Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review
Abstract
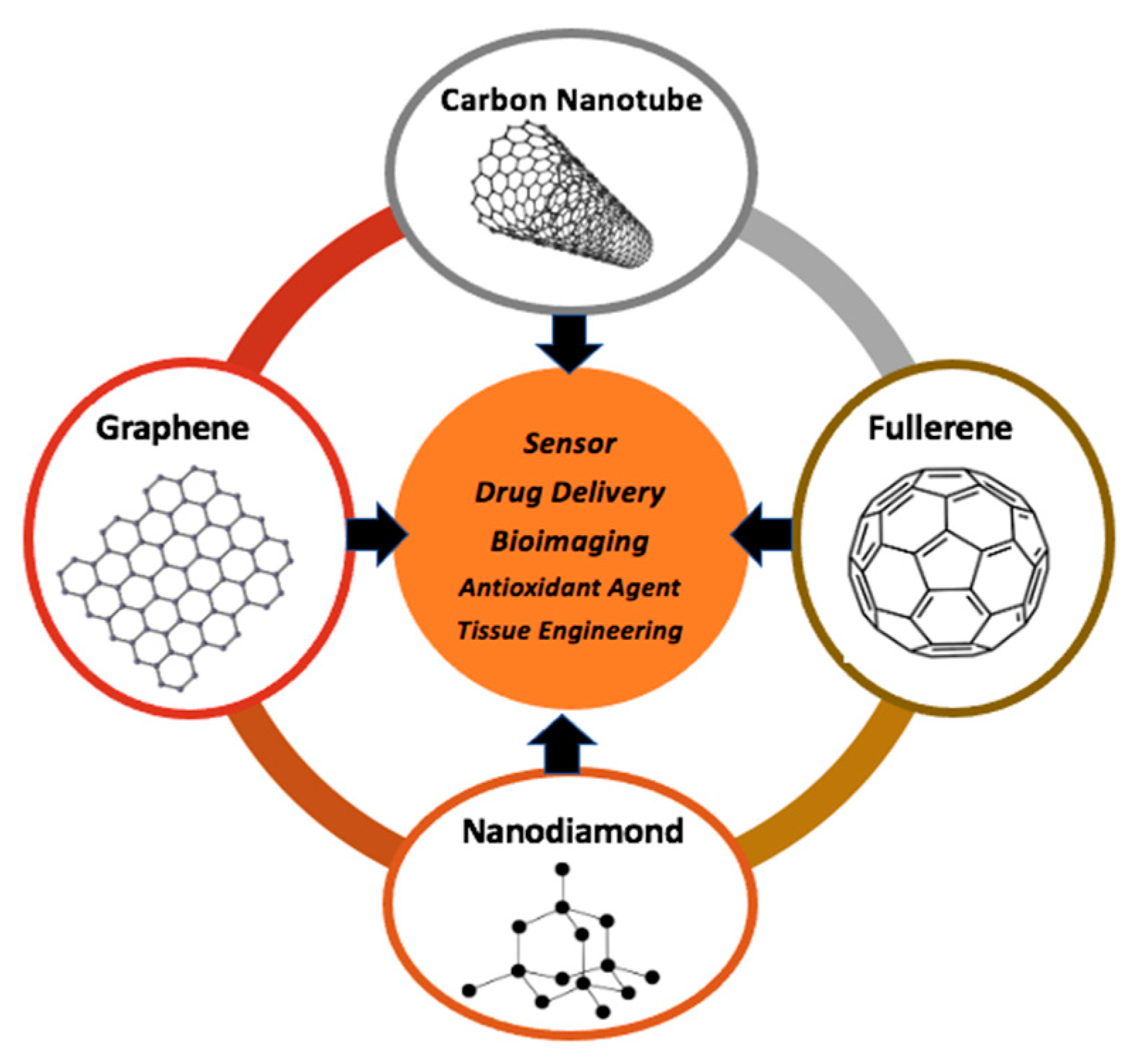
:1. Introduction
2. Sensor Applications
2.1. CNTs
| Electrode | Modifier | Detection Method | Target | Sample | Electrolyte | pH | % Recovery | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| GCE | WS2/CNTs | CV, DPV | Isoniazid | Urine | 0.24 | 10.0–80.0 | KCl, HCLO4 (only for hydroquinone) | 7 | 96.9–104.5 | [43] |
| FePc/f-MWCNT | CV | Saliva, Blood | 0.56 | 5–476 | NA. | 7.4 | 97.3–104.0 | [29] | ||
| Zn@S-FeNC/f-CNT | CV, DPV | Human Blood Serum, Urine | 0.00501 | 0.05–230.5 | PBS | 7 | 96.12–99.1 | [26] | ||
| Amperomety | 0.00854 | 0.07–233.4 | ||||||||
| GCE | MWCNTs–LGH–EG | CV, LSV, DPV | N-(4-hydroxyphenyl) acetamide (paracetamol) | Urine | 0.1 | 0.100–7.510 | PBS, BBS | 10 | 90–92 | [31] |
| SPE | NNC-PPY/SWCNTs | CV. LSV, EIS | Paracetamol (PCM) | Lake Water | 0.000072 | 0.05–40.0 | PBS | 7 | 98–105 | [44] |
| Ciprofloxacin | 0.000196 | 1.00–50.00 | PBS | 7 | 99–102 | |||||
| GCE | PANI–β–CD/fMWCNTs | CV | Water sample | 0.05 | 10.00–80.00 | PBS | 6 | 98.2–107.0 | [39] | |
| PEI/FE3O4/CNTs | DPV | Drug tablets, urine, serum | 0.003 | 0.03–70.00 | B-R Buffer (Britton Robinson), KCl | 6.5 | 97–108 | [38] | ||
| COOH-CNTs/Ag/NH2-CNTs) | CV | Amlodipine | Tablets, tap/drinking water, sweat/saliva, urine/serum sample | 7.76 × 10−8 | 6 nM–50 pM | PBS | 6 | 95–102 | [37] | |
| GC/MWCNT/ILC/RGO/CW | CV | Human serum | 0.000139 | 0.008–30 | PBS | 7.4 | 99.77 | [36] | ||
| Carbon Paste Electrode (CPE) | AgNPs/fMWCNT/Cu-NPs | CV, EIS, AdSWV | Drug tablets, urine, serum, plasma | 0.000516 | 0.02–6.3 | Britton-Robinson (BR) buffer | 10.5 | 99.00–100.75 | [45] | |
| POA/CA | CV, EIS, DPV | Acetaminophen | Blood serum, tablet | 0.015 | 2.0–10.0 & 15.0–50.0 | PBS | 7 | 98–101 | [35] | |
| GCE | GC/MWCNT/ILC/RGO/CW | CV | Human serum | 0.0000906 | 0.001–20 | PBS | 7.4 | 98.96 | [36] | |
| MWCNTs/β-cyclodextrin (β-CD). | CV, LSV | Drinking Water, Urine | 0.0033 | 0.005–20 | PBS | 7.4 | 98–101 | [32] | ||
| Pd-MWCNT | CV | Real sample | 0.13 | 0.5–100 | PBS | 7 | 96.0–101.1 | [33] | ||
| SPE | Nafion-SWCNT | CV, DPV | Plasma | 0.8 | 1.00–2000 | PBS | 7.4 | 79 | [34] | |
| finger-prick whole blood | 74 |
2.2. Fullerene
2.3. Graphene
2.4. Nanodiamond
| Electrode | Modifier | Detection Method | Target | Sample | Electrolyte | pH | % Recovery | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BDD | NG-BDD film | EIS, CV, DNPV | Acetaminophen | Tablet | 0.005 | 0.02–50 | PBS | 7.4 | 98–103 | [126] |
| GCE | ND-Graphite/chitosan | CV, SWV | Codeine | Human Serum, Urine | 0.0545 | 0.299–10.8 | PBS | 5 | 87.8–100.8 | [127] |
| Carbon-fiber microelectrodes (CFMEs) | ND-COOH | FSCV, EIS | Dopamine | Brain slice tissue | 0.003 | 0–5.0 | PBS | 7.4 | NA. | [134] |
| SPEs | ND-SPE | CV, DPV | Real samples | 0.57 | NA. | PBS | 7.4 | [132] | ||
| GCE | NOND | CV | Human Blood | 0.054 | 0.1–100 | PBS | 7.2 | [133] | ||
| 15 | 0.01–0.2 | |||||||||
| 0.12 | 0.5–10 | |||||||||
| SPEs | ND/AuNPs/PEDOT:PSS | SWV | Tryptophan | Milk, 70% Dark Chocolate, Synthetic urine | 0.2 | 0.8–18 | B-R Buffer (Britton Robinson) | 4 | 95–103 | [128] |
| GCE | ND/MS | CV, adtDPV | Tetracycline | Water sample | 2:0 × 10−6 mol L−1 | 5.0 × 10−6 to 1.8 × 10−4 mol L−1 | PBS | 6.3 | 86–112 | [129] |
3. Drug Delivery Application
3.1. Carbon Nanodots (C-Dots)
3.2. Fullerene
3.3. CNTs
3.4. Graphene
3.5. Nanodiamonds
4. Other Applications
4.1. Antioxidant Agent
4.2. Tissue Engineering
4.3. Bioimaging Application
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, N.; Waldvogel, S.R.; Jiang, X. Electrochemistry of Carbon Dioxide on Carbon Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28357–28371. [Google Scholar] [CrossRef] [PubMed]
- Uslu, B.; Ozkan, S.A. Electroanalytical Application of Carbon Based Electrodes to the Pharmaceuticals. Anal. Lett. 2007, 40, 817–853. [Google Scholar] [CrossRef]
- Jia, C.; Dastafkan, K.; Ren, W.; Yang, W.; Zhao, C. Carbon-Based Catalysts for Electrochemical CO2 Reduction. Sustain. Energy Fuels 2019, 3, 2890–2906. [Google Scholar] [CrossRef]
- Jiwanti, P.K.; Aritonang, R.P.; Abdullah, I.; Einaga, Y.; Ivandini, T.A. Copper-Nickel-Modified Boron-Doped Diamond Electrode for CO2 Electrochemical Reduction Application: A Preliminary Study. Makara J. Sci. 2019, 23, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Jiwanti, P.K.; Sultana, S.; Wicaksono, W.P.; Einaga, Y. Metal Modified Carbon-Based Electrode for CO2 Electrochemical Reduction: A Review. J. Electroanal. Chem. 2021, 898, 115634. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Ariani, J.; Jiwanti, P.K.; Gunlazuardi, J.; Saepudin, E.; Einaga, Y. Electrochemical Detection of Neuraminidase Based on Zanamivir Inhibition Reaction at Platinum and Platinum-Modified Boron-Doped Diamond Electrodes. Makara J. Sci. 2017, 21, 34–42. [Google Scholar] [CrossRef]
- Ren, Y.; Ji, J.; Sun, J.; Pi, F.; Zhang, Y.; Sun, X. Rapid Detection of Antibiotic Resistance in Salmonella with Screen Printed Carbon Electrodes. J. Solid State Electrochem. 2020, 24, 1539–1549. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Wu, L.; Zhi, J. Electrochemical Biosensor Based on Boron-Doped Diamond Electrodes with Modified Surfaces. Int. J. Electrochem. 2012, 2012, 567171. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with Powerful Ability for Drug Delivery and Biomedical Applications: Recent Updates on in Vivo Study and Patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Debnath, S.K.; Srivastava, R. Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon Materials for Drug Delivery & Cancer Therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Petersen, R. Carbon Fiber Biocompatibility for Implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, R.; Badea, I. Nanodiamonds as Novel Nanomaterials for Biomedical Applications: Drug Delivery and Imaging Systems. Int. J. Nanomed. 2013, 8, 203–220. [Google Scholar]
- Liu, X.W.; Sun, X.F.; Huang, Y.X.; Sheng, G.P.; Wang, S.G.; Yu, H.Q. Carbon Nanotube/Chitosan Nanocomposite as a Biocompatible Biocathode Material to Enhance the Electricity Generation of a Microbial Fuel Cell. Energy Environ. Sci. 2011, 4, 1422–1427. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, H.; Won, S.; Kim, H.; Kwon, W. Biocompatible Nitrogen-Doped Carbon Dots: Synthesis, Characterization, and Application. J. Mater. Chem. B 2020, 8, 8935–8951. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like Carbon Coatings as Biocompatible Materials-An Overview. Diam. Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Tagi, S.; Solhi, E.; Mokhtarzadeh, A.; Shadjou, N.; Eftekhari, A.; Mahboob, S. An Innovative Immunosensor for Ultrasensitive Detection of Breast Cancer Specific Carbohydrate (CA 15-3) in Unprocessed Human Plasma and MCF-7 Breast Cancer Cell Lysates Using Gold Nanospear Electrochemically Assembled onto Thiolated Graphene Quantum Dots. Int. J. Biol. Macromol. 2018, 114, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-Bacterial Activity of Graphene Oxide as a New Weapon Nanomaterial to Combat Multidrug-Resistance Bacteria. Mater. Sci. Eng. C 2017, 74, 568–581. [Google Scholar] [CrossRef]
- Rezaee, M.; Behnam, B.; Banach, M.; Sahebkar, A. The Yin and Yang of Carbon Nanomaterials in Atherosclerosis; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 36. [Google Scholar]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical Applications of Carbon Nanomaterials: Drug and Gene Delivery Potentials. J. Cell. Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.R.; Govindhan, M.; Chen, A. Carbon Nanomaterials Based Electrochemical Sensors/Biosensors for the Sensitive Detection of Pharmaceutical and Biological Compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef] [Green Version]
- Porto, L.S.; Silva, D.N.; de Oliveira, A.E.F.; Pereira, A.C.; Borges, K.B. Carbon Nanomaterials: Synthesis and Applications to Development of Electrochemical Sensors in Determination of Drugs and Compounds of Clinical Interest. Rev. Anal. Chem. 2020, 38, 3. [Google Scholar] [CrossRef]
