Organic/Inorganic Species Synergistically Supported Unprecedented Vanadomolybdates
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Compounds 1 and 2
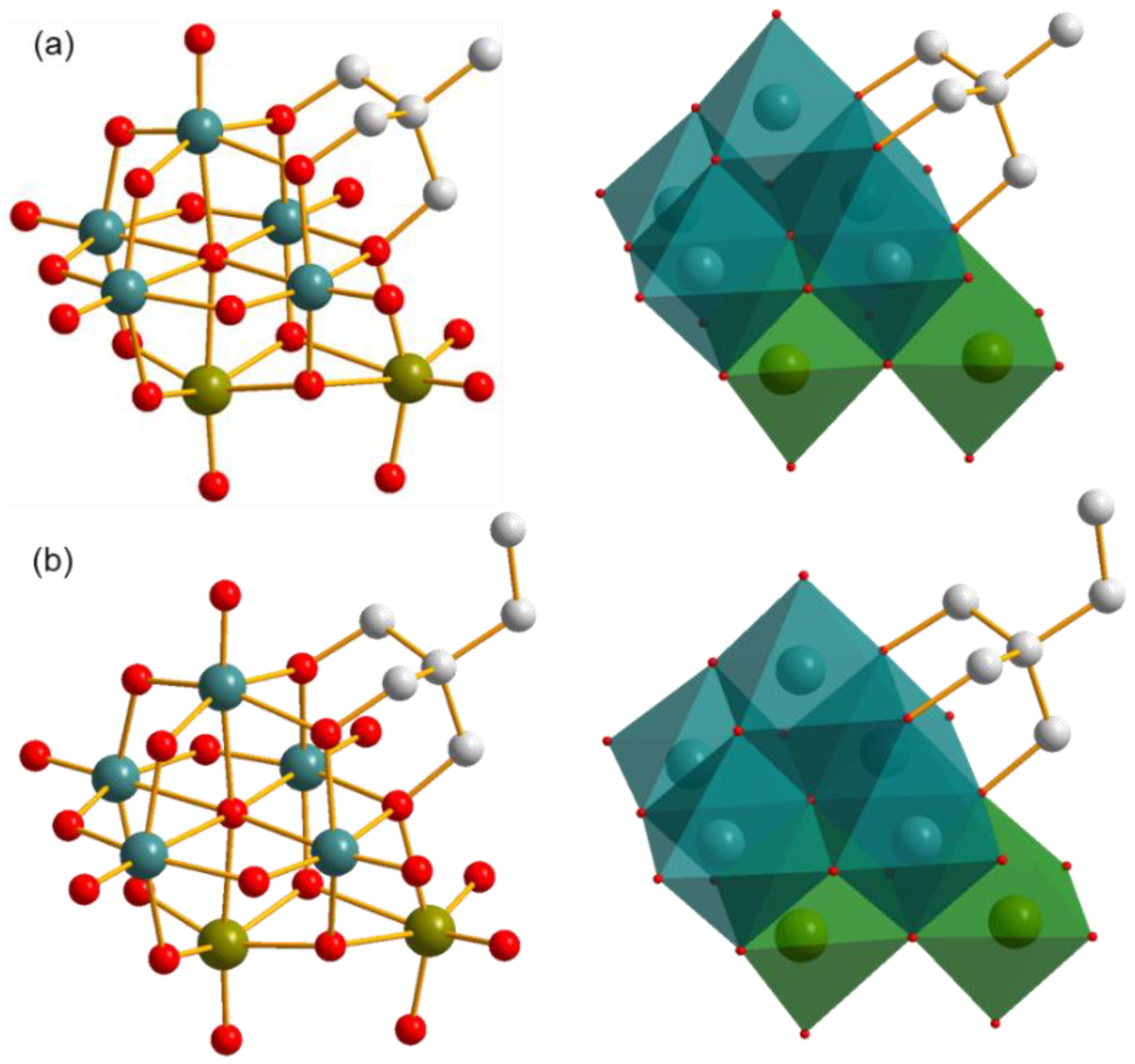
2.2. Structures of Compounds 1 and 2
2.2.1. Inorganic Architecture
2.2.2. The Decoration of Triol Ligand on the Cluster
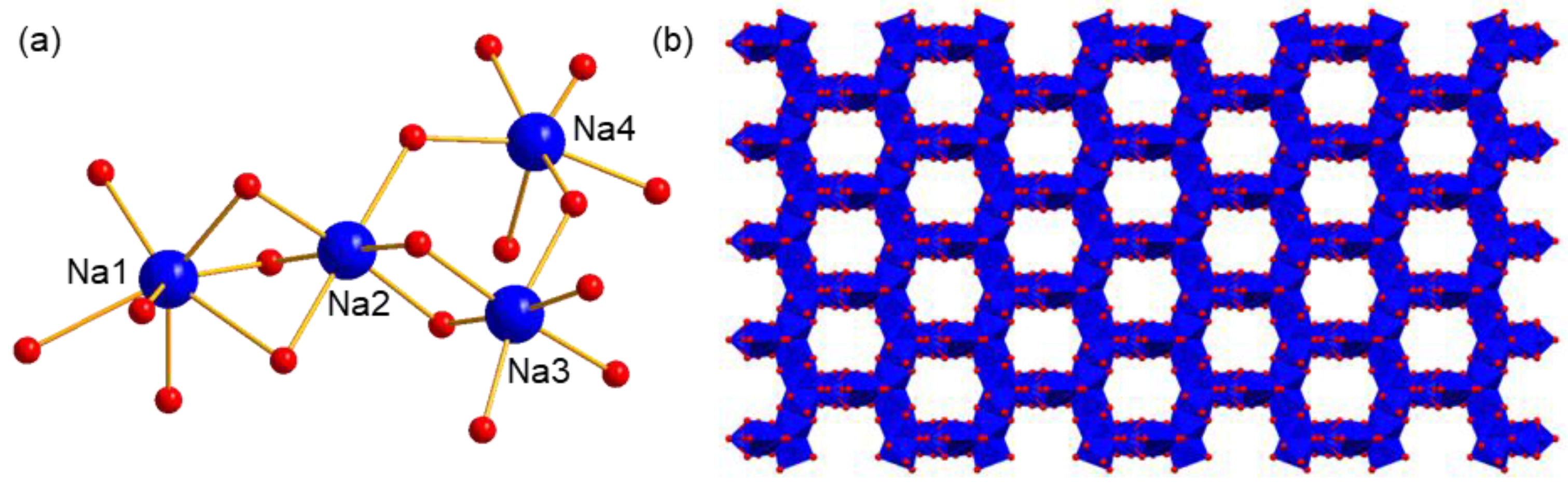
2.2.3. The Assembly Structure of Cations in Compounds 1 and 2
2.2.4. FT-IR, XPS and TGA Curves of Compounds 1 and 2
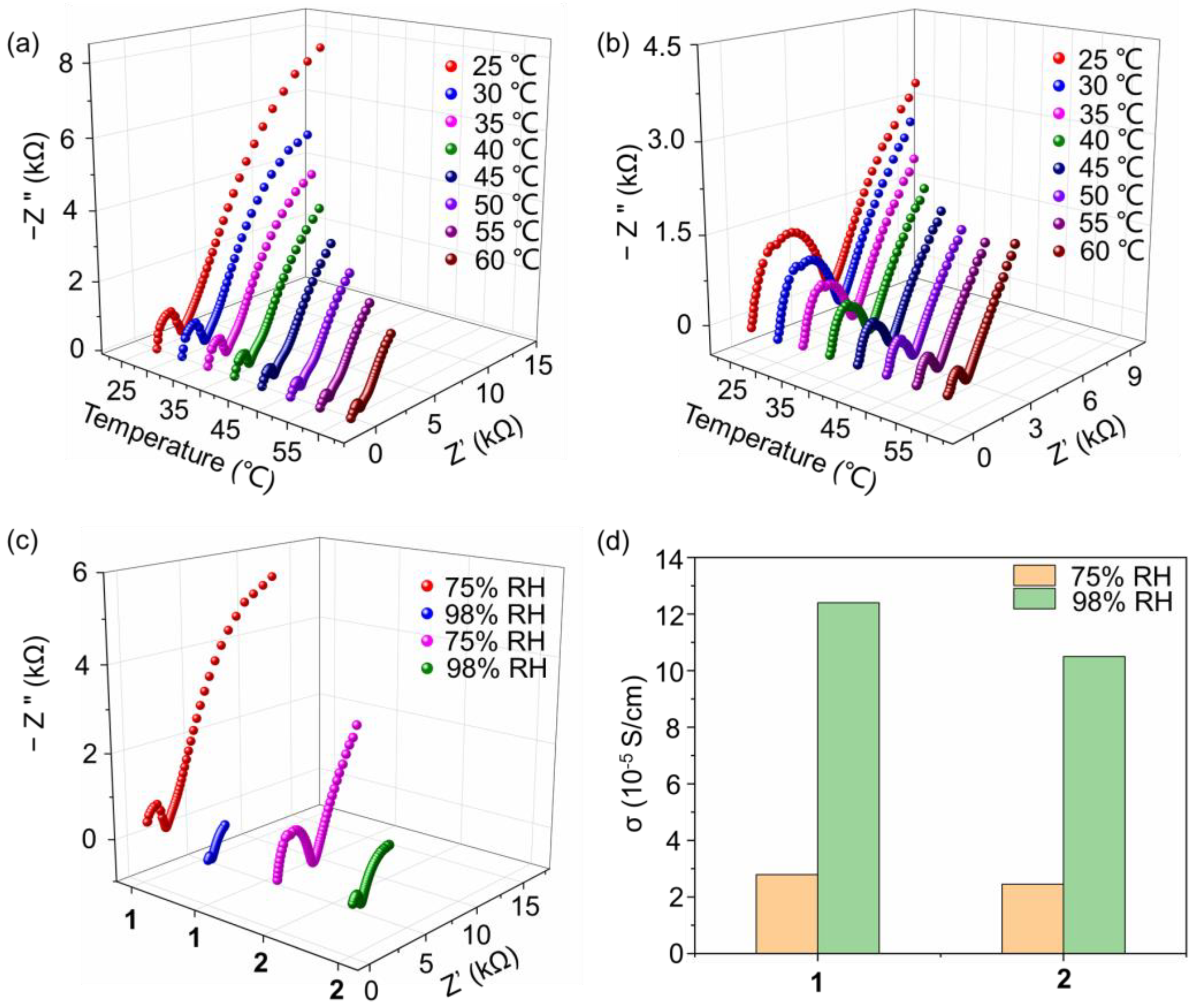
2.2.5. Stability of Compounds 1 and 2 in Aqueous Solution
2.3. Proton Conductivity of Compounds 1 and 2
3. Materials and Methods
3.1. Instruments and Materials
3.2. Synthesis of Compounds 1 and 2
3.2.1. Synthesis of Na4{V5Mo2O19[CH3C(CH2O)3]}∙13H2O (1)
3.2.2. Synthesis of Na4{V5Mo2O19[CH3CH2C(CH2O)3]}∙13H2O (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wei, Z.; Wang, J.; Yu, H.; Han, S.; Wei, Y. Recent Advances of Anderson-Type Polyoxometalates as Catalysts Largely for Oxidative Transformations of Organic Molecules. Molecules 2022, 27, 5212. [Google Scholar] [CrossRef] [PubMed]
- Thorimbert, S.; Hasenknopf, B.; Lacôte, E. Cross-Linking Organic and Polyoxometalate Chemistries. Isr. J. Chem. 2011, 51, 275–280. [Google Scholar] [CrossRef]
- Pardiwala, A.; Kumar, S.; Jangir, R. Insights into organic-inorganic hybrid molecular materials: Organoimido functionalized polyoxomolybdates. Dalton Trans. 2022, 51, 4945–4975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, F.; Hao, J.; Wei, Y. The chemistry of organoimido derivatives of polyoxometalates. Dalton Trans. 2012, 41, 3599–3615. [Google Scholar] [CrossRef]
- Cameron, J.M.; Guillemot, G.; Galambos, T.; Amin, S.S.; Hampson, E.; Mall Haidaraly, K.; Newton, G.N.; Izzet, G. Supramolecular assemblies of organo-functionalised hybrid polyoxometalates: From functional building blocks to hierarchical nanomaterials. Chem. Soc. Rev. 2022, 51, 293–328. