The Taste-Masking Mechanism of Chitosan at the Molecular Level on Bitter Drugs of Alkaloids and Flavonoid Glycosides from Traditional Chinese Medicine
Abstract
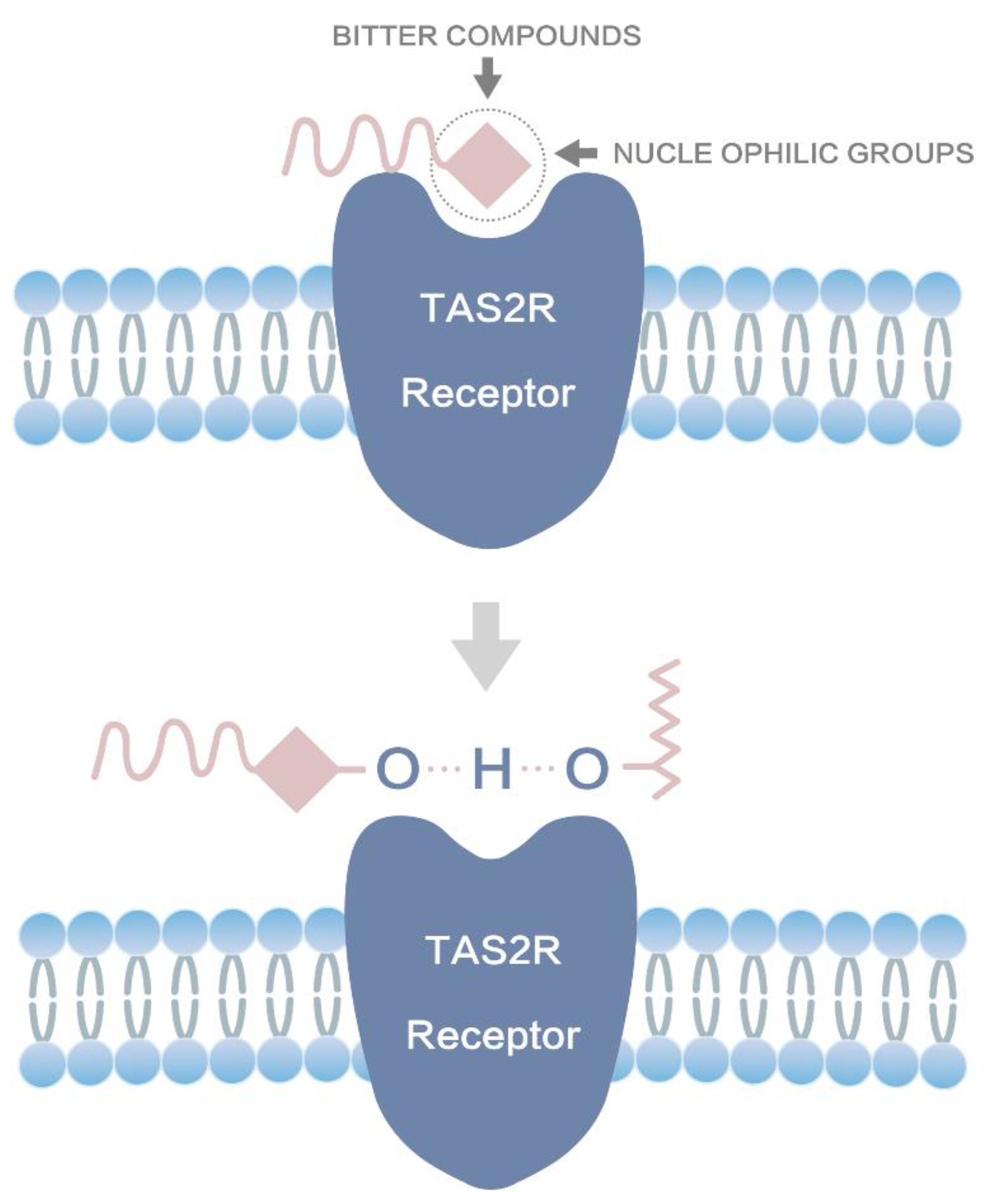
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethics Statement
2.3. Animals
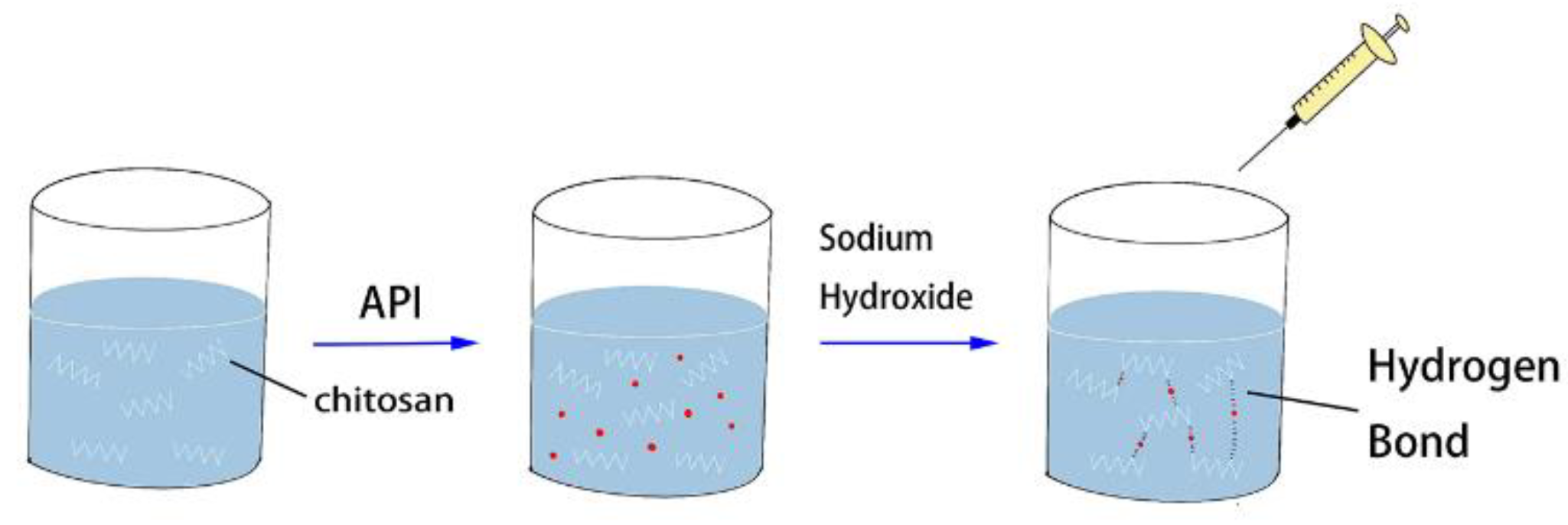
2.4. Preparation and Characterization of Taste-Masked BER/CS, PHI/CS, and BER-PHI/CS Compounds
2.4.1. Preparation of BER/CS, PHI/CS, and BER-PHI/CS
2.4.2. Binding Efficiency
2.4.3. Morphology
2.4.4. Powder X-ray Diffraction (PXRD) Analysis
2.4.5. Differential Scanning Calorimetry (DSC)
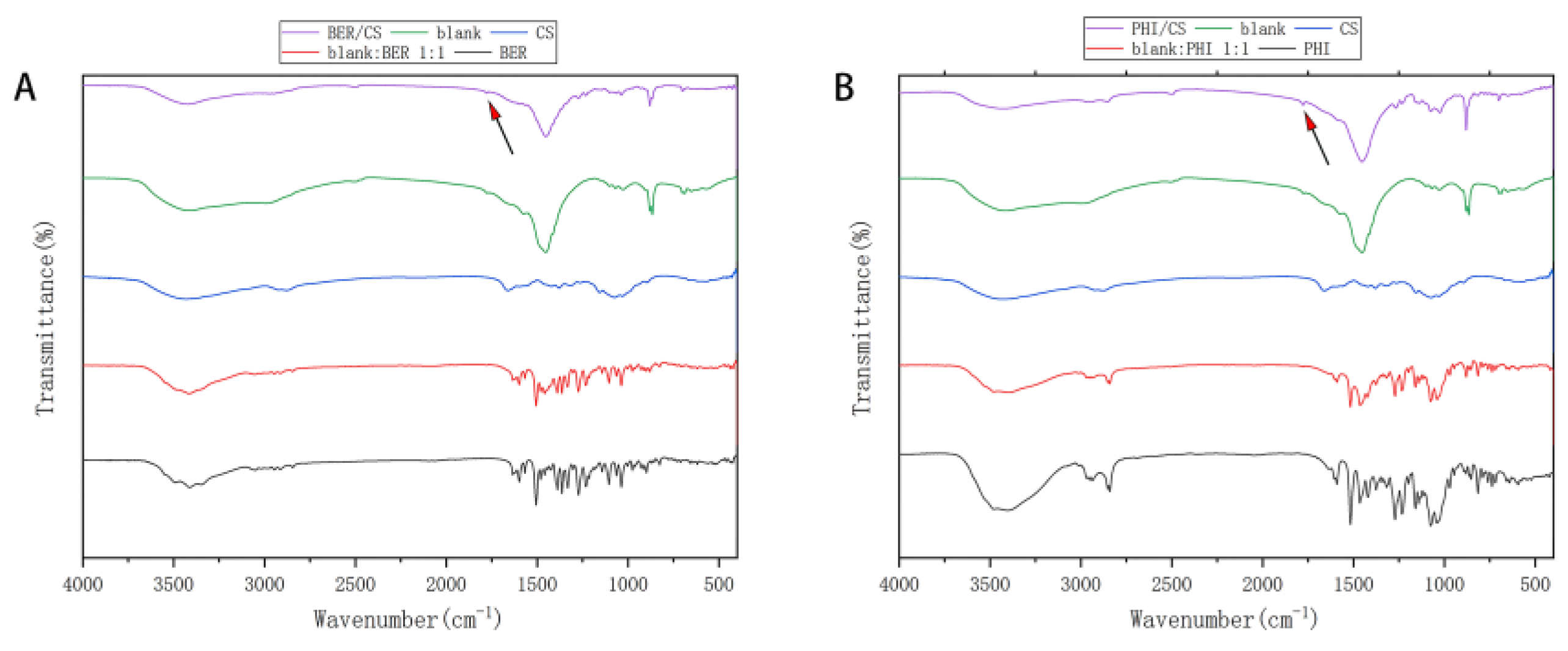
2.4.6. Fourier Transform Infrared (FTIR) Spectrometry
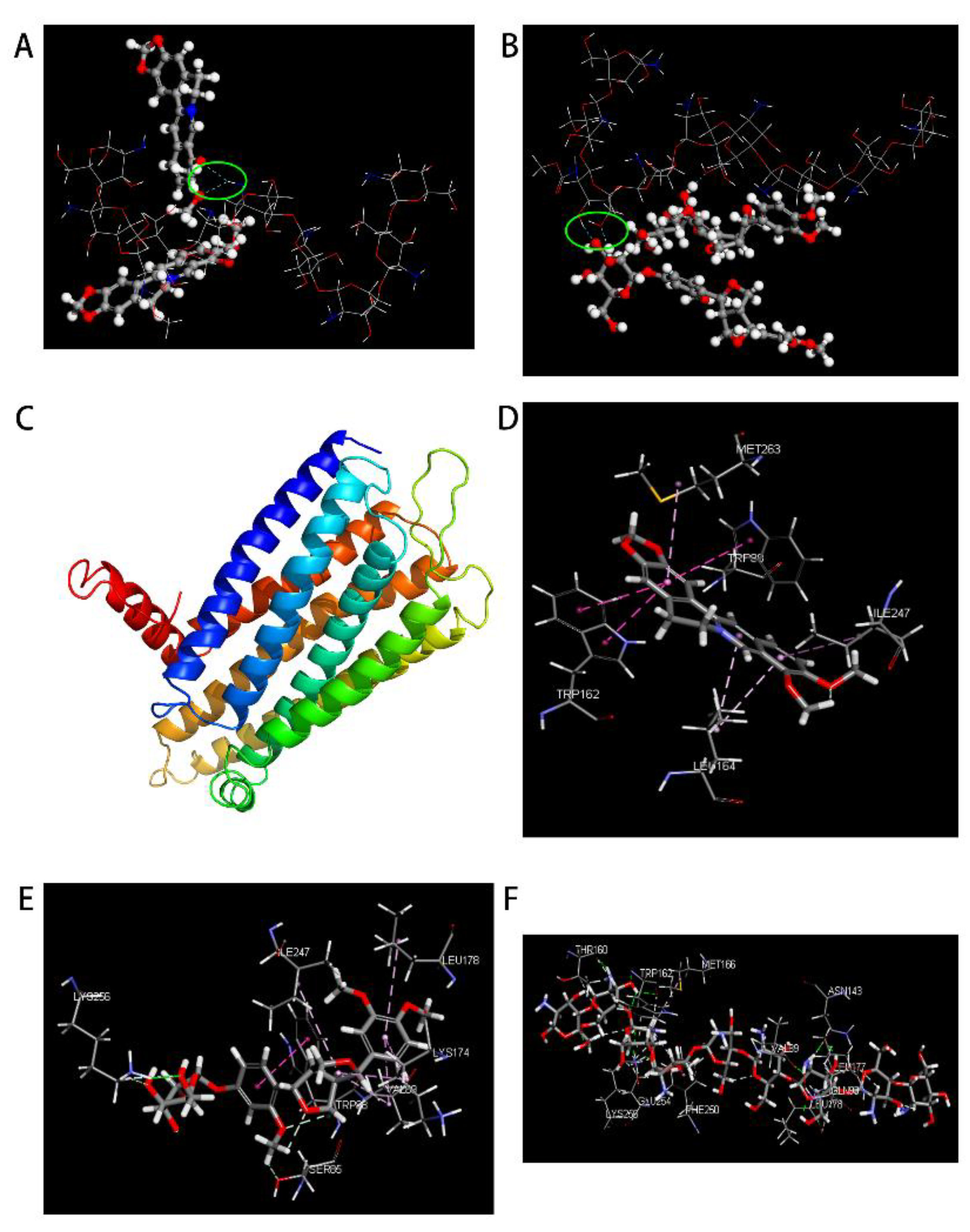
2.5. Molecular Docking
2.5.1. Materials Studio (MS)
2.5.2. Discovery Studio (DS)
2.6. The Electronic Tongue Test
2.7. In Vitro Drug Release
2.7.1. Simulated Saliva
2.7.2. Simulated Gastric Acid
2.8. In Vivo Pharmacokinetics of BER and BER/CS
2.9. Statistical Analysis
3. Results
3.1. Binding Efficiency
3.2. Physiochemical Characterization of BER/CS and PHI/CS
3.3. PXRD Analysis
3.4. DSC Analysis
3.5. FTIR Analysis
3.6. Molecule Docking
3.6.1. MS
3.6.2. DS
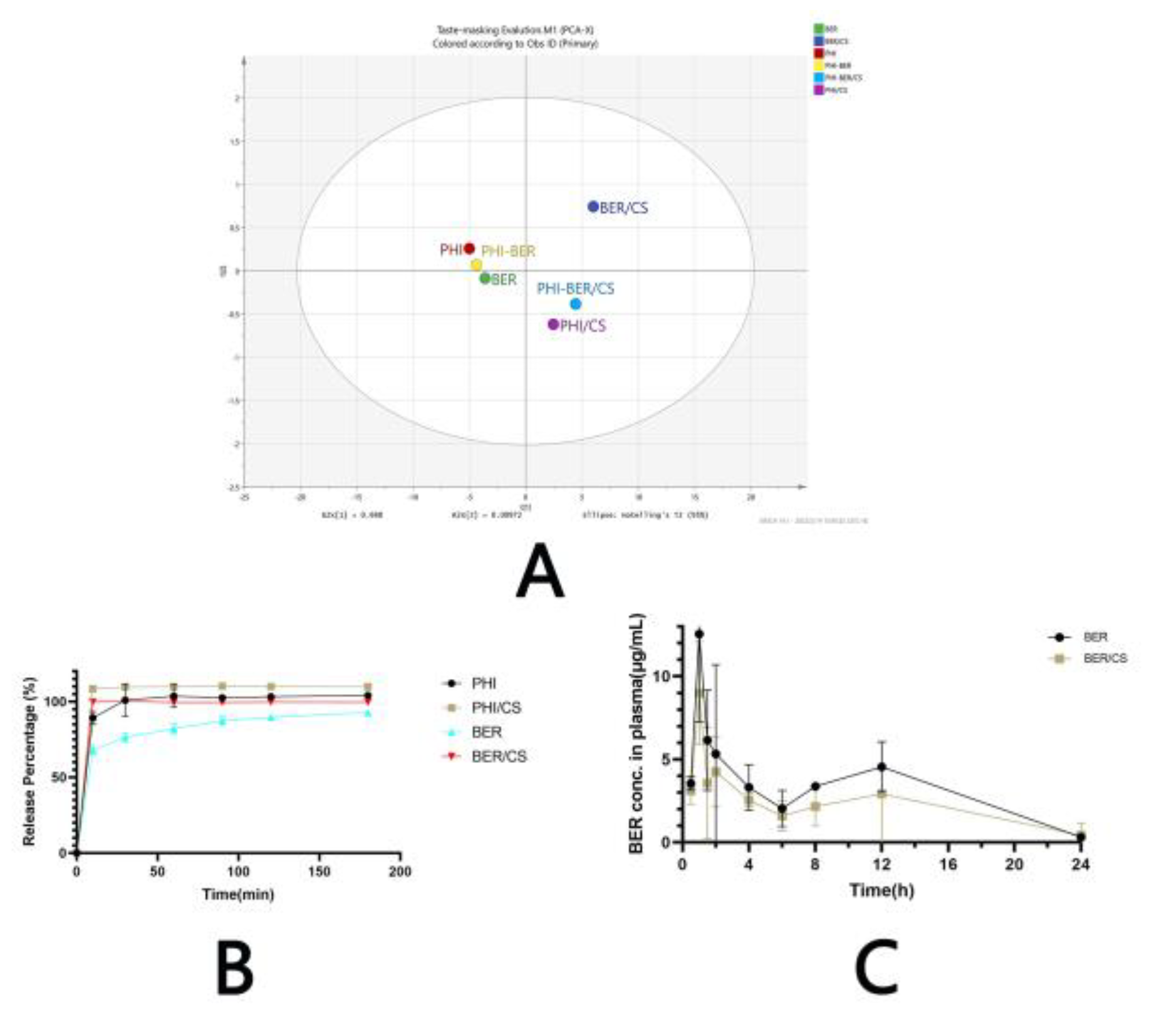
3.7. The Electronic Tongue Test
3.8. In Vitro Drug Release
3.8.1. Simulated Saliva
3.8.2. Simulated Gastric Acid
