Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease
Abstract
1. Introduction
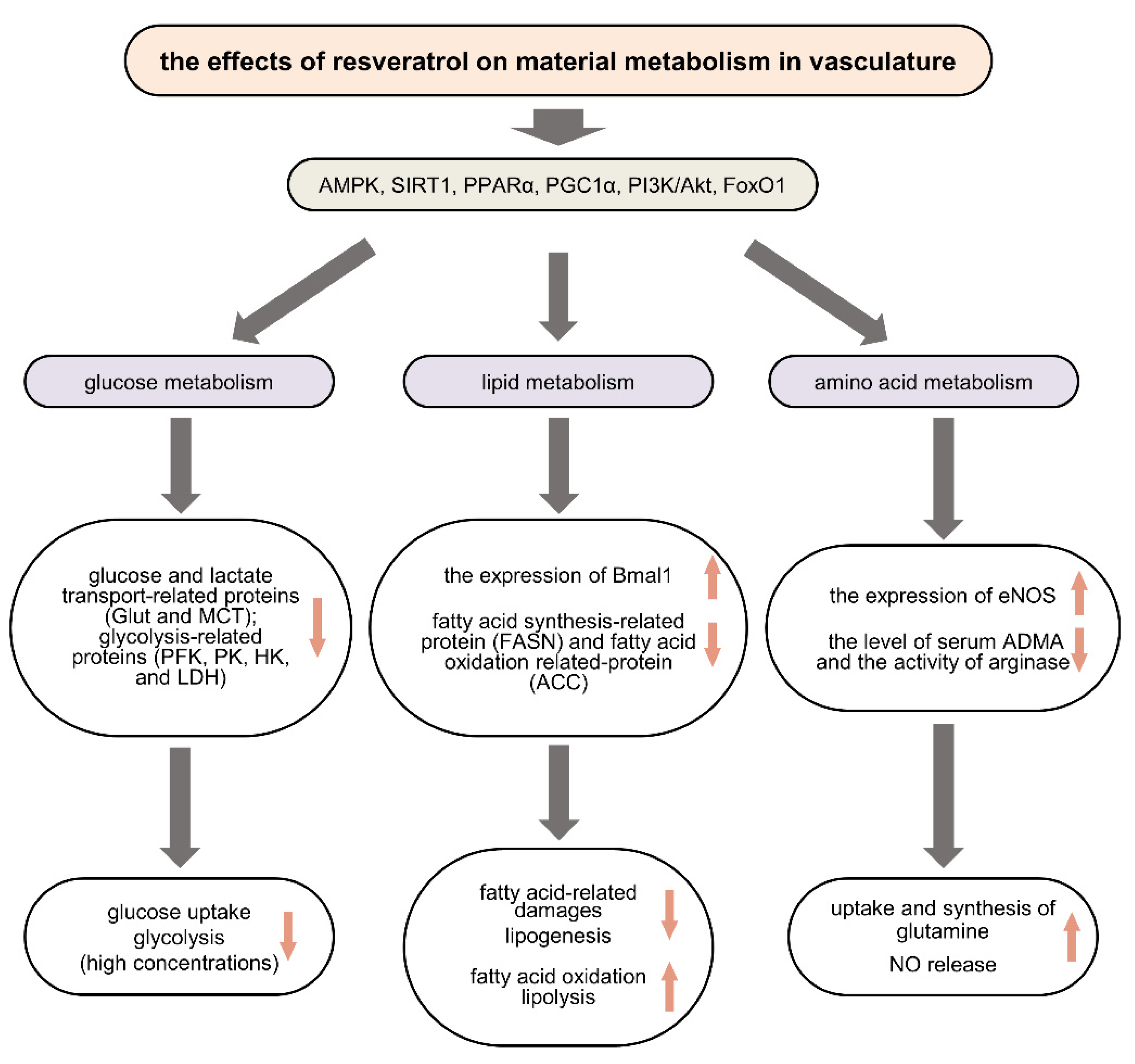
2. The Protective Effects of RSV on Vasculature in Vascular Metabolic Diseases
2.1. Regulating Glucose Metabolism in Vascular Metabolic Diseases
2.2. Regulating Lipid Metabolism in Vascular Metabolic Diseases
2.3. Regulating Amino Acid Metabolism in Vascular Metabolic Diseases
3. Clinical Trials of RSV in the Treatment of Vascular Metabolic Diseases
3.1. The Effect of RSV on Atherosclerosis
3.2. The Effect of RSV on Hypertension
3.3. The Effect of RSV on Ischemia
3.4. The Effect of RSV on Vascular Complications of Metabolic Disease
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.H.; Zhou, M.S. Vascular insulin resistance: A potential link between cardiovascular and metabolic diseases. Curr. Hypertens. Rep. 2009, 11, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Memariani, Z.; Abbas, S.Q.; Ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Helmlinger, G.; Endo, M.; Ferrara, N.; Hlatky, L.; Jain, R.K. Formation of endothelial cell networks. Nature 2000, 405, 139–141. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Carmeliet, P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013, 18, 634–647. [Google Scholar] [CrossRef]
- Butler, T.M.; Siegman, M.J. High-energy phosphate metabolism in vascular smooth muscle. Annu. Rev. Physiol. 1985, 47, 629–643. [Google Scholar] [CrossRef]
- Lambert, C.M.; Roy, M.; Robitaille, G.A.; Richard, D.E.; Bonnet, S. HIF-1 inhibition decreases systemic vascular remodelling diseases by promoting apoptosis through a hexokinase 2-dependent mechanism. Cardiovasc. Res. 2010, 88, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, J.; Liu, M.; He, L.; Zhang, K.; Yang, Y.; Yang, X.; Zhou, H.; Tang, M.; Lu, L.; et al. Warburg effect is involved in apelin-13-induced human aortic vascular smooth muscle cells proliferation. J. Cell. Physiol. 2019, 234, 14413–14421. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, L.; Nickel, T.; Yang, J.; Zhou, J.; Gilbertsen, A.; Geng, Z.; Johnson, C.; Young, B.; Henke, C.; et al. Lactate Promotes Synthetic Phenotype in Vascular Smooth Muscle Cells. Circ. Res. 2017, 121, 1251–1262. [Google Scholar] [CrossRef]
- Werle, M.; Kreuzer, J.; Höfele, J.; Elsässer, A.; Ackermann, C.; Katus, H.A.; Vogt, A.M. Metabolic control analysis of the Warburg-effect in proliferating vascular smooth muscle cells. J. Biomed. Sci. 2005, 12, 827–834. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Bahrami, A.; Ayen, E.; Razi, M.; Behfar, M. Effects of atorvastatin and resveratrol against the experimental endometriosis; evidence for glucose and monocarboxylate transporters, neoangiogenesis. Life Sci. 2021, 272, 119230. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, L.; Shi, J.; Hou, X.; Zhang, H.; Zhang, X.; An, Q.; Fan, F. Resveratrol inhibits VEGF-induced angiogenesis in human endothelial cells associated with suppression of aerobic glycolysis via modulation of PKM2 nuclear translocation. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1265–1273. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Zhan, L.; Wang, M.; Shi, R.; Yuan, X.; Gao, X.; Liu, X.; Zang, J.; Liu, W.; et al. Resveratrol-induced Sirt1 phosphorylation by LKB1 mediates mitochondrial metabolism. J. Biol. Chem. 2021, 297, 100929. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Z.; Ke, L.; Li, Z.; Li, W.; Zhang, Z.; Zhou, Y.; Feng, X.; Zhu, W. Resveratrol improves glucose uptake in insulin-resistant adipocytes via Sirt1. J. Nutr. Biochem. 2018, 55, 209–218. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Chen, H. Resveratrol ameliorates the glucose uptake and lipid metabolism in gestational diabetes mellitus mice and insulin-resistant adipocytes via miR-23a-3p/NOV axis. Mol. Immunol. 2021, 137, 163–173. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Chen, G.; Niu, C.; Wang, Y.; Zhao, C.; Sun, J.; Huang, H.; Huang, S.; Liang, Y.; et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharmacol. 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Schneider, J.G.; Shenouda, S.M.; Lee, A.; Towler, D.A.; Chakravarthy, M.V.; Vita, J.A.; Semenkovich, C.F. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J. Biol. Chem. 2011, 286, 2933–2945. [Google Scholar] [CrossRef]
