Rust Conversion of Proanthocyanidins to Archaeological Steel: A Case Study of Lingzhao Xuan in the Forbidden City
Abstract
1. Introduction
2. Results and Discussion
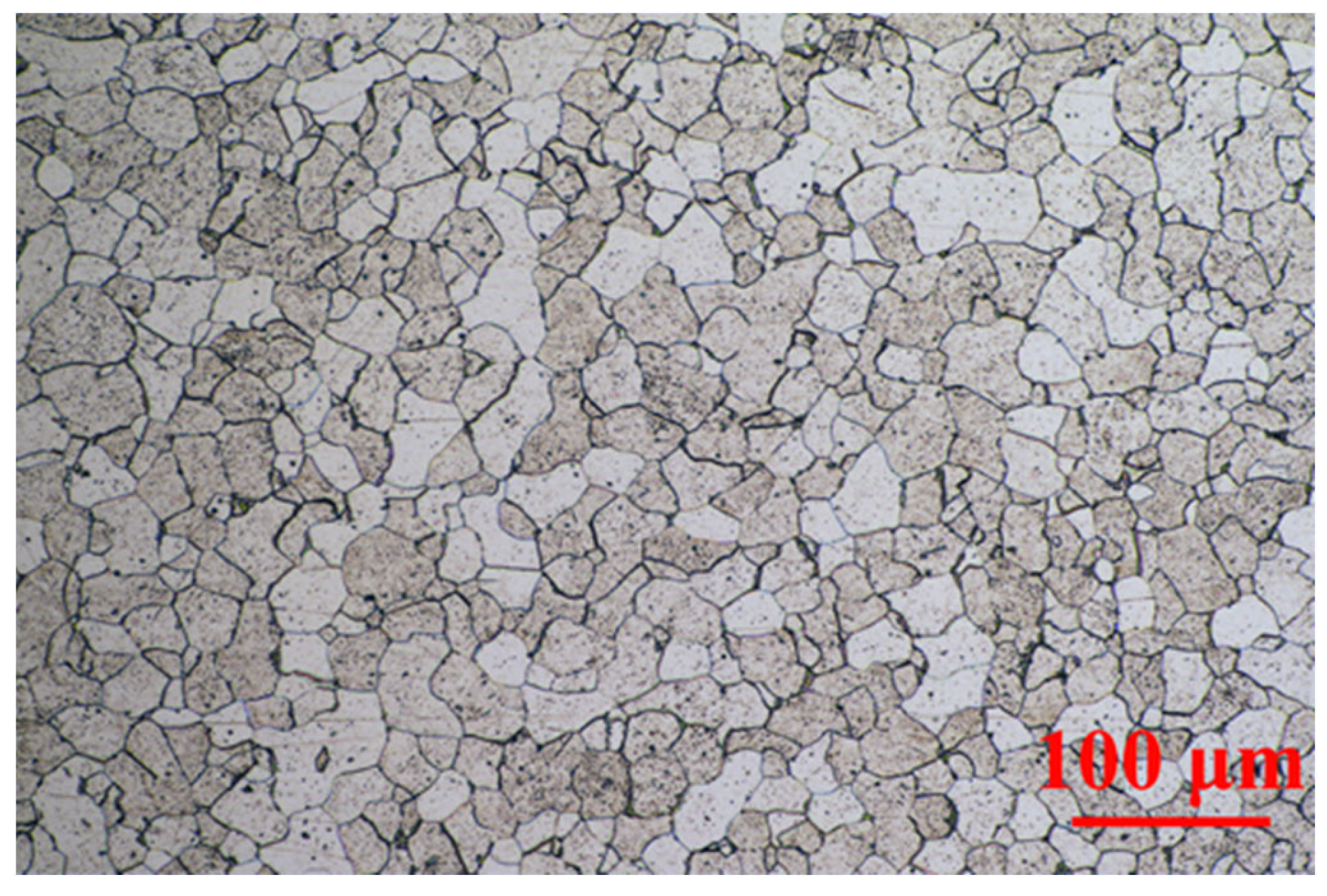
2.1. Metallographic Structure
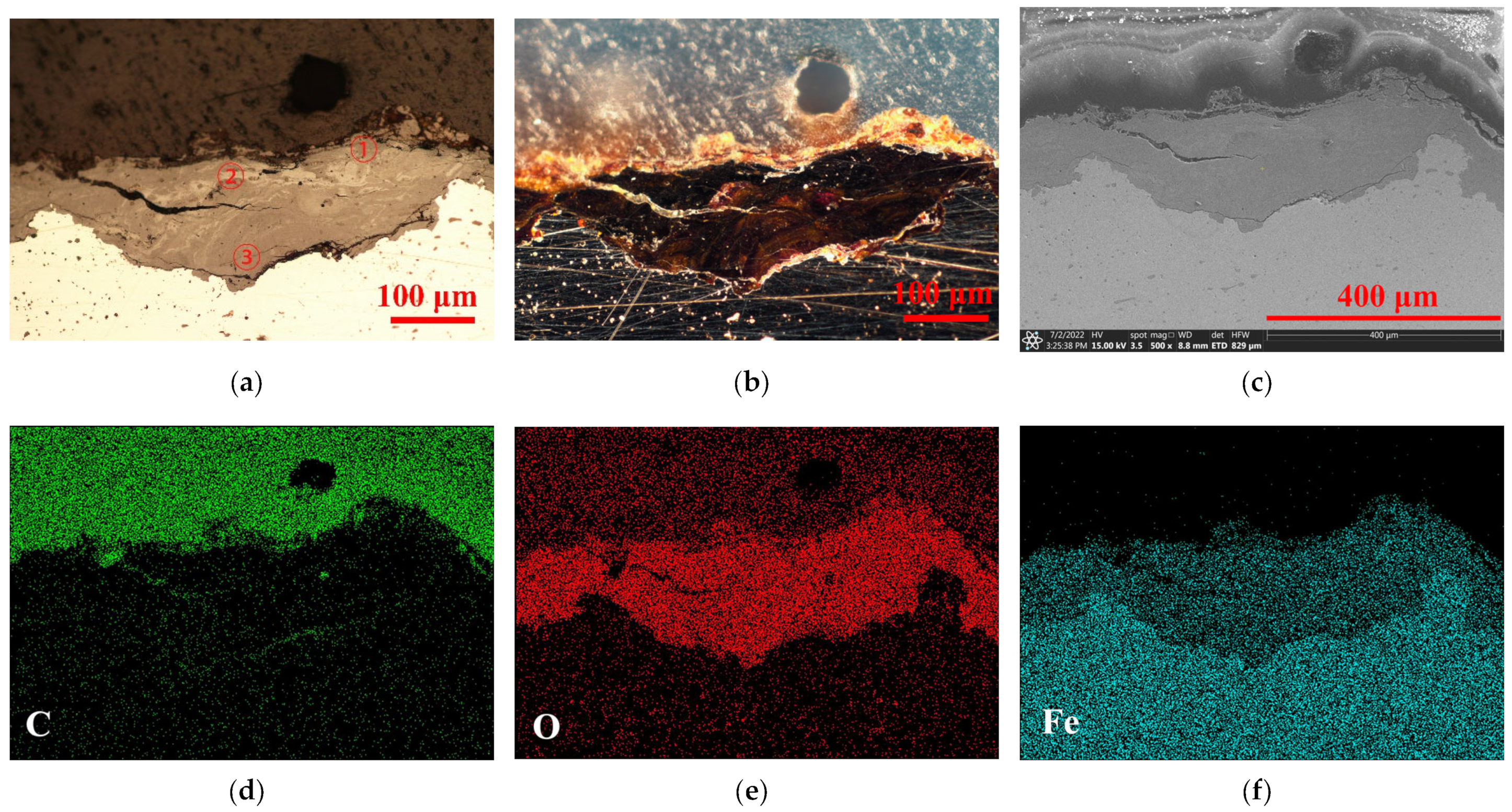
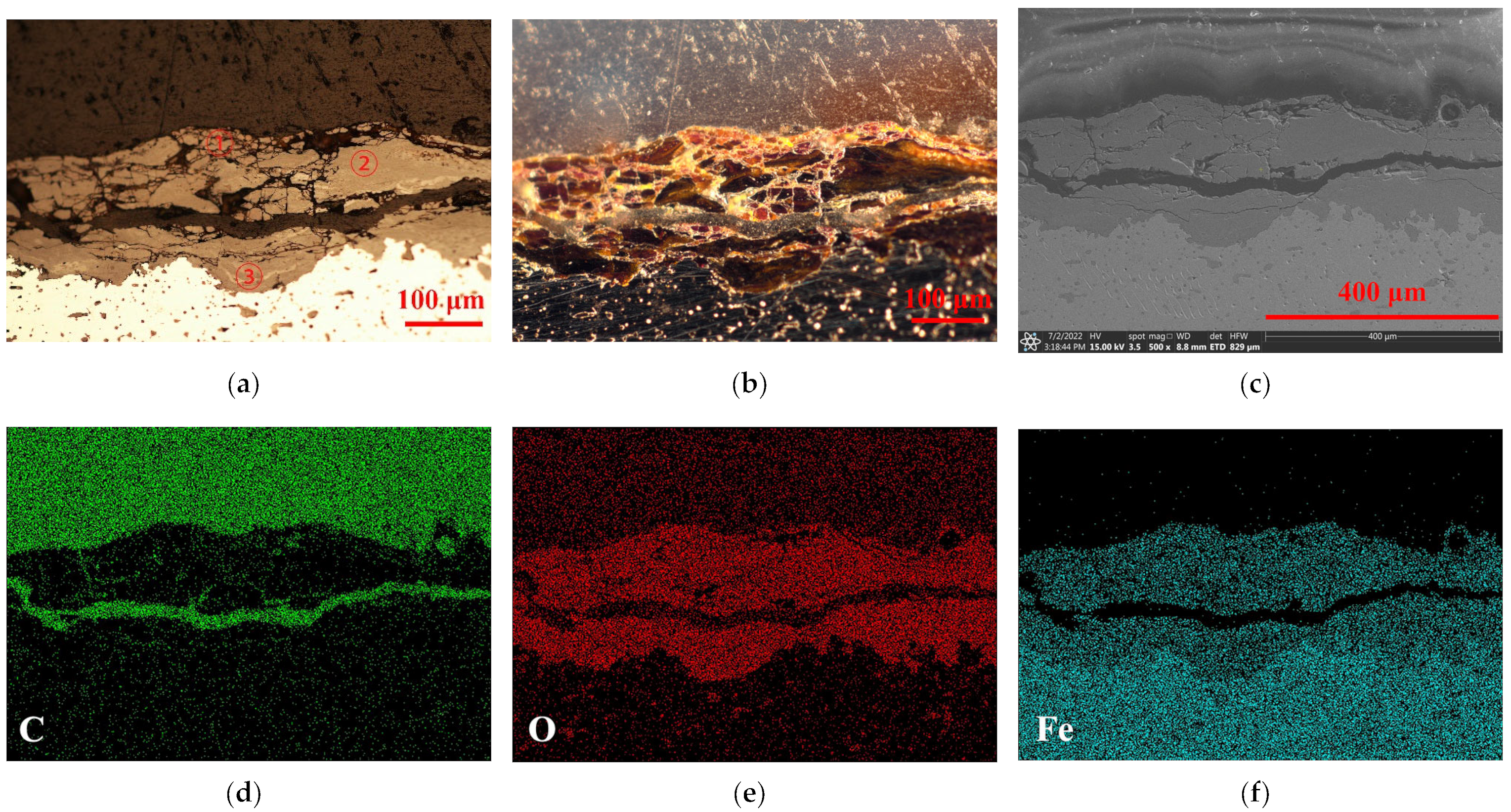
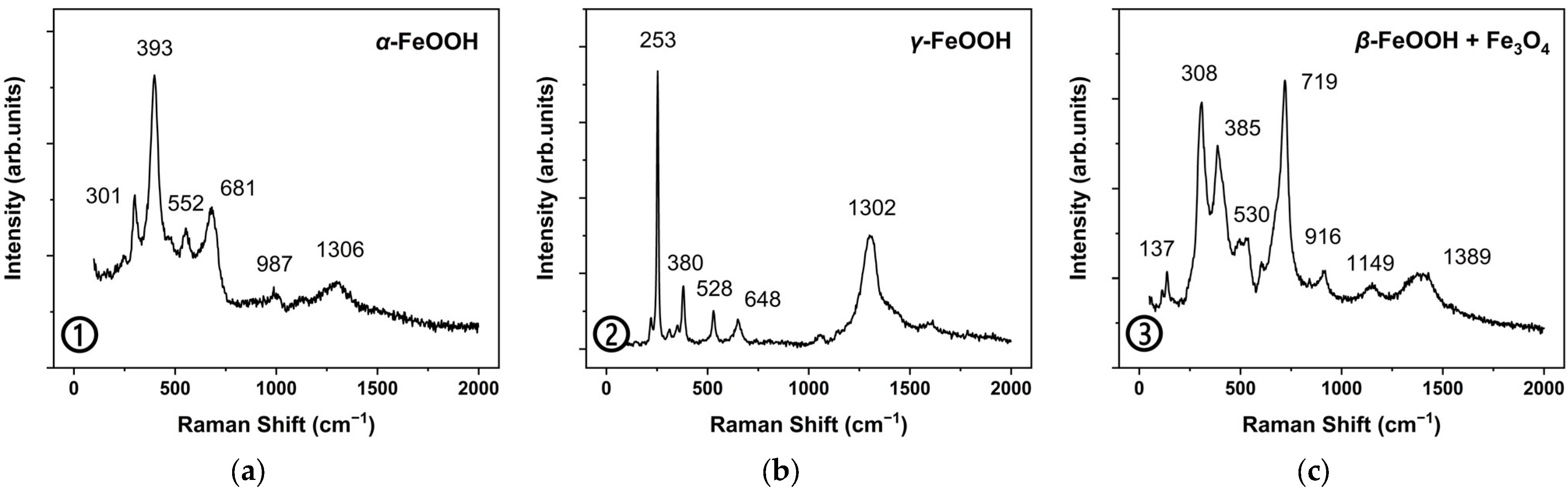
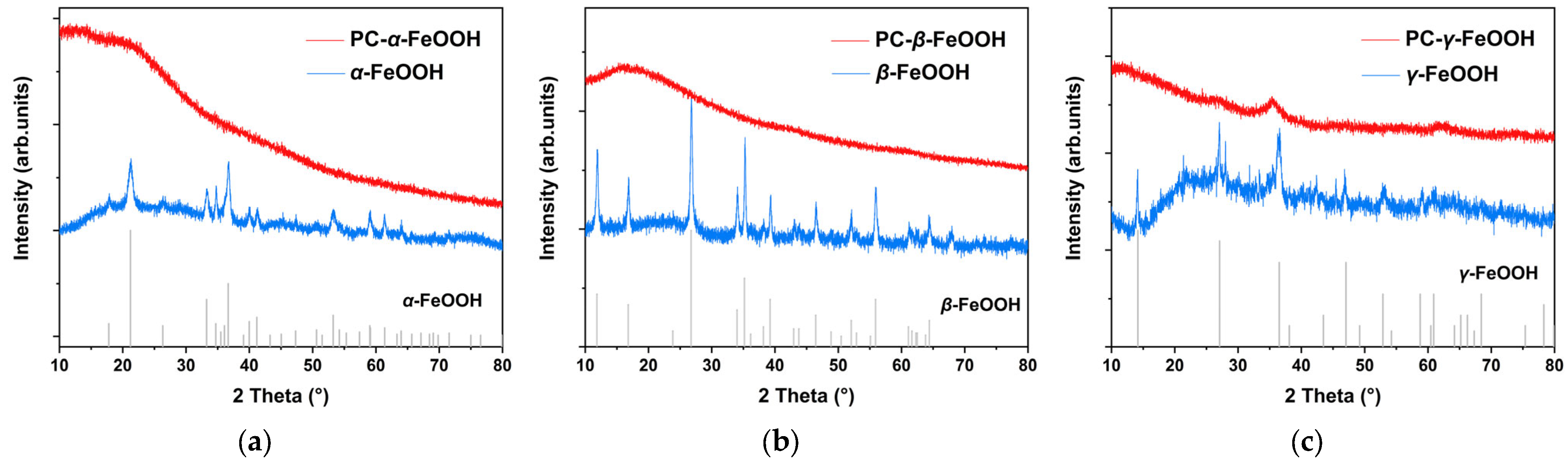
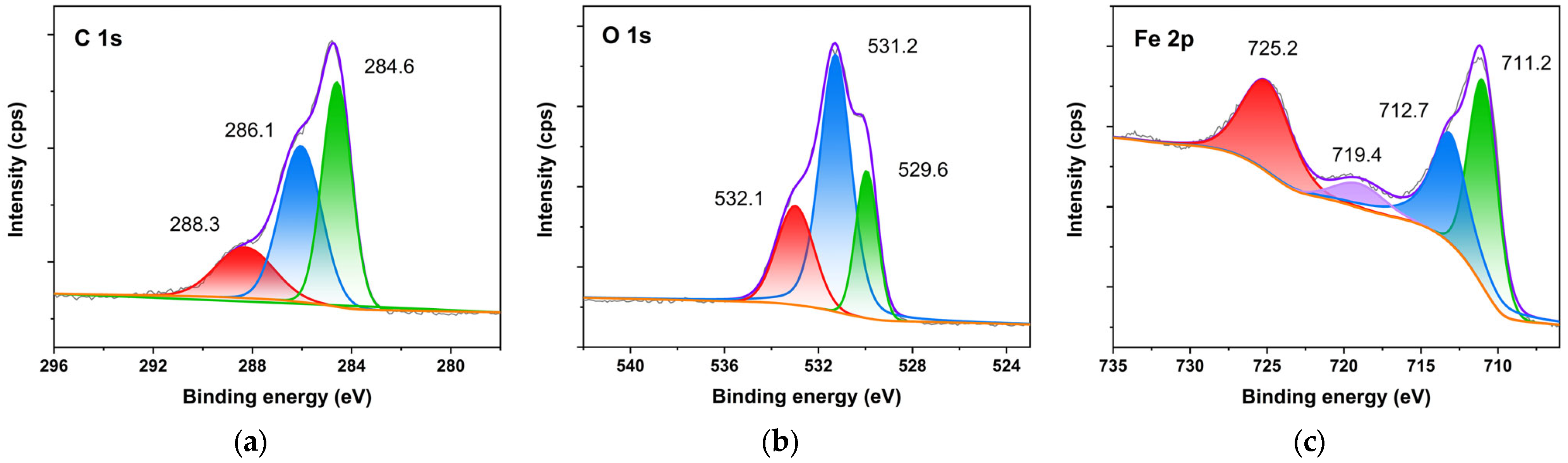
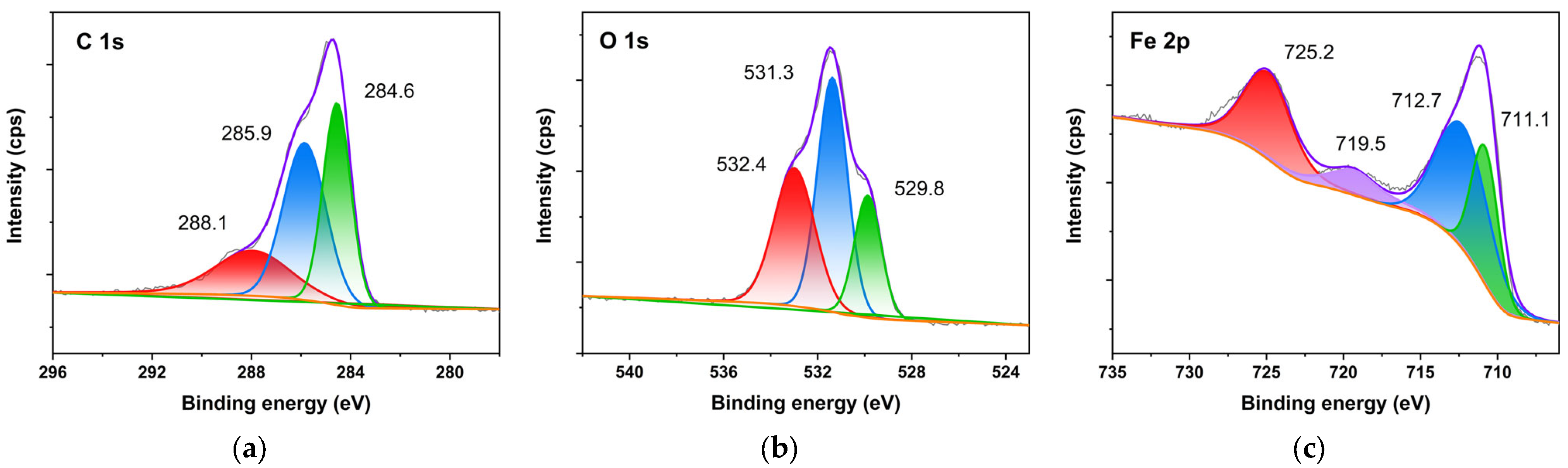
2.2. Original Rust Composition
2.3. Rust Conversion
2.4. Surface Morphology
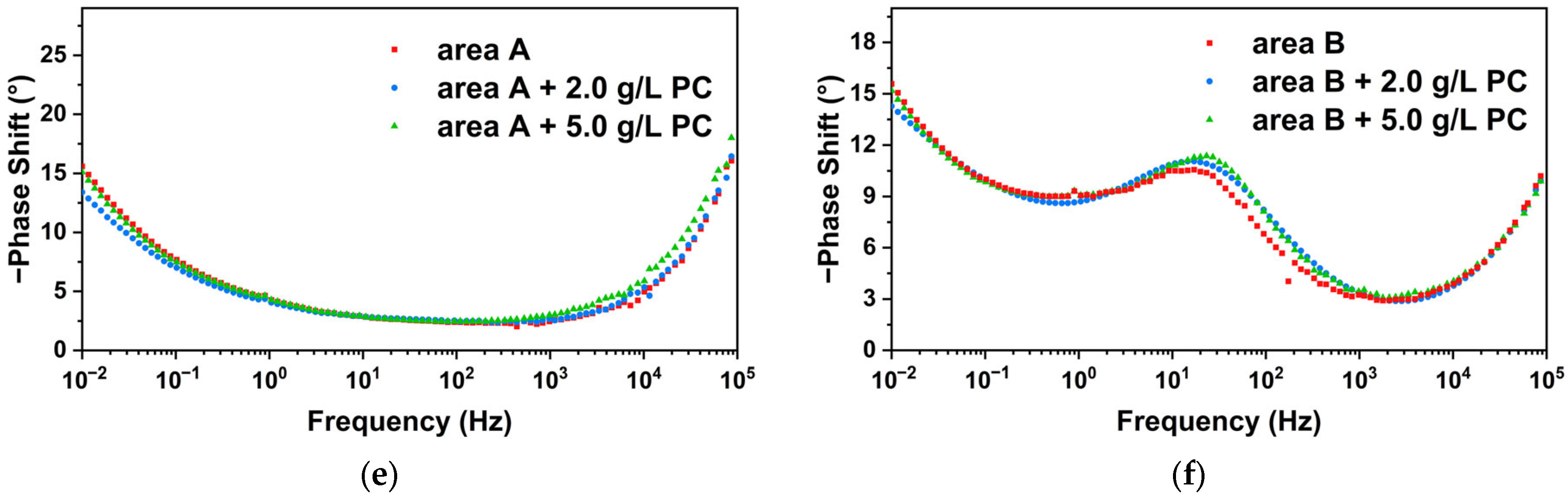
2.5. Electrochemical Analysis
3. Materials and Methods
3.1. Archaeological Objects
3.2. Samples Preparation
3.2.1. Rust Powder
Synthesis of PC-FeOOH
Modification of Original Rust
3.2.2. Metallographic Samples
3.2.3. Working Electrodes
3.3. Morphology Observation
3.4. Determination of Rust Conversion
3.5. Electrochemical Impedance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jasniok, T.; Jasniok, M.; Skorkowski, A. Diagnostics of Large Non-Conductive Anti-Corrosion Coatings on Steel Structures by Means of Electrochemical Impedance Spectroscopy. Materials 2021, 14, 3539. [Google Scholar] [CrossRef] [PubMed]
- Vasconez-Maza, M.D.; Martinez-Pagan, P.; Aktarakci, H.; Garcia-Nieto, M.C.; Martinez-Segura, M.A. Enhancing Electrical Contact with a Commercial Polymer for Electrical Resistivity Tomography on Archaeological Sites: A Case Study. Materials 2020, 13, 5012. [Google Scholar] [CrossRef] [PubMed]
