Capsaicin Decreases Kidney Iron Deposits and Increases Hepcidin Levels in Diabetic Rats with Iron Overload: A Preliminary Study
Abstract
1. Introduction
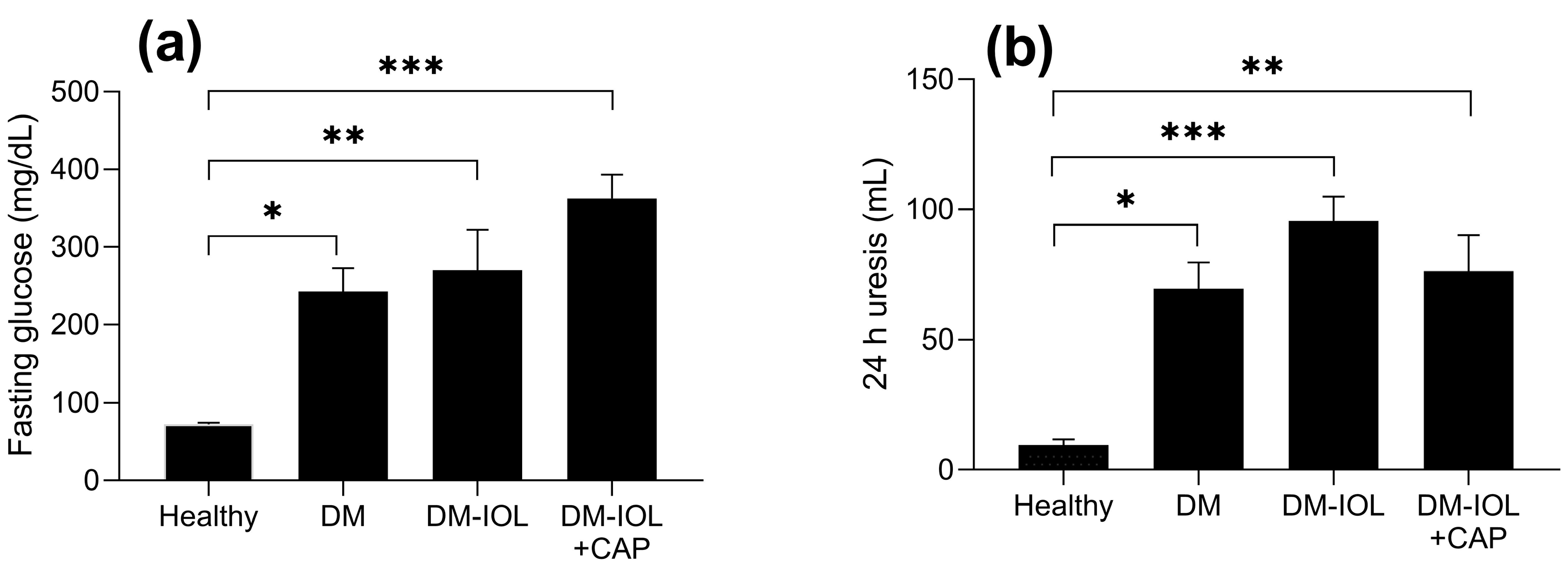
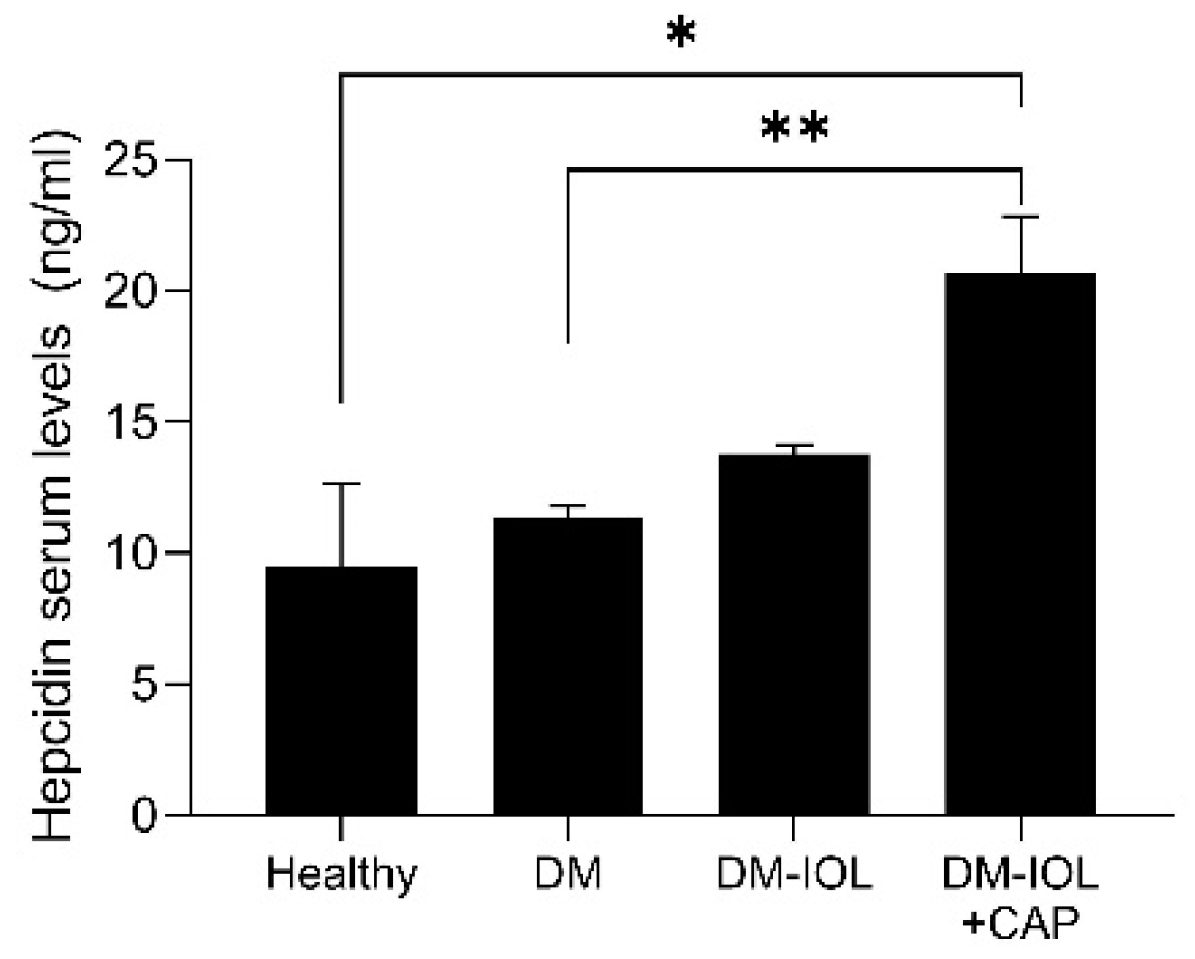
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
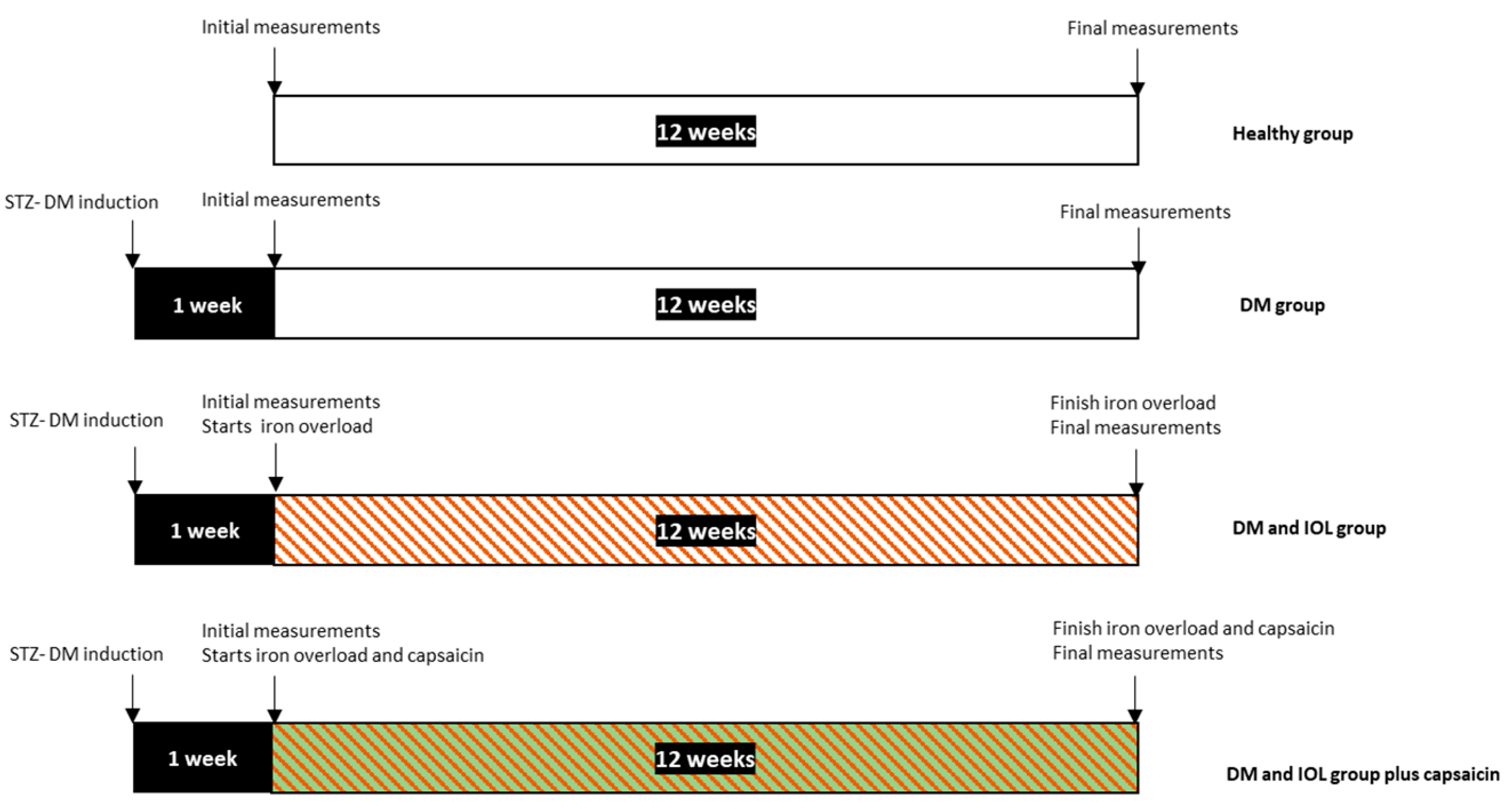
4.2. Experimental Protocol
4.3. Analysis of Iron Tissue Content
4.4. Measurements of Kidney Disease Biomarkers
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Jones, D.; Luo, B.; Sanderson, M.; Soto, J.; Abel, E.D.; Cooksey, R.C.; McClain, D.A. Iron Overload and Diabetes Risk: A Shift From Glucose to Fatty Acid Oxidation and Increased Hepatic Glucose Production in a Mouse Model of Hereditary Hemochromatosis. Diabetes 2011, 60, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, L.; Tan, Y.; Wang, G.; Lin, X.; Cai, L. Role of Iron Deficiency and Overload in the Pathogenesis of Diabetes and Diabetic Complications. Curr. Med. Chem. 2009, 16, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.V.; Lorenzo, F.R.; McClain, D.A. Iron and the Pathophysiology of Diabetes. Annu. Rev. Physiol. 2022, 85, 2.1–2.24. [Google Scholar] [CrossRef] [PubMed]
- Martines, A.-C.M.; Masereeuw, R.; Tjalsma, H.; Hoenderop, J.G.; Wetzels, J.F.M.; Swinkels, D.W. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat. Rev. Nephrol. 2013, 9, 385–398. [Google Scholar] [CrossRef]
- Lv, J.-C.; Zhang, L.-X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Dominguez, J.H.; Liu, Y.; Kelly, K.J. Renal iron overload in rats with diabetic nephropathy. Physiol. Rep. 2015, 3, e12654. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Gao, Z.; Li, H. Iron Increases Diabetes-Induced Kidney Injury and Oxidative Stress in Rats. Biol. Trace Elem. Res. 2014, 160, 368–375. [Google Scholar] [CrossRef]