- Patella, F.; Schug, Z.T.; Persi, E.; Neilson, L.J.; Erami, Z.; Avanzato, D.; Maione, F.; Hernandez-Fernaud, J.R.; Mackay, G.; Zheng, L.; et al. Proteomics-based metabolic modeling reveals that fatty acid oxidation (FAO) controls endothelial cell (EC) permeability. Mol. Cell. Proteom. 2015, 14, 621–634. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Yang, M.; Li, X.; Tan, J.; Wu, Y.; Zhang, Y.; Li, Y.; Hu, B.; Deng, S.; et al. Pigment Epithelial-Derived Factor Deficiency Accelerates Atherosclerosis Development via Promoting Endothelial Fatty Acid Uptake in Mice With Hyperlipidemia. J. Am. Heart Assoc. 2019, 8, e013028. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Cacicedo, J.M.; Itani, S.; Yagihashi, N.; Saha, A.K.; Ye, J.M.; Chen, K.; Zou, M.; Carling, D.; Boden, G.; et al. Malonyl-CoA and AMP-activated protein kinase (AMPK): Possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem. Soc. Trans. 2003, 31, 202–206. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhao, H.; Dong, L.; Zhen, Y.F.; Xing, H.Y.; Ma, H.J.; Song, G.Y. Resveratrol ameliorates high-fat diet-induced insulin resistance and fatty acid oxidation via ATM-AMPK axis in skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9117–9125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, L.; Zhao, C.; Li, J.; Liu, Z.; Zhang, M.; Wang, Y. Resveratrol Maintains Lipid Metabolism Homeostasis via One of the Mechanisms Associated with the Key Circadian Regulator Bmal1. Molecules 2019, 24, 2916. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Peng, Y.; Xiang, R.; Li, X.; Li, Z. Ox-LDL Causes Endothelial Cell Injury Through ASK1/NLRP3-Mediated Inflammasome Activation via Endoplasmic Reticulum Stress. Drug Des. Dev. Ther. 2020, 14, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2009, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ke, L.; Sun, Y.; Li, W.; Feng, X.; Zhu, W.; Chen, S. Resveratrol promotes white adipocytes browning and improves metabolic disorders in Sirt1-dependent manner in mice. FASEB J. 2020, 34, 4527–4539. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Li, W.; Shan, Z.; Zhu, W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can. J. Physiol. Pharmacol. 2011, 89, 793–799. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, S.; Li, Z.; Zhao, X.; Li, W.; Sun, Y.; Zhang, Z.; Ling, W.; Feng, X. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr. Metab. 2014, 11, 35. [Google Scholar] [CrossRef]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, H.S.; Ha, J.M.; Jin, S.Y.; Kum, H.J.; Vafaeinik, F.; Ha, H.K.; Song, S.H.; Kim, C.D.; Bae, S.S. Modulation of Vascular Smooth Muscle Cell Phenotype by High Mobility Group AT-Hook 1. J. Lipid Atheroscler. 2021, 10, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Araim, O.; Ballantyne, J.; Waterhouse, A.L.; Sumpio, B.E. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J. Vasc. Surg. 2002, 35, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Mnjoyan, Z.H.; Fujise, K. Profound negative regulatory effects by resveratrol on vascular smooth muscle cells: A role of p53-p21(WAF1/CIP1) pathway. Biochem. Biophys. Res. Commun. 2003, 311, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lim, S.C.; Lee, M.Y.; Lee, J.W.; Oh, W.K.; Kim, S.K.; Kang, K.W. Inhibition of neointimal formation by trans-resveratrol: Role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol. Nutr. Food. Res. 2010, 54, 1497–1505. [Google Scholar] [CrossRef]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Brüning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Osman, I.; He, X.; Liu, J.; Dong, K.; Wen, T.; Zhang, F.; Yu, L.; Hu, G.; Xin, H.; Zhang, W.; et al. TEAD1 (TEA Domain Transcription Factor 1) Promotes Smooth Muscle Cell Proliferation Through Upregulating SLC1A5 (Solute Carrier Family 1 Member 5)-Mediated Glutamine Uptake. Circ. Res. 2019, 124, 1309–1322. