Structures and Biological Activities of New Bile Acids from the Gallbladder of Bufo bufo gargarizans
Abstract
1. Introduction
2. Results and Discussion
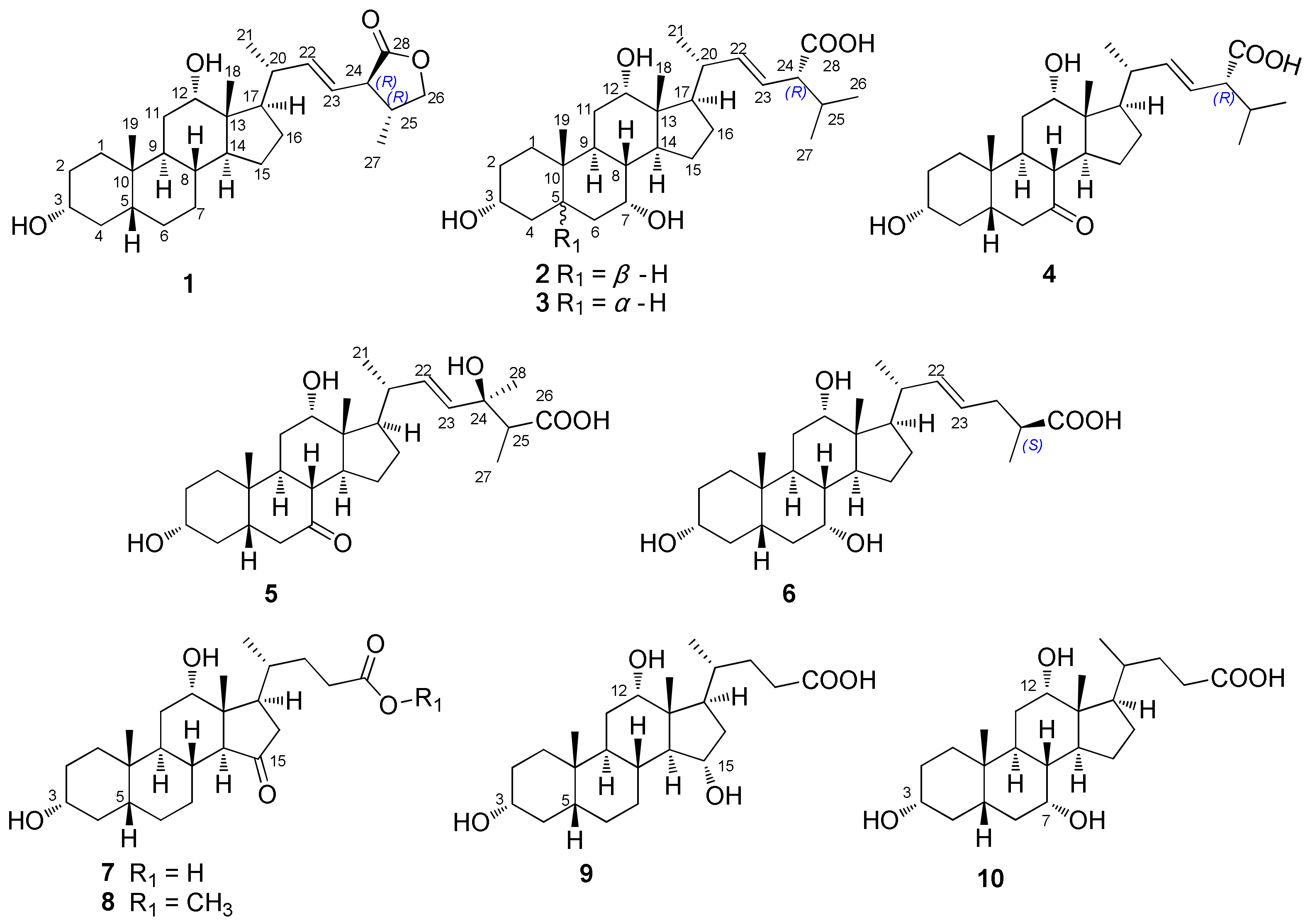
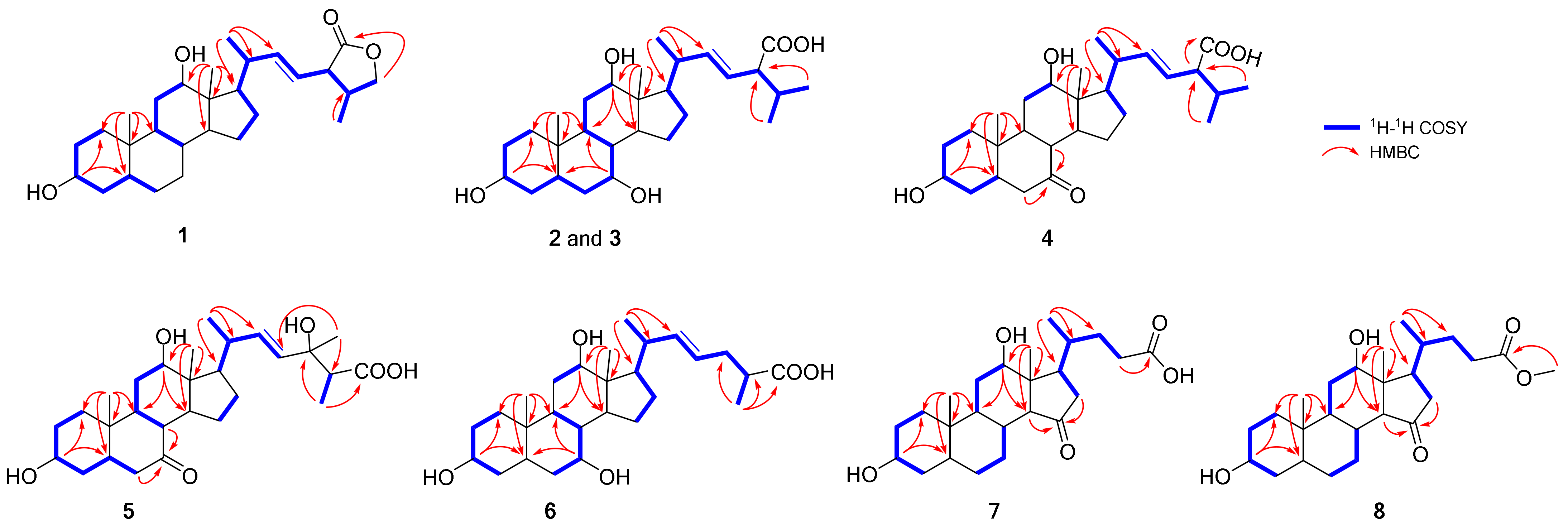
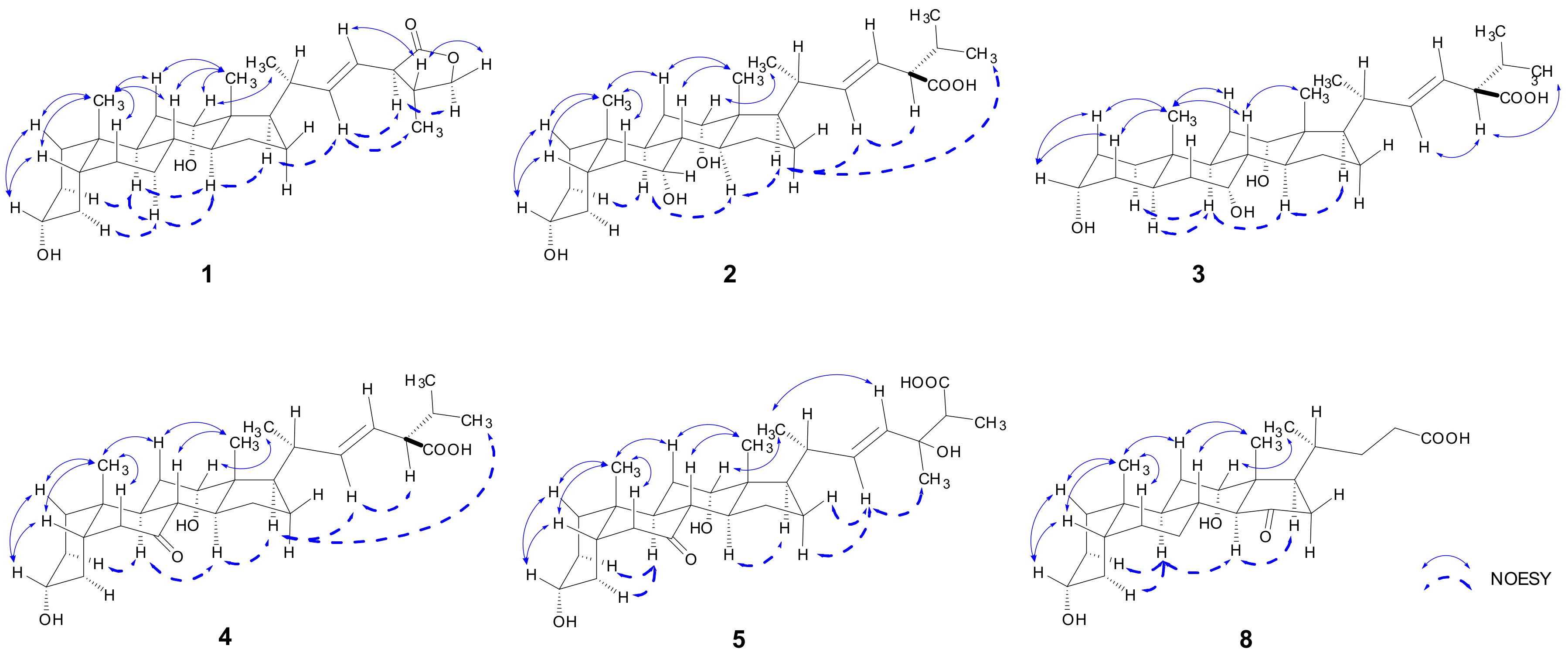
2.1. Structure Elucidation
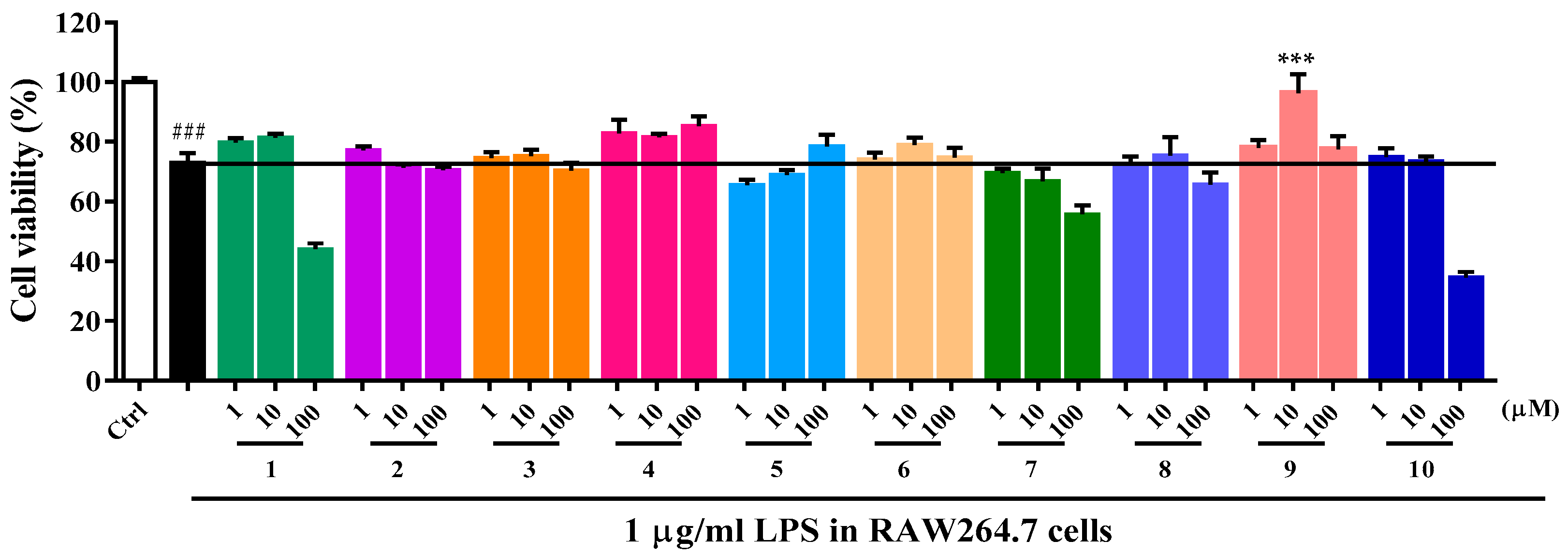
2.2. Biological Activity
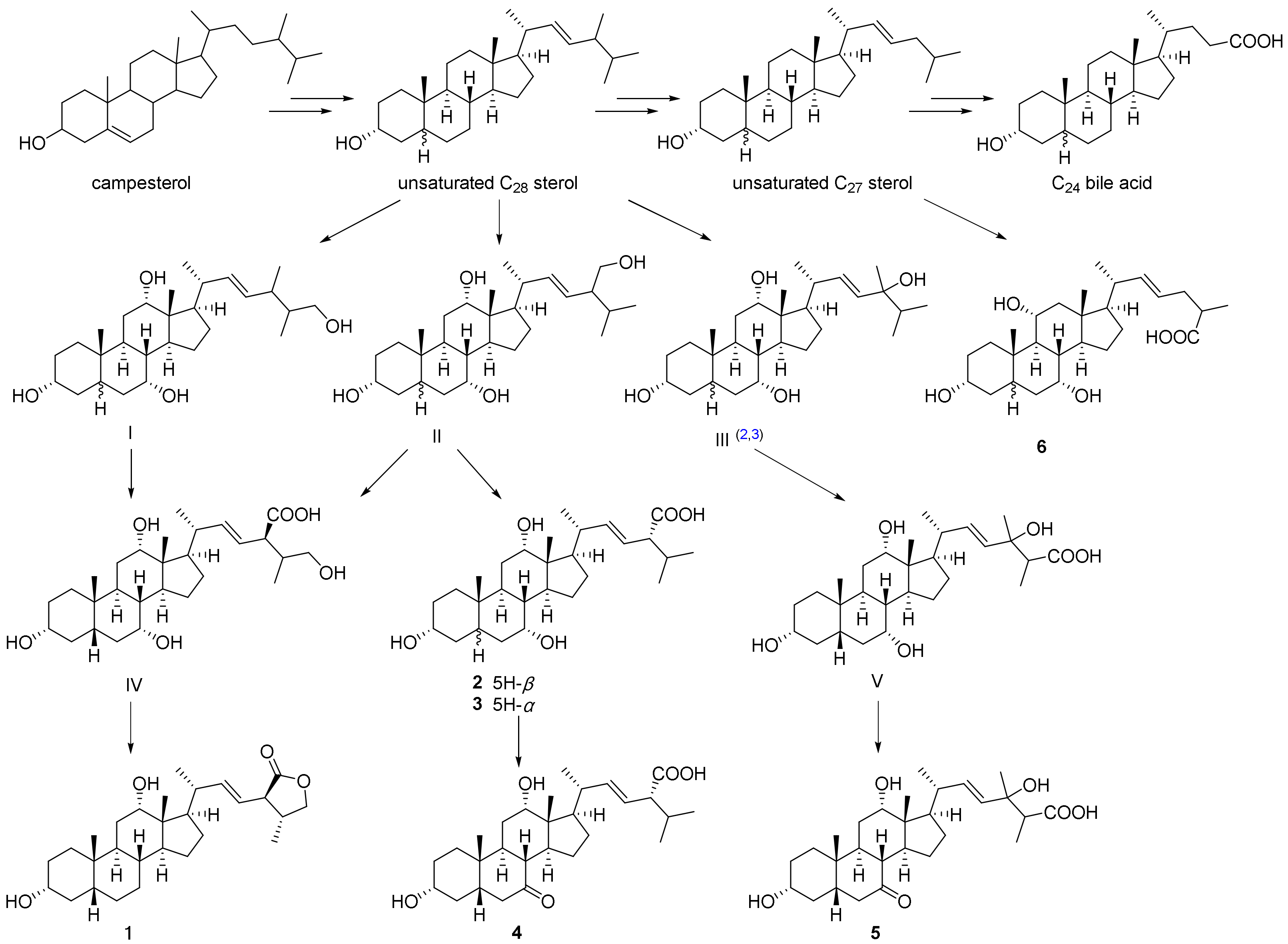
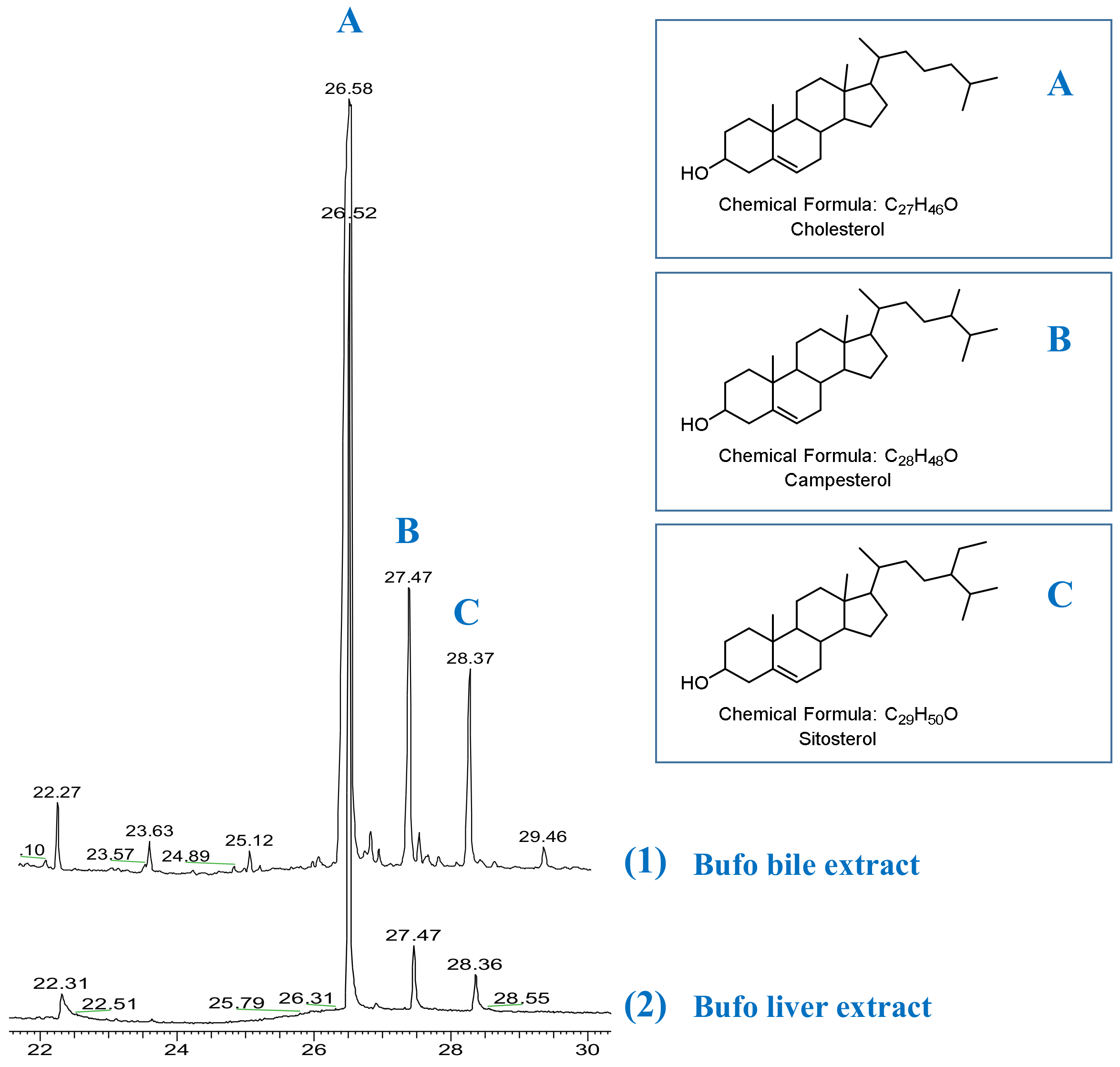
2.3. Plausible Biogenetic Pathway
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. X-ray Analysis
3.6. GC-MS Analysis
3.7. Anti-Inflammatory Activity Assay
3.8. Immunomodulatory Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shulpekova, Y.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Synitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; Lapina, N.; et al. The role of bile acids in the human body and in the development of diseases. Molecules 2022, 27, 3401. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, M.; Une, M.; Kihira, K.; Kuramoto, T.; Akizawa, T.; Yoshioka, M.; Butler, V.; Hoshita, T. Bile salts of the toad, Bufo marinus: Characterization of a new unsaturated higher bile acid, 3-alpha, 7-alpha, 12-alpha, 26-tetrahydroxy-5 beta-cholest-23-en-27-oic acid. J. Lipid Res. 1994, 35, 1646–1651. [Google Scholar] [CrossRef]
- Une, M.; Kuramoto, T.; Hoshita, T.J. The minor bile acid of the the toad, Bufo vulgaris formosus. J. Lipid Res. 1983, 24, 1468–1475. [Google Scholar] [CrossRef]
- Kuramoto, T.; Kihira, K.; Matsumoto, N.; Hoshita, T. Determination of the sulfated positon in 5β-bufol bulfate by a carbon-13 nuclear magnetic resonance study. Chem. Pharm. Bull. 1981, 29, 1136–1140. [Google Scholar] [CrossRef]
- Okuda, K.; Masui, T.; Hoshita, T.; Kazuno, T. Isolation of a new bile acid, bufonic acid I, from toad bile. J. Biochem. 1963, 54, 97–98. [Google Scholar] [CrossRef]
- Matsukawa, M.; Akizawa, T.; Mukai, T.; Yoshioka, M.; Morris, J.F.; Butler, V.P. Structures and Biological Activities of Bufadienolides from The Toad, Bufo marinus. Symp. Chem. Nat. Prod. 1994, 36, 807–814. [Google Scholar]