- Kondo, T. Electrochemical Applications of Conductive Diamond Powders; Springer International Publishing: Switzerland, 2019; Volume 121. [Google Scholar]
- Hassanpour, S.; Behnam, B.; Baradaran, B.; Hashemzaei, M.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Carbon Based Nanomaterials for the Detection of Narrow Therapeutic Index Pharmaceuticals. Talanta 2021, 221, 121610. [Google Scholar] [CrossRef] [PubMed]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon Nanotubes: Sensor Properties. A Review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Chen, S.M.; Vinothkumar, V.; Balu, S.; Hung, S.T.; Lin, K.C. Zinc and Sulfur Codoped Iron Oxide Nanocubes Anchored on Carbon Nanotubes for the Detection of Antitubercular Drug Isoniazid. ACS Appl. Nano Mater. 2021, 4, 4562–4575. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Van Der Zalm, J.; Chen, A. Graphene Oxide-Based Nanomaterials for the Electrochemical Sensing of Isoniazid. ACS Appl. Nano Mater. 2021, 4, 3696–3706. [Google Scholar] [CrossRef]
- Farokhi-Fard, A.; Golichenari, B.; Mohammadi Ghanbarlou, M.; Zanganeh, S.; Vaziri, F. Electroanalysis of Isoniazid and Rifampicin: Role of Nanomaterial Electrode Modifiers. Biosens. Bioelectron. 2019, 146, 111731. [Google Scholar] [CrossRef]
- Spindola, R.F.; Zanin, H.; Macena, C.S.; Contin, A.; de Cássia Silva Luz, R.; Damos, F.S. Evaluation of a Novel Composite Based on Functionalized Multi-Walled Carbon Nanotube and Iron Phthalocyanine for Electroanalytical Determination of Isoniazid. J. Solid State Electrochem. 2017, 21, 1089–1099. [Google Scholar] [CrossRef]
- Pandolfi, S.; Simonetti, V.; Ricevuti, G.; Chirumbolo, S. Paracetamol In The Home Treatment Of Early COVID-19 Symptoms: A Possible Foe Rather Than A Friend For Elderly Patients? J. Med. Virol. 2021, 93, 5704–5706. [Google Scholar] [CrossRef]
- Teglia, C.M.; Gutierrez, F.A.; Goicoechea, H.C. Natural Deep Eutectic Solvent: A Novelty Alternative as Multi-Walled Carbon Nanotubes Dispersing Agent for the Determination of Paracetamol in Urine. Talanta 2022, 242, 123290. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Catalano, M.; Wang, L.; Kim, M.J.; Howlader, M.M.R.; Hu, N.X.; Deen, M.J. Tailoring MWCNTs and β-Cyclodextrin for Sensitive Detection of Acetaminophen and Estrogen. ACS Appl. Mater. Interfaces 2018, 10, 21411–21427. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Lv, X.; Lei, W.; Ding, Y.; Chen, C.; Lv, J.; Feng, S.; Chen, S.M.; Hao, Q. A Sensitive Sensing Platform for Acetaminophen Based on Palladium and Multi-Walled Carbon Nanotube Composites and Electrochemical Detection Mechanism. Mater. Chem. Phys. 2020, 239, 121977. [Google Scholar] [CrossRef]
- Wester, N.; Mikladal, B.F.; Varjos, I.; Peltonen, A.; Kalso, E.; Lilius, T.; Laurila, T.; Koskinen, J. Disposable Nafion-Coated Single-Walled Carbon Nanotube Test Strip for Electrochemical Quantitative Determination of Acetaminophen in a Finger-Prick Whole Blood Sample. Anal. Chem. 2020, 92, 13017–13024. [Google Scholar] [CrossRef] [PubMed]
- Charithra, M.M.; Manjunatha, J.G. Electroanalytical Determination of Acetaminophen Using a Polymerised Carbon Nanotube Based Sensor. J. Electron. Mater. 2021, 50, 6929–6940. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, Y.M.; El-Ads, E.H. Design Strategy and Preparation of a Conductive Layered Electrochemical Sensor for Simultaneous Determination of Ascorbic Acid, Dobutamine, Acetaminophen and Amlodipine. Sens. Actuators B Chem. 2019, 297, 126648. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Khan, M.A.; Nisar, J.; Ashiq, M.N. Electrochemical Sensing Platform for the Simultaneous Femtomolar Detection of Amlodipine and Atorvastatin Drugs. RSC Adv. 2021, 11, 27135–27151. [Google Scholar] [CrossRef]
- Jalal, N.R.; Madrakian, T.; Afkhami, A.; Ghamsari, M. Polyethylenimine@Fe3O4@carbon Nanotubes Nanocomposite as a Modifier in Glassy Carbon Electrode for Sensitive Determination of Ciprofloxacin in Biological Samples. J. Electroanal. Chem. 2019, 833, 281–289. [Google Scholar] [CrossRef]
- Garrido, J.M.P.J.; Melle-franco, M.; Strutyński, K.; Brett, C.M.A.; Garrido, E.M.P.J. β–Cyclodextrin Carbon Nanotube-Enhanced Sensor for Ciprofloxacin Detection. J. Environ. Sci. Health Part A 2017, 52, 313–319. [Google Scholar] [CrossRef]
- Tubati, V.; Murthy, T.; Rao, A. Comparision of Different Techniques Involved in the Development of Ivabradine HCL Floating Pulsatile Multiparticulate Systems for Chronotherapeutic Delivery. Br. J. Pharm. Res. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. T-Butyl Calixarene/Fe2O3@MWCNTs Composite-Based Potentiometric Sensor for Determination of Ivabradine Hydrochloride in Pharmaceutical Formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Saad, M.; Barhoum, A.; Bechelany, M.; Rizk, M.S. PVC Membrane, Coated-Wire, and Carbon-Paste Ion-Selective Electrodes for Potentiometric Determination of Galantamine Hydrobromide in Physiological Fluids. Mater. Sci. Eng. C 2018, 89, 140–148. [Google Scholar] [CrossRef]
- Santos, B.G.; Gonçalves, J.M.; Rocha, D.P.; Higino, G.S.; Yadav, T.P.; Pedrotti, J.J.; Ajayan, P.M.; Angnes, L. Electrochemical Sensor for Isoniazid Detection by Using a WS2/CNTs Nanocomposite. Sens. Actuators Rep. 2022, 4, 100073. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Jeffrey Basirun, W.; Sanghiran Lee, V.; Rafie Johan, M. A Metal Free Nanosensor Based on Nanocellulose-Polypyrrole Matrix and Single-Walled Carbon Nanotube: Experimental Study and Electroanalytical Application for Determination of Paracetamol and Ciprofloxacin. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100691. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Pandit, U.J.; Sheikh, M.U.D.; Hassan, I.U.; Khan, G.A.; Bhat, A.R.; Das, R.; Horchani, R. Synergistic Effect of Carbon Nanotubes, Copper and Silver Nanoparticles as an Efficient Electrochemical Sensor for the Trace Recognition of Amlodipine Besylate Drug. SN Appl. Sci. 2020, 2, 983. [Google Scholar] [CrossRef]
- Rather, J.A.; De Wael, K. Fullerene-C60 Sensor for Ultra-High Sensitive Detection of Bisphenol-A and Its Treatment by Green Technology. Sens. Actuators B Chem. 2013, 176, 110–117. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Stathis, P.; Permuth, S.F.; Tokes, L.; Feldman, D. Bisphenol-A: An Estrogenic Substance Is Released from Polycarbonate Flasks during Autoclaving. Endocrinology 2018, 132, 2279–2286. [Google Scholar] [CrossRef]
- Palanza, P.; Gioiosa, L.; vom Saal, F.S.; Parmigiani, S. Effects of Developmental Exposure to Bisphenol A on Brain and Behavior in Mice. Environ. Res. 2008, 108, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.Y.; Park, H.Y.; Lee, J.W.; Jung, I.O.; Choi, K.H.; Kim, K.; Cho, K.H. Evaluation of the Immune Response Following Exposure of Mice to Bisphenol A: Induction of Th1 Cytokine and Prolactin by BPA Exposure in the Mouse Spleen Cells. Arch. Pharm. Res. 2002, 25, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Khoshroo, A. High Performance Electrochemical Sensor Based on Fullerene-Functionalized Carbon Nanotubes/Ionic Liquid: Determination of Some Catecholamines. Electrochem. Commun. 2014, 42, 9–12. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Khoshroo, A.; Hosseinzadeh, L. Simultaneous Determination of Hydrazine and Hydroxylamine Based on Fullerene-Functionalized Carbon Nanotubes/Ionic Liquid Nanocomposite. Sens. Actuators B Chem. 2015, 214, 132–137. [Google Scholar] [CrossRef]
- Filanovsky, B.; Granot, E.; Presman, I.; Kuras, I.; Patolsky, F. Long-Term Room-Temperature Hydrazine/Air Fuel Cells Based on Low-Cost Nanotextured Cu-Ni Catalysts. J. Power Source 2014, 246, 423–429. [Google Scholar] [CrossRef]
- Evans, D.M. Two Cases of Hydrazine Hydrate Dermatitis without Systemic Intoxication. Br. J. Ind. Med. 1959, 16, 126–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.M.; Ali, M.A.; Al-Hemaid, F.M.A. Palladium Nanoparticles Decorated on Activated Fullerene Modified Screen Printed Carbon Electrode for Enhanced Electrochemical Sensing of Dopamine. J. Colloid Interface Sci. 2015, 448, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Babazadeh, M.; Momeni, H.R. A Sensor for Simultaneous Determination of Dopamine and Morphine in Biological Samples Using a Multi-Walled Carbon Nanotube/Chitosan Composite Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2011, 6, 1382–1395. [Google Scholar]
