Abstract
Plants rich in hydrolyzable tannins were traditionally used all over the world for a variety of chronic inflammatory disorders, including arthritis, colitis, and dermatitis. However, the knowledge of their immunological targets is still limited though fundamental for their rational use in phytotherapy. The recent advances regarding the pathogenesis of inflammatory-based diseases represent an opportunity to elucidate the pharmacological mechanism of plant-derived metabolites with immunomodulatory activity. This review collects recent articles regarding the role of hydrolyzable tannins and their gut metabolites in Th1, Th2, and Th17 inflammatory responses. In line with the traditional use, rheumatoid arthritis (RA), inflammatory bowel diseases (IBDs), psoriasis, atopic dermatitis (AD), and asthma were the most investigated diseases. A substantial body of in vivo studies suggests that, beside innate response, hydrolyzable tannins may reduce the levels of Th-derived cytokines, including IFN-γ, IL-17, and IL-4, following oral administration. The mode of action is multitarget and may involve the impairment of inflammatory transcription factors (NF-κB, NFAT, STAT), enzymes (MAPKs, COX-2, iNOS), and ion channels. However, their potential impact on pathways with renewed interest for inflammation, such as JAK/STAT, or the modulation of the gut microbiota demands dedicate studies.
1. Introduction
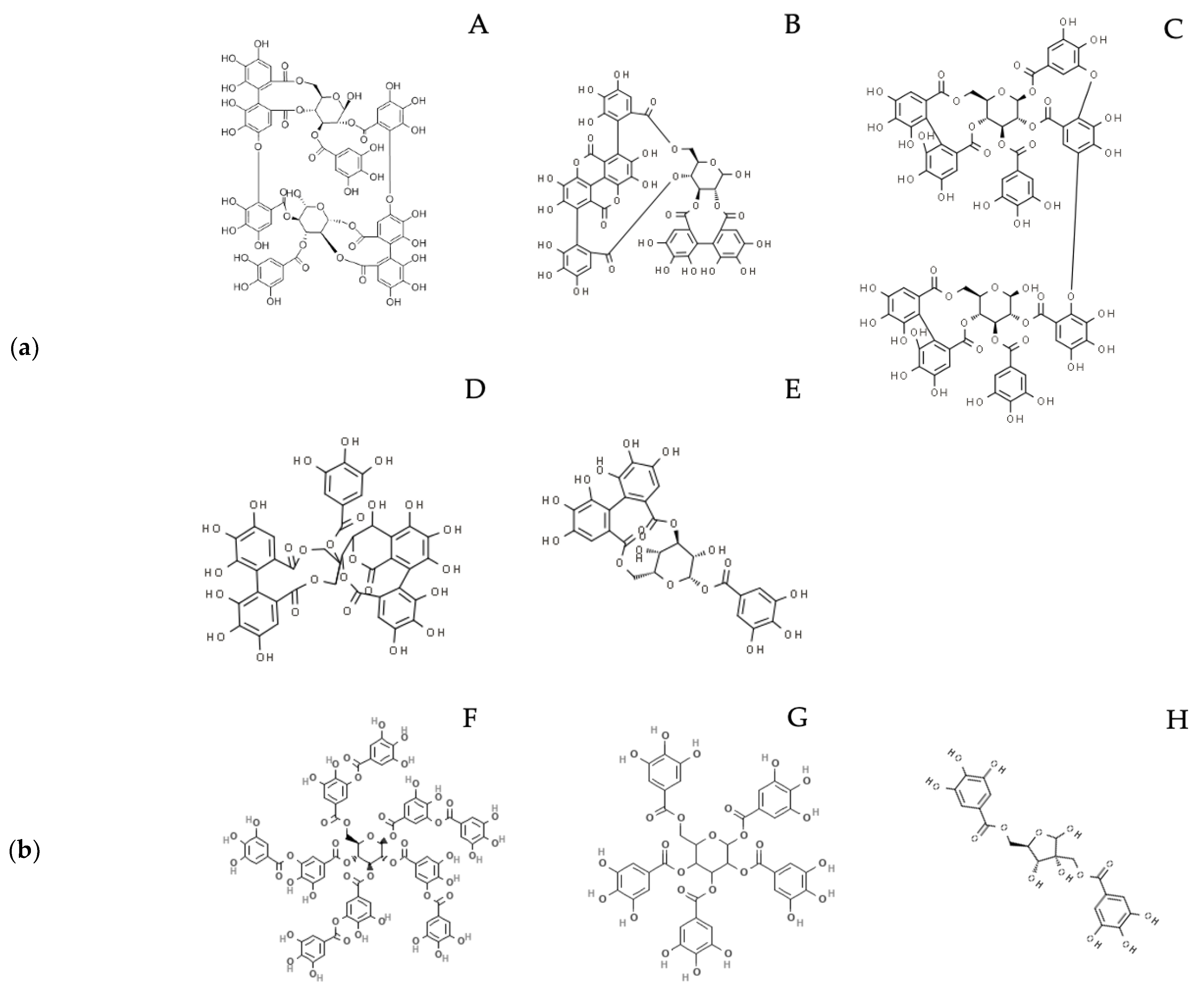
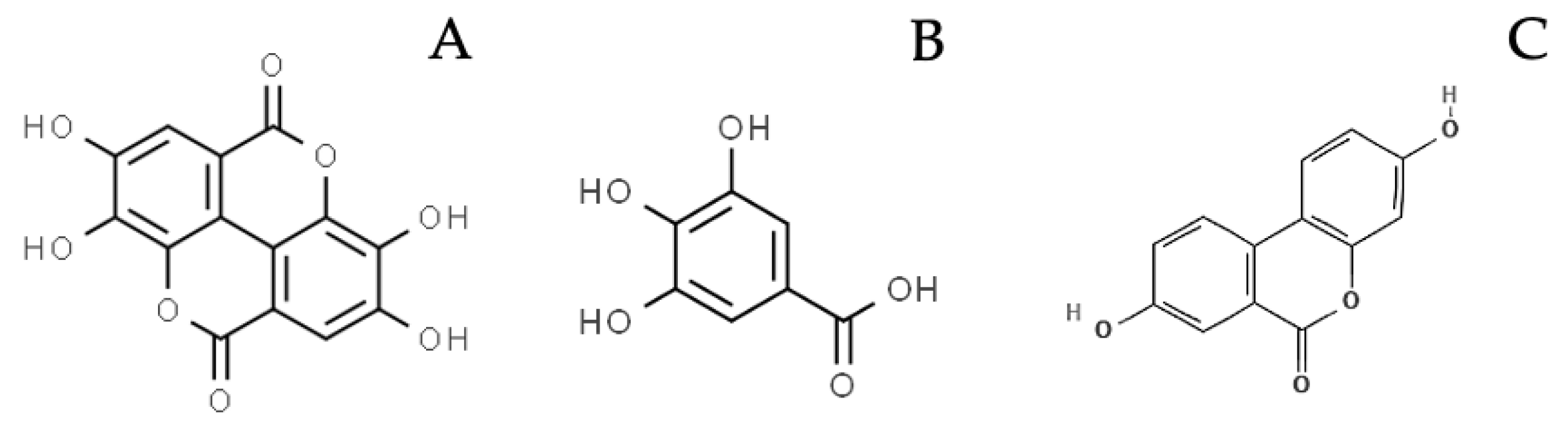
Hydrolyzable tannins (HTs) consist of gallotannins (GTs) and ellagitannins (ETs): the first are composed by a variable number of galloyl-moieties linked to a glycosylic unit, while the latter also contain hexahydroxydiphenic acid (HHDP) moieties (Figure 1a,b). After chemical or enzymatic hydrolysis, gallotannins release gallic acid (GA), while ellagitannins release also HHDP, spontaneously converted to ellagic acid (EA). GA and EA are available for absorption in their form or after gut microbiota metabolism, which transforms GA to pyrogallol derivatives and EA to urolithins (Figure 2) [1,2].
Figure 1.
(a) Examples of ellagitannins cited in the main text: A. Oenothein B; B. Punicalagin; C. Tamarixinin A; D. Casuarinin; E. Corilagin. (b) Examples of gallotannins cited in the main text: F. Tannic acid; G. 1,2,3,4,6 Pentagalloyl glucose; H. Hamamelitannin.
Figure 2.
Examples of compounds deriving from HTs hydrolysis and gut metabolism, cited in the main text: A. Ellagic acid; B. Gallic acid; C. Urolithin A.
Plants containing HTs were reported by traditional medicine belonging to different geographical areas on the basis of their common astringent properties [3,4,5]. The ethnopharmacology from the Mediterranean area includes rich sources of GTs and ETs, such as pomegranate (Punica granatum L.) and other berries (Fragaria spp., Rubus spp.), walnut (Juglans regia L.), sumac (Rhus coriaria L.), sweet chestnut (Castanea sativa Mill.), and oak (Quercus spp.). Despite growing knowledge on the metabolism and pharmacological properties of HTs, their application for the control of inflammatory-based diseases is still limited. In 2018, Kiss and Piwowarski summarized the pre-clinical evidence regarding the anti-inflammatory activity of HTs and derived metabolites from various sources, with a particular focus on cardiovascular and inflammatory bowel diseases (IBDs) [6]. Similarly, our research group sustained the evidence regarding the role of berries in skin and gastro-intestinal inflammation [7,8,9,10]. This body of pre-clinical studies demonstrated that HTs and their metabolites reduce the release of innate inflammatory mediators (TNF-α, IL-6, IL-1β, PGE2, MMPs), mainly through NF-κB impairment.
Of note, the research for novel therapeutical strategies, often inspired by natural compounds, has been renewed by recent discoveries regarding the pathogenesis of inflammatory-based diseases, such as rheumatic disorders, inflammatory bowel diseases, psoriasis, atopic dermatitis (AD), and asthma. The number of publications on PubMed regarding immune-mediated inflammatory diseases increased from 309 to 1059 per year during the last 10 years. Cytokines involved in Th polarization and response, such as IFN-γ, IL-23, IL-17, and IL-4, represent renewed targets for novel pharmacological therapies [11]. Accordingly, the inhibition of their molecular signaling, with a particular reference to the JAK/STAT pathway, is an emerging strategy to control several inflammatory diseases [12].
The potential role of HTs against inflammatory-based diseases might also involve their impact on gut microbiota: generally speaking, these molecules have poor bioavailability and undergo extensive metabolism at the gut level, thus leading to the formation of small metabolites with prebiotic effects [6,8,13]. From this point of view, HTs can be considered potential pro-drugs and prebiotics; from another point of view, their poor absorption is exploitable to conceive an in situ action on human epithelia, with minor side effects at the systemic level. To this end, it is relevant to consider that several inflammatory-based diseases are triggered by the interaction among the environment and the immune system at the level of physiological barriers, such as respiratory, intestinal, and cutaneous.
On this basis, our review aims to update the evidence on the inflammatory targets of plant extracts rich in HTs, with a focus on Th1, Th2, and Th17 responses. For this purpose, the most common inflammatory diseases in which Th-derived cytokines play a key role in pathogenesis have been considered. Of note, the role of HTs in rheumatoid arthritis (RA), psoriasis, AD, and asthma was not recently revised. The bibliographic research took into consideration the crucial role of gut metabolism and bioavailability after oral consumption.
