M-Carboxylic Acid Induced Formation of New Coordination Polymers for Efficient Photocatalytic Degradation of Ciprofloxacin
Abstract
1. Introduction
2. Results
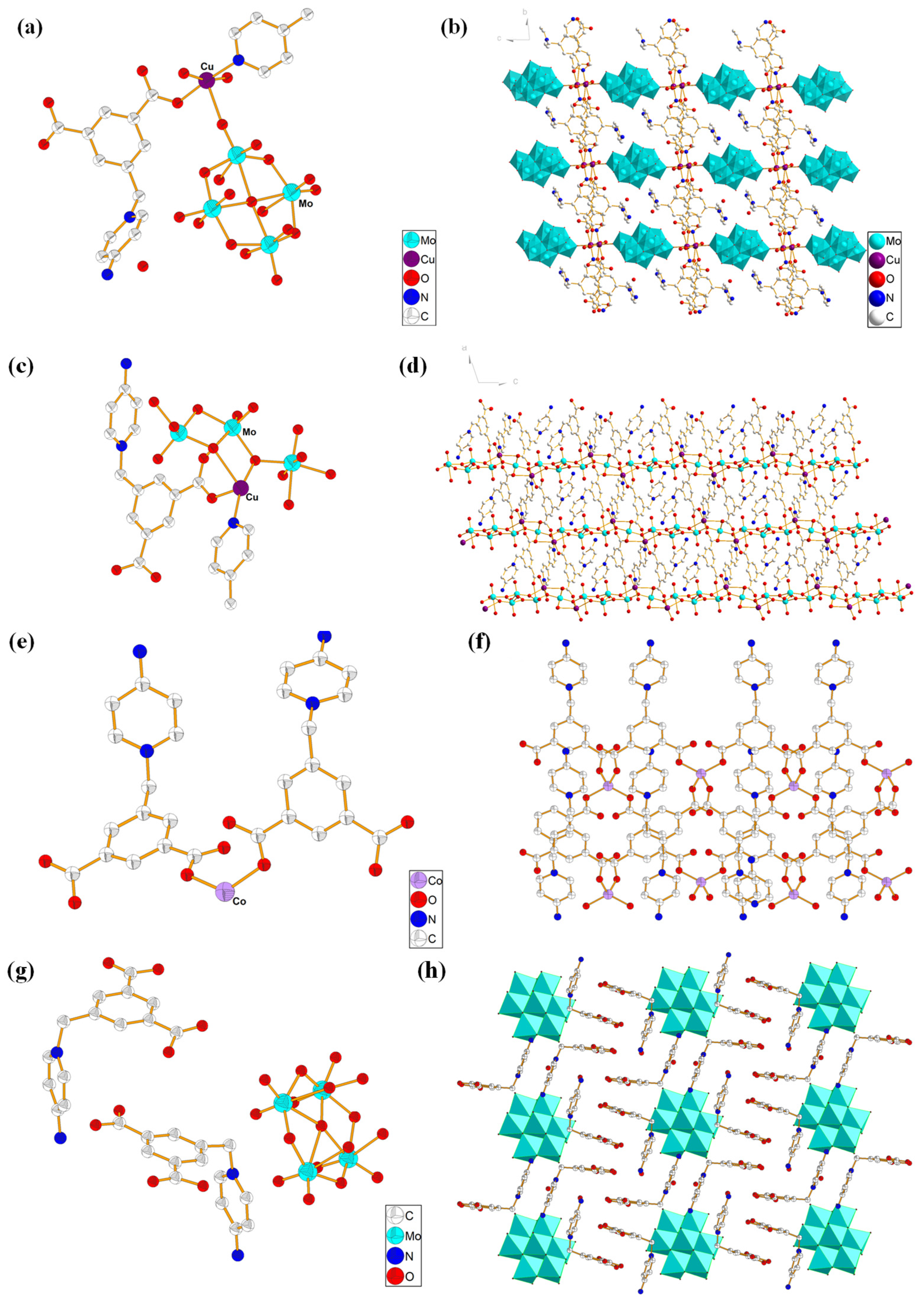
2.1. Crystal Structure
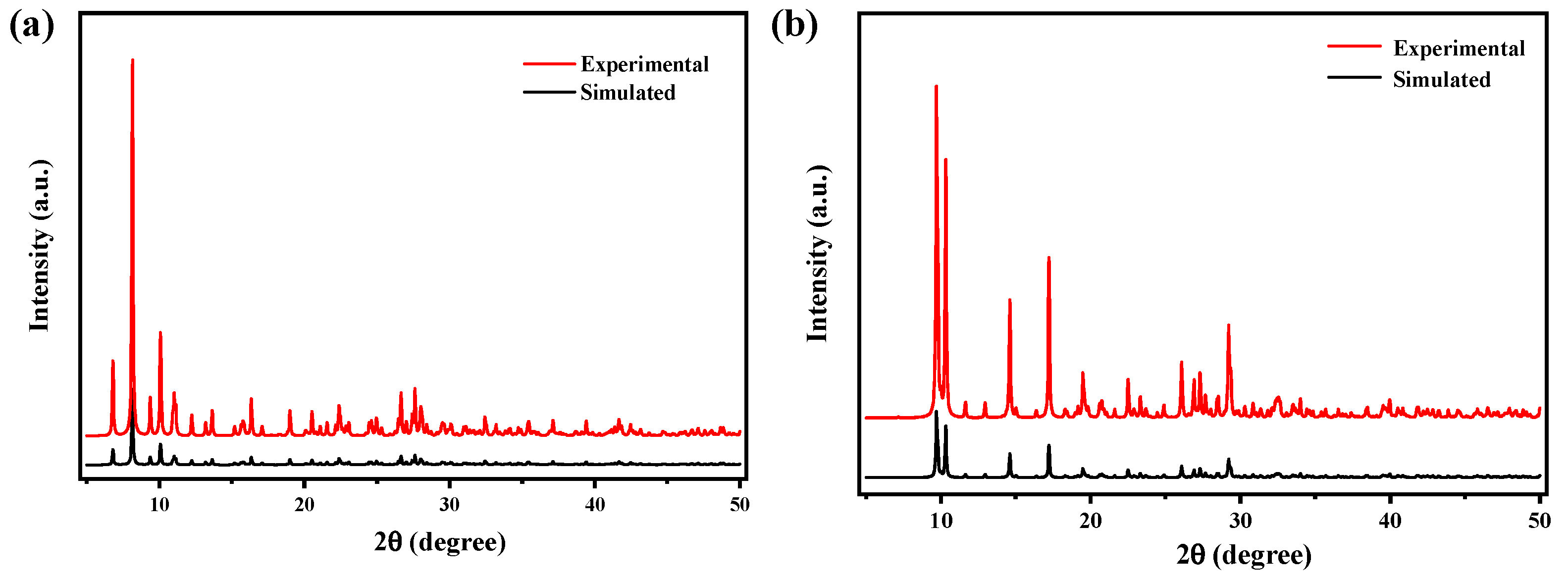
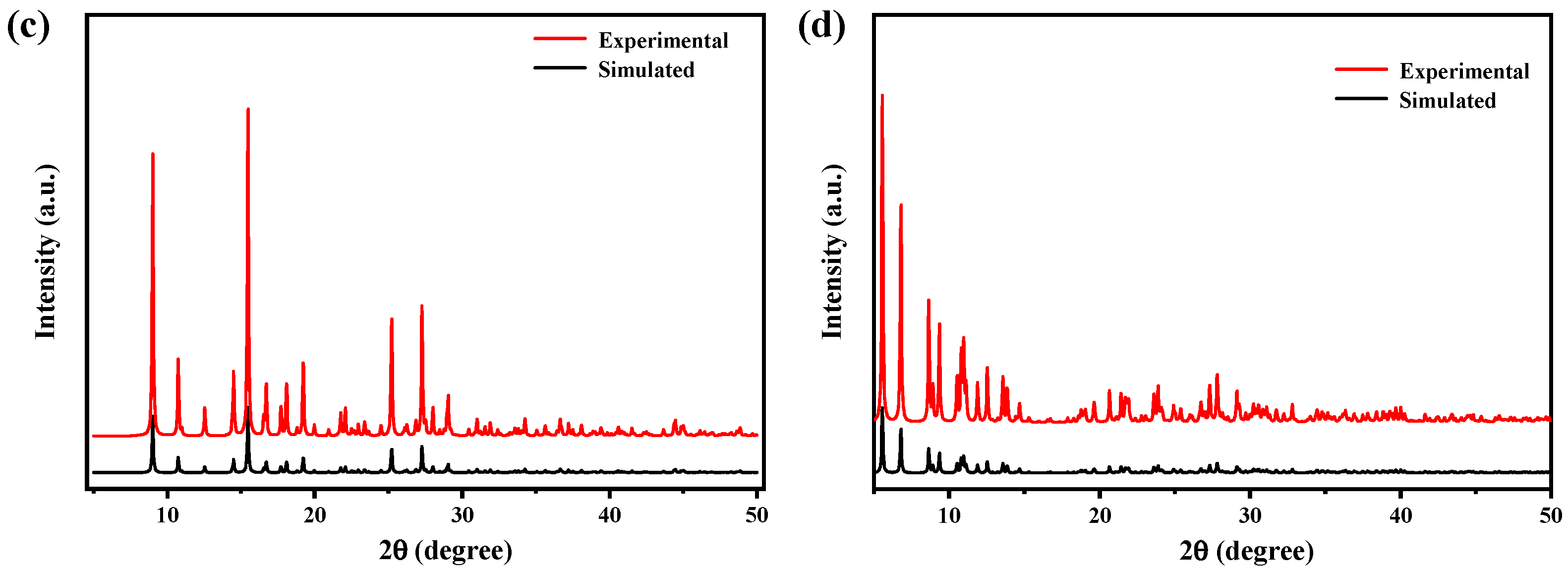
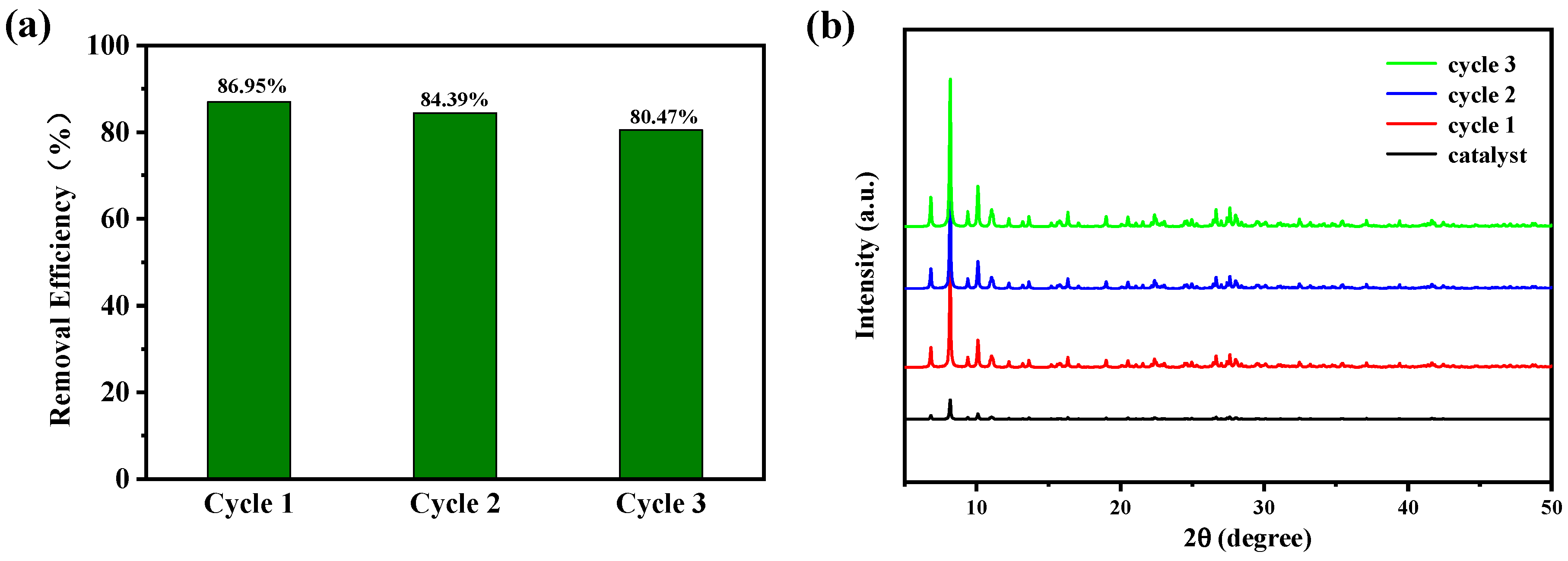
2.2. XRD Analysis
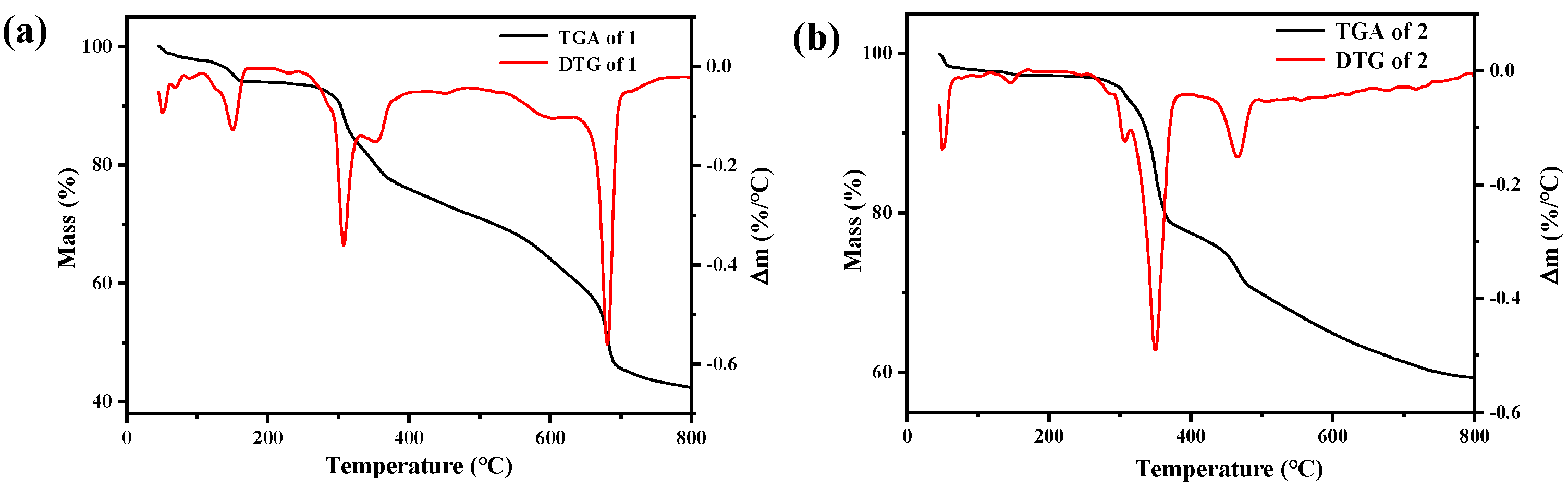
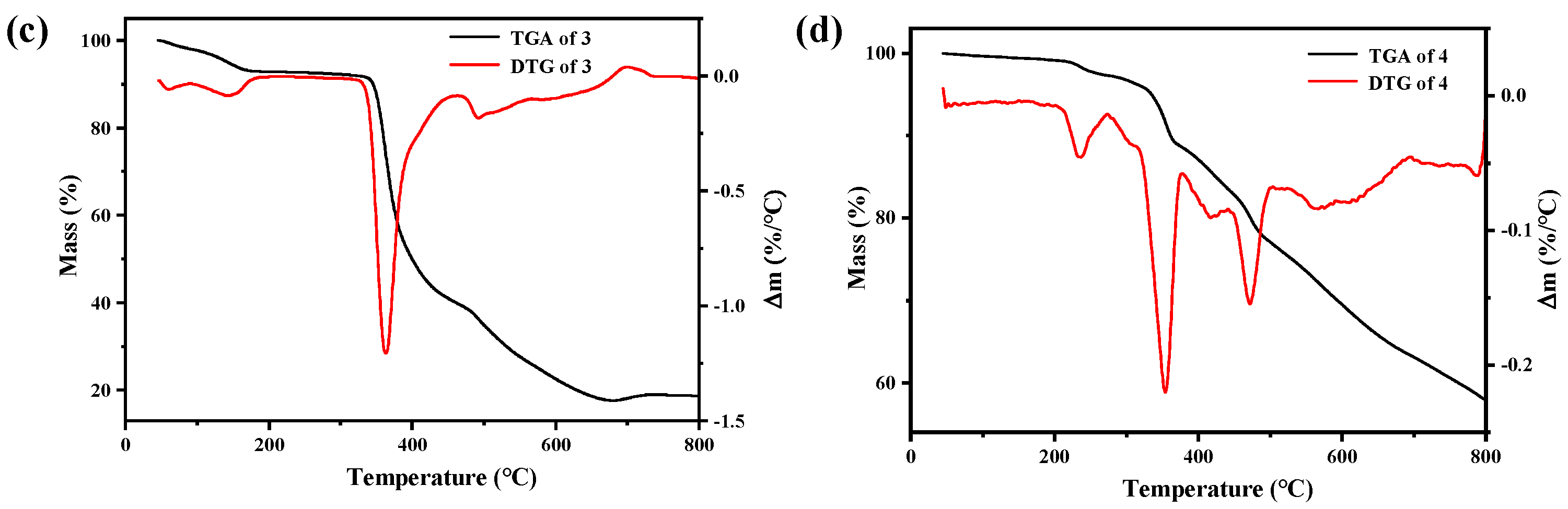
2.3. TG Analysis
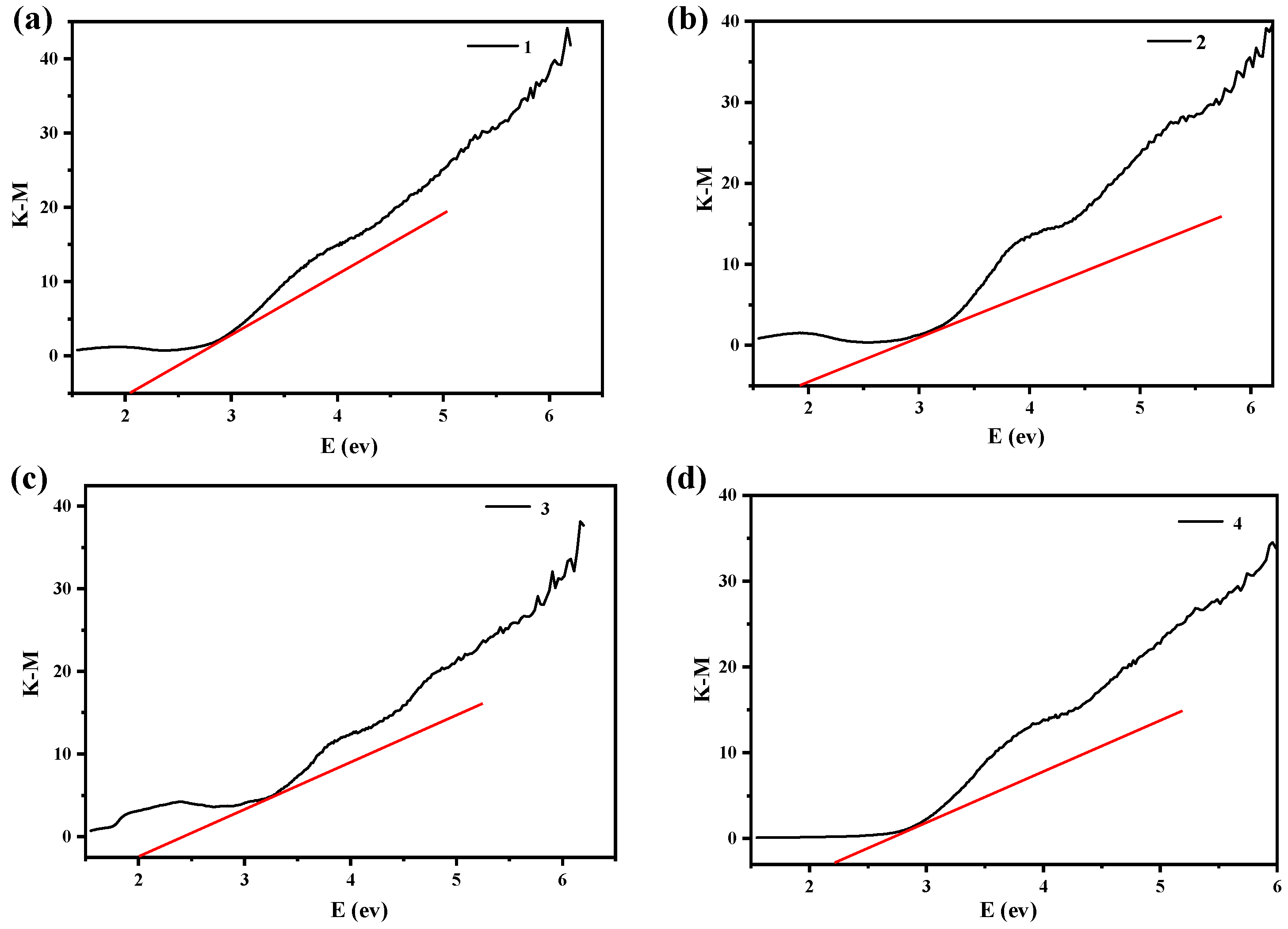
2.4. Band Gap Analysis
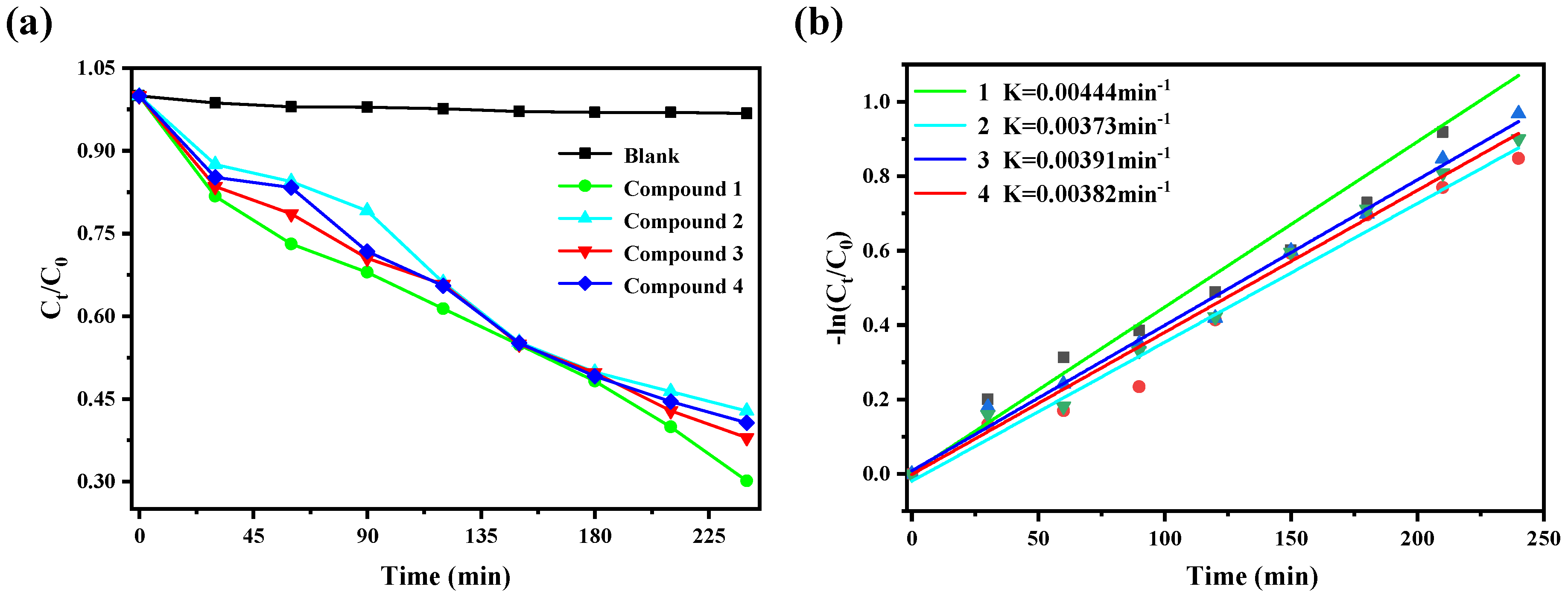
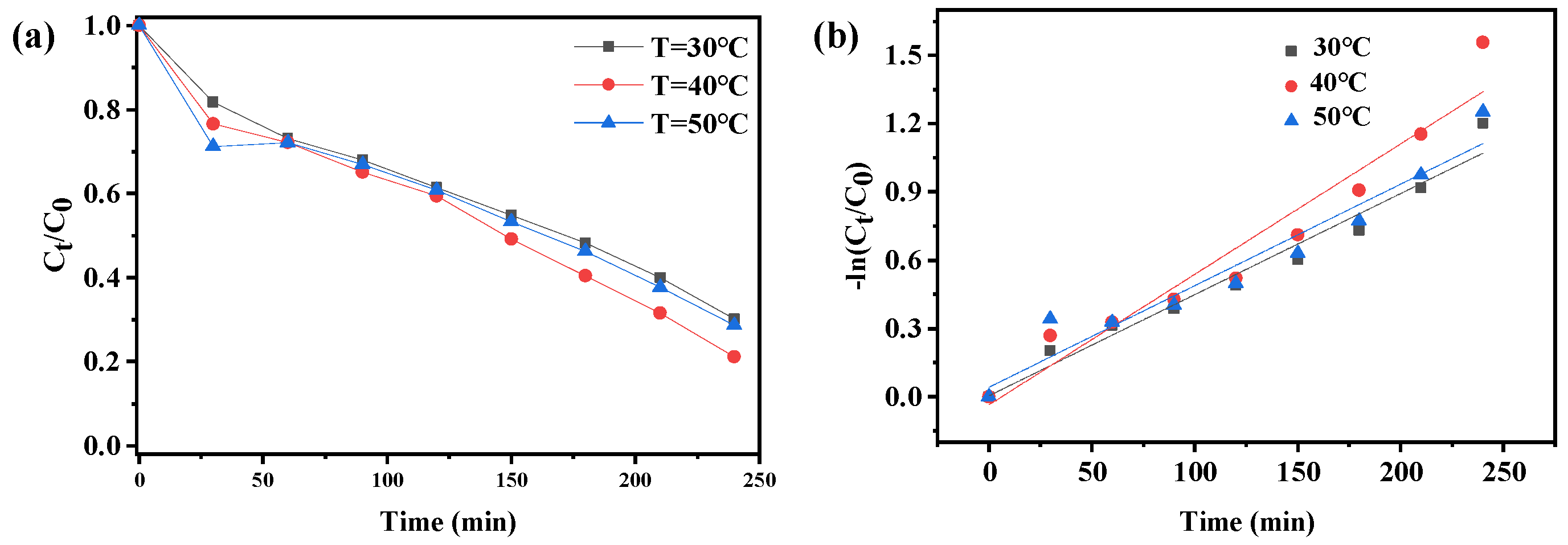
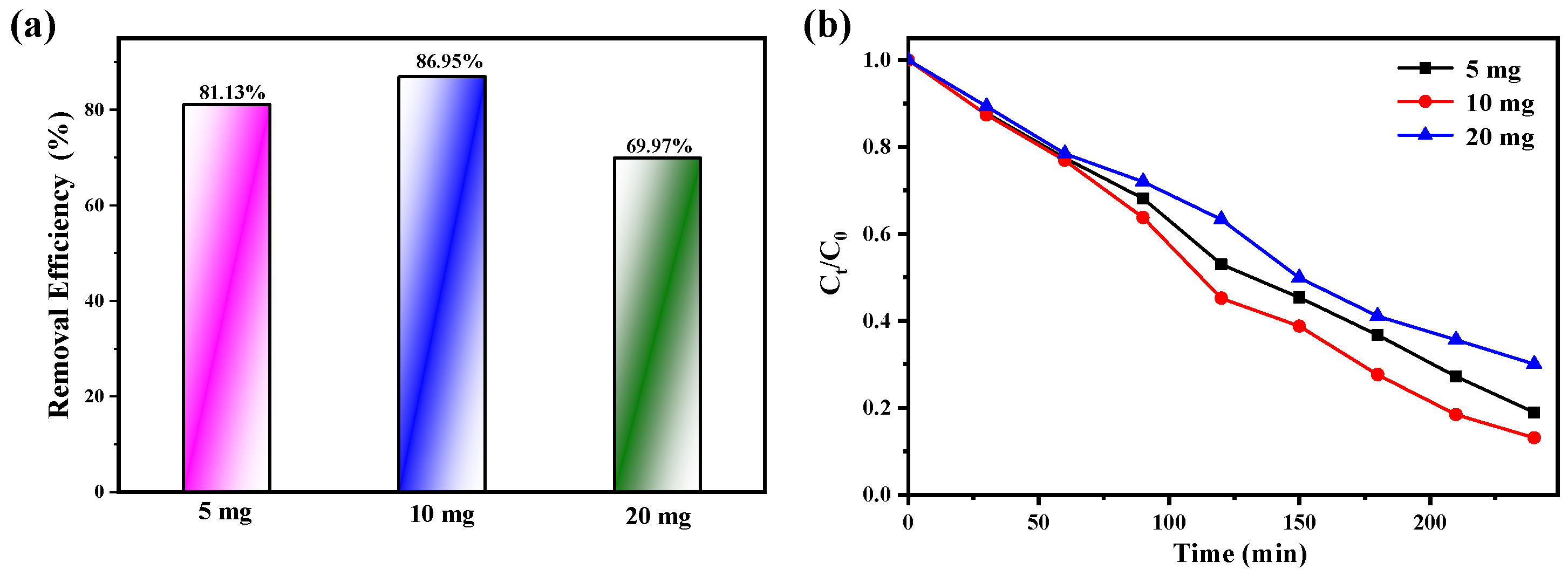
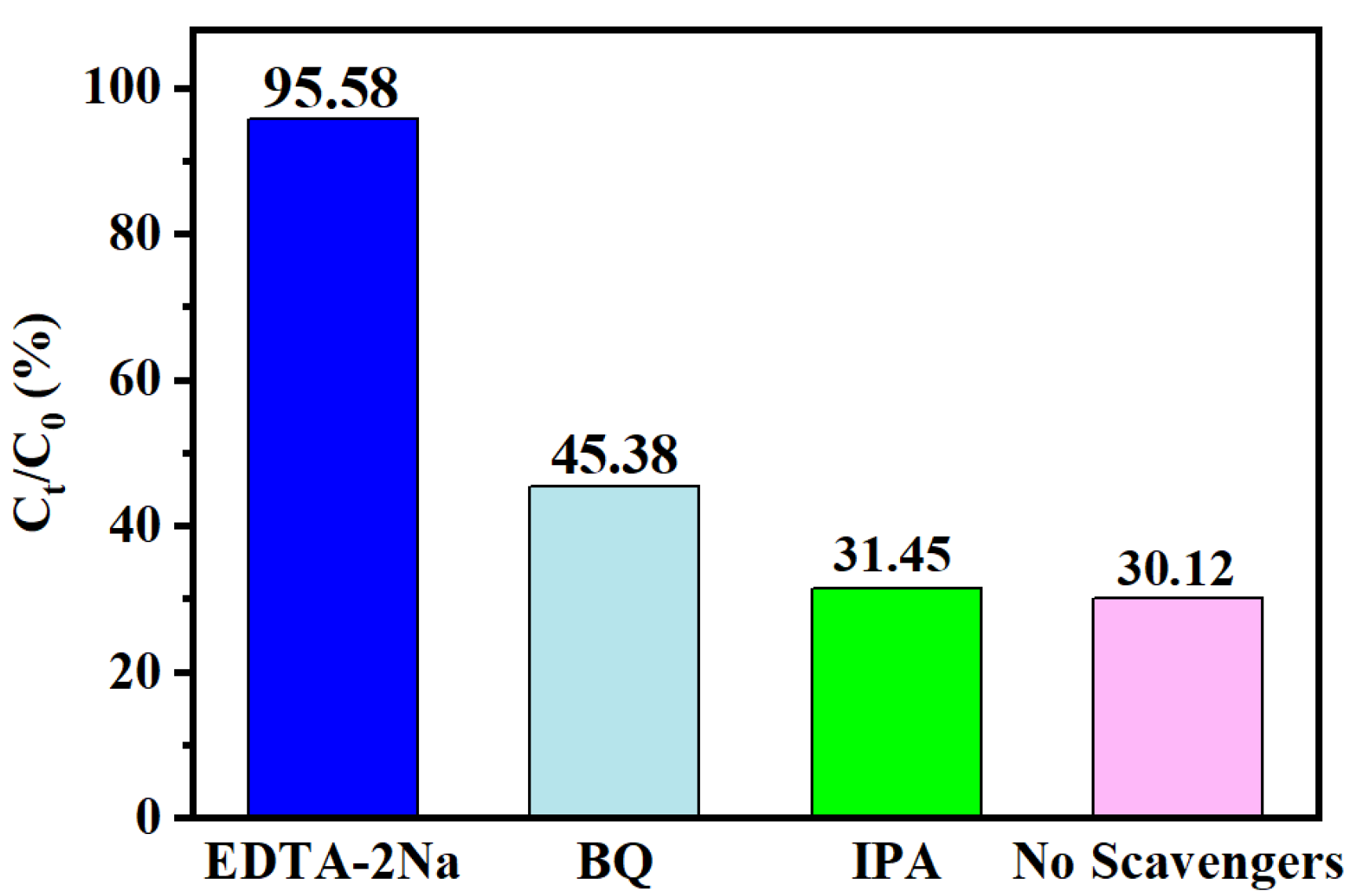
2.5. Photocatalytic Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis Methods
Synthesis of Compound 1
4.2.2. Characterization Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babić, S.; Periša, M.; Škorić, I. Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 2013, 91, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Antonin, V.S.; Santos, M.C.; Garcia-Segura, S.; Brillas, E. Electrochemical incineration of the antibiotic ciprofloxacin in sulfate medium and synthetic urine matrix. Water Res. 2015, 83, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Porras, J.; Bedoya, C.; Silva-Agredo, J.; Santamaría, A.; Fernández, J.J.; Torres-Palma, R.A. Role