Nutritional and Therapeutic Properties of Fermented Camel Milk Fortified with Red Chenopodium quinoa Flour on Hypercholesterolemia Rats
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition and Phytochemical Properties of Red Quinoa Seed
2.2. Gross Chemical Composition and Sensory Evaluation of Different Fermented Milks
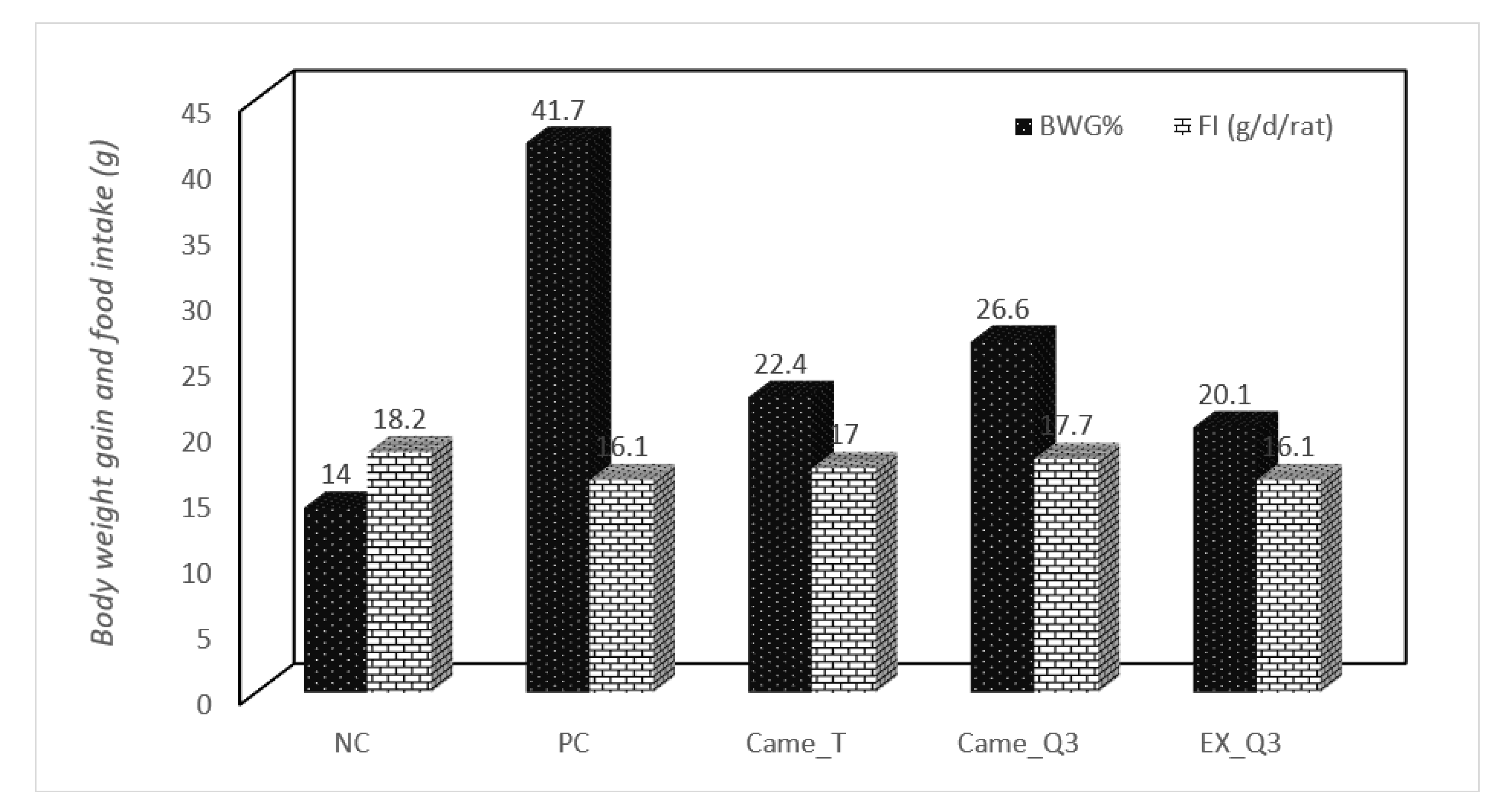
2.3. Effect of Fermented Milk on the Body Weight Gain in Rats
2.4. Effect of Fermented Camel Milk on Fasting Serum Insulin and Glucose Levels in Obese Rats
2.5. Effect of Fermented Camel Milk on Fasting Serum Insulin and Glucose Levels in Obese Rats
2.6. Effect of Fermented Camel Milk Fortified with Red Quinoa on Serum AST, ALT, ALP, Liver, and Kidney Functions in Obese Rats
2.7. Effect of Fermented Camel Milk Fortified with Red Quinoa Seed Flour on the Activity of Serum MDA, GSH, SOD and TAC Level Enzymes in Hypercholesterolemic Rats
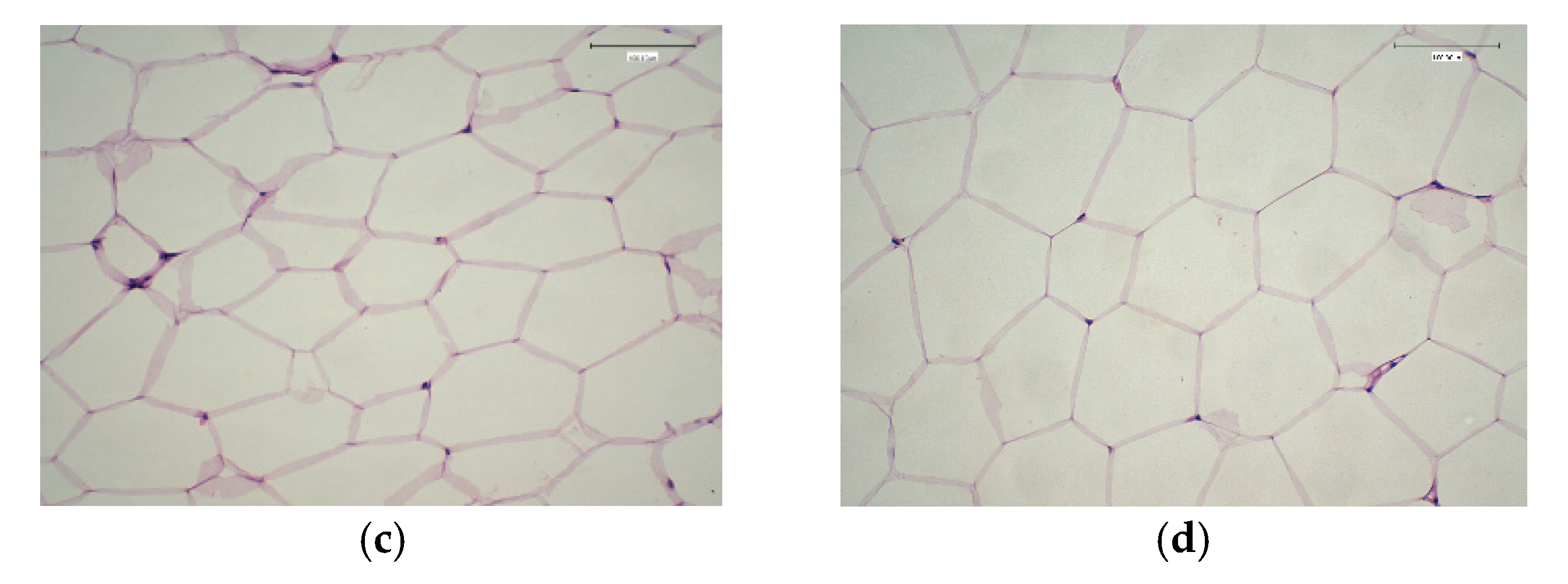
2.8. Effect of Fermented Camel Milk Fortified with Red Quinoa on Adipose Tissue Weight and Adipocyte Volume in Hypercholesterolemic Rats
2.9. Histological Changes in Experimental Rats Fed on Fermented Camel Milk Fortified with Red Quinoa
3. Material and Methods
3.1. Sample Collection
3.2. Chemicals
3.3. Experimental Animals
3.4. High Fat, High Cholesterol Diet (HF-Diet)
3.5. Red Quinoa Flour Preparation and Aquoes Extract
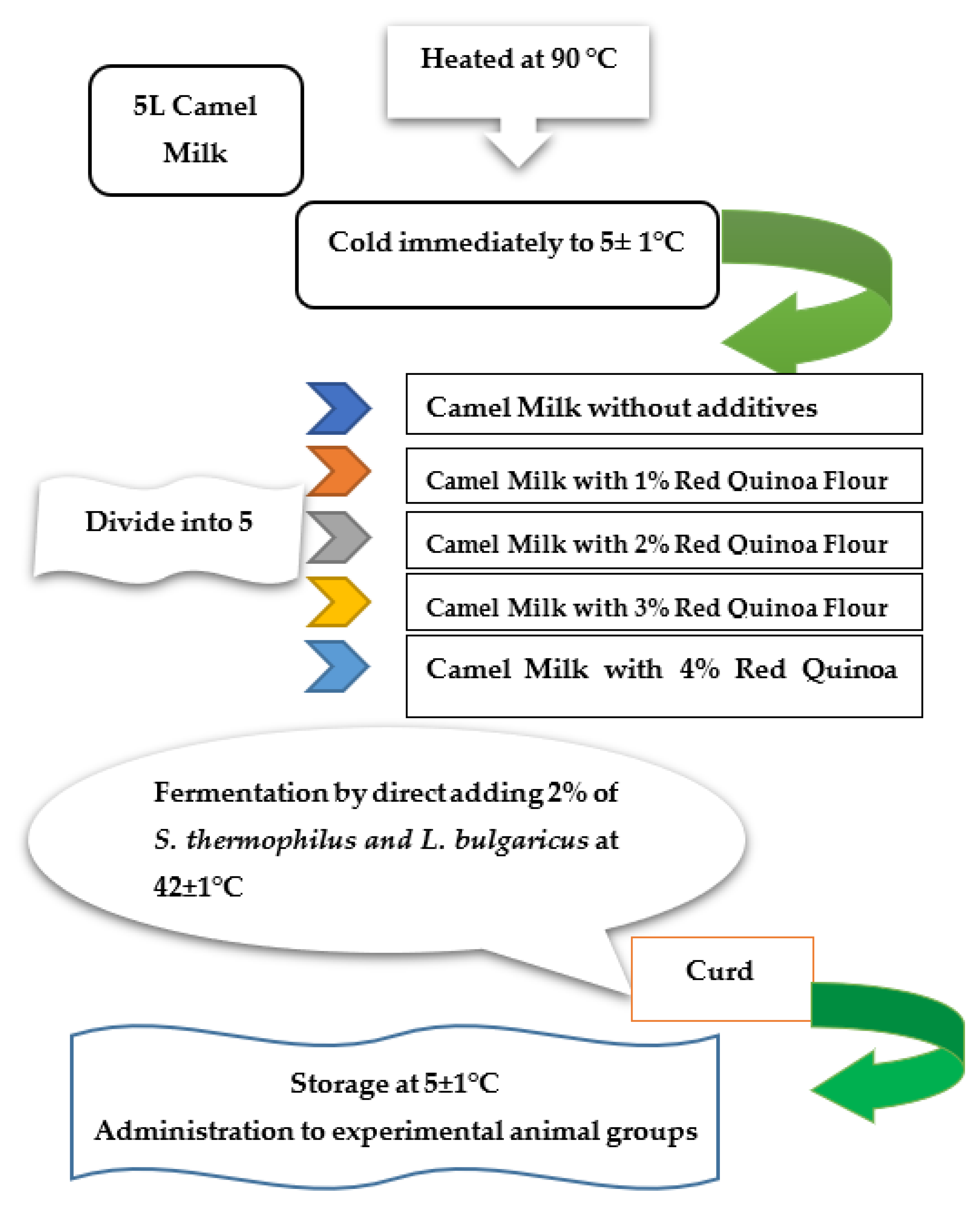
3.6. Starter Cultures and Fermented Milk Manufacture
3.7. Organoleptic Properties
3.8. Chemical Analysis
3.9. Experimental Design
3.10. Measurement of Adipocyte Size
3.11. Histopathological Examination
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sumaira, A.M.S.; Solangi, G.A.; Anwar, I.; Kalwar, Q. Composition and beneficial impact of camel milk on human health. Punjab. Univ. J. Zool. 2020, 35, 179–189. [Google Scholar] [CrossRef]
- Izadi, A.; Khedmat, L.; Mojtahedi, S.Y. Nutritional and therapeutic perspectives of camel milk and its protein hydrolysates: A review on versatile bio functional properties. J. Funct. Foods 2019, 60, 103441. [Google Scholar] [CrossRef]
- Gordillo-Bastidas, E.; Díaz-Rizzolo, D.A.; Roura, E.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An Integrative review. J. Nutr. Food Sci. 2016, 6, 497–510. [Google Scholar] [CrossRef]
- Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa secondary metabolites and their biological activities or functions. Molecules 2019, 24, 2512. [Google Scholar] [CrossRef]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem. 2019, 30, 107–114. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeón, M.E.; Raskin, I. Innovations in health value and functional food development of quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Selma-Gracia, R.; Megušar, P.; Haros, C.M.; Laparra-Llopis, J.M. Immuno-nutritional bioactive from Chenopodium quinoa and Salvia hispanica L. flour positively modulate insulin resistance and preserve alterations in peripheral myeloid population. Nutrients 2021, 13, 1537. [Google Scholar] [CrossRef]
- Farajzadeh, Z.; Shakerian, A.; Rahimi, E.; Bagheri, M. Chemical, antioxidant, total phenolic and flavonoid components and antimicrobial effects of different species of quinoa seeds. Egypt. J. Vet. Sci. 2020, 51, 43–54. [Google Scholar] [CrossRef]
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Bimolecular content of camel milk: A traditional superfood towards future healthcare industry. Trend. Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Bahobail, A.S.; Ali, A.A.; Alyan, A.A. Effect of fermentation process on the improvement of nutrition value of camel milk. Int. J. Multidiscip. Curr. Res. 2014, 2, 78–82. [Google Scholar]
- Hailu, Y.; Hansen, E.B.; Seifu, E.; Eshetu, M.; Ipsen, R.; Stefan, K. Functional and technological properties of camel milk proteins: A review. J. Dairy Res. 2016, 83, 422–429. [Google Scholar] [CrossRef]
- Ashraf, A.; Mudgil, P.; Palakkott, A.; Iratni, R.; Gan, C.Y.; Maqsood, S.; Ayoub, M.A. Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. J. Dairy Sci. 2021, 104, 61–77. [Google Scholar] [CrossRef]
- Olorunnisola, O.; Bradley, G.; Afolayan, A. Protective effect of T. violacea Rhizome extract against hypercholesterolemia-induced oxidative stress in Wistar rats. Molecules 2012, 17, 6033–6045. [Google Scholar] [CrossRef]
- Moslehishad, M.; Ehsani, M.R.; Salami, M.; Mirdamadi, S.; Ezzatpanah, H.; Naslaji, A.N.; Moosavi-Movahedi, A.A. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int. Dairy J. 2013, 29, 82–87. [Google Scholar] [CrossRef]
- El-Sayed, M.; Awad, S. Milk bioactive peptides: Antioxidant, antimicrobial and anti-diabetic activities. Adv. Biochem. 2019, 7, 22–33. [Google Scholar] [CrossRef]
- El-Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Relationship between heart disease and liver disease: A Two-way street. Cells 2020, 9, 567. [Google Scholar] [CrossRef]