- Peña-Montes, D.J.; Huerta-Cervantes, M.; Ríos-Silva, M.; Trujillo, X.; Cortés-Rojo, C.; Huerta, M.; Saavedra-Molina, A. Effects of dietary iron restriction on kidney mitochondria function and oxidative stress in streptozotocin-diabetic rats. Mitochondrion 2020, 54, 41–48. [Google Scholar] [CrossRef]
- Karamzad, N.; Eftekhari, A.; Ashrafi-Asgarabad, A.; Sullman, M.J.; Sahebkar, A.; Safiri, S. Serum Hepcidin, the Hepcidin/Ferritin Ratio and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Curr. Med. Chem. 2021, 28, 1224–1233. [Google Scholar] [CrossRef]
- Islam, S.; Choi, H. Dietary red chilli (Capsicum frutescens L.) is insulinotropic rather than hypoglycemic in type 2 diabetes model of rats. Phytother. Res. 2008, 22, 1025–1029. [Google Scholar] [CrossRef]
- Johnson, W. Final Report on the Safety Assessment of Capsicum Annuum Extract, Capsicum Annuum Fruit Extract, Capsicum Annuum Resin, Capsicum Annuum Fruit Powder, Capsicum frutescens Fruit, Capsicum frutescens Fruit Extract, Capsicum frutescens Resin, and Capsaicin. Int. J. Toxicol. 2007, 26, 3–106. [Google Scholar] [CrossRef]
- Feng, N.-H.; Lee, H.-H.; Shiang, J.-C.; Ma, M.-C. Transient receptor potential vanilloid type 1 channels act as mechanoreceptors and cause substance P release and sensory activation in rat kidneys. Am. J. Physiol. Physiol. 2008, 294, F316–F325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Wang, N.H. Diuresis and Natriuresis Caused by Activation of VR1-Positive Sensory Nerves in Renal Pelvis of Rats. Hypertension 2005, 46, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.H. Protective Effect of TRPV1 against Renal Fibrosis via Inhibition of TGF-β/Smad Signaling in DOCA-Salt Hypertension. Mol. Med. 2011, 17, 1204–1212. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, L.; Xu, H.; Liu, S.; Zhu, F.; Yan, F.; Shen, S.; Zhu, M. TRPV1 agonism inhibits endothelial cell inflammation via activation of eNOS/NO pathway. Atherosclerosis 2017, 260, 13–19. [Google Scholar] [CrossRef]