2. Results
2.1. Th1 and Th17 Inflammation: Rheumatoid Arthritis, Psoriasis, and IBDs
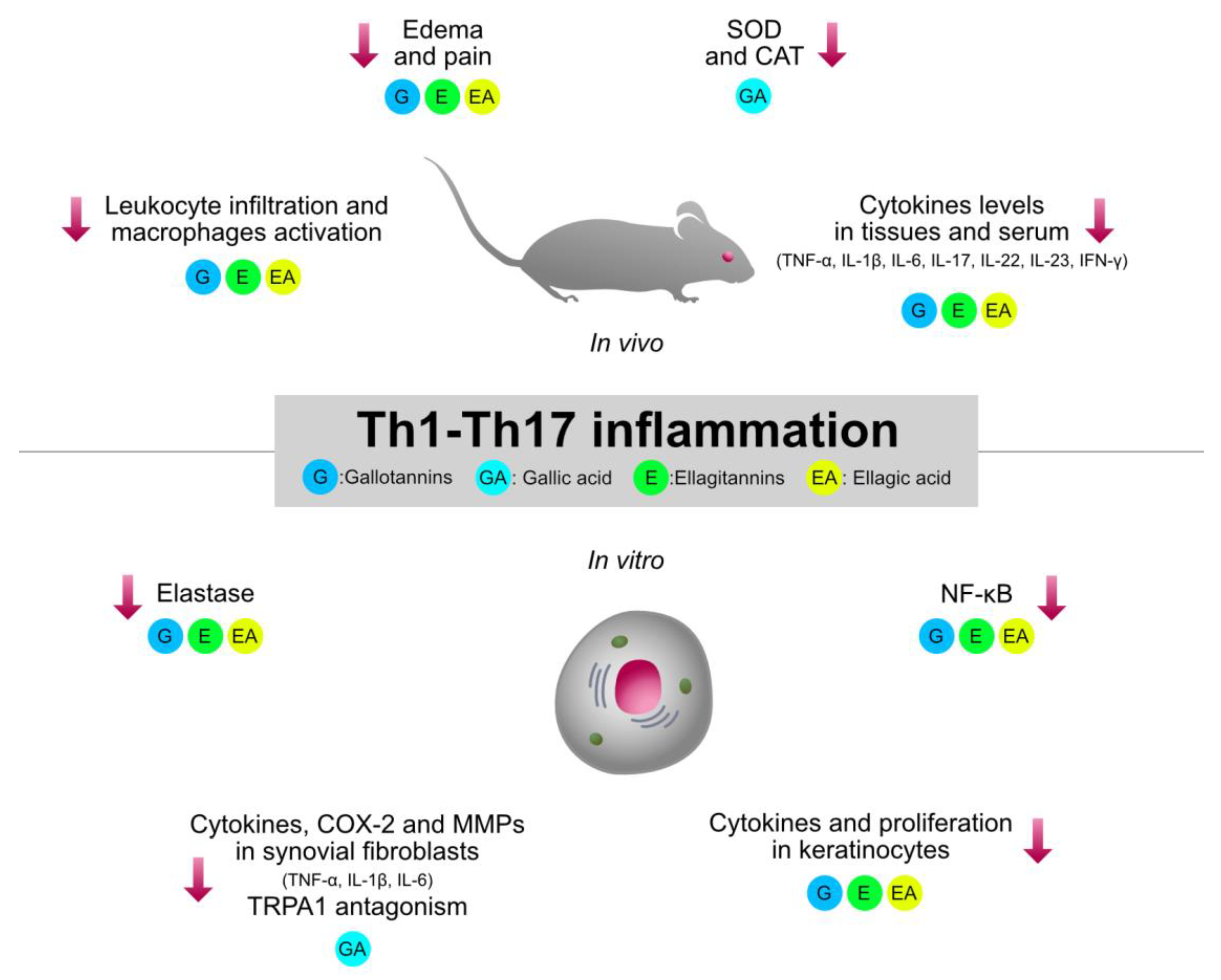
Type 1 inflammation, orchestrated by Th1 cells, plays a crucial role in the pathogenesis of immune-mediated inflammatory diseases, such as rheumatoid arthritis (RA), IBDs, and psoriasis. Traditionally used HTs-containing plants and relative isolated compounds were investigated by several pre-clinical models involving type 1 inflammation. The collection of the experimental evidence allowed a better clarification of the structure–activity relationship regarding the immunomodulatory mechanisms shared by HTs, which are summarized in Figure 3.
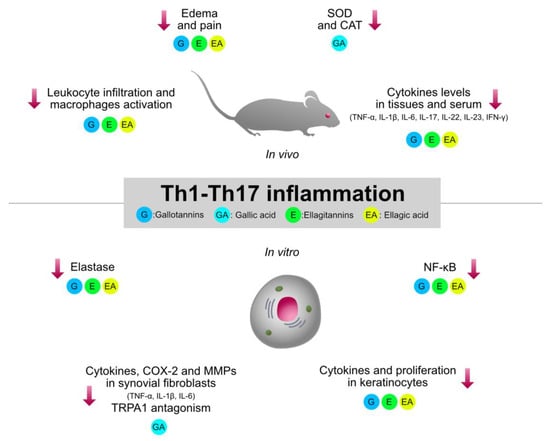
Figure 3.
Graphical summary of the anti-inflammatory mechanisms of HTs and their hydrolysis derivatives (GA, EA), investigated in type 1 inflammation models (RA, IBDs, psoriasis). The compounds share immunosuppressive properties on innate cytokines (TNF-α, IL-1β, IL-6) and type 1 cytokines (IL-23, IL-17, IFN-γ), which are modulated by NF-κB and STAT pathways. G, gallotannins; E, ellagitannins; GA, gallic acid; EA, ellagic acid. Red arrows indicate inhibition.
2.1.1. Rheumatoid Arthritis (RA)
The etiologies of RA, psoriasis, and Crohn’s diseases (CD) share several common pathogenetic pathways and are frequently associated as co-morbidities [14]. RA is a chronic autoimmune inflammatory disorder, influenced by genetic and environmental factors. Mediators from innate immunity (TNF-α, IL-6) and adaptive immunity, namely type 1 cytokines (IL-12, IL-17A, IFN-γ), are crucial for the pathogenesis of RA [15]. The main aim of pharmacological treatment is to relieve inflammation and to recover motor function to prevent long-term complications. Over the past decades, the knowledge regarding the pathophysiology of RA drove the discovery of several efficacious agents to cure patients unresponsive to DMARDs therapy, such as JAK/STAT inhibitors and novel biological drugs (i.e., anti-IL-17 and anti-IL-6 antibodies). However, the need for personalized medicine and the safety of chronic immunosuppressive treatments both remain a major issue [16].
Traditional medicine from different countries, including Mediterranean countries, is also based on plants containing HTs to attenuate articular pain and inflammation. Pomegranate (Punica granatum L.) is one of the most investigated dietary sources of ETs and free EA; this crop is widespread in the Mediterranean area, with an especially long tradition in Middle-Eastern countries. The pre-clinical evidence on the potential anti-inflammatory role of pomegranate for RA was recently reviewed by Mahdavi et al., in 2021 [17]; thus, only unreviewed articles or studies regarding the role of specific pomegranate tannins were included in the present review. The authors reported eight in vivo studies, mainly conducted on polar extracts from pomegranate peel (10–320 mg/kg) administered per os (p.o.) in a rodent model of Freund’s complete adjuvant (FCA)-induced arthritis. In three articles, homogeneous doses of extracts (10–50 mg/kg) were shown to decrease the arthritis score; the mechanisms that explain the biological effects include the inhibition of pro-inflammatory cytokines (IL-6, TNF-α) and the increase of antioxidant enzymes (MDA, GSH, SOD) [18,19,20]. Another in vivo study also demonstrated that the topical application of a standardized pomegranate peel extract can counteract the FCA-induced arthritis as much as 1% diclofenac. The inhibitory effect on edema and pain was observed at doses ranging from 1 to 5%, containing EA 0.13 to 0.65% w/w, respectively. The administration of EA alone was at least partially able to explain the bioactivity. Similarly, the histological analysis showed the reduction of leukocyte infiltration and cytokines levels (TNF-α, IL-1β) [21].
The bioactivity of pomegranate is often attributed to free EA and to its main ET, punicalagin. In vivo studies confirmed that the intra-peritoneal administration (i.p.) of punicalagin (10–50 mg/kg) inhibited joint edema in adjuvant-induced and collagen-induced models of arthritis [22,23,24]. In vitro experiments showed that punicalagin may act on M1 macrophages and fibroblast-like synoviocytes by inhibiting the NF-κB pathway and cytokine release (TNF-α, IL-1β, IL-6) [22,24]. From this investigation, punicalagin resulted in the most investigated pure ET for a potential anti-arthritis effect, although i.p. administration limited the speculation on the role of ET biotransformation at the gut level.
A similar limitation exists in the study from Zhuang et al., in which another ET, tamarixinin A (12.5 or 50 mg/kg/d), was administered via subcutaneous injection to collagen- or FCA-induced arthritis [25]. According to previous results, the compound showed the suppression of the progression and development of arthritis in both models. Again, tamarixinin A (5, 10, 20 μM) impaired the release of NO and cytokines (TNF-α, IL-6) in LPS-activated peritoneal macrophages; the mechanism of action was ascribed to NF-κB and p38 MAPK inhibition. Similarly, oenothein B (1–60 μg/mL, corresponding to 1.27–76.2 μM) inhibited cytokines (TNF-α, IL-1β, and IL-6) and iNOS expression in stimulated RAW 264.7 macrophages: the inflammatory model was TLR2- or TLR4-dependent, since selective agonists or LPS were used for the stimulation. Once again, the activity correlated with the inhibition of p65 and p50 (NF-κB) translocation. Oenothein B was unable to counteract IFN-γ-induced iNOS, thus excluding the interference with the IFN-γ pathway. Of note, the GA moiety was not responsible for the bioactivity, thus suggesting the importance of the whole chemical structure [26]. On the other hand, the plausibility of the interaction among unmetabolized ETs and circulating immune cells is poor, according to their low bioavailability. On the other hand, the potential anti-inflammatory role of low μM (<2.5 μM) oenothein B, isolated from Myrtus communis L., was also demonstrated in human gastric epithelial cells stimulated by TNF-α or IL-1β [27].
Sources of ETs other than pomegranate were less investigated. However, relevant information regarding the bioactivity of dietary products containing ETs comes from few studies performing oral administration. Javed et al. evaluated the effect of walnut (Juglans regia L.), in comparison to methotrexate (0.5 mg/kg), in a model of FCA-induced arthritis. The oral consumption of raw walnut (10% w/w of the total feed) or hydroalcoholic extract (900 mg/kg) increased the serum total antioxidant capacity (TAC) by elevating the antioxidant enzyme level (SOD, CAT). Moreover, RA-related renal and hepatic damage, measured by classical biochemical markers (AST, ALT, creatinine) and histological evaluation, were reverted by both treatments. Unfortunately, no data regarding inflammatory markers were collected [28].
Another example of an ET-containing plant used in traditional Chinese medicine (TCM) with anti-inflammatory purposes, is Cleidion brevipetiolatum Pax & Hoffm (Euphorbiaceae). Zhao et al. isolated six new ETs called brevipetins (B–G) from the ethanolic extract and attributed to brevipetin E (50 mg/kg, p.o.) an anti-arthritis effect in the model of collagen-induced arthritis (CIA). The compound also impaired the NF-κB activity, COX-2, and iNOS expression in LPS-stimulated murine macrophages (RAW 264.7) [29].