of humic substances in the degradation pathways and residual antibacterial activity during the photodecomposition of the antibiotic ciprofloxacin in water. Water Res. 2016, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.J.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B: Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, G.; Wang, C.; Niu, Y. Effective degradation of tetracycline by organic-inorganic hybrid materials induced by triethylenediamine. Environ. Res. 2021, 198, 111253. [Google Scholar] [CrossRef]
- Ali, I.; Han, G.; Kim, J. Reusability and photocatalytic activity of bismuth-TiO2 nanocomposites for industrial wastewater treatment. Environ. Res. 2019, 170, 222–229. [Google Scholar] [CrossRef]
- Qiu, H.; Fang, S.; Huang, G.; Bi, J. A novel application of In2S3 for visible-light-driven photocatalytic inactivation of bacteria: Kinetics, stability, toxicity and mechanism. Environ. Res. 2020, 190, 110018. [Google Scholar] [CrossRef]
- Li, W.; Zuo, Y.; Jiang, L.; Yao, D.; Chen, Z.; He, G.; Chen, H. Bi2Ti2O7/TiO2/RGO composite for the simulated sunlight-driven photocatalytic degradation of ciprofloxacin. Mater. Chem. Phys. 2020, 256, 123650. [Google Scholar] [CrossRef]
- Núñez-Salas, R.E.; Hernández-Ramírez, A.; Santos-Lozano, V.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Gracia-Pinilla, M.Á.; Maya-Treviño, M.d.L. Synthesis, characterization, and photocatalytic performance of FeTiO3/ZnO on ciprofloxacin degradation. J. Photochem. Photobiol. A Chem. 2021, 411, 113186. [Google Scholar] [CrossRef]
- Alhokbany, N.S.; Mousa, R.; Naushad, M.; Alshehri, S.M.; Ahamad, T. Fabrication of Z-scheme photocatalysts g-C3N4/Ag3PO4/chitosan for the photocatalytic degradation of ciprofloxacin. Int. J. Biol. Macromol. 2020, 164, 3864–3872. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Liu, C.; Wang, D.; Yan, G.; Liu, B.; Che, G. Enhanced visible-light-induced photocatalytic degradation of tetracycline using BiOI/MIL-125(Ti) composite photocatalyst. J. Alloy. Compd. 2021, 854, 157166. [Google Scholar] [CrossRef]
- Qiao, G.-Y.; Li, S.-M.; Niu, Y.-Y. One new copper iodide coordination polymer directed by 4-pyridyl dithioether ligand: Syntheses, structures, and photocatalysis. Main Group Chem. 2020, 19, 217–225. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, C.; Niu, Y. N-Benzyl HMTA induced self-assembly of organic-inorganic hybrid materials for efficient photocatalytic degradation of tetracycline. J. Hazard. Mater. 2020, 391, 122121. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New Strategies for Novel MOF-Derived Carbon Materials Based on Nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, G.; Yang, R.; Chu, F.; Chen, J.; Wang, L.; Huang, X. 2D metal-organic framework-based materials for electrocatalytic, photocatalytic and thermocatalytic applications. Nanoscale 2021, 13, 3911–3936. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.; Zhang, J.; Hu, Y. The development and application of metal-organic frameworks in the field of photocatalysis. Res. Chem. Intermed. 2021, 47, 325–343. [Google Scholar] [CrossRef]
- Dao, X.-Y.; Sun, W.-Y. Single- and mixed-metal–organic framework photocatalysts for carbon dioxide reduction. Inorg. Chem. Front. 2021, 8, 3178–3204. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, G.; Zhu, G.; Li, J.; Guo, X.; Liang, Y.; Niu, Y. Preparation of 2D supramolecular material doping with TiO2 for degradation of tetracycline. Environ. Res. 2021, 202, 111689. [Google Scholar] [CrossRef]
- Yu, D.; Shao, Q.; Song, Q.; Cui, J.; Zhang, Y.; Wu, B.; Ge, L.; Wang, Y.; Zhang, Y.; Qin, Y.; et al. A solvent-assisted ligand exchange approach enables metal-organic frameworks with diverse and complex architectures. Nat. Commun. 2020, 11, 927. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Alam Kabir, N.; Zhou, K.; Verpoort, F. Metal–organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy. Chem. Soc. Rev. 2013, 42, 9304–9332. [Google Scholar] [CrossRef]