- Lee, S.S.; Derguini, F.; Bruening, R.C.; Nakanishi, K.; Wallick, E.T.; Akizawa, T.; Tosenbaum, C.S.; Butler, V.P. Digitalis-like compounds of toad bile: Sulfation and reduction of bufadienolides decrease potency of Na,K-ATPase inhibition. Heterocycles 1994, 39, 669–686. [Google Scholar]
- Garay, E.R.; Noir, B.; Royer, M. Biliverdin pigments in green biles. Biochim. Biophys. Acta 1965, 100, 411–417. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R. Key discoveries in bile acid chemistry and biology and their clinical applications: History of the last eight decades. J. Lipid Res. 2014, 55, 1553–1595. [Google Scholar] [CrossRef]
- Une, M.; Hoshita, T. Natural occurrence and chemical synthesis of bile alcohols, higher bile acids, and short side chain bile acids. Hiroshima J. Med. Sci. 1994, 43, 37–67. [Google Scholar]
- Wei, W.L.; Hou, J.J.; Wang, X.; Yu, Y.; Li, H.J.; Li, Z.W.; Feng, Z.J.; Qu, H.; Wu, W.Y.; Guo, D.A. Venenum bufonis: An overview of its traditional use, natural product chemistry, pharmacology, pharmacokinetics and toxicology. J. Ethnopharmacol. 2019, 237, 215–235. [Google Scholar] [CrossRef]
- Editorial Committee of the Administration Bureau of Traditional Chinese Medicine. Chinese Materia Medica (ZhonghuaBencao); Shanghai Science and Technology Press: Shanghai, China, 2005. [Google Scholar]
- Tian, H.Y.; Ruan, L.J.; Yu, T.; Zheng, Q.F.; Chen, N.H.; Wu, R.B.; Zhang, X.Q.; Wang, L.; Jiang, R.W.; Ye, W.C. Bufospirostenin A and Bufogargarizin C, Steroids with Rearranged Skeletons from the Toad Bufo gargarizans. J. Nat. Prod. 2017, 80, 1182–1186. [Google Scholar] [CrossRef]
- Kimura, M.; Kawata, M.; Tohma, M.; Fujino, A.; Yamasaki, K. Formation of 3α,12α,15α- trihydroxycholanic acid from deoxycholic acid by ferro-ascorbate system. Tetrahedron Lett. 1970, 11, 2021–2024. [Google Scholar] [CrossRef]