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.T.; Xu, W.; Yue, Y.; Li, B.; Wu, L.X. Triol-ligand modification and structural transformation of Anderson-Evans oxomolybdates via modulating oxidation state of Co-heteroatom. Inorg. Chem. 2017, 56, 7019–7028. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.L.; Jiang, F.R.; Li, B.; Wu, L.X. A closed hollow capsule structure assembled by double acetate-decorated Anderson-like polyanions. J. Coord. Chem. 2017, 70, 25–35. [Google Scholar] [CrossRef]
- Ma, P.T.; Hu, F.; Wang, J.P.; Niu, J.Y. Carboxylate covalently modified polyoxometalates: From synthesis, structural diversity to applications. Coord. Chem. Rev. 2019, 378, 281–309. [Google Scholar] [CrossRef]
- Wang, Y.R.; Duan, F.X.; Liu, X.T.; Li, B. Cations Modulated Assembly of Triol-Ligand Modified Cu-Centered Anderson-Evans Polyanions. Molecules 2022, 27, 2933. [Google Scholar] [CrossRef]
- Duan, F.X.; Liu, X.T.; Qu, D.; Li, B.; Wu, L.X. Polyoxometalate-based ionic frameworks for highly selective CO2 capture and separation. CCS Chem. 2020, 2, 2676–2687. [Google Scholar] [CrossRef]
- Zhang, J.W.; Huang, Y.C.; Li, G.; Wei, Y.G. Recent advances in alkoxylation chemistry of polyoxometalates: From synthetic strategies, structural overviews to functional applications. Coord. Chem. Rev. 2019, 378, 395–414. [Google Scholar] [CrossRef]
- Jia, H.; Li, Q.; Bayaguud, A.; Huang, Y.; She, S.; Chen, K.; Wei, Y. Diversified polyoxovanadate derivatives obtained by copper(i)-catalysed azide-alkyne cycloaddition reaction: Their synthesis and structural characterization. Dalton Trans. 2018, 47, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.P.; Pal, A.K.; Hanan, G.S.; Tang, M.C.; Furtos, A.; Hasenknopf, B. A light-harvesting polyoxometalate-polypyridine hybrid induces electron transfer as its Re(I) complex. Dalton Trans. 2014, 43, 6990–6993. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Li, B.; Wu, L.X. Layered supramolecular network of cyclodextrin triplets with azobenzene-grafting polyoxometalate for dye degradation and partner-enhancement. Chem. Commun. 