Valorization of Different Fractions from Butiá Pomace by Pyrolysis: H2 Generation and Use of the Biochars for CO2 Capture
Abstract
1. Introduction
2. Results and Discussion
2.1. Features of the Butiá Precursors
2.2. Results of the Pyrolysis Process
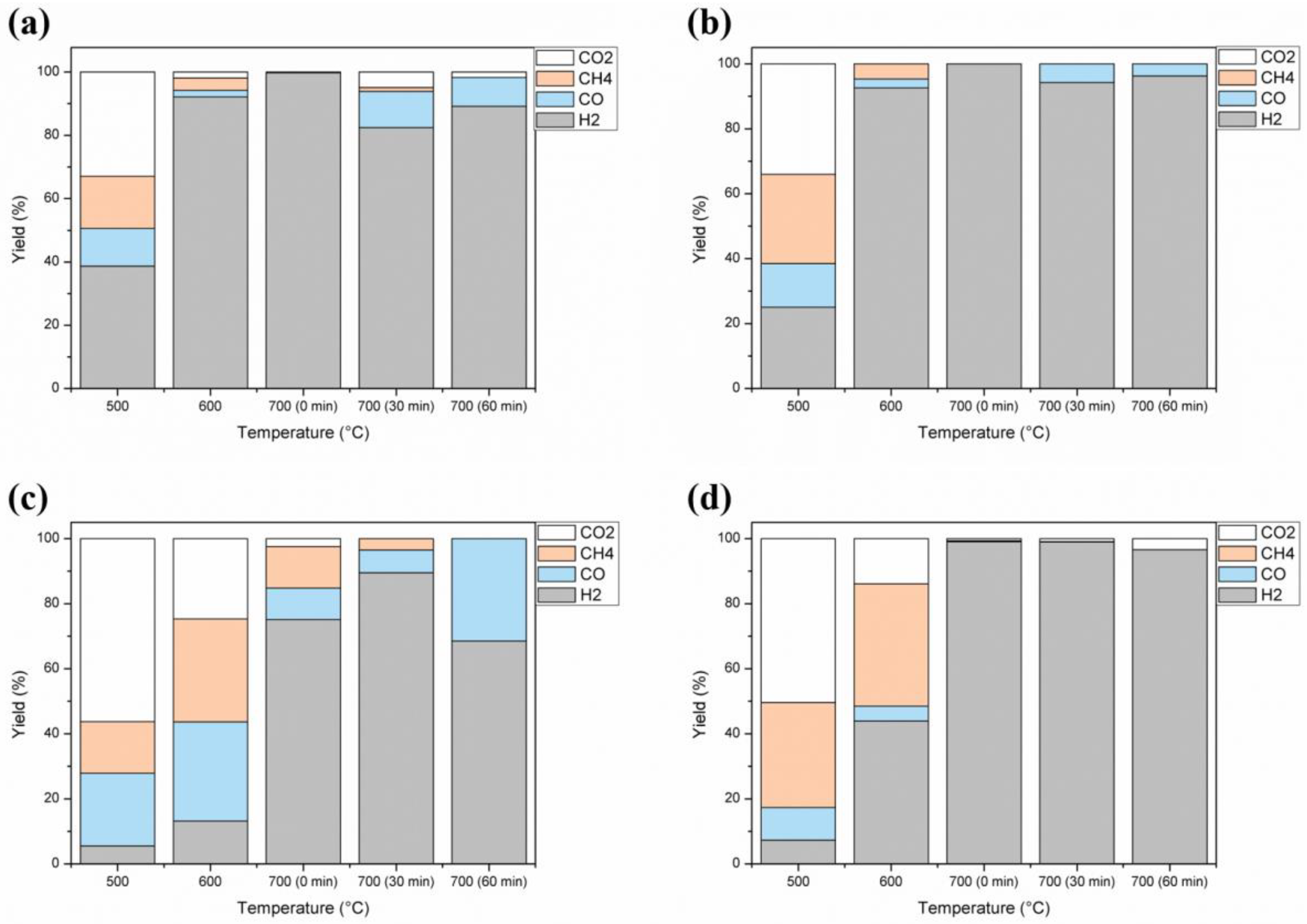
2.3. Non-Condensable Gases Generation
2.4. Biochars Characteristics
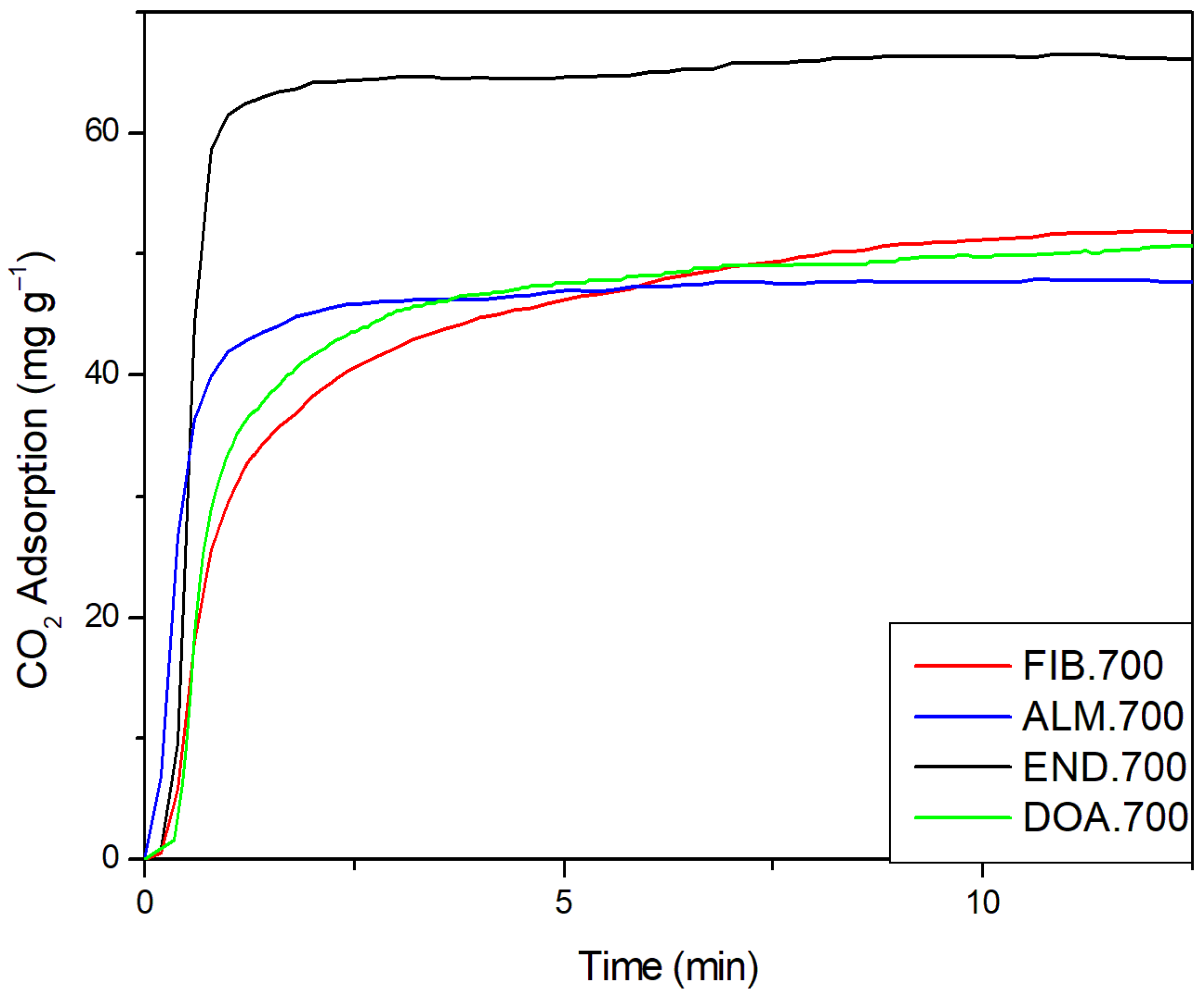
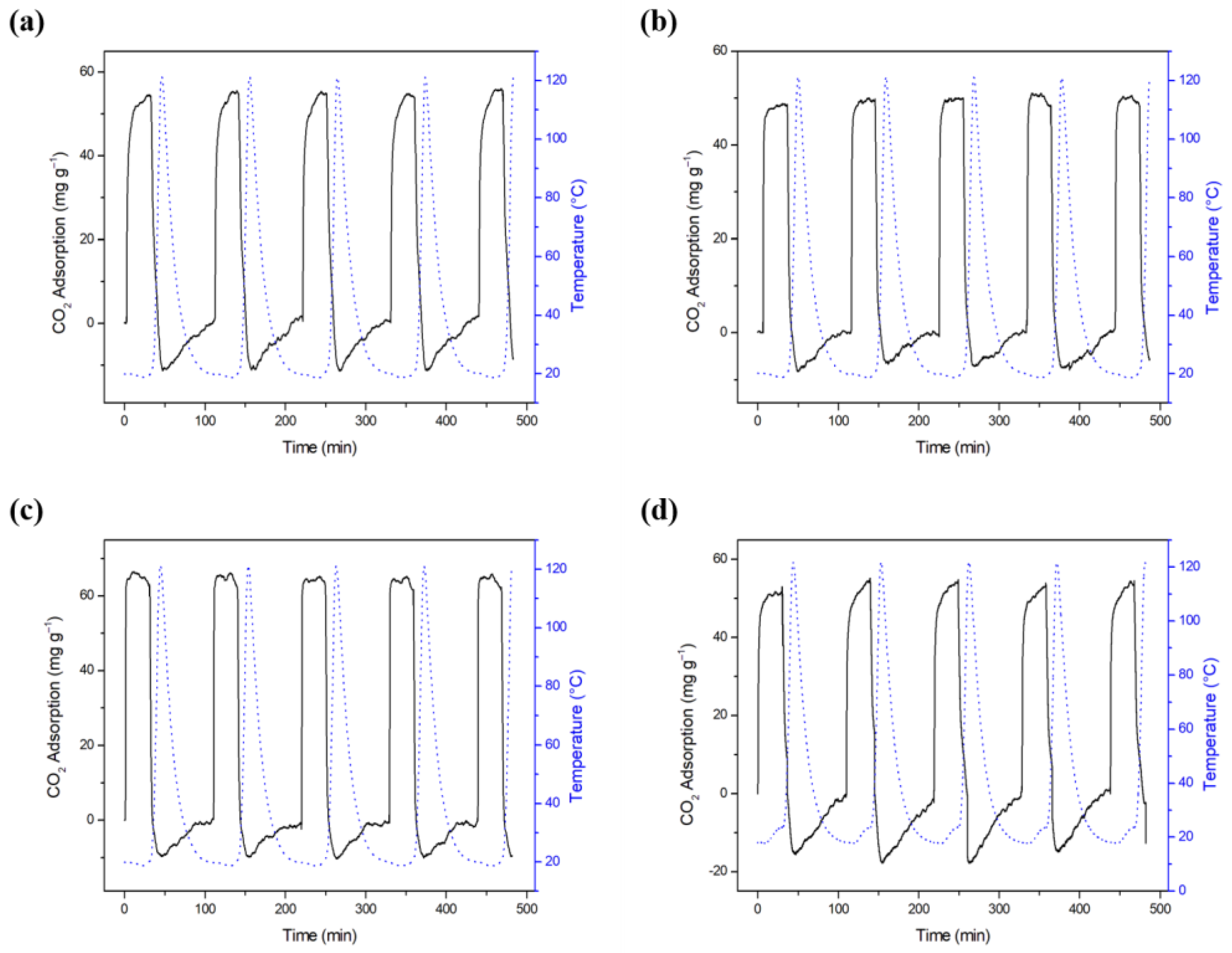
2.5. Results of CO2 Capture on the Biochars
3. Material and Methods
3.1. Obtainment and Pre-Treatment of the Precursors
3.2. Characterization of the Precursors
3.3. Pyrolysis Process
3.4. Non-Condensable Gases Characterization
3.5. Biochars Characterization
3.6. Biochars Potential for CO2 Capture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ibrahim, M.; Vo, X.V. Exploring the Relationships among Innovation, Financial Sector Development and Environmental Pollution in Selected Industrialized Countries. J. Environ. Manage. 2021, 284, 112057. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Fang, M.; Liu, B.; Chen, R.; Shi, R.; Wu, Q.; Zeng, Z.; Li, L. In-Situ Activated Ultramicroporous Carbon Materials Derived from Waste Biomass for CO2 Capture and Benzene Adsorption. Biomass Bioenergy 2022, 158, 106353. [Google Scholar] [CrossRef]
- Bergstra, A.D.; Brunekreef, B.; Burdorf, A. The Influence of Industry-Related Air Pollution on Birth Outcomes in an Industrialized Area. Environ. Pollut. 2021, 269, 115741. [Google Scholar] [CrossRef]
- Bernstein, A.S.; Rice, M.B. Lungs in a Warming World: Climate Change and Respiratory Health. Chest 2013, 143, 1455–1459. [Google Scholar] [CrossRef]
- Horemans, J.A.; Janssens, I.A.; Gielen, B.; Roland, M.; Deckmyn, G.; Verstraeten, A.; Neirynck, J.; Ceulemans, R. Weather, Pollution and Biotic Factors Drive Net Forest—Atmosphere Exchange of CO2 at Different Temporal Scales in a Temperate-Zone Mixed Forest. Agric. For. Meteorol. 2020, 291, 108059. [Google Scholar] [CrossRef]
- Lelandais, L.; Xueref-Remy, I.; Riandet, A.; Blanc, P.E.; Armengaud, A.; Oppo, S.; Yohia, C.; Ramonet, M.; Delmotte, M. Analysis of 5.5 Years of Atmospheric CO2, CH4, CO Continuous Observations (2014–2020) and Their Correlations, at the Observatoire de Haute Provence, a Station of the ICOS-France National Greenhouse Gases Observation Network. Atmos. Environ. 2022, 277, 119020. [Google Scholar] [CrossRef]
- Bao, J.; Lu, W.H.; Zhao, J.; Bi, X.T. Greenhouses for CO2 Sequestration from Atmosphere. Carbon Resour. Convers. 2018, 1, 183–190. [Google Scholar] [CrossRef]
- Khalidy, R.; Santos, R.M. The Fate of Atmospheric Carbon Sequestrated through Weathering in Mine Tailings. Miner. Eng. 2021, 163, 106767. [Google Scholar] [CrossRef]
- Breuer, J.L.; Samsun, R.C.; Stolten, D.; Peters, R. How to Reduce the Greenhouse Gas Emissions and Air Pollution Caused by Light and Heavy Duty Vehicles with Battery-Electric, Fuel Cell-Electric and Catenary Trucks. Environ. Int. 2021, 152, 106474. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Chu, C.; Fu, T.; Ma, Y. Mass Transfer and Capture of Carbon Dioxide Using Amino Acids Sodium Aqueous Solution in Microchannel. Chem. Eng. Process.-Process Intensif. 2022, 173, 108831. [Google Scholar] [CrossRef]
- Nasrifar, K.; Moshfeghian, M. Thermodynamics of Carbon Dioxide Mixtures at Cryogenic Conditions. Cryogenics 2022, 121, 103404. [Google Scholar] [CrossRef]
- Singh, S.; Varghese, A.M.; Reinalda, D.; Karanikolos, G.N. Graphene—Based Membranes for Carbon Dioxide Separation. J. CO2 Util. 2021, 49, 101544. [Google Scholar] [CrossRef]
- Pu, Q.; Zou, J.; Wang, J.; Lu, S.; Ning, P.; Huang, L.; Wang, Q. Systematic Study of Dynamic CO2 Adsorption on Activated Carbons Derived from Different Biomass. J. Alloys Compd. 2021, 887, 161406. [Google Scholar] [CrossRef]
- Pires, J.; Juźków, J.; Pinto, M.L. Amino Acid Modified Montmorillonite Clays as Sustainable Materials for Carbon Dioxide Adsorption and Separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 105–110. [Google Scholar] [CrossRef]
- Surra, E.; Ribeiro, R.P.P.L.; Santos, T.; Bernardo, M.; Mota, J.P.B.; Lapa, N.; Esteves, I.A.A.C. Evaluation of Activated Carbons Produced from Maize Cob Waste for Adsorption-Based CO2 Separation and Biogas Upgrading. J. Environ. Chem. Eng. 2022, 10, 107065. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, L.; Wang, W.; Shen, Z.; Liu, S.; Li, X.; Wang, Y. Constructing Highly Porous Carbon Materials from Porous Organic Polymers for Superior CO2 Adsorption and Separation. J. Colloid Interface Sci. 2022, 609, 775–784. [Google Scholar] [CrossRef]