- Montague, P.R.; Hyman, S.E.; Cohen, J.D. Computational Roles for Dopamine in Behavioural Control. Nat. Publ. Gr. 2004, 431, 760–767. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.M.; Lou, B.S. Preparation of Highly Stable Fullerene C60 Decorated Graphene Oxide Nanocomposite and Its Sensitive Electrochemical Detection of Dopamine in Rat Brain and Pharmaceutical Samples. J. Colloid Interface Sci. 2016, 462, 375–381. [Google Scholar] [CrossRef]
- Ma, X.; Chao, M.; Wang, Z. Electrochemical Detection of Dopamine in the Presence of Epinephrine, Uric Acid and Ascorbic Acid Using a Graphene-Modified Electrode. Anal. Methods 2012, 4, 1687–1692. [Google Scholar] [CrossRef]
- Smythies, J. The Neurotoxicity of Glutamate, Dopamine, Iron and Reactive Oxygen Species: Functional Interrelationships in Health and Disease: A Review—Discussion. Neurotox. Res. 1999, 1, 27–39. [Google Scholar] [CrossRef]
- Brahman, P.K.; Suresh, L.; Lokesh, V.; Nizamuddin, S. Fabrication of Highly Sensitive and Selective Nanocomposite Film Based on CuNPs/Fullerene-C60/MWCNTs: An Electrochemical Nanosensor for Trace Recognition of Paracetamol. Anal. Chim. Acta 2016, 917, 107–116. [Google Scholar] [CrossRef]
- Lourenção, B.C.; Medeiros, R.A.; Rocha-Filho, R.C.; Mazo, L.H.; Fatibello-Filho, O. Simultaneous Voltammetric Determination of Paracetamol and Caffeine in Pharmaceutical Formulations Using a Boron-Doped Diamond Electrode. Talanta 2009, 78, 748–752. [Google Scholar] [CrossRef]
- El Bouabi, Y.; Farahi, A.; Labjar, N.; El Hajjaji, S.; Bakasse, M.; El Mhammedi, M.A. Square Wave Voltammetric Determination of Paracetamol at Chitosan Modified Carbon Paste Electrode: Application in Natural Water Samples, Commercial Tablets and Human Urines. Mater. Sci. Eng. C 2016, 58, 70–77. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Ahmadi, S.H.; Safaei Mahmoudabadi, Z.; Khoshroo, A. Nano Composite System Based on Fullerene-Functionalized Carbon Nanotubes for Simultaneous Determination of Levodopa and Acetaminophen. Meas. J. Int. Meas. Confed. 2016, 91, 162–167. [Google Scholar] [CrossRef]
- Hardy, J.; Gwinn-Hardy, K. Genetic Classification of Primary Neurodegenerative Disease. Science 1998, 282, 1075–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzenschlager, R.; Poewe, W. Intestinal Levodopa Infusion in PD-the First Randomized Trial. Nat. Rev. Neurol. 2014, 10, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Thanvi, B.R.; Lo, T.C.N. Long Term Motor Complications of Levodopa: Clinical Features, Mechanisms, and Management Strategies. Postgrad. Med. J. 2004, 80, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi-Nasrabadi, M.; Khoshroo, A.; Mazloum-Ardakani, M. Electrochemical Determination of Diazepam in Real Samples Based on Fullerene-Functionalized Carbon Nanotubes/Ionic Liquid Nanocomposite. Sens. Actuators B Chem. 2017, 240, 125–131. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Aitchison, K.; Bateson, A.; Curran, H.V.; Davies, S.; Leonard, B.; Nutt, D.J.; Stephens, D.N.; Wilson, S. Benzodiazepines: Risks and Benefits. A Reconsideration. J. Psychopharmacol. 2013, 27, 967–971. [Google Scholar] [CrossRef]
- Anusha, T.; Bhavani, K.S.; Kumar, J.V.S.; Brahman, P.K. Designing and Fabrication of Electrochemical Nanosensor Employing Fullerene-C60 and Bimetallic Nanoparticles Composite Film for the Detection of Vitamin D3 in Blood Samples. Diam. Relat. Mater. 2020, 104, 107761. [Google Scholar] [CrossRef]
- Scott, M.G.; Gronowski, A.M.; Reid, I.R.; Holick, M.F.; Thadhani, R.; Phinney, K. Vitamin D: The More We Know, the Less We Know. Clin. Chem. 2015, 61, 462–465. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, S.; Yang, R.; Zhou, W.; Li, H.; Dong, J.; Chen, W. A Simple and Precise LC-MS/MS Method for Simultaneous Determination of Serum 25-Hydroxyvitamin D3 and D2 without Interference from the C3 Epimer. Anal. Methods 2015, 7, 5254–5261. [Google Scholar] [CrossRef]
- Zhu, Y.; Huai, S.; Jiao, J.; Xu, Q.; Wu, H.; Zhang, H. Fullerene and Platinum Composite-Based Electrochemical Sensor for the Selective Determination of Catechol and Hydroquinone. J. Electroanal. Chem. 2020, 878, 114726. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Chen, S.M.; Huang, L.H.; Ali, M.A.; Al-Hemaid, F.M.A. Simultaneous Determination of Catechol and Hydroquinone Using a Pt/ZrO 2-RGO/GCE Composite Modified Glassy Carbon Electrode. Electrochim. Acta 2014, 125, 503–509. [Google Scholar] [CrossRef]
- Du, H.; Ye, J.; Zhang, J.; Huang, X.; Yu, C. A Voltammetric Sensor Based on Graphene-Modified Electrode for Simultaneous Determination of Catechol and Hydroquinone. J. Electroanal. Chem. 2011, 650, 209–213. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. Simultaneous Determination of Catechol, Hydroquinone, and Resorcinol on CTAB Functionalized Graphene Oxide/ Multiwalled Carbon Nanotube Modified Electrode. Fuller. Nanotub. Carbon Nanostructures 2015, 23, 410–417. [Google Scholar] [CrossRef]
- Tajeu, K.Y.; Dongmo, L.M.; Tonle, I.K. Fullerene/MWCNT/Nafion Modified Glassy Carbon Electrode for the Electrochemical Determination of Caffeine. Am. J. Anal. Chem. 2020, 11, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Furtado, L.D.A.; Goncąlves, M.C.D.O.; Inocêncio, C.V.M.; Pinto, E.M.; Martins, D.D.L.; Semaan, F.S. Electrodeposition of 4-Benzenesulfonic Acid onto a Graphite-Epoxy Composite Electrode for the Enhanced Voltammetric Determination of Caffeine in Beverages. J. Anal. Methods Chem. 2019, 2019, 8596484. [Google Scholar] [CrossRef] [Green Version]
- Rostagno, M.A.; Manchón, N.; D’Arrigo, M.; Guillamón, E.; Villares, A.; García-Lafuente, A.; Ramos, A.; Martínez, J.A. Fast and Simultaneous Determination of Phenolic Compounds and Caffeine in Teas, Mate, Instant Coffee, Soft Drink and Energetic Drink by High-Performance Liquid Chromatography Using a Fused-Core Column. Anal. Chim. Acta 2011, 685, 204–211. [Google Scholar] [CrossRef]
- Khoo, W.Y.H.; Pumera, M.; Bonanni, A. Graphene Platforms for the Detection of Caffeine in Real Samples. Anal. Chim. Acta 2013, 804, 92–97. [Google Scholar] [CrossRef]
- Svítková, J.; Machková, M.; Šatkovská, P.; Cinková, K.; Švorc, Ľ. Utilization of Electrochemical Methods in Determination of Trace Elements in Beverages. Acta Chim. Slovaca 2012, 5, 42–46. [Google Scholar] [CrossRef]
- Smith, A.; Sutherland, D.; Christopher, G. Effects of Repeated Doses of Caffeine on Mood and Performance of Alert and Fatigued Volunteers. J. Psychopharmacol. 2005, 19, 620–626. [Google Scholar] [CrossRef]
- Abdellatef, R.; Khaled, E.; Hendawy, H.A.; Hassan, R.Y.A. Manganese Dioxide (MnO2)/Fullerene-C60-Modified Electrodes for the Voltammetric Determination of Rifaximin. J. Anal. Test. 2021, 5, 341–349. [Google Scholar] [CrossRef]
- Materón, E.M.; Wong, A.; Freitas, T.A.; Faria, R.C.; Oliveira, O.N. A Sensitive Electrochemical Detection of Metronidazole in Synthetic Serum and Urine Samples Using Low-Cost Screen-Printed Electrodes Modified with Reduced Graphene Oxide and C60. J. Pharm. Anal. 2021, 11, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic Uses of Metronidazole and Its Side Effects: An Update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [PubMed]
- Mallikarjuna Rao, C.; Ghosh, A.; Raghothama, C.; Bairy, K.L. Does Metronidazole Reduce Lipid Peroxidation in Burn Injuries to Promote Healing? Burns 2002, 28, 427–429. [Google Scholar] [CrossRef]
- Xi, X.; Ming, L. A Voltammetric Sensor Based on Electrochemically Reduced Graphene Modified Electrode for Sensitive Determination of Midecamycin. Anal. Methods 2012, 4, 3013–3018. [Google Scholar] [CrossRef]
- Arvand, M.; Ghodsi, N. A Voltammetric Sensor Based on Graphene-Modified Electrode for the Determination of Trace Amounts of l-Dopa in Mouse Brain Extract and Pharmaceuticals. J. Solid State Electrochem. 2013, 17, 775–784. [Google Scholar] [CrossRef]