3.9. Pharmacokinetics of BER and BER/CS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behrens, M.; Gu, M.; Fan, S.J.; Huang, C.; Meyerhof, W. Bitter substances from plants used in traditional Chinese medicine exert biased activation of human bitter taste receptors. Chem. Biol. Drug Des. 2018, 91, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, C.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Jeon, A.; Han, E.; Lee, K.; Sommerfield, A.; Lim, L.Y.; Sommerfield, D.; Von Ungern-Sternberg, B.S. Parents’ perspectives towards pediatric confectionary masked medications: A qualitative study. Int. J. Clin. Pharm. 2022, 44, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.D.; Li, J.X.; Lu, C.H.; Zhao, L.J.; Lin, X.; Wang, Y.J.; Yang, X.M. Data mining-based detection of the physical and chemical characteristics of Chinese medical herbs aqueous decoction in spray drying yield. Dry. Technol. 2021, 39, 896–904. [Google Scholar] [CrossRef]
- Muoka, L.C.; Ross, S.A.; Mithu, M.S.H.; Nandi, U.; Douroumis, D. Comparative taste-masking evaluation of microencapsulated bitter drugs using Smartseal 30D and ReadyMix for paediatric dosage forms. AAPS Pharmscitech 2021, 22, 141. [Google Scholar] [CrossRef]
- Dashevskiy, A.; Mohylyuk, V.; Ahmed, A.R.; Kolter, K.; Guth, F.; Bodmeier, R. Micropellets coated with Kollicoat (R) Smart seal 30D for taste masking in liquid oral dosage forms. Drug Dev. Ind. Pharm. 2017, 43, 1548–1556. [Google Scholar] [CrossRef]
- Dagan-Wiener, A.; Nissim, I.; Ben Abu, N.; Borgonovo, G.; Bassoli, A.; Niv, M.Y. Bitter or not? Bitter Predict, a tool for predicting taste from chemical structure. Sci. Rep. 2017, 7, 12074. [Google Scholar] [CrossRef]