- An, X.; Zhao, K.; Zhang, W.; Yang, J.; Liao, Y.; Wang, L.; Fu, D. Tailoring the Pore Structure Modified with Functional Groups for Superior CO2 Adsorption Capacity and the Selectivity of Separation. Fuel 2022, 309, 122175. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Feng, Y.; Chen, Y.; Wang, X.; Chen, H. Nitrogen Enriched Biochar Modified by High Temperature CO2-Ammonia Treatment: Characterization and Adsorption of CO2. Chem. Eng. J. 2014, 257, 20–27. [Google Scholar] [CrossRef]
- Li, S.; Yuan, X.; Deng, S.; Zhao, L.; Lee, K.B. A Review on Biomass-Derived CO2 Adsorption Capture: Adsorbent, Adsorber, Adsorption, and Advice. Renew. Sustain. Energy Rev. 2021, 152, 111708. [Google Scholar] [CrossRef]
- Goel, C.; Mohan, S.; Dinesha, P. CO2 Capture by Adsorption on Biomass-Derived Activated Char: A Review. Sci. Total Environ. 2021, 798, 149296. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Yusoff, R. Adsorption of CO2 on Palm Shell Based Activated Carbon Modified by Deep Eutectic Solvent: Breakthrough Adsorption Study. J. Environ. Chem. Eng. 2021, 9, 105333. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Venkatesh, K.; N, K.; Gundanna, S.K.; Bhatta, U.M. Synthesis and Characterization of Activated Carbon from Delonix Regia Seeds for CO2 Adsorption. Energy Clim. Chang. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bud, J.; Byambajav, E.; Tsubouchi, N. Influence of Ammonia Treatment on the CO2 Adsorption of Activated Carbon. J. Environ. Chem. Eng. 2022, 10, 107273. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.J. Valorization of Shrimp Shell Biowaste for Environmental Remediation: Efficient Contender for CO2 Adsorption and Separation. J. Environ. Manag. 2021, 299, 113661. [Google Scholar] [CrossRef]
- Li, J.; Bao, A.; Chen, J.; Bao, Y. A Green Route to CO2 Adsorption on Biomass Chitosan Derived Nitrogen-Doped Micropore-Dominated Carbon Nanosheets by Different Activators. J. Environ. Chem. Eng. 2022, 10, 107021. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.W.; Kim, S.; Kang, Y.T. CO2 Adsorption on Zeolite 13X Modified with Hydrophobic Octadecyltrimethoxysilane for Indoor Application. J. Clean. Prod. 2022, 337, 130597. [Google Scholar] [CrossRef]
- Gan, F.; Wang, B.; Jin, Z.; Xie, L.; Dai, Z.; Zhou, T.; Jiang, X. From Typical Silicon-Rich Biomass to Porous Carbon-Zeolite Composite: A Sustainable Approach for Efficient Adsorption of CO2. Sci. Total Environ. 2021, 768, 144529. [Google Scholar] [CrossRef]
- Aquatar, M.O.; Bhatia, U.; Rayalu, S.S.; Krupadam, R.J. Reduced Graphene Oxide -MnO2 Nanocomposite for CO2 Capture from Flue Gases at Elevated Temperatures. Sci. Total Environ. 2022, 816, 151522. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A Review on Production of Metal Organic Frameworks (MOF) for CO2 Adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Daud, N.K.; Najib, N.H.I.M. Adsorption of CO2 on ZSM-5 and Cu-MOF at Room Temperature and Low Pressure Conditions for Carbon Capture and Storage (CCS) Application. Mater. Today Proc. 2022, 57, 1345–1355. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, X.; Xu, Y.; Xiang, W.; Wang, R.; Ding, F.; Hong, P.; Gao, B. Straw and Wood Based Biochar for CO2 Capture: Adsorption Performance and Governing Mechanisms. Sep. Purif. Technol. 2022, 287, 120592. [Google Scholar] [CrossRef]
- Lampropoulos, A.; Kaklidis, N.; Athanasiou, C.; Montes-Morán, M.A.; Arenillas, A.; Menéndez, J.A.; Binas, V.D.; Konsolakis, M.; Marnellos, G.E. Effect of Olive Kernel Thermal Treatment (Torrefaction vs. Slow Pyrolysis) on the Physicochemical Characteristics and the CO2 or H2O Gasification Performance of as-Prepared Biochars. Int. J. Hydrogen Energy 2021, 46, 29126–29141. [Google Scholar] [CrossRef]
- Xie, W.-H.; Li, H.; Yang, M.; He, L.-N.; Li, H.-R. CO2 Capture and Utilization with Solid Waste. Green Chem. Eng. 2022, 3, 199–209. [Google Scholar] [CrossRef]
- Perondi, D.; Poletto, P.; Restelatto, D.; Manera, C.; Silva, J.P.; Junges, J.; Collazzo, G.C.; Dettmer, A.; Godinho, M.; Vilela, A.C.F. Steam Gasification of Poultry Litter Biochar for Bio-Syngas Production. Process Saf. Environ. Prot. 2017, 109, 478–488. [Google Scholar] [CrossRef]
