Abstract
Aryl-C-glycosides, of both synthetic and natural origin, are of great significance in medicinal chemistry owing to their unique structures and stability towards enzymatic and chemical hydrolysis as compared to O-glycosides. They are well-known antibiotics and potent enzyme inhibitors and possess a wide range of biological activities such as anticancer, antioxidant, antiviral, hypoglycemic effects, and so on. Currently, a number of aryl-C-glycoside drugs are on sale for the treatment of diabetes and related complications. This review summarizes the findings on aryl-C-glycoside scaffolds over the past 20 years, concerning new structures (over 200 molecules), their bioactivities—including anticancer, anti-inflammatory, antioxidant, antivirus, glycation inhibitory activities and other pharmacological effects—as well as their synthesis.
1. Introduction
Aryl-C-glycosides (ACGs), natural secondary metabolites, are glycosides in which the anomeric center is covalently linked to the carbon atom of arenes or heterocycles (Csp3-Csp2) [1]. They tend to have high oral bioavailability and reach high plasma levels without needing to be converted to a prodrug. The stable linkage between the sugar and the arene or heterocycle moieties is resistant to enzymatic hydrolysis, allowing these compounds to interfere with DNA and RNA synthases more efficiently [2]. Both natural and synthetic aryl-C-glycosides are of marked pharmaceutical interest, and many of them have proven to be efficient antibiotics, antitumor agents, and antidiabetics. Therefore, aryl-C-glycosides have received considerable attention [3].
Beginning with the structural elucidation of the first aryl-C-glycoside in 1970 [4], the study of aryl-C-glycosides has continued to flourish through the constant isolation/characterization of new aryl-C-glycoside natural products, the characterization of the corresponding biosynthetic pathways/enzymes, and the development of aryl-C-glycoside synthetic methods. As their name suggests, the core structure of aryl-C-glycosides is often abundantly decorated with substituents such as aromatic acids (e.g., gallic acid, caffeic acid, vanillic acid, benzoic acid, and ferulic acid) and various saccharides (e.g., L-rhamnose, D-xylose, D-glucose, D-galactose, and L-arabinose) through ester or glycosidic linkages, respectively. The outstanding activity of aryl-C-glycosides against diverse diseases proves their importance in medicinal chemistry research. Several reviews on aryl C-glycosides regarding their isolation and purification, structure elucidation, synthesis and biosynthesis, and pharmacological activities have been published [5,6,7]. Recently, interest in aryl-C-glycoside has been growing, as shown by a significantly increasing volume of literature describing novel structures, diverse bioactivities, general synthesis, and evident roles of these molecules in the prevention and treatment of various human diseases [8]. Such rich information prompted me to review papers on novel aryl-C-glycoside structures, pharmacological activities, and chemical synthesis published in the last two decades. This review will highlight the new structures (over 200 new aryl-C-glycoside molecules), bioactivities, and synthetic approaches to the preparation of aryl-C-glycosides, which were published in prestigious journals such as Journal of the American Chemical Society, Angewandte Chemie International Edition, Journal of Natural Products, Organic Letters, Phytochemistry and in related peer-reviewed natural product research journals, from 2002 to 2022. A summary of the structures, bioactivities, and origins of recently discovered aryl-C-glycoside molecules is provided in Table 1.

Table 1.
Structures, Bioactivities, Sources of Aryl-C-glycoside Molecules.
2. Structures
2.1. Flavonoid C-Glycosides
Apigenin and luteolin represent the major parent nuclei of flavonoid C-glycosides; the most common glycan moieties linked to C-glycosides are D-glucose, D-galactose, D-xylose, D-mannose, D-ribose, L-fucose, L-arabinose, and L-rhamnose.
2.1.1. Flavonoid C-Glucosides