Other authors treated CIA mice with a HT-rich fraction from the pericarp of Terminalia chebula Retz. fruit, a plant (fam. Combretaceae) belonging to the Ayurvedic tradition. It contained peculiar HTs known as chebulinic and chebulagic acid but also more common ETs such as corilagin, casuarinin, or GTs, such as 1,2,3,4,6 pentagalloyl glucose; 1,2,3,6 tetragalloyl β d-glucose; and 2,4,6 tri-O-galloyl d-glucose. The tannin-rich fraction (100, 200, and 400 mg/kg p.o.) was compared with methotrexate (2 mg/kg) and reduced paw volume, arthritic score, spleen index, and serum cytokine level (TNF-α, IL-1β, and IL-6) in a dose-dependent fashion. All doses impaired TNF-α and IL-1β release and preserved the articular texture at the level of arthritic joints [30]. In line with these results, Lu et al. evaluated the role of pure chebulinic acid administered p.o. (50 mg/kg/d) in collagen-induced arthritis mice, observing a reduction in paw swelling associated with impaired inflammatory infiltration and cartilage erosion at the joint level. The authors also suggested that chebulinic acid may counteract synovial angiogenesis, since VEGFR-related genes were downregulated [31].
The role of corilagin as an individual compound was evaluated in the FCA model (20, 40 mg/kg, p. o.) in comparison to methotrexate. The in vivo results showed that corilagin significantly reduced paw swelling and arthritis score and inhibited joint erosion and the infiltration of inflammatory cells; pro-inflammatory cytokines were also inhibited at the serum level (IL-6, TNF-α, IL-1β, and IL-17). In fibroblast-like synoviocyte (FLS) cells challenged by IL-1β, corilagin (6.25 or 12.5 μM) impaired COX-2, iNOS, and MMPs expression through MAPKs and NF-κB inhibition [32].
Other interesting indications were derived from experiments in which the efficacy of pure EA was investigated, considering the high presence of free EA in pomegranate and its production at the gut level following the metabolization of ETs. Three in vivo studies demonstrated that the oral administration of EA reduced joint swelling and articular disruption in an AIA model [33,34]. The efficacy of EA (50 mg/kg/d) was comparable to the reference compound celecoxib (5 mg/kg). The mechanism of action was attributed to NF-κB impairment and a reduction in MMP-9, VEGF, and TNF-α levels. Moreover, an improvement in oxidative markers was observed, such as MPO activity, NO release, GSH levels, and lipid peroxidation. Similar doses of EA were used by Song et al. (25, 50, 100 mg/kg/d), who confirmed the reduction of paw swelling, synovial hyperplasia, and inflammation in a collagen-induced arthritis (CIA) model [35]. Moreover, EA was treated in TNF-α-induced FLS cells, at the range of 10 to 100 μM, thus inhibiting the levels of cytokines (IL-6, IL-1β) and oxidative markers (MDA). The authors suggested that EA may interfere with the metastasis-associated gene 1 (MTA1)/HDAC-1 pathway, involved in RA development, reflecting the inhibition of Nur77 deacetylation [35].
Despite the different way of administration (i.p.), Allam et al. obtained similar results with EA 175 mg/kg/week in the AIA model. EA significantly inhibited paw edema and reduced serum levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-17). On the contrary, increased serum levels of IL-10 were measured, thus suggesting a re-balancing of the inflammatory status [36].
Another key metabolite of HTs is GA. Several studies investigated the effect of GA-containing plant extracts in rodent models of arthritis. Polar extracts from Alternanthera bettzickiana (Regel) G. Nicholson and Sarcococca saligna Müll. Arg., characterized for the presence of GA as one the main compounds (around 0.5 and 1.7 mg/g, respectively), were administered orally at doses of 250, 500, and 1000 mg/kg [37,38]. Both studies reported a reduction in paw swelling and pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. The expression of COX-2 was also reduced, and the molecular docking analysis from Manan et al. suggested that GA might also interfere with enzymatic activity. Moreover, an increase in anti-inflammatory mediators (IL-10, IL-4) and the impairment of the NF-κB pathway were observed.
The anti-inflammatory effect of GA was sustained by another in vivo study in which the pure compound was administered orally (3–100 mg/kg) in pain and arthritis models. In particular, the reduction of TRPA1-mediated edema or allodynic and neuropathic pain was observed at a dose of 10 mg/kg. Accordingly, gallic acid (10 μM) inhibited calcium influx in mouse spinal cord synaptosomes induced by the TRPA1 agonist cinnamaldehyde [39]. A subsequent study confirmed the effect of a GA-rich ethyl acetate fraction (0.01–100 mg/kg) from Tabernaemontana catharinensis A. DC. in TRPA1-induced paw inflammation and FCA-induced arthritis. The same fraction potently inhibited TRPA1-induced calcium influx (IC50 = 0.023 μg/mL) but not TRPV1, thus suggesting a specific activity on TRPA1 [40].
From a structure–activity relationship point of view, it is also interesting to note that methyl gallate (0.7 to 70 mg/kg, p.o.) exhibited similar effects with respect to GA in the AIA model. In fact, a dose of 7 mg/kg was enough to reduce leukocyte infiltration, osteoclast activity, and pannus formation at the joint level [41].
An in vitro study suggested that GA might also act at the joint level on synoviocytes: the authors demonstrated that GA (0.1–1 μM) was able to counteract the proliferation of FLS from RA patients through pro-apoptotic mechanisms; moreover, it suppressed the expression of cytokines (IL-1β, IL-6), chemokines (MCP-1, MCP-3), MMP-9, and COX-2 versus the TNF-α challenge [42].
Along with synoviocytes and macrophages, dendritic cells (DCs) play a well-recognized role in the pathogenesis of RA and other inflammatory-based diseases. The role of HTs and their relative metabolites, such as GA, EA, and urolithins (Uro), on this cell population is still unclear. Given the poor bioavailability of unmetabolized HTs, their potential bioactivity might strictly regard resident DCs orchestrating epithelial inflammation, as occurs in psoriasis, AD, and IBDs.
Two in vitro studies investigated the impact of GA or oenothein B treatment on the activation of human DCs: in LPS or cytokine-induced cells, GA (50 μg/mL, corresponding to 300 μM) and oenothein B (10, 25, 50 μM) inhibited the expression of markers with a key role in DCs–T cells interaction, such as CD40, CD80, CD83, and CD86. The effect of GA was also demonstrated in addition to hydrocortisone (0.2 μM), thus suggesting its possible complementary use. GA and oenothein B also inhibited the release of cytokines involved in Th1 polarization, such as IL-12p40 and IL-6, respectively [43,44].
Differently from EA and GA, urolithins were not investigated in models of RA as isolated compounds. However, a recent study attributed anti-inflammatory properties to UroA through in vitro and in vivo experiments, involving surgically induced osteoarthritis; the main mechanism was ascribed to NF-κB pathway impairment [45]. Accordingly, our group reported that urolithins (UroA, UroB, UroA-8Me) at 25 μM can counteract MMP-9 release in human macrophages (THP-1), challenged by TNF-α [46]. Shen et al. managed a work by means of the experimental model of autoimmune encephalomyelitis, suggesting that also urolithin A (UroA) may counteract Th1 inflammation. In fact, UroA (25 mg/kg/d) reduced the activation of DCs and the Th1/Th17 infiltrate at the CNS level. In vitro, UroA (25 μM) inhibited bone-marrow-derived DCs activation and Th17 differentiation in a co-culture model, thus leading to a IL-10 increase and IL-1β, IL-6, and IL-17 reductions, plausibly by acting on the AHR receptor [47].
Despite the concentrations used in several studies, which appear quite high, studies in the literature suggest that HTs may exert an immunosuppressive effect on activated macrophages and dendritic cells after gut metabolization, thus reflecting anti-inflammatory and analgesic effects in animal models of RA (Table 1).

Table 1.
Collection of recent articles concerning the biological activity of HTs and their metabolites in RA models.
2.1.2. Psoriasis
As previously mentioned, psoriasis and RA are frequently associated in patients with autoimmune disorders, and it is not surprising that psoriatic arthritis often resembles RA symptoms. The cytokine TNF-α is a well-known pathogenetic mediator of many immune-mediated diseases, including psoriasis, targeted by the first biological drugs, such as etanercept and infliximab. However, clinical trials demonstrated the heterogeneous response of psoriasis patients to anti-TNF-α treatments, thus stimulating the search for novel therapeutical targets [48]. In this context, the discoveries regarding the role of IL-23/Th17 axis represent an important advance for pharmacology, as reviewed by Girolomoni et al. [49].
Despite the inhibition of cytokine signaling, including TNF-α, as one of the main bioactivities attributed to HTs [50], few studies have been carried out to elucidate their potential role in psoriasis.
Recently, our research group published an article regarding the potential anti-inflammatory activity of Rhus coriaria L. fruit at the skin level, namely a tannin-rich spice from the Mediterranean and Middle-Eastern regions. Water and ethanolic extracts (1–50 μg/mL) from Rhus coriaria L. fruit exhibited an inhibitory effect on TNF-α-induced cytokines in human keratinocytes, with IC50s lower than 20 μg/mL. Ethanolic extracts exhibited more promising effects and inhibited MMP-9, ICAM-1, IL-8, and NF-κB-driven transcription. The major compounds identified were GTs (4.54%), followed by a lower content of flavonoids (0.23%) [51]. On the contrary, we excluded the role of the main GT from Hamamelis virginiana L., namely hamamelitannin, in the impairment of NF-κB and cytokine release in human keratinocytes challenged by TNF-α [52].
Other authors investigated the biological activity of an ethanolic extract from Woodfordia fruticosa (L.) Kurz flower, a source of tannins with traditional uses, from tropical to subtropical areas. The extract was formulated as gold nanoparticles Carbopol® 934 ointment gel (500 μg/kg) or Carbopol® 934 only (2000 mg/kg) and applied topically in a mice model of imiquimod (IMQ)-induced psoriasis [53]. Both formulations reduced the disease score, the serum level of TNF-α and IL-23, and the hyperproliferation of keratinocytes. EA was suggested as the main bioactive compound.
Few works reported the role of ETs in psoriatic inflammation. Punicalagin was administered topically (25 mg/kg) in IMQ-induced mice in comparison to dexamethasone, thus improving psoriasis symptoms. The level of cytokines such as CXCL1, CCL20, and IL-1β was reduced in skin tissue and paralleled NF-κB impairment. Moreover, punicalagin inhibited the release of IL-1β in TNF-α and IL-17A challenged human keratinocytes (HaCaT cells), once again by NF-κB impairment [54]. Similarly, agrimoniin and pedunculagin inhibited enzymatic activity but not the release of human neutrophil elastase ex vivo with low IC50s (<3 μM). The direct interaction of ETs with the enzyme was supported by docking analysis. Moreover, agrimoniin exhibited an anti-proliferative effect in the ATP assay in HaCaT cells (IC50 = 3.4 μM) [55]. To the best of our knowledge, HTs are poorly permeable across skin barrier after topical application and plausibly reach nM concentration at the dermal level [56]; however, μM concentrations might be potentially reached through adequate formulative strategies [57]. The impact of orally administrated ETs and GTs in psoriatic models has rarely been investigated.
According to the literature, the efficacy of HTs and their metabolites on psoriasis has not been fully elucidated. Papers on this subject are limited, thus making it impossible to draw clearcut conclusions (Table 2).

Table 2.
Collection of recent articles concerning the biological activity of hydrolyzable tannins and their metabolites in psoriasis models.
2.1.3. Inflammatory Bowel Diseases (IBDs)
IBDs are represented by ulcerative colitis (UC) and Crohn’s disease (CD). The paradigm of CD and UC as Th1 and Th2 diseases, respectively, was recently criticized by many experts in the field, including Ramos et al. [58]. Since the IL23/Th17 axis became crucial to signaling in the development of novel effective biological drugs, such as ustekinumab, in both IBDs [59,60], we inserted a common paragraph regarding the role of plant extracts in IBDs pathogenesis in this section.