- Alayoubi, A.; Daihom, B.; Adhikari, H.; Mishra, S.; Helms, R.; Almoazen, H. Development of a taste-masked oral suspension of clindamycin HCl using ion exchange resin Amberlite IRP 69 for use in pediatrics. Drug Dev. Ind. Pharm. 2016, 42, 1579–1589. [Google Scholar] [CrossRef]
- Li, P.; Tian, Y.; Ke, X.M.; Tan, Q.C.; Han, X.; Ma, H.Y.; Pei, J.; Lin, J.Z.; Xu, R.C.; Han, L.; et al. Amphiphilic Block Copolymers: A Novel Substance for Bitter-Masking in Aqueous Solutions. Mol. Pharm. 2020, 17, 1586–1595. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Leonida, M.D.; Belbekhouche, S.; Benzecry, A.; Peddineni, M.; Suria, A.; Carbonnier, B. Antibacterial hop extracts encapsulated in nanochitosan matrices. Int. J. Biol. Macromol. 2018, 120, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Yuan, H.Q.; Liu, B.S.; He, J.X.; Fan, Q.; Deng, K.; Song, D.P.; Bao, G.M. Facile one-pot synthesis of chitosan-based nanoparticles for pH-responsive enrofloxacin delivery. Mater. Today Commun. 2021, 29, 102883. [Google Scholar] [CrossRef]
- Liu, M.X.; Yin, D.P.; Fu, H.L.; Deng, F.Y.; Peng, G.N.; Shu, G.; Yuan, Z.X.; Shi, F.; Lin, J.C.; Zhao, L.; et al. Double-coated enrofloxacin microparticles with chitosan and alginate: Preparation, characterization and taste-masking effect study. Carbohydr. Polym. 