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Kovamees, O.; Shemyakin, A.; Eriksson, M.; Angelin, B.; Pernow, J. Arginase inhibition improves endothelial function in patients with familial hypercholesterolaemia irrespective of their cholesterol levels. J. Intern. Med. 2016, 279, 477–484. [Google Scholar] [CrossRef]
- Villalba, N.; Sackheim, A.M.; Nunez, I.A.; Hill-Eubanks, D.C.; Nelson, M.T.; Wellman, G.C.; Freeman, K. Traumatic Brain Injury Causes Endothelial Dysfunction in the Systemic Microcirculation through Arginase-1-Dependent Uncoupling of Endothelial Nitric Oxide Synthase. J. Neurotrauma 2017, 34, 192–203. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Cheng, A.; Xu, G.; He, F. Metabolism of vascular smooth muscle cells in vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H613–H631. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; Yang, Y. Resveratrol Prevents Retinal Dysfunction by Regulating Glutamate Transporters, Glutamine Synthetase Expression and Activity in Diabetic Retina. Neurochem. Res. 2016, 41, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, W.; Liu, B.; Li, S.; Zhang, B.; Xu, Y. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Med. Sci. Monit. Basic Res. 2014, 20, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, Z.; Yuan, J.; Chen, X.; Huang, Z.; Wu, Z. Resveratrol improves endothelial dysfunction and attenuates atherogenesis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2019, 67, 63–71. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Kurose, I.; Wolf, R.; Grisham, M.B.; Granger, D.N. Effects of an endogenous inhibitor of nitric oxide synthesis on postcapillary venules. Am. J. Physiol. 1995, 268, H2224–H2231. [Google Scholar] [CrossRef]
- Tabatabaie, M.; Abdollahi, S.; Salehi-Abargouei, A.; Clark, C.C.T.; Karimi-Nazari, E.; Fallahzadeh, H.; Rahmanian, M.; Mozaffari-Khosravi, H. The effect of resveratrol supplementation on serum levels of asymmetric de-methyl-arginine and paraoxonase 1 activity in patients with type 2 diabetes: A randomized, double-blind controlled trial. Phytother. Res. 2020, 34, 2023–2031. [Google Scholar] [CrossRef]
- Choi, C.I.; Koo, B.H.; Hong, D.; Kwon, H.J.; Hoe, K.L.; Won, M.H.; Kim, Y.M.; Lim, H.K.; Ryoo, S. Resveratrol is an arginase inhibitor contributing to vascular smooth muscle cell vasoconstriction via increasing cytosolic calcium. Mol. Med. Rep. 2019, 19, 3767–3774. [Google Scholar] [CrossRef]
- Göçmen, A.Y.; Burgucu, D.; Gümüşlü, S. Effect of resveratrol on platelet activation in hypercholesterolemic rats: CD40-CD40L system as a potential target. Appl. Physiol. Nutr. Metab. 2011, 36, 323–330. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Ren, B.; Zhao, Q.; Zhang, F. The Effect of Resveratrol on Blood Glucose and Blood Lipids in Rats with Gestational Diabetes Mellitus. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 2956795. [Google Scholar] [CrossRef]
- Jeon, S.M.; Lee, S.A.; Choi, M.S. Antiobesity and vasoprotective effects of resveratrol in apoE-deficient mice. J. Med. Food 2014, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2020, 242, 117212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Chen, D.W.; He, J.; Zheng, P.; Yu, J.; Mao, X.B.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Yu, B. Long-term dietary resveratrol supplementation decreased serum lipids levels, improved intramuscular fat content, and changed the expression of several lipid metabolism-related miRNAs and genes in growing-finishing pigs1. J. Anim. Sci. 2019, 97, 1745–1756. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Asghari, S.; Rafraf, M.; Asghari-Jafarabadi, M.; Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2020, 90, 279–289. [Google Scholar] [CrossRef]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.; Schechtman, K.B.; Gu, C.; Kunz, I.; Rossi Fanelli, F.; Patterson, B.W.; et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef]
