Abstract
Synthesis of nanomaterials with specific morphology is an essential aspect for the optimisation of its properties and applications. The application of nanomaterials is being discussed in a wide range of areas, one of which is directly relevant to the environment through photocatalysis. To produce an effective photocatalyst for environmental applications, morphology plays an important role as it affects the surface area, interfaces, crystal facets and active sites, which ultimately affects efficiency. The method of synthesis and synthesis temperature can be the basic considerations for the evaluation of a particular nanomaterial. In this study, we have considered the aspects of morphology with a basic understanding and analyzed them in terms of nanomaterial efficacy in photocatalysis. Different morphologies of specific nanomaterials such as titanium dioxide, zinc oxide, silver phosphate, cadmium sulphide and zinc titanate have been discussed to come to reasonable conclusions. Morphologies such as nanorods, nanoflower, nanospindles, nanosheets, nanospheres and nanoparticles were compared within and outside the domain of given nanomaterials. The different synthesis strategies adopted for a specific morphology have been compared with the photocatalytic performance. It has been observed that nanomaterials with similar band gaps show different performances, which can be linked with the reaction conditions and their nanomorphology as well. Materials with similar morphological structures show different photocatalytic performances. TiO2 nanorods appear to have the best features of efficient photocatalyst, while the nanoflowers show very low efficiency. For CdS, the nanoflower is the best morphology for photocatalysis. It appears that high surface area is the key apart from the morphology, which controls the efficiency. The overall understanding by analyzing all the available information has enumerated a path to select an effective photocatalyst amongst the several nanomaterials available. Such an analysis and comparison is unique and has provided a handle to select the effective morphology of nanomaterials for photocatalytic applications.
1. Introduction
The nanotechnology industry has been envisioned to grow to the tune of 50 billion USD in the year 2022 [1]. The market survey done by Allied Market Research Group projected a compound annual growth rate (CAGR) of 20.7% from 2016 to 2022 for nanomaterials (NMs) that are widely used in industries [2]. It has become crucial for every country to invest in the development of NMs and their applications to keep pace with the growing technological advancement. This has happened because of the unique properties possessed by the devices based on patterns that one could engineer at atomic and molecular levels. Applications in nanotechnology are in wide-ranging fields like water purification, medicine, cosmetics, energy storage, healthcare, aerospace, consumer goods, agriculture, electronics, plastics, coatings, and electronic and electrical industries [3,4]. Due to the enormous need in the future for nanomaterials, it is crucial to understand the synthesis and growth of these nanostructures [5]. Besides considering the role of surface area, crystal structure, grain boundaries, chemical nature, density, and interfacial interaction of NMs with other materials, the properties of NMs are regulated to a large extent through the morphology of the NMs.
According to Pokropivny and Skorokhod, NMs can be classified as 0D, 1D, 2D and 3D, respectively [6]. It was considered that the characteristics of the NMs depend on the shape of the particle and its dimensionality. Thus, the application of the NMs can be devised based on their morphology and dimensions, which control, to a large extent, the movement of electrons within the NMs. The term 0D is often used for the NMs in which the electrons are entrapped in the dimensionless space. In 1D type NMs, electrons can move along a particular direction, whereas in 2D and 3D type NMs, the electrons conduct in the plane and all directions, respectively. Various morphologies of NMs such as rods, spheres, flowers, wires, tubes, and belts have already been reported in the literature and also had a scope of different structures to be synthesized in future. Synthesis processes and the parameters such as time and temperature regulate the synthesis of different morphologies of NMs. On the other hand, different morphologies of the same nanomaterial display varying performance depending upon the type of application intended. Presently there are no reports/reviews which have focused entirely on this specific aspect of the role of varying morphologies of a nanomaterial concerning its synthesis and efficiency towards a specific application.
Environmental contamination is growing at an alarming rate due to rapid growth in population and industrialization. Organic pollutants are contaminating air, water and soil at catastrophic rates. Conventional technologies such as adsorption, ultrafiltration, coagulation and photocatalysis are routinely used for the decontamination of anthropogenic organic contaminants. The efficiency and economic cost of these technologies govern their wide-scale application in the detoxification of the environment. Consideration of efficiency in terms of the performance of the catalyst and time/energy required in its synthesis plays a vital role in its commercialization and application in the large-scale treatment of pollutants. The photocatalytic performance of NMs is the most researched topic in the present context. The past two decades have witnessed extensive research on semiconductor nanoparticles with unique photoinduced polarization properties and size-dependent band gap expansion. Due to their ability to oxidize the organic and inorganic substrates, these NMs find their use as photocatalysts for the removal of organic and inorganic pollutants either from the aqueous or gas phase. Compared to conventional chemical oxidation methods, semiconductor catalysts are low-cost, nontoxic oxides that do not lose photocatalytic activity on extended use. The photocatalytic activity of the semiconductor material is due to the generation of electrons/holes on the absorption of photons, which are influenced by a band gap. Photoexcitation of the electrons occurs when the incident energy of the photon equals or is larger than the band gap of the particular semiconductor catalyst. It triggers the photochemical reaction on the surface of the NMs. In this process, the performance of the semiconductor material is governed by optical absorption, charge separation and, finally, surface reactions. The morphology of the photocatalyst dramatically affects the efficiency of photocatalytic reactions. The unusual structural and optical properties of these NMs are controlled by the change in morphology, which in turn is governed by the synthesis process adopted, the time required for synthesis and reaction temperature. Hence, to employ the best morphology of a particular photocatalyst, these factors are required to be viewed together for process optimization. Interest in strategies to control particle morphology has increased in the past decade because of its potential in various applications. For the production of NMs with controlled morphology, many alternative methods like lyophilization, precipitation, hydrothermal, freeze-drying, spray-drying, emulsion-based, mechanical milling, and sol-gel methods have been proposed [7,8,9]. Of all these synthesis methods, Ghoderao et al. [10] found the hydrothermal method to be the most efficient synthetic method not only due to its simplicity and low-cost feasibility but due to the crystalline nature of the product obtained with a large surface of the photocatalyst resulting in a higher % of photodegradation. Nandiyanto et al. [11] reported that the aerosol-assisted self-assembly, besides being rapid and economically produced spherical particles that were free from agglomeration with a relatively monodispersed size. It was observed that a change in the process conditions could produce various particle morphologies. For instance, the spray method was used to generate spherical-shaped particles with the advantage of maximum structural stability [12].
Even though an impressive variation of morphologies ranging from spheres to rods, needles, cubes, hollow rods, flowers, square plates, ribbons and belts are available in the literature [13,14,15,16], in most of the studies, a clear correlation between the morphology and the photocatalytic efficiency is not adequately explained. Different NMs have been evaluated in terms of photocatalytic efficiency using different morphologies under different experimental degradation conditions; however, due to the absence of an elaborate description, clear reasoning for their activity may not be completely evident. This may be related to the difficulties in obtaining a well-defined surface and, consequently, a lack of clarity of the exposed crystallographic faces. Moreover, it is a tough task to compare various morphologies whose efficiencies are different due to different photocatalysis procedures adopted. Being a multi-step process, the photocatalytic degradation of a pollutant may impact differently depending on the relative reactivity of the exposed surfaces of NMs. For an efficient photocatalyst, electrons and holes must first be efficiently photogenerated within the bulk material, followed by their migration to the surface, where their surface trapping may depend on the crystalline quality and the shape of the particle morphology. However, it is also possible while migrating to the surface of the photocatalyst, the photoinduced electrons and holes might become inactive through recombination. A short distance from the core of the photocatalyst to its surface and a suitable concentration gradient reduces this recombination. The concentration gradient has a close correlation to the morphology and surface properties of the NMs [17].
The morphology of the semiconducting photocatalytic materials plays a vital role as the irradiated surface initiate reactions such as oxidation or reduction of the contaminants in the desired process. It is the relative reactivity of the exposed surfaces of NMs that directs further surface reactions directly with the pollutant to yield active radicals that are prone to degrade pollutants. It is, therefore, imperative to consider these parameters for selecting the catalyst amongst the different morphologies of the available photocatalytic materials. Semiconductor materials such as TiO2, ZnO, Ag3PO4, CdS and ZnTiO3 are emerging as some of the most popular materials for the treatment of contaminants by the photocatalytic process. It can be regarded as a potential material of the future required in large quantities. This is due to their environmental adaptability and ability to harness solar energy, which is abundantly available on earth for pollution control. Since the scope of metal oxide-based photocatalysts is broad, selective examples of the widely accepted photocatalysts such as TiO2, ZnO, Ag3PO4, CdS, and ZnTiO3 have been compared based on their photocatalytic performance, the time required for synthesis and reaction temperature. This review is significant for the energy and environment sectors, providing a perspective on the assessment of key photocatalysts, and will provide a direction to the material scientist, chemist and technologist working in the area of environmental mitigation.
2. Discussion on Various Nanomaterials
2.1. Titanium Dioxide (TiO2) Nanostructures
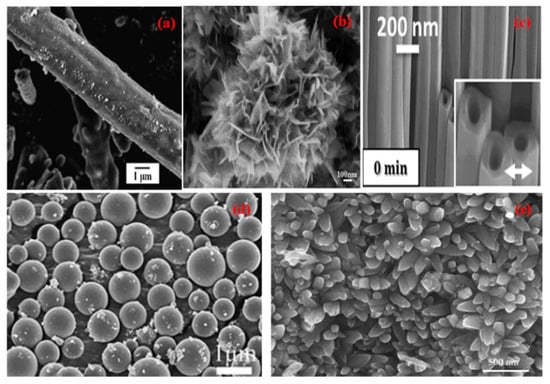
Figure 1.
SEM micrographs of different nano morphologies of TiO2 (a) Nanorod (Reprinted with permission from [18]. Copyright 2014 Elsevier), (b) Nanoflower (Reprinted with permission from [19]. Copyright 2012 Elsevier), (c) Nanotubes (Reprinted with permission from [20]. Copyright 2014 Springer Nature), (d) Nanospheres (Reprinted with permission from [21]. Copyright 2011 Royal Society of Chemistry), (e) Nanospindles (Reprinted with permission from [22]. Copyright 2018 Elsevier).
Due to the nontoxic nature, natural and abundant availability, wide spectrum application range, and attractive physical properties, titanium dioxide-based nanomaterials of different morphologies (Figure 1) are of tremendous interest in the field of photocatalysis [23], photovoltaics [24], hydrogen production [25], self-cleaning coatings [26], energy storage [27], and antifogging coatings [28]. Different morphologies of titania NMs including nanorods, nano spindles, nanotubes, nanospheres, and nanoflowers, can be prepared using different approaches such as sol-gel method, electrochemical route, hydrothermal method, atomic layer deposition approach (ALD) etc. It becomes imperative to understand the effect of various morphologies of TiO2 nanomaterial on the surface physiochemical properties during its growth and end application. The performance of TiO2 as a photocatalyst primarily depends upon ion transport across the catalyst material, mass transfer to catalytic sites and charge transfer at the available surface of the material, and all of the above depends on the morphology of TiO2. The photocatalytic process is controlled to a large extent by the morphology of the titania nanomaterials and can be improved by the large surface area and nano-size of the nanoparticles [29]. It can ultimately control nature, density, crystallinity and defect concentration in the nanomaterials. It has a profound effect on charge transfer and ion transport properties. In contrast to the bulk titania phase, the morphology of nanostructures such as nanorods, nanoflowers, nano spindles, nanotubes, and nanospheres show high surface area and ease of mass transfer.

Table 1.
Photocatalytic activity study of different nano morphologies of TiO2.
Table 1.
Photocatalytic activity study of different nano morphologies of TiO2.
| S. NO. | Material & Morphology | Method | Application | Photocatalytic Activity | Reference |
|---|---|---|---|---|---|
| Nanorod | |||||
| 1 | TiO2/Nanorod | Sol-gel | P-nitrophenol, under 15 W UV Philips lamp | 69% in 20 min | [30] |
| 2 | TiO2/Nanorod | Chemical vapour deposition | Methyl Orange (MO) & Methylene Blue (MB) under 100 W UV mercury lamp | 97% of MO in 100 min 99% of MB in 50 min | [18] |
| 3 | TiO2/Nanorod | Sol-gel | Phenol under18 W UV lamp, | 48% in 360 min | [23] |
| 4 | TiO2/Nanorod | Hydrothermal Method at 120 °C for 15 h | Phenol under UV light | 87% in 360 min | [31] |
| 5 | TiO2/Nanorod | Hydrothermal Method at 200 °C for 12 h | MO under 300 W UV Xenon lamp | 100% in 95 min | [32] |
| 6 | TiO2/Nanorod | Hydrothermal Method at 225 °C for 24 h | MO & MB under 6 W UV lamp | 100% of MO & 88% of MB in 120 min | [33] |
| 7 | TiO2/Nanorod | Hydrothermal Method at 180 °C for 12 h | Phenol, 20 W UV lamp, 365 | 55% in 360 min | [21] |
| 8 | TiO2/rod | Hydrothermal, 200 °C for 18 h | MB, under 6 W UV lamp | 80% in 100 min | [34] |
| 9 | TiO2/Nanorod | Hydrothermal Method at 180 °C for 24 h | MO, under UV mercury lamp | 51% in 150 min | [35] |
| Flower | |||||
| 10 | TiO2/Flower | Sol-gel | Phenol, under 18 W UV lamp | 70% within 360 min | [23] |
| 11 | TiO2/Flower | Hydrothermal, 180 °C for 12 h | Phenol, 20 W UV lamp | 97% in120 min | [21] |
| 12 | TiO2/Flower | Hydrothermal, 180 °C for 6 h | Rhodamine B (RhB), under 450 W UV Xenon lamp | 69% in 160 min | [36] |
| 13 | TiO2/Flower | Sol-gel | RhB under 300 W UV lamp | 91.4% in 50 min | [37] |
| 14 | TiO2/Flower | Hydrothermal, 150 °C for 24 h | RhB, under 350 W Xenon Visible lamp | 63% in 60 min | [38] |
| 15 | TiO2/Flower | Hydrothermal, 150 °C for 3 h | MB under UV lamp | 78% in 60 min | [39] |
| 16 | TiO2/Flower | Hydrothermal, 120 °C for 48 h | MO under sunlight | 60% in 60 min | [19] |
| 17 | TiO2/Flower | Hydrothermal, 150 °C for 24 h | MB under UV 300 W high-pressure mercury (Hg) lamp | 75% in 60 min | [40] |
| Tube | |||||
| 18 | TiO2/Tube | Furnace 500 °C for 4 h | Papermaking wastewater, under 375 W high-pressure Hg lamp | 99.5% in 720 min | [41] |
| 19 | TiO2/Tube | Electrochemical Method | MB, under UV lamp | 98% in 60 min | [20] |
| 20 | TiO2/Tube | Hydrothermal, 160 °C for 24 h | MO, under 300 W UV lamp | 50.2% in 60 min | [42] |
| 21 | TiO2/Tube | Sol-gel stirring at 40 °C for 24 h | RhB & Dibutyl phthalate (DBP) 125 W high-pressure Hg UV lamp | 20% of RhB in 60 min & 15% of DBP in 60 min | [43] |
| 22 | TiO2/Tube | Solvothermal, 180 °C for 24 h | Orange II, under 18 high-pressure Hg lamps | 97.98% in 3000 min | [44] |
| 23 | TiO2/Tube | Electrochemical method | MB, under UV light lamp | 72% in 200 min | [45] |
| 24 | TiO2/Tube | Electrochemical Method | Phenol, under 1000 W Xenon lamp visible light lamp | 99.5% in 40 min | [46] |
| Sphere | |||||
| 25 | TiO2/Sphere | Hydrothermal, 180 °C for 24 h | Phenol, under 20 W UV lamp, | 60% in 120 min | [21] |
| 26 | TiO2/Sphere | Hydrothermal, 130 °C for 48 h | MB, under UV lamp | 96% in 80 min | [47] |
| 27 | TiO2/Sphere | Hydrothermal, 200 °C for 18 h | MB, under 6 W UV lamp | 90% in 100 min | [34] |
| 28 | TiO2/Sphere | Hydrothermal, 80 °C for 24 h | MB, under UV light | 96% in 100 min | [48] |
| 29 | TiO2/Sphere | Hydrothermal, 160 °C for 24 h | MO, under 4 W UV lamp | 50% in 60 min | [49] |
| 30 | TiO2/Sphere | Hydrothermal, 150 °C for 72 h | MO, under 8 W UV lamp | 91.6% in 60 min | [50] |
| Spindle | |||||
| 31 | TiO2/spindle | Hydrothermal, 180 °C for 12 h | MO, under 300 W visible light | 38% in 120 min, | [51] |
| 32 | TiO2/spindle | Hydrothermal, 200 °C for 24 h | RhB, under 350 W Xenon visible lamp | 23% in 60 min | [52] |
| 33 | TiO2/spindle | Reverse micellar method | RhB, under UV lamp | 90% in 130 min | [53] |
| 34 | TiO2/spindle | Hydrothermal, 180 °C for 12 h | MO, under 250 W UV high-voltage Hg lamp | 91% in 300 min | [54] |
| 35 | TiO2/spindle | Hydrothermal, 200 °C for 24 h | RhB, visible light | 25% in 60 min | [55] |
Various morphologies of TiO2 were synthesized using different approaches (Table 1), such as sol-gel [56,57], hydrothermal, electrochemical, solvothermal and solid-state methods. The nanorods were synthesized using sol-gel [30], solid-state [58], and hydrothermal methods [34]. Generally, it takes two hours for the complete mineralization of organic pollutants using TiO2 nanorods. TiO2 nanoflowers were prepared by using the sol-gel [34] and hydrothermal methods [21,36]. It takes more than an hour to remove organic contaminants by a photocatalytic process using TiO2 nanoflowers. The nanotubes were prepared by using electrochemical, hydrothermal [59], sol-gel [60], and solvothermal methods [61]. The 99.5% removal of organic pollutants was achieved within 40 min using TiO2 nanotubes [46]. TiO2 nanospheres were synthesized mostly by hydrothermal technique. It takes more than 2 h for the total mineralization of organic contaminants photocatalytically by using TiO2 nanospheres. TiO2 nano spindles were obtained by using hydrothermal [52] and reverse micellar [20] approaches. Nanospindle morphology was seen to be the slowest among all morphologies for photocatalytic mineralization of organic pollutants. It takes more than two hours to complete the mineralization of organic contaminants by using TiO2 nano spindles.