2017, 170, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Ji, R.R.; Chen, X.; You, B.M.; Pan, Y.H.; Su, J.C. Cetirizine dihydrochloride loaded microparticles design using ionotropic cross-linked chitosan nanoparticles by spray-drying method. Arch. Pharmacal. Res. 2010, 33, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, A.M.; Bhatt, S.A.; Brahmbhatt, H.; Anand, P.S.; Popat, K.M. Taste masking of ciprofloxacin by ion-exchange resin and sustain release at gastric-intestinal through interpenetrating polymer network. Asian J. Pharm. Sci. 2015, 10, 331–340. [Google Scholar] [CrossRef]
- Bora, D.; Borude, P.; Bhise, K. Taste masking by spray-drying technique. AAPS Pharmscitech 2008, 9, 1159–1164. [Google Scholar] [CrossRef]
- Stagner, W.C.; Iyer, M.; Rathod, V.; Meruva, S.; Staton, S.; Haware, R.V. Human volunteer, in vitro, and molecular level evaluation of an optimized taste-masked isoniazid-chitosan spray-dried microparticle matrix. Int. J. Pharm. 2019, 572, 118774. [Google Scholar] [CrossRef]
- Yehia, S.A.; El-Ridi, M.S.; Tadros, M.I.; El-Sherif, N.G. Phenylalanine-free taste-masked orodispersible tablets of fexofenadine hydrochloride: Development, in vitro evaluation and in vivo estimation of the drug pharmacokinetics in healthy human volunteers. Pharm. Dev. Technol. 2015, 20, 528–539. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yue, T.L.; Yuan, Y.H.; Cai, R.; Niu, C.; Guo, C.X. Kinetics of adsorption of bovine serum albumin on magnetic carboxymethyl chitosan nanoparticles. Int. J. Biol. Macromol. 2013, 58, 57–65. [Google Scholar] [CrossRef]