- Kumar, M.; Rakesh, S.; Nagpal, R.; Hemalatha, R.; Ramakrishna, A.; Sudarshan, V.; Ramagoni, R.; Shujauddin, M.; Verma, V.; Kumar, A.; et al. Probiotic Lactobacillus rhamnosus GG and Aloe vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition 2013, 29, 574–579. [Google Scholar] [CrossRef]
- Shehata, M.G.; El-Sahn, M.A.; El Sohaimy, S.A.; Youssef, M.M. Role and Mechanisms lowering cholesterol by dietary of probiotics and prebiotics: A Review. J. Appl. Sci. 2019, 19, 737–746. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Grover, S.; Batish, V.K. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. Br. J. Nutr. 2011, 105, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Lin, P.P.; Hsieh, Y.M.; Zhang, Z.Y.; Wu, H.C.; Huang, C.C. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci. World J. 2014, 690752. [Google Scholar] [CrossRef] [PubMed]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Diaz-Valencia, Y.K.; Alca, J.J.; Calori-Domingues, M.A.; Zanabria-Galvez, S.J.; Cruz, S.H.D. Nutritional composition, total phenolic compounds and antioxidant activity of quinoa (Chenopodium quinoa Willd.) of different colours. Nova Biotechnol. Chim. 2018, 17, 74–85. [Google Scholar] [CrossRef]
- Abugoch-James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef]
- Atwaa, E.H.; Hassan, M.A.A.; Ramadan, M.F. Production of probiotic stirred yoghurt from camel milk and oat milk. J. Food Dairy Sci. 2020, 11, 259–264. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Inds. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Fernandes, Â.; Pereira, C.; Calhelha, R.C.; Sokovic, M.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Nutritional value, physicochemical characterization and bioactive properties of the Brazilian quinoa BRS Piabiru. Food Funct. 2020, 11, 2969–2977. [Google Scholar] [CrossRef]
- Al-Harbi, Y.M.; El-Zahar, K.M.; Mousa, H.M. Beneficial effects of fermented camel and cow’s milk in lipid profile, liver, and renal function in hypercholesterolemic rats. Fermentation 2022, 8, 171. [Google Scholar] [CrossRef]
- El-Zahar, K.M.; Hassan, M.F.Y.; Al-Qaba, S.F. Protective effect of fermented camel milk containing bifidobacterium longum bb536 on blood lipid profile in hypercholesterolemic rats. J. Nutr. Metabol. 2021, 2021, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.H.; Amal, M.; El-Nimer, M.; Ahmed, M.A.; Hassaan, H.M.H. Production of functional bio-yoghurt made from camel milk, skim milk retentate and fortified with sweet potato powder. Egypt. J. Agric. Res. 2019, 97, 441–458. [Google Scholar] [CrossRef]