- Misu, Y.; Goshima, Y.; Miyamae, T. Is DOPA a Neurotransmitter? Trends Pharmacol. Sci. 2002, 23, 262–268. [Google Scholar] [CrossRef]
- Laitinen, L.V.; Bergenheim, A.T.; Hariz, M.I. Leksell’s Posteroventral Pallidotomy in the Treatment of Parkinson’s Disease. J. Neurosurg. 1992, 76, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Arvand, M.; Gholizadeh, T.M. Gold Nanorods-Graphene Oxide Nanocomposite Incorporated Carbon Nanotube Paste Modified Glassy Carbon Electrode for Voltammetric Determination of Indomethacin. Sens. Actuators B Chem. 2013, 186, 622–632. [Google Scholar] [CrossRef]
- Ali, A.M.M. Cathodic Adsorptive Stripping Voltammetric Determination of the Anti-Inflammatory Drug Indomethacin. J. Pharm. Biomed. Anal. 1999, 18, 1005–1012. [Google Scholar] [CrossRef]
- Hajjizadeh, M.; Jabbari, A.; Heli, H.; Moosavi-Movahedi, A.A.; Haghgoo, S. Electrocatalytic Oxidation of Some Anti-Inflammatory Drugs on a Nickel Hydroxide-Modified Nickel Electrode. Electrochim. Acta 2007, 53, 1766–1774. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Asadi, M.H.; Biparva, P. Application of a Modified Graphene Nanosheet Paste Electrode for Voltammetric Determination of Methyldopa in Urine and Pharmaceutical Formulation. J. Anal. Sci. Technol. 2014, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Rosy; Yadav, S.K.; Agrawal, B.; Oyama, M.; Goyal, R.N. Graphene Modified Palladium Sensor for Electrochemical Analysis of Norepinephrine in Pharmaceuticals and Biological Fluids. Electrochim. Acta 2014, 125, 622–629. [Google Scholar] [CrossRef]
- LeWitt, P.A. Norepinephrine: The next Therapeutics Frontier for Parkinson’s Disease. Transl. Neurodegener. 2012, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Plasma Norepinephrine during Stress in Essential Hypertension. Hypertension 1981, 3, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavikova, J.; Kuncova, J.; Topolcan, O. Plasma Catecholamines and Ischemic Heart Disease. Clin. Cardiol. 2007, 30, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.N.; Aziz, M.A.; Oyama, M.; Chatterjee, S.; Rana, A.R.S. Nanogold Based Electrochemical Sensor for Determination of Norepinephrine in Biological Fluids. Sens. Actuators B Chem. 2011, 153, 232–238. [Google Scholar] [CrossRef]
- Ganguly, P.K.; Beamish, R.E.; Dhalla, K.S.; Innes, I.R. Norepinephrine Storage, Distribution, and Release in Diabetic Cardiomyopathy. Am. J. Physiol.-Endocrinol. Metab. 1987, 252, 734–739. [Google Scholar] [CrossRef]
- Shrivastava, R.; Saxena, S.; Satsangee, S.P.; Jain, R. Graphene/TiO2/Polyaniline Nanocomposite Based Sensor for the Electrochemical Investigation of Aripiprazole in Pharmaceutical Formulation. Ionics 2015, 21, 2039–2049. [Google Scholar] [CrossRef]
- DeLeon, A.; Patel, N.C.; Crismon, M.L. Aripiprazole: A Comprehensive Review of Its Pharmacology, Clinical Efficacy, and Tolerability. Clin. Ther. 2004, 26, 649–666. [Google Scholar] [CrossRef]
- Lieberman, J.A. Dopamine Partial Agonists: A New Class of Antipsychotic. CNS Drugs 2004, 18, 251–267. [Google Scholar] [CrossRef]
- Mallikaarjun, S.; Salazar, D.E.; Bramer, S.L. Pharmacokinetics, Tolerability, and Safety of Aripiprazole Following Multiple Oral Dosing in Normal Healthy Volunteers. J. Clin. Pharmacol. 2004, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Krishnamoorthy, K.; Sekar, C.; Wilson, J.; Kim, S.J. A Promising Electrochemical Sensing Platform Based on Ternary Composite of Polyaniline-Fe2O3-Reduced Graphene Oxide for Sensitive Hydroquinone Determination. Chem. Eng. J. 2015, 259, 594–602. [Google Scholar] [CrossRef]
- Jin, P.; Chang, R.; Liu, D.; Zhao, K.; Zhang, L.; Ouyang, Y. Phenol Degradation in an Electrochemical System with TiO 2/Activated Carbon Fiber as Electrode. J. Environ. Chem. Eng. 2014, 2, 1040–1047. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption Characteristics of Natural Zeolites as Solid Adsorbents for Phenol Removal from Aqueous Solutions: Kinetics, Mechanism, and Thermodynamics Studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R.; Chen, S.M.; Kohilarani, K.; Ramachandran, R. A Sensitive Amperometric Sensor for the Determination of Dopamine at Graphene and Bismuth Nanocomposite Film Modified Electrode. Int. J. Electrochem. Sci. 2015, 10, 1199–1207. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yiǧit, A.; Yardim, Y.; Çelebi, M.; Levent, A.; Şentürk, Z. Graphene/Nafion Composite Film Modified Glassy Carbon Electrode for Simultaneous Determination of Paracetamol, Aspirin and Caffeine in Pharmaceutical Formulations. Talanta 2016, 158, 21–29. [Google Scholar] [CrossRef]
- Jozwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of Action, Applications and Safety Concern. Acta Pol. Pharm.-Drug Res. 2014, 71, 11–23. [Google Scholar]
- Amann, R.; Peskar, B.A. Anti-Inflammatory Effects of Aspirin and Sodium Salicylate. Eur. J. Pharmacol. 2002, 447, 1–9. [Google Scholar] [CrossRef]
- Benowitz, N.L. Clinical Pharmacology of Caffeine. Annu. Rev. Med. 1990, 41, 277–288. [Google Scholar] [CrossRef]
- Lipton, R.B.; Stewart, W.F.; Ryan, R.E.; Saper, J.; Silberstein, S.; Sheftell, F. Efficacy and Safety of Acetaminophen, Aspirin, and Caffeine in Alleviating Migraine Headache Pain. Arch. Neurol. 1998, 55, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. Am. Chem. Soc. 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Afkhami, A.; Bahiraei, A.; Madrakian, T. Application of Nickel Zinc Ferrite/Graphene Nanocomposite as a Modifier for Fabrication of a Sensitive Electrochemical Sensor for Determination of Omeprazole in Real Samples. J. Colloid Interface Sci. 2017, 495, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.G.; Vega Hissi, E.G.; Rizzi, A.C.; Brondino, C.D.; Salinas Ibañez, Á.G.; Vega, A.E.; Silva, H.J.; Mercader, R.; Narda, G.E. Synthesis, Physicochemical Characterization, DFT Calculation and Biological Activities of Fe(III) and Co(II)-Omeprazole Complexes. Potential Application in the Helicobacter Pylori Eradication. J. Mol. Struct. 2014, 1061, 5–13. [Google Scholar] [CrossRef]
- El-Nezhawy, A.O.H.; Biuomy, A.R.; Hassan, F.S.; Ismaiel, A.K.; Omar, H.A. Design, Synthesis and Pharmacological Evaluation of Omeprazole-like Agents with Anti-Inflammatory Activity. Bioorganic Med. Chem. 2013, 21, 1661–1670. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. A Modified Nanostructured Graphene-Gold Nanoparticle Carbon Screen-Printed Electrode for the Sensitive Voltammetric Detection of Rutin. Meas. J. Int. Meas. Confed. 2018, 114, 37–43. [Google Scholar] [CrossRef]
- Freitas, K.H.G.; Fatibello-Filho, O.; de Mattos, I.L. Square-Wave Voltammetric Determination of Rutin in Pharmaceutical Formulations Using a Carbon Composite Electrode Modified with Copper (II) Phosphate Immobilized in Polyester Resin. Brazilian J. Pharm. Sci. 2012, 48, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Petkova Pencheva, I.; Nikolova Maslarska, V.; Christova Stoimenova, A.; Metodieva Manova, M.; Antonova Andonova, L.; Krumova Zdraveva, P. Quality Control Optimization Solutions for Determination of Rutin in Supplements Containing Ginkgo Biloba Extract. Curr. Pharm. Anal. 2016, 12, 386–390. [Google Scholar] [CrossRef]
- Chua, L.S. A Review on Plant-Based Rutin Extraction Methods and Its Pharmacological Activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Oghli, A.H.; Soleymanpour, A. Polyoxometalate/Reduced Graphene Oxide Modified Pencil Graphite Sensor for the Electrochemical Trace Determination of Paroxetine in Biological and Pharmaceutical Media. Mater. Sci. Eng. C 2020, 108, 110407. [Google Scholar] [CrossRef] [PubMed]
- Richelson, E. Synaptic Effects of Antidepressants. J. Clin. Psychopharmacol. 1996, 16, 1S–7S. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Chue, P.; Guillon, Y. Paroxetine: A Review. CNS Drug Rev. 2006, 7, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, X.; Cui, Z.; Xu, C.; Sun, Z.; Li, J.; Liu, J.; Tian, Y.; Li, H. A Nanometer-Sized Graphite/Boron-Doped Diamond Electrochemical Sensor for Sensitive Detection of Acetaminophen. ACS Omega 2021, 6, 6326–6334. [Google Scholar] [CrossRef] [PubMed]