- Shah, P.P.; Mashru, R.C. Influence of Chitosan Crosslinking on Bitterness of Mefloquine Hydrochloride Microparticles Using Central Composite Design. J. Pharm. Sci. 2009, 98, 690–703. [Google Scholar] [CrossRef]
- Banerjee, P.; Preissner, R. BitterSweet Forest: A Random Forest Based Binary Classifier to Predict Bitterness and Sweetness of Chemical Compounds. Front. Chem. 2018, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Nissim, I.; Dagan-Wiener, A.; Niv, M.Y. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wu, Y.H.; Li, J.S.; Liu, M.H. Comparative modeling of improved synthesis of energetic dinitrobenzofuroxan (DNBF) derivatives. J. Mol. Model. 2020, 26, 240. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.F.; Zhang, K.R.; Zhou, X.; Ma, J.F.; Liu, X.J.; Xiang, A.Y.; Ge, F.H. Berberine-loaded solid proliposomes prepared using solution enhanced dispersion by supercritical CO2: Sustained release and bioavailability enhancement. J. Drug Deliv. Sci. Technol. 2019, 51, 356–363. [Google Scholar] [CrossRef]
- Guan, X.S.; Jiang, L.; Cai, L.H.; Zhang, L.; Hu, X.N. A New Co-Crystal of Synthetic Drug Rosiglitazone with Natural Medicine Berberine: Preparation, Crystal Structures, and Dissolution. Molecules 2020, 25, 4288. [Google Scholar] [CrossRef]
- Temussi, P.A.; Lelj, F.; Tancredi, T. 3-dimensional mapping of sweet taste receptor-site. J. Med. Chem. 1978, 21, 1154–1158. [Google Scholar] [CrossRef]