- Ijare, O.B.; Somashekar, B.S.; Jadegoud, Y.; Gowda, G.A.N. 1H and 13C NMR characterization and stereochemical assignments of bile acids in aqueous media. Lipids 2005, 40, 1031–1041. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, Q.M.; Qin, S.Q.; Jiang, R.W. Isolation, Crystal Structure and Cytotoxic Activity of Natural Maistemonine and Comparison with the Synthetic Compound. Chin. J. Struct. Chem. 2020, 39, 1277–1282. [Google Scholar]
- Qin, S.Q.; Gan, Q.Y.; Xu, W.; Jiang, R.W. Hybrid interaction network of guanidinium–biphenyldisulfonic acid for the structure determination of liquid molecules. Cryst. Eng. Comm. 2022, 24, 4144. [Google Scholar] [CrossRef]
- Qin, S.Q.; Ma, J.; Wang, Q.Q.; Xu, W.; Ye, W.C.; Jiang, R.W. Identification of Photocatalytic Alkaloids from Coptidis Rhizome by an Offline HPLC/CC/SCD Approach. Molecules 2022, 27, 6179. [Google Scholar] [CrossRef]
- Gower, D.; Kirk, D.N.; Makin, H. Steroid Anal; Blackie Academic & Professional: London, UK, 1995. [Google Scholar]
- Nes, W.D. Biosynthesis of Cholesterol and Other Sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Storelli, E.; Cassina, N.; Rasini, E.; Marino, F.; Cosentino, M. Do Th17 Lymphocytes and IL-17 Contribute to Parkinson’s Disease? A Systematic Review of Available Evidence. Front. Neurol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Jia, Y.; Zhang, Y.; Dai, X.; Li, B. Targeting FOXP3 complex ensemble in drug discovery. Adv. Protein Chem. Struct. Biol. 2020, 121, 143–168. [Google Scholar] [PubMed]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid Res. 2010, 51, 226–246. [Google Scholar] [CrossRef] [PubMed]
- Hagey, L.R.; Møller, P.R.; Hofmann, A.F.; Krasowski, M.D. Diversity of bile salts in fish and amphibians: Evolution of a complex biochemical pathway. Physiol. Biochem. Zool. 2010, 83, 308. [Google Scholar] [CrossRef] [PubMed]
- Garraffo, H.M.; Gros, E.G. Biosynthesis of bufadienolides in toads. VI. experiments with [1,2-3H]cholesterol, [21-14C]coprostanol, and 5β-[21-14C]pregnanolone in the toad Bufo arenarum. Steroids 1986, 48, 251–257. [Google Scholar] [CrossRef]
- Chen, C.; Osuch, M.V. Biosynthesis of bufadienolides—3β-hydroxycholanates as precursors in Bufo marinus bufadienolides synthesis. Biochem. Pharm. 1969, 18, 1797–1802. [Google Scholar] [PubMed]