- Simioni, N.B.; Oliveira, G.G.; Vicentini, F.C.; Lanza, M.R.V.; Janegitz, B.C.; Fatibello-Filho, O. Nanodiamonds Stabilized in Dihexadecyl Phosphate Film for Electrochemical Study and Quantification of Codeine in Biological and Pharmaceutical Samples. Diam. Relat. Mater. 2017, 74, 191–196. [Google Scholar] [CrossRef]
- Wong, A.; Materón, E.M.; Freitas, T.A.; Faria, R.C.; Gonçalves, D. Voltammetric Sensing of Tryptophan in Dark Chocolate Bars, Skimmed Milk and Urine Samples in the Presence of Dopamine and Caffeine. J. Appl. Electrochem. 2022, 52, 1249–1257. [Google Scholar] [CrossRef]
- Fernandes-Junior, W.S.; Zaccarin, L.F.; Oliveira, G.G.; De Oliveira, P.R.; Kalinke, C.; Bonacin, J.A.; Prakash, J.; Janegitz, B.C. Electrochemical Sensor Based on Nanodiamonds and Manioc Starch for Detection of Tetracycline. J. Sens. 2021, 2021, 6622612. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [Green Version]
- Simioni, N.B.; Silva, T.A.; Oliveira, G.G.; Fatibello-Filho, O. A Nanodiamond-Based Electrochemical Sensor for the Determination of Pyrazinamide Antibiotic. Sens. Actuators B Chem. 2017, 250, 315–323. [Google Scholar] [CrossRef]
- Baccarin, M.; Rowley-Neale, S.J.; Cavalheiro, É.T.G.; Smith, G.C.; Banks, C.E. Nanodiamond Based Surface Modified Screen-Printed Electrodes for the Simultaneous Voltammetric Determination of Dopamine and Uric Acid. Microchim. Acta 2019, 186, 200. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Lee, C.Y.; Tai, N.H. Nitrogen-Incorporated Ovoid-Shaped Nanodiamond Films for Dopamine Detection. ACS Appl. Nano Mater. 2020, 3, 11970–11978. [Google Scholar] [CrossRef]
- Puthongkham, P.; Venton, B.J. Nanodiamond Coating Improves the Sensitivity and Antifouling Properties of Carbon Fiber Microelectrodes. ACS Sens. 2019, 4, 2403–2411. [Google Scholar] [CrossRef]
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological Considerations When Creating Nanoparticle-Based Drugs and Drug Delivery Systems. Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold Nanoparticles: Distribution, Bioaccumulation and Toxicity. In Vitro and in Vivo Studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Kurth, S.; Marques, M.A.L.; Gross, E.K.U. Density-Functional Theory. In Encyclopedia of Condensed Matter Physics; Bassani, F., Liedl, G.L., Wyder, P., Eds.; Elsevier: Oxford, UK, 2005; pp. 395–402. [Google Scholar]
- Crocombette, J.P.; Willaime, F. Ab Initio Electronic Structure Calculations for Nuclear Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Ross, S.; Wu, R.-S.; Wei, S.-C.; Ross, G.M.; Chang, H.-T. The Analytical and Biomedical Applications of Carbon Dots and Their Future Theranostic Potential: A Review. J. Food Drug Anal. 2020, 28, 677–695. [Google Scholar] [CrossRef]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural Carbon-Based Quantum Dots and Their Applications in Drug Delivery: A Review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Silva, S.; Duarte, D.; Crista, D.M.A.; Pinto da Silva, L.; Esteves da Silva, J.C.G. Normal Breast Epithelial MCF-10A Cells to Evaluate the Safety of Carbon Dots. RSC Med. Chem. 2021, 12, 245–253. [Google Scholar] [CrossRef]
- Rajendran, S.; Zichri, S.B.; Usha Vipinachandran, V.; Jelinek, R.; Bhunia, S.K. Triphenylphosphonium-Derived Bright Green Fluorescent Carbon Dots for Mitochondrial Targeting and Rapid Selective Detection of Tetracycline. ChemNanoMat 2021, 7, 545–552. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hu, Y.; Wang, G.; Dong, S.; Hao, J. Magnetic Networks of Carbon Quantum Dots and Ag Particles. J. Colloid Interface Sci. 2019, 539, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Koduru, J.R. Perspectives of Magnetic Nature Carbon Dots in Analytical Chemistry: From Separation to Detection and Bioimaging. Trends Environ. Anal. Chem. 2021, 33, e00153. [Google Scholar] [CrossRef]
- Du, F.; Zhang, M.; Gong, A.; Tan, Y.; Miao, J.; Gong, Y.; Zou, S.; Zhang, L.; Zhang, L.; Wu, C.; et al. Engineered Gadolinium-Doped Carbon Dots for Magnetic Resonance Imaging-Guided Radiotherapy of Tumors. Biomaterials 2017, 121, 109–120. [Google Scholar] [CrossRef]
- Dehvari, K.; Chiu, S.H.; Lin, J.S.; Girma, W.M.; Ling, Y.C.; Chang, J.Y. Heteroatom Doped Carbon Dots with Nanoenzyme like Properties as Theranostic Platforms for Free Radical Scavenging, Imaging, and Chemotherapy. Acta Biomater. 2020, 114, 343–357. [Google Scholar] [CrossRef]
- Wang, H.; Li, F.; Du, C.; Wang, H.; Mahato, R.I.; Huang, Y. Doxorubicin and Lapatinib Combination Nanomedicine for Treating Resistant Breast. Cancer. Mol. Pharm. 2014, 11, 2600–2611. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An Update on Anticancer Molecular Action, Toxicity and Novel Drug Delivery Systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Haris, A.; Permana, A.J.; Wibowo, D.L.N.; Purwanto, B.; Nikmah, Y.L.; Idris, A. Bamboo Leaf-Based Carbon Dots for Efficient Tumor Imaging and Therapy. RSC Adv. 2018, 8, 38376–38383. [Google Scholar] [CrossRef] [Green Version]
- Ankireddy, S.R.; Vo, V.G.; An, S.S.A.; Kim, J. Solvent-Free Synthesis of Fluorescent Carbon Dots: An Ecofriendly Approach for the Bioimaging and Screening of Anticancer Activity via Caspase-Induced Apoptosis. ACS Appl. Bio Mater. 2020, 3, 4873–4882. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Merlo, L.; Caffo, M. Nanoparticles Potential: Types, Mechanisms of Action, Actual in Vitro and Animal Studies, Recent Patents. In Innovative Brain Tumor Therapy; Caruso, G., Merlo, L., Caffo, M., Eds.; Woodhead Publishing: Oxford, UK, 2014; pp. 53–150. [Google Scholar]
- Pelinovskaya, N.; Bottero, J.; Masion, A. Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Nayak, T.R.; Zhang, Y.; Cai, W. Cancer Theranostics with Carbon-Based Nanoplatforms. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Academic Press: Oxford, UK, 2014; pp. 347–361. [Google Scholar]
- Maleki, R.; Khoshoei, A.; Ghasemy, E.; Rashidi, A. Molecular Insight into the Smart Functionalized TMC-Fullerene Nanocarrier in the PH-Responsive Adsorption and Release of Anti-Cancer Drugs. J. Mol. Graph. Model. 2020, 100, 107660. [Google Scholar] [CrossRef] [PubMed]
- Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Fullerene Derivatives in Photodynamic Inactivation of Microorganisms. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 413–433. [Google Scholar]
- Ye, L.; Kollie, L.; Liu, X.; Guo, W.; Ying, X.; Zhu, J.; Yang, S.; Yu, M. Antitumor Activity and Potential Mechanism of Novel Fullerene Derivative Nanoparticles. Molecules 2021, 26, 3252. [Google Scholar] [CrossRef]
- Caruso, G.; Merlo, L.; Tot, E.; Pignataro, C.; Caffo, M. Nanotechnology and the New Frontiers of Drug Delivery in Cerebral Gliomas. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 95–112. [Google Scholar]
- Alipour, E.; Alimohammady, F.; Yumashev, A.; Maseleno, A. Fullerene C60 Containing Porphyrin-like Metal Center as Drug Delivery System for Ibuprofen Drug. J. Mol. Model. 2020, 26, 7. [Google Scholar] [CrossRef]
- Christy, P.A.; Peter, A.J.; Arumugam, S.; Lee, C.W.; Prakash, P.S. Superparamagnetic Behavior of Sulfonated Fullerene (C60SO3H): Synthesis and Characterization for Biomedical Applications. Mater. Chem. Phys. 2020, 240, 122207. [Google Scholar] [CrossRef]
- Rahimah, R.; Fadli, A.; Yelmida, Y.; Nurfajriani, N.; Zakwan, Z. Synthesis and Characterization Nanomagnetite by Co-Precipitation. Indones. J. Chem. Sci. Technol. 2019, 2, 90–96. [Google Scholar] [CrossRef]
- Jauhari, S.; Singh, S.; Dash, A.K. Paclitaxel. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2009; Volume 34, pp. 299–344. [Google Scholar]
- Xu, H.; Tu, X.; Fan, G.; Wang, Q.; Wang, X.; Chu, X. Adsorption Properties Study of Boron Nitride Fullerene for the Application as Smart Drug Delivery Agent of Anti-Cancer Drug Hydroxyurea by Density Functional Theory. J. Mol. Liq. 2020, 318, 114315. [Google Scholar] [CrossRef]
- Stevens, M.R. Hydroxyurea: An Overview. J. Biol. Regul. Homeost. Agents 1999, 13, 172–175. [Google Scholar]
- Madaan, K.; Kaushik, D.; Verma, T. Hydroxyurea: A Key Player in Cancer Chemotherapy. Expert Rev. Anticancer Ther. 2012, 12, 19–29. [Google Scholar] [CrossRef]