- Tan, D.C.T.; Ong, J.J.; Gokhale, R.; Heng, P.W.S. Hot melt extrusion of ion-exchange resin for taste masking. Int. J. Pharm. 2018, 547, 385–394. [Google Scholar] [CrossRef]
- Yokoyama, T.; Mizuguchi, M.; Nabeshima, Y.; Kusaka, K.; Yamada, T.; Hosoya, T.; Niimura, N. Hydrogen-bond network and pH sensitivity in transthyretin: Neutron crystal structure of human transthyretin. J. Struct. Biol. 2012, 177, 283–290. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and Its Use as a Pharmaceutical Excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Ye, M.Z.; Fu, S.; Pi, R.B.; He, F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J. Pharm. Pharmacol. 2009, 61, 831–837. [Google Scholar] [CrossRef]
- De León, G.; Fröhlich, E.; Salar-Behzadi, S. Bitter taste in silico: A review on virtual ligand screening and characterization methods for TAS2R-bitterant interactions. Int. J. Pharm. 2021, 600, 120486. [Google Scholar] [CrossRef] [PubMed]
- Almurisi, S.H.; Akkawi, M.E.; Chatterjee, B.; Sarker, M.Z.I. Taste masking of paracetamol encapsulated in chitosan-coated alginate beads. J. Drug Deliv. Sci. Technol. 2020, 56, 101520. [Google Scholar] [CrossRef]
- Al-Kasmi, B.; Alsirawan, M.B.; Paradkar, A.; Nattouf, A.H.; El-Zein, H. Aqueous and pH dependent coacervation method for taste masking of paracetamol via amorphous solid dispersion formation. Sci. Rep. 2021, 11, 8907. [Google Scholar] [CrossRef]
- Spaggiari, G.; Di Pizio, A.; Cozzini, P. Sweet, umami and bitter taste receptors: State of the art of in silico molecular modeling approaches. Trends Food Sci. Technol. 2020, 96, 21–29. [Google Scholar] [CrossRef]