Therefore, it is important to study the parameters involved, such as surface area, the temperature of crystal growth, the time required for synthesis as well as photocatalytic activity in unison and collectively along with the range of different morphologies for a holistic understanding. The data collected from existing literature indicates that on all counts, TiO2 nanotube morphology proved to be excellent amongst different morphologies compared, as stated above. We have considered the best results from the available literature for comparison and analysis (Figure 2).
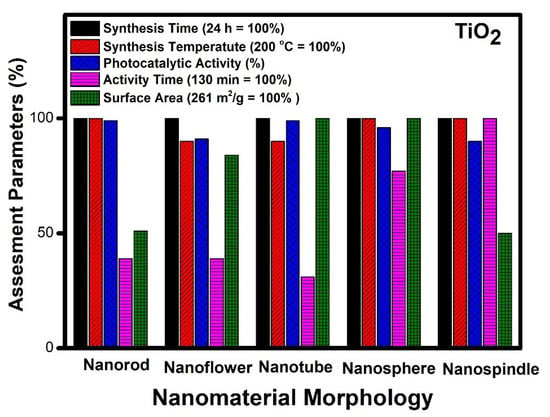
Figure 2.
Assessment of different morphologies of TiO2 based on various parameters.
It can be observed from Figure 2 that different morphologies can be compared based on various parameters. The temperature of synthesis varies for obtaining different morphologies of TiO2. Nanorods, nanospindle, and nanospheres show the highest temperature requirement (200 °C), whereas nanoflower and nanotubes can be synthesized at a twenty-degree lower temperature. In the case of photocatalytic performance, nanorods, nanotubes, and nanospheres show the highest performance, whereas nanoflowers and nanospindle show a 10% lesser performance. The catalytic activity time taken by nanospindle is the highest, and nanotubes show the lowest among the reported. The nanorod and nanoflower show moderate time, but nanosphere time is a little higher comparatively. The surface area of nanotubes and nanospheres is the highest among the reported, whereas nanorods and nanospindle show a 50% lesser surface area as compared to nanotubes and nanospheres. The surface area of nanoflower shows a moderate value comparatively.
The remarkable catalytic activity (99%) with minimum activity time (40 min) was observed in the case of TiO2 with nanotube morphology [46]. This can be related to the high surface area (261 m2/g) and ease of access to the reactants during the catalytic process. Thus, it can be inferred that surface area and easy access to reactants are essential factors which govern the performance of the different morphologies in TiO2. The increased surface area makes available microchannels for the free flow of reactants and degradation products during the photocatalytic process. These microchannel increases the possibility of interaction with a more catalytic active center, thereby increasing the performance of the catalyst. The highlight of this tube-like morphology is its low-temperature synthesis (180 °C) by solvothermal route [61]. The different morphologies of TiO2, such as nanorods and nanospheres, also show excellent performance in terms of photocatalytic activity but have less surface area and more activity time as compared to the nanotube morphology. However, the efficiency of nanospheres is close to nanotubes and can be considered as the next best material matched with nanotubes [62]. Further, nanoflower morphology with a high surface area lacks the desired catalytic performance, probably due to poor accessibility of reactive species to active centers during the photochemical process [23]. The different morphologies studied in decreasing order of their efficiency are nanotubes [47], nanospheres [48], nanorods [18], nanospindle [53] and nanoflowers [37].
2.2. Zinc Oxide (ZnO) Nanostructures
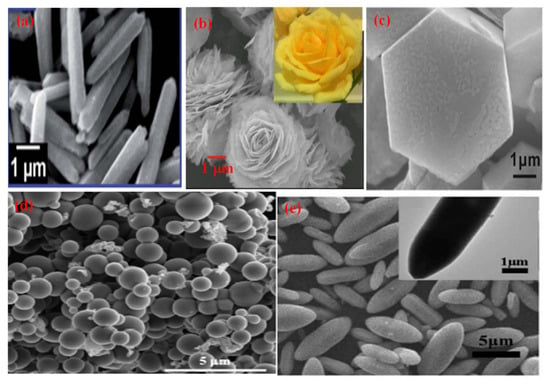
Figure 3.
SEM micrographs of different morphologies of ZnO (a) Nanorod (Reprinted with permission from [63]. Copyright 2014 Royal Society of Chemistry), (b) Nanoflower (Reprinted with permission from [64]. Copyright 2016 Elsevier), (c) Hexagonal (Reprinted with permission from [65]. Copyright 2017 Royal Society of Chemistry), (d) Nanospheres (Reprinted with permission from [66]. Copyright 2019 Elsevier), (e) Nanospindles (Reprinted with permission from [67] Copyright 2017 Elsevier).
Different morphologies of zinc oxide (ZnO) are (Figure 3) considered a vital semiconductor catalyst with a wide band gap of 3.37 eV with high excitation binding energy of 60 eV and have been studied extensively for photocatalytic reactions. The performance of ZnO as a semiconductor photocatalyst can be related to the absorption of light, which excites electrons from the valence band (VB) to the conduction band, which creates a hole (h+) in VB and initiates the photo redox reaction. Zinc oxide occurs in nature as a zincite mineral which has a hexagonal structure with lattice parameters a and c of 3.25 Å and 5.20 Å, respectively. It is used for making pigments, transducers, lasers, diodes, sensors and catalysts. The most common use of ZnO in the nanoscale range is for making sunscreen additives for protection from UV radiation, and ZnO thin films are used in making devices. Thus, ZnO can be used in a broad range of applications starting with catalytic activity to optoelectronics and to antimicrobial activity as well, which leads to a variety of products manufactured. ZnO is preferred as a semiconductor catalyst due to its low cost, efficiency, nontoxic nature, high redox potential and ease of availability. When compared to TiO2, the photocatalytic performance of ZnO is much superior. It is due to the absorption of a more significant portion of the UV spectrum by ZnO with greater electron mobility (200–300 cm2V−1s−1) and higher oxidation potential of the •OH radical generated [68]. This results in a rapid electron transfer leading to a higher quantum yield. ZnO has a higher recombination rate of electrons and holes generated during photocatalysis which reduces its performance as a photocatalyst.

Table 2.
Comparative study of different nano morphologies of nanostructured ZnO.
Table 2.
Comparative study of different nano morphologies of nanostructured ZnO.
| S. NO. | Material & Morphology | Method | Application | Performance | Reference |
|---|---|---|---|---|---|
| Rod | |||||
| 1 | ZnO/Rod | Microwave reactor (heated to 80 °C for 10 min) | MO, under 300 W Hg lamp | 86.3% in 180 min | [69] |
| 2 | ZnO/Rod | Hydrothermal, 180 °C for 24 h | Resorcinol, under 15 W UV lamp | 100% in 120 min | [70] |
| 3 | ZnO/Rod | Atmospheric self-induction method | RhB, under 400 W Xenon visible lamp | 36.8% in 300 min | [71] |
| 4 | ZnO/Rod | Solvothermal, 80 °C for 5 h | MB under 300 W UV lamp | 100% in 20 min | [72] |
| 5 | ZnO/Rod | Hydrothermal, 140 °C for 12 h | MB under 6 W UV lamp | 98.5% in 100 min | [73] |
| 6 | ZnO/Rod | Hydrothermal, 120 °C for 20 h | Phenol under l 15 W UV lamp | 100% in 40 min | [74] |
| 7 | ZnO/Rod | Hydrothermal, 95 °C for 30 h | RhB under 500 W visible Xenon lamp | 50% in 300 min | [75] |
| Flower | |||||
| 8 | ZnO/Flower | Hydrothermal, 100 °C for 12 h | RhB, under 300 W Hg lamp | 99.84% in 25 min | [64] |
| 9 | ZnO/Flower | Thermal decomposition at 300 °C for 20 min | RhB, under 36 W UV lamp | 100% in 90 min | [76] |
| 10 | ZnO/Flower | Sol-gel at 80 °C | RhB, under 200 W high-pressure Hg UV lamp | 99.8% in 100 min | [77] |
| 11 | ZnO/Flower | Hydrothermal, 140 °C for 12 h | MB, under 6 W UV lamp | 94% in 100 min | [73] |
| 12 | ZnO/Flower | Precipitation method | RhB, under Hg UV lamp | 30% in 180 min | [78] |
| 13 | ZnO/Flower | Hydrothermal, at 190 °C for 1 h | MB, 125 W Hg UV lamp | 98% in 60 min | [77] |
| 14 | ZnO/Flower | Solution based at 97 °C for 4 h. | MB, under 30 W Hg UV lamp | 99.9% in 180 min | [79] |
| 15 | ZnO/Flower | Microwave, at 300 W for 12 s | MB, under high-pressure Hg UV lamp | 80% in 60 min | [63] |
| 16 | ZnO/Flower | Hydrothermal, at 90 ° C for 24 h | MB, under BLB UV lamp | 100% in 105 min | [80] |
| 17 | ZnO/Flower | Sol-gel at room temperature for 16 h. | RhB, under 300 W Xenon UV lamp | 100% in 100 min | [81] |
| Sphere | |||||
| 18 | ZnO/Sphere | Hydrothermal, at 140 °C for 12 h | MB, under 6 W UV lamp | 74% in 100 min | [73] |
| 19 | ZnO/Sphere | Hydrothermal, at 180 °C for 24 h | RhB, under 15 W UV lamp | 100% in 240 min | [82] |
| 20 | ZnO/Sphere | Heated in a silicone bath at 120−140 °C for 4 h | MO, under 24 W UV lamp | 90% in 300 min | [83] |
| 21 | ZnO/Sphere | Hydrothermal, at 180 °C for 4 h | Congo Red, under 30 W UV lamp | 99.2%, in 90 min | [84] |
| 22 | ZnO/Sphere | Hydrothermal, at 120 °C for 6 h | MB, under 80 W UV lamp | 95% in 60 min | [85] |
| Hexagonal | |||||
| 23 | ZnO/Hexagonal | Heated at 150 °C on a hotplate | MB, under 450 W medium pressure Hg UV lamp | 100% in 16 min | [68] |
| 24 | ZnO/Hexagonal | Calcined at 400 °C | MB, under 16 W UV lamp | 100% in 75 min | [86] |
| 25 | ZnO/Hexagonal | Solvothermal, at 110 °C for 10 h | RhB, under Hg UV lamp | 80% in 60 min | [87] |
| 26 | ZnO/Hexagonal | Sol-gel at 80 °C for 3 h | MB, under 100 W UV lamp | 100% in 20 min | [88] |
| 27 | ZnO/Hexagonal | Hydrothermal, 120 °C for 20 h | MB, under UV lamp | 100% in 60 min | [74] |
| 28 | ZnO/Hexagonal | solid-phase method | MO, under 300 W UV lamp | 96.4% in 60 min | [89] |
| 29 | ZnO/Hexagonal | Hydrothermal, at 200 °C for 24 h | MB, under 300 W Hg UV lamp | 60% in 180 min | [90] |
| 30 | ZnO/Hexagonal | Sol-gel at 80 °C for 12 h | MB, under UV lamp | 95% in 60 min | [65] |
| 31 | ZnO/Hexagonal | Sonochemical method | MB, under 400 W Xenon UV lamp | 97% in 30 min | [91] |
| Spindle | |||||
| 32 | ZnO/Spindle | Hydrothermal, 140 °C for 12 h | MB, under 365 UV lamp | 62% in 100 min | [73] |
| 33 | ZnO/Spindle | Hydrothermal, 120 °C for 8 h | MO, under UV lamp | 55% in 180 min | [67] |
| 34 | ZnO/Spindle | Hydrothermal, 150 °C for 3 h | RhB, under 8W HG UV lamp | 73% in 120 min | [92] |
| 35 | ZnO/Spindle | Microwave, at 110 °C for 17 min | MB, under 300 W high-pressure Hg UV lamp | 98% in 120 min | [93] |
| 36 | ZnO/Spindle | Hydrothermal, at 140 °C for 12 h | MB, under 6 W high-pressure Hg UV lamp | 72% in 100 min | [73] |
| 37 | ZnO/Spindle | Hydrothermal, 95 °C for 24 h | MB, under 60 W Hg UV lamp | 83% in 55 min | [94] |
There are different methods, such as hydrothermal, sol-gel, coprecipitation, microwave, sonochemical and solid-state approaches which have been used in the preparation of zinc oxide with varying morphologies (Table 2). Zinc oxide nanorods were synthesized using microwave irradiation [69], hydrothermal [73,75] and solvothermal methods [72]. The average time taken for complete mineralization is around two hours. Zinc oxide nanoflowers were prepared using hydrothermal [64,73,77,80], sol-gel [79,81,95], coprecipitation [78], and microwave irradiation method [63]. It is reported to take about two hours for the nanoflower morphology to completely remove organic contaminants photocatalytically from an aqueous medium. Zinc oxide nanospheres were synthesized mostly using the hydrothermal route, and when applied for environmental photocatalysis, it takes less than 2 h for the complete mineralization of organic contaminants from water. The hexagonal morphology of zinc oxide was made using solvothermal [87], sol-gel [65,88,96], hydrothermal [90], solid-state [89] and sonochemical [91] approaches. It takes less than 1 h for the removal of organic pollutants by using hexagonal morphology. Nanospindle morphology was prepared mostly using hydrothermal routes [72,94]; however, the microwave technique [93] was also applied in its synthesis. The complete mineralization of organic contaminants achieved by using nano spindles was less than two hours.
Thus, it will be quite interesting to know as to which factors are responsible for the best performance of ZnO morphologies as a photocatalyst. ZnO nanomaterials show different morphologies such as nanorods [72], nanoflowers [97], nanospheres [85], nanospindle [94] and hexagonal morphology [91]. To analyze these different morphologies, the best results available in the literature were compared (Figure 4).
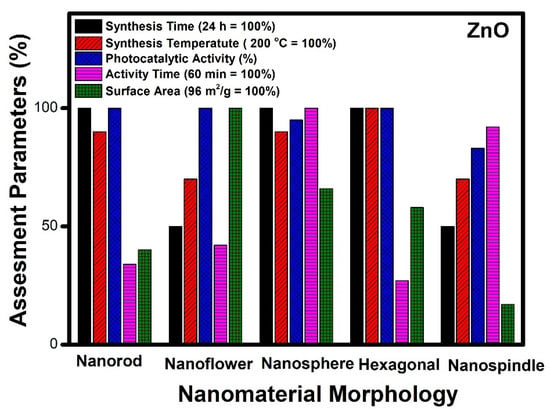
Figure 4.
Assessment of different morphologies of ZnO based on various parameters.
Figure 4 indicates that the nanorods, nanospheres and hexagonal morphology of ZnO required more time for synthesis (24 h), whereas nanoflower and nanospindle morphologies need 50% lesser time, comparatively. The temperature needed for the synthesis of hexagonal, nanorod and nanospheres is maximum (200 °C); however, nanoflower and nanospindle morphology require thirty per cent lesser temperature relatively. In the case of photocatalytic performance, nanorod, nanoflower, and hexagonal morphologies show the highest value, while the performance of nanospindle and nanospheres shows a little lesser value comparatively. The photocatalytic activity time taken by nanospheres and nanospindle morphology is the highest amongst reported morphologies, while nanorods, nanoflowers and hexagonal morphology show moderate values. The surface area of nanoflowers shows the highest values, whereas nanorods, nanospheres, and hexagons have modest values, but nanospindle reveal the lowest among the reported morphologies.
It is observed that ZnO nanoflower morphology shows the best results [98] in terms of surface area, time of synthesis, time of catalytic activity, and performance, amongst all other reported morphologies. Figure 3b shows the highest surface area (96 m2/g) for nanoflower morphology which has a direct impact on the performance of the catalyst. The surface area plays a vital role in mass transfer and charge transfer on the active catalytic surface. The time required (12 h) for the synthesis of nanoflower is also the lowest among other reported morphologies. The nanoflower morphology shows the removal of pollutants within 25 min, and the temperature of synthesis is the lowest compared to other morphologies. Thus, the nanoflower morphology of the ZnO stands out to be the best photocatalyst on all counts as equated with other reported morphologies of ZnO. The other morphology next to nanoflower is hexagonal in terms of surface area (56 m2/g), which enables the complete removal of pollutants within 16 min by the photocatalytic route. However, considering the time for synthesis of (24 h at 200 °C) of the hexagonal structure needs further optimization [68]. The nanorod morphology of ZnO shows complete removal of the pollutants within 20 min, whereas, in the case of nanosphere morphology, it takes an hour to achieve the same. The nano spindle morphology shows poor results in terms of catalytic performance (83%) and time taken to remove pollutants. However, the good thing is that the time and temperature of synthesis, i.e., 12 h and 140 °C, respectively, are more economical. Thus, the order of the morphologies for ZnO in terms of significance may be considered (from most significant to the lowest) as nanoflowers, nanospheres, nanorods, nano hexagons and lastly, nanospindle.
2.3. Cadmium Sulphide (CdS) Nanostructures

Figure 5.
SEM micrographs of different morphologies of CdS (a) Nanorod (Reprinted with permission from [99]. Copyright 2018 Elsevier), (b) Nanoflower (Reprinted with permission from [100]. Copyright 2017 Elsevier), (c) Particles (Reprinted with permission from [101]. Copyright 2019 Elsevier), (d) Nanosphere (Reprinted with permission from [102]. Copyright 2013 Elsevier), (e) Sheet (Reprinted with permission from [103]. Copyright 2018 Elsevier).