- Hasani, S.; Sari, A.A.; Heshmati, A.; Karami, M. Physicochemical and sensory attributes assessment of functional low-fat yogurt produced by incorporation of barley bran and Lactobacillus acidophilus. Food Sci. Nutr. 2017, 5, 875–880. [Google Scholar] [CrossRef]
- Soliman, T.N.; Shehata, S.H. Characteristics of fermented camel’s milk fortified with kiwi or avocado fruits. Acta Sci. Pol. Technol. Aliment. 2019, 18, 53–63. [Google Scholar] [CrossRef]
- Tak, L.; Bais, B.; Singh, R.; Singh, S.; Nayak, T. Assessment of probiotic and neutraceutical properties of camel milk yoghurt. Int. J. Cur. Micro. App. Sci. 2018, 7, 3351–3357. [Google Scholar] [CrossRef]
- Curti, C.A.; Vidal, P.M.; Curti, R.N.; RamÓn, A.N. Chemical characterization, texture and consumer acceptability of yogurts supplemented with quinoa flour. Food Sci. Technol. 2017, 37, 627–631. [Google Scholar] [CrossRef]
- Halaby, M.S.; Abdel-Rahman, M.K.; Hassan, R.A. Protective influence of quinoa on hypercholesterolemia in male rats. Cur. Sci. Int. 2017, 6, 259–270. [Google Scholar]
- Repo-Carrasco-Valencia, R.R.; Serna, L.A. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci. Technol. 2011, 31, 225–230. [Google Scholar] [CrossRef]
- Foucault, A.S.; Even, P.; Lafont, R.; Dioh, W.; Veillet, S.; Tomé, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulangé, A. Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet. Physiol. Behav. 2014, 128, 226–231. [Google Scholar] [CrossRef]
- Yahya, M.H.; Alhaj, O.A.; AL-Khalifah, A.S.; Ahmad, T.; Almnaizel, A.T. Hypocholesterolemic effect of camel milk on rats fed a high-cholesterol diet. Emir. J. Food Agric. 2018, 30, 288–294. [Google Scholar] [CrossRef]
- Zhang, M.; Hang, X.; Fan, X.; Li, D.; Yang, H. Characterization and selection of Lactobacillus strains for their effect on bile tolerance, taurocholate deconjugation and cholesterol removal. World J. Microbiol. Biotechnol. 2008, 24, 7–14. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.L.; Dekker, M.; Verkerk, R.; van-Boekel, M.A. Health-promoting compounds in Cape gooseberry (Physalis peruviana L.): Review from a supply chain perspective. Trends Food Sci. Technol. 2016, 57, 83–92. [Google Scholar] [CrossRef]