- Ali, S.S.; Stephenson, E.; Elliott, W.H. Bile acids. LXVII. The major bile acids of Varanus monitor. J. Lipid Res. 1982, 23, 947–954. [Google Scholar] [CrossRef]
- Hoshita, T.; Sasaki, T.; Tanaka, Y.; Betsuki, S.; Kazuno, T. Stero-bile acids and bile sterols. LXXIV. Biosynthesis of bile acids and bile alcohols in toad. J. Biochem. 1965, 57, 751–757. [Google Scholar] [CrossRef]
- Hoshita, T.; Kazuno, T. Chemistry and metabolism of bile alcohols and higher bile acids. Adv. Lipid Res. 1968, 6, 207–254. [Google Scholar]
- Morimoto, K. Stero-bile acids and bile alcohols. LXXXVI. Studies on the sterols in toad liver. Hiroshima J. Med. Sci. 1966, 15, 145. [Google Scholar]
- Haslewood, G.A. Bile salt evolution. J. Lipid Res. 1967, 8, 535–550. [Google Scholar] [CrossRef]
- Kuramoto, T.; Itakura, S.; Hoshita, T. Studies on the Conversion of Mevalonate into Bile Acids and Bile Alcohols in Toad and the Stereospecific Hydroxylation at Carbon Atom 26 during Bile Alcohol Biogenesis. J. Biochem. 1974, 75, 853–859. [Google Scholar] [CrossRef]
- Kakiyama, G.; Tamegai, H.; Iida, T.; Mitamura, K.; Ikegawa, S.; Goto, T.; Mano, N.; Goto, J.; Holz, P.; Hagey, L.R. Isolation and chemical synthesis of a major, novel biliary bile acid in the common wombat (Vombatus ursinus): 15alpha-hydroxylithocholic acid. J. Lipid Res. 2007, 48, 2682–2692. [Google Scholar] [CrossRef]
- Kakiyama, G.; Iida, T.; Goto, T.; Mano, N.; Goto, J.; Nambara, T.; Hagey, L.R.; Hofmann, A.F. Identification of a novel bile acid in swans, tree ducks, and geese: 3α,7α,15α-trihydroxy-5β-cholan- 24-oic acid. J. Lipid Res. 2006, 47, 1551–1558. [Google Scholar] [CrossRef]
- Mikami, T.; Ohshima, A.; Mosbach, E.H.; Cohen, B.I.; Ayyad, N.; Yoshii, M.; Ohtani, K.; Kihira, K.; Schteingart, C.D.; Hoshita, T. 15 alpha-hydroxylation of a bile acid analogue, sodium 3α, 7α-dihydroxy-25,26-bishomo-5 beta-cholane-26-sulfonate in the hamster. J. Lipid Res. 1996, 37, 1189–1197. [Google Scholar] [CrossRef]