Synthesis of various morphologies of CdS nanostructures has been achieved by employing different approaches such as the solvothermal method, hydrothermal method, irradiation technique, electrodeposition process, liquid crystal template route [104] etc. Systematic research on the influence of phase behaviour has opened up new avenues for the generation of different morphologies, such as nanorods, nanospheres, and nanoflowers. According to Onsager [105], at low volume fractions, nanorods can form a distinct phase of anisotropic crystals having the positional disorder and leading to a nematic phase. Entropy drives the phase transition in the synthesis of such morphology. The contact of the photocatalyst surface with reactants has a remarkable influence on photocatalytic reactions, and the interfacial contact depends upon the morphology of the catalyst and the surface area available. In the case of CdS, nanomaterials morphology and particle size have an enormous impact on the photocatalytic property, and hence researchers are working on controlling the morphology of CdS nanomaterials to enhance the photocatalytic activity. Different morphologies such as nanosheets [103], nanorods [106], nanospheres [107], nano particles [108], and nanoflower [109] have been explored in this context (Figure 5). Several synthetic routes, such as chemical vapor deposition, thermal deposition, electrodeposition, template approach, microwave-assisted process, and solvothermal and hydrothermal methods, were applied to achieve the desired morphology of CdS nanomaterials. Out of these synthesis approaches hydrothermal route was found to be the most preferred and yielded the best results for optimal morphology. CdS has a narrow and direct band gap of 2.42 eV and can be considered an ideal II-VI semiconductor photocatalyst in the visible region. Thus, it is the most widely studied photocatalyst for several photocatalytic transformations, such as water splitting, reduction of nitro compounds, dye degradation, and photoelectric conversions. Tri-n-octyl phosphine oxide (TOPO) was used by Alivisatos [110] as a size and shape-controlling ligand for obtaining CdS nanomaterials. CdS nanowires were synthesized by Lieber et al. [111]., by using a laser ablation technique. Nanorods, nanowires and nanotubes were synthesized by using a thermal approach or a CVD method. The Ethylenediamine template approach was used [112] in the synthesis of nanorods and nanowires via a solvothermal route.
The Nanoflower morphology of semiconductor materials (CdS) is being applied as a chemo-sensor, photocatalyst, and catalyst for microbiological processes. As compared to other materials like ZnS, ZnO, TiO2, CdSe and similar other semiconductor materials, CdS has shown an increase in photocatalytic activity under visible light irradiation due to its narrow bandgap of 2.4 eV [113]. The nanomorphology and surface area are mainly responsible for its light absorption ability and charge carrier’s characteristics. Further, the ordered flower-like microstructure also boosts the photocatalytic properties of CdS. The nucleation and growth of different shapes of nanoflowers decide the order of construction of CdS nanoflowers. The actual morphology of the CdS microstructure can be tuned by adding surfactants or capping agents. The active surface of nanocrystals, due to the addition of surfactants, reduces interfacial activation energy and induces secondary nucleation during synthesis. There are many methods available in the literature for the synthesis of nanoflowers, such as rose-like flowers, nanocomposite flowers, petal-like structures and spindles. The CdS nanoflower morphology is applicable over a wide range of wavelengths and can be useful for the degradation of dyes like Rhodamine B and methylene blue under visible light. The structure of CdS nanoflowers contains more oxygen vacancies than that of other morphologies. The hydrothermal method is the most preferred in the synthesis of hierarchical CdS nanostructures due to its cost-effectiveness, scale-up, easy control and better crystalline quality of the product obtained. Hierarchical morphologies of CdS play an essential role during photocatalytic activity. It enhances the performance of the photocatalyst by increasing the absorption efficiency of the incident radiation and further transferring the energy to reactant molecules [114]. The charge separation in the hierarchical structure is much more efficient. To attain the maximum efficiency of CdS nanostructures, the synthesis of controllable hierarchical nano morphologies become important.

Table 3.
Details of the synthesis and photocatalytic performance of various morphologies of CdS.
Table 3.
Details of the synthesis and photocatalytic performance of various morphologies of CdS.
| S. NO. | Material & Morphology | Method | Application | Performance | Reference |
|---|---|---|---|---|---|
| Rod | |||||
| 1 | CdS/Rod | Hydrothermal, 180 °C for 6 h | MB, under 300 W Xenon visible lamp | 70% in 80 min | [115] |
| 2 | CdS/Rod | Reflux method for 13 h | MB, under 300 W Xenon visible lamp | 95% in 50 min | [106] |
| 3 | CdS/Rod | Hydrothermal, 160 °C for 48 h | Malachite green (MG) & MO, under 300 W Xenon visible lamp | 67% of MG in 30 min & 58% of MO in 45 min | [116] |
| 4 | CdS/Rod | Hydrothermal, 120 °C for 10 h | Salicylic acid and p-nitrophenol under 125 W Hg UV lamp | 70% Salicylic acid & 43.7% p-nitrophenol in 240 min | [117] |
| 5 | CdS/Rod | Hydrothermal, 180 °C for 12 h | Congo red (CR), under a visible tungsten lamp | 40% in 25 min | [118] |
| 6 | CdS/Rod | Hydrothermal, 180 °C for 24 h | MB, under a 100 W visible lamp | 62% in 180 min | [119] |
| 7 | CdS/Rod | Hydrothermal, 160 °C for 12 h | MB, under Hg UV lamp | 35% in 120 min | [114] |
| 8 | CdS/Rod | Hydrothermal, 160 °C for 24 h | Cr (VI), under a 1 kW Xenon visible light lamp | 19% in 120 min | [120] |
| 9 | CdS/Rod | 400 °C for 1 h in an N2 atmosphere | RhB, under a 200 W tungsten halogen visible lamp | 100% in 55 min | [121] |
| 10 | CdS/Rod | Hydrothermal, 200 °C for 10 h | MB, under a Xenon visible lamp | 50% in 120 min | [122] |
| 11 | CdS/Rod | Hydrothermal, 180 °C for 1 h | Ciprofloxacin (CIP), under a 300 W Xenon visible lamp | 57% in 60 min | [123] |
| 12 | CdS/Rod | Wet chemical method under reflux condition (100 °C for 7 h) | MO under a 300 W UV mercury lamp | 93% within 40 min | [124] |
| Flower | |||||
| 13 | CdS/Flower | Hydrothermal 200 °C for 12 h | MB, under a 125 W Hg visible lamp | 100% in 220 min | [125] |
| 14 | CdS/Flower | Sol-gel method | MB, under a 300 W Xenon visible lamp | 80% in 60 min | [126] |
| 15 | CdS/Flower | Hydrothermal 260 °C for 12 h | RhB, under a 300 W Xenon visible lamp | 70%, in 180 min | [127] |
| 16 | CdS/Flower | Hydrothermal, 180 °C for 12 h, | Acid fuchsine, under a 125 W Hg UV lamp | 100% in 40 min | [109] |
| 17 | CdS/Flower | Hydrothermal, 160 °C for 12 h | MB, under a 300 W Xenon visible lamp | 100% in 180 min | [128] |
| 18 | CdS/Flower | Hydrothermal, 160 °C for 4 h | MB, MO & RhB, under a 500 W Xenon visible lamp | 100% of MB, 91% of MO, and 85% of RhB in 150 min | [113] |
| 19 | CdS/Flower | Hydrothermal, 200 °C for 5 h | RhB, under visible light irradiation | 93% in 120 min | [100] |
| Sheet | |||||
| 20 | CdS/Sheet | Hydrothermal, 80 °C for 72 h | H2 production under a visible-light, AM 1.5 G solar simulator | 20 μmol within 480 min | [129] |
| 21 | CdS/Sheet | Electrochemical deposition for 15 min | CO2 reduction under sunlight | 2.1 μmol/g of C2H5OH and 62.8 μmol/g of HCOOH, 0.25% in 300 min | [130] |
| 22 | CdS/Sheet | Microwave method, at 80 °C for 30 min | H2 production, under visible light irradiation | 27.4 μmol/g in 240 min | [131] |
| 23 | CdS/Sheet | Heated in an oil bath at 60 °C for 3 h | H2 production under a 350 W Xenon visible lamp | 582 μmol/g in 240 min | [103] |
| 24 | CdS/Sheet | Ultrasonication at 90 °C for 2.5 h | RhB, under a 500 W Xenon visible lamp | 50% in 180 min | [112] |
| Sphere | |||||
| 25 | CdS/Sphere | Hydrothermal, at 120 °C for 10 h | Salicylic acid & p-nitrophenol, under a 125 W Hg UV lamp | 20% Salicylic acid & 6.25% p-nitrophenol in 240 min | [117] |
| 26 | CdS/Sphere | Hydrothermal, at 160 °C for 12 h | MB, under a Hg UV lamp | 38% in 120 min | [114] |
| 27 | CdS/Sphere | Hydrothermal, 180 °C for 4 h | Eosin Y, under a 500 W iodine tungsten lamp | 100% in 120 min | [107] |
| 28 | CdS/Sphere | Ultrasonic method | MB, under a 125 W UV lamp | 87% in 90 min | [132] |
| 29 | CdS/Sphere | Hydrothermal, at 100 °C for 2 h | 4-Chlorophenol, under 65 W fluorescent visible lamps | 52% in 150 min | [133] |
| 30 | CdS/Sphere | Microwave for 30 min | MB & RhB, under a 300 W Xenon visible lamp | 95% of MB in 150 min, 90% of RhB in 180 min | [102] |
| 31 | CdS/Sphere | Hydrothermal, at 200 °C for 3.5 h | RhB, under a 300 W tungsten halide visible lamp | 90% in 180 min | [134] |
| Particles | |||||
| 32 | CdS/Particle | Hydrothermal, at 160 °C for 12 h | MO, under a 350 W Xenon visible lamp | 12% in 60 min | [101] |
| 33 | CdS/Particle | Microwave for 20 s | Selective oxidation of alcohols to corresponding aldehydes under a 300 W Xenon visible lamp | 94% in 60 min | [108] |
| 34 | CdS/Particle | Hydrothermal, at 160 °C for 12 h | MB, under a Hg UV lamp | 29% in 120 min | [114] |
| 35 | CdS/Particle | Heating at 120 °C in an N2 environment | RhB, MB, & Cr (VI), under a 300 W Xenon visible lamp | 21% of RhB, 16% of Cr (VI) in 20 min & 24% of MB in 40 min | [135] |
| 36 | CdS/Particle | Hydrothermal, at 160 °C for 24 h | RhB, under a 250 W visible lamp | 72% in 240 min | [136] |
| 37 | CdS/Particle | Sol-gel method | MB, under a 300 W Xenon visible lamp | 48% in 60 min | [126] |
Herein, different morphologies such as nanorods, nanoflowers, nanosheets, nanospheres and nanoparticles of CdS have been reviewed. From Table 3, it can be observed that CdS nanorods were mainly obtained by the hydrothermal method. The time required in the synthesis by hydrothermal route varies from 1 h to 48 h. It was later applied in the mineralisation of organic pollutants and was reported to take an hour for the complete degradation of organic dye [121]. Generally, the temperature for the hydrothermal synthesis of the nanorods varies between 120 and 180 °C. CdS nanoflowers can be synthesised by using a hydrothermal route (most commonly used) where synthesis time and temperature vary between 4–12 h and 160–260 °C, respectively. The removal of organic dye photo catalytically was done within less than an hour [109]. The CdS nanosheets have been prepared by different methods, such as hydrothermal [129], electrochemical [130], microwave [131] and ultrasonication methods. It is reported that CdS nanosheets require three hours for the complete degradation of organic pollutants [112]. CdS nanosheets can also be applied in hydrogen production and CO2 reduction. Synthesis of CdS nanospheres was reported mostly by using hydrothermal routes [134], whereas other methods such as ultrasonication [132] and microwave irradiation [102] were also used. The removal of organic pollutants was carried out within three hours photocatalytically by using CdS nanospheres. CdS nanoparticles were synthesized by hydrothermal method [136], microwave technique [108] and sol-gel method [126]. The CdS nanoparticles required around 1 h for the complete degradation of organic dye [108].
CdS nanoparticles have been stabilized in different nano morphologies such as nanorods, nanoflowers, nanosheets, nanospheres and nanoparticles etc. We need to understand the morphology and its efficiency in terms of its end application and the time taken for photocatalytic activity, the time required for synthesis and the temperature of catalyst synthesis.
CdS nanosheet morphology requires 72 h for synthesis; however, nanorods, nanoflowers, and nanoparticles can be prepared in 17 h, and nanosphere morphology takes only 6 h for synthesis. For nanoflowers, nanospheres and nanorods need the highest reported temperature for synthesis, while nanoparticles can be prepared with a twenty per cent lesser value, and nanosheets can be synthesized at the lowest reported value of temperature. The photocatalytic performance of nanorods, nanoflowers, nanospheres and nanoparticles are the highest amongst those reported, while nanosheet shows a 50% lower value, comparatively. The time taken for the degradation of organic compounds photo catalytically is the maximum for nanosheet morphology; however other morphologies show an average of 1 h. The surface area of nanorods and nanoflowers is the highest among the reported, whereas nanosheets, nanospheres, and nanoparticles show moderate values.
It can be seen from Figure 6 that the surface area of CdS nanoflower (47 m2/g) is the highest among all the reported morphologies. The time required for the complete degradation of organic pollutants is also comparatively less than (40 min). It can be observed that the temperature of synthesis of various morphologies varies between 160 to 200 °C. In the case of CdS nanosheets, nanospheres, and nanoparticles, the reported surface area are 28, 18, and 19 m2/g, respectively. This seems to be the primary reason for the poor performance in terms of catalytic activity for these morphologies as compared to nanoflowers. Thus, based on the assessment parameters such as surface area, activity time, and photocatalytic performance, it can be seen that nanoflower morphology emerges as the best CdS nanostructure to be used as a photocatalyst. The order of preference of the different CdS morphologies for photocatalysis can be assigned as nanoflowers, nanorods, nanosheets, nanospheres and nanoparticles.
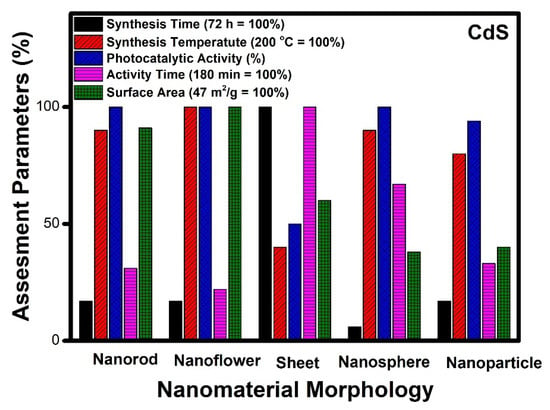
Figure 6.
Comparative study of different morphologies of CdS based on various assessment parameters.
2.4. Silver Phosphate (Ag3PO4) Nanostructures
The treatment of pollutants in wastewater by heterogeneous photocatalysis in the presence of semiconductors has been regarded as a promising and efficient approach due to the high efficiency, eco-friendly nature, and easy recycling carried out under mild conditions. Among various photoactive materials, silver phosphate (Ag3PO4) nanostructure with different morphologies (Figure 7) has attracted much attention and has been found to be a fascinating material. It is an excellent photocatalyst in the visible region and has a high quantum efficiency [137] of about 90%. It has superior semiconductor properties for direct water splitting and photodecomposition of organic dyes [138]. It has been reported that the direct band gap of Ag3PO4 is 2.43 eV which can absorb wavelengths up to 530 nm [139]. The photocatalytic activity of Ag3PO4 depends upon its electronic structure, and its valence band (VB) is made up of 2p orbitals of oxygen and 4d orbitals of silver. However, the conduction band (CB) is made up of hybridized 5s and 5p orbitals of silver favoring electron transfer and energy dispersion in all directions. The presence of d states in the conduction band in other semiconductors increases electron-hole recombination and also hinders the mobility of electrons, resulting in a reduction in photocatalytic activity. However, here in the case of Ag3PO4, the effect of d orbital in CB is inhibited. Therefore, the high photocatalytic activity is also attributed to the absence of d orbitals in the CB of Ag3PO4. However, the practical application of Ag3PO4 is still not satisfactory due to the formation of Ag0 particles on the surface of the photocatalyst during the photocatalysis process. Recently, some studies revealed that the photocatalytic activity of Ag3PO4 is directly influenced by size, morphology and presence of highly reactive facets [140]. To understand the properties of Ag3PO4 semiconductors, the surface and interfaces are crucial for photocatalytic activity. Hence, significant attention has been paid to the synthesis of morphology-controlled Ag3PO4, including cubes, dodecahedrons, tetrahedrons, spheres and polyhedrons.
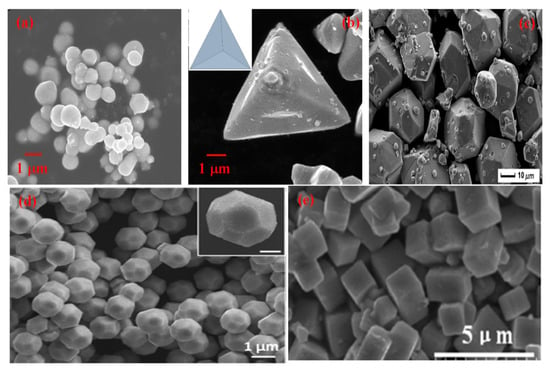
Figure 7.
SEM micrographs of different morphologies of Ag3PO4 (a) Sphere (Reprinted with permission from [141]. Copyright 2018 Elsevier), (b) Tetrahedral (Reprinted with permission from [142]. Copyright 2018 Elsevier), (c) Polyhedral (Reprinted with permission from [137] Copyright 2013 American Chemical Society), (d) Dodecahedral (Reprinted with permission from [143]. Copyright 2017 American Chemical Society), (e) Cubic (Reprinted with permission from [144]. Copyright 2015 Elsevier).

Table 4.
Details of different morphologies of nanostructured Ag3PO4.
Table 4.