- Martinez, O.D.M.; Toledo, R.C.L.; Queiroz, V.A.V.; Pirozi, M.R.; Martino, H.S.D.; de-Barros, F.A.R. Mixed sorghum and quinoa flour improves protein quality and increases antioxidant capacity in vivo. LWT-Food Sci. Technol. 2020, 129, 109597. [Google Scholar] [CrossRef]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M. Effect of diet supplemented with quinoa seeds on oxidative status in plasma and selected tissues of high fructose-fed rats. Plant Foods Human Nut. 2010, 65, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lietz, G.; Bal, W.; Watson, A.; Morfey, B.; Seal, C. Effects of quinoa (Chenopodium quinoa Willd.) consumption on markers of CVD risk. Nutrients 2018, 10, 777. [Google Scholar] [CrossRef]
- Pilvi, T.K.; Storvik, M.; Louhelainen, M.; Merasto, S.; Korpela, R.; Mervaala, E.M. Effect of dietary calcium and dairy proteins on the adipose tissue gene expression profile in diet-induced obesity. Lifestyle Genom. 2008, 1, 240–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Althwab, S.A.; Alsudais, M.A.; Mousa, H.M.; Ashoush, I.S.; Hamad, E.M. Reduction of lipid profile and adipocyte size in rats fed on high-fat diet using camel milk and whey protein mixture. Food Sci. Technol. Res. 2020, 26, 527–534. [Google Scholar] [CrossRef]
- Saleem, M.; Ahmed, B.; Qadir, M.I.; Rafiq, M.; Ahmad, M.; Ahmad, B. Hepatoprotective effect of Chenopodium murale in mice. Bangladesh J. Pharmacol. 2014, 9, 124–128. [Google Scholar] [CrossRef][Green Version]
- Aleisa, A.M.; Abouhashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S.; Alkhamees, O.A.; Alroujayee, A.S. Ameliorative effects of rutin and ascorbic acid combination on hypercholesterolemia induced hepatotoxicity in female rats. Afr. J. Pharm. Pharmacol. 2013, 7, 280–288. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Abbasmanthiri, R.; Al-Elawi, A.M.; Al-Horaib, G.; Al-Sadoon, K.; Al-Asmari, B.A. Effect of camel milk against renal toxicity in experimental rats. Pak. J. Pharm. Sci. 2017, 2, 561–565. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Nistor, A.; Bulla, A.; Filip, D.A.; Radu, A. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis 1987, 8, 159–173. [Google Scholar] [CrossRef]