- Calero, J.; Alcantara, J.; Chico, B.; Diaz, I.; Simancas, J.; de la Fuente, D.; Morcillo, M. Wet/dry accelerated laboratory test to simulate the formation of multilayered rust on carbon steel in marine atmospheres. Corros. Eng. Sci. Technol. 2017, 52, 178–187. [Google Scholar] [CrossRef]
- Qian, B.; Hou, B.; Zheng, M. The inhibition effect of tannic acid on mild steel corrosion in seawater wet/dry cyclic conditions. Corros. Sci. 2013, 72, 1–9. [Google Scholar] [CrossRef]
- Aourabi, S.; Driouch, M.; Sfaira, M.; Mahjoubi, F.; Hammouti, B.; Verma, C.; Ebenso, E.E.; Guo, L. Phenolic fraction of Ammi visnaga extract as environmentally friendly antioxidant and corrosion inhibitor for mild steel in acidic medium. J. Mol. Liq. 2021, 323, 114950. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.-R.; Li, H.-B.; Wu, D.-T.; Geng, F.; Corke, H.; Wei, X.-L.; Gan, R.-Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Domenech, A.; Lastras, M.; Rodriguez, F.; Osete, L. Mapping of corrosion products of highly altered archeological iron using voltammetry of microparticles. Microchem. J. 2013, 106, 41–50. [Google Scholar] [CrossRef]
- Reitzer, F.; Allais, M.; Ball, V.; Meyer, F. Polyphenols at interfaces. Adv. Colloid Interface Sci. 2018, 257, 31–41. [Google Scholar] [CrossRef]
- Chun, Q.; Zhang, J.W.; Zhao, P.; Han, Y.D.; Meng, Z. Research on damage to and structural performance of Lingzhao Xuan in the Forbidden City. Sci. Consev. Archaeol. 2018, 30, 40–46. [Google Scholar]
- Zhou, Q.; Yan, W.M.; Ji, J.B. Dynamic characteristic and seismic responses of steel structure of Ling-zhao Veranda in the Palace Museum. J. Shandong Univ. Eng. Sci. 2016, 46, 70–79. [Google Scholar]