Details of different morphologies of nanostructured Ag3PO4.
| S. NO. | Material & Morphology | Method | Application | Performance | Reference |
|---|---|---|---|---|---|
| Spherical | |||||
| 1 | Ag3PO4/Spherical | Continuous flow synthesis | Microfluidic photocatalytic dye-degradation, microreactor under visible light illumination | 97% within 15 min | [145] |
| 2 | Ag3PO4/Spherical | Precipitation method | Phenol, BSP, visible light, 400-W metal halide lamp | 82% phenol within 12 min, 81% Bisphenol within 10 min | [146] |
| 3 | Ag3PO4/Spherical | Precipitation | Rh.B. Xenon lamp (15 W), visible light | 88% within 35 min, | [147] |
| 4 | Ag3PO4/Spherical | Sol-gel | phenol under visible light irradiation with a 1000 W Xenon lamp | 42% within 60 min, | [142] |
| 5 | Ag3PO4/Spherical | Precipitation method at room temperature, 500 W Xenon lamp | MO under visible light irradiation | 35%, within 15 min | [141] |
| 6 | Ag3PO4/Spherical | Precipitation method | MB, 5 W compact fluorescent lamp, visible light | 78%, within 70 min | [13] |
| 7 | Ag3PO4/Spherical | Sol-gel | 6 W/649 fluorescent lamp | Sulfamethoxazole, 100% after 15 min | [148] |
| 8 | Ag3PO4/Spherical | Coprecipitation method at 20 °C | RhB, visible light, 300 W Xenon lamp | 66% within 6 min | [149] |
| 9 | Ag3PO4/Spherical | Precipitation method | Congo red (CR) under visible light irradiation, 400 W metal halogen lamp | 96%, within 210 min | [150] |
| 10 | Ag3PO4/Spherical | Precipitation method | CR, visible light irradiation with a 350 W Xenon lamp | 91%, within 14 min | [151] |
| 11 | Ag3PO4/Spherical | Precipitation method | RhB and MO dyes in 10 mgL−1, WLED with a luminous flux (Φv) of 85 l m | 100% within 30 min | [152] |
| Tetrahedral | |||||
| 12 | Ag3PO4/Tetrahedral | Sol-gel | phenol under visible light irradiation with a 1000 W Xenon lamp | 70% within 60 min, | [142] |
| 13 | Ag3PO4/Tetrahedral | Sol-gel method | MO, visible light, 500 W Xenon lamp | 100% within 90 min | [143] |
| 14 | Ag3PO4/Tetrahedral | Precipitation method | MB, MO, RhB Visible light, 500 W Xenon lamp | 100% MB, 93% MO, 100% RhB within 6 min | [153] |
| 15 | Ag3PO4/Tetrahedral | Precipitation method | MB, under visible light irradiation, 300 W Xenon lamp | 88% within 12 min | [154] |
| 16 | Ag3PO4/Tetrahedral | Ion exchange in the ethanol-water solvent at room temperature | RhB, visible-light provided by a 250 W Xenon lamp | 100% within 24 min | [155] |
| Dodecahedral | |||||
| 17 | Ag3PO4/dodecahedral | Sol-gel | phenol under visible light irradiation with a 1000 W Xenon lamp | 100% within 60 min, | [142] |
| 18 | Ag3PO4/dodecahedral | Precipitation method | CR under visible light irradiation, 400 W metal halogen lamp | 85%, within 210 min | [150] |
| 19 | Ag3PO4/dodecahedral | Precipitation method | MB), RhB, and reactive orange (RO), visible light, TL-D/35 fluorescent tube (18 W, Philips) | 90% MB, 82% RhB, 22% RO within 60 min | [156] |
| 20 | Ag3PO4/dodecahedral | Sol-gel method | MO, visible light, 500 W Xenon lamp | 78% within 90 min | [143] |
| 21 | Ag3PO4/dodecahedral | Hydrothermally processed at 150 °C for 24 h | RhB, UV illumination, 15 W UV germicidal irradiation lamps | 99.55% within 120 min | [157] |
| 22 | Ag3PO4/dodecahedral | Precipitation method | MB, MO, RhB Visible light, 500 W Xenon lamp | 93% MB, 62% MO, 100% RhB within 18 min | [153] |
| Polyhedral | |||||
| 23 | Ag3PO4/Polyhedral | Hydrothermal at 120 °C for 12 h | RhB, visible light, 300 W Xenon lamp | 97.83% in 6 min | [149] |
| 24 | Ag3PO4/Polyhedral | Sol-gel | RhB, visible light, 350 W Xenon lamp | 100% in 4 min | [137] |
| 25 | Ag3PO4/Polyhedral | Precipitation method | MO, 300 W halogen lamp | 85% of MO within 15 min | [158] |
| 26 | Ag3PO4/Polyhedral | Sol-gel | phenol under visible light irradiation with a 1000 W Xenon lamp | 100% within 60 min | [142] |
| 27 | Ag3PO4/Polyhedral | Conventional ion exchange/precipitation method | phenol under visible light irradiation with a 35 W Xenon lamp | 100% within 120 min | [159] |
| Cubic | |||||
| 28 | Ag3PO4/Cubic | Ion exchange method | RhB, Sunlight | 100% within 10 min | [144] |
| 29 | Ag3PO4/Cubic | Sol-gel method | MO, visible light, 500 W Xenon lamp | 65% within 90 min | [143] |
| 30 | Ag3PO4/Cubic | Precipitation method | crystal violet (CV) and MB, MO and orange G (OG) with visible irradiation, 125 W high-pressure HG lamp | 93.0% of CV, within 30 min, 98% MB, MO 79.4%, OG 57.3% within 50 min | [160] |
| 31 | Ag3PO4/Cubic | Precipitation method | MB, MO, RhB Visible light, 500 W Xenon lamp | 91% MB, 32% MO, 78% RhB within 18 min | [153] |
| 32 | Ag3PO4/Cubic | Simple ion-exchange deposition method | RhB, Visible light, 300 W Xenon | 92% within 30 min | [161] |
| 33 | Ag3PO4/Cubic | Hydrothermal treatment at 160 °C for 3 h | MB and RhB, sunny light between 10 am to 2 pm in the summer | 81% within 90 min | [162] |
Variation in the synthesis methodology acts as an important aspect of the development of a particular morphology. Different morphologies of silver phosphate have been synthesized by various approaches, such as coprecipitation, sol-gel, hydrothermal, and ion exchange methods (Table 4). Nanospheres of silver phosphate were prepared mostly using the coprecipitation method [13,133,141,147,149,152,163]; however, the sol-gel route was also employed [142]. In less than 1 h, the complete mineralization was achieved using silver phosphate nanospheres catalytically. Tetrahedral morphology of silver phosphate was obtained using sol-gel method [143], coprecipitation [153] and ion exchange method [155]. The removal of aromatic dye contaminant was accomplished within an hour using tetrahedral nanocrystals. Dodecahedral morphology of silver phosphate was synthesized using sol-gel [142,143], coprecipitation [153] and hydrothermal route [157]. It takes about two hours for the complete mineralization of organic pollutants from an aqueous medium photocatalytically. Polyhedral morphology was obtained using the hydrothermal [149], sol-gel [142] and coprecipitation methods [158]. It takes less than an hour for the degradation of organic contaminants from an aqueous medium by employing the polyhedral morphology of silver phosphate. It has been reported [137] that Ag3PO4 with different morphology shows entirely different exposed facets. Hence it is important to compare the efficiency of photocatalysis by different morphologies such as spheres, tetrahedral, dodecahedral, polyhedral and cubic morphologies of Ag3PO4 (Figure 8).
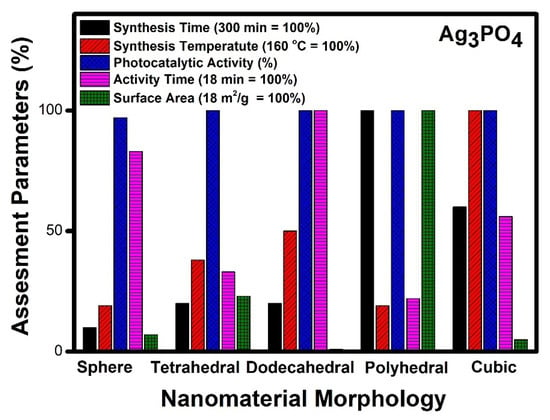
Figure 8.
Comparative study of different morphologies of Ag3PO4 based on various assessment parameters.
Figure 8 denotes a comparative account of different morphologies of silver phosphate. It can be seen that polyhedral morphology takes five hours for synthesis, whereas nanospheres, tetrahedral, and dodecahedral morphologies of silver phosphate can be prepared within an hour. The temperature of synthesis required by cubic morphology was reported to be the highest (160 °C), while other morphologies show moderate values. The photocatalytic performance of all the reported morphologies of silver phosphate was good. In the case of time required for photocatalytic degradation of organic compounds, dodecahedral and nanospheres shows the highest value, whereas tetrahedral, polyhedral and cubic morphologies denote moderate value. Surface area values of polyhedral morphology are the highest among the reported, while dodecahedral and nanospheres had the lowest values among reported.
Our analysis indicates that polyhedral morphology exhibits the highest activity compared to any other morphology. The photocatalytic activity follows the order of polyhedral > tetrahedral > cubic > dodecahedral and sphere. It can be seen from Figure 8 that polyhedral morphology shows 100% activity within 4 min and probably due to its higher surface area (18 m2/g). The polyhedral morphology was obtained using a simple eco-friendly sol-gel method at room temperature [142]. Other morphologies also show good photocatalytic activity, but the required time is high, and this may be due to low surface area and poor accessibility of reactants to reach the active sites.
2.5. Zinc Titanate (ZnTiO3) Nanostructures
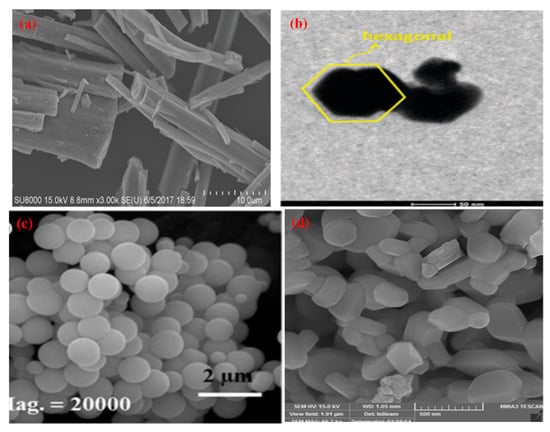
Figure 9.
SEM & TEM micrographs of different morphologies of ZnTiO3 (a) Nanorod (Reprinted with permission from [164]. Copyright 2018 Royal Society of Chemistry), (b) Hexagonal (Reprinted with permission from [165]. Copyright 2014 Royal Society of Chemistry), (c) Nanosphere (Reprinted with permission from [166]. Copyright 2016 Wiley), (d) Particle (Reprinted with permission from [167]. Copyright 2016 Elsevier).
Metal titanates-based dielectric materials with the general formula ATiO3 (A denotes metal) are known for different values of permittivity ranging from 7 to more than 5000 under temperature variations. ZnTiO3 system (hexagonal ilmenite) has been studied earlier mainly due to its striking features for practical applications in microwave resonators, mobile phones, and satellite communication systems. However, the above studies need well-sintered materials with large particle sizes. Various morphologies of ZnTiO3 NMs have been developed (Figure 9) due to their special features for their application as photocatalysts with a lower band gap (3.08 eV) than TiO2 (3.2 eV). Three compounds are known in the ZnO-TiO2 system such as Zn2TiO4 (cubic spinel), ZnTiO3 (hexagonal ilmenite) and Zn2Ti3O8 (cubic defect spinel). The thermodynamically stable phase for ZnTiO3 at low temperatures is a cubic phase which changes to a hexagonal phase at high temperatures (800 °C) and also decomposes into Zn2TiO4 and TiO2 at a temperature of 900 °C. ZnTiO3 decomposes at high temperatures since Zn volatilizes and creates non-stoichiometry. Thus, it is challenging to synthesize pure ZnTiO3.
ZnTiO3 has two types of crystal structures, i.e., cubic and hexagonal, out of which this hexagonal structure shows the best photocatalytic performance. The pure form of hexagonal ZnTiO3 can be obtained by using high-temperature solid-state reactions as well as low-temperature methods such as sol-gel, hydrothermal, modified alcoholysis, carbothermal and molten salt methods. Hexagonal ZnTiO3 is a UV-active photocatalyst with a band gap of 3.08 eV. The hydrothermal method was used in the synthesis of zinc titanate [168]. Alternately pure zinc titanate can also be prepared by the conventional solid-state method at high temperatures. Other methods, such as mechanochemical activation, molten salt synthesis, and microbead milling, were also applied in the synthesis of ZnTiO3 [169], but they resulted in irregular size, morphology, and grain size. Sol-gel technique has been preferred mostly by researchers in the synthesis of zinc titanate due to its low cost, high yield, small processing time, homogeneity and high purity of the end product. It can be useful in making thin films, membranes and multicomponent oxide materials. Generally, metal alkoxides are used as precursors in the sol-gel process. Further, the metal alkoxide is subjected to hydrolysis and polycondensation to obtain the desired combinations. The resultant material obtained by the sol-gel process has a large specific surface area and pore volume, which is the essential requirement of the material to be used as a catalyst.

Table 5.
Details of different morphologies of nanostructured ZnTiO3.
Table 5.
Details of different morphologies of nanostructured ZnTiO3.
| S. NO. | Material & Morphology | Method | Application | Performance | Reference |
|---|---|---|---|---|---|
| Particles | |||||
| 1 | ZnTiO3/Particle | Sol-gel | Phenol, under a 100 W incandescent visible lamp | 100% in 300 min | [170] |
| 2 | ZnTiO3/Particle | Hydrothermal, at 180 °C for 8 h | MB, under a 350 W Xenon visible lamp | 29.7% in 120 min | [168] |
| 3 | ZnTiO3/Particle | Sol-gel | 4-chlorophenol, under natural sunlight | 67% in 45 min | [171] |
| 4 | ZnTiO3/Particle | Sonochemical method | Rh), under a 70 W LED visible lamp | 36% in 150 min | [172] |
| 5 | ZnTiO3/Particle | Sol-gel | MO, under a 400 W UV lamp | 70% in 60 min | [167] |
| 6 | ZnTiO3/Particle | Solvothermal, at 180 °C 24 h | RhB & MO, under a 400 W halide visible lamp | 17% of Rh B, 3% of MO in 90 min | [157] |
| Rod | |||||
| 7 | ZnTiO3/Rod | Microwave | RhB, under a 150 W Xenon visible lamp | 93% in 180 min | [173] |
| 8 | ZnTiO3/Rod | Sol-gel | RhB, under a 50 W high-pressure Hg lamp | 97% in 70 min | [174] |
| 9 | ZnTiO3/Rod | Precipitation method | RhB, under sunlight | 71% in 60 min | [164] |
| 10 | ZnTiO3/Rod | Hydrothermal, 120 °C for 24 h | MO, under a 500 W Xenon lamp | 99.3% in 20 min | [175] |
| 11 | ZnTiO3/Rod | Sol-gel | RhB & crystal violet, under sunlight | 98% of CV in 60 min & 77% of RhB in 90 min | [166] |
| Spherical | |||||
| 12 | ZnTiO3/Spherical | Sol-gel | Methyl violet, under sunlight | 97% in 120 min | [169] |
| 13 | ZnTiO3/Spherical | Sol-gel | H2 production, under a 125 W high-pressure Hg UV lamp | 110 µmol/h, 60% in 3600 min | [176] |
| 14 | ZnTiO3/Spherical | Sol-gel | MB, under a 150 W UV lamp | 33% in 3600 min | [177] |
| 15 | ZnTiO3/Spherical | Sol-gel | Norfloxacin (NOR) and MO, under a 300 W Xenon visible lamp | 95% of NOR & 46% of MB in 60 min | [178] |
| 16 | ZnTiO3/Spherical | Sol-gel | MB, under sunlight | 76% in 60 min | [179] |
| Hexagonal | |||||
| 17 | ZnTiO3/Hexagonal | Sol-gel | p-nitrophenol, under sonocatalytic activity | 74.8% in 180 min | [180] |
| 18 | ZnTiO3/Hexagonal | Sol-gel | RhB, under sunlight | 35% in 180 min | [165] |
Varying morphologies of zinc titanates were synthesized using several low-temperature methods. It can be observed from Table 5 that the method of synthesis plays an essential role in the formation of different morphologies such as nanorods, nanoparticles, nanospheres and hexagonal nanostructures. Zinc titanate nanoparticles were synthesized by using a sol-gel approach [167,170,171], hydrothermal/solvothermal process [168,181] sonochemical method [172]. The temperature of synthesis of nanoparticles was reported as 180 °C in general for the hydrothermal method, whereas sol-gel and sonochemical methods require a lower temperature. Zinc titanate nanoparticles normally take an hour to degrade 70% of the organic pollutants. The nanorods were obtained using sol-gel [166,174], microwave technique [173], coprecipitation method [164], and the hydrothermal process [173]. The hydrothermal process consumes more energy as compared to sol-gel and coprecipitation techniques. The photocatalytic performance shows that the nanorod structure of zinc titanate requires 20 min [182] for the complete mineralization of organic pollutants. Zinc titanate nanospheres have been synthesized using the sol-gel technique in general. This morphology, as reported by Kong et al. [169], takes 2 h for the complete mineralization of aromatic compounds photocatalytically. In the case of hexagonal nanostructures synthesis by the sol-gel technique, [165,180] is mostly preferred. As per the available report, the time taken for the degradation of aromatic compounds is more than three hours by zinc titanate hexagonal nanostructures. Thus, considering the ease of synthesis by the sol-gel method and the efficiency of the photocatalytic performance of zinc titanate nanorods, it should be the most preferred morphology.
To analyze the different reported morphologies of zinc titanate and to decide the optimal one which is the best for the photocatalytic application, we have chosen nanorods, hexagonal nanostructures, nanospheres and nanoparticles for our assessment (Figure 10). The standard parameters such as surface area, time of synthesis, photocatalytic and catalytic activity time and synthesis temperature have been considered for the assessment.
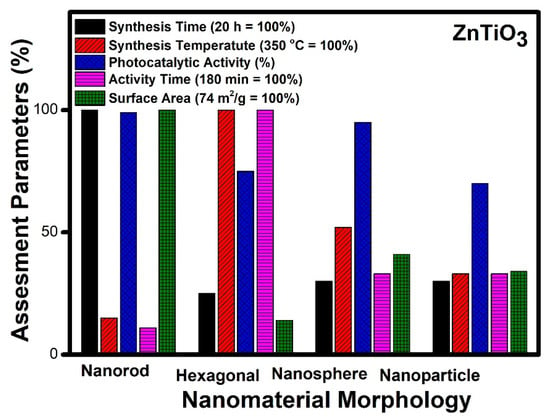
Figure 10.
Comparative study of different morphologies of ZnTiO3 based on various assessment parameters.
Figure 10 reveals an assessment of various morphologies of zinc titanate. The nanorod morphology requires maximum time for synthesis (20 h), while other morphologies such as nanospheres, hexagonal and nanoparticles can be prepared in an average of five hours. Hexagonal morphology needs the highest temperature for synthesis (350 °C), whereas nanorods need the lowest values, and nanospheres show the highest values, whereas hexagonal and nanoparticles show a 30% lesser value, comparatively. The time taken for photocatalytic degradation is highest (3 h) for hexagonal morphology; however other morphologies need an hour for complete degradation. The value of the surface area is the highest for nanorod morphology, while nanoparticles and nanospheres show moderate value, and hexagonal morphology denotes a lower surface area, comparatively.