Mango fruit (Mangifera indica L.), a well-known source of GTs and free GA, was investigated by several in vivo studies involving UC mice models (DSS-induced colitis). A mango beverage (89.74 mg/kg/day of gallic acid eq.) reduced colon inflammation and cytokine expression (TNF-α, IL-1β, IL-6); moreover, the expression of COX-2 and iNOS and the NF-κB pathway were impaired. The results were sustained by in vitro studies on colon fibroblasts and epithelial cells (CCD-18Co and HT-29 cells, respectively), in which a polar mango extract (10 μg/mL) inhibited iNOS and the mTOR pathway [61]. A mango beverage (475.90 mg/L gallic acid eq.) was compared with a pomegranate beverage (2504.74 mg/L gallic acid eq.) in another study involving a DSS-induced colitis model. Mice received the respective beverage ad libitum (about 90 mL/day) for 3 weeks before colitis induction, showing reduced inflammatory and ulceration scores. Cytokines such as TNF-α, IL-1β, IL-6, and MAPKs were inhibited in the intestinal mucosa; moreover, the mTOR pathway was equally modulated by mango and pomegranate, although different genes were involved in the mechanisms exerted by each beverage. In addition, the expression of IGF-1R and EGFR, known to activate the mTOR pathway, was impaired by mango, pomegranate (10 μg/mL), and the respective major compounds GA and EA (4 μg/mL) in CCD-18Co cells [62].
Of note, Barnes et al. measured relevant levels of GTs and GA metabolites in the urine of healthy volunteers consuming 400 mg/day of mango pulp (Mangifera indica L. cv. Keitt) for 10 days. The metabolites pyrogallol-O-sulfate and deoxypyrogallol-O-sulfate were the main metabolites excreted across the considered period, ranging from 28.5 to 55.4 mg and 23.6 to 47.7 mg of total excretion, respectively [1].
Other in vivo studies evaluated the effect of pomegranate peel extract after oral consumption in rodent models of colitis. A decoction of pomegranate peel (300 mg/kg/day), containing 45.3 mg of total ETs, reduced the visceral sensitivity measured by a visceromotor response test. The decoction was compared with the corresponding ETs-enriched fraction (45 mg/kg/day), which was considered responsible for the improvement of visceral pain and the intestinal damage score. Both preparations reduced the infiltration of mast cells and the density of collagen fibers as an index of fibrosis in mucosal stroma [63]. Another work showed that pomegranate extract (250 mg/kg/day), characterized for punicalagin (35%), punicalin (13%), and EA (8.9%) content, caused a slight reduction in the inflammatory infiltrate in DSS-injured intestinal mucosa, while UroA (15 mg/kg/day) showed a statistically relevant inhibitory effect. Both the extracts and UroA prevented the upregulation of COX-2 and iNOS expression, leading to lower NO and PGE2 tissue levels [64].
Pomegranate extract (250–500 mg/kg/die) was compared with pure EA (10–20 mg/kg/die) by Rosillo et al. in a model of TNBS-induced colitis [65,66]. The authors observed similar effects, with reduced neutrophil infiltration and intestinal injury. The anti-inflammatory mechanism consisted of lower iNOS and COX-2 expression and the impairment of MAPKs and the NF-κB pathway.
Two papers investigated the impact of walnut administration in colitis models. The first observed that the addition of 7–14% of walnut to the mouse diet caused partial protection against DSS-induced mucosal damage. The formation of urolithin A and EA was confirmed in fecal samples [67]. In the same model, a phenolic extract from walnut (10–20 mg/kg/day), characterized for the presence of GA and EA, significantly prevented colitis with respect to either acute or chronic DSS-induced damage. In vitro experiments, conducted on intestinal epithelial cells (COLO 205) challenged with TNF-α, attributed an anti-inflammatory effect to walnut phenols (10–20 μg/mL), since NF-κB activity and IL-8 expression were impaired [68].
One author aimed to validate the traditional use of the root bark from Paeonia × suffruticosa Andrews (called “Moutan Cortex Radicis”) in TCM. The aqueous extract is known to contain a relevant amount of the GT pentagalloyl–glucose (PGG), which was investigated as a main bioactive compound. The oral administration of 5% aqueous extract reduced the inflammation score and the infiltration of macrophages in the intestinal mucosa of DSS-induced mice. PGG (5–10 µM) inhibited NF-κB and IRF in THP-1 cells challenged by TRL2 ligand or poly (I:C) [69].
Similarly, corilagin (7.5–30 mg/kg/day, i.p.) counteracted the shortening of the colon length, caused by DSS induction, in a dose-dependent manner. Moreover, the level of cytokines (TNF-α, IL-1β, IL-6) and MPO activity were reduced in colon tissues [70].
The contribution of pure GA for colitis relief was evaluated by two in vivo studies. In the first, GA (10 mg/kg/day, p.o.) prevented a reduction in body weight and a shortening in colon length and also reduced tissue inflammation. The level of cytokines and MPO was reduced and associated with NF-κB inhibition. In LPS-induced RAW 264.7 cells, high concentrations of GA (100–200 µg/mL, corresponding to 589–1178 µM) decreased p65, iNOS, and COX-2 expression, as well as STAT3 phosphorylation [71]. Accordingly, Zhu et al. showed that GA (20–60 mg/kg, intragastric injection) improved the histological alteration in TNSB-induced colitis, by anti-inflammatory mechanisms which include the NF-κB impairment. In intestinal epithelial cells (HIEC-6) stimulated by IL-1β, GA (20–60 µg/mL) inhibited the cytokines release (IL-6, IL-12, IL-17, IL-23, TGF-β, and TNF-α) and exhibited anti-apoptotic activity [72]. A summary of commented articles is reported in Table 3.

Table 3.
Collection of recent articles concerning the biological activity of hydrolyzable tannins and their metabolites in IBD models.
2.2. Th2 Inflammation: Asthma, Atopic Dermatitis
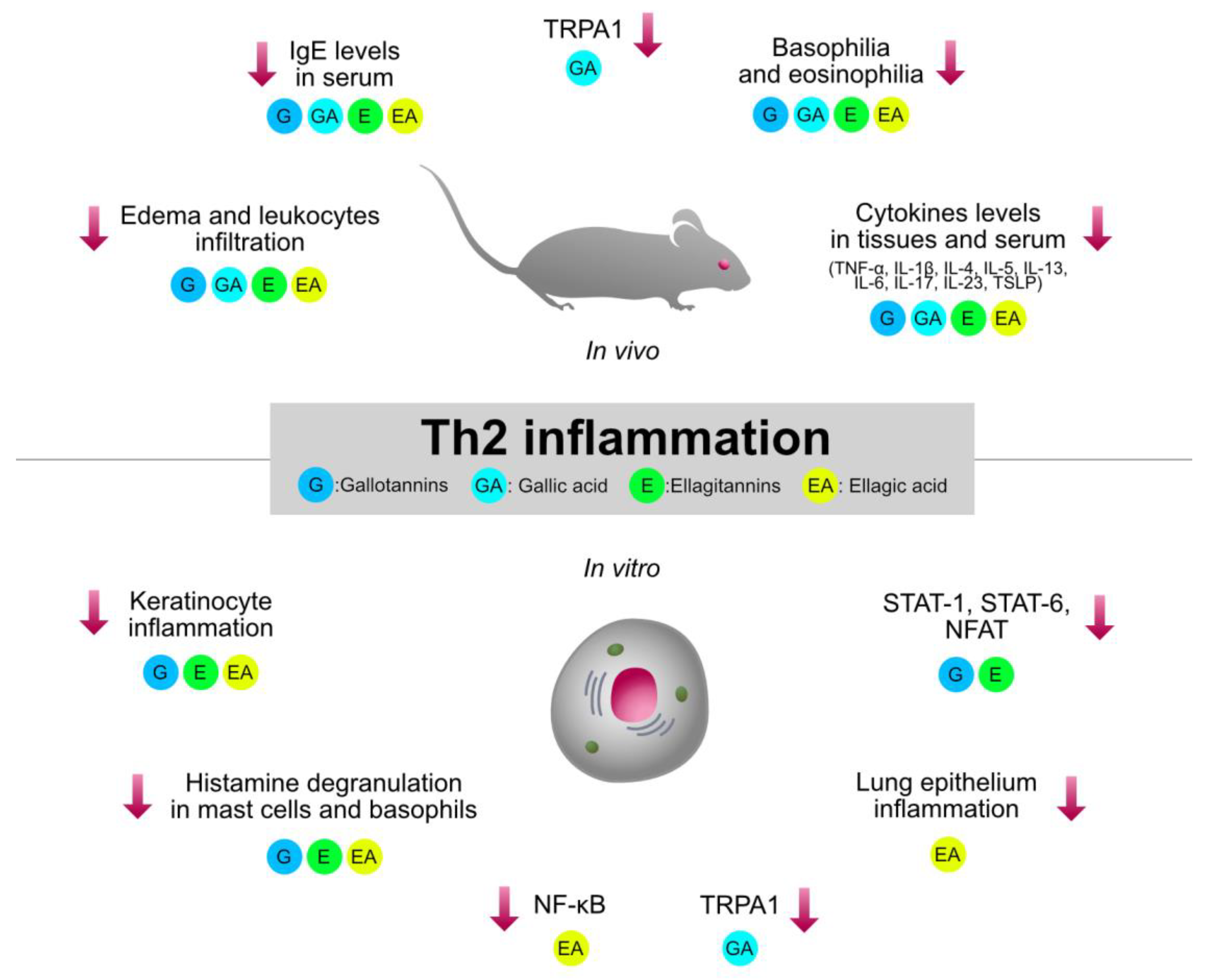
Type 2 inflammation, orchestrated by Th2 cells, plays a crucial role in the pathogenesis of immune-mediated and allergic disorders, such as asthma, AD, and allergic rhinitis. HTs-containing plants and the relative isolated compounds were investigated by several pre-clinical models involving type 2 inflammation. The immunomodulatory mechanisms shared by HTs are summarized in Figure 4.
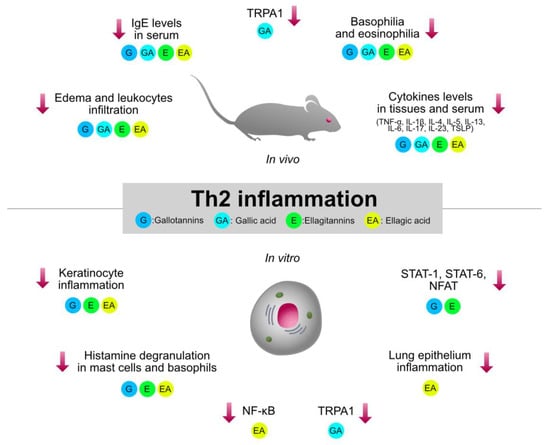
Figure 4.
Graphical summary of the anti-inflammatory mechanisms of HTs and their hydrolysis derivatives (GA, EA), investigated in type 2 inflammation models (asthma, AD, allergic rhinitis). The compounds share immunosuppressive properties on innate cytokines (TNF-α, IL-1β, IL-6) and type 2 cytokines (TSLP, IL-4, IL-5, IL-13), which are modulated by the NF-κB and STAT pathways. G, gallotannins; E, ellagitannins; GA, gallic acid; EA, ellagic acid. Red arrows indicate inhibition.