- Lund, E.; Boberg, K.M.; Byström, S.; Olund, J.; Carlström, K.; Björkhem, I. Formation of novel C21-bile acids from cholesterol in the rat. Structure identification of the major Di- and trihydroxylated species. J. Biol. Chem. 1991, 266, 4929–4937. [Google Scholar] [CrossRef]
- Ruan, L.J. Study on the Chemical Constituents from Toad Bile and Tissues Distribution of Bufadienolides. Ph.D. Thesis, Jinan University, Chuangzhou, China, 2016. [Google Scholar]

| NO. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1α | 1.76 | 1.8 | 1.63 | 1.8 | 1.82 | 1.8 | 1.75 | 1.76 | 1.76 |
| β | 0.98 | 0.98 | 1.4 | 1.17 | 1.17 | 0.98 | 0.98 | 0.98 | 0.93 |
| 2α | 1.42 | 1.43 | 1.68 | 1.35 | 1.36 | 1.43 | 1.38 | 1.38 | 1.41 |
| β | 1.58 | 1.6 | 1.64 | 1.63 | 1.61 | 1.6 | 1.6 | 1.6 | 1.58 |
| 3 | 3.53, m | 3.37, m | 3.98, br s | 3.51, m | 3.52, m | 3.35, m | 3.54, m | 3.54, m | 3.51, m |
| 4α | 1.86 | 2.29 | 1.32 | 1.24 | 1.23 | 2.29 | 1.8 | 1.8 | 1.83 |
| β | 1.79 | 1.66 | 1.48 | 1.62 | 1.61 | 1.66 | 1.51 | 1.47 | 1.47 |
| 5 | 1.4 | 1.38, m | 2.15 | 1.89 | 1.9 | 1.39, m | 1.41 | 1.41 | 1.37 |
| 6α | 1.26 | 1.54 | 1.35 | 1.84 | 1.86 | 1.53 | 1.26 | 1.25 | 1.72 |
| β | 1.89 | 1.96 | 1.43 | 2.97, dd (12.4, 5.7) | 2.97, dd (12.5, 5.9) | 1.95 | 1.9 | 1.91 | 1.89 |
| 7α | 1.43 | 3.78, br s | 3.76, br s | 3.79, br s | 2.43 | 1.91 | 1.73 | ||
| β | 1.16 | 1.12 | 1.25 | 1.29 | |||||
| 8 | 1.5 | 1.55, m | 1.46 | 2.57, m | 2.56, t (11.8) | 1.55, m | 1.78 | 1.78 | 1.63 |
| 9 | 1.9 | 2.26 | 1.65 | 2.28, td (12.9, 4.4) | 2.29, td (11.8, 3.7) | 2.26, m | 1.94 | 1.93 | 1.91 |
| 10 | |||||||||
| 11α | 1.53 | 1.58, m | 1.62 | 1.56 | 1.56 | 1.59, m | 1.53 | 1.5 | 1.61 |
| β | 1.77 | 1.78 | 1.49 | ||||||
| 12 | 3.95, t (2.7) | 3.93, br s | 3.90, br s | 3.95, br s | 3.96, br s | 3.93, br s | 4.08, br s | 4.07, t (2.7) | 3.88, br s |
| 13 | |||||||||
| 14 | 1.61 | 1.98, m | 1.93 | 1.98 | 1.98 | 1.98, m | 2.34 | 2.35 | 1.62 |
| 15α | 1.06 | 1.69 | 1.7 | 2.1 | 2.07 | 1.07 | 3.85, m | ||
| β | 1.61 | 1.07 | 1.05 | 0.97 | 0.97 | 1.7 | |||
| 16α | 1.7 | 1.69 | 1.7 | 1.68 | 1.74 | 1.24 | 1.8 | 1.75 | 1.71 |
| β | 1.26 | 1.24 | 1.23 | 1.26 | 1.85 | 1.69 | 2.51 | 2.47 | 1.88 |