- Ghasemi, A.S.; Ashrafi, F.; Babanejad, S.A.; Elyasi, A. Study of the Physicochemical Properties of Anti-Cancer Drug Gemcitabine on the Surface of Al Doped C60 and C70 Fullerenes: A DFT Computation. J. Struct. Chem. 2019, 60, 13–19. [Google Scholar] [CrossRef]
- Silverman, E.; Eddy, A. Systemic Lupus Erythematosus. In Textbook of Pediatric Rheumatology, 6th ed.; Cassidy, J.T., Laxer, R.M., Petty, R.E., Lindsley, C.B., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; Chapter 21; pp. 315–343. [Google Scholar]
- Newton, H.B. Neurological Complications of Chemotherapy to the Central Nervous System. In Neuro-Oncology Part II; Grisold, W., Soffietti, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 105, Chapter 59; pp. 903–916. [Google Scholar]
- Van Laar, J.M. Immunosuppressive Drugs. In Kelley and Firestein’s Textbook of Rheumatology, Tenth ed.; Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes, I.B., O’Dell, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 62; pp. 983–998. [Google Scholar]
- Meor, M.; Manap, D. DFT Study of Boron-Fullerene Carrier for Delivery of Anticancer Drugs. Medbiotech J. 2021, 5, 6–9. [Google Scholar]
- Kakaei, A.; Mirzaei, M. Cyclophosphamide@CNT: In Silico Exploration of Nano Drug Delivery System. Lab-in-Silico 2021, 2, 9–14. [Google Scholar]
- Kraemer, T.; Maurer, H.H. Forensic Science. In Handbook of Analytical Separations; Bogusz, M.J., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2008; Volume 6, pp. 319–356. [Google Scholar]
- Esrafili, M.D.; Khan, A.A. Alkali Metal Decorated C60 fullerenes as Promising Materials for Delivery of the 5-Fluorouracil Anticancer Drug: A DFT Approach. RSC Adv. 2022, 12, 3948–3956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, P.; Javed Ansari, M.; Aldawsari, M.F.; Alalaiwe, A.S.; Kaur, J.; Kumar, R.; Ng Kay Lup, A.; Enayati, A.; Mirzaei, H.; et al. Electrostatic Interaction Assisted Ca-Decorated C20 Fullerene Loaded to Anti-Inflammatory Drugs to Manage Cardiovascular Disease Risk in Rheumatoid Arthritis Patients. J. Mol. Liq. 2022, 350, 118564. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Bagheri Novir, S.; Aram, M.R. Quantum Mechanical Simulation of Chloroquine Drug Interaction with C60 Fullerene for Treatment of COVID-19. Chem. Phys. Lett. 2020, 757, 137869. [Google Scholar] [CrossRef]
- Karimi, M.; Solati, N.; Amiri, M.; Mirshekari, H.; Mohamed, E.; Taheri, M.; Hashemkhani, M.; Saeidi, A.; Estiar, M.A.; Kiani, P.; et al. Carbon Nanotubes Part I: Preparation of a Novel and Versatile Drug-Delivery Vehicle. Expert Opin. Drug Deliv. 2015, 12, 1071–1087. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. Biomed Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef]
- Mousa, M.S. Comparison between Single-Walled CNT, Multi-Walled CNT, and Carbon Nanotube-Fiber Pyrograf III. IOP Conf. Ser. Mater. Sci. Eng. 2018, 305, 012025. [Google Scholar] [CrossRef] [Green Version]
- Foldvari, M.; Bagonluri, M. Carbon Nanotubes as Functional Excipients for Nanomedicines: II. Drug Delivery and Biocompatibility Issues. Nanomedicine 2008, 4, 183–200. [Google Scholar] [CrossRef]
- Endo, M.; Strano, M.; Ajayan, P. Potential Applications of Carbon Nanotubes. In Carbon Nanotubes Advanced Topics in the Synthesis, Structure, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2008; pp. 13–61. [Google Scholar]
- Kushwaha, S.K.S.; Ghoshal, S.; Rai, A.K.; Singh, S. Carbon Nanotubes as a Novel Drug Delivery System for Anticancer Therapy: A Review. Brazilian J. Pharm. Sci. 2013, 49, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Karimzadeh, S.; Safaei, B.; Jen, T.C. Prediction Effect of Ethanol Molecules on Doxorubicin Drug Delivery Using Single-Walled Carbon Nanotube Carrier through POPC Cell Membrane. J. Mol. Liq. 2021, 330, 115698. [Google Scholar] [CrossRef]
- Lyra, K.M.; Kaminari, A.; Panagiotaki, K.N.; Spyrou, K.; Papageorgiou, S.; Sakellis, E.; Katsaros, F.K.; Sideratou, Z. Multi-Walled Carbon Nanotubes Decorated with Guanidinylated Dendritic Molecular Transporters: An Efficient Platform for the Selective Anticancer Activity of Doxorubicin. Pharmaceutics 2021, 13, 858. [Google Scholar] [CrossRef]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific Targeting Cancer Cells with Nanoparticles and Drug Delivery in Cancer Therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Wolski, P.; Nieszporek, K.; Panczyk, T. Cytosine-Rich Dna Fragments Covalently Bound to Carbon Nanotube as Factors Triggering Doxorubicin Release at Acidic Ph. A Molecular Dynamics Study. Int. J. Mol. Sci. 2021, 22, 8466. [Google Scholar] [CrossRef] [PubMed]
- Dahri, M.; Akbarialiabad, H.; Jahromi, A.M.; Maleki, R. Loading and Release of Cancer Chemotherapy Drugs Utilizing Simultaneous Temperature and PH-Responsive Nanohybrid. BMC Pharmacol. Toxicol. 2021, 22, 41. [Google Scholar] [CrossRef]
- Azqhandi, M.H.A.; Farahani, B.V.; Dehghani, N. Encapsulation of Methotrexate and Cyclophosphamide in Interpolymer Complexes Formed between Poly Acrylic Acid and Poly Ethylene Glycol on Multi-Walled Carbon Nanotubes as Drug Delivery Systems. Mater. Sci. Eng. C 2017, 79, 841–847. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon N. Y. 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Bagheri Novir, S.; Aram, M.R. Quantum Mechanical Studies of the Adsorption of Remdesivir, as an Effective Drug for Treatment of COVID-19, on the Surface of Pristine, COOH-Functionalized and S-, Si- and Al- Doped Carbon Nanotubes. Phys. E Low-Dimensional Syst. Nanostructures 2021, 129, 114668. [Google Scholar] [CrossRef]
- Zandler, M.E.; D’Souza, F. The Remarkable Ability of B3LYP/3-21G(*) Calculations to Describe Geometry, Spectral and Electrochemical Properties of Molecular and Supramolecular Porphyrin-Fullerene Conjugates. Comptes Rendus Chim. 2006, 9, 960–981. [Google Scholar] [CrossRef]
- Al-Sawaff, Z.H.; Dalgic, S.S.; Kandemirli, F.; Monajjemi, M.; Mollaamin, F.; Dalgic, S. DFT Study Adsorption of Hydroxychloroquine for Treatment COVID-19 by Al, Si and Si-C Doping on Carbon Nanotube Surface: A Drug Delivery Simulation. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Zobir, S.A.M.; Rashid, S.A.; Tan, T. Recent Development on the Synthesis Techniques and Properties of Graphene Derivatives; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Holkar, C.R.; Jain, S.S.; Jadhav, A.J.; Pinjari, D.V. Scale-up Technologies for Advanced Nanomaterials for Green Energy: Feasibilities and Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Vovusha, H.; Banerjee, D.; Yadav, M.K.; Perrozzi, F.; Ottaviano, L.; Sanyal, S.; Sanyal, B. Binding Characteristics of Anticancer Drug Doxorubicin with Two-Dimensional Graphene and Graphene Oxide: Insights from Density Functional Theory Calculations and Fluorescence Spectroscopy. J. Phys. Chem. C 2018, 122, 21031–21038. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Saebfar, H.; Gholami, M.H.; Hushmandi, K.; Zabolian, A.; Bikarannejad, P.; Hashemi, M.; Daneshi, S.; Mirzaei, S.; Sharifi, E. Doxorubicin-Loaded Graphene Oxide Nanocomposites in Cancer Medicine: Stimuli-Responsive Carriers, Co-Delivery and Suppressing Resistance. Expert Opin. Drug Deliv. 2022, 19, 355–382. [Google Scholar] [CrossRef]
- Song, J.; Cui, N.; Sun, S.; Lu, X.; Wang, Y.; Shi, H.; Lee, E.S.; Jiang, H.B. Controllability of Graphene Oxide Doxorubicin Loading Capacity Based on Density Functional Theory. Nanomaterials 2022, 12, 479. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Ladani, R.B.; Ghorbani, K.; Mouritz, A.P.; Kinloch, A.J.; Wang, C.H. A Novel Route for Tethering Graphene with Iron Oxide and Its Magnetic Field Alignment in Polymer Nanocomposites. Polymer 2016, 97, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Pourjavadi, A.; Kohestanian, M.; Yaghoubi, M. Poly(Glycidyl Methacrylate)-Coated Magnetic Graphene Oxide as a Highly Efficient Nanocarrier: Preparation, Characterization, and Targeted DOX Delivery. New J. Chem. 2019, 43, 18647–18656. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated Nano-Graphene Oxide as a Nanocarrier for Delivering Mixed Anticancer Drugs to Improve Anticancer Activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, M.; Fattahi, A.; Tajkhorshid, E.; Nouranian, S. Molecular Insights into the Loading and Dynamics of Doxorubicin on PEGylated Graphene Oxide Nanocarriers. ACS Appl. Bio Mater. 2020, 3, 1354–1363. [Google Scholar] [CrossRef]