2.2.1. Atopic Dermatitis (AD)
AD, asthma, and allergic rhinitis are among the most common diseases occurring in children [73]. The complex pathogenesis of asthma and AD were better clarified in recent years, thus suggesting novel therapeutical strategies, such as anti-TSLP (tezepelumab) and anti-IL-4 (dupilumab) antibodies, respectively [74,75]. Asthma and AD are frequently associated with atopy and are characterized by elevated levels of type 2 cytokines (IL-4, IL-5, IL-13), which cause IgE production, mast cell activation, and basophil and eosinophil recruitment, well-known cellular actors in IgE-mediated inflammatory response [76,77]. These recent advances represented winsome tools for the discovery of novel small molecules and the validation of potential anti-allergic natural products.
A plant diffused in the Mediterranean area with traditional usage against mucosal and epithelial inflammation is oak (Quercus spp.). Galls occurring in oak are a well-known rich source of GTs, with medicinal and industrial uses in different areas of the world. Tannic acid and tannin-like synthetic polymers (such as Tamol PP) have been used to relieve allergic itch and inflammation [78]. However, the potential role of HTs-rich extracts in allergic diseases is still poorly investigated.
An important effort toward the clarification of AD pathogenesis and the validation of plant traditional medicines was made in Korean research. An ethanolic extract from acorn shells from Quercus mongolica Fisch. ex Ledeb. (1% topically), collected in Korea, inhibited the epidermal edema in mice models of oxazolone- and DNCB-induced dermatitis. The level of cytokines involved in AD (TNF-α, IL-1β, IL-33, IL-4) and serum IgE were reduced. The fractionation of the extract correlated with the bioactivity with the EA and GA content. Accordingly, EA and GA were active on IL-4 release and degranulation in RBL-2H3 cells (10–30 μg/mL, corresponding to concentrations < 10 μM) [79]. Similarly, the tannin fraction from common oak bark (Quercus robur L.) showed a concentration-dependent inhibition of basophilic degranulation capacity (58–580 μg/mL) in RBL-2H3 cells. Moreover, it inhibited cytokine release (IL-8, IL-6, TNF-α) in human mast cells (HMC-1) [80].
Quercus spp. are also known to contain tannic acid, investigated as a pure compound (80 mg/kg/day p.o.) in a NC/Nga mice model of AD induced by Dermatophagoides farinae (DfE) cream. Tannic acid strongly lowered the dermatitis score, while moderately inhibiting epidermal thickness and neutrophil infiltration. Several cytokines, such as TNF-α, IFN-γ, and IL-1β, were inhibited at the tissue level, while IL-4, IgE, and IFN-γ were inhibited at the serum level. PPARγ agonism and NF-κB inhibition were involved as potential anti-inflammatory mechanisms [81].
Another important source of GTs is witch hazel (Hamamelis virginiana L.), traditionally used to treat skin inflammation and hemorrhoids [82]. Stems and leaves were extracted with ethanol and characterized for the presence of hexagalloylglucose (HGG) as the main compound. The extract (2% in the cell media) inhibited calcium influx induced by particulate matter in human keratinocytes (HaCaT cells). The expression of the PAR-2 receptor and the consequent activation of NF-κB were also inhibited. In addition, the expression of occludin, a protein involved in epithelial integrity, was recovered. HGG (100 μg/mL) resembled the observed inhibition of calcium influx [83].
Recently, our group of researchers attributed an anti-inflammatory activity to a polar extract from witch hazel bark and twigs (0.5–125 μg/mL) in HaCaT cells challenged with TNF-α and IL-4 [52,84]. The extract was characterized by the presence of oligomeric proanthocyanidins (0.29%) and hamamelitannin (0.30%) as the main compounds. The latter (at a concentration lower than 10 μM) showed a specific role in the inhibition of IL-4 targets, such as the release of TSLP and eotaxin-3 (CCL26), and the impairment of cytokeratin-10 (CK-10) and involucrin (INV) expression.
Several articles suggest that GA may represent the anti-allergic moiety of HTs or an anti-allergic molecule itself. GA (0.01–10 μM) inhibited histamine release, and the elevation of [Ca2+]i induced cAMP increase and inhibited NF-κB and MAPK activity in rat mast cells (RPMC) induced by IgE or 48/80. Moreover, it lowered IL-6 and TNF-α after PMA induction in human mast cells (HMC-1). In vivo, GA (1–100 mg/kg) showed 50% inhibition in a IgE-mediated passive cutaneous anaphylaxis model (PCA) and inhibited serum histamine at 10 mg/kg p.o. [85]. Similarly, GA (20–40–80 mg/kg, p.o.) inhibited the TNF-α and IgE serum level in a dose-concentration manner in DNCB-induced mice. The effect was associated with reduced leucocyte infiltration and ear thickness in histopathological evaluation. It also reduced lymph node weight and the expression of TNF-α, IL-4, IFN-γ, and IL-17. In the ear, topical GA reduced the expression of IL-4, IL-5, IL-17, and IL-23, while the expression of IL-10 and TGF-β were increased. In line with this data, the expression of the pro-inflammatory factor ROR-γt was decreased and the suppressor SOCS3 was increased [86].
ETs were even less investigated in AD models. Casuarinin isolated from Hippophae rhamonoides L., a plant widely distributed in the Mediterranean region, was investigated in HaCaT cells challenged by TNF-α or IFN-γ. The concentrations of 5–20 μM impaired the proallergic chemokines CCL17 and CCL22, plausibly through STAT-1 and NF-κB inhibition [87]. Of note, the hydrolysis product EA was demonstrated to improve IgE-mediated cutaneous anaphylaxis in SD rats, at doses (10–50 mg/kg, p.o.) comparable with azelastine (10 mg/kg). EA (50–100–200 μM) was also able to impair histamine and cytokine (TNF-α, IL-6) release in ex vivo rat mast cells, by controlling [Ca2+]i and NF-κB activation [88]. EA was also considered the main bioactive compounds in Rubus coreanus Miquel root extract (100 mg/kg, topically), which was shown to control the severity of diseases in a DNCB-induced NC/Nga mouse model. The extract had comparable effect to tacrolimus ointment, used as positive control at the same concentration (100 mg/kg, topically) [89]. The summary of commented articles is reported in Table 4.

Table 4.
Collection of recent articles concerning the biological activity of HTs and their metabolites in AD models.
2.2.2. Asthma and Allergic Rhinitis
The major cause of asthma and allergic rhinitis, in genetically predisposed subjects, is exposure to respiratory allergens [77]. HTs were traditionally used to link and trap allergic aptens to avoid respiratory disorders: environmental sprays based on tannic acid (3%) were investigated by clinical trials for their ability to reduce exposure to typical house allergens in children [90].
However, tannic acid also exhibits pharmacological activity, according to several in vivo studies. The intratracheal application of tannic acid (25 mg/kg) significantly decreased OVA-induced airway hyperresponsiveness in BALB/c mice. The authors observed a reduction of leukocyte infiltration and reduced levels of pro-allergic mediators (Th2 and Th1 cytokines, eotaxin, and total IgE). Moreover, the compound attenuated the expression of mucins (Muc5ac and Muc5b), mucus production in airway goblet cells, mast cell infiltration, and airway remodeling. The mechanism was attributed to the impairment of NF-κB and adhesion molecules at the lung epithelial level [91].
Tannic acid also showed anti-allergic properties after oral administration (40 mg/kg) in an OVA-induced rhinitis model in comparison to dexamethasone (5 mg/kg), based on another study [92]. Tannic acid diminished the number of rushes, histamine and IgE levels, Th2 cytokines (TSLP, IL-4, IL-5, IL-13, IL-33), and innate cytokines (IL-1β, TNF-α). In addition, protein expression levels of caspase-1, MCP-2, and ICAM-1 were reduced. Accordingly, the oral administration of tannic acid (4 mg/mL in water ad libitum) isolated from Rhus javanica L. extract showed an anti-allergic effect in a general model of OVA-sensitization (i.p. injection). It lowered IgE production by inhibiting IL-4-induced ε germline transcript (εGT), known to guide the IgE switch [93]. The same authors also investigated the in vitro mechanism of tannic acid (1 μg/mL) in comparison to PGG (1, 10, 25 μM). Both compounds inhibited the IL-4 pathway by acting on IL-4Rα, JAK3, and STAT6 activation in lymphoma cells (DND39) and fibroblasts (NIH3T3). Of note, PGG exhibited the preferential inhibition of IL-4 signaling rather than IFN-γ, thus suggesting selectivity in type 2 inflammation.
The role of PGG was then sustained by an in vivo study, in which oral administration (10 mg/kg) caused a decrease in IgE levels in the serum of OVA-sensitized mice. The production of Th2 (IL-4 and IL-13), Th1 (IFN-γ), and pro-inflammatory cytokines (IL-6, TNF-α), but not anti-inflammatory cytokine (IL-10), from splenocytes was strongly suppressed. In contrast with the observation regarding IL-10 levels, PGG enhanced the proliferation of Treg cells, known for their immunosuppressive properties.
Finally, PGG administration reduced the expression of eotaxin, TIMP-1 (marker of mast cell infiltration), but also increased the expression of IGFBP-3, which inhibits IgE production [94].
Another group evaluated the in vitro bioactivity of eight GTs from Euphorbia spp. (E. jolkini Bioss and E. fisheriana Steud.), showing different sugar moieties and degrees of galloylation (1-O-galloyl-b-D-glucose, 1,2,3-tri-O-galloyl-b-D-glucose, 1,2,3,4,6-penta-O-galloyl-b-D-glucose, 3-O-galloylquinic acid, 2-O-galloyl-D-galactose, 1,3,6-tri-O-galloyl-b-D-allose, 1,2,6-tri-O-galloyl-b-D-allopyanose, and 1,2,3,6- tetra-O-galloyl-b-D-allopyranose). Their effect was evaluated at different concentrations (0.1, 1, 10 μg/mL) in human mast cells (HMC-1) induced by PMA+A23187 (calcium ionophore) in comparison with GA (10 μg/mL), used as positive control. The highly galloylated compounds inhibited the gene expression and secretion of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in a concentration-dependent manner, acting on the transcriptional activity of NF-κB [95].
In line with the potential role of GTs, several studies investigated the bioactivity of GA: Fan et al. suggested that the oral administration (20–80 mg/kg) alleviated nasal allergic symptoms in an OVA-induced allergic rhinitis model. The compound decreased the levels of interleukin IL-4, IL-5, IL-13, and IL-17 in nasal lavage fluid and diminished the levels of OVA-specific IgE, IgG1, and IgG2a in serum. In this study, the observed increase in type 1 cytokines (IFN-γ and IL-12) in nasal fluids suggested an inflammatory-state re-balance [96]. Accordingly, GA (10, 50, 100 μg/mL) exhibited suppressor activity on IL-33-induced human basophils (KU812 cells), as is evident from the inhibition of adhesion molecules (ICAM-1) and pro-inflammatory mediators (CCL2, CCL5, CXCL8, IL-6) [97].
The anti-allergic properties of ETs were poorly investigated. An in vivo study demonstrated the anti-inflammatory effect of pomegranate leaf extract (20 mg/kg, intranasal) in a model OVA-induced asthma [98]. The authors suggested the presence of HTs according to HPLC/UV analysis, but their nature was not elucidated. Two additional studies evaluated the role of corilagin: the first reported a decrease in leukocyte and eosinophil counts in the blood of milk-sensitized mice after the oral administration of corilagin (10, 20, 40 mg/kg/day, p.o.); moreover, in another model, corilagin attenuated the anaphylactic reaction, IgE levels, and the degranulation of mast cells. The mechanism of action was also ascribed to the antagonism of Ach- and histamine-induced tracheal contraction ex vivo [99]. In the second, corilagin (10–50 μg/mL) was not able to impair histamine release in rat basophils (RBL-2H3), but the cell line and induction protocol were not comparable with the previous study [100].