- Caponetti, E.; Francesco, A.; Martino, D.C.; Saladino, M.L.; Ridolfi, S.; Chirco, G.; Berrettoni, M.; Conti, P.; Bruno, N.; Tusa, S. First discovery of orichalcum ingots from the remains of a 6th century bc shipwreck near Gela (Sicily) seabed. Mediterr. Archaeol. Archaeom. 2017, 17, 11–18. [Google Scholar]
- Quaranta, M.; Catelli, E.; Prati, S.; Sciutto, G.; Mazzeo, R. Chinese archaeological artefacts: Microstructure and corrosion behaviour of high-leaded bronzes. J. Cult. Herit. 2014, 15, 283–291. [Google Scholar] [CrossRef]
- Ingo, G.M.; De Caro, T.; Riccucci, C.; Angelini, E.; Grassini, S.; Balbi, S.; Bernardini, P.; Salvi, D.; Bousselmi, L.; Cilingiroglu, A.; et al. Large scale investigation of chemical composition, structure and corrosion mechanism of bronze archeological artefacts from Mediterranean basin. Appl. Phys. A 2006, 83, 513–520. [Google Scholar] [CrossRef]
- Grevey, A.L.; Vignal, V.; Krawiec, H.; Ozga, P.; Peche-Quilichini, K.; Rivalan, A.; Maziere, F. Microstructure and long-term corrosion of archaeological iron alloy artefacts. Herit. Sci. 2020, 8, 57. [Google Scholar] [CrossRef]
- Bernabale, M.; Nigro, L.; Vaccaro, C.; Nicoli, M.; Montanari, D.; Bigini, P.; De Vito, C. Micro-Raman spectroscopy and complementary techniques for the study of iron weapons from Motya and Lilybaeum (Sicily, Italy): Corrosion patterns in lagoon-like and calcarenitic hypogea environments. J. Raman Spectrosc. 2022, 53, 272–287. [Google Scholar] [CrossRef]