- Li, Z.; Xing, B.; Ding, Y.; Li, Y.; Wang, S. A High-Performance Biochar Produced from Bamboo Pyrolysis with in-Situ Nitrogen Doping and Activation for Adsorption of Phenol and Methylene Blue. Chinese J. Chem. Eng. 2020, 28, 2872–2880. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Ahmad Farid, M.A.; Tengku Yasim-Anuar, T.A.; Samsudin, M.H.; Mohd Yusoff, M.Z.; Zakaria, M.R.; Mokhtar, M.N.; Shirai, Y. Adsorption Mechanism and Effectiveness of Phenol and Tannic Acid Removal by Biochar Produced from Oil Palm Frond Using Steam Pyrolysis. Environ. Pollut. 2021, 269, 116197. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar Carbon Materials for CO2 Capture and Sequestration by Cyclic Adsorption Processes: A Review and Prospects for Future Directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Shafawi, A.N.; Mohamed, A.R.; Lahijani, P.; Mohammadi, M. Recent Advances in Developing Engineered Biochar for CO2 Capture: An Insight into the Biochar Modification Approaches. J. Environ. Chem. Eng. 2021, 9, 106869. [Google Scholar] [CrossRef]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.S.A.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Ok, Y.S.; Wu, K.C.W.; Hou, C.H. A Critical Review on Biochar-Based Engineered Hierarchical Porous Carbon for Capacitive Charge Storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Wan, J.; Liu, L.; Ayub, K.S.; Zhang, W.; Shen, G.; Hu, S.; Qian, X. Characterization and Adsorption Performance of Biochars Derived from Three Key Biomass Constituents. Fuel 2020, 269, 117142. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Removal of Copper and Lead Using Banana Biochar in Batch Adsorption Systems: Isotherms and Kinetic Studies. Arab. J. Sci. Eng. 2018, 43, 5711–5722. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Silva-Gordillo, M.D.M.; Muñoz, G.A.; Arboleda-Faini, X.; Almeida Streitwieser, D. Comparison of the Adsorption Capacity of Organic Compounds Present in Produced Water with Commercially Obtained Walnut Shell and Residual Biomass. J. Environ. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, C.; Yan, Z.; Jiang, H.; Xu, H. Magnetic Particles Modification of Coconut Shell-Derived Activated Carbon and Biochar for Effective Removal of Phenol from Water. Chemosphere 2018, 211, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; da Boit Martinello, K.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Yilmaz, M.; Silva, L.F.O.; Dotto, G.L. Residual Peel of Pitaya Fruit (Hylocereus Undatus) as a Precursor to Obtaining an Efficient Carbon-Based Adsorbent for the Removal of Metanil Yellow Dye from Water. J. Environ. Chem. Eng. 2022, 10, 107006. [Google Scholar] [CrossRef]
- Lang, J.; Matějová, L.; Cuentas-Gallegos, A.K.; Lobato-Peralta, D.R.; Ainassaari, K.; Gómez, M.M.; Solís, J.L.; Mondal, D.; Keiski, R.L.; Cruz, G.J.F. Evaluation and Selection of Biochars and Hydrochars Derived from Agricultural Wastes for the Use as Adsorbent and Energy Storage Materials. J. Environ. Chem. Eng. 2021, 9, 105979. [Google Scholar] [CrossRef]
- El-gamal, E.H.; Saleh, M.; Elsokkary, I.; Rashad, M.; El-latif, M.M.A. Comparison between Properties of Biochar Produced by Traditional and Controlled Pyrolysis Comparison between Properties of Biochar Produced by Traditional and Controlled Pyrolysis. Alex. Sci. Exch. J. 2017, 38, 412–425. [Google Scholar]
- Tomul, F.; Arslan, Y.; Kabak, B.; Trak, D.; Kendüzler, E.; Lima, E.C.; Tran, H.N. Peanut Shells-Derived Biochars Prepared from Different Carbonization Processes: Comparison of Characterization and Mechanism of Naproxen Adsorption in Water. Sci. Total Environ. 2020, 726, 137828. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, Y.; Dang, J.; Zhao, J.; Chen, S. Magnetic Apple Pomace Biochar: Simple Preparation, Characterization, and Application for Enriching Ag(I) in Effluents. Sci. Total Environ. 2019, 668, 115–123. [Google Scholar] [CrossRef]
- Mohammed, N.A.S.; Abu-Zurayk, R.A.; Hamadneh, I.; Al-Dujaili, A.H. Phenol Adsorption on Biochar Prepared from the Pine Fruit Shells: Equilibrium, Kinetic and Thermodynamics Studies. J. Environ. Manag. 2018, 226, 377–385. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar Derived from Non-Customized Matamba Fruit Shell as an Adsorbent for Wastewater Treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Zakaria, M.R.; Yusoff, M.Z.M.; Norrrahim, M.N.F.; Mokhtar, M.N.; Shirai, Y. Effect of Oil Palm Biomass Cellulosic Content on Nanopore Structure and Adsorption Capacity of Biochar. Bioresour. Technol. 2021, 332, 125070. [Google Scholar] [CrossRef] [PubMed]
- Albalasmeh, A.; Gharaibeh, M.A.; Mohawesh, O.; Alajlouni, M.; Quzaih, M.; Masad, M.; El Hanandeh, A. Characterization and Artificial Neural Networks Modelling of Methylene Blue Adsorption of Biochar Derived from Agricultural Residues: Effect of Biomass Type, Pyrolysis Temperature, Particle Size. J. Saudi Chem. Soc. 2020, 24, 811–823. [Google Scholar] [CrossRef]