2021, 57, 10512–10515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Q.; Tao, R.; Li, F.; Sun, Z.; Xu, L. Molybdovanadate [VMo7O26]5− cluster directed inorganic-organic hybrid: The highest coordination linkage and In Situ isoniazid dimerization. Inorg. Chem. Commun. 2018, 89, 94–98. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, D.H.; Duan, M.H.; Li, D.H. A novel organic-inorganic hybrid built upon both [VMo6O24]6− and [Mo7O24]6− units: Synthesis, crystal structure, surface photovoltage and electrocatalytic activities. J. Mol. Struct. 2019, 1184, 400–404. [Google Scholar] [CrossRef]
- Krivosudsky, L.; Roller, A.; Rompel, A. Regioselective synthesis and characterization of monovanadium-substituted beta-octamolybdate [VMo7O26]5−. Acta Cryst. C 2019, 75, 872–876. [Google Scholar] [CrossRef]
- Amini, M.; Sheykhi, A.; Naslhajian, H.; Bayrami, A.; Bagherzadeh, M.; Hołyńska, M. A novel 12-molybdovanadate nanocluster: Synthesis, structure investigation and its application as an efficient heterogeneous sulfoxidation catalyst. Inorg. Chem. Commun. 2017, 83, 103–108. [Google Scholar] [CrossRef]
- Odyakov, V.F.; Zhizhina, E.G.; Rodikova, Y.A.; Gogin, L.L. Mo-V-Phosphoric Heteropoly Acids and Their Salts: Aqueous Solution Preparation—Challenges and Perspectives. Eur. J. Inorg. Chem. 2015, 2015, 3618–3631. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Gao, G.; Li, F.; Liu, X.; Guo, W. An Unexpected Ferromagnetic Coupling in a Dinuclear Manganese(II) Linked Trivacant Heteropolymolybdate Derivative. Eur. J. Inorg. Chem. 2009, 2009, 1460–1463. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Ge, J.; Sui, C.; Hao, J.; Wei, Y. [V4Mo3O14(NAr)3(μ2-NAr)3]2−: The first polyarylimido-stabilized molybdovanadate cluster. Chem. Commun. 2017, 53, 2551–2554. [Google Scholar] [CrossRef]