- Lee, C.-Y.J.; Kim, M.; Yoon, S.-W.; Lee, C.-H. Short-term control of capsaicin on blood and oxidative stress of ratsin vivo. Phytotherapy Res. 2003, 17, 454–458. [Google Scholar] [CrossRef]
- Ríos-Silva, M.; Santos-Álvarez, R.; Trujillo, X.; Cárdenas-María, R.Y.; López-Zamudio, M.; Bricio-Barrios, J.A.; Leal, C.; Saavedra-Molina, A.; Huerta-Trujillo, M.; Espinoza-Mejía, K.; et al. Effects of Chronic Administration of Capsaicin on Biomarkers of Kidney Injury in Male Wistar Rats with Experimental Diabetes. Molecules 2018, 24, 36. [Google Scholar] [CrossRef]
- Márquez-Ibarra, A.; Huerta, M.; Villalpando-Hernández, S.; Ríos-Silva, M.; Díaz-Reval, M.I.; Cruzblanca, H.; Mancilla, E.; Trujillo, X. The Effects of Dietary Iron and Capsaicin on Hemoglobin, Blood Glucose, Insulin Tolerance, Cholesterol, and Triglycerides, in Healthy and Diabetic Wistar Rats. PLoS ONE 2016, 11, e0152625. [Google Scholar] [CrossRef]
- Manjunatha, H.; Srinivasan, K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. FEBS J. 2006, 273, 4528–4537. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Chen, Y.; Wu, H.; Zhang, S.; Chen, X. Prediction Value of Serum NGAL in the Diagnosis and Prognosis of Experimental Acute and Chronic Kidney Injuries. Biomolecules 2020, 10, 981. [Google Scholar] [CrossRef]