- Mansur, A.P.; Roggerio, A.; Goes, M.F.S.; Avakian, S.D.; Leal, D.P.; Maranhão, R.C.; Strunz, C.M.C. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int. J. Cardiol. 2017, 227, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Amato, B.; Compagna, R.; Amato, M.; Gallelli, L.; de Franciscis, S.; Serra, R. Aterofisiol(®) in carotid plaque evolution. Drug Des. Dev. Ther. 2015, 9, 3877–3884. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Diaz, M.; Avila, A.; Degens, H.; Coeckelberghs, E.; Vanhees, L.; Cornelissen, V.; Azzawi, M. Acute resveratrol supplementation in coronary artery disease: Towards patient stratification. Scand. Cardiovasc. J. SCJ 2020, 54, 14–19. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Han, X.; Fu, S.; Zhu, C.; Chen, Q. Efficacy of Resveratrol Supplementation on Glucose and Lipid Metabolism: A Meta-Analysis and Systematic Review. Front. Physiol. 2022, 13, 795980. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.; Zhang, X.; Liu, Z.; Zhang, W.; Mao, W.; Wang, W.; Cui, W.; Zhang, X.; Jia, X.; et al. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin. Exp. Pharmacol. Physiol. 2005, 32, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Fenning, A.; Iyer, A.; Hoey, A.; Brown, L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr. Pharm. Biotechnol. 2011, 12, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Lisboa, P.C.; Lima, N.S.; Amaral, T.A.; Peixoto-Silva, N.; Resende, A.C.; Oliveira, E.; Passos, M.C.; Moura, E.G. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013, 24, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.N.; Luksha, L.; Logman, H.; Poston, L.; Agewall, S.; Kublickiene, K. Acute responses to phytoestrogens in small arteries from men with coronary heart disease. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1969–H1975. [Google Scholar] [CrossRef]
- Rakici, O.; Kiziltepe, U.; Coskun, B.; Aslamaci, S.; Akar, F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int. J. Cardiol. 2005, 105, 209–215. [Google Scholar] [CrossRef]
- Wong, R.H.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef]
- Marques, B.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef]
- Biesinger, S.; Michaels, H.A.; Quadros, A.S.; Qian, Y.; Rabovsky, A.B.; Badger, R.S.; Jalili, T. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur. J. Clin. Nutr. 2016, 70, 10–16. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef]
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F.G. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Téllez, V.; Villegas-Romero, M.; Rubio-Ruiz, M.E.; Pérez-Torres, I.; Carreón-Torres, E.; Díaz-Díaz, E.; Guarner-Lans, V. Effect of a Resveratrol/Quercetin Mixture on the Reversion of Hypertension Induced by a Short-Term Exposure to High Sucrose Levels Near Weaning and a Long-Term Exposure That Leads to Metabolic Syndrome in Rats. Int. J. Mol. Sci. 2020, 21, 2231. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, S.; Tan, C.H.; Cao, S.; Xu, X.; Saw, P.E.; Tao, W. Nanocarriers in the Enhancement of Therapeutic Efἀcacy of Natural Drugs. BIO Integr. 2021, 2, 40–49. [Google Scholar] [CrossRef]
- Arrick, D.M.; Sun, H.; Patel, K.P.; Mayhan, W.G. Chronic resveratrol treatment restores vascular responsiveness of cerebral arterioles in type 1 diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H696–H703. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.M.; Su, M.J.; Chen, J.K. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic. Biol. Med. 2004, 36, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Liu, C.; Guo, Z.; Huang, K.; Peng, M.; Li, N.; Luo, H.; Wang, T.; Cen, Z.; Cai, W.; et al. Resveratrol Promotes Angiogenesis in a FoxO1-Dependent Manner in Hind Limb Ischemia in Mice. Molecules 2021, 26, 7528. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Chen, J.; Bai, Q.; Zhao, Z.; Sui, H.; Xie, X. Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurol. Scand. 2016, 134, 54–60. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef]
