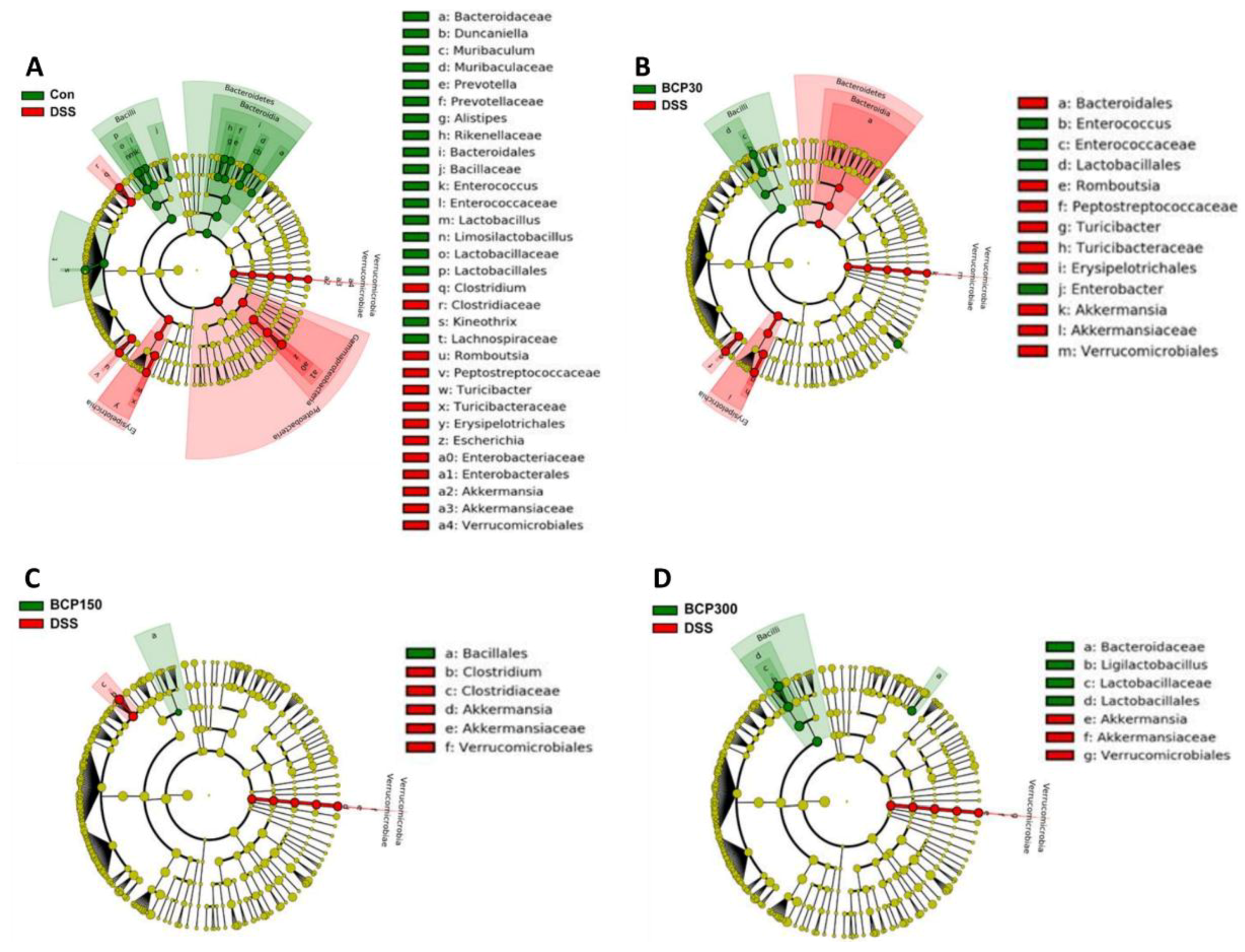
2.6. Analysis of Changes in the Gut Microbiota
3. Methods
3.1. Materials
3.2. Ethics
3.3. Mice and Experimental Protocol
3.4. Observation of General Symptoms and Blood Collection by Autopsy
3.5. Feces Weight Measurement
3.6. Assessment of Colon Length and Contraction Reaction
3.7. Histopathological Examination
3.8. Immunological Evaluation
3.9. Pyrosequencing Analysis of the Gut Microbiota Based on the 16S rRNA Gene
3.10. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Curr. Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Lee, A.S.; Lee, K.M.; Lee, J.A.; Choi, I. Peanut shell extract inhibits the development of dextran sulfate sodium (DSS)-induced colitis. Int. Immunopharmacol. 2019, 70, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Valatas, V.; Vakas, M.; Kolios, G. The value of experimental models of colitis in predicting efficacy of biological therapies for inflammatory bowel diseases. Am. J. Physiol.-Gastrointest. 2013, 305, G763–G785. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Oiyama, Y.; Mizoguchi, A.; Okugawa, Y.; Koike, Y.; Morimoto, Y.; Araki, T.; Uchida, K.; Tanaka, K.; Nakashima, H.; Hibi, M.; et al. Intravital imaging of DSS-induced cecal mucosal damage in GFP-transgenic mice using two-photon microscopy. J. Gastroenterol. 2010, 45, 544–553. [Google Scholar]
- Jeon, Y.D.; Bang, K.S.; Shin, M.K.; Lee, J.H.; Chang, Y.N.; Jin, J.S. Regulatory effects of glycyrrhizae radix extract on DSS-induced ulcerative colitis. BMC Complement. Med. Ther. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V. Syzygium aromaticum. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 611–625. [Google Scholar]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Andra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef]
- Beg, A.Z.; Ahmad, I. In vitro fungitoxicity of the essential oil of Syzygium aromaticum. World J. Microbiol. Biotechnol. 2002, 18, 317–319. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef]
- Agarwal, R.B.; Rangari, V.D. Phytochemical investigation and evaluation of anti-inflammatory and anti-arthritic activities of essential oil of Strobilanthus ixiocephala Benth. Indian J. Exp. Biol. 2003, 41, 890–894. [Google Scholar] [PubMed]
- Demirci, B.; Baser, K.H.; Demirci, F.; Hamann, M.T. New caryophyllene derivatives from Betula litwinowii. J. Nat. Prod. 2000, 63, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Chaudhuri, S.K.; Kubo, Y.; Sanchez, Y.; Ogura, T.; Saito, T.; Shikawa, H.; Haraguchi, H. Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides. Planta Med. 1996, 62, 427–430. [Google Scholar] [CrossRef]
- Lourens, A.C.; Reddy, D.; Baser, K.H.; Viljoen, A.M.; Van, S.F. Vuuren, In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J. Ethnopharmacol. 2004, 95, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C.A. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components, J. Agric. Food Chem. 2006, 54, 174–181. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Tambe, Y.; Tsujiuchi, H.; Honda, G.; Ikeshiro, Y.; Tanaka, S. Gastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, β -caryophyllene. Planta Med. 1996, 62, 469–470. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, H.Y.; Kim, S.K.; Park, J.H.Y.; Lee, H.J.; Chun, H.S. β-Caryophyllene attenuates dextran sulfate sodium-induced colitis in mice via modulation of gene expression associated mainly with colon inflammation. Toxicol Rep. 2015, 2, 1039–1045. [Google Scholar] [CrossRef]