- Mohammad, G.; Matakidou, A.; Robbins, P.A.; Lakhal-Littleton, S. The kidney hepcidin/ferroportin axis controls iron reabsorption and determines the magnitude of kidney and systemic iron overload. Kidney Int. 2021, 100, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, X.; Zhang, J.; Zhao, S.; Wang, Z.; Wang, F.; Shang, W.; Barasch, J.; Qiu, A. Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury. Am. J. Physiol. Physiol. 2018, 315, F1042–F1057. [Google Scholar] [CrossRef] [PubMed]
- Moulouel, B.; Houamel, D.; Delaby, C.; Tchernitchko, D.; Vaulont, S.; Letteron, P.; Thibaudeau, O.; Puy, H.; Gouya, L.; Beaumont, C.; et al. Hepcidin regulates intrarenal iron handling at the distal nephron. Kidney Int. 2013, 84, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Hamilton, K.; Burnand, R.; Smith, C.; Tomlinson, D.; Riccardi, D. Altered expression of iron transport proteins in streptozotocin-induced diabetic rat kidney. Biochim. Biophys. Acta 2005, 1740, 79–84. [Google Scholar] [CrossRef]
- Ikeda, Y.; Enomoto, H.; Tajima, S.; Izawa-Ishizawa, Y.; Kihira, Y.; Ishizawa, K.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Dietary iron restriction inhibits progression of diabetic nephropathy in db/db mice. Am. J. Physiol. Physiol. 2013, 304, F1028–F1036. [Google Scholar] [CrossRef]
- Wareing, M.; Smith, C.P. Iron Is Filtered by the Kidney and Is Reabsorbed by the Proximal Tubule. Front. Physiol. 2021, 12, 740716. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.V.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Xu, Y.; Alfaro-Magallanes, V.M.; Babitt, J.L. Physiological and pathophysiological mechanisms of hepcidin regulation: Clinical implications for iron disorders. Br. J. Haematol. 2020, 193, 882–893. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of in-flammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Daniele, G.; Mendoza, R.G.; Winnier, D.; Fiorentino, T.V.; Pengou, Z.; Cornell, J.; Andreozzi, F.; Jenkinson, C.; Cersosimo, E.; Federici, M.; et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Geol. Rundsch. 