- Giri, D.D.; Jha, J.M.; Srivastava, N.; Hashem, A.; Abd_Allah, E.F.; Shah, M.; Pal, D.B. Sustainable Removal of Arsenic from Simulated Wastewater Using Solid Waste Seed Pods Biosorbents of Cassia Fistula L. Chemosphere 2022, 287, 132308. [Google Scholar] [CrossRef] [PubMed]
- Streit, A.F.M.; Côrtes, L.N.; Druzian, S.P.; Godinho, M.; Collazzo, G.C.; Perondi, D.; Dotto, G.L. Development of High Quality Activated Carbon from Biological Sludge and Its Application for Dyes Removal from Aqueous Solutions. Sci. Total Environ. 2019, 660, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fu, L.; Li, H.; Liu, X.; Wan, C. Using Biochar to Strengthen the Removal of Antibiotic Resistance Genes: Performance and Mechanism. Sci. Total Environ. 2022, 816, 151554. [Google Scholar] [CrossRef]
- Yıldız, Z.; Kaya, N.; Topcu, Y.; Uzun, H. Pyrolysis and Optimization of Chicken Manure Wastes in Fluidized Bed Reactor: CO2 Capture in Activated Bio-Chars. Process Saf. Environ. Prot. 2019, 130, 297–305. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Kalsoom, T.; Yasmeen, S.; Kalsoom, A.; Raina, S.; Zhuang, Q.; Kong, J. Animal Manure-Derived Biochars Produced via Fast Pyrolysis for the Removal of Divalent Copper from Aqueous Media. J. Environ. Manag. 2018, 213, 109–118. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-Based Adsorbents for Postcombustion CO2 Capture: A Critical Review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Hoffmann, J.F.; Barbieri, R.L.; Rombaldi, C.V.; Chaves, F.C. Butia Spp. (Arecaceae): An Overview. Sci. Hortic. 2014, 179, 122–131. [Google Scholar] [CrossRef]
- Sosinski, Ê.E.; Urruth, L.M.; Barbieri, R.L.; Marchi, M.M.; Martens, S.G. On the Ecological Recognition of Butia Palm Groves as Integral Ecosystems: Why Do We Need to Widen the Legal Protection and the in Situ/on-Farm Conservation Approaches? Land Use Policy 2019, 81, 124–130. [Google Scholar] [CrossRef]
- de Jesus Matias Ventura, L.; Pereira, G.S.L.; Mazzottini-dos-Santos, H.C.; de Lima, J.P.; Mercadante-Simões, M.O.; Lopes, P.S.N.; Ribeiro, L.M. Cytological Aspects of Butia Capitata (Arecaceae) Fruit Maturation and Senescence. Sci. Hortic. 2022, 297, 110938. [Google Scholar] [CrossRef]
- dos Santos Cruxen, C.E.; Hoffmann, J.F.; Zandoná, G.P.; Fiorentini, Â.M.; Rombaldi, C.V.; Chaves, F.C. Probiotic Butiá (Butia Odorata) Ice Cream: Development, Characterization, Stability of Bioactive Compounds, and Viability of Bifidobacterium Lactis during Storage. LWT 2017, 75, 379–385. [Google Scholar] [CrossRef]
- Camboim Rockett, F.; de Oliveira Schmidt, H.; Schmidt, L.; Rodrigues, E.; Tischer, B.; Ruffo de Oliveira, V.; Lima da Silva, V.; Rossini Augusti, P.; Hickmann Flôres, S.; Rios, A. Phenolic Compounds and Antioxidant Activity in Vitro and in Vivo of Butia and Opuntia Fruits. Food Res. Int. 2020, 137, 109740. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L.; Gomes, J.C.C.; Alercia, A.; Padulosi, S. Agricultural Biodiversity in Southern Brazil: Integrating Efforts for Conservation and Use of Neglected and Underutilized Species. Sustainability 2014, 6, 741–757. [Google Scholar] [CrossRef]
- Haubert, L.; Zehetmeyr, M.L.; Pereira, Y.M.N.; Kroning, I.S.; Maia, D.S.V.; Sehn, C.P.; Lopes, G.V.; de Lima, A.S.; da Silva, W.P. Tolerance to Benzalkonium Chloride and Antimicrobial Activity of Butia Odorata Barb. Rodr. Extract in Salmonella Spp. Isolates from Food and Food Environments. Food Res. Int. 2019, 116, 652–659. [Google Scholar] [CrossRef]
- Maia, D.S.V.; Haubert, L.; Kroning, I.S.; Soares, K.; dos, S.; Oliveira, T.L.; da Silva, W.P. Biofilm Formation by Staphylococcus Aureus Isolated from Food Poisoning Outbreaks and Effect of Butia Odorata Barb. Rodr. Extract on Planktonic and Biofilm Cells. LWT 2020, 117, 108685. [Google Scholar] [CrossRef]
- Vieira, B.M.; Elicker, C.; Nunes, C.F.P.; Bairros, A.V.; Becker, E.M.; de Oliveira, D.M.; Piva, E.; Fontoura, L.A.M.; Pereira, C.M.P. The Synthesis and Characterization of Butia Capitata Seed Oil as a FAME Feedstock. Fuel 2016, 184, 533–535. [Google Scholar] [CrossRef]
- Zanuttini, M.S.; Pisarello, M.L.; Querini, C.A. Butia Yatay Coconut Oil: Process Development for Biodiesel Production and Kinetics of Esterification with Ethanol. Energy Convers. Manag. 2014, 85, 407–416. [Google Scholar] [CrossRef]
- Peralta, S.L.; de Carvalho, P.H.A.; Ccahuana-Vásquez, R.A.; de Pereira, C.M.P.; Cury, J.A.; Piva, E.; Lund, R.G. Cytotoxicity, Genotoxicity and Antibiofilm Activity on Streptococcus Mutans of an Experimental Self-Etching Adhesive System Containing Natural Butia Capitata Oil. Int. J. Adhes. Adhes. 2017, 78, 95–101. [Google Scholar] [CrossRef]