- El Sohaimy, S.A. Chemical composition, antioxidant and antimicrobial potential of artichoke. Open Nutraceuticals J. 2014, 7, 15–20. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology, 2nd ed.; Woodhead Publishing Ltd.: Cambridge, UK, 1999; pp. 420–432. ISBN 0-8493-1785-1. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 20th ed.; Latimer, G.W., Jr., Ed.; AOAC International: Rockville, MD, USA, 2016; ISBN 0935584870. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Seasotiya, L.; Siwach, P.; Bai, S.; Malik, A.; Bharti, P. Free radical scavenging activity and phytochemical analysis of seeds of Trigonella foenum-graecum. Asian Pac. J. Health Sci. 2014, 1, 219–226. [Google Scholar] [CrossRef]
- Anwar, M.; Khan, D.A.; Khan, F.A. Comparison of fried-Ewald formula and modified fried-Ewald formula with direct homogeneous assay for low density lipoprotein cholesterol estimation. J. Coll. Physicians Surg. Pak. 2014, 24, 8–12. [Google Scholar]
- Ike, C.; Arome, O.; Affiong, E.; Ogechukwu, A.; Chimere, U. Liver Enzymes and Total Protein Levels as Index of Hepatotoxicity of Naphthalene. IOSR J. Pharm. Biol. Sci. 2016, 11, 28–31. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Schänzer, W.; Thevis, M. Determination of human insulin and its analogues in human blood using liquid chromatography coupled to ion mobility mass spectrometry (LC-IM-MS). Drug Test. Anal. 2014, 6, 1125–1132. [Google Scholar] [CrossRef]
- Namıduru, E.S.; Tarakçıoğlu, M.; Namıduru, M.; Kocabaş, R.; Erbağcı, B.; Meram, I.; Karaoğlan, I.; Yılmaz, N.; Çekmen, M. Increased serum nitric oxide and malondialdehyde levels in patients with acute intestinal amebiasis. Asian Pac. J. Trop. Biomed. 2011, 1, 478–481. [Google Scholar] [CrossRef]
- Banchroft, J.D.; Alton, D.; Floyd, G. Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Science: New York, NY, USA, 2019. [Google Scholar] [CrossRef]




| (a) | |
| Chemical Composition | Chemical Composition (g/100g) on Dry Weight |
| Protein (%) | 15.0 ± 0.24 |
| Fiber (%) | 8.8 ± 0.05 |
| Ash (%) | 3.2 ± 0.2 |
| Carbohydrates (%) | 57.58 ± 0.21 |
| Fat (%) | 5.2 ± 0.14 |
| Moisture (10) | 10.22 ± 0.21 |
| Energy (kcal/100g) | 352.12 ± 0.7 |
| (b) | |
| Chemical Composition | Eliminates Instead of Mineral (mg/100 g) |
| Calcium | 148.7 ± 1.2 |
| Phosphorous | 383.9 ± 2.3 |
| Magnesium | 246.9 ± 2.2 |
| Sodium | 12.2 ± 0.2 |
| Potassium | 926.7 ± 4 |
| Iron | 13.2 ± 0.3 |
| Manganese | 10.1 ± 0.24 |
| Copper | 5.1 ± 0.2 |
| Zinc | 4.4 ± 0.16 |
| (c) | |
| Phytochemical Properties | |
| Yield g/100 g DW Vitamin C (mg/100 g DW) | 16.86 ± 1.5 16.4 ± 0.24 |