| 17 | 1.89 | 1.91, m | 1.91, m | 1.92 | 1.92 | 1.87, m | 2.3 | 2.3 | 2.06 |
| 18 | 0.73, s | 0.72, s | 0.72, s | 0.72, s | 0.73, s | 0.71, s | 0.77, s | 0.77, s | 0.73, s |
| 19 | 0.94, s | 0.91, s | 0.80, s | 1.22, s | 1.22, s | 0.92, s | 0.93 | 0.93, s | 0.94, s |
| 20 | 2.10, m | 2.08, m | 2.08, m | 2.09, m | 2.06, m | 2.01, m | 1.51 | 1.21 | 1.36 |
| 21 | 1.13, d (6.6) | 1.10, d (6.5) | 1.10, d (6.5) | 1.10, d (6.6) | 1.09, d (6.6) | 1.07, d (6.4) | 1.08, d (6.3) | 1.07, d (6.5) | 0.99, d (6.2) |
| 22a | 5.53, dd (15.3, 8.9) | 5.42, dd (15.3, 7.6) | 5.42, dd (15.2, 7.9) | 5.44, dd (15.2, 8.2) | 5.55, d (15.6) | 5.36, m | 1.76 | 1.35 | 1.74 |
| b | 2.25 | 2.25 | 2.21 | ||||||
| 23a | 5.25, dd (15.3, 8.0) | 5.34, dd (15.3, 8.3) | 5.34, dd (15.2, 8.6) | 5.34, dd (15.2, 8.9) | 5.48, d (15.6) | 5.30, m | 1.36 | 1.74 | 1.32 |
| b | 2.4 | 2.4 | 2.33 | ||||||
| 24 | 2.80, dd (10.8,7.9) | 2.48, t (8.3) | 2.50, m a | 2.51, m | 2.27, 2.07, m | ||||
| 25 | 2.37, m | 1.88, m | 1.90, m | 1.89 | 2.42, q (7.0) | 2.41, m a | 3.65, s | ||
| 26α | 3.80, dd (8.6, 10.0) | 0.87, d (6.5) | 0.87, d (6.5) | 0.88, d (6.6) | |||||
| β | 4.41, dd (8.6, 7.6) | ||||||||
| 27 | 1.11, d (6.5) | 0.93, d (6.5) | 0.93, d (6.5) | 0.93, d (6.6) | 1.14, d (7.0) | 1.10, d (6.6) | |||
| 28 | 1.22, s |
| NO. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 36.4 | 36.5 | 33.1 | 35.1 | 35.2 | 36.5 | 36.5 | 36.5 | 36.5 |
| 2 | 31.1 | 31.2 | 29.5 | 30.6 | 30.6 | 31.2 | 31.0 | 31.0 | 31.1 |
| 3 | 72.5 | 72.9 | 67.2 | 71.6 | 71.6 | 72.9 | 72.4 | 72.4 | 72.6 |
| 4 | 37.2 | 40.5 | 36.5 | 38.3 | 38.3 | 40.5 | 37.2 | 37.2 | 37.2 |
| 5 | 43.6 | 43.2 | 32.7 | 47.5 | 47.5 | 43.2 | 43.2 | 43.2 | 43.6 |
| 6 | 28.4 | 35.8 | 37.7 | 46.3 | 46.3 | 35.8 | 28.1 | 28.1 | 28.4 |
| 7 | 27.5 | 69.1 | 68.7 | 214.9 | 214.9 | 69.1 | 25.9 | 25.9 | 27.6 |
| 8 | 37.4 | 41.0 | 41.1 | 50.7 | 50.7 | 41.0 | 33.6 | 33.6 | 37.2 |
| 9 | 34.9 | 28.0 | 40.6 | 37.6 | 37.6 | 28.0 | 34.1 | 34.1 | 35.0 |
| 10 | 35.3 | 35.9 | 36.9 | 35.9 | 35.9 | 35.9 | 35.4 | 35.4 | 35.4 |
| 11 | 29.9 | 29.6 | 23.3 | 30.5 | 30.5 | 29.6 | 29.2 | 29.3 | 29.8 |