- Cruz, P.N.; Pereira, T.C.S.; Guindani, C.; Oliveira, D.A.; Rossi, M.J.; Ferreira, S.R.S. Antioxidant and Antibacterial Potential of Butia (Butia Catarinensis) Seed Extracts Obtained by Supercritical Fluid Extraction. J. Supercrit. Fluids 2017, 119, 229–237. [Google Scholar] [CrossRef]
- Kerkhoff, C.M.; da Boit Martinello, K.; Franco, D.S.P.; Netto, M.S.; Georgin, J.; Foletto, E.L.; Piccilli, D.G.A.; Silva, L.F.O.; Dotto, G.L. Adsorption of Ketoprofen and Paracetamol and Treatment of a Synthetic Mixture by Novel Porous Carbon Derived from Butia Capitata Endocarp. J. Mol. Liq. 2021, 339, 117184. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Lima, D.R.; da Silva, R.S.; Thue, P.S.; Seliem, M.K.; Sher, F.; dos Reis, G.S.; Larsson, S.H. Removal of Captopril Pharmaceutical from Synthetic Pharmaceutical-Industry Wastewaters: Use of Activated Carbon Derived from Butia Catarinensis. J. Environ. Chem. Eng. 2020, 8, 104506. [Google Scholar] [CrossRef]
- Mishra, R.K. Pyrolysis of Low-Value Waste Switchgrass: Physicochemical Characterization, Kinetic Investigation, and Online Characterization of Hot Pyrolysis Vapours. Bioresour. Technol. 2022, 347, 126720. [Google Scholar] [CrossRef] [PubMed]
- Selvarajoo, A.; Oochit, D. Effect of Pyrolysis Temperature on Product Yields of Palm Fibre and Its Biochar Characteristics. Mater. Sci. Energy Technol. 2020, 3, 575–583. [Google Scholar] [CrossRef]
- de Godois Baroni, É.; Tannous, K.; Rueda-Ordóñez, Y.J.; Tinoco-navarro, L.K. The Applicability of Isoconversional Models in Estimating the Kinetic Parameters of Biomass Pyrolysis. J. Ther. Anal. Calorim. 2015, 123, 909–917. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of Granular Activated Carbon from Fruit Stones and Nutshells and Evaluation of Their Physical, Chemical and Adsorption Properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Santos, V.O.; Queiroz, L.S.; Araujo, R.O.; Ribeiro, F.C.P.; Guimarães, M.N.; Carlos, E.F.; Chaar, J.S.; De Souza, L.K.C. Pyrolysis of Acai Seed Biomass: Kinetics and Thermodynamic Parameters Using Thermogravimetric Analysis. Bioresour. Technol. Reports 2020, 12, 100553. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Al Ayed, O.S.; Ye, G.; Luo, H.; Ibrahim, M.; Rashid, U.; Arbi Nehdi, I.; Qadir, G. Kinetic Analyses and Pyrolytic Behavior of Para Grass (Urochloa Mutica) for Its Bioenergy Potential. Bioresour. Technol. 2017, 224, 708–713. [Google Scholar] [CrossRef]
- Toribio-cuaya, H.; Pedraza-segura, L.; Macías-bravo, S. Biological and Physical Sciences Characterization of Lignocellulosic Biomass Using Five Simple Steps. J. Chem. Biol. Phys. Sci. 2014, 4, 28–49. [Google Scholar]
- Siqueira, P.; Mabel, M.; Helena, R.; De Oliveira, J.; Prado, A.; Matias, S.; Alencar, D. Açaí Seeds: An Unexplored Agro-Industrial Residue as a Potential Source of Lipids, Fibers, and Antioxidant Phenolic Compounds. Ind. Crops Prod. 2021, 161, 113204. [Google Scholar]
- Rasool, T.; Najar, I.; Chandra, V.; Pandey, A. Pyrolysis of Almond (Prunus Amygdalus) Shells: Kinetic Analysis, Modelling, Energy Assessment and Technical Feasibility Studies. Bioresour. Technol. 2021, 337, 125466. [Google Scholar] [CrossRef] [PubMed]
- Hansted, A.L.S.; Cacuro, T.A.; Nakashima, G.T.; Costa, V.E.; Yamamoto, H.; Yamaji, F.M. Industrial Crops & Products Use of a Lignocellulosic Residue as Solid Fuel: The e Ff Ect of Ash Content in the Energy Potential. Ind. Crops Prod. 2018, 116, 209–214. [Google Scholar] [CrossRef]
- Reshad, A.S.; Tiwari, P.; Goud, V.V. Thermo-Chemical Conversion of Waste Rubber Seed Shell to Produce Fuel and Value-Added Chemicals. J. Energy Inst. 2018, 91, 940–950. [Google Scholar] [CrossRef]
- Sangaré, D.; Bostyn, S.; Santillán, M.M.; García-Alamilla, P.; Belandria, V.; Gökalp, I. Comparative Pyrolysis Studies of Lignocellulosic Biomasses: Online Gas Quantification, Kinetics Triplets, and Thermodynamic Parameters of the Process. Bioresour. Technol. 2022, 346, 126598. [Google Scholar] [CrossRef] [PubMed]
- Rambo, M.K.D.; Alexandre, G.P.; Rambo, M.C.D.; Alves, A.R.; Garcia, W.T.; Baruque, E. Characterization of Biomasses from the North and Northeast Regions of Brazil for Processes in Biorefineries. Food Sci. Technol. 2015, 35, 605–611. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Xu, J. Investigating the Release Behavior of Biomass and Coal during the Co-Pyrolysis Process. Int. J. Hydrog. Energy 2021, 46, 34652–34662. [Google Scholar] [CrossRef]