- Cheng, M.; Xiao, Z.; Yu, L.; Lin, X.; Wang, Y.; Wu, P. Direct syntheses of nanocages and frameworks based on Anderson-type polyoxometalates via one-pot reactions. Inorg. Chem. 2019, 58, 11988–11992. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, F.; Wang, Y.; Xu, L.; Bai, J.; Wang, Y. Organic functionalization of polyoxometalate in aqueous solution: Self-assembly of a new building block of {VMo6O25} with triethanolamine. Dalton Trans. 2014, 43, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Liu, X.T.; Duan, F.X.; Xue, R.; Li, B.; Wu, L.X. {VMo9O31[RC(CH2O)3]}6–: The first class of triol ligand covalently-decorated Keggin-type polyoxomolybdates. Dalton Trans. 2020, 49, 12950–12954. [Google Scholar] [CrossRef]
- Chen, Q.; Goshorn, D.P.; Scholes, C.P.; Tan, X.L.; Zubieta, J. Coordination compounds of polyoxovanadates with a hexametalate core. Chemical and structural characterization of [VV6O13[(OCH2)3CR]2]2−, [VV6O11(OH)2[(OCH2)3CR]2], [VIV4VV2O9(OH)4[(OCH2)3CR]2]2-, and [VIV6O7(OH)6](OCH2)3CR]2]2. J. Am. Chem. Soc. 1992, 114, 4667–4681. [Google Scholar] [CrossRef]
- Howarth, O.W.; Pettersson, L.; Andersson, I. Aqueous molybdovanadates at high Mo: V ratio. J. Chem. Soc. Dalton Trans. 1991, 1799–1812. [Google Scholar] [CrossRef]
- Tucher, J.; Wu, Y.; Nye, L.C.; Ivanovic-Burmazovic, I.; Khusniyarov, M.M.; Streb, C. Metal substitution in a Lindqvist polyoxometalate leads to improved photocatalytic performance. Dalton Trans. 2012, 41, 9938–9943. [Google Scholar] [CrossRef] [PubMed]
- Himeno, S.; Ishio, N. A voltammetric study on the formation of V(V)- and V(IV)-substituted molybdophosphate(V) complexes in aqueous solution. J. Electroanal. Chem. 1998, 451, 203–209. [Google Scholar] [CrossRef]
- Zhang, S.W.; Huang, G.Q.; Wei, Y.G.; Shao, M.C.; Tang, Y.Q. Structure of Na3[VMo12O40]·19H2O. Acta Cryst. C 1993, 49, 1446–1448. [Google Scholar] [CrossRef]