- Ma, K.; Li, W.; Zhu, G.; Chi, H.; Yin, Y.; Li, Y.; Zong, Y.; Guo, Z.; Wang, L.; Xu, W.; et al. PEGylated DOX-Coated Nano Graphene Oxide as PH-Responsive Multifunctional Nanocarrier for Targeted Drug Delivery. J. Drug Target. 2021, 29, 884–891. [Google Scholar] [CrossRef]
- Pooja, D.; Kadari, A.; Kulhari, H.; Sistla, R. Lipid-Based Nanomedicines: Current Clinical Status and Future Perspectives; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hosseini, S.M.; Mazinani, S.; Abdouss, M.; Kalhor, H.; Kalantari, K.; Amiri, I.S.; Ramezani, Z. Designing Chitosan Nanoparticles Embedded into Graphene Oxide as a Drug Delivery System. Polym. Bull. 2022, 79, 541–554. [Google Scholar] [CrossRef]
- Shen, J.W.; Li, J.; Dai, J.; Zhou, M.; Ren, H.; Zhang, L.; Hu, Q.; Kong, Z.; Liang, L. Molecular Dynamics Study on the Adsorption and Release of Doxorubicin by Chitosan-Decorated Graphene. Carbohydr. Polym. 2020, 248, 116809. [Google Scholar] [CrossRef] [PubMed]
- Khoshoei, A.; Ghasemy, E.; Poustchi, F.; Shahbazi, M.A.; Maleki, R. Engineering the PH-Sensitivity of the Graphene and Carbon Nanotube Based Nanomedicines in Smart Cancer Therapy by Grafting Trimetyl Chitosan. Pharm. Res. 2020, 37, 160. [Google Scholar] [CrossRef] [PubMed]
- Gooneh-Farahani, S.; Naghib, S.M.; Naimi-Jamal, M.R.; Seyfoori, A. A PH-Sensitive Nanocarrier Based on BSA-Stabilized Graphene-Chitosan Nanocomposite for Sustained and Prolonged Release of Anticancer Agents. Sci. Rep. 2021, 11, 17404. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Mitra, S. Development of Nano Structured Graphene Oxide Incorporated Dexamethasone with Enhanced Dissolution. Colloid Interface Sci. Commun. 2022, 47, 100599. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical Applications of Nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Houshyar, S.; Sarker, S.R.; Pyreddy, S.; Dekiwadia, C.; Nasa, Z.; Padhye, R.; Wang, X. Nanodiamond-Chitosan Functionalized Hernia Mesh for Biocompatibility and Antimicrobial Activity. J. Biomed. Mater. Res.-Part A 2021, 109, 2449–2461. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Gao, G.; Liu, R.; Einaga, Y.; Zhi, J. Nanodiamonds Inhibit Cancer Cell Migration by Strengthening Cell Adhesion: Implications for Cancer Treatment. ACS Appl. Mater. Interfaces 2021, 13, 9620–9629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, W.; Ahmad, K.Z.; Wang, X.; Zhang, T.; Yu, Y.; Chow, E.K.-H.; Ho, D.; Ding, X. Safety Evaluation of Nanodiamond-Doxorubicin Complexes in a Naïve Beagle Canine Model Using Hematologic, Histological, and Urine Analysis. Nano Res. 2022, 15, 3356–3366. [Google Scholar] [CrossRef]
- Dai, L.; Liu, M.; Long, W.; Hu, X.; Ouyang, H.; Feng, Y.; Deng, F.; Wen, Y.; Zhang, X.; Wei, Y. Synthesis of Water Dispersible and Biocompatible Nanodiamond Composite via Photocatalytic Surface Grafting of Zwitterionic Polymers for Intracellular Delivery of DOX. Mater. Today Commun. 2022, 30, 103010. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Yoo, W.; Lee, W.; Kim, H.N.; Jeong, J.; Park, H.H.; Ahn, J.H.; Jung, D.; Lee, J.; Kim, J.; Lee, S.W.; et al. Nanodiamond as a Cytokine Sponge in Infectious Diseases. Front. Bioeng. Biotechnol. 2022, 504. [Google Scholar] [CrossRef] [PubMed]
- Toński, M.; Paszkiewicz, M.; Dołżonek, J.; Flejszar, M.; Bielicka-Giełdoń, A.; Stepnowski, P.; Białk-Bielińska, A. Regeneration and Reuse of the Carbon Nanotubes for the Adsorption of Selected Anticancer Drugs from Water Matrices. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 618, 126355. [Google Scholar] [CrossRef]
- Nattagh, F.; Hosseini, S.; Esrafili, M.D. Effects of B and N Doping/Codoping on the Adsorption Behavior of C60 Fullerene towards Aspirin: A DFT Investigation. J. Mol. Liq. 2021, 342, 117459. [Google Scholar] [CrossRef]
- Li, W.; Zhao, T. Hydroxyurea Anticancer Drug Adsorption on the Pristine and Doped C70 Fullerene as Potential Carriers for Drug Delivery. J. Mol. Liq. 2021, 340, 117226. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Safaei, B.; Jen, T.C. Theorical Investigation of Adsorption Mechanism of Doxorubicin Anticancer Drug on the Pristine and Functionalized Single-Walled Carbon Nanotube Surface as a Drug Delivery Vehicle: A DFT Study. J. Mol. Liq. 2021, 322, 114890. [Google Scholar] [CrossRef]
- Yuan, S.J.; Wang, C.; Xu, H.Z.; Liu, Y.; Zheng, M.Y.; Li, K.; Sun, S.K.; Komatsu, N.; Zhao, L.; Chen, X. Conjugation with Nanodiamonds via Hydrazone Bond Fundamentally Alters Intracellular Distribution and Activity of Doxorubicin. Int. J. Pharm. 2021, 606, 120872. [Google Scholar] [CrossRef]
- Cheng, X.; Ni, X.; Wu, R.; Chong, Y.; Gao, X.; Ge, C.; Yin, J.J. Evaluation of the Structure-Activity Relationship of Carbon Nanomaterials as Antioxidants. Nanomedicine 2018, 13, 733–747. [Google Scholar] [CrossRef]
- Huq, R.; Samuel, E.L.G.; Sikkema, W.K.A.; Nilewski, L.G.; Lee, T.; Tanner, M.R.; Khan, F.S.; Porter, P.C.; Tajhya, R.B.; Patel, R.S.; et al. Preferential Uptake of Antioxidant Carbon Nanoparticles by T Lymphocytes for Immunomodulation. Sci. Rep. 2016, 6, 33808. [Google Scholar] [CrossRef] [Green Version]
- Wang, I.C.; Tai, L.A.; Lee, D.D.; Kanakamma, P.P.; Shen, C.K.F.; Luh, T.Y.; Cheng, C.H.; Hwang, K.C. C60 and Water-Soluble Fullerene Derivatives as Antioxidants against Radical-Initiated Lipid Peroxidation. J. Med. Chem. 1999, 42, 4614–4620. [Google Scholar] [CrossRef]
- Dugan, L.L.; Gabrielsen, J.K.; Yu, S.P.; Lin, T.S.; Choi, D.W. Buckminsterfullerenol Free Radical Scavengers Reduce Excitotoxic and Apoptotic Death of Cultured Cortical Neurons. Neurobiol. Dis. 1996, 3, 129–135. [Google Scholar] [CrossRef] [Green Version]
- González-García, Y.; López-Vargas, E.R.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; González-Morales, S.; Robledo-Olivo, A.; Alpuche-Solís, Á.G.; Juárez-Maldonado, A. Impact of Carbon Nanomaterials on the Antioxidant System of Tomato Seedlings. Int. J. Mol. Sci. 2019, 20, 5858. [Google Scholar] [CrossRef] [Green Version]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-Based Tissue Engineering: Progress, Challenges, and Opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Liu, Z. Biomedical Applications of Carbon Nanomaterials. In Biomedical Applications and Toxicology of Carbon Nanomaterials; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 131–162. [Google Scholar]
- Shi, X.; Sitharaman, B.; Pham, Q.P.; Liang, F.; Wu, K.; Edward Billups, W.; Wilson, L.J.; Mikos, A.G. Fabrication of Porous Ultra-Short Single-Walled Carbon Nanotube Nanocomposite Scaffolds for Bone Tissue Engineering. Biomaterials 2007, 28, 4078–4090. [Google Scholar] [CrossRef] [Green Version]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Nukavarapu, S.P.; Kumbar, S.G. Carbon Nanotube Composite Scaffolds for Bone Tissue Engineering. U.S. Patent No. 8614189, 24 December 2013. [Google Scholar]
- Ahmed, M.K.; Mansour, S.F.; Al-Wafi, R. Nanofibrous Scaffolds of ε-Polycaprolactone Containing Sr/Se_hydroxyapatite/ Graphene Oxide for Tissue Engineering Applications. Biomed. Mater. 2021, 16, 045030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent PLLA-Nanodiamond Composites for Bone Tissue Engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef]
- Malik, N.; Arfin, T.; Khan, A.U. Graphene Nanomaterials: Chemistry and Pharmaceutical Perspectives; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tang, Z.; Wu, H.; Cort, J.R.; Buchko, G.W.; Zhang, Y.; Shao, Y.; Aksay, I.A.; Liu, J.; Lin, Y. Constraint of DNA on Functionalized Graphene Improves Its Biostability and Specificity. Small 2010, 6, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Chen, Y.W.; Hung, W.T.; Chen, I.W.; Chen, S.Y. Quantum-Dot-Tagged Reduced Graphene Oxide Nanocomposites for Bright Fluorescence Bioimaging and Photothermal Therapy Monitored in Situ. Adv. Mater. 2012, 24, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wu, T.; Tang, Z.W.; Xiao, J.Y.; Zhuo, R.X.; Shi, B.; Liu, C.J. Water-Soluble Photoluminescent Fullerene Capped Mesoporous Silica for PH-Responsive Drug Delivery and Bioimaging. Nanotechnology 2016, 27, 315104. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Okazaki, T.; Iizumi, Y.; Kataura, H.; Yudasaka, M. Semiconductor SWCNT Dispersion Fluid for Bioimaging and Production Method Therefor. WO2019097697, 23 May 2019. [Google Scholar]