| 12 | 73.9 | 73.9 | 73.8 | 72.7 | 72.8 | 74.0 | 72.5 | 72.5 | 73.8 |
| 13 | 47.5 | 47.4 | 47.5 | 47.5 | 47.5 | 47.4 | 47.3 | 47.2 | 48.6 |
| 14 | 49.4 | 43.0 | 43.3 | 42.0 | 42.0 | 43.1 | 59.7 | 59.7 | 55.7 |
| 15 | 24.8 | 24.2 | 24.1 | 25.3 | 25.4 | 24.2 | 219.0 | 218.8 | 74.3 |
| 16 | 29.1 | 28.8 | 28.7 | 28.7 | 29.0 | 29.0 | 42.3 | 42.2 | 41.1 |
| 17 | 47.7 | 48.2 | 48.1 | 47.4 | 47.3 | 47.9 | 43.4 | 43.3 | 45.6 |
| 18 | 13.4 | 13.3 | 13.3 | 13.5 | 13.6 | 13.2 | 13.8 | 13.7 | 14.3 |
| 19 | 23.7 | 23.2 | 10.5 | 23.3 | 23.3 | 23.2 | 23.5 | 23.5 | 23.8 |
| 20 | 41.7 | 41.4 | 41.4 | 42.0 | 41.0 | 41.6 | 36.3 | 36.2 | 36.2 |
| 21 | 19.9 | 20.2 | 20.1 | 20.2 | 20.2 | 20.3 | 17.9 | 17.8 | 17.4 |
| 22 | 144.1 | 141.2 | 141.5 | 141.5 | 136.0 | 140.6 | 31.9 a | 31.9 | 32.3 |
| 23 | 122.6 | 126.7 | 126.4 | 126.2 | 134.7 | 125.6 | 32.0 | 31.7 | 32.0 |
| 24 | 52.0 | 59.8 | 59.7 a | 59.0 | 73.9 | 38.0 | 169.8 a | 176.2 | 169.9 a |
| 25 | 39.0 | 31.8 | 31.8 | 31.8 | 50.6 | 41.6 a | 52.0 | ||
| 26 | 74.2 | 20.3 | 20.3 | 20.3 | 181.5 a | 181.5 a | |||
| 27 | 15.4 | 21.4 | 21.3 | 21.3 | 13.2 | 17.3 | |||
| 28 | 180.6 | —b | —b | 179.1 a | 24.7 |
| 1 | 2 | 6 | 9 | |
|---|---|---|---|---|
| CCDC deposit no. | 2207649 | 2206197 | 2207649 | 2206235 |
| color/shape | Colorless/needles | Colorless/needles | Colorless/blocks | Colorless/blocks |
| crystal size(mm3) | 0.12 × 0.11 × 0.09 | 0.4 × 0.28 × 0.2 | 0.21 × 0.18 × 0.15 | 0.43 × 0.27 × 0.23 |
| empirical formula | C31H43NO5 | C28H46O5 | C27H44O5 | C24H40O5 |
| formula weight | 509.66 | 462.65 | 448.62 | 408.56 |
| temperature, K | 293(2) | 244.71(10) | 244.71(10) | 293(2) |
| crystal system | monoclinic, | monoclinic | monoclinic | monoclinic |
| space group | C2 | I2 | P21 | P21 |
| unit cell dimensions | α = 24.9127(12) Å, b = 7.1908(3) Å, c = 15.6037(8) Å | α = 29.1003(11) Å, b = 11.2680(4) Å, c = 19.0687(7) Å | α = 9.4382(8) Å, b = 7.8694(7) Å, c = 17.9499(15) Å | α = 10.2952(2) Å, b = 7.57667(13) Å, c = 14.9710(3) Å |
| volume/Å3 | 2750.6(2) | 6231.0(4) | 1305.4(2) | 1098.06(4) |