- Cui, T.; Xu, J.; Fan, W.; Chang, Q.; Yu, G.; Wang, F. Experimental Study on Fragmental Behavior of Coals and Biomasses during Rapid Pyrolysis. Bioresour. Technol. 2016, 222, 439–447. [Google Scholar] [CrossRef]
- Vasudev, V.; Ku, X.; Lin, J. Kinetic Study and Pyrolysis Characteristics of Algal and Lignocellulosic Biomasses. Bioresour. Technol. 2019, 288, 121496. [Google Scholar] [CrossRef]
- Sahoo, A.; Kumar, S.; Kumar, J.; Bhaskar, T. A Detailed Assessment of Pyrolysis Kinetics of Invasive Lignocellulosic Biomasses (Prosopis Juliflora and Lantana Camara) by Thermogravimetric Analysis. Bioresour. Technol. 2021, 319, 124060. [Google Scholar] [CrossRef]
- Aguiar, M.C.S.; Silvério, F.O.; de Pinho, G.P.; Lopes, P.S.N.; Fidêncio, P.H.; Ventura, S.J. Volatile Compounds from Fruits of Butia Capitata at Different Stages of Maturity and Storage. Food Res. Int. 2014, 62, 1095–1099. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S.; Upadhyay, S.N.; Mishra, P.K. Analysis of Thermal Degradation of Banana (Musa Balbisiana) Trunk Biomass Waste Using Iso-Conversional Models. Bioresour. Technol. 2020, 310, 123393. [Google Scholar] [CrossRef] [PubMed]
- Hernowo, P.; Steven, S.; Restiawaty, E.; Irawan, A.; Borromeus, C.; Marno, S.; Meliana, Y.; Bindar, Y. Chemicals Component Yield Prediction and Kinetic Parameters Determination of Oil Palm Shell Pyrolysis through Volatile State Approach and Experimental Study. J. Anal. Appl. Pyrolysis 2022, 161, 105399. [Google Scholar] [CrossRef]
- Raza, M.; Abu-jdayil, B.; Al-marzouqi, A.H.; Inayat, A. Kinetic and Thermodynamic Analyses of Date Palm Surface Fi Bers Pyrolysis Using Coats-Redfern Method. Renew. Energy 2022, 183, 67–77. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of Pyrolysis Characteristics and Kinetics of Palm Kernel Shell Using TGA—FTIR and Model-Free Integral Methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Salgado-ramos, M.; Martí-quijal, F.J.; Huertas-alonso, A.J.; Barba, F.J. Almond Hull Biomass: Preliminary Characterization and Development of Two Alternative Valorization Routes by Applying Innovative and Sustainable Technologies. Ind. Crops Prod. 2022, 179, 114697. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal Decomposition of Wood: Influence of Wood Components and Cellulose Crystallite Size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef]
- Zazycki, M.A.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. New Biochar from Pecan Nutshells as an Alternative Adsorbent for Removing Reactive Red 141 from Aqueous Solutions. J. Clean. Prod. 2018, 171, 57–65. [Google Scholar] [CrossRef]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 Activated Biochar from Solid Wastes of a Beer Industry and Its Application for Methylene Blue Adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef]
- Rijo, B.; Paula, A.; Dias, S.; Ramos, M.; Ameixa, M. Valorization of Forest Waste Biomass by Catalyzed Pyrolysis. Energy 2022, 243, 122766. [Google Scholar] [CrossRef]
- Mishra, R.K.; Lu, Q.; Mohanty, K. Thermal Behaviour, Kinetics and Fast Pyrolysis of Cynodon Dactylon Grass Using Py-GC/MS and Py-FTIR Analyser. J. Anal. Appl. Pyrolysis 2020, 150, 104887. [Google Scholar] [CrossRef]
- Shi, J.; Xing, D.; Li, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Bentes, V.L.I.; Nobre, F.X.; Barros, I.C.L.; Couceiro, P.R.C. Composite of Iron Phosphate-Supported Carbon from the Açaí (Euterpe Oleracea) as a Solid Catalyst for Photo-Fenton Reactions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100520. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Yi, Z.; Zhang, L.; Zhang, S.; Hu, X. Co-Pyrolysis of Coke Bottle Wastes with Cellulose, Lignin and Sawdust: Impacts of the Mixed Feedstock on Char Properties. Renew. Energy 2022, 181, 1126–1139. [Google Scholar] [CrossRef]
- Faria, J.P.; Arellano, D.B.; Grimaldi, R.; Silva, L.D.C.R.; Vieira, R.F.; Silva, D.D.B.; Agostini-costa, T.D.S. Chemical Characterization of Nut of Butia Capitata Var Capitata. Rev. Bras. Frutic. 2008, 30, 549–552. [Google Scholar] [CrossRef]
- Kobelnik, M.; Fontanari, G.G.; Marques, M.R.; Ribeiro, C.A.; Crespi, M.S. Thermal Behavior and Chromatographic Characterization of Oil Extracted from the Nut of the Butia (Butia Capitata). J. Therm. Anal. Calorim. 2016, 123, 2517–2522. [Google Scholar] [CrossRef][Green Version]
- Peralta, S.L.; Carvalho, P.H.A.; van de Sande, F.H.; Pereira, C.M.P.; Piva, E.; Lund, R.G. Self-Etching Dental Adhesive Containing a Natural Essential Oil: Anti-Biofouling Performance and Mechanical Properties. Biofouling 2013, 29, 345–355. [Google Scholar] [CrossRef]