- McDermott, M.M.; Leeuwenburgh, C.; Guralnik, J.M.; Tian, L.; Sufit, R.; Zhao, L.; Criqui, M.H.; Kibbe, M.R.; Stein, J.H.; Lloyd-Jones, D.; et al. Effect of Resveratrol on Walking Performance in Older People With Peripheral Artery Disease: The RESTORE Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Gögebakan, Ö.; Redlich, U.; Tautenhahn, J.; Ricke, J.; Halloul, Z.; Meyer, D.R.; Waliszewski, M.; Schnorr, B.; Zeller, T.; et al. Angiographic and Clinical Outcomes After Treatment of Femoro-Popliteal Lesions with a Novel Paclitaxel-Matrix-Coated Balloon Catheter. Cardiovasc. Interv. Radiol. 2017, 40, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Waliszewski, M.; Roca, C.; Redlich, U.; Tautenhahn, J.; Pech, M.; Halloul, Z.; Gögebakan, Ö.; Meyer, D.R.; Gemeinhardt, I.; et al. Two-Year Clinical Outcomes of the CONSEQUENT Trial: Can Femoropopliteal Lesions be Treated with Sustainable Clinical Results that are Economically Sound? Cardiovasc. Interv. Radiol. 2018, 41, 1008–1014. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhang, Z.; Li, W.; Sun, Y.; Zhang, Q.; Feng, X.; Zhu, W. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can. J. Physiol. Pharmacol. 2012, 90, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, X.; Feng, X.; Li, W.; Zhou, N.; Zheng, L.; Sun, Y.; Zhang, Z.; Zhu, W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J. Nutr. Biochem. 2012, 23, 1100–1112. [Google Scholar] [CrossRef]
- Knop, F.K.; Konings, E.; Timmers, S.; Schrauwen, P.; Holst, J.J.; Blaak, E.E. Thirty days of resveratrol supplementation does not affect postprandial incretin hormone responses, but suppresses postprandial glucagon in obese subjects. Diabet. Med. 2013, 30, 1214–1218. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Abdollahi, S.; Salehi-Abargouei, A.; Toupchian, O.; Sheikhha, M.H.; Fallahzadeh, H.; Rahmanian, M.; Tabatabaie, M.; Mozaffari-Khosravi, H. The Effect of Resveratrol Supplementation on Cardio-Metabolic Risk Factors in Patients with Type 2 Diabetes: A Randomized, Double-Blind Controlled Trial. Phytother. Res. 2019, 33, 3153–3162. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.K.; Nellemann, B.; Bibby, B.M.; Stødkilde-Jørgensen, H.; Pedersen, S.B.; Grønbaek, H.; Nielsen, S. No effect of resveratrol on VLDL-TG kinetics and insulin sensitivity in obese men with nonalcoholic fatty liver disease. Diabetes Obes. Metab. 2018, 20, 2504–2509. [Google Scholar] [CrossRef]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Storgaard, J.H.; Løkken, N.; Madsen, K.L.; Voermans, N.C.; Laforêt, P.; Nadaj-Pakleza, A.; Tard, C.; van Hall, G.; Vissing, J.; Ørngreen, M.C. No effect of resveratrol on fatty acid oxidation or exercise capacity in patients with fatty acid oxidation disorders: A randomized clinical cross-over trial. J. Inherit. Metab. Dis. 2022, 45, 517–528. [Google Scholar] [CrossRef]
- Boswijk, E.; de Ligt, M.; Habets, M.J.; Mingels, A.M.A.; van Marken Lichtenbelt, W.D.; Mottaghy, F.M.; Schrauwen, P.; Wildberger, J.E.; Bucerius, J. Resveratrol treatment does not reduce arterial inflammation in males at risk of type 2 diabetes: A randomized crossover trial. Nukl. Nucl. Med. 2022, 61, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, L.; Zoller, T.; Atkinson, R.L.; Nisi, F.; Antoniazzi, F.; Cavarzere, P.; Piacentini, G.; Pietrobelli, A. Supportive treatment of vascular dysfunction in pediatric subjects with obesity: The OBELIX study. Nutr. Diabetes 2022, 12, 2. [Google Scholar] [CrossRef]