It can be seen from Figure 10 that the nanorod [182] morphology possesses the highest surface area (74 m2/g), whereas hexagonal morphology has the lowest surface area (10 m2/g) amongst the reported structures [180]. Complete mineralization of the organic pollutants was achieved photo-catalytically within 20 min in the case of nanorod morphology, while various other morphologies require more than an hour to achieve the same target. The time of synthesis of the nanorod morphology was 24 h which is the highest amongst the other reported morphologies, whereas nanospheres can be prepared within one hour by using a similar hydrothermal technique. It is the lowest reported time of synthesis compared to other morphologies mentioned. Thus, by taking into account the variation in the assessment parameter reported, it can be seen that nanorod morphology shows the best result and can be considered the best photocatalytic material in the case of zinc titanate. The order of preference based on efficiencies is nanorods [182], nanospheres [178], nanoparticles [167] and lastly, hexagonal nanostructures [180] of zinc titanate.
Figure 11 shows the comparative analysis of photocatalytic performance and mineralization time of TiO2, ZnO, CdS, Ag3PO4 and ZnTiO3 nanomaterials at a glance. It can be seen that silver phosphate shows the best photocatalytic activity amongst the studied nanomaterials based on the minimum time taken (less than ten minutes) for the complete mineralization of organic compounds. Though the band gap of silver phosphate (2.28 eV) and cadmium sulphide (2.3 eV) are similar, their performances are poles apart, and the reason may be the morphology formed by these nanomaterials. Further, based on an overall comparison with the other photocatalyst, the order of preference can be assigned as silver phosphate (Ag3PO4), zinc oxide (ZnO), zinc titanate (ZnTiO3), titanium dioxide (TiO2) and cadmium sulphide (CdS).
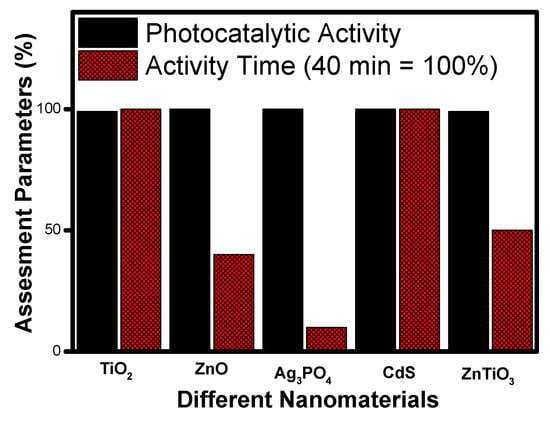
Figure 11.
Comparative photocatalytic activity among different photocatalysts based on degradation time.

Table 6.
Mechanism of photocatalysis for TiO2, ZnO, Ag3PO4, CdS and ZnTiO3 photocatalysts nanomaterials photocatalysts.
Table 6.
Mechanism of photocatalysis for TiO2, ZnO, Ag3PO4, CdS and ZnTiO3 photocatalysts nanomaterials photocatalysts.
| S. No. | Materials | Photocatalysis Mechanism | Ref. |
|---|---|---|---|
| 1. | TiO2 | 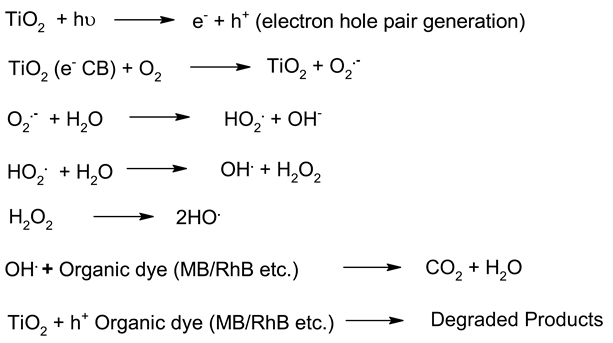 | [183] |
| 2. | ZnO | 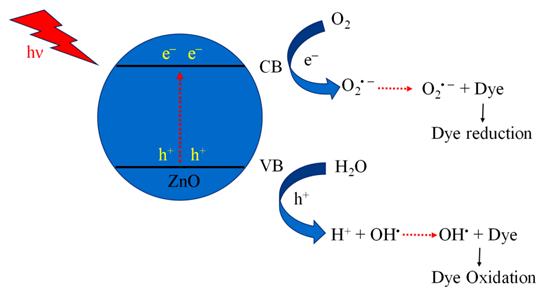 | [184] |
| 3. | CdS | 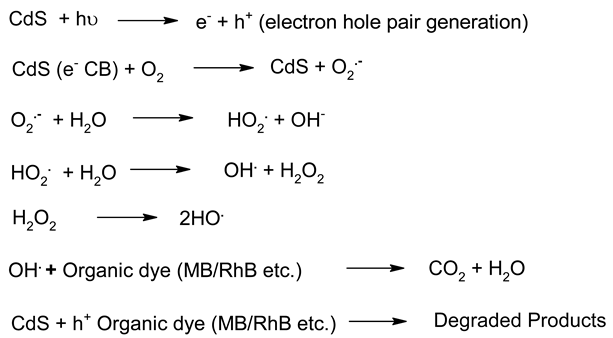 | [185] |
| 4. | Ag3PO4 | 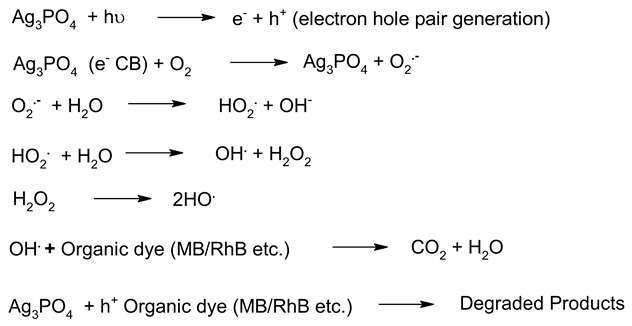 | [186] |
| 5. | ZnTiO3 | 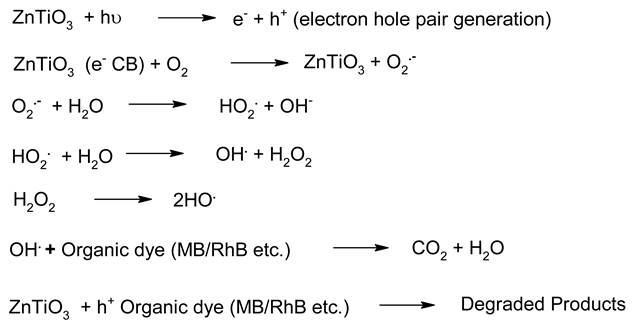 | [187] |

Table 7.
Important characteristics of photocatalyst TiO2, ZnO, Ag3PO4, CdS and ZnTiO3.
Table 7.
Important characteristics of photocatalyst TiO2, ZnO, Ag3PO4, CdS and ZnTiO3.
| S. No. | Photocatalytic Materials | Band Gap Energy (Eg) eV | Photocatalytic Performances | Ref. |
|---|---|---|---|---|
| 1. | TiO2 | Eg ~ 3.1 | The synthesised TiO2 nanotubes possess better photocatalytic activity than the as-prepared counterparts because of the larger surface area and good crystallinity. The emission stability of the catalyst also validates that TiO2 nanotubes could find potential applications in cold-cathode-based electronics. Literature shows that the decoration of TiO2 nanotubes by noble metal nanoparticles (such as Au, Ag, and Pt) also enhances its photocatalytic activity. | [45,188,189] |
| 2. | ZnO | Eg ~ 3.35 | Qu et al. Synthesised various shapes and morphology of ZnO nanomaterials using different ultrasonic processes. The study revealed that ZnO nanoflower morphology shows excellent photocatalytic activity. | [97,190] |
| 3. | Ag3PO4 | Eg ~ 2.45 | Morales et al. synthesised silver phosphate microcrystals with polyhedral morphologies and its higher surface area played an important role in higher photocatalytic activity.Geng et al. successfully prepared the polyhedral morphology of the Ag3PO4 microcrystal structure. The photocatalytic activity study confirmed its excellent photocatalytic ability. | [191,192] |
| 4. | CdS | Eg ~ 2.4 | Ganesh et al. synthesised CdS nanoflowers. The nano flowers of the CdS materials showed better photocatalytic activity in visible light. Wang et al. recently studied Ti3C2 MXene@CdS based nanoflowers composites heterostructures. It shows steady photoluminescence intensity and a longer fluorescence lifetime. | [100,193] |
| 5. | ZnTiO3 | Eg ~ 2.9 (indirect) 3.59 (direct) | Dutta et al. reported the Ag-doped ZnTiO3 nanorods. The photocatalytic results show improved photocatalytic activity. Chuaicham et al. recently studied ZnTiO3 mixed metal oxide. The higher photocatalytic activity was observed for phenol photodegradation. | [173,194] |

Table 8.
Photocatalytic Stability and biocompatibility of different NP photocatalysts.
Table 8.
Photocatalytic Stability and biocompatibility of different NP photocatalysts.
| Type of NPs | Stability and Recyclability | Biocompatibility | Ref. |
|---|---|---|---|
| TiO2 | The photostability of TiO2 for phenol degradation in four cycles remained constant. | Biocompatible, supports osteoblast-like cell formation, and can be used in biomedical applications. | [195,196,197] |
| ZnO | RhB aqueous solution for five cycles, good recyclability. | high bactericidal efficacy along with good cytocompatibility | [64,76,98,198] |
| Ag3PO4 | Photocatalytic efficiency remained consistently high after four cycles [11]. | Spectacular biocompatibility and good immunosensor sensitivity, low toxicity. | [137,199] |
| CdS | The catalytic activity of the photocatalyst remained constant. | Environment-friendly and economical photocatalyst | [200,201] |
| ZnTiO3 | The efficacy of the photocatalyst remained constant. | Excellent antitumor ability and good biocompatibility | [202,203,204] |
2.6. Challenges during the Application of NPs for Photochemical Reactions
Irrespective of the worldwide interest in the use of NPs in photocatalysis for the conversion of visible light energy for chemical conversions, this technology has not been successful in its commercialisation and large-scale applications. The long-term exposure of photocatalytic NPs under constant irradiation may lead to changes in the morphology of the material, which may affect the performance of the photocatalysts. To enhance the efficiency of the photocatalysts, in the majority of cases, the photocatalysis process requires a constant light source, which can be enabled through artificial setups. It has been observed that the supported NPs perform better than the bare ones during photocatalysis [205]. The challenges in the area of photocatalysis can only be addressed through research and a sustainable approach. There is a need for an effective implementation strategy to be worked for MNPs’ visible light photocatalysis. The use of scavengers in photocatalysis may help in improving the efficiency of the photocatalysts. Effective protocols are required for the large-scale synthesis of MNPs, and their further disposal after use may encourage sustainable practices in this area of work. The noteworthy research on greener processes for the synthesis of MNPs can be a significant step in the large-scale implementation of photocatalysis processes for environmental mitigation [206,207]. The typical mechanistic pathway of the photochemical reactions (Table 6) reveals the role of different nanomaterial photocatalysts during dye degradation. The generation of free radicals by the photochemical effect initiates dye degradation, and it depends on the band gap of the nanomaterial photocatalyst. It can be seen from Table 7 that the recyclability and band gap of the different nanomaterial photocatalysts should be considered for the selection of the catalyst for photochemical reactions. Reusability, photochemical stability and biocompatibility (Table 8) are the essential characteristics of sustainable photochemical catalysts.
In photocatalysis, the efficacy of the catalyst depends on its ability to lower the activation energy by offering an efficient reaction pathway. The metal oxide-based photocatalysts work on these characteristics for the degradation of organic contaminants [208]. The environmentally benign photocatalyst materials with low cost and availability of other chemicals such as solvents, dopants etc., and their environmental impact is a challenging task. But the phenomenal growth in this field of work can make available smart and sustainable materials for environmental photocatalysis [209].
2.7. Future Direction for the Application of Nanomaterial Photocatalyst
Presently, nanotechnology manipulates and creates matter to fabricate materials on the nanometre scale. Nanomaterials are used in the interdisciplinary field, and they have societal implications for a wide range of scientific and engineering disciplines. In the past few decades, nanotechnology has been applied in various sectors like; environment, materials science, energy, electronics, agriculture, healthcare, biotechnology, and information technology. Certain nanomaterials have the excellent potential to revolutionize wastewater treatment through photocatalysis and other techniques. Because of their remarkable characteristics, such as effective adsorption and photocatalytic properties, nanomaterial oxides are being used commercially for wastewater treatment extensively. For technology related to clean environment, and health care, like drug delivery and cancer therapy, nanotechnology has commercially provided scope for existing firms to upgrade their products and services. Several oxides, sulfides, tantalates, phosphates and niobates are used commercially for multiple cures [210,211]. Silver phosphate and cadmium sulfide are used commercially as photocatalysts in novel photoreactors for better catalytic activity [212].
3. Conclusions
Effective morphology of the nanomaterial is an essential requirement in photocatalysis for application in dye degradation or conversion of solar energy into chemical energy. The morphology and size of nanomaterial not only reduce the band gap of the photocatalyst but also enhances its photocatalytic performance in the degradation of complex organic contaminants. The activity of photocatalysts solely relies upon band gap reduction and effective utilisation of photons into photocatalytic conversions when subjected to the required wavelength. Morphology played an important role in the band gap reduction of nanomaterials. Various morphologies of a particular nanomaterial show different photocatalytic performances.
- (i)
- The analysis of literature data indicates that titanium oxide (TiO2) nanotube morphology emerged as the best material. It has excellent photocatalytic performance and takes minimum time for the degradation of contaminants. Due to its high surface area, the active centres are easily accessible during photocatalysis;
- (ii)
- Similar criteria, when applied to other nanomaterials, show zinc oxide (ZnO) and cadmium sulphide (CdS) nanomaterial having nanoflower morphology as the best among other reported;
- (iii)
- Nanorod morphology appeared as the best morphology for zinc titanate (ZnTiO3) for photocatalytic applications;
- (iv)
- Silver phosphate (Ag3PO4) shows polyhedral morphology as the best performer on all given counts and appears to be the best morphology for a photocatalyst.
Thus, based on its impact on photocatalytic performance, the morphology of the nanomaterials should be considered an essential aspect in the selection of a photocatalyst. We are positive that the analysis applied in this research article can be useful in the study of other photocatalytic systems and bridge the gap between academia and industry in terms of the selection of photocatalysts for industrial applications.
Author Contributions
Conceptualization, A.K.G. and G.B.K.; methodology, A.K.G. and G.B.K.; software, G.B.K. and W.R.; validation, A.K.G., G.B.K. and W.R.; formal analysis, A.K.G. and G.B.K.; investigation, A.K.G., G.B.K. and W.R.; resources, A.K.G. and G.B.K.; data curation, G.B.K. and W.R.; writing—original draft preparation, A.K.G., G.B.K. and W.R.; writing—review and editing, G.B.K., S.K. and P.Y.; visualization, G.B.K., S.K. and P.Y.; supervision, A.K.G. and G.B.K.; project administration, A.K.G. and G.B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any external dataset link.
Acknowledgments
The author GBK is thankful to UGC, New Delhi, India, for postdoctoral research fellowship No PDFSS-2017-18-MAH-7304.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Inshakova, E.; Inshakov, O. World Market for Nanomaterials: Structure and Trends. MATEC Web Conf. 2017, 129, 02013. [Google Scholar] [CrossRef]
- Yashwant, S. Nanomaterials Market-Global Opportunity Analysis and Industry Forecast 2014–2022. Allied Market Research report No. A01419. 2016. Available online: https://www.alliedmarketresearch.com/europe-nanomaterials-market (accessed on 1 October 2022).