- Wang, X.-J.; Qiao, X.-Y.; Niu, Y.-Y. Synthesis, characterization, adsorption and catalytic activity of a polyoxometalate supramolecule templated by arylmethylamine. Main Group Chem. 2020, 19, 187–198. [Google Scholar] [CrossRef]
- Niu, Y.; Song, Y.; Hou, H.; Zhu, Y. Synthesis, Structure, and Large Optical Limiting Effect of the First Coordination Polymeric Cluster Based on an {I@[AgI(inh)]6} Hexagram Block. Inorg. Chem. 2005, 44, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.; Liu, Y.; Liu, J.; Li, Y.; Qiao, X.; Huang, W.; Niu, Y. POM-based metal-organic compounds: Assembly, structures and properties. Main Group Chem. 2021, 20, 575–592. [Google Scholar] [CrossRef]
- Fang, H.; Oberoi, A.S.; He, Z.; Khanal, S.K.; Lu, H. Ciprofloxacin-degrading Paraclostridium sp. isolated from sulfate-reducing bacteria-enriched sludge: Optimization and mechanism. Water Res. 2021, 191, 116808. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean Prod. 2021, 291, 125880. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Sun, J.; Yao, F.; Wang, S.; Wang, Y.; Wang, X.; Li, X.; Niu, C.; Wang, D.; et al. Enhanced Photocatalytic Degradation of Tetracycline by AgI/BiVO4 Heterojunction under Visible-Light Irradiation: Mineralization Efficiency and Mechanism. ACS Appl. Mater. Interfaces 2016, 8, 32887–32900. [Google Scholar] [CrossRef]