- Kodama, S.; Nomoto, A.; Yano, S.; Ueshima, M.; Ogawa, A. Novel Heterotetranuclear V2Mo2 or V2W2 Complexes with 4,4′-Di-tert-butyl-2,2′-bipyridine: Syntheses, Crystal Structures, and Catalytic Activities. Inorg. Chem. 2011, 50, 9942–9947. [Google Scholar] [CrossRef]
- Maksimovskaya, R.I.; Chumachenko, N.N. 51V and 17O NMR studies of the mixed metal polyanions in aqueous V—Mo solutions. Polyhedron 1987, 6, 1813–1821. [Google Scholar] [CrossRef]
- Björnberg, A. Multicomponent polyanions. 26. The crystal structure of Na6Mo6V2O26(H2O)16, a compound containing sodium-coordinated hexamolybdodivanadate anions. Acta Cryst. B 1979, 35, 1995–1999. [Google Scholar] [CrossRef]
- Li, F.; Meng, F.; Ma, L.; Xu, L.; Sun, Z.; Gao, Q. 3D pure inorganic framework based on polymolybdovanadate possessing photoelectric properties. Dalton Trans. 2013, 42, 12079–12082. [Google Scholar] [CrossRef]
- Liang, D.-D.; Liu, S.-X.; Ren, Y.-H.; Zhang, C.-D.; Xu, L. [Mo18O54(VO4)2]6−: A conventional Dawson structure with unpredicted transition metal hetero-atoms based on VVO4 tetrahedra. Inorg. Chem. Commun. 2007, 10, 933–935. [Google Scholar] [CrossRef]
- Gao, Q.; Li, F.; Xu, L. 1D pure inorganic helical chain framework built upon banana-shaped molybdovanadates: Synthesis, crystal structure, and magnetic properties. Inorg. Chem. Commun. 2015, 59, 50–52. [Google Scholar] [CrossRef]
- Karoui, H.; Ritchie, C. Microwave-assisted synthesis of organically functionalized hexa-molybdovanadates. New J. Chem. 2018, 42, 25–28. [Google Scholar] [CrossRef]
- Cindrić, M.; Kamenar, B.; Strukan, N.; Veksli, Z. Synthesis, structure and ESR spectrum of (Hmorph)6[VIV, VV, Mo10)VO40]·3H2O. Polyhedron 1995, 14, 1045–1049. [Google Scholar] [CrossRef]
- Miras, H.N.; Long, D.L.; Kogerler, P.; Cronin, L. Bridging the gap between solution and solid state studies in polyoxometalate chemistry: Discovery of a family of [V1M17]-based cages encapsulating two {VVO4} moieties. Dalton Trans. 2008, 214–221. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; Dillinger, S.; Bögge, H.; Plass, W.; Proust, A.; Dloczik, L.; Menke, C.; Meyer, J.; Rohlfing, R. New Perspectives in Polyoxometalate Chemistry by isolation of compounds containing very large moieties as transferable building blocks: (NMe4)5[As2Mo8V4AsO40]·3H2O, (NH4)21[H3Mo57V6(NO)6O183(H2O)18]·65H2O, (NH2Me2)18(NH4)6[Mo57V6(NO)6O183(H2O)18]·14H2O, and (NH4)12[Mo36(NO)4O108(H2O)16]·33H2O. Z. Anorg. Allg. Chem. 1994, 620, 599–619. [Google Scholar] [CrossRef]
- Kamenar, B.; Cindrić, M.; Strukan, N. Synthesis and structure of a molybdovanadate with the asymmetric [Mo4V5O27]5− anion. Polyhedron 1994, 13, 2271–2275. [Google Scholar] [CrossRef]
- Björnberg, A. Multicomponent polyanions. 28. The structure of K7Mo8V5O40·~8H2O, a compound containing a structurally new potassium-coordinated octamolybdopentavanadate anion. Acta Cryst. B 1980, 36, 1530–1536. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Huang, G.-Q.; Shao, M.-C.; Tang, Y.-Q. Crystal structure of a novel mixed valence MoV–MoVI heteropolymolybdate cluster: [H3O+]6[Mo57V6O183(NO)6(H2O)18]6–·89H2O. J. Chem. Soc. Chem. Commun. 1993, 37–38. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, Z.; Li, Y.; Wang, E. Two dumbbell-like polyoxometalates constructed from capped molybdovanadate and transition metal complexes. Inorg. Chim. Acta 2010, 363, 2131–2136. [Google Scholar] [CrossRef]
- Corella-Ochoa, M.N.; Miras, H.N.; Long, D.L.; Cronin, L. Controlling the self-assembly of a mixed-metal Mo/V-selenite family of polyoxometalates. Chem. Eur. J. 2012, 18, 13743–13754. [Google Scholar] [CrossRef] [PubMed]
- Björnberg, A. Multicomponent polyanions. 22. The molecular and crystal structure of K8Mo4V8O36·12H2O, a compound containing a structurally new heteropolyanion. Acta Cryst. B 1979, 35, 1989–1995. [Google Scholar] [CrossRef]
- Zhang, Y.; Haushalter, R.C.; Clearfield, A. A heteropolyanion containing two linked mixed Mo/V pentadecaoxometalate clusters: Structure of [Mo16V14O84]14−. J. Chem. Soc. Chem. Commun. 1995, 1149–1150. [Google Scholar] [CrossRef]