- Zhao, C.; Xi, M.; Huo, J.; He, C. B-Doped 2D-InSe as a Bifunctional Catalyst for CO2/CH4separation under the Regulation of an External Electric Field. Phys. Chem. Chem. Phys. 2021, 23, 23219–23224. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.R.; Deng, L.; Liu, G.C.; Wen, L.; Wang, J.G.; Huang, K.B.; Tang, H.T.; Pan, Y.M. Porous Organic Polymer-Derived Nanopalladium Catalysts for Chemoselective Synthesis of Antitumor Benzofuro[2,3-b]Pyrazine from 2-Bromophenol and Isonitriles. Org. Lett. 2019, 21, 4929–4932. [Google Scholar] [CrossRef] [PubMed]
- Nikazar, S.; Barani, M.; Rahdar, A.; Zoghi, M.; Kyzas, G. Photo-and Magnetothermally Responsive Nanomaterials for Therapy, Controlled Drug Delivery and Imaging Applications. ChemistrySelect 2020, 5, 12590–12609. [Google Scholar] [CrossRef]
- Pokropivny, V.V.; Skorokhod, V.V. Classification of Nanostructures by Dimensionality and Concept of Surface Forms Engineering in Nanomaterial Science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Ji, Y.; Ma, X.; Xu, J.; Que, D. Selenium Nanotubes Synthesized by a Novel Solution Phase Approach. J. Phys. Chem. B 2004, 108, 1179–1182. [Google Scholar] [CrossRef]
- Lao, J.Y.; Wen, J.G.; Ren, Z.F. Hierarchical ZnO Nanostructures. Nano Lett. 2002, 2, 1287–1291. [Google Scholar] [CrossRef]
- Ye, C.; Meng, G.; Wang, Y.; Jiang, Z.; Zhang, L. On the Growth of CdS Nanowires by the Evaporation of CdS Nanopowders. J. Phys. Chem. B 2002, 106, 10338–10341. [Google Scholar] [CrossRef]
- Ghoderao, K.P.; Jamble, S.N.; Kale, R.B. PEG—Assisted Morphological Transformation of 3D Flower-like ZnO to 1D Micro-/Nanorods and Nanoparticles for Enhanced Photocatalytic Activity. Mater. Res. Express 2017, 4, 105009. [Google Scholar] [CrossRef][Green Version]
- Nandiyanto, A.B.D.; Iskandar, F.; Okuyama, K. Macroporous Anatase Titania Particle: Aerosol Self-Assembly Fabrication with Photocatalytic Performance. Chem. Eng. J. 2009, 152, 293–296. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Yang, Z.M.; Liu, Y.Y.; Xu, L.; Huang, G.F.; Huang, W.Q. Facile Shape-Controllable Synthesis of Ag3PO4 Photocatalysts. Mater. Lett. 2014, 133, 139–142. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-Based Hybrid Photocatalyst with Broad Spectrum Photocatalytic Properties under UV, Visible, and near-Infrared Irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Hu, H.; Jiao, Z.; Yu, H.; Lu, G.; Ye, J. Two-Dimensional Dendritic Ag3PO4 Nanostructures and Their Photocatalytic Properties. Phys. Chem. Chem. Phys. 2012, 14, 14486–14488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 Nanobelts Heterostructure with Enhanced Ultraviolet and Visible Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Miao, J.; Tao, H.B.; Hung, S.F.; Wang, H.Y.; Yang, H.B.; Chen, J.; Chen, R.; Liu, B. One-Dimensional Hybrid Nanostructures for Heterogeneous Photocatalysis and Photoelectrocatalysis. Small 2015, 11, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Y.; Li, Y.; Jia, D.; Xie, J. Directed Synthesis of TiO2 Nanorods and Their Photocatalytic Activity. Ceram. Int. 2014, 40, 11735–11742. [Google Scholar] [CrossRef]
- Tao, Y.G.; Xu, Y.Q.; Pan, J.; Gu, H.; Qin, C.Y.; Zhou, P. Glycine Assisted Synthesis of Flower-like TiO2 Hierarchical Spheres and Its Application in Photocatalysis. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 1664–1671. [Google Scholar] [CrossRef]
- Lamberti, A.; Chiodoni, A.; Shahzad, N.; Bianco, S.; Quaglio, M.; Pirri, C.F. Ultrafast Room-Temperature Crystallization of TiO2 Nanotubes Exploiting Water-Vapor Treatment. Sci. Rep. 2015, 5, 7808. [Google Scholar] [CrossRef]
- Tian, G.; Chen, Y.; Zhou, W.; Pan, K.; Tian, C.; Huang, X.R.; Fu, H. 3D Hierarchical Flower-like TiO2 Nanostructure: Morphology Control and Its Photocatalytic Property. CrystEngComm 2011, 13, 2994–3000. [Google Scholar] [CrossRef]
- Zhao, Z.; Kou, T.; Zhang, L.; Zhai, S.; Wang, W.; Wang, Y. Dealloying Induced N-Doping in Spindle-like Porous Rutile TiO2 for Enhanced Visible Light Photocatalytic Activity. Corros. Sci. 2018, 137, 204–211. [Google Scholar] [CrossRef]
- Zhao, Q.E.; Wen, W.; Xia, Y.; Wu, J.M. Photocatalytic Activity of TiO2 Nanorods, Nanowires and Nanoflowers Filled with TiO2 Nanoparticles. Thin Solid Films 2018, 648, 103–107. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Molecular Photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A Review on Self-Cleaning Coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Orilall, M.C.; Wiesner, U. Block Copolymer Based Composition and Morphology Control in Nanostructured Hybrid Materials for Energy Conversion and Storage: Solar Cells, Batteries, and Fuel Cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef]
- Ghezelbash, A.; Koo, B.; Korgel, B.A. Self-Assembled Stripe Patterns of CdS Nanorods. Nano Lett. 2006, 6, 1832–1836. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Liu, J.; Zhai, Z.; Yang, Z.; Xia, J.; Deng, S.; Qu, X.; Zhang, H.; Wu, D.; et al. Mo-Modified Band Structure and Enhanced Photocatalytic Properties of Tin Oxide Quantum Dots for Visible-Light Driven Degradation of Antibiotic Contaminants. J. Environ. Chem. Eng. 2021, 10, 107091. [Google Scholar] [CrossRef]
- Sarkar, S.; Biswas, S.; Sarkar, M.; Ray, T.; Sharma, A.; Won, S.O.; Saha, A.; De, S. Ag Deposition Effects on the Photocatalytic Activity of Nanoparticulate TiO2—Comparison of Gamma Irradiation and UV Irradiation Methods. Nano-Struct. Nano-Objects 2018, 16, 134–143. [Google Scholar] [CrossRef]
- Liao, J.; Shi, L.; Yuan, S.; Zhao, Y.; Fang, J. Solvothermal Synthesis of TiO2 Nanocrystal Colloids from Peroxotitanate Complex Solution and Their Photocatalytic Activities. J. Phys. Chem. C 2009, 113, 18778–18783. [Google Scholar] [CrossRef]
- Zheng, X.; Kuang, Q.; Yan, K.; Qiu, Y.; Qiu, J.; Yang, S. Mesoporous TiO2 Single Crystals: Facile Shape-, Size-, and Phase-Controlled Growth and Efficient Photocatalytic Performance. ACS Appl. Mater. Interfaces 2013, 5, 11249–11257. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Park, Y.; Sohn, Y.; Leung, K.T.; Pradhan, D. Green Synthesis of Anatase TiO2 Nanocrystals with Diverse Shapes and Their Exposed Facets-Dependent Photoredox Activity. ACS Appl. Mater. Interfaces 2014, 6, 16498–16507. [Google Scholar] [CrossRef] [PubMed]
- Degabriel, T.; Colaço, E.; Domingos, R.F.; El Kirat, K.; Brouri, D.; Casale, S.; Landoulsi, J.; Spadavecchia, J. Factors impacting the aggregation/agglomeration and photocatalytic activity of highly crystalline spheroid- and rod-shaped TiO2 nanoparticles in aqueous solutions. Phys. Chem. Chem. Phys. 2018, 20, 12898–12907. [Google Scholar] [CrossRef] [PubMed]
- Santhi, K.; Navaneethan, M.; Harish, S.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of TiO2 nanorods by hydrothermal method with different pH conditions and their photocatalytic activity. Appl. Surf. Sci. 2019, 500, 144058. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C.; Ni, Y.; Su, M.; Xu, Z. Hydrothermal synthesis and enhanced photocatalytic activity of flower-like TiO2 on carbon nanotubes. Mater. Lett. 2012, 79, 11–13. [Google Scholar] [CrossRef]
- Song, H.; Chen, T.; Sun, Y.-L.; Zhang, X.-Q.; Jia, X.-H. Controlled synthesis of porous flower-like TiO2 nanostructure with enhanced photocatalytic activity. Ceram. Int. 2014, 40, 11015–11022. [Google Scholar] [CrossRef]
- Ge, M.; Li, J.W.; Liu, L.; Zhou, Z. Template-Free Synthesis and Photocatalytic Application of Rutile TiO2 Hierarchical Nanostructures. Ind. Eng. Chem. Res. 2011, 50, 6681–6687. [Google Scholar] [CrossRef]
- Liu, M.; Lu, W.-M.; Zhao, L.; Zhou, C.-L.; Li, H.-L.; Wang, W.-J. Fabrication and photocatalytical properties of flower-like TiO2 nanostructures. Trans. Nonferrous Met. Soc. China 2010, 20, 2299–2302. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Z.; Zhang, C.; Zhang, P.; Guo, R.; Gou, H.; Hu, R.; Wei, X. Morphology-controllable 3D flower-like TiO2 for UV shielding application. J. Ind. Eng. Chem. 2015, 32, 172–177. [Google Scholar] [CrossRef]
- Shao, C.; Zhou, G.; Li, Z.; Wu, Y.; Xu, D.; Sun, B. Fabrication of large-diameter tube-like mesoporous TiO2 via homogeneous precipitation and photocatalytic decomposition of papermaking wastewater. Chem. Eng. J. 2013, 230, 227–235. [Google Scholar] [CrossRef]
- Huang, F.; Guo, Y.; Wang, S.; Zhang, S.; Cui, M. Solgel-hydrothermal synthesis of Tb/Tourmaline/TiO2 nano tubes and enhanced photocatalytic activity. Solid State Sci. 2017, 64, 62–68. [Google Scholar] [CrossRef]
- He, G.; Zhang, J.; Hu, Y.; Bai, Z.; Wei, C. Dual-template synthesis of mesoporous TiO2 nanotubes with structure-enhanced functional photocatalytic performance. Appl. Catal. B Environ. 2019, 250, 301–312. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Chowdhury, S.; Sufian, S.; Omar, A.A. Enhanced photocatalytic activity of Orange II in aqueous solution using solvent-based TiO2 nanotubes: Kinetic, equilibrium and thermodynamic studies. J. Clean. Prod. 2018, 203, 848–859. [Google Scholar] [CrossRef]
- Xu, X.; Tang, C.; Zeng, H.; Zhai, T.; Zhang, S.; Zhao, H.; Bando, Y.; Golberg, D. Structural Transformation, Photocatalytic, and Field-Emission Properties of Ridged TiO2 Nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Pancielejko, A.; Mazierski, P.; Lisowski, W.; Zaleska-Medynska, A.; Kosek, K.; Łuczak, J. Facile Formation of Self-Organized TiO2 Nanotubes in Electrolyte Containing Ionic Liquid-Ethylammonium Nitrate and Their Remarkable Photocatalytic Properties. ACS Sustain. Chem. Eng. 2018, 6, 14510–14522. [Google Scholar] [CrossRef]
- Manjunath, K.; Yadav, L.S.R.; Jayalakshmi, T.; Reddy, V.; Rajanaika, H.; Nagaraju, G. Ionic liquid assisted hydrothermal synthesis of TiO2 nanoparticles: Photocatalytic and antibacterial activity. J. Mater. Res. Technol. 2018, 7, 7–13. [Google Scholar] [CrossRef]
- Mohammadi, N.N.; Pajootan, E.; Bahrami, H.; Arami, M. Magnetization of TiO2 nanofibrous spheres by one-step ultrasonic-assisted electrochemical technique. J. Mol. Liq. 2018, 265, 251–259. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, Z.; Xie, L.; Fang, Z.; Feng, W.; Huang, M.; Liu, P. Synthesis of single-crystal-like TiO2 hierarchical spheres with exposed {101} and {111} facets via lysine-inspired method. Appl. Surf. Sci. 2015, 353, 714–722. [Google Scholar] [CrossRef]
- An, T.; Chen, J.; Nie, X.; Li, G.; Zhang, H.; Liu, X.; Zhao, H. Synthesis of Carbon Nanotube-Anatase TiO2 Sub-Micrometer-Sized Sphere Composite Photocatalyst for Synergistic Degradation of Gaseous Styrene. ACS Appl. Mater. Interfaces 2012, 4, 5988–5996. [Google Scholar] [CrossRef]
- Sun, D.; Liu, J.; Li, J.; Feng, Z.; He, L.; Zhao, B.; Wang, T.; Li, R.; Yin, S.; Sato, T. Solvothermal synthesis of spindle-like WO3–TiO2 particles with enhanced photocatalytic activity. Mater. Res. Bull. 2014, 53, 163–168. [Google Scholar] [CrossRef]
- Tang, H.; Chang, S.; Jiang, L.; Tang, G.; Liang, W. Novel spindle-shaped nanoporous TiO2 coupled graphitic g-C3N4 nanosheets with enhanced visible-light photocatalytic activity. Ceram. Int. 2016, 42, 18443–18452. [Google Scholar] [CrossRef]
- Das, D.; Shivhare, A.; Saha, S.; Ganguli, A.K. Room temperature synthesis of mesoporous TiO2 nanostructures with high photocatalytic efficiency. Mater. Res. Bull. 2012, 47, 3780–3785. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, J.; Shang, C.; Ye, R.; Wang, Y. Dealloying-driven synthesis of sea-urchin like titanate nanowires and hierarchically porous anatase TiO2 nanospindles with enhanced photocatalytic performance. Corros. Sci. 2015, 98, 651–660. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Zhang, T.; Sun, D.D.; Ng, W. Hierarchical TiO2/CdS “spindle-like” composite with high photodegradation and antibacterial capability under visible light irradiation. J. Hazard. Mater. 2012, 229, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Haque, M.; Muneer, M.; Fleisch, M.; Hakki, A.; Bahnemann, D.B.D. Photocatalytic degradation of different chromophoric dyes in aqueous phase using La and Mo doped TiO2 hybrid carbon spheres. J. Alloys Compd. 2015, 632, 837–844. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2nanoparticles under visible-light irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Allende-González, P.; Laguna-Bercero, M.Á.; Barrientos, L.; Valenzuela, M.L.; Díaz, C. Solid State Tuning of TiO2 Morphology, Crystal Phase, and Size through Metal Macromolecular Complexes and Its Significance in the Photocatalytic Response. ACS Appl. Energy Mater. 2018, 1, 3159–3170. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal Synthesis of Graphene-TiO2 Nanotube Composites with Enhanced Photocatalytic Activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Jeon, S.J.; Kim, H.T. Inexpensive Sol-Gel Synthesis of Multiwalled Carbon Nanotube-TiO2 Hybrids for High Performance Antibacterial Materials. Mater. Sci. Eng. C 2016, 68, 780–788. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Muthukumarasamy, N.; Balraju, P.; Pitchaiya, S.; Velauthapillai, D.; Pugazhendhi, A. Transformation of TiO2 Nanoparticles to Nanotubes by Simple Solvothermal Route and Its Performance as Dye-Sensitized Solar Cell (DSSC) Photoanode. Int. J. Hydrog. Energy 2020, 45, 15441–15452. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A Review on TiO2-Based Nanotubes Synthesized via Hydrothermal Method: Formation Mechanism, Structure Modification, and Photocatalytic Applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Anwar, M.S.; Danish, R.; Koo, B.H. Morphological Evolution of ZnO Nanostructures and Their Aspect Ratio-Induced Enhancement in Photocatalytic Properties. RSC Adv. 2014, 4, 29249–29263. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, H.; Yuan, S.; Jiao, Z.; Zhu, X. Preparation of Flower-like ZnO Architectures Assembled with Nanosheets for Enhanced Photocatalytic Activity. J. Colloid Interface Sci. 2016, 462, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Zhang, Q.; Li, T. Adjusting the Proportions of {0001} Facets and High-Index Facets of ZnO Hexagonal Prisms and Their Photocatalytic Activity. RSC Adv. 2017, 7, 3515–3520. [Google Scholar] [CrossRef]
- Patrinoiu, G.; Hussien, M.D.; Calderón-Moreno, J.M.; Atkinson, I.; Musuc, A.M.; Ion, R.N.; Cimpean, A.; Chifiriuc, M.C.; Carp, O. Eco-Friendly Synthesized Spherical ZnO Materials: Effect of the Core-Shell to Solid Morphology Transition on Antimicrobial Activity. Mater. Sci. Eng. C 2019, 97, 438–450. [Google Scholar] [CrossRef]
- Lv, X.; Du, Y.; Li, Z.; Chen, Z.; Yang, K.; Liu, T.; Zhu, C.; Du, M.; Feng, Y. High Photocatalytic Property and Crystal Growth of Spindle-like ZnO Microparticles Synthesized by One-Step Hydrothermal Method. Vacuum 2017, 144, 229–236. [Google Scholar] [CrossRef]
- Messali, M.; Al Wadaani, F.; Oudghiri-Hassani, H.; Rakass, S.; Al Amri, S.; Benaissa, M.; Abboudi, M. Preparation, Characterization and Photocatalytic Activity of Hexagonal ZnO Nanoparticles. Mater. Lett. 2014, 128, 187–190. [Google Scholar] [CrossRef]
- Tong, L.; Li, W.; Shen, O.; Rong, H.; Gong, L. Microwave-Assisted the Facile Synthesis and Photocatalytic Properties of Rhombic ZnO Microstructures. Mater. Lett. 2018, 218, 1–4. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C. A Green and Facile Hydrothermal Synthesis of ZnO Nanorods for Photocatalytic Application. JOJ Mater. Sci. 2018, 4, 8–10. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.K.; Wang, J.D.; Luo, H.X.; Lu, Y.; Yang, X.H. Atmospheric Self-Induction Synthesis and Enhanced Visible Light Photocatalytic Performance of Fe3+ Doped Ag-ZnO Mesocrystals. Ind. Eng. Chem. Res. 2014, 53, 13236–13246. [Google Scholar] [CrossRef]
- Deng, Q.; Duan, X.; Ng, D.H.L.; Tang, H.; Yang, Y.; Kong, M.; Wu, Z.; Cai, W.; Wang, G. Ag Nanoparticle Decorated Nanoporous ZnO Microrods and Their Enhanced Photocatalytic Activities. ACS Appl. Mater. Interfaces 2012, 4, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Wan, Q.; Dai, G.; Zhou, C.; Zou, B. Controllable ZnO Architectures by Ethanolamine-Assisted Hydrothermal Reaction for Enhanced Photocatalytic Activity. J. Phys. Chem. C 2011, 115, 2769–2775. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.-L.; Pelligra, C.; Chen, C.-H.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joesten, R.; Suib, S.L. ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity. Chem. Mater. 2009, 21, 2875–2885. [Google Scholar] [CrossRef]
- Wang, G.; Chen, D.; Zhang, H.; Zhang, J.Z.; Li, J. Tunable Photocurrent Spectrum in Well-Oriented Zinc Oxide Nanorod Arrays with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2008, 112, 8850–8855. [Google Scholar] [CrossRef]