| Phenolic compounds (mg GAE/100 g DW) | 488.32 ± 2.91 |
| Total Flavonoid (mg CE/100 g DW) | 367.11 ± 4.22 |
| DPPH* Inhibition (%) | 47.0 ± 2.4 |
| (a) | |||||
| Attribute | Fermented Milk Types | ||||
| Cam_T | Cam_Q1 | Cam_Q2 | Cam_Q3 | Cam_Q4 | |
| Total Solids (%) | 14.60 e ± 0.55 | 15.30 d ± 0.48 | 16.14 c ± 0.68 | 17.02 b ± 0.88 | 17.98 a ± 0.78 |
| Protein (%) | 5.02 c ± 0.16 | 5.14 bc ± 0.20 | 5.26 b ± 0. 24 | 5.36 ab ± 0. 22 | 5.44 a ± 0.24 |
| Fat (%) | 4.04 a ± 0.12 | 4.18 a ± 0.16 | 4.24 a ± 0.14 | 4.30 a ± 0.16 | 4.38 a ± 0.18 |
| Ash (%) | 0.96 a ± 0.09 | 1.00 a ± 0.07 | 1.05 a ± 0.10 | 1.09 a ± 0.08 | 1.14 a ± 0.05 |
| Fiber (%) | 0.00 | 0.12 d ± 0.02 | 0.23 c ± 0.03 | 0.34 b ± 0.02 | 0.46 a ± 0.03 |
| Acidity (%) | 0.78 c ± 0.04 | 0.82 b ± 0.03 | 0.88 ab ± 0.06 | 0.90 a ± 0.02 | 0.92 a ± 0.02 |
| pH | 4.80 a ± 0.08 | 4.76 b ± 0.06 | 4.70 c ± 0.09 | 4.66 d ± 0.06 | 4.62 e ±0.05 |
| (b) | |||||
| Sensory Properties | Fermented Milk Types | ||||
| Cam_T | Cam_Q1 | Cam_Q2 | Cam_Q3 | Cam_Q4 | |
| Flavor (30) | 21.18 e ± 1.2 | 25.30 c ± 1.4 | 26.60 b ± 1.3 | 27.10 a ± 1.1 | 24.60 d ± 1.3 |
| Color (10) | 30.20 e ± 4.5 | 32.24 d ± 3.7 | 34.2 b ± 4.6 | 35.40 a ± 4.92 | 36.20 c ± 3.8 |
| Body and Texture (40) | 8.2 d ± 0.3 | 8.6 a ± 0.4 | 8.4 b ± 0.4 | 8.3 c ± 0.6 | 7.9 e ± 0.5 |
| Acidity (10) | 8.82 c ± 0.2 | 8.93 bc ± 0.2 | 9.02 b ± 0.2 | 9.17 a ± 0.1 | 8.58 d ± 0.2 |
| Overall acceptability (10) | 9.01 bc ± 0.1 | 9.11 b ± 0.1 | 9.14 ab ± 0.2 | 9.25 a ± 0.1 | 8.85 c ± 0.2 |
| Total (100) | 77.41 d ± 3.12 | 84.18 c ± 4.22 | 87.36 b ± 3.70 | 89.22 a ± 3.82 | 86.13 ab ± 3.70 |
| Groups | TG mg/dL | TC mg/dL | HDL mg/dL | LDL mg/dL | VLDL mg/dL |
|---|---|---|---|---|---|
| NC | 65.9 d ± 1.1 | 64.9 d ± 2.7 | 37.2 a ± 0.9 | 14.5 e ± 1.3 | 13.2 c ± 0.1 |
| PC | 146.3 a ± 5.7 | 152.4 a ± 6.2 | 24.5 d ± 0.8 | 98.5 a ± 5.8 | 29.3 a ± 1.1 |
| Cam_T | 97.7 b ± 2.3 | 105.1 b ± 5.6 | 32.6 b ± 0.8 | 53.0 b ± 3.4 | 19.5 b ± 0.6 |
| Cam_Q3 | 93.6 c ± 1.9 | 96.1 c ± 4.6 | 36.2 ab ± 0.9 | 41.2 d ± 2.7 | 18.7 bc ± 0.5 |
| EX_Q3 | 96.4 b ± 2.1 | 99.3 bc ± 5.1 | 30.5 cd ± 0.8 | 49.3 c ± 2.9 | 19.3 b ± 0.6 |
| Groups | ALP (U/L) | ALT (U/L) | AST (U/L) | Total Protein (g/dL) | Albumin (g/dL) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|
| NC | 105.2 b ± 7.1 | 25.2 d ± 1.74 | 52.3 d± 5.4 | 7.3 b ± 0.1 | 3.85 a ± 0.04 | 17.54 d ± 0.1 | 0.89 e ± 0.3 |