- Faryal, S.; Ulllah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-capped nanoapatites amplify osmotic stress toleranc in Zea mays L. by conserving photosynthetic pigments, osmolytes biosynthesis and antioxidant biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Akhter, M.H.; Ahmad, I.; Muzammil, K.; Dawria, A.; Zeyaullahm, M.; Altamimi, A.S.A.; Khaliullah, H.; Ullah, S.N.M.N.; Rahman, M.A.; et al. A ß-Sitosterol encapsulated biocompatible Alginate/Chitosan polymer Nanocomposite for the treatment of Breast Cancer. Pharmaceurics 2022, 14, 1711. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; M Al Kaabi, J.; M Nurulain, S.; N Goyal, S.; Amjad Kamal, M.; Ojha, S. Polypharmacological properties and therapeutic potential of β-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, E.; Taddei, G.; Vetuschi, A.; Sferra, R.; Frieri, G.; Ricciardi, G.; Caprilli, R. Dextran sulfate sodium (DSS) colitis in rats (clinical, structural, and ultrastructural aspects). Dig. Dis. Sci. 1999, 44, 1458–1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. JoVE 2012, 60, 3678. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.C.; Kirsner, J.B. Immunological apparatus of the gut and inflammatory bowel disease. Gastroenterology 1971, 60, 922–951. [Google Scholar] [CrossRef]
- Iwamoto, M.; Koji, T.; Makiyama, K.; Kobayashi, N.; Nakane, P.K. Apoptosis of crypt epithelial cells in ulcerative colitis. J. Pathol. 1996, 180, 152–159. [Google Scholar] [CrossRef]
- Boltin, D.; Perets, T.T.; Vilkin, A.; Niv, Y. Mucin function in inflammatory bowel disease: An update. J. Clin. Gastroenterol. 2013, 47, 106–111. [Google Scholar] [CrossRef]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015, 6, 81. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Stappenbeck, T.S. Host–microbe interactions shaping the gastrointestinal environment. Trends Immunol. 2014, 35, 538–548. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase. Proc. Assoc. Am. Physicians 1999, 111, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Ridwan, B.U.; Tennyson, G.S.; Beagley, K.W.; Bucy, R.P.; Elson, C.O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 1994, 107, 1643–1652. [Google Scholar] [CrossRef]
- Tomoyose, M.; Mitsuyama, K.; Ishida, H.; Toyonaga, A.; Tanikawa, K. Role of interleukin-10 in a murine model of dextran sulfate sodium-induced colitis. Scand. J. Gastroenterol. 1998, 33, 435–440. [Google Scholar]
- Elinav, E.; Strowig, T.; Kau, A.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.; Gordon, J.I.; et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The role of Enterobacteriaceae in gut microbiota dysbiosis in inflammatory bowel diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Lavelle, A.; Lennon, G.; O’Sullivan, O.; Docherty, N.; Balfe, A.; Maguire, A.; Mulcahy, H.E.; Doherty, G.; O’Donoghue, D.; Hyland, J.; et al. Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 2015, 64, 1553–1561. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeom, J.E.; Kim, S.-K.; Park, S.-Y. Regulation of the Gut Microbiota and Inflammation by β-Caryophyllene Extracted from Cloves in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Molecules 2022, 27, 7782. https://doi.org/10.3390/molecules27227782
Yeom JE, Kim S-K, Park S-Y. Regulation of the Gut Microbiota and Inflammation by β-Caryophyllene Extracted from Cloves in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Molecules. 2022; 27(22):7782. https://doi.org/10.3390/molecules27227782
Chicago/Turabian StyleYeom, Ji Eun, Sung-Kyu Kim, and So-Young Park. 2022. "Regulation of the Gut Microbiota and Inflammation by β-Caryophyllene Extracted from Cloves in a Dextran Sulfate Sodium-Induced Colitis Mouse Model" Molecules 27, no. 22: 7782. https://doi.org/10.3390/molecules27227782
APA StyleYeom, J. E., Kim, S.-K., & Park, S.-Y. (2022). Regulation of the Gut Microbiota and Inflammation by β-Caryophyllene Extracted from Cloves in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Molecules, 27(22), 7782. https://doi.org/10.3390/molecules27227782