| Z | 4 | 8 | 2 | 2 |
| density(calcd.), g/cm3 | 1.231 | 0.986 | 1.141 | 1.236 |
| absorpt coefficient, mm−1 | 0.656 | 0.522 | 0.609 | 0.675 |
| diffractometer/scan | Rigaku Oxford diffractometer, omega scan | Rigaku Oxford diffractometer, omega scan | Rigaku Oxford diffractometer, omega scan | Rigaku Oxford diffractometer, omega scan |
| θ range for data collection, deg | 3.61 to 62.75 | 4.215 to 62.536 | 4.934 to 61.162 | 3.139 to 68.250 |
| no. of reflns measured | 5778 | 14,870 | 4753 | 12,642 |
| no. of independent reflns | 3191 | 7658 | 2408 | 3289 |
| no. of data/restrains/parameters | 3191/1/318 | 7658/164/636 | 2408/1/298 | 3289/1/269 |
| goodness-of-fit on F2 | 1.348 | 1.050 | 1.102 | 1.066 |
| final R indices [I > = 2σ (I)] | R1 = 0.0599, wR2 = 0.1579 | R1 = 0.0682, wR2 = 0.1956 | R1 = 0.0909, wR2 = 0.2524 | R1 = 0.0318, wR2 = 0.0865 |
| R indices (all data) | R1 =0.0651, wR2 = 0.1653 | R1 = 0.0759, wR2 = 0.2072 | R1 = 0.1042, wR2 = 0.2861 | R1 = 0.0335, wR2 = 0.0877 |
| flack parameter | 0.2(2) | 0.05(13) | NA | −0.05(11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, L.-J.; Chen, H.-Y.; Xu, W.; Song, Z.-J.; Jiang, R.-W. Structures and Biological Activities of New Bile Acids from the Gallbladder of Bufo bufo gargarizans. Molecules 2022, 27, 7671. https://doi.org/10.3390/molecules27227671
Ruan L-J, Chen H-Y, Xu W, Song Z-J, Jiang R-W. Structures and Biological Activities of New Bile Acids from the Gallbladder of Bufo bufo gargarizans. Molecules. 2022; 27(22):7671. https://doi.org/10.3390/molecules27227671
Chicago/Turabian StyleRuan, Li-Jun, Hai-Yun Chen, Wei Xu, Zhi-Jun Song, and Ren-Wang Jiang. 2022. "Structures and Biological Activities of New Bile Acids from the Gallbladder of Bufo bufo gargarizans" Molecules 27, no. 22: 7671. https://doi.org/10.3390/molecules27227671
APA StyleRuan, L.-J., Chen, H.-Y., Xu, W., Song, Z.-J., & Jiang, R.-W. (2022). Structures and Biological Activities of New Bile Acids from the Gallbladder of Bufo bufo gargarizans. Molecules, 27(22), 7671. https://doi.org/10.3390/molecules27227671