- Vors, C.; Couillard, C.; Paradis, M.E.; Gigleux, I.; Marin, J.; Vohl, M.C.; Couture, P.; Lamarche, B. Supplementation with Resveratrol and Curcumin Does Not Affect the Inflammatory Response to a High-Fat Meal in Older Adults with Abdominal Obesity: A Randomized, Placebo-Controlled Crossover Trial. J. Nutr. 2018, 148, 379–388. [Google Scholar] [CrossRef]
- van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef]
- van der Made, S.M.; Plat, J.; Mensink, R.P. Trans-Resveratrol Supplementation and Endothelial Function during the Fasting and Postprandial Phase: A Randomized Placebo-Controlled Trial in Overweight and Slightly Obese Participants. Nutrients 2017, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef] [PubMed]
- Sansbury, B.E.; Spite, M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef] [PubMed]
| Cohort (No.) | Dose and Duration of RSV | Main Outcome after RSV Administration | First Author, Year, Reference |
|---|---|---|---|
| Healthy, obese men (n = 11) treated with placebo and RSV | 150 mg/day, 30 days | plasma TG ↓ (2.16 ± 0.21 vs. 2.29 ± 0.23 mmol/L in RSV vs. placebo); intrahepatic lipid content ↓; non-esterified fatty acids →. | Timmers, 2011, [65] |
| Healthy smokers (n = 50) treated with placebo and RSV | 500 mg/day, 30 days | plasma TG ↓; plasma TC and HDL-C →. | Bo, 2013, [66] |
| Patients at risk for cardiovascular disease randomized into placebo (n = 25), grape extract (n = 25) and RSV-enriched grape extract (n = 25) groups | containing ~8 mg RSV/day, 6 months | plasma ox-LDL and LDL-C ↓ (decreasing oxidized-LDL by 20% and decreasing LDL-C by 4.5%); plasma TG, TC and HDL-C →. | Tomé-Carneiro, 2012, [67] |
| Type 2 diabetes mellitus patients randomized into control (n = 29) and RSV intervention (n = 28) groups | 250 mg/day, 3 months | plasma TC and LDL-C ↓ (4.70 ± 0.90 vs. 4.33 ± 0.76 mmol/L and 2.58 ± 0.83 vs. 2.26 ± 0.65 mmol/L after RSV intervention, respectively); plasma TG and HDL-C →. | Bhatt, 2012, [68] |
| Patients with nonalcoholic fatty liver disease randomized into placebo (n = 25) and RSV (n = 25) groups | 600 mg/day, 12 weeks | plasma ox-LDL, LDL-C/HDL-C and LDL-C/ox-LDL →. | Farzin, 2020, [69] |
| Nonobese women randomized into placebo (n = 14) and RSV (n = 15) groups | 75 mg/day, 12 weeks | intra-abdominal fat volume, intrahepatic triglyceride content, plasma lipids, and insulin sensitivity in the liver, skeletal muscle and adipose tissue →. | Yoshino, 2012, [70] |
| Healthy participants randomized into RSV (n = 24) and caloric restriction (n = 24) groups | 500 mg/day, 30 days | plasma TG, HDL-C, LDL-C, and apolipoprotein A1 →, plasma TC and non-HDL cholesterol ↑. | Mansur, 2017, [71] |
| Patients with carotid stenosis >70% and in a surgical intervention request randomized to Cardioaspirin® and Aterofisiol® (n = 107) and Cardioaspirin® and placebo (n = 107) groups | containing 20 mg RSV/day, 25 days | dry weight of lipid and cholesterol in removed plaques ↓ (0.232 ± 0.018 vs. 0.356 ± 0.022; 0.036 ± 0.006 vs. 0.053 ± 0.007 mg/mg dry weight, respectively). | Amato, 2015, [72] |
| Patients with type 2 diabetes mellitus and coronary heart disease randomized into placebo (n = 28) and RSV (n = 28) groups | 500 mg/day, 4 weeks | plasma HDL-C ↑; plasma TC/HDL-C ↓; plasma TG, TC and LDL-C →. | Hoseini, 2019, [73] |
| Patients with stable coronary artery disease randomized into placebo (n = 20) and RSV (n = 20) groups | 10 mg/day, 3 months | plasma LDL-C ↓; FMD ↑; plasma TG, TC and LDL-C →. | Magyar, 2012, [74] |
| Patients with stable coronary artery disease randomized into placebo (n = 25), grape extract (n = 25) and RSV-containing grape extract (n = 25) groups | containing ~8 mg RSV/day for 6 months and ~16 mg RSV/day for following 6 months | plasma TC and non-HDL-C ↓; inflammation ↓. | To-mé-Carneiro, 2013, [75] |
| Patients with stable coronary artery disease (n = 10) treated with placebo and RSV | 330 mg/day, 3 days | FMD in patients who had undergone coronary artery bypass grafting ↑; FMD in those who had undergone percutaneous coronary intervention →. | Diaz, 2020, [76] |