- Morcillo, M.; Wolthuis, R.; Alcantara, J.; Chico, B.; Diaz, I.; de la Fuente, D. Scanning Electron Microscopy/Micro-Raman: A Very Useful Technique for Characterizing the Morphologies of Rust Phases Formed on Carbon Steel in Atmospheric Exposures. Corrosion 2016, 72, 1044–1054. [Google Scholar]
- Letardi, P.; Salvadori, B.; Galeotti, M.; Cagnini, A.; Porcinai, S.; Santagostino Barbone, A.; Sansonetti, A. An in situ multi-analytical approach in the restoration of bronze artefacts. Microchem. J. 2016, 125, 151–158. [Google Scholar] [CrossRef]
- Hoerle, S.; Mazaudier, F.; Dillmann, P.; Santarini, G. Advances in understanding atmospheric corrosion of iron. II. Mechanistic modelling of wet-dry cycles. Corros. Sci. 2004, 46, 1431–1465. [Google Scholar] [CrossRef]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Estalayo, E.; Aramendia, J.; Mates Luque, J.M.; Manuel Madariaga, J. Chemical study of degradation processes in ancient metallic materials rescued from underwater medium. J. Raman Spectrosc. 2019, 50, 289–298. [Google Scholar] [CrossRef]
- Hu, P.; Jia, M.H.; Li, M.H.; Sun, J.; Cui, Y.; Hu, D.B.; Hu, G. Corrosion Behavior of Ancient White Cast Iron Artifacts from Marine Excavations at Atmospheric Condition. Metals 2022, 12, 921. [Google Scholar] [CrossRef]
- Lair, V.; Antony, H.; Legrand, L.; Chausse, A. Electrochemical reduction of ferric corrosion products and evaluation of galvanic coupling with iron. Corros. Sci. 2006, 48, 2050–2063. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage-An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Flores Merino, S.; Jose Caprari, J.; Vasquez Torres, L. Inhibitive action of tara tannin in rust converter formulation. Anti-Corros. Methods Mater. 2017, 64, 136–147. [Google Scholar] [CrossRef]