| Electrode | Modifier | Detection Method | Target | Sample | LOD (µM) | Linear Range (µM) | Electrolyte | pH | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| GCE | C60 | CV, SWV, DPV | BPA | Wastewater | 0.0037 | 0.074–0.23 | KCl | 8.0 | 94.0–104.0 | [46] |
| C60-CNT/IL | CV | NE | Serum, urine | 0.018 | 0.07–30.0, 30.0–750.0 | ACN | 7.0 | 98.0 | [50] | |
| IP | 0.022 | 0.1–25.0, 25.0–700.0 | 101.0 | |||||||
| DA | 0.015 | 0.06–25.0, 25.0–800.0 | 98.5 | |||||||
| C60-CNT/IL | CV, DPV | Hydrazine, hydroxylamine | Tap water | 0.017 | 0.05–700.0 | ACN | 7.0 | 98.0–101.0 | [51] | |
| Auxiliary cooling water | 0.028 | 1.0–300.0 | 99.0–101.0 | |||||||
| AC60-PdNPs | CV, DPV | DA | Pharmaceutical formulations | 0.056 | 0.35–133.35 | PBS | 7.0 | 97.0–97.7 | [54] | |
| GO-C60 | CV, DPV | DA | Rat brain | 0.008 | 0.02–73.5 | PBS | 7.0 | 101.8 | [57] | |
| DA injection | 94.0–100.16 | |||||||||
| C60-CNT | CV, DPV | L-dopa | Real samples | 0.035 | 0.5–2000.0 | PBS | 7.0 | 99.8–100.4 | [63] | |
| AC | 99.25–100.16 | |||||||||
| C60-CNT/IL | CV, DPV | Diazepam | Urine | 0.087 | 0.3–700.0 | PBS | 7.0 | 96.0–103.6 | [67] | |
| Serum | 96.3–102.8 | |||||||||
| NiNPs-CuNPs@reduced-C60 | SWV | Vitamin D3 | Serum | 0.0025 | 1.25–475.0 | KCl, LiClO4 | 7.0 | 98.6–100.5 | [69] | |
| Urine | 98.2–99.8 | |||||||||
| Tablet | 96.8–98.1 | |||||||||
| Pt/C60 | CV, DPV, EIS | CC | Local river water, sanitary wastewater | 2.97 | 50.0–1500.0 | PBS | 7.0 | 96.2–103.8 | [72] | |
| HQ | 2.19 | 50.0–1100.0 | 95.7–104.4 | |||||||
| C60/MWCNT/Nafion | CV, DPV, EIS | CAF | Brupanas | 0.07289 | 10.0–1000.0 | HClO4 | 1.0 | 97.71 | [76] | |
| Pipadol Extra | 95.66 | |||||||||
| CPE | CuNPs/C60/MWCNTs | SWV | PT | Human blood serum, plasma, urine | 0.000073 | 0.004–0.04 | PBS | 6.8 | 99.21–103.0 | [60] |
| MnO2/C60 | CV, DPV, EIS | RFX | Pharmaceutical formulations | 0.000968 | 0.001–0.04 | BR buffer | 3.0 | 98.98–101.52 | [82] | |
| SPE | C60-rGO-Nafion | SWV | MTZ | Synthetic serum and urine | 0.21 | 0.25–34.0 | PBS | 7.0 | 92.0–100.0 | [83] |
| Electrode | Modifier | Detection Method | Target | Sample | LOD (µM) | Linear Range (µM) | Electrolyte | pH | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| GCE | GO | CV, LSV | MD | Serum, urine | 0.1 | 0.3–200.0 | PBS | 7.4 | 95.6–104.3 | [86] |
| graphene | DPV | L-dopa | Tablet, mouse brain extract | 0.022 | 0.04–79.0 | PBS | 6.2 | 101.4–109.42 | [87] | |
| GNRs-GO-CNTP | SWV | Indomethacin | Human blood serum, urine, pharmaceutical | 0.017 | 0.2–0.9, 2.5–91.5 | PBS | 8.0 | 98.0–103.5 | [90] | |
| graphene/TiO2/PANI | SWV | ARP | Pharmaceutical formulations | 0.0022 | 0.0112–0.0893 | BR buffer | 2.5 | 99.0–101.6 | [100] | |
| PANI-Fe2O3-rGO | CV, LSV, DPV | HQ | Tap water | 0.06 | 0.1–550.0 | PBS | 2.5 | - | [104] | |
| graphene nanoparticles-Bi | CV, LSV | DA | Commercial injection | 0.35 | 1.0–30.0 | PBS | 7.0 | 98.2–98.4 | [107] | |
| graphene-Nafion | CV, SWAdASV | PT | Commercial tablet | 0.0012 | 0.0083–0.51 | PBS | 2.5 | - | [109] | |
| ASA | 0.065 | 0.087–17.0 | ||||||||
| CAF | 0.038 | 0.26–10.0 | ||||||||
| Ni0.5Zn0.5Fe2O4/graphene | CV, DPV | OMZ | Serum | 0.015 | 0.03–100.0 | PBS | 6.0 | 97.5–101.1 | [116] | |
| OMZ capsule | 98.6 | |||||||||
| GPE | graphene/2,7-BF | CV, SWV, CA | Methyldopa | Tablet | 0.05 | 0.09–500.0 | PBS | 7.0 | 97.6–102.0 | [93] |
| Urine | 98.7–103.2 | |||||||||
| Pd | graphene | EIS, CV, SWV | NE | Human urine | 0.06744 | 0.5–500.0 | PBS | 7.2 | 97.2–98.86 | [94] |
| SPCE | graphene-AuNPs | CV, SWV | Rutin | Tablet | 0.011 | 0.1–15.0 | ABS | 5.0 | 96.52–102.97 | [119] |
| PGE | rGO/PWA | DPV | PRX | Tablet | 0.0009 | 0.008–1.0 | BR buffer | 7.0 | 97.6 | [123] |
| Urine | 98.4–101.6 | |||||||||
| Serum | 97.7–101.3 |
| Carbon Materials | Incorporated Molecule | Drug | Usage | Ref |
|---|---|---|---|---|
| C-dot | C, N, and O | DOX | Evaluate the safety of three different carbon dots. | [146] |
| TPP(PEG-di-NH2) | Anticancer | [147] | ||
| Gd3+ | Bladder cleansing. | [149] | ||
| Mn, N, S, and HA | Anticancer, an option for PDT. | [151] | ||
| CBBA | Anticancer | [154] | ||
| EDC | DOX | Antitumor | [155] | |
| GEM | ||||
| Fullerene C20 | Ca | SSZ | Reduce cardiovascular risk in rheumatoid arthritis | [178] |
| CUR | ||||
| NPX | ||||
| Fullerene C55 | FeN4 | Ibuprofen | Nonsteroidal anti-inflammatory drug | [163] |
| CoN4 | ||||
| NiN4 | ||||
| Fullerene C58 | BN | Aspirin | Anti-inflammatory and antiplatelet drug | [221] |
| Fullerene C59 | B | CQ | Treatment of COVID-19 | [194] |
| Al | ||||
| Si | ||||
| B | Aspirin | Anti-inflammatory and antiplatelet drug | [221] | |
| N | ||||
| B | CTX | Anti-cancer | [174] | |
| Fullerene C60 | SO3H | Ibuprofen | Nonsteroidal anti-inflammatory drug | [163] |
| Discovery of new superparamagnetic materials | [164] | |||
| TMC | DOX | Anti-cancer | [159] | |
| TMC | PAX | |||
| B36N36 | HU | Anti-cancer | [167] | |
| CQ | Treatment of COVID-19 | [180] | ||
| Aspirin | Anti-inflammatory and antiplatelet drug | [221] | ||
| Li | 5FU | Anti-cancer | [177] | |
| Na | ||||
| K | ||||
| Fullerene C69 | B | HU | Anti-cancer | [222] |
| Al | ||||
| Si | ||||
| Fullerene C70 | HU | Anti-cancer | [222] | |
| CNT | - | CPP | Anti-cancer | [175] |
| - | RDV | Treatment of COVID-19 | [194] | |
| S | ||||
| Si | ||||
| Al | ||||
| COOH | ||||
| - | CPP | Anti-cancer | [175] | |
| ssDNA | DOX | Anti-cancer | [190] | |
| Al- | HCQ | Treatment of COVID-19 | [196] | |
| Si- | ||||
| SiC- | ||||
| SWCNT | −COOH and −OH | DOX | Anti-cancer | [223] |
| TMC | DOX | Anti-cancer | [210] | |
| PAX | ||||
| DMAA-TMC | DOX | Anti-cancer | [191] | |
| PAX | ||||
| Ethanol | DOX | Anti-cancer | [187] | |
| MWCNT | PAA/PEG | CPP | Anti-cancer | [192] |
| MTX | ||||
| oxCNTs | GPEI | DOX | Anti-cancer | [188] |
| Graphene | - | DOX | Anti-cancer | [199] |
| Chi | [209] | |||
| TMC | [210] | |||
| BSA and Chi | [211] | |||
| TMC | PAX | [210] | ||
| GO | - | DOX | Anti-cancer | [199] |
| OH | DOX | Anti-cancer | [201] | |
| OH-O | ||||
| O | ||||
| PGMA | DOX | Anti-cancer | [202] | |
| PEG | DOX | Anti-cancer | [205] | |
| Sh-PEG | ||||
| L-PEG | ||||
| hy-PEG-FA | DOX | Anti-tumor | [206] | |
| hy-PEG | ||||
| PEG | Pt | Anti-tumor hypoxia | [204] | |
| Pt-DOX | Dual drug anti-tumor hypoxia | |||
| - | DXM | Treatment of COVID-19 | [212] | |
| Nanodiamond | - | - | The safety of ND in large mammals | [216] |
| MPC | DOX | Anti-tumor | [217] | |
| polyglycerol | DOX | Anti-cancer | [224] | |
| - | Cytokine | Treatment of COVID-19 | [219] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiwanti, P.K.; Wardhana, B.Y.; Sutanto, L.G.; Dewi, D.M.M.; Putri, I.Z.D.; Savitri, I.N.I. Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review. Molecules 2022, 27, 7578. https://doi.org/10.3390/molecules27217578
Jiwanti PK, Wardhana BY, Sutanto LG, Dewi DMM, Putri IZD, Savitri INI. Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review. Molecules. 2022; 27(21):7578. https://doi.org/10.3390/molecules27217578
Chicago/Turabian StyleJiwanti, Prastika K., Brasstira Y. Wardhana, Laurencia G. Sutanto, Diva Meisya Maulina Dewi, Ilmanda Zalzabhila Danistya Putri, and Ilmi Nur Indira Savitri. 2022. "Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review" Molecules 27, no. 21: 7578. https://doi.org/10.3390/molecules27217578
APA StyleJiwanti, P. K., Wardhana, B. Y., Sutanto, L. G., Dewi, D. M. M., Putri, I. Z. D., & Savitri, I. N. I. (2022). Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review. Molecules, 27(21), 7578. https://doi.org/10.3390/molecules27217578