- Han, Z.; Liao, L.; Wu, Y.; Pan, H.; Shen, S.; Chen, J. Synthesis and photocatalytic application of oriented hierarchical ZnO flower-rod architectures. J. Hazard. Mater. 2012, 217, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Chandrashekar, B.N.; Sadasivuni, K.K.; Yamani, Z.H.; Cheng, C.; Byrappa, K. Heterogeneous Growth Mechanism of ZnO Nanostructures and the Effects of Their Morphology on Optical and Photocatalytic Properties. CrystEngComm 2017, 19, 3299–3312. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, N.; Li, L.; Li, R.; Ji, G.; Gan, S. Facile Synthesis of Ag/ZnO Micro-Flowers and Their Improved Ultraviolet and Visible Light Photocatalytic Activity. New J. Chem. 2015, 40, 1587–1594. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Kumar, R.T.R. Regeneration of an efficient, solar active hierarchical ZnO flower photocatalyst for repeatable usage: Controlled desorption of poisoned species from active catalytic sites. RSC Adv. 2017, 7, 4983–4992. [Google Scholar] [CrossRef]
- Silva, H.; Mateos-Pedrero, C.; Magén, C.; Tanaka, D.A.P.; Mendes, A. Simple hydrothermal synthesis method for tailoring the physicochemical properties of ZnO: Morphology, surface area and polarity. RSC Adv. 2014, 4, 31166–31176. [Google Scholar] [CrossRef]
- Sun, H.; Yu, Y.; Luo, J.; Ahmad, M.; Zhu, J. Morphology-controlled synthesis of ZnO 3D hierarchical structures and their photocatalytic performance. CrystEngComm 2012, 14, 8626–8632. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X. Hydrothermal Synthesis and Photocatalytic Activity of Zinc Oxide Hollow Spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- de Lucas-Gil, E.; Leret, P.; Monte-Serrano, M.; Reinosa, J.J.; Enríquez, E.; Del Campo, A.; Cañete, M.; Menéndez, J.; Fernández, J.F.; Rubio-Marcos, F. ZnO Nanoporous Spheres with Broad-Spectrum Antimicrobial Activity by Physicochemical Interactions. ACS Appl. Nano Mater. 2018, 1, 3214–3225. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Mahjoub, A.R.; Abazari, R. Green synthesis of ZnO hollow sphere nanostructures by a facile route at room temperature with efficient photocatalytic dye degradation properties. RSC Adv. 2015, 5, 107378–107388. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhou, G.-Q.; Guo, J.; Liu, T.-Q. Controllable preparation of porous ZnO microspheres with a niosome soft template and their photocatalytic properties. Ceram. Int. 2016, 42, 12467–12474. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile solid state synthesis of ZnO hexagonal nanogranules with excellent photocatalytic activity. Appl. Surf. Sci. 2014, 292, 520–530. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Y.; Zou, Y.; Shen, X.; Zhu, L.; Liao, G. Solvothermal synthesis and photocatalytic properties of ZnO micro/nanostructures. Ceram. Int. 2018, 45, 1724–1729. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, L.; Wang, C.; Lun, N.; Qi, Y. Sol−Gel Growth of Hexagonal Faceted ZnO Prism Quantum Dots with Polar Surfaces for Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 1769–1773. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Hao, C.; Feng, F.; Yin, H.; Si, N. Solid-Phase Synthesis of Mesoporous ZnO Using Lignin-Amine Template and Its Photocatalytic Properties. Ind. Eng. Chem. Res. 2014, 53, 6585–6592. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S.; Wu, X.; Wei, Q. Controllable Synthesis of Hexagonal, Bullet-Like ZnO Microstructures and Nanorod Arrays and Their Photocatalytic Property. Ind. Eng. Chem. Res. 2012, 51, 4922–4926. [Google Scholar] [CrossRef]
- Warule, S.S.; Chaudhari, N.S.; Kale, B.B.; More, M.A. Novel sonochemical assisted hydrothermal approach towards the controllable synthesis of ZnO nanorods, nanocups and nanoneedles and their photocatalytic study. CrystEngComm 2009, 11, 2776–2783. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K.; Babu, R.R.; Sethuraman, K.; Babu, S.M. Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl. Surf. Sci. 2017, 418, 138–146. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Wang, R.; Li, G.; Yang, H.; Chen, R. Controllable microwave and ultrasonic wave combined synthesis of ZnO micro-/nanostructures in HEPES solution and their shape-dependent photocatalytic activities. J. Alloys Compd. 2013, 567, 1–9. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S. Fabrication of different morphologies of ZnO superstructures in presence of synthesized ethylammonium nitrate (EAN) ionic liquid: Synthesis, characterization and analysis. Dalton Trans. 2012, 42, 1645–1656. [Google Scholar] [CrossRef]
- Arzac, G.M.; Fernández, A. Hydrogen Production through Sodium Borohydride Ethanolysis. Int. J. Hydrogen Energy 2015, 40, 5326–5332. [Google Scholar] [CrossRef]
- Raza, W.; Faisal, S.M.; Owais, M.; Bahnemann, D.; Muneer, M. Facile Fabrication of Highly Efficient Modified ZnO Photocatalyst with Enhanced Photocatalytic, Antibacterial and Anticancer Activity. RSC Adv. 2016, 6, 78335–78350. [Google Scholar] [CrossRef]
- Qu, Y.; Huang, R.; Qi, W.; Shi, M.; Su, R.; He, Z. Controllable Synthesis of ZnO Nanoflowers with Structure-Dependent Photocatalytic Activity. Catal. Today 2020, 355, 397–407. [Google Scholar] [CrossRef]
- Temel, S.; Gökmen, F.Ö.; Yaman, E. Investigation of Structural and Morphological Properties of ZnO Nanoflowers on Biocompatible Polymeric Substrate. Acad. Platf. J. Eng. Sci. 2020, 8, 36–40. [Google Scholar] [CrossRef]
- Li, Q.; Shi, T.; Li, X.; Lv, K.; Li, M.; Liu, F.; Li, H.; Lei, M. Remarkable Positive Effect of Cd(OH)2 on CdS Semiconductor for Visible-Light Photocatalytic H2 Production. Appl. Catal. B Environ. 2018, 229, 8–14. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Sharma, S.K.; Durgadevi, E.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Hayakawa, Y.; Kim, D.Y. Growth, Microstructure, Structural and Optical Properties of PVP-Capped CdS Nanoflowers for Efficient Photocatalytic Activity of Rhodamine, B. Mater. Res. Bull. 2017, 94, 190–198. [Google Scholar] [CrossRef]
- Alomar, M.; Liu, Y.; Chen, W.; Fida, H. Controlling the Growth of Ultrathin MoS2 Nanosheets/CdS Nanoparticles by Two-Step Solvothermal Synthesis for Enhancing Photocatalytic Activities under Visible Light. Appl. Surf. Sci. 2019, 480, 1078–1088. [Google Scholar] [CrossRef]
- Deng, C.; Tian, X. Facile Microwave-Assisted Aqueous Synthesis of CdS Nanocrystals with Their Photocatalytic Activities under Visible Lighting. Mater. Res. Bull. 2013, 48, 4344–4350. [Google Scholar] [CrossRef]
- Bie, C.; Fu, J.; Cheng, B.; Zhang, L. Ultrathin CdS Nanosheets with Tunable Thickness and Efficient Photocatalytic Hydrogen Generation. Appl. Surf. Sci. 2018, 462, 606–614. [Google Scholar] [CrossRef]
- Ouyang, L.; Maher, K.N.; Yu, C.L.; McCarty, J.; Park, H. Catalyst-Assisted Solution-Liquid-Solid Synthesis of CdS/CdSe Nanorod Heterostructures. J. Am. Chem. Soc. 2007, 129, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Onsager, L. The Effects Of Shape On The Interaction Of Colloidal Particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Chen, J.; Li, N.; Zheng, J.; Zhao, J.; Zhu, Z. Diethylenetriamine-Assisted Synthesis of CdS Nanorods under Reflux Condition and Their Photocatalytic Performance. J. Alloys Compd. 2012, 535, 15–20. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, L.; Wang, L.; Wang, H. Urchin-like CdS microspheres self-assembled from CdS nanorods and their photocatalytic properties. Solid State Sci. 2011, 13, 970–975. [Google Scholar] [CrossRef]
- She, H.; Li, L.; Sun, Y.; Wang, L.; Huang, J.; Zhu, G.; Wang, Q. Facile preparation of mixed-phase CdS and its enhanced photocatalytic selective oxidation of benzyl alcohol under visible light irradiation. Appl. Surf. Sci. 2018, 457, 1167–1173. [Google Scholar] [CrossRef]
- Yao, W.-T.; Yu, S.-H.; Liu, S.-J.; Chen, J.-P.; Liu, X.-M.; Li, F.-Q. Architectural Control Syntheses of CdS and CdSe Nanoflowers, Branched Nanowires, and Nanotrees via a Solvothermal Approach in a Mixed Solution and Their Photocatalytic Property. J. Phys. Chem. B 2006, 110, 11704–11710. [Google Scholar] [CrossRef]
- Alivisatos, A.P.; Kadavanich, A.V.; Schlamp, M.C.; Peng, X. Epitaxial Growth of Highly Luminescent CdSe/CdS Core/Shell Nanocrystals with Photostability and Electronic Accessibility. J. Am. Chem. Soc. 1997, 119, 7019–7029. [Google Scholar] [CrossRef]
- Duan, X.; Lieber, C.M. General synthesis of compound semiconductor nanowires. Adv. Mater. 2000, 12, 298–302. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; Wang, X. High Efficient Photodegradation and Photocatalytic Hydrogen Production of CdS/BiVO4 Heterostructure through Z-Scheme Process. ACS Sustain. Chem. Eng. 2016, 5, 303–309. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Xu, Y.; Wu, K.; Huang, J.; Ji, C.; Ryu, S.O. A facile template-free approach for fabrication of flower-like CdS: The evolutionary process of the structure and the performance of photocatalytic activity. CrystEngComm 2016, 18, 4681–4687. [Google Scholar] [CrossRef]
- Ahmed, B.; Kumar, S.; Kumar, S.; Ojha, A.K. Shape induced (spherical, sheets and rods) optical and magnetic properties of CdS nanostructures with enhanced photocatalytic activity for photodegradation of methylene blue dye under ultra-violet irradiation. J. Alloys Compd. 2016, 679, 324–334. [Google Scholar] [CrossRef]
- Wang, X.; Mu, B.; An, X.; Wang, A. Insights into the Relationship between the Color and Photocatalytic Property of Attapulgite/CdS Nanocomposites. Appl. Surf. Sci. 2018, 439, 202–212. [Google Scholar] [CrossRef]
- Liang, Q.; Cui, S.; Liu, C.; Xu, S.; Yao, C.; Li, Z. Construction of CdS@UIO-66-NH2 core-shell nanorods for enhanced photocatalytic activity with excellent photostability. J. Colloid Interface Sci. 2018, 524, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pal, B. Study of excited charge carrier’s lifetime for the observed photoluminescence and photocatalytic activity of CdS nanostructures of different shapes. J. Mol. Catal. A Chem. 2013, 371, 77–85. [Google Scholar] [CrossRef]
- Kumar, P.S.; Prabavathi, S.L.; Indurani, P.; Karuthapandian, S.; Muthuraj, V. Light assisted synthesis of hierarchically structured Cu/CdS nanorods with superior photocatalytic activity, stability and photocatalytic mechanism. Sep. Purif. Technol. 2017, 172, 192–201. [Google Scholar] [CrossRef]
- Shen, Q.; Xue, J.; Zhao, H.; Shao, M.; Liu, X.; Jia, H. The role of crystalline TiO2 nanoparticle in enhancing the photocatalytic and photoelectrocatalytic properties of CdS nanorods. J. Alloys Compd. 2017, 695, 1080–1087. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Y.; Zhou, M.; Li, Z.; Xu, S.; Yao, C. Facile synthesis of novel ZnFe2O4/CdS nanorods composites and its efficient photocatalytic reduction of Cr(VI) under visible-light irradiation. J. Ind. Eng. Chem. 2018, 58, 64–73. [Google Scholar] [CrossRef]
- Maji, S.K.; Dutta, A.K.; Dutta, S.; Srivastava, D.N.; Paul, P.; Mondal, A.; Adhikary, B. Single-Source Precursor Approach for the Preparation of CdS Nanoparticles and Their Photocatalytic and Intrinsic Peroxidase like Activity. Appl. Catal. B Environ. 2012, 126, 265–274. [Google Scholar] [CrossRef]
- Singh, S.; Khare, N. Magnetically Separable, CoFe2O4 Decorated CdS Nanorods for Enhanced Visible Light Driven Photocatalytic Activity. Mater. Lett. 2015, 161, 64–67. [Google Scholar] [CrossRef]
- Huo, P.; Zhou, M.; Tang, Y.; Liu, X.; Ma, C.; Yu, L.; Yan, Y. Incorporation of N–ZnO/CdS/Graphene oxide composite photocatalyst for enhanced photocatalytic activity under visible light. J. Alloys Compd. 2016, 670, 198–209. [Google Scholar] [CrossRef]
- Chen, F.; Jia, D.; Cao, Y.; Jin, X.; Liu, A. Facile synthesis of CdS nanorods with enhanced photocatalytic activity. Ceram. Int. 2015, 41, 14604–14609. [Google Scholar] [CrossRef]
- Jamble, S.N.; Ghoderao, K.P.; Kale, R.B. Studies on growth mechanism and physical properties of hydrothermally synthesized CdS with novel hierarchical superstructures and their photocatalytic activity. J. Phys. Chem. Solids 2018, 114, 109–120. [Google Scholar] [CrossRef]
- Bera, R.; Kundu, S.; Patra, A. 2D Hybrid Nanostructure of Reduced Graphene Oxide–CdS Nanosheet for Enhanced Photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 13251–13259. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, B.; Liang, Y.; Li, Y.; Li, J. Preparation of CdS/g-C3N4/MOF composite with enhanced visible-light photocatalytic activity for dye degradation. J. Solid State Chem. 2019, 274, 32–39. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Qin, Y.; Rao, J.; Chen, G.; Lv, C.; Liu, B. The synthesis of elegant hierarchical CdS via a facile hydrothermal method assisted by inorganic salt, with photocorrosion inhibition. CrystEngComm 2016, 18, 7523–7529. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Fu, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl. Catal. B Environ. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Kandy, M.M.; Gaikar, V.G. Enhanced photocatalytic reduction of CO2 using CdS/Mn2O3 nanocomposite photocatalysts on porous anodic alumina support with solar concentrators. Renew. Energy 2019, 139, 915–923. [Google Scholar] [CrossRef]
- Ke, X.; Dai, K.; Zhu, G.; Zhang, J.; Liang, C. In situ photochemical synthesis noble-metal-free NiS on CdS-diethylenetriamine nanosheets for boosting photocatalytic H2 production activity. Appl. Surf. Sci. 2019, 481, 669–677. [Google Scholar] [CrossRef]
- Mohanraj, V.; Jayaprakash, R.; Sangaiya, P.; Gopi, S.; Dilip, R.; Suresh, R. Study of photocatalytic activity and inherent property of surfactant (PVA) on CdS nanoparticles by ultrasonic wave irradiation method. Int. J. Hydrogen Energy 2017, 42, 28266–28277. [Google Scholar] [CrossRef]
- Lavand, A.B.; Malghe, Y.S. Visible light photocatalytic degradation of 4-chlorophenol using C/ZnO/CdS nanocomposite. J. Saudi Chem. Soc. 2015, 19, 471–478. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.-X.; Zhang, W.-D. Carbon quantum dots-doped CdS microspheres with enhanced photocatalytic performance. J. Alloys Compd. 2013, 569, 102–110. [Google Scholar] [CrossRef]
- Saha, M.; Ghosh, S.; De, S.K. Nanoscale Kirkendall Effect Driven Au Decorated CdS/CdO Colloidal Nanocomposites for Efficient Hydrogen Evolution, Photocatalytic Dye Degradation and Cr (VI) Reduction. Catal. Today 2018, 340, 253–267. [Google Scholar] [CrossRef]
- Xiong, S.; Xi, B.; Qian, Y. CdS Hierarchical Nanostructures with Tunable Morphologies: Preparation and Photocatalytic Properties. J. Phys. Chem. C 2010, 114, 14029–14035. [Google Scholar] [CrossRef]
- Yang, X.; Cui, H.; Li, Y.; Qin, J.; Zhang, R.; Tang, H. Fabrication of Ag3PO4-Graphene Composites with Highly Efficient and Stable Visible Light Photocatalytic Performance. ACS Catal. 2013, 3, 363–369. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, L.; Lu, H.; Li, Y.; Hu, C.; Yu, H. One-Pot Pyridine-Assisted Synthesis of Visible-Light-Driven Photocatalyst Ag/Ag3PO4. Appl. Catal. B Environ. 2012, 115, 245–252. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An Orthophosphate Semiconductor with Photooxidation Properties under Visible-Light Irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Wang, X. Methods and Mechanism for Improvement of Photocatalytic Activity and Stability of Ag3PO4: A Review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Wang, Y.; Zhang, X.; Duan, D.; Fan, C.; Wang, Y. Insight into the Enhanced Photocatalytic Performance of Ag3PO4 Modified Metastable Hexagonal WO3. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 541, 145–153. [Google Scholar] [CrossRef]
- Zwara, J.; Grabowska, E.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. Shape-Dependent Enhanced Photocatalytic Effect under Visible Light of Ag3PO4 Particles. J. Photochem. Photobiol. A Chem. 2018, 367, 240–252. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Su, H.J.; Hsieh, P.L.; Chiang, Y.W.; Huang, M.H. Synthesis of Ag3PO4 Crystals with Tunable Shapes for Facet-Dependent Optical Property, Photocatalytic Activity, and Electrical Conductivity Examinations. ACS Appl. Mater. Interfaces 2017, 9, 39086–39093. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Yin, S.; Huang, L.; Qin, W. Controlled synthesis and photocatalytic properties of Ag3PO4 microcrystals. J. Alloys Compd. 2015, 619, 293–297. [Google Scholar] [CrossRef]