- Alsabagh, A.M.; Migahed, M.A.; Abdelraouf, M.; Khamis, E.A. Utilization of Green Tea as Environmentally Friendly Corrosion Inhibitor for Carbon Steel in acidic media. Inter. J. Electrochem. Sci. 2015, 10, 1855–1872. [Google Scholar]
- Wang, D.Y.; Nie, B.L.; Li, H.J.; Zhang, W.W.; Wu, Y.C. Anticorrosion performance of grape seed proanthocyanidins extract and Tween-80 for mild steel in hydrochloric acid medium. J. Mol. Liq. 2021, 331, 115799. [Google Scholar] [CrossRef]
- Grigsby, W.J. Simulating the protective role of bark proanthocyanidins in surface coatings: Unexpected beneficial photo-stabilisation of exposed timber surfaces. Prog. Org. Coat. 2017, 110, 55–61. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.X.; Zheng, Y.F.; Zhao, J.W.; Yu, H.L.; Zhu, J.J. The Relationship between Procyanidin Structure and Their Protective Effect in a Parkinson’s Disease Model. Molecules 2022, 27, 5007. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape seed proanthocyanidin extract inhibits DNA and protein damage and labile iron, enzyme, and cancer cell activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef]
- Wang, D.Y.; Li, H.J.; Chen, X.; Nie, B.L.; Wu, Y.C. Fabricating of grape seed proanthocyanidins loaded Zein-NaCas composite nanoparticles to exert effective inhibition of Q235 steel corrosion in seawater. J. Mol. Liq. 2022, 348, 118467. [Google Scholar] [CrossRef]
- Huang, L.; Yang, K.P.; Zhao, Q.; Li, H.J.; Wang, J.Y.; Wu, Y.C. Corrosion resistance and antibacterial activity of procyanidin B2 as a novel environment-friendly inhibitor for Q235 steel in 1 M HCl solution. Bioelectrochemistry 2022, 143, 107969. [Google Scholar] [CrossRef]