Another ET (putranjivain A), occurring in E. jolkini Bioss, showed an inhibitory effect in another in vitro study on RBL-2H3 and HMC-1 cells, at a concentration range of 0.1 to 10 μM. The compound inhibited the expression of pro-inflammatory cytokines (TNF-α, IL-6, and IL-4) in IgE-induced or antigen-induced cells, through the impairment of NF-κB and NFAT activity. The effect was also observed at the in vivo level, in which oral administration (10 mg/kg) reduced systemic and cutaneous anaphylaxis, the release of serum histamine, and the expression of the histamine H1 receptor [101].
Finally, three papers investigated the role of EA and EA-rich extracts in allergic models. Lafoensia pacari A.St.-Hil. ethanol extract (200 mg/kg, p.o.), a Paraguayan plant belonging to Lythraceae, and isolated EA (10 mg/kg, p.o.) exhibited anti-asthma activity in OVA-induced models. The mechanism was attributed to Th2 cytokine inhibition and reduced granulocyte count. In human bronchial epithelial cells, EA (100 μM) significantly reduced the levels of pro-inflammatory mediators (IL-6, IL-8, and CCL-2) induced by house dust mites [102,103]. Similar effects on asthma were observed by Zhou et al., by treating OVA-induced mice with EA (10 mg/kg, p.o.): the compound was shown to impair lung eosinophilia and Th2 cytokines and to counteract NF-κB activation in the respiratory tissue [104]. A summary of commented articles is reported in Table 5.

Table 5.
Collection of recent articles concerning the biological activity of HTs and their metabolites in asthma and allergic rhinitis models.
3. Discussion
Natural products containing HTs have been widely applied for anti-inflammatory purpose by physicians from different countries all over the world, and their bioactivity is sustained by many pre-clinical studies. However, the limited knowledge regarding how they could modulate the immune system limits the rational use.
Several Mediterranean plants are well-known sources of HTs, such as Rhus spp. Punica spp., and Quercus spp., with documented traditional use against different inflammatory disorders. Previous in vitro and in vivo studies sustained the anti-inflammatory effect of HTs and their metabolites on macrophages, fibroblasts, and epithelial cells, which resulted in the inhibition of innate mediators, such as TNF-α, IL-6, IL-1β, PGE2, and MMPs [6,7,8]. The main putative mechanism was ascribed to the impairment of the NF-κB pathway and enzymatic function (i.e., COX-2, MAPKs). Although strongly involved in inflammatory-based diseases, these inflammatory mediators have been mainly investigated in animal models of generic inflammation.
The recent advances regarding the pathogenesis of immune-mediated inflammatory diseases represent an opportunity for the discovery of pharmacological mechanisms beyond innate immunity, such as the modulation of the Th-driven response.
The present review aimed to collect evidence regarding the role of HTs-rich plants in Th inflammatory function, with a particular focus on plants traditionally used. The article collection allowed the summarization of the efficacy of HTs and their gut metabolites (GA, EA, urolithins) in animal models of RA, psoriasis, IBDs, AD, and asthma, which are characterized by the strong involvement of the Th1, Th2, and Th17 responses. The impact on other common autoimmune and inflammatory disorders has been scarcely evaluated by scientific articles: for this reason, no available data were found regarding contact dermatitis, multiple sclerosis, systemic lupus erythematosus, or celiac disease.
Regardless the pathological context, the biological activity of HTs is likely to involve the suppression of both innate and adaptive responses, although the latter has still been poorly investigated. On the other hand, among selected inflammatory-based diseases, psoriasis was poorly considered in pharmacological studies regarding HTs, as already discussed in our previous review in 2020 [7].
The role of gut metabolites in the modulation of specific subsets of Th cells and related cytokines is still unclear, although the increase of Treg response and the reduction of type 1 and type 2 cytokines (such as IFN-γ, IL-17, and IL-4) were both documented by several authors [47,92,94,104]. In this regard, interesting work from Zhang et al. may suggest a potential immunosuppressive effect for urolithin A, regardless the type of T cell [105]. Despite the reduction of cytokines involved in the Th response being observed by many authors, the modulation of the JAK/STAT pathway was rarely investigated. However, few articles suggested that HTs may interfere with STAT1, STAT3, and STAT6 [87,93,103].
The body of literature herein suggests the possibility that HTs may act as mild immunosuppressors, with a generally safe profile after oral or topical administration. Plausibly, products from hydrolysis and metabolism, such as GA, EA, and UroA, participate in the biological effect, although their formation at the gut level has rarely been addressed. This evidence is supported by several in vivo experiments, in which HTs and metabolites were administered as individual compounds through the oral route at comparable doses: EA (50 mg/kg) ameliorated the inflammatory profile in RA models [31,32,33,34,35]; similarly EA, GA, and UroA (10–20 mg/kg) inhibited gut inflammation in IBD models [64,65,66,70,71,72], while EA (10 mg/kg) and GA (20–80 mg/kg) reduced allergic inflammation in AD, asthma, and rhinitis [86,88,96,102]. Remarkably, HTs have frequently been mentioned for direct antibacterial and antiviral activity [106], which may suggest a restrained impact on infection risk.
Several methodological concerns regarding the experimental design of collected articles were underlined during the search process. For example, the route of administration was often neither oral nor topical, which are the most common routes in traditional medicine. Moreover, the doses of extracts and compounds were highly variable among different in vivo studies, thus limiting the potential translation to humans: experiments regarding RA and IBDs were conducted in the broad range of 10 to 1000 mg/kg p.o. On the contrary, doses in the range of 10 to 80 mg/kg were plausibly effective in allergic inflammation models.
The route and dose of administration are extremely relevant issues for any consideration regarding the pharmacokinetic fate, which has been poorly reported in terms of the potential role of gut metabolites or skin-permeable compounds. Another criticism regards several articles in which excessive doses were used, thus leading to implausible biological effects.
4. Materials and Methods
The literature was collected from the main database from the biomedical area (MEDLINE) and Google Scholar. The collection was updated in May 2022, and no limit was applied to the year of publication. The search methodology was focused on the detection of articles regarding the role of HTs and inflammatory diseases characterized by type 1 or type 2 inflammation. Consequently, the following two classes of words, linked by using the “and” logic conjunction, were searched in article titles and abstracts:
- Class 1: “tannin”, “hydrolyzable tannin”, “gallotannin”, “ellagitannin”, “gallic acid”, “ellagic acid”, “urolithin”.
- Class 2: “inflammation”, “Th1”, “Th2”, “Th17”, “arthritis”, “dermatitis”, “psoriasis”, “Crohn”, “asthma”, “ulcerative colitis”, “rheumatoid”, “IBD”, “multiple sclerosis”, “lupus”, “celiac diseases”.
The review process included peer-reviewed articles written in English, regardless of the nature of the evidence (in vivo, in vitro, in silico, or clinical trials). All of the articles included a comparison with a reference anti-inflammatory drug. Papers regarding other inflammatory contexts but demonstrating molecular mechanisms of potential interest for inflammatory-based diseases (i.e., cancer, degenerative diseases, etc.) were also included. On the contrary, papers lacking information related to doses, botanical name of plants, or chemical characterization were excluded.
5. Conclusions
HTs were shown to counteract either innate and adaptive cytokines in classical type 1 or type 2 inflammatory diseases. However, the evidence was not always homogeneous in terms of doses and routes of administration, which were highly variable among the selected studies, thus limiting general conclusions. RA and IBDs were more investigated than other diseases, such as psoriasis. Moreover, ETs were predominantly evaluated in models of RA, while GTs were predominantly evaluated in models of AD and asthma. These observations might suggest a preferential use of different classes of HTs in different inflammatory contexts, according to traditional use. Of note, most of the pre-clinical studies were driven by ethnopharmacological indications, which represent an empirical filter. For all of these reasons, this review may prompt further investigation regarding the mechanism and the efficacy of HTs in specific inflammatory diseases.
Author Contributions
All authors (S.P., M.F., G.M., C.P., N.M., M.A., E.S. and M.D.) contributed to the review article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from MIUR “Progetto Eccellenza”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barnes, R.C.; Krenek, K.A.; Meibohm, B.; Mertens-Talcott, S.U.; Talcott, S.T. Urinary metabolites from mango (Mangifera indica L. cv. Keitt) galloyl derivatives and in vitro hydrolysis of gallotannins in physiological conditions. Mol. Nutr. Food Res. 2016, 60, 542–550. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid. Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Duo, L.; Wang, J.; Zhula, G.; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, A.; Zhang, Y.; Han, T.; Guan, L.; Fan, D.; Liu, J.; Xu, Y. A comprehensive review on ethnobotanical, phytochemical and pharmacological aspects of Rhus chinensis Mill. J. Ethnopharmacol. 2022, 293, 115288. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, Gallotannins and their Metabolites—The Contribution to the Anti-Inflammatory Effect of Food Products and Medicinal Plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef]
- Colombo, E.; Sangiovanni, E.; Dell’agli, M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid. Based Complement. Altern. Med. 2013, 2013, 247145. [Google Scholar] [CrossRef]
- Martinelli, G.; Angarano, M.; Piazza, S.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Khalilpour, S.; Dell’Agli, M.; Sangiovanni, E. The Nutraceutical Properties of Sumac (Rhus coriaria L.) against Gastritis: Antibacterial and Anti-Inflammatory Activities in Gastric Epithelial Cells Infected with H. pylori. Nutrients 2022, 14, 1757. [Google Scholar] [CrossRef]
- Fumagalli, M.; Sangiovanni, E.; Vrhovsek, U.; Piazza, S.; Colombo, E.; Gasperotti, M.; Mattivi, F.; De Fabiani, E.; Dell’Agli, M. Strawberry tannins inhibit IL-8 secretion in a cell model of gastric inflammation. Pharm. Res. 2016, 111, 703–712. [Google Scholar] [CrossRef]