- Salazar Marcano, D.E.; Moussawi, M.A.; Anyushin, A.V.; Lentink, S.; Van Meervelt, L.; Ivanović-Burmazović, I.; Parac-Vogt, T.N. Versatile post-functionalisation strategy for the formation of modular organic–inorganic polyoxometalate hybrids. Chem. Sci. 2022, 13, 2891–2899. [Google Scholar] [CrossRef]
- Fernandez-Navarro, L.; Nunes-Collado, A.; Artetxe, B.; Ruiz-Bilbao, E.; San Felices, L.; Reinoso, S.; San Jose Wery, A.; Gutierrez-Zorrilla, J.M. Isolation of the Elusive Heptavanadate Anion with Trisalkoxide Ligands. Inorg. Chem. 2021, 60, 5442–5445. [Google Scholar] [CrossRef] [PubMed]

| Atomic V/Mo Ratio a | Formula of Polyanion b | Valence of V | Valence of Mo | Ref. |
|---|---|---|---|---|
| 1:4 | [VMo4O17]5− | +5 | +6 | [26] |
| 1:5 | [VMo5O19]3− | +5 | +6 | [27] |
| 1:6 | [VMo6O24]6− | +5 | +6 | [16] |
| 1:7 | [VMo7O26]5− | +5 | +6 | [15] |
| 1:9 | {VMo9O31[CH3C(CH2O)3]}6− | +5 | +6 | [24] |
| 1:11 | [P(VMo11)O40]4− | +5 | +6 | [28] |
| 1:12 | [VMo12O40]3− | +5 | +6 | [29] |
| 2:2 | [V2O2(μ-MeO)2(μ-MoO4)2(4,4′-tBubpy)2] | +5 | +6 | [30] |
| 2:4 | [V2Mo4O19]4− | +5 | +6 | [31] |
| 2:6 | [V2Mo6O26]6− | +5 | +6 | [32] |
| 2:8 | [HV2Mo8O32]5− | +5 | +6 | [26] |
| 2:10 | [HV2Mo10O38]5− | +5 | +6 | [26] |
| 2:16 | [V2Mo16O58]10− | +5 | +6 | [33] |
| 2:18 | [V2Mo18O62]6− | +5 | +6 | [34] |
| 2:22 | [Fe5CoMo22V2O87(H2O)]}12− | +5 | +6 | [35] |
| 3:3 | [V3Mo3O16(C5H9O3)]2− | +5 | +6 | [36] |
| 3:9 | [V3Mo9O38]7− | +5 | +6 | [26] |
| 3:10 | [V(VIVVVMo10O40)]6− | +4, +5 | +6 | [37] |
| 3:17 | [H2VIVMo17O54(VVO4)2]6− | +4, +5 | +6 | [38] |
| 4:3 | [V4Mo3O14(NAr)3(μ2-NAr)3]9− | +5 | +6 | [21] |
| 4:8 | [As2V4Mo8AsO40]5− | +5 | +6 | [39] |
| 5:2 | {V5Mo2O19[CH3C(CH2O)3]}4− | +5 | +6 | This work |
| 5:4 | [V5Mo4O27]5− | +5 | +6 | [40] |
| 5:8 | [V5Mo8O40]7− | +5 | +6 | [41] |
| 6:57 | [V6Mo57O183(NO)6(H2O)18]6− | +5 | +6 | [42] |
| 7:8 | [(VVMo8V4IVO40)(VIVO)2]7− | +4, +5 | +6 | [43] |