- Guedes, D.; Martins, G.R.; Jaramillo, L.Y.A.; Bernardes Dias, D.S.; da Silva, A.J.R.; Lutterbach, M.T.S.; Reznik, L.Y.; Servulo, E.F.C.; Alviano, C.S.; Alviano, D.S. Proanthocyanidins with Corrosion Inhibition Activity for AISI 1020 Carbon Steel under Neutral pH Conditions of Coconut (Cocos nucifera L.) Husk Fibers. ACS Omega 2021, 6, 6893–6901. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1 M HCl. Mater. Chem. Phys. 2011, 125, 461–468. [Google Scholar] [CrossRef]
- Abdallah, M. Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 2002, 44, 717–728. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Zhao, X.D.; Cheng, Y.F.; Fan, W.; Vladimir, C.; Volha, V.; Alla, T. Inhibitive Performance of a Rust Converter on Corrosion of Mild Steel. J. Mater. Eng. Perform. 2014, 23, 4102–4108. [Google Scholar] [CrossRef]
- Mohapatra, J.N.; Babu, T.S.; Dabbiru, S.K.; Balachandran, G. Magnetic Hysteresis Loop as a Tool for the Evaluation of Mechanical Properties of Hypoeutectoid Pearlitic Steels with Spheroidization Heat Treatmemt. J. Nondestr. Eval. 2021, 40, 73. [Google Scholar] [CrossRef]
- Saleh, S.A. Corrosion Mechanism of Iron Objects in Marine Environment an Analytical Investigation Study by Raman Spectrometry. Eur. J. Sci. Theol. 2017, 13, 185–206. [Google Scholar]
- Rocca, E.; Faiz, H.; Dillmann, P.; Neff, D.; Mirambet, F. Electrochemical behavior of thick rust layers on steel artefact: Mechanism of corrosion inhibition. Electrochim. Acta 2019, 316, 219–227. [Google Scholar] [CrossRef]