- Singh, A.; Baruah, A.; Katoch, V.; Vaghasiya, K.; Prakash, B.; Ganguli, A.K. Continuous Flow Synthesis of Ag3PO4 Nanoparticles with Greater Photostability and Photocatalytic Dye Degradation Efficiency. J. Photochem. Photobiol. A Chem. 2018, 364, 382–389. [Google Scholar] [CrossRef]
- Liu, L.; Hu, P.; Li, Y.; An, W.; Lu, J.; Cui, W. P3HT-Coated Ag3PO4 Core-Shell Structure for Enhanced Photocatalysis under Visible Light Irradiation. Appl. Surf. Sci. 2018, 466, 928–936. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, S.; Huang, H.; Huang, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X. Construction of an Ag3PO4 Morphological Homojunction for Enhanced Photocatalytic Performance and Mechanism Investigation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 99–106. [Google Scholar] [CrossRef]
- Petala, A.; Spyrou, D.; Frontistis, Z.; Mantzavinos, D.; Kondarides, D.I. Immobilized Ag3PO4 photocatalyst for micro-pollutants removal in a continuous flow annular photoreactor. Catal. Today 2018, 328, 223–229. [Google Scholar] [CrossRef]
- Cui, X.; Zheng, Y.F.; Zhou, H.; Yin, H.Y.; Song, X.C. The effect of synthesis temperature on the morphologies and visible light photocatalytic performance of Ag3POJ. Taiwan Inst. Chem. Eng. 2016, 60, 328–334. [Google Scholar] [CrossRef]
- Song, X.; Li, R.; Xiang, M.; Hong, S.; Yao, K.; Huang, Y. Morphology and photodegradation performance of Ag3PO4 prepared by (NH4)3PO4, (NH4)2HPO4 and NH4H2PO. Ceram. Int. 2017, 43, 4692–4701. [Google Scholar] [CrossRef]
- Li, R.; Song, X.; Huang, Y.; Fang, Y.; Jia, M.; Ma, W. Visible-light photocatalytic degradation of azo dyes in water by Ag3PO4: An unusual dependency between adsorption and the degradation rate on pH value. J. Mol. Catal. A Chem. 2016, 421, 57–65. [Google Scholar] [CrossRef]
- Cruz-Filho, J.; Costa, T.; Lima, M.; Silva, L.; Santos, R.; Cavalcante, L.; Longo, E.; Luz, G. Effect of different synthesis methods on the morphology, optical behavior, and superior photocatalytic performances of Ag3PO4 sub-microcrystals using white-light-emitting diodes. J. Photochem. Photobiol. A Chem. 2019, 377, 14–25. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, X.; Liu, C.; Tan, K.; Xie, Z.; Zheng, L. High-efficiently visible light-responsive photocatalysts: Ag3PO4 tetrahedral microcrystals with exposed {111} facets of high surface energy. J. Mater. Chem. A 2013, 1, 12635–12640. [Google Scholar] [CrossRef]
- Tang, C.; Liu, E.; Fan, J.; Hu, X.; Ma, Y.; Wan, J. A graphitic-C3N4-hybridized Ag3PO4 tetrahedron with reactive {111} facets to enhance the visible-light photocatalytic activity. RSC Adv. 2015, 5, 91979–91987. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Wei, X.-M.; Bai, X.; Liu, C.-M.; Wang, B.-Y.; Liu, J.-W. Ethanol–water ambient precipitation of {111} facets exposed Ag3PO4 tetrahedra and its hybrid with graphene oxide for outstanding photoactivity and stability. Inorg. Chem. Front. 2018, 5, 951–961. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Intarasuwan, K.; Suwanboon, S.; Baltrusaitis, J. Effect of Phosphate Salts (Na3PO4, Na2HPO4, and NaH2PO4) on Ag3PO4 Morphology for Photocatalytic Dye Degradation under Visible Light and Toxicity of the Degraded Dye Products. Ind. Eng. Chem. Res. 2013, 52, 17369–17375. [Google Scholar] [CrossRef]
- Krungchanuchat, S.; Ekthammathat, N.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. High UV-Visible Photocatalytic Activity of Ag3PO4 Dodecahedral Particles Synthesized by a Simple Hydrothermal Method. Mater. Lett. 2017, 201, 58–61. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, H.; Liu, Y.; Guan, W.; Long, J.; Li, W.; You, J. Fabrication of Robust M/Ag3PO4 (M = Pt, Pd, Au) Schottky-Type Heterostructures for Improved Visible-Light Photocatalysis. RSC Adv. 2014, 4, 37220–37230. [Google Scholar] [CrossRef]
- Hewer, T.L.R.; Machado, B.C.; Freire, R.S.; Guardani, R. Ag3PO4 Sunlight-Induced Photocatalyst for Degradation of Phenol. RSC Adv. 2014, 4, 34674–34680. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Hou, J.; Yang, Y. Visible Photocatalysis and Photostability of Ag3PO4 Photocatalyst. Appl. Surf. Sci. 2014, 319, 332–338. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, G.; Yang, H.; Zhang, H.; Suo, R.; Xie, Y.; Liu, G. Fabrication of Ag3PO4/GO/NiFe2O4 Composites with Highly Efficient and Stable Visible-Light-Driven Photocatalytic Degradation of Rhodamine, B. RSC Adv. 2018, 8, 28179–28188. [Google Scholar] [CrossRef]
- Abroushan, E.; Farhadi, S.; Zabardasti, A. Ag3PO4/CoFe2O4 Magnetic Nanocomposite: Synthesis, Characterization and Applications in Catalytic Reduction of Nitrophenols and Sunlight-Assisted Photocatalytic Degradation of Organic Dye Pollutants. RSC Adv. 2017, 7, 18293–18304. [Google Scholar] [CrossRef]
- Ren, Y.; Li, H.; Yang, W.; Shi, D.; Wu, Q.; Zhao, Y.; Feng, C.; Liu, H.; Jiao, Q. Alkaline Ionic Liquids Immobilized on Protective Copolymers Coated Magnetic Nanoparticles: An Efficient and Magnetically Recyclable Catalyst for Knoevenagel Condensation. Ind. Eng. Chem. Res. 2019, 58, 2824–2834. [Google Scholar] [CrossRef]
- Li, J.; Cui, H.; Mu, D.; Liu, Y.; Guan, T.; Xia, Z.; Jiang, L.; Zuo, J.; Tan, C.; You, H. Synthesis and Characterization of RGO Decorated Cubic ZnTiO3 Rods for Solar Light-Induced Photodegradation of Rhodamine B. New J. Chem. 2019, 43, 3374–3382. [Google Scholar] [CrossRef]
- Surendar, T.; Kumar, S.; Shanker, V. Influence of La-Doping on Phase Transformation and Photocatalytic Properties of ZnTiO3 nanoparticles Synthesized via Modified Sol-Gel Method. Phys. Chem. Chem. Phys. 2013, 16, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Jeevanandam, P. Synthesis Temperature Dependent Morphological Evolution in Zinc Titanate Heteronanostructures and Their Application in Environmental Remediation. ChemistrySelect 2016, 1, 6382–6395. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Soofivand, F.; Sobhani-Nasab, A.; Shakouri-Arani, M.; Yeganeh Faal, A.; Bagheri, S. Synthesis, Characterization, and Morphological Control of ZnTiO3 Nanoparticles through Sol-Gel Processes and Its Photocatalyst Application. Adv. Powder Technol. 2016, 27, 2066–2075. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Huang, J.; Feng, Z.; Luo, J. Novel g-C3N4/h′ZnTiO3-a′TiO2 direct Z-scheme heterojunction with significantly enhanced visible-light photocatalytic activity. J. Alloys Compd. 2018, 774, 768–778. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Li, A.-D.; Zhai, H.; Li, H.; Yan, Q.-Y.; Ma, J.; Wu, D. Preparation, characterization and photocatalytic properties of ZnTiO3 powders. J. Hazard. Mater. 2009, 171, 918–923. [Google Scholar] [CrossRef]
- Perween, S.; Ranjan, A. Improved visible-light photocatalytic activity in ZnTiO3 nanopowder prepared by sol-electrospinning. Sol. Energy Mater. Sol. Cells 2017, 163, 148–156. [Google Scholar] [CrossRef]
- Ozturk, B.; Soylu, G.S.P. Promoting role of transition metal oxide on ZnTiO3–TiO2 nanocomposites for the photocatalytic activity under solar light irradiation. Ceram. Int. 2016, 42, 11184–11192. [Google Scholar] [CrossRef]
- Wattanawikkam, C.; Kansa-Ard, T.; Pecharapa, W. X-ray absorption spectroscopy analysis and photocatalytic behavior of ZnTiO3 nanoparticles doped with Co and Mn synthesized by sonochemical method. Appl. Surf. Sci. 2019, 474, 169–176. [Google Scholar] [CrossRef]
- Dutta, D.P.; Singh, A.; Tyagi, A.K. Ag Doped and Ag Dispersed Nano ZnTiO3: Improved Photocatalytic Organic Pollutant Degradation under Solar Irradiation and Antibacterial Activity. J. Environ. Chem. Eng. 2014, 2, 2177–2187. [Google Scholar] [CrossRef]
- Chi, Y.; Yuan, Q.; Hou, S.; Zhao, Z. Synthesis and Characterization of Mesoporous ZnTiO3 Rods via a Polyvinylpyrrolidone Assisted Sol-Gel Method. Ceram. Int. 2016, 42, 5094–5099. [Google Scholar] [CrossRef]
- Xin, Y.A.N.; Zhao, C.L.; Zhou, Y.L.; Wu, Z.J.; Yuan, J.M.; Li, W.S. Synthesis and characterization of ZnTiO3 with high photocatalytic activity. Trans. Nonferrous Met. Soc. China 2015, 25, 2272–2278. [Google Scholar] [CrossRef]
- Reddy, K.H.; Martha, S.; Parida, K.M. Erratic charge transfer dynamics of Au/ZnTiO3 nanocomposites under UV and visible light irradiation and their related photocatalytic activities. Nanoscale 2018, 10, 18540–18554. [Google Scholar] [CrossRef]
- Yang, J.; Akbarzadeh, J.; Maurer, C.; Peterlik, H.; Schubert, U. Sol–gel synthesis of ZnTiO3 using a single-source precursor based on p-carboxybenzaldehyde oxime as a linker. J. Mater. Chem. 2012, 22, 24034–24041. [Google Scholar] [CrossRef]
- Wu, H.; Min, Y.; Zhang, Q.; Li, W.; Yuan, J.; Wu, Z.; Wang, S. Low-temperature synthesis of mesoporous ZnTiO3–graphene composite for the removal of norfloxacin in aqueous solution. RSC Adv. 2016, 6, 103822–103829. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of the graphene-ZnTiO3 nanocomposite for solar light assisted photodegradation of methylene blue. J. Phys. D Appl. Phys. 2015, 48, 415305. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Tavakoli, A.; Ziarani, G.M. Ultrasonic-assisted synthesis of Ce doped cubic–hexagonal ZnTiO3 with highly efficient sonocatalytic activity. Ultrason. Sonochem. 2016, 29, 258–269. [Google Scholar] [CrossRef]
- Yu, C.; Chen, F.; Zeng, D.; Xie, Y.; Zhou, W.; Liu, Z.; Wei, L.; Yang, K.; Li, D. A facile phase transformation strategy for fabrication of novel Z-scheme ternary heterojunctions with efficient photocatalytic properties. Nanoscale 2019, 11, 7720–7733. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.; Muneer, M. Synthesis of visible light driven ZnO: Characterization and photocatalytic performance. Appl. Surf. Sci. 2014, 322, 215–224. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeifard, Z.; Shariatinia, Z.; Jourshabani, M.; Rezaei Darvishi, S.M. ZnO Photocatalyst Revisited: Effective Photocatalytic Degradation of Emerging Contaminants Using S-Doped ZnO Nanoparticles under Visible Light Radiation. Ind. Eng. Chem. Res. 2020, 59, 15894–15911. [Google Scholar] [CrossRef]
- Yang, X.; Ma, J.; Wang, T.; Wang, B.; Meng, D.; Wang, Y. Synthesis, growth mechanism and photocatalytic property of CdS with different kinds of surfactants. New J. Chem. 2019, 43, 10126–10133. [Google Scholar] [CrossRef]
- Santos, R.K.; Martins, T.A.; Silva, G.N.; Conceição, M.V.S.; Nogueira, I.C.; Longo, E.; Botelho, G. Ag3PO4/NiO Composites with Enhanced Photocatalytic Activity under Visible Light. ACS Omega 2020, 5, 21651–21661. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Lu, S.; He, L.; Zhu, X.; Feng, W.; Zhang, W. Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance. Nanomaterials 2022, 12, 1345. [Google Scholar] [CrossRef]
- Esrafili, A.; Salimi, M.; Jafari, A.J.; Sobhi, H.R.; Gholami, M.; Kalantary, R.R. Pt-based TiO2 photocatalytic systems: A systematic review. J. Mol. Liq. 2022, 352, 118685. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Vinayagam, R.; Pai, S.; Varadavenkatesan, T.; Pugazhendhi, A.; Selvaraj, R. Characterization and photocatalytic activity of ZnO nanoflowers synthesized using Bridelia retusa leaf extract. Appl. Nanosci. 2021, 1–10. [Google Scholar] [CrossRef]
- Morales, M.; Fernández-Cervantes, I.; Agustín-Serrano, R.; Ruíz-Salgado, S.; Sampedro, M.; Varela-Caselis, J.; Portillo, R.; Rubio, E. AgPO microcrystals with complex polyhedral morphologies diversity obtained by microwave-hydrothermal synthesis for MB degradation under sunlight. Results Phys. 2018, 12, 1344–1356. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, S.; Chen, Y. Study on the synthesis and photocatalysis of Ag3PO4 polyhedral microcrystals. Bull. Mater. Sci. 2020, 43, 223. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Ji, Y.; Bian, R.; Li, J.; Zhang, X.; Tian, J.; Yang, Q.; Shi, F. Ti3C2 MXene coupled with CdS nanoflowers as 2D/3D heterostructures for enhanced photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2022, 47, 22045–22053. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Song, J.T.; Ishihara, T.; Ohtani, B.; Sasaki, K. Importance of ZnTiO3 Phase in ZnTi-Mixed Metal Oxide Photocatalysts Derived from Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2020, 12, 9169–9180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, C.; Hodgson, P.; Li, Y. Biocompatibility of TiO2 nanotubes with different topographies. J. Biomed. Mater. Res. Part A 2013, 102, 743–751. [Google Scholar] [CrossRef]
- Fontes, A.C.C.A.; Sopchenski, L.; Laurindo, C.A.H.; Torres, R.D.; Popat, K.C.; Soares, P. Annealing Temperature Effect on Tribocorrosion and Biocompatibility Properties of TiO2 Nanotubes. J. Bio- Tribo-Corros. 2020, 6, 64. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Hubler, R.; Shinkai, R.S.A.; Teixeira, E.R. Application of TiO2 Nanotubes as a Drug Delivery System for Biomedical Implants: A Critical Overview. ChemistrySelect 2018, 3, 11180–11189. [Google Scholar] [CrossRef]
- Ray, P.G.; Biswas, S.; Roy, T.; Ghosh, S.; Majumder, D.; Basak, P.; Roy, S.; Dhara, S. Sonication Assisted Hierarchical Decoration of Ag-NP on Zinc Oxide Nanoflower Impregnated Eggshell Membrane: Evaluation of Antibacterial Activity and in Vitro Cytocompatibility. ACS Sustain. Chem. Eng. 2019, 7, 13717–13733. [Google Scholar] [CrossRef]
- Khan, M.S.; Ameer, H.; Ali, A.; Zhu, W.; Li, X.; Yang, L.; Wang, H.; Feng, R.; Wei, Q. Label-free electrochemiluminescent immunosensor for prostate specific antigen ultrasensitive detection based on novel luminophore Ag3PO4 decorated GO. J. Electroanal. Chem. 2019, 847, 113266. [Google Scholar] [CrossRef]
- Bhavsar, K.; Labhane, P.; Dhake, R.; Sonawane, G. Solvothermal synthesis of activated carbon loaded CdS nanoflowers: Boosted photodegradation of dye by adsorption and photocatalysis synergy. Chem. Phys. Lett. 2020, 744, 137202. [Google Scholar] [CrossRef]
- Al-Jawad, S.M.H.; Imran, N.J.; Aboud, K.H. Synthesis and characterization of Mn:CdS nanoflower thin films prepared by hydrothermal method for photocatalytic activity. J. Sol-Gel Sci. Technol. 2021, 100, 423–439. [Google Scholar] [CrossRef]
- Mathew, A.M.; Chukwuike, V.; Venkatesan, K.; Raveendran, S.; Barik, R.C.; Pattanayak, D.K. Zinc oxide decorated titania nanostructured layer over Ti metal as a biocompatible and antimicrobial surface for biomedical application. Surf. Interfaces 2022, 33, 102275. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, Z.; Zhang, J.; Cheng, H.; Chen, J.; Zeng, Y.; Zhu, Z.; Wan, Y. Engineered Zinc Titanate Coatings on the Titanium Surface with Enhanced Antitumor Properties and Biocompatibility. ACS Biomater. Sci. Eng. 2019, 5, 5935–5946. [Google Scholar] [CrossRef] [PubMed]
- Abirami, R.; Senthil, T.; Keerthana, S.; Yuvakkumar, R.; Ravi, G.; Pannipara, M.; Al-Sehemi, A.G. An approach to enhance the photocatalytic activity of ZnTiO3. Ceram. Int. 2021, 47, 18122–18131. [Google Scholar] [CrossRef]
- Grilli, M.L. Metal Oxides. Metals 2020, 10, 820. [Google Scholar] [CrossRef]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; Ben Zina, M.; Galvez, M.E.; Da Costa, P. Photocatalytic Degradation of Methyl Green Dye in Aqueous Solution over Natural Clay-Supported ZnO-TiO2 Catalysts. J. Photochem. Photobiol. A Chem. 2016, 315, 25–33. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Chandrasekaran, S.; Salla, S.; Elakkiya, V.; Senthil, R.A.; Nithyadharseni, P.; Maiyalagan, T.; Micheal, K.; Ayeshamariam, A.; Arasu, M.V.; et al. Recent Developments of Metal Oxide Based Heterostructures for Photocatalytic Applications towards Environmental Remediation. J. Solid State Chem. 2018, 267, 35–52. [Google Scholar] [CrossRef]
- Yumashev, A.; Ślusarczyk, B.; Kondrashev, S.; Mikhaylov, A. Global Indicators of Sustainable Development: Evaluation of the Influence of the Human Development Index on Consumption and Quality of Energy. Energies 2020, 13, 2768. [Google Scholar] [CrossRef]
- Huang, T.; Crews, J.B. Wastewater Purification with Nanoparticle Treated Bed. PCT/US2009/040597, 15 April 2009. [Google Scholar]
- Shalabayev, Z.; Baláž, M.; Khan, N.; Nurlan, Y.; Augustyniak, A.; Daneu, N.; Tatykayev, B.; Dutková, E.; Burashev, G.; Casas-Luna, M.; et al. Sustainable Synthesis of Cadmium Sulfide, with Applicability in Photocatalysis, Hydrogen Production, and as an Antibacterial Agent, Using Two Mechanochemical Protocols. Nanomaterials 2022, 12, 1250. [Google Scholar] [CrossRef]
- Li, X.; Xu, P.; Chen, M.; Zeng, G.; Wang, D.; Chen, F.; Tang, W.; Chen, C.; Zhang, C.; Tan, X. Application of Silver Phosphate-Based Photocatalysts: Barriers and Solutions. Chem. Eng. J. 2019, 366, 339–357. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).