- De Conto, D.; Silvestre, W.P.; Baldasso, C.; Godinho, M. Performance of Rotary Kiln Reactor for the Elephant Grass Pyrolysis. Bioresour. Technol. 2016, 218, 153–160. [Google Scholar] [CrossRef]
- Chang, G.; Huang, Y.; Xie, J.; Yang, H.; Liu, H.; Yin, X.; Wu, C. The Lignin Pyrolysis Composition and Pyrolysis Products of Palm Kernel Shell, Wheat Straw, and Pine Sawdust. Energy Convers. Manag. 2016, 124, 587–597. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; González, L.A. Review on the Physicochemical Treatments of Rice Husk for Production of Advanced Materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Teixeira, V.G.; Coutinho, F.M.B.; Gomes, A.S. Principais Métodos de Caracterização Da Porosidade de Resinas à Base de Divinilbenzeno. Quim. Nova 2001, 24, 808–818. [Google Scholar] [CrossRef]
- IUPAC. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Darmawan, S.; Wistara, N.J.; Pari, G.; Maddu, A.; Syafii, W. Characterization of Lignocellulosic Biomass as Raw Material for the Production of Porous Carbon-Based Materials. BioResources 2016, 11, 3561–3574. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of Hemicellulose, Cellulose and Lignin to the Mass and the Porous Properties of Chars and Steam Activated Carbons from Various Lignocellulosic Precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef]
- Daud, W.M.A.W.; Ali, W.S.W. Comparison on Pore Development of Activated Carbon Produced from Palm Shell and Coconut Shell. Bioresour. Technol. 2004, 93, 63–69. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.N.M. Optimized Preparation for Large Surface Area Activated Carbon from Date (Phoenix dactylifera L.) Stone Biomass. Biomass Bioenergy 2014, 61, 167–178. [Google Scholar] [CrossRef]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing Biochar Surface Area: Optimization of Ball Milling Parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, J.; Yi, Y. Biochar and Hydrochar Derived from Freshwater Sludge: Characterization and Possible Applications. Sci. Total Environ. 2021, 763, 144550. [Google Scholar] [CrossRef]
- Manera, C.; Perondi, D.; Godinho, M. Production of Micro-Mesoporous Activated Carbons from Various Citrus Waste. In Proceedings of the IV Congresso Internacional de Biomassa, Curitiba, Brazil, 25–27 June 2019. [Google Scholar]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U. Modeling and Evaluation of Chromium Remediation from Water Using Low Cost Bio-Char, a Green Adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Q.; Yao, Q.; Wen, S.-E.; Chi, Y.; Yan, J.-H. Properties of Pyrolytic Chars and Activated Carbons Derived from Pilot-Scale Pyrolysis of Used Tires. J. Air Waste Manag. Assoc. 2005, 55, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Botomé, M.L.; Poletto, P.; Junges, J.; Perondi, D.; Dettmer, A.; Godinho, M. Preparation and Characterization of a Metal-Rich Activated Carbon from CCA-Treated Wood for CO2 Capture. Chem. Eng. J. 2017, 321, 614–621. [Google Scholar] [CrossRef]
- Sabri, M.A.; Al Jitan, S.; Bahamon, D.; Vega, L.F.; Palmisano, G. Current and Future Perspectives on Catalytic-Based Integrated Carbon Capture and Utilization. Sci. Total Environ. 2021, 790, 148081. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, L.; Xiang, W.; Xu, Y.; Gao, B. Preparation and Evaluation of Fine-Tuned Micropore Biochar by Lignin Impregnation for CO2 and VOCs Adsorption. Sep. Purif. Technol. 2022, 295, 121295. [Google Scholar] [CrossRef]
- Wjihi, S.; Aouaini, F.; Erto, A.; Balsamo, M.; Lamine, A. Ben Advanced Interpretation of CO2 Adsorption Thermodynamics onto Porous Solids by Statistical Physics Formalism. Chem. Eng. J. 2021, 406, 126669. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Rashidi, A.; Ghoreyshi, A.A. Adsorptive Removal of CO2 on Highly Microporous Activated Carbons Prepared from Eucalyptus Camaldulensis Wood: Effect of Chemical Activation. J. Taiwan Inst. Chem. Eng. 2014, 45, 579–588. [Google Scholar] [CrossRef]
- Du, X.; Cheng, Y.; Liu, Z.; Hou, Z.; Wu, T.; Lei, R.; Shu, C. Study on the Adsorption of CH4, CO2 and Various CH4/CO2 Mixture Gases on Shale. Alex. Eng. J. 2020, 59, 5165–5178. [Google Scholar] [CrossRef]
- Singh, J.; Basu, S.; Bhunia, H. CO2 Capture by Modified Porous Carbon Adsorbents: Effect of Various Activating Agents. J. Taiwan Inst. Chem. Eng. 2019, 102, 438–447. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a Dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- TAPPI T 204 Cm-97; Solvent Extractives of Wood and Pulp. Tappi Press: Atlanta, GA, USA, 1997.