- Lei, X.; Wang, J.; Shi, Y.; Yao, W.; Wu, Q.; Wu, Q.; Zou, R. Constructing novel red phosphorus decorated iron-based metal organic framework composite with efficient photocatalytic performance. Appl. Surf. Sci. 2020, 528, 146963. [Google Scholar] [CrossRef]
- Li, C.; Che, H.; Huo, P.; Yan, Y.; Liu, C.; Dong, H. Confinement of ultrasmall CoFe2O4 nanoparticles in hierarchical ZnIn2S4 microspheres with enhanced interfacial charge separation for photocatalytic H2 evolution. J. Colloid Interface Sci. 2021, 581, 764–773. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Tian, Y.; Song, C.; Qiu, H.; Xue, H. Piezotronic effect boosted photocatalytic performance of heterostructured BaTiO3/TiO2 nanofibers for degradation of organic pollutants. Nano Energy 2020, 77, 105122. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Jiang, L.; Yuan, X.; Fei, J.; Zhang, W. Photocatalytic removal of antibiotics by MOF-derived Ti3+- and oxygen vacancy-doped anatase/rutile TiO2 distributed in a carbon matrix. Chem. Eng. J. 2022, 427, 130945. [Google Scholar] [CrossRef]
- Ji, Z.; Di, Z.; Li, H.; Zou, S.; Wu, M.; Hong, M. A flexible Zr-MOF with dual stimulus responses to temperature and guest molecules. Inorg. Chem. Commun. 2021, 128, 108597. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Kansal, S.K. CdS-Decorated MIL-53(Fe) Microrods with Enhanced Visible Light Photocatalytic Performance for the Degradation of Ketorolac Tromethamine and Mechanism Insight. J. Phys. Chem. C 2019, 123, 16857–16867. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chai, J.; Xue, S.; Wang, R.; Jiang, L.; Wang, J.; Zhang, Z.; Dionysiou, D.D. Construction of novel symmetric double Z-scheme BiFeO3/CuBi2O4/BaTiO3 photocatalyst with enhanced solar-light-driven photocatalytic performance for degradation of norfloxacin. Appl. Catal. B. 2020, 272, 119017. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; Zeng, G.; Xue, W.; Chen, S.; Li, Z.; Deng, R.; Yang, Y.; Cheng, M. Facile construction of hierarchical flower-like Z-scheme AgBr/Bi2WO6 photocatalysts for effective removal of tetracycline: Degradation pathways and mechanism. Chem. Eng. J. 2019, 375, 121991. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.; Na, F.; Wei, J.; Xu, S.; Li, Y.; Guo, F. Fabrication of CdS/titanium-oxo-cluster nanocomposites based on a Ti32 framework with enhanced photocatalytic activity for tetracycline hydrochloride degradation under visible light. Appl. Catal. B: Environ. 2019, 254, 541–550. [Google Scholar] [CrossRef]