| 7:11 | [VV5VIV2Mo11O52(SeO3)]7− | +4, +5 | +6 | [44] |
| 8:2 | [V8Mo2O28]4− | +5 | +6 | [26] |
| 8:4 | [V8Mo4O36]8− | +5 | +6 | [45] |
| 9:1 | [V9MoO28]5− | +5 | +6 | [31] |
| 9:8 | [(VVMoVI4MoV4V4IVO40)(VIVO)4]7− | +4, +5 | +6 | [43] |
| 10:12 | [Mo12V10O58(SeO3)8]10− | +5 | +6 | [44] |
| 14:16 | [V12IVVV2Mo16O84]14− | +4, +5 | +6 | [46] |
| Item | Compound 1 | Compound 2 |
|---|---|---|
| Formula | Na4{V5Mo2O19[CH3C-(CH2O)3]}∙13H2O | Na4{V5Mo2O19[CH3CH2C-(CH2O)3]}∙13H2O |
| Formula weight | 1193.87 | 1207.89 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/m | C2/m |
| a (Å) | 22.294 (5) | 22.170 (1) |
| b (Å) | 10.180 (2) | 10.146 (1) |
| c (Å) | 16.394 (4) | 16.777 (1) |
| α (deg.) | 90 | 90 |
| β (deg.) | 114.233 (8) | 112.257 (2) |
| γ (deg.) | 90 | 90 |
| V (Å3) | 3392.6 (12) | 3492.5 (3) |
| Z | 4 | 4 |
| Dc (g cm−3) | 2.337 | 2.297 |
| F (000) | 2352 | 2384 |
| Reflections coll./unique | 24947/4045 | 21017/3261 |
| Rint | 0.0785 | 0.0315 |
| GOOF on F2 | 1.029 | 1.111 |
| a R1 [I > 2σ(I)] | 0.0468 | 0.0410 |
| b wR2 (all data) | 0.1154 | 0.1218 |
| CCDC no. | 2206198 | 2206199 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.; Qu, D.; Li, B.; Wu, L. Organic/Inorganic Species Synergistically Supported Unprecedented Vanadomolybdates. Molecules 2022, 27, 7447. https://doi.org/10.3390/molecules27217447
Chang T, Qu D, Li B, Wu L. Organic/Inorganic Species Synergistically Supported Unprecedented Vanadomolybdates. Molecules. 2022; 27(21):7447. https://doi.org/10.3390/molecules27217447
Chicago/Turabian StyleChang, Tian, Di Qu, Bao Li, and Lixin Wu. 2022. "Organic/Inorganic Species Synergistically Supported Unprecedented Vanadomolybdates" Molecules 27, no. 21: 7447. https://doi.org/10.3390/molecules27217447
APA StyleChang, T., Qu, D., Li, B., & Wu, L. (2022). Organic/Inorganic Species Synergistically Supported Unprecedented Vanadomolybdates. Molecules, 27(21), 7447. https://doi.org/10.3390/molecules27217447