- ASTM. Standard Practice for Proximate Analysis of Coal and Coke. In Annual Book of ASTM Standards; ASTM International: West Conshohoken, PA, USA, 1993; pp. D3172–D3189. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Determination of Lignin and Cellulose in Acid-Detergent Fiber with Permanganate. J. AOAC Int. 1968, 51, 780–785. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Srivastava, P.; Naidu, R.; Vinu, A. Single Step Synthesis of Activated Bio-Carbons with a High Surface Area and Their Excellent CO2 Adsorption Capacity. Carbon N. Y. 2017, 116, 448–455. [Google Scholar] [CrossRef]
- Nasri, N.S.; Hamza, U.D.; Ismail, S.N.; Ahmed, M.M.; Mohsin, R. Assessment of Porous Carbons Derived from Sustainable Palm Solid Waste for Carbon Dioxide Capture. J. Clean. Prod. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Wedler, C.; Span, R. A Pore-Structure Dependent Kinetic Adsorption Model for Consideration in Char Conversion—Adsorption Kinetics of CO2 on Biomass Chars. Chem. Eng. Sci. 2021, 231, 116281. [Google Scholar] [CrossRef]
- Ello, A.S.; De Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Coconut Shell-Based Microporous Carbons for CO2 Capture. Microporous Mesoporous Mater. 2013, 180, 280–283. [Google Scholar] [CrossRef]
- Conte, G.; Stelitano, S.; Policicchio, A.; Minuto, F.D.; Lazzaroli, V.; Galiano, F.; Agostino, R.G. Assessment of Activated Carbon Fibers from Commercial Kevlar® as Nanostructured Material for Gas Storage: Effect of Activation Procedure and Adsorption of CO2 and CH4. J. Anal. Appl. Pyrolysis 2020, 152, 104974. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Joseph, S.; Srivastava, P.; Naidu, R.; Vinu, A. Heteroatom Functionalized Activated Porous Biocarbons and Their Excellent Performance for CO2 Capture at High Pressure. J. Mater. Chem. A 2017, 5, 21196–21204. [Google Scholar] [CrossRef]
- Choi, S.W.; Tang, J.; Pol, V.G.; Lee, K.B. Pollen-Derived Porous Carbon by KOH Activation: Effect of Physicochemical Structure on CO2 Adsorption. J. CO2 Util. 2019, 29, 146–155. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly Microporous Activated Carbons from Biomass for CO2 Capture and Effective Micropores at Different Conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Bae, J.S.; Su, S. Macadamia Nut Shell-Derived Carbon Composites for Post Combustion CO2 Capture. Int. J. Greenh. Gas Control 2013, 19, 174–182. [Google Scholar] [CrossRef]
- Zhu, X.L.; Wang, P.Y.; Peng, C.; Yang, J.; Yan, X. Bin Activated Carbon Produced from Paulownia Sawdust for High-Performance CO2 Sorbents. Chin. Chem. Lett. 2014, 25, 929–932. [Google Scholar] [CrossRef]
- Labus, K.; Gryglewicz, S.; Machnikowski, J. Granular KOH-Activated Carbons from Coal-Based Cokes and Their CO2 Adsorption Capacity. Fuel 2014, 118, 9–15. [Google Scholar] [CrossRef]
- Quan, C.; Wang, H.; Jia, X.; Gao, N. Effect of Carbonization Temperature on CO2 Adsorption Behavior of Activated Coal Char. J. Energy Inst. 2021, 97, 92–99. [Google Scholar] [CrossRef]
- Ello, A.S.; De Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Development of Microporous Carbons for CO2 Capture by KOH Activation of African Palm Shells. J. CO2 Util. 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Cong, H.; Zhang, M.; Chen, Y.; Chen, K.; Hao, Y.; Zhao, Y.; Feng, L. Highly Selective CO2 Capture by Nitrogen Enriched Porous Carbons. Carbon N. Y. 2015, 92, 297–304. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated Carbons Prepared from Hydrothermally Carbonized Waste Biomass Used as Adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Pramanik, P.; Patel, H.; Charola, S.; Neogi, S.; Maiti, S. High Surface Area Porous Carbon from Cotton Stalk Agro-Residue for CO2 adsorption and Study of Techno-Economic Viability of Commercial Production. J. CO2 Util. 2021, 45, 101450. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass Derived Low-Cost Microporous Adsorbents for Efficient CO2 Capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Song, J.; Shen, W.; Wang, J.; Fan, W. Superior Carbon-Based CO2 Adsorbents Prepared from Poplar Anthers. Carbon N. Y. 2014, 69, 255–263. [Google Scholar] [CrossRef]



| Proximate Analysis * | ||||
|---|---|---|---|---|
| FIB | END | ALM | DOA | |
| Moisture (%wt.) | 5.48 ± 0.08 | 5.19 ± 0.20 | 5.22 ± 1.44 | 6.06 ± 0.19 |
| Ash (%wt.) | 1.72 ± 0.08 | 0.51 ± 0.02 | 1.15 ± 0.06 | 2.25 ± 0.14 |
| Fixed carbon (%wt.) | 22.25 ± 0.58 | 25.6 ± 0.41 | 7.62 ± 0.42 | 12.13 ± 0.06 |
| Volatile matter (%wt.) | 76.03 ± 0.51 | 74.06 ± 0.27 | 91.23 ± 0.41 | 85.62 ± 0.18 |
| Chemical Composition * | ||||
| FIB | END | ALM | DOA | |
| Cellulose (%wt.) | 5.94 ± 0.68 | 6.14 ± 1.35 | 10.57 ± 0.38 | 57.79 ± 0.39 |
| Hemicellulose (%wt.) | 29.00 ± 1.14 | 24.34 ± 1.17 | 8.03 ± 0.36 | 11.81 ± 0.44 |
| Lignin (%wt.) | 15.32 ± 0.34 | 48.14 ± 1.44 | 19.12 ± 1.14 | 10.80 ± 0.85 |
| Extractive (%wt.) | 11.10 ± 0.65 | 7.44 ± 0.38 | 36.62 ± 1.14 | 1.35 ± 0.35 |
| Sample | Surface Area BET (m2 g−1) | Average Pore Size (nm) | Micropores Volume (cm3 g−1) | Mesopores Volume (cm3 g−1) | Total Volume Pores (cm3 g−1) | pHPCZ |
|---|---|---|---|---|---|---|
| FIB.700 | 183.59 | 2.527 | 0.109 | 0.0 | 0.109 | 6.52 |
| ALM.700 | 1.92 | 5.628 | 0.0 | 0.00194 | 0.00194 | 7.20 |
| END.700 | 58.39 | 3.336 | 0.065 | 0.0 | 0.065 | 7.34 |
| DOA.700 | 220.43 | 2.456 | 0.123 | 0.0 | 0.123 | 7.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, I.d.S.; Schnorr, C.; Perondi, D.; Godinho, M.; Diel, J.C.; Machado, L.M.M.; Dalla Nora, F.B.; Silva, L.F.O.; Dotto, G.L. Valorization of Different Fractions from Butiá Pomace by Pyrolysis: H2 Generation and Use of the Biochars for CO2 Capture. Molecules 2022, 27, 7515. https://doi.org/10.3390/molecules27217515
Nunes IdS, Schnorr C, Perondi D, Godinho M, Diel JC, Machado LMM, Dalla Nora FB, Silva LFO, Dotto GL. Valorization of Different Fractions from Butiá Pomace by Pyrolysis: H2 Generation and Use of the Biochars for CO2 Capture. Molecules. 2022; 27(21):7515. https://doi.org/10.3390/molecules27217515
Chicago/Turabian StyleNunes, Isaac dos S., Carlos Schnorr, Daniele Perondi, Marcelo Godinho, Julia C. Diel, Lauren M. M. Machado, Fabíola B. Dalla Nora, Luis F. O. Silva, and Guilherme L. Dotto. 2022. "Valorization of Different Fractions from Butiá Pomace by Pyrolysis: H2 Generation and Use of the Biochars for CO2 Capture" Molecules 27, no. 21: 7515. https://doi.org/10.3390/molecules27217515
APA StyleNunes, I. d. S., Schnorr, C., Perondi, D., Godinho, M., Diel, J. C., Machado, L. M. M., Dalla Nora, F. B., Silva, L. F. O., & Dotto, G. L. (2022). Valorization of Different Fractions from Butiá Pomace by Pyrolysis: H2 Generation and Use of the Biochars for CO2 Capture. Molecules, 27(21), 7515. https://doi.org/10.3390/molecules27217515