- Gan, H.; Yi, F.; Zhang, H.; Qian, Y.; Jin, H.; Zhang, K. Facile ultrasonic-assisted synthesis of micro-nanosheet structure Bi4Ti3O12/g-C3N4 composites with enhanced photocatalytic activity on organic pollutants. Chin. J. Chem. Eng. 2018, 26, 2628–2635. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, X.; Niu, Y. M-Carboxylic Acid Induced Formation of New Coordination Polymers for Efficient Photocatalytic Degradation of Ciprofloxacin. Molecules 2022, 27, 7731. https://doi.org/10.3390/molecules27227731
Li J, Wang X, Niu Y. M-Carboxylic Acid Induced Formation of New Coordination Polymers for Efficient Photocatalytic Degradation of Ciprofloxacin. Molecules. 2022; 27(22):7731. https://doi.org/10.3390/molecules27227731
Chicago/Turabian StyleLi, Jian, Xiaojia Wang, and Yunyin Niu. 2022. "M-Carboxylic Acid Induced Formation of New Coordination Polymers for Efficient Photocatalytic Degradation of Ciprofloxacin" Molecules 27, no. 22: 7731. https://doi.org/10.3390/molecules27227731
APA StyleLi, J., Wang, X., & Niu, Y. (2022). M-Carboxylic Acid Induced Formation of New Coordination Polymers for Efficient Photocatalytic Degradation of Ciprofloxacin. Molecules, 27(22), 7731. https://doi.org/10.3390/molecules27227731