- Ji, M.F.; Su, X.B.; Su, X.H.; Chen, Y.Y.; Huang, W.K.; Zhang, J.; Gao, Z.B.; Li, C.G.; Lu, X.F. Identification of Novel Compounds for Human Bitter Taste Receptors. Chem. Biol. Drug Des. 2014, 84, 63–74. [Google Scholar] [CrossRef] [PubMed]



| No. | Amount of CS (mg) | Amount of BER (mg) | NaOH Volume (10% w/v) (mL) | Binding Efficiency (%) |
|---|---|---|---|---|
| 1 | 30 | 54 | 15 | 54.10 ± 2.98 |
| 2 | 40 | 54 | 15 | 47.99 ± 9.99 |
| 3 | 50 | 54 | 15 | 53.27 ± 6.21 |
| 4 | 30 | 20 | 15 | 51.58 ± 3.84 |
| 5 | 30 | 54 | 15 | 48.25 ± 4.39 |
| 6 | 30 | 80 | 15 | 50.15 ± 2.63 |
| 7 | 30 | 54 | 5 | 48.65 ± 7.17 |
| 8 | 30 | 54 | 15 | 56.39 ± 4.50 |
| 9 | 30 | 54 | 30 | 42.79 ± 4.16 |
| No. | Amount of CS (mg) | Amount of PHI (mg) | NaOH Volume (10% w/v) (mL) | Binding Efficiency (%) |
|---|---|---|---|---|
| 1 | 30 | 50 | 15 | 75.96 ± 3.69 |
| 2 | 40 | 50 | 15 | 60.18 ± 1.96 |
| 3 | 50 | 50 | 15 | 48.67 ± 4.02 |
| 4 | 30 | 20 | 15 | 80.43 ± 0.33 |
| 5 | 30 | 50 | 15 | 76.03 ± 3.51 |
| 6 | 30 | 80 | 15 | 67.10 ± 2.52 |
| 7 | 30 | 50 | 5 | 61.46 ± 3.33 |
| 8 | 30 | 50 | 15 | 74.06 ± 2.31 |
| 9 | 30 | 50 | 30 | 75.56 ± 0.31 |
| Group | C-Docker Energy | C-Docker Interaction Energy |
|---|---|---|
| PHI | −38.2429 | 49.0511 |
| BER | −23.7863 | 30.6670 |
| CS | −397.9064 | 22.1711 |
| Group | Cmax (mg/L) | Tmax (h) | t1/2 (h) | AUC (0–t) (mg/L·h) |
|---|---|---|---|---|