| Cohort (No.) | Dose and Duration of RSV | Main Outcome after RSV Administration | First Author, Year, Reference |
|---|---|---|---|
| Overweight/obese individuals with untreated borderline hypertension (n = 19) treated with 30, 90, and 270 mg RSV doses and placebo | 30, 90, and 270 mg, 1 h | FMD ↑ in a dose-dependent manner | Wong, 2011, [86] |
| Hypertensive patients (n = 24) treated with placebo and RSV | 300 mg, acute supplementation | FMD in women and individuals with higher LDL-C ↑. | Marques, 2018, [87] |
| Obese but otherwise healthy adults (n = 28) treated with placebo and RSV | 75 mg/day, 6 weeks | FMD ↑; blood pressure →. | Wong, 2013, [88] |
| Hypertensive individuals (n = 18) treated with placebo and isolated phytochemicals | containing ~60 mg RSV/day, 28 days | diastolic blood pressure ↓; diastolic blood pressure →. | Biesinger, 2016, [89] |
| Cohort (No.) | Dose and Duration of RSV | Main Outcome after RSV Administration | First Author, Year, Reference |
|---|---|---|---|
| Ischemic stroke patients with a clearly defined time of onset randomized to r-tPA plus placebo (early onset-to-treatment time, n = 78; delayed onset-to-treatment time, n = 80) and r-tPA plus RSV (early onset-to-treatment time, n = 77; delayed onset-to-treatment time, n = 77) groups | 2.5 mg RSV/kg of body weight (maximum 250 mg), simultaneously with r-tPA | treatment outcomes in patients receiving delayed r-tPA treatment ↑; plasma matrix metalloproteinase-2 and matrix metalloproteinase-9 in patients receiving early or delayed r-tPA treatment ↓. | Chen, 2016, [98] |
| Healthy individuals (n = 22) treated with placebo and 2 doses of RSV | 250 and 500 mg/day, 45 min | cerebral blood flow during task performance ↑ in a dose-dependent manner | Kennedy, 2010, [99] |
| Patients with stable angina pectoris randomized to RSV (n = 29), calcium fructoborate (n = 29) and RSV plus calcium fructoborate (n = 29) groups | 20 mg/day, 60 days | plasma high-sensitivity C reactive protein, n-terminal prohormone of brain natriuretic peptide, TC, TG and LDL-C ↓; plasma HDL-C ↑. | Militaru, 2013, [100] |
| Older people with peripheral artery disease randomized to placebo (n = 22), 125 mg of RSV (n = 21) and 500 mg of RSV (n = 23) groups | 125 and 500 mg/day, 6 months | 6-min walk distance →. | McDermott, 2017, [101] |
| Patients with peripheral artery disease randomized to plain old balloon angioplasty (n = 75) and RSV drug-coated balloon (n = 78) groups | containing 0.9 µg/mm2, 6 and 12 months | in-lesion late lumen loss at 6 months and target lesion revascularization at 12 months compared to plain old balloon angioplasty group ↓; censored walking distance ↑. | Tepe, 2017, [102] |
| Patients with peripheral artery disease randomized to plain old balloon angioplasty (n = 75) and RSV drug-coated balloon (n = 78) groups | containing 0.9 µg/mm2, 24 months | target lesion revascularization ↓ and walking distance ↑ compared to plain old balloon angioplasty group | Albrecht, 2018, [103] |
| Cohort (No.) | Dose and Duration of RSV | Main Outcome after RSV Administration | First Author, Year, Reference |
|---|---|---|---|
| Obese but otherwise healthy men (n = 10) treated with placebo and RSV | 150 mg/day, 30 days | postprandial plasma glucagon responses ↓; fasting plasma glucagon, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide levels →. | Knop, 2013, [108] |
| Patients with non-alcoholic fatty liver disease randomized into placebo (n = 30) and RSV (n = 30) groups | 600 mg/day, 3 months | plasma TC, LDL-C, glucose, aspartate aminotransferase and alanine aminotransferase ↓; plasma insulin, TG and HDL-C →. | Chen, 2015, [109] |
| Patients with type 2 diabetes randomized into placebo (n = 38) and RSV (n = 38) groups | 1000 mg/day, 8 weeks | plasma glucose ↓; plasma HDL-C ↑; TG, TC and LDL-C →. | Abdollahi, 2019, [110] |