- Selvi, I.K.; Nagarajan, S. Separation of catechins from green tea (Camellia sinensis L.) by microwave assisted acetylation, evaluation of antioxidant potential of individual components and spectroscopic analysis. LWT 2018, 91, 391–397. [Google Scholar] [CrossRef]
- Zhang, R.N.; Li, L.; Liu, J.X. Synthesis and characterization of ferric tannate as a novel porous adsorptive-catalyst for nitrogen removal from wastewater. RSC Adv. 2015, 5, 40785–40791. [Google Scholar] [CrossRef]
- Xu, W.H.; Han, E.H.; Wang, Z.Y. Effect of tannic acid on corrosion behavior of carbon steel in NaCl solution. J. Mater. Sci. Technol. 2019, 35, 64–75. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.W.; Zhang, W.H.; Guo, H.; He, X.L. Influence of outer rust layers on corrosion of carbon steel and weathering steel during wet-dry cycles. Corros. Sci. 2014, 82, 165–172. [Google Scholar] [CrossRef]
- Han, D.; Jiang, R.J.; Cheng, Y.F. Mechanism of electrochemical corrosion of carbon steel under deoxygenated water drop and sand deposit. Electrochim. Acta 2013, 114, 403–408. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pebere, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric Properties of Materials Showing Constant-Phase-Element (CPE) Impedance Response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef]







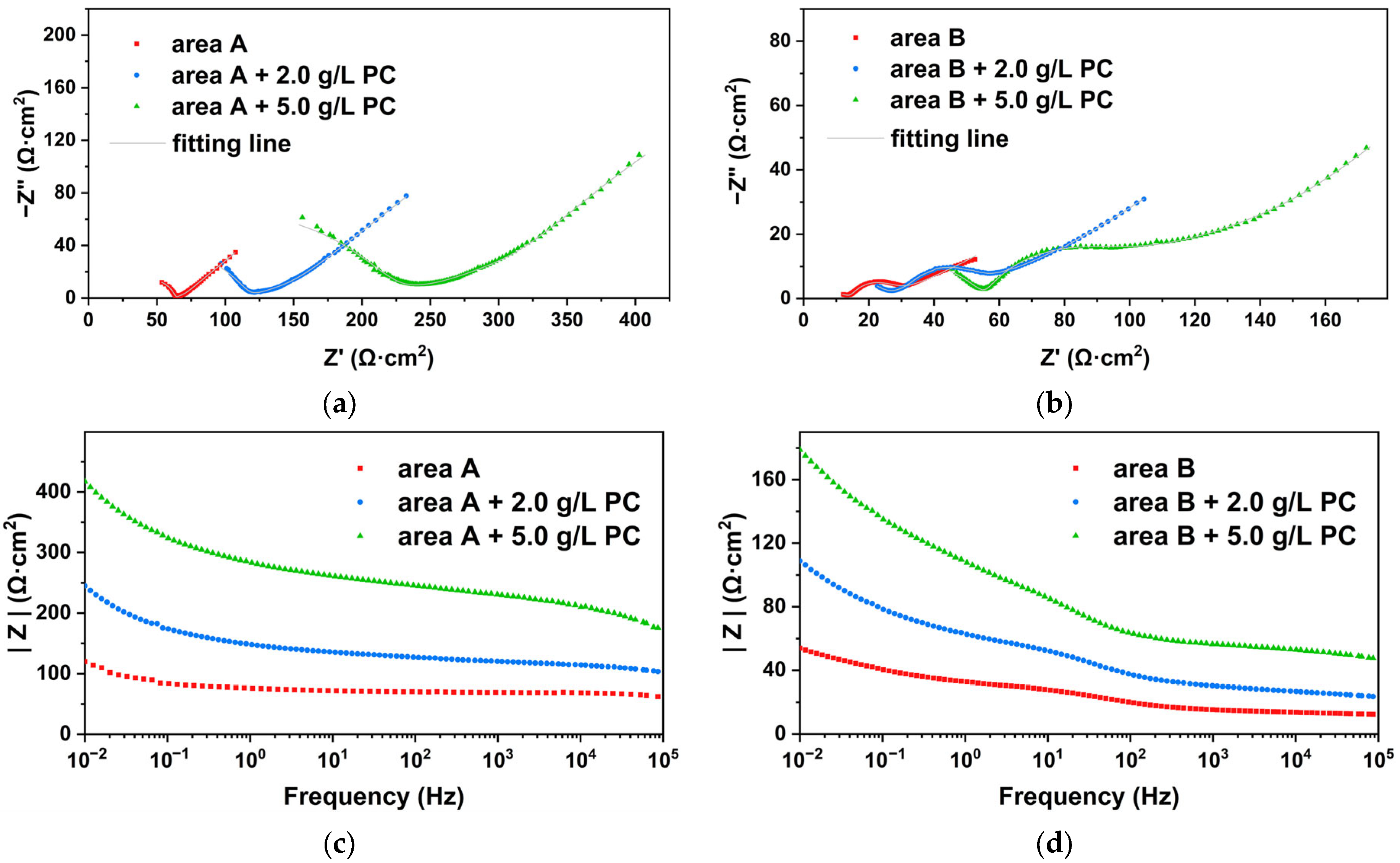


| Samples | Rs (Ω·cm2) | Rr (Ω·cm2) | Rct (Ω·cm2) | Rw (Ω·cm2) | CPEr (sn·Ω−1·cm−2) | CPEdl (sn·Ω−1·cm−2) | nr | ndl | χ2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| area A | blank | 11.69 | 34.39 | 15.32 | 27.23 | 1.73 × 10−7 | 16.91 × 10−4 | 0.84 | 0.42 | 2.03 × 10−4 |