2014, 51, 123–131. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Sun, Z.; Dong, H.; Yu, H.; Huang, M.; Gao, X. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice. Front. Endocrinol. 2021, 12, 626390. [Google Scholar] [CrossRef] [PubMed]
- Dairam, A.; Fogel, R.; Daya, S.; Limson, J.L. Antioxidant and Iron-Binding Properties of Curcumin, Capsaicin, and S-Allylcysteine Reduce Oxidative Stress in Rat Brain Homogenate. J. Agric. Food Chem. 2008, 56, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhao, B.-C.; Yang, X.; Lin, Z.-B.; Sun, Q.-S.; Wang, Y.-F.; Yan, Z.-Z.; Liu, W.-F.; Li, C.; Hu, J.-J.; et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes 2021, 13, 1902719. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Jun, J.H.; Shim, J.-K.; Oh, J.E.; Lee, C.; Kwak, Y.-L. Changes in Hepcidin Levels in an Animal Model of Anemia of Chronic Inflammation: Mechanistic Insights Related to Iron Supplementation and Hepcidin Regulation. Oxidative Med. Cell. Longev. 2021, 2021, 4357756. [Google Scholar] [CrossRef]
- Ortega, L.; Ladero, J.M.; Carreras, M.P.; Alvarez, T.; Taxonera, C.; Oliván, M.P.; Sanz-Esponera, J.; Díaz-Rubio, M. A computer-assisted morphometric quantitative analysis of iron overload in liver biopsies. A comparison with histological and biochemical methods. Pathol. Res. Pr. 2005, 201, 673–677. [Google Scholar] [CrossRef]


| Characteristic | Healthy | DM | DM-IOL | DM-IOL + CAP |
|---|---|---|---|---|
| Initial body weight (g) | 291.5 ± 1.9 | 288 ± 1.58 | 278.6 ± 6.8 | 271.2 ± 10.2 |
| Final body weight (g) | 455.6 ± 13.6 £ | 370 ± 29.3 £ | 281.1 ± 9.1 | 268.4 ± 9.8 |
| Kidney weight (g) | 1.19 ± 0.06 | 1.4 ± 0.09 | 1.3 ± 0.02 | 1.2 ± 0.07 |
| Kidney hypertrophy index (mg/g) | 2.6 ± 0.2 £ | 4.1 ± 0.3 | 4.8 ± 2.2 | 4.6 ± 0.2 |