| BER | 13.872 ± 3.655 | 1.333 ± 0.577 | 4.667 ± 1.949 | 76.897 ± 7.851 |
| BER/CS | 8.996 ± 3.086 | 1.000 ± 0.000 | 5.127 ± 3.555 | 53.847 ± 37.237 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Sun, Q.; Chen, W.; Han, Y.; Gao, Y.; Ye, J.; Wang, H.; Gao, L.; Liu, Y.; Yang, Y. The Taste-Masking Mechanism of Chitosan at the Molecular Level on Bitter Drugs of Alkaloids and Flavonoid Glycosides from Traditional Chinese Medicine. Molecules 2022, 27, 7455. https://doi.org/10.3390/molecules27217455
Xu Y, Sun Q, Chen W, Han Y, Gao Y, Ye J, Wang H, Gao L, Liu Y, Yang Y. The Taste-Masking Mechanism of Chitosan at the Molecular Level on Bitter Drugs of Alkaloids and Flavonoid Glycosides from Traditional Chinese Medicine. Molecules. 2022; 27(21):7455. https://doi.org/10.3390/molecules27217455
Chicago/Turabian StyleXu, Yaqi, Qianwen Sun, Wei Chen, Yanqi Han, Yue Gao, Jun Ye, Hongliang Wang, Lili Gao, Yuling Liu, and Yanfang Yang. 2022. "The Taste-Masking Mechanism of Chitosan at the Molecular Level on Bitter Drugs of Alkaloids and Flavonoid Glycosides from Traditional Chinese Medicine" Molecules 27, no. 21: 7455. https://doi.org/10.3390/molecules27217455
APA StyleXu, Y., Sun, Q., Chen, W., Han, Y., Gao, Y., Ye, J., Wang, H., Gao, L., Liu, Y., & Yang, Y. (2022). The Taste-Masking Mechanism of Chitosan at the Molecular Level on Bitter Drugs of Alkaloids and Flavonoid Glycosides from Traditional Chinese Medicine. Molecules, 27(21), 7455. https://doi.org/10.3390/molecules27217455