| 2.0 g/L | 12.52 | 63.69 | 56.23 | 83.34 | 1.64 × 10−7 | 4.73 × 10−4 | 0.71 | 0.44 | 2.45 × 10−4 | |
| 5.0 g/L | 11.05 | 207.76 | 98.34 | 275.65 | 0.31 × 10−7 | 4.40 × 10−4 | 0.66 | 0.39 | 2.61 × 10−4 | |
| area B | blank | 13.31 | 15.74 | 12.38 | 84.42 | 5.30 × 10−4 | 7.98 × 10−4 | 0.75 | 0.37 | 4.51 × 10−4 |
| 2.0 g/L | 10.75 | 31.71 | 23.44 | 148.32 | 1.91 × 10−4 | 6.27 × 10−4 | 0.73 | 0.41 | 1.62 × 10−4 | |
| 5.0 g/L | 11.22 | 56.16 | 31.92 | 182.36 | 0.04 × 10−4 | 6.10 × 10−4 | 0.71 | 0.54 | 1.14 × 10−4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Hu, P.; Zhang, X.; Hu, G. Rust Conversion of Proanthocyanidins to Archaeological Steel: A Case Study of Lingzhao Xuan in the Forbidden City. Molecules 2022, 27, 7711. https://doi.org/10.3390/molecules27227711
Jia M, Hu P, Zhang X, Hu G. Rust Conversion of Proanthocyanidins to Archaeological Steel: A Case Study of Lingzhao Xuan in the Forbidden City. Molecules. 2022; 27(22):7711. https://doi.org/10.3390/molecules27227711
Chicago/Turabian StyleJia, Minghao, Pei Hu, Xiaogu Zhang, and Gang Hu. 2022. "Rust Conversion of Proanthocyanidins to Archaeological Steel: A Case Study of Lingzhao Xuan in the Forbidden City" Molecules 27, no. 22: 7711. https://doi.org/10.3390/molecules27227711
APA StyleJia, M., Hu, P., Zhang, X., & Hu, G. (2022). Rust Conversion of Proanthocyanidins to Archaeological Steel: A Case Study of Lingzhao Xuan in the Forbidden City. Molecules, 27(22), 7711. https://doi.org/10.3390/molecules27227711