| PC | 180.4 a ± 9.4 | 58.8 a ± 3.72 | 115.2 a ± 6.9 | 5.5 c ± 0.11 | 2.85 c ± 0.01 | 40.89 a ± 0.3 | 2.4 a ± 0.12 |
| Cam_T | 141.5 c ± 6.8 | 36.4 c ± 1.80 | 79.8 b ± 5.12 | 7.3 bc ± 0.12 | 3.81 ab ± 0.02 | 22.1 b ± 0.13 | 1.96 b ± 0.5 |
| Cam_Q3 | 128.6 d ± 5.7 | 33.8 c ± 3.18 | 73.2 c ± 2.7 | 7.6 b ± 0.09 | 4.15 a ± 0.03 | 19.75 c ± 0.9 | 1. 5 d ± 0.11 |
| EX_Q3 | 135.6 cd ± 5.7 | 41.7 b ± 1.2 | 84.3 b ± 5.2 | 7.9 a ± 0.08 | 3.70 b ± 0.04 | 23.1 b ± 0.19 | 1.75 c ± 0.1 |
| Groups | MDA (μmol/L) | TAC (μmol/L) | SOD (U/g Hb) | GSH-px (U/g Hb) |
|---|---|---|---|---|
| NC | 1.27 d ± 0.05 | 780 a ± 14 | 5.34 a ± 0.85 | 22.14 a ± 0.88 |
| PC | 2.13 a ± 0.07 | 550 e ± 21 | 3.28 d ± 0.40 | 15.12 d ± 0.76 |
| Cam_T | 1.56 b ± 0.03 | 655 d ± 18 | 4.08 c ± 0.55 | 17.28 c ± 0.85 |
| Cam_Q3 | 1.32 c ± 0.02 | 775 b ± 16 | 4.60 b ± 0.68 | 18.22 b ± 0.80 |
| EX_Q3 | 1.30 cd ± 0.01 | 735 c ± 15 | 3.98 c ± 0.55 | 16.82 c ± 0.85 |
| Groups | Liver (g) | White Adipose Tissue (g) | Adipocyte Size (μm2 × 103) |
|---|---|---|---|
| NC | 8.59 b ± 0.2 | 1.66 d ± 0.2 | 3.1 d ± 0.4 |
| PC | 9.99 a ± 0.3 | 5.45 a ± 0.6 | 10.96 a ± 0.7 |
| Cam_T | 7.9 cd ± 0.8 | 2.72 b ± 0.5 | 6.3 b ± 0.4 |
| Cam_Q3 | 8.1 c ± 0.3 | 2.3 c ± 0.4 | 4.55 c ± 0.2 |
| EX_Q3 | 7.5 d ± 0.8 | 2.62 b ± 0.5 | 5.9 bc ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Anazi, M.S.; El-Zahar, K.M.; Rabie, N.A.-H. Nutritional and Therapeutic Properties of Fermented Camel Milk Fortified with Red Chenopodium quinoa Flour on Hypercholesterolemia Rats. Molecules 2022, 27, 7695. https://doi.org/10.3390/molecules27227695
Al-Anazi MS, El-Zahar KM, Rabie NA-H. Nutritional and Therapeutic Properties of Fermented Camel Milk Fortified with Red Chenopodium quinoa Flour on Hypercholesterolemia Rats. Molecules. 2022; 27(22):7695. https://doi.org/10.3390/molecules27227695
Chicago/Turabian StyleAl-Anazi, Mohamed Saleh, Khaled Meghawry El-Zahar, and Nourhan Abdel-Hamid Rabie. 2022. "Nutritional and Therapeutic Properties of Fermented Camel Milk Fortified with Red Chenopodium quinoa Flour on Hypercholesterolemia Rats" Molecules 27, no. 22: 7695. https://doi.org/10.3390/molecules27227695
APA StyleAl-Anazi, M. S., El-Zahar, K. M., & Rabie, N. A.-H. (2022). Nutritional and Therapeutic Properties of Fermented Camel Milk Fortified with Red Chenopodium quinoa Flour on Hypercholesterolemia Rats. Molecules, 27(22), 7695. https://doi.org/10.3390/molecules27227695