| Glomerular diameter (μm) | 211.8 ± 8.1 | 206.4 ± 14.6 | 221.4 ± 6.7 | 96.2 ± 1.4 £ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, M.; Quintero-Macías, L.; Huerta, M.; Rodríguez-Hernández, A.; Melnikov, V.; Cárdenas, Y.; Bricio-Barrios, J.A.; Sánchez-Pastor, E.; Gamboa-Domínguez, A.; Leal, C.; et al. Capsaicin Decreases Kidney Iron Deposits and Increases Hepcidin Levels in Diabetic Rats with Iron Overload: A Preliminary Study. Molecules 2022, 27, 7764. https://doi.org/10.3390/molecules27227764
López M, Quintero-Macías L, Huerta M, Rodríguez-Hernández A, Melnikov V, Cárdenas Y, Bricio-Barrios JA, Sánchez-Pastor E, Gamboa-Domínguez A, Leal C, et al. Capsaicin Decreases Kidney Iron Deposits and Increases Hepcidin Levels in Diabetic Rats with Iron Overload: A Preliminary Study. Molecules. 2022; 27(22):7764. https://doi.org/10.3390/molecules27227764
Chicago/Turabian StyleLópez, Marisa, Laura Quintero-Macías, Miguel Huerta, Alejandrina Rodríguez-Hernández, Valery Melnikov, Yolitzy Cárdenas, Jaime Alberto Bricio-Barrios, Enrique Sánchez-Pastor, Armando Gamboa-Domínguez, Caridad Leal, and et al. 2022. "Capsaicin Decreases Kidney Iron Deposits and Increases Hepcidin Levels in Diabetic Rats with Iron Overload: A Preliminary Study" Molecules 27, no. 22: 7764. https://doi.org/10.3390/molecules27227764
APA StyleLópez, M., Quintero-Macías, L., Huerta, M., Rodríguez-Hernández, A., Melnikov, V., Cárdenas, Y., Bricio-Barrios, J. A., Sánchez-Pastor, E., Gamboa-Domínguez, A., Leal, C., Trujillo, X., & Ríos-Silva, M. (2022). Capsaicin Decreases Kidney Iron Deposits and Increases Hepcidin Levels in Diabetic Rats with Iron Overload: A Preliminary Study. Molecules, 27(22), 7764. https://doi.org/10.3390/molecules27227764