| Individuals at high risk of cardiovascular disease randomized into placebo (n = 25), RSV-rich grape supplement (n = 25) and grape supplement lacking RSV (n = 25) groups | 8 mg/day for 6 months; 16 mg/day for following 6 months | high-sensitivity C-reactive protein, tumor necrosis factor-α, plasminogen activator inhibitor type 1 and interleukin-6/interleukin-10 ratio ↓; anti-inflammatory interleukin-10 ↑. | To-mé-Carneiro, 2012, [111] |
| Obese individuals with nonalcoholic fatty liver disease randomized into placebo (n = 8) and RSV (n = 8) groups | 1500 mg/day, 6 months | basal and insulin-mediated very low-density lipoprotein-TG secretion, oxidation and clearance rates →. | Poulsen, 2018, [112] |
| Patients with well-controlled type 2 diabetes (n = 17) treated with placebo and RSV | 150 mg/day, 30 days | hepatic and peripheral insulin sensitivity and intrahepatic lipid content →. | Timmers, 2016, [113] |
| Patients with type 2 diabetes (n = 14) treated with placebo and RSV | 1000 mg/day, 5 weeks | glucagon-like peptide 1 secretion and glycemic control →. | Thazhath, 2016, [114] |
| Older glucose-intolerant individuals (n = 30) treated with placebo and RSV | 2–3 g/day, 6 weeks | reactive hyperemia index ↑, plasma lipid profiles and blood pressure →. | Pollack, 2017, [115] |
| Patients with fatty acid oxidation (n = 9) treated with placebo and RSV | 1000 mg/day, 4 weeks | fatty acid oxidation and exercise capacity →. | Storgaard, 2022, [116] |
| Patients at risk of developing type 2 diabetes mellitus (n = 8) treated with placebo and RSV | 150 mg/day, 34 days | arterial 18F-fluoroxyglucose uptake and arterial inflammation →. | Boswijk, 2022, [117] |
| Overweight and obese pediatric subjects randomized to placebo (n = 11) and antioxidant supplementation (n = 16) groups | containing 20 mg RSV/day, 6 months | post-occlusive release hyperemic delta flow at 6 months ↑. | Pecoraro, 2022, [118] |
| Older adults with abdominal obesity (n = 22) treated with placebo and RSV plus curcumin | containing 200 mg RSV, 30 min before consuming the high-fat meal | cumulative postprandial response of soluble vascular cell adhesion molecule-1 ↓; circulating inflammatory markers and adhesion molecules →. | Vors, 2018, [119] |
| Overweight and slightly obese individuals (n = 45) treated with placebo and RSV | 150 mg/day, 4 weeks | plasma lipid profiles, glucose, insulin, and markers for inflammation and endothelial function →. | van der Made, 2015, [120] |
| Overweight and slightly obese individuals (n = 45) treated with placebo and RSV | 150 mg/day, 4 weeks | plasma lipid profiles, glucose, insulin, and markers for inflammation and endothelial function in the fasting state or postprandial phase →. | van der Made, 2017, [121] |
| Overweight older individuals randomized into placebo (n = 10), 300 mg RSV (n = 10), and 1000 mg RSV (n = 9) groups | 300 and 1000 mg/day, 90 days | soluble vascular cell adhesion molecule-1 and total plasminogen activator inhibitor ↑ in the 1000 mg RSV vs. 300 mg RSV and placebo groups | Mankowski, 2020, [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Liu, C.; Zhang, Z.; Huang, K.; Wang, T.; Chen, S.; Li, Z. Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease. Molecules 2022, 27, 7524. https://doi.org/10.3390/molecules27217524
Fan D, Liu C, Zhang Z, Huang K, Wang T, Chen S, Li Z. Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease. Molecules. 2022; 27(21):7524. https://doi.org/10.3390/molecules27217524
Chicago/Turabian StyleFan, Dongxiao, Chenshu Liu, Zhongyu Zhang, Kan Huang, Tengyao Wang, Sifan Chen, and Zilun Li. 2022. "Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease" Molecules 27, no. 21: 7524. https://doi.org/10.3390/molecules27217524
APA StyleFan, D., Liu, C., Zhang, Z., Huang, K., Wang, T., Chen, S., & Li, Z. (2022). Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease. Molecules, 27(21), 7524. https://doi.org/10.3390/molecules27217524