- Hirahara, K.; Nakayama, T. CD4+ T-cell subsets in inflammatory diseases: Beyond the Th1/Th2 paradigm. Int. Immunol. 2016, 28, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Barrett, J.C.; Parkes, M.; Satsangi, J. New IBD genetics: Common pathways with other diseases. Gut 2011, 60, 1739–1753. [Google Scholar] [CrossRef]
- Giannini, D.; Antonucci, M.; Petrelli, F.; Bilia, S.; Alunno, A.; Puxeddu, I. One year in review 2020: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 387–397. [Google Scholar]
- Ferro, F.; Elefante, E.; Luciano, N.; Talarico, R.; Todoerti, M. One year in review 2017: Novelties in the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35, 721–734. [Google Scholar] [PubMed]
- Malek Mahdavi, A.; Seyedsadjadi, N.; Javadivala, Z. Potential effects of pomegranate (Punica granatum) on rheumatoid arthritis: A systematic review. Int. J. Clin. Pract. 2021, 75, e13999. [Google Scholar] [CrossRef] [PubMed]
- Karwasra, R.; Singh, S.; Sharma, D.; Sharma, S.; Sharma, N.; Khanna, K. Pomegranate supplementation attenuates inflammation, joint dysfunction via inhibition of NF-kappaB signaling pathway in experimental models of rheumatoid arthritis. J. Food Biochem. 2019, 43, e12959. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.; Sharma, K.; Gupta, G. Evaluation of Antiarthritic Activity of Butanol Fraction of Punica granatum Linn. Rind Extract Against Freund’s Complete Adjuvant-Induced Arthritis in Rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 53–62. [Google Scholar] [CrossRef]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition 2008, 24, 733–743. [Google Scholar] [CrossRef]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Topical anti-inflammatory and analgesic activities of standardized pomegranate rind extract in comparison with its marker compound ellagic acid in vivo. J. Ethnopharmacol. 2013, 148, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Bai, J.; Wang, Q.; Liang, X.; Tao, H.; Chen, H.; Wei, M.; Niu, J.; Yang, H.; Xu, Y.; et al. Punicalagin ameliorates collagen-induced arthritis by downregulating M1 macrophage and pyroptosis via NF-kappaB signaling pathway. Sci. China Life Sci. 2022, 65, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.; Vaidyanathan, V.G.; King, R.; Cho, B.; Worthen, D.R.; Chichester, C.O., 3rd; Seeram, N.P. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem. Biol. Interact. 2013, 205, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, K.; Zeng, S.; Liu, W.; Cui, T.; Chen, Z.; Lin, L.; Chen, D.; Ouyang, H. Punicalagin Inhibited Inflammation and Migration of Fibroblast-Like Synoviocytes Through NF-kappaB Pathway in the Experimental Study of Rheumatoid Arthritis. J. Inflamm. Res. 2021, 14, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Liu, J.; Ma, P.; Bai, J.; Ding, Y.; Yang, H.; Fan, Y.; Lin, M.; Li, S.; Hou, Q. Tamarixinin A Alleviates Joint Destruction of Rheumatoid Arthritis by Blockade of MAPK and NF-kappaB Activation. Front. Pharm. 2017, 8, 538. [Google Scholar] [CrossRef]
- Schmid, D.; Gruber, M.; Piskaty, C.; Woehs, F.; Renner, A.; Nagy, Z.; Kaltenboeck, A.; Wasserscheid, T.; Bazylko, A.; Kiss, A.K.; et al. Inhibition of NF-kappaB-dependent cytokine and inducible nitric oxide synthesis by the macrocyclic ellagitannin oenothein B in TLR-stimulated RAW 264.7 macrophages. J. Nat. Prod. 2012, 75, 870–875. [Google Scholar] [CrossRef]
- Franco, A.M.; Tocci, N.; Guella, G.; Dell’Agli, M.; Sangiovanni, E.; Perenzoni, D.; Vrhovsek, U.; Mattivi, F.; Manca, G. Myrtle Seeds (Myrtus communis L.) as a Rich Source of the Bioactive Ellagitannins Oenothein B and Eugeniflorin D2. ACS Omega 2019, 4, 15966–15974. [Google Scholar] [CrossRef]
- Javed, K.; Rakha, A.; Butt, M.S.; Faisal, M.N. Probing the antioxidant potential of Juglans regia (walnut) against arthritis-induced oxidative stress in Sprague Dawley rats. J. Food Biochem. 2022, 46, e14082. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, X.; Pei, Y.H.; Ye, H.Y.; Peng, A.H.; Tang, M.H.; Guo, D.L.; Deng, Y.; Chen, L.J. Anti-inflammatory Ellagitannins from Cleidion brevipetiolatum for the Treatment of Rheumatoid Arthritis. J. Nat. Prod. 2019, 82, 2409–2418. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Erusappan, T.; Srinivasan, A. Hydrolysable tannin-rich fraction from Terminalia chebula Retz. fruits ameliorates collagen-induced arthritis in BALB/c mice. Inflammopharmacology 2020, 28, 275–287. [Google Scholar] [CrossRef]
- Lu, K.; Iwenofu, O.H.; Mitra, R.; Mo, X.; Dasgupta, P.S.; Basu, S. Chebulinic acid is a safe and effective antiangiogenic agent in collagen-induced arthritis in mice. Arthritis Res. Ther. 2020, 22, 273. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Teng, L.; Qu, Y.; Liu, J.; Zhu, X.; Chen, S.; Yang, L.; Huang, Y.; Song, Q.; Fu, Q. Anti-proliferation and anti-inflammation effects of corilagin in rheumatoid arthritis by downregulating NF-kappaB and MAPK signaling pathways. J. Ethnopharmacol. 2022, 284, 114791. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Gad, A.M.; Fikry, E.M.; Eid, A.H. Ellagic acid attenuates testicular disruption in rheumatoid arthritis via targeting inflammatory signals, oxidative perturbations and apoptosis. Life Sci. 2019, 239, 117012. [Google Scholar] [CrossRef]
- Fikry, E.M.; Gad, A.M.; Eid, A.H.; Arab, H.H. Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed. Pharm. 2019, 110, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wu, H.; Dong, J.; Huang, S.; Ye, J.; Liu, R. Ellagic Acid Alleviates Rheumatoid Arthritis in Rats through Inhibiting MTA1/HDAC1-Mediated Nur77 Deacetylation. Mediat. Inflamm. 2021, 2021, 6359652. [Google Scholar] [CrossRef] [PubMed]
- Allam, G.; Mahdi, E.A.; Alzahrani, A.M.; Abuelsaad, A.S. Ellagic acid alleviates adjuvant induced arthritis by modulation of pro- and anti-inflammatory cytokines. Cent. Eur. J. Immunol. 2016, 41, 339–349. [Google Scholar] [CrossRef]
- Manan, M.; Saleem, U.; Akash, M.S.H.; Qasim, M.; Hayat, M.; Raza, Z.; Ahmad, B. Antiarthritic Potential of Comprehensively Standardized Extract of Alternanthera bettzickiana: In Vitro and In Vivo Studies. ACS Omega 2020, 5, 19478–19496. [Google Scholar] [CrossRef]
- Farrukh, M.; Saleem, U.; Qasim, M.; Manan, M.; Shah, M.A. Sarcococca saligna extract attenuates formaldehyde-induced arthritis in Wistar rats via modulation of pro-inflammatory and inflammatory biomarkers. Inflammopharmacology 2022. [Google Scholar] [CrossRef]
- Trevisan, G.; Rossato, M.F.; Tonello, R.; Hoffmeister, C.; Klafke, J.Z.; Rosa, F.; Pinheiro, K.V.; Pinheiro, F.V.; Boligon, A.A.; Athayde, M.L.; et al. Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 679–689. [Google Scholar] [CrossRef]
- Brum, E.D.S.; Becker, G.; Fialho, M.F.P.; Casoti, R.; Trevisan, G.; Oliveira, S.M. TRPA1 involvement in analgesia induced by Tabernaemontana catharinensis ethyl acetate fraction in mice. Phytomedicine 2019, 54, 248–258. [Google Scholar] [CrossRef]
- Correa, L.B.; Padua, T.A.; Alabarse, P.V.G.; Saraiva, E.M.; Garcia, E.B.; Amendoeira, F.C.; Ferraris, F.K.; Fukada, S.Y.; Rosas, E.C.; Henriques, M.G. Protective effect of methyl gallate on murine antigen-induced arthritis by inhibiting inflammatory process and bone erosion. Inflammopharmacology 2022, 30, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Chung, S.J.; Lee, S.W.; Park, Y.B.; Lee, S.K.; Park, M.C. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Jt. Bone Spine 2013, 80, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.; Li, L.F.; Hu, S.Q.; Wat, E.; Wong, E.C.; Zhang, V.X.; Lau, C.B.; Wong, C.K.; Hon, K.L.; Hui, P.C.; et al. Gallic Acid Is the Major Active Component of Cortex Moutan in Inhibiting Immune Maturation of Human Monocyte-Derived Dendritic Cells. Molecules 2015, 20, 16388–16403. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Akiyama, H.; Kondo, K.; Sakata, K.; Matsuoka, H.; Amakura, Y.; Teshima, R.; Yoshida, T. Immunological effects of Oenothein B, an ellagitannin dimer, on dendritic cells. Int J. Mol. Sci. 2012, 14, 46–56. [Google Scholar] [CrossRef]
- Fu, X.; Gong, L.F.; Wu, Y.F.; Lin, Z.; Jiang, B.J.; Wu, L.; Yu, K.H. Urolithin A targets the PI3K/Akt/NF-kappaB pathways and prevents IL-1beta-induced inflammatory response in human osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 6135–6146. [Google Scholar] [CrossRef]
- Dell’agli, M.; Galli, G.V.; Bulgari, M.; Basilico, N.; Romeo, S.; Bhattacharya, D.; Taramelli, D.; Bosisio, E. Ellagitannins of the fruit rind of pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanisms involved in the onset of malaria. Malar. J. 2010, 9, 208. [Google Scholar] [CrossRef]
- Shen, P.X.; Li, X.; Deng, S.Y.; Zhao, L.; Zhang, Y.Y.; Deng, X.; Han, B.; Yu, J.; Li, Y.; Wang, Z.Z.; et al. Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. EBioMedicine 2021, 64, 103227. [Google Scholar] [CrossRef]
- De Simone, C.; Caldarola, G.; Maiorino, A.; Tassone, F.; Campana, I.; Sollena, P.; Peris, K. Clinical predictors of nonresponse to anti-TNF-alpha agents in psoriatic patients: A retrospective study. Dermatol Ther. 2016, 29, 372–376. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef]
- Senobari, Z.; Karimi, G.; Jamialahmadi, K. Ellagitannins, promising pharmacological agents for the treatment of cancer stem cells. Phytother. Res. 2022, 36, 231–242. [Google Scholar] [CrossRef]
- Khalilpour, S.; Sangiovanni, E.; Piazza, S.; Fumagalli, M.; Beretta, G.; Dell′Agli, M. In vitro evidences of the traditional use of Rhus coriaria L. fruits against skin inflammatory conditions. J. Ethnopharmacol. 2019, 238, 111829. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Canilli, L.; Terno, M.; Angarano, M.; et al. Anti-Inflammatory and Anti-Acne Effects of Hamamelis virginiana Bark in Human Keratinocytes. Antioxidants (Basel) 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, N.; Yadav, T.C.; Srivastava, A.K.; Raj, U.; Varadwaj, P.; Pruthi, V. Structure-based drug designing and identification of Woodfordia fruticosa inhibitors targeted against heat shock protein (HSP70-1) as suppressor for Imiquimod-induced psoriasis like skin inflammation in mice model. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 57–71. [Google Scholar] [CrossRef]
- Tang, L.; Li, T.; Zhang, B.; Zhang, Z.; Sun, X.; Zhu, Y.; Feng, B.; Su, Z.; Yang, L.; Li, H.; et al. Punicalagin Alleviates Psoriasis by Inhibiting NF-kappaB-Mediated IL-1beta Transcription and Caspase-1-Regulated IL-1beta Secretion. Front. Pharm. 2022, 13, 817526. [Google Scholar] [CrossRef]
- Hrenn, A.; Steinbrecher, T.; Labahn, A.; Schwager, J.; Schempp, C.M.; Merfort, I. Plant phenolics inhibit neutrophil elastase. Planta Med. 2006, 72, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.M.J.; Robins, B.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. In vitro permeation and biological activity of punicalagin and zinc (II) across skin and mucous membranes prone to Herpes simplex virus infection. Eur. J. Pharm. Sci. 2017, 96, 99–106. [Google Scholar] [CrossRef]
- Garcia, S.A.S.; da Rocha, P.B.R.; Souza, B.D.S.; Paz, A.T.S.; Negris, A.L.C.; Marreto, R.N.; da Conceicao, E.C.; Bara, M.T.F.; Taveira, S.F. Enhanced Skin Permeation of Punicalagin after Topical Application of Pluronic Micelles or Vesicles Loaded with Lafoensia pacari Extract. Planta Med. 2022, 88, 479–488. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Rowan, C.R.; Boland, K.; Harewood, G.C. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Eng. J. Med. 2020, 382, 91. [Google Scholar]
- Fradkov, E.; Sheehan, J.; Cushing, K.; Higgins, P.D.R. Efficacy of Ustekinumab in Crohn’s Disease With and Without Concurrent Autoimmune Skin Disease. Inflamm. Bowel Dis. 2021. [Google Scholar]
- Kim, H.; Banerjee, N.; Barnes, R.C.; Pfent, C.M.; Talcott, S.T.; Dashwood, R.H.; Mertens-Talcott, S.U. Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog. 2017, 56, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Ivanov, I.; Pfent, C.M.; Prudhomme, K.R.; Bisson, W.H.; Dashwood, R.H.; Talcott, S.T.; Mertens-Talcott, S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol. Nutr. Food Res. 2016, 60, 1912–1923. [Google Scholar] [CrossRef] [PubMed]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Khatib, M.; Mulinacci, N.; Calosi, L.; Bani, D.; Di Cesare Mannelli, L.; Ghelardini, C. Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions. Int. J. Mol. Sci. 2020, 21, 4304. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Gonzalez-Sarrias, A.; Yanez-Gascon, M.J.; Selma, M.V.; Azorin-Ortuno, M.; Toti, S.; Tomas-Barberan, F.; Dolara, P.; Espin, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cardeno, A.; de la Lastra, C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharm. 2011, 82, 737–745. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cardeno, A.; Aparicio-Soto, M.; Sanchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharm. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef]
- Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef]
- Koh, S.J.; Choi, Y.I.; Kim, Y.; Kim, Y.S.; Choi, S.W.; Kim, J.W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor kappaB signaling in intestinal epithelial cells, and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2019, 58, 1603–1613. [Google Scholar] [CrossRef]
- Chen, T.F.; Hsu, J.T.; Wu, K.C.; Hsiao, C.F.; Lin, J.A.; Cheng, Y.H.; Liu, Y.H.; Lee, D.Y.; Chang, H.H.; Cho, D.Y.; et al. A systematic identification of anti-inflammatory active components derived from Mu Dan Pi and their applications in inflammatory bowel disease. Sci. Rep. 2020, 10, 17238. [Google Scholar] [CrossRef]
- Xiao, H.T.; Lin, C.Y.; Ho, D.H.; Peng, J.; Chen, Y.; Tsang, S.W.; Wong, M.; Zhang, X.J.; Zhang, M.; Bian, Z.X. Inhibitory effect of the gallotannin corilagin on dextran sulfate sodium-induced murine ulcerative colitis. J. Nat. Prod. 2013, 76, 2120–2125. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-kappaB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mortz, C.G.; Lauritsen, J.M.; Bindslev-Jensen, C.; Andersen, K.E. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br. J. Derm. 2001, 144, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Steenkamp, J.; Singh, S.; Erhardt, W.; Rowell, J.; Rane, P.; Martin, N.; Llanos Ackert, J.P.; Quinton, A. Tezepelumab compared with other biologics for the treatment of severe asthma: A systematic review and indirect treatment comparison. J. Med. Econ. 2022, 25, 679–690. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suarez-Farinas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular Mechanisms of Cutaneous Inflammatory Disorder: Atopic Dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Folster-Holst, R.; Latussek, E. Synthetic tannins in dermatology—A therapeutic option in a variety of pediatric dermatoses. Pediatr. Derm. 2007, 24, 296–301. [Google Scholar] [CrossRef]
- Lee, S.; Jegal, H.; Bong, S.K.; Yoon, K.N.; Park, N.J.; Shin, M.S.; Yang, M.H.; Kim, Y.K.; Kim, S.N. Anti-Atopic Effect of Acorn Shell Extract on Atopic Dermatitis-Like Lesions in Mice and Its Active Phytochemicals. Biomolecules 2019, 10, 57. [Google Scholar] [CrossRef]
- Lorenz, P.; Heinrich, M.; Garcia-Kaufer, M.; Grunewald, F.; Messerschmidt, S.; Herrick, A.; Gruber, K.; Beckmann, C.; Knoedler, M.; Huber, R.; et al. Constituents from oak bark (Quercus robur L.) inhibit degranulation and allergic mediator release from basophils and mast cells in vitro. J. Ethnopharmacol. 2016, 194, 642–650. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Tannic acid modulates NFkappaB signaling pathway and skin inflammation in NC/Nga mice through PPARgamma expression. Cytokine 2015, 76, 206–213. [Google Scholar] [CrossRef] [PubMed]
- HMPC. European Medicines Agency Committeeon Herbal Medicinal Products. Assessment re-port on Hamamelis virginiana L., cortex; Hamamelis virginiana L., folium; Hamamelis virginiana L., folium et cortex aut ramunculus destillatum, EMA/HMPC/114585/2008. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2010/04/WC500089242.pdf (accessed on 11 July 2022).
- Choi, J.; Yang, D.; Moon, M.Y.; Han, G.Y.; Chang, M.S.; Cha, J. The Protective Effect of Hamamelis virginiana Stem and Leaf Extract on Fine Dust-Induced Damage on Human Keratinocytes. Cosmetics 2021, 8, 119. [Google Scholar] [CrossRef]
- Piazza, S.; Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Pozzoli, C.; Terno, M.; Canilli, L.; Angarano, M.; Maranta, N.; Dell’Agli, M.; et al. Unveiling the Ability of Witch Hazel (Hamamelis virginiana L.) Bark Extract to Impair Keratinocyte Inflammatory Cascade Typical of Atopic Eczema. Int. J. Mol. Sci. 2022, 23, 9279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jun, C.D.; Suk, K.; Choi, B.J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.Y.; Kim, D.K.; Shin, T.Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhou, X. Gallic Acid Ameliorates Atopic Dermatitis-Like Skin Inflammation Through Immune Regulation in a Mouse Model. Clin. Cosmet. Investig. Derm. 2021, 14, 1675–1683. [Google Scholar] [CrossRef]
- Kwon, D.J.; Bae, Y.S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-kappaB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yan, G.H. Ellagic Acid attenuates immunoglobulin E-mediated allergic response in mast cells. Biol. Pharm. Bull. 2009, 32, 1118–1121. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, W. Alleviation effects of Rubus coreanus Miquel root extract on skin symptoms and inflammation in chronic atopic dermatitis. Food Funct. 2022, 13, 2823–2831. [Google Scholar] [CrossRef]
- El-Ghitany, E.M.; Abd El-Salam, M.M. Environmental intervention for house dust mite control in childhood bronchial asthma. Env. Health Prev. Med. 2012, 17, 377–384. [Google Scholar] [CrossRef]
- Rajasekar, N.; Sivanantham, A.; Kar, A.; Mukhopadhyay, S.; Mahapatra, S.K.; Paramasivam, S.G.; Rajasekaran, S. Anti-asthmatic effects of tannic acid from Chinese natural gall nuts in a mouse model of allergic asthma. Int. Immunopharmacol. 2021, 98, 107847. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.; Jeong, H.J.; Kim, H.M. Potential anti-inflammatory effect of Madi-Ryuk and its active ingredient tannic acid on allergic rhinitis. Mol. Immunol. 2019, 114, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yoshimoto, M.; Nakayama, K.; Tanino, S.; Fujimura, Y.; Yamada, K.; Tachibana, H. Tannic acid, a higher galloylated pentagalloylglucose, suppresses antigen-specific IgE production by inhibiting varepsilon germline transcription induced by STAT6 activation. FEBS Open Bio. 2013, 3, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yang, X.; Yamashita, S.; Kumazoe, M.; Huang, Y.; Nakahara, K.; Won, Y.S.; Murata, M.; Lin, I.C.; Tachibana, H. 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose increases a population of T regulatory cells and inhibits IgE production in ovalbumin-sensitized mice. Int. Immunopharmacol. 2015, 26, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, H.H.; Kim, J.E.; Kim, J.A.; Kim, Y.H.; Jun, C.D.; Kim, S.H. Allose gallates suppress expression of pro-inflammatory cytokines through attenuation of NF-kappaB in human mast cells. Planta Med. 2007, 73, 769–773. [Google Scholar] [CrossRef]
- Fan, Y.; Piao, C.H.; Hyeon, E.; Jung, S.Y.; Eom, J.E.; Shin, H.S.; Song, C.H.; Chai, O.H. Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis. Int. Immunopharmacol. 2019, 70, 512–519. [Google Scholar] [CrossRef]
- Liu, K.Y.; Hu, S.; Chan, B.C.; Wat, E.C.; Lau, C.B.; Hon, K.L.; Fung, K.P.; Leung, P.C.; Hui, P.C.; Lam, C.W.; et al. Anti-inflammatory and anti-allergic activities of Pentaherb formula, Moutan Cortex (Danpi) and gallic acid. Molecules 2013, 18, 2483–2500. [Google Scholar] [CrossRef]
- de Oliveira, J.F.; Garreto, D.V.; da Silva, M.C.; Fortes, T.S.; de Oliveira, R.B.; Nascimento, F.R.; Da Costa, F.B.; Grisotto, M.A.; Nicolete, R. Therapeutic potential of biodegradable microparticles containing Punica granatum L. (pomegranate) in murine model of asthma. Inflamm. Res. 2013, 62, 971–980. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Sun, Y.; Wang, L.; Zhang, J.; Li, F.; Mao, W. Corilagin Attenuates Allergy and Anaphylactic Reaction by Inhibiting Degranulation of Mast Cells. Med. Sci. Monit. 2018, 24, 891–896. [Google Scholar] [CrossRef]
- Abd Rani, N.Z.; Lam, K.W.; Jalil, J.; Mohamad, H.F.; Mat Ali, M.S.; Husain, K. Mechanistic Studies of the Antiallergic Activity of Phyllanthus amarus Schum. & Thonn. and Its Compounds. Molecules 2021, 26, 695. [Google Scholar]
- Kim, H.H.; Park, S.B.; Lee, S.; Kwon, T.K.; Shin, T.Y.; Park, P.H.; Lee, S.H.; Kim, S.H. Inhibitory effect of putranjivain A on allergic inflammation through suppression of mast cell activation. Toxicol. Appl. Pharm. 2014, 274, 455–461. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Fontanari, C.; Borducchi, E.; Keller, A.C.; Russo, M.; Soares, E.G.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur. J. Pharm. 2008, 580, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Julio de Souza, A.L.; Beatriz Mahler Pereira, A.; Robison de Oliveira, J.; Santos Ramalho, L.; Ismarsi de Souza, H.; Lacerda Nascimento, A.; Uddin, M.; Sergio Pereira, P.; Nascimento Silva Teixeira, D.; Roberto da Silva, P.; et al. Dermatophagoides pteronyssinus-induced pro-inflammatory responses mediated via STAT3 and NF-kappaB signaling pathways in human bronchial epithelial cells—Inhibitory effects of Lafoensia pacari and ellagic acid. J. Pharm. Sci. 2020, 142, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Fu, Y.; Wei, Z.; Yang, Z. Inhibition of allergic airway inflammation through the blockage of NF-kappaB activation by ellagic acid in an ovalbumin-Induc. mouse asthma model. Food Funct. 2014, 5, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Al-Maghout, T.; Cao, H.; Pelzl, L.; Salker, M.S.; Veldhoen, M.; Cheng, A.; Lang, F.; Singh, Y. Gut Bacterial Metabolite Urolithin A (UA) Mitigates Ca(2+) Entry in T Cells by Regulating miR-10a-5p. Front. Immunol. 2019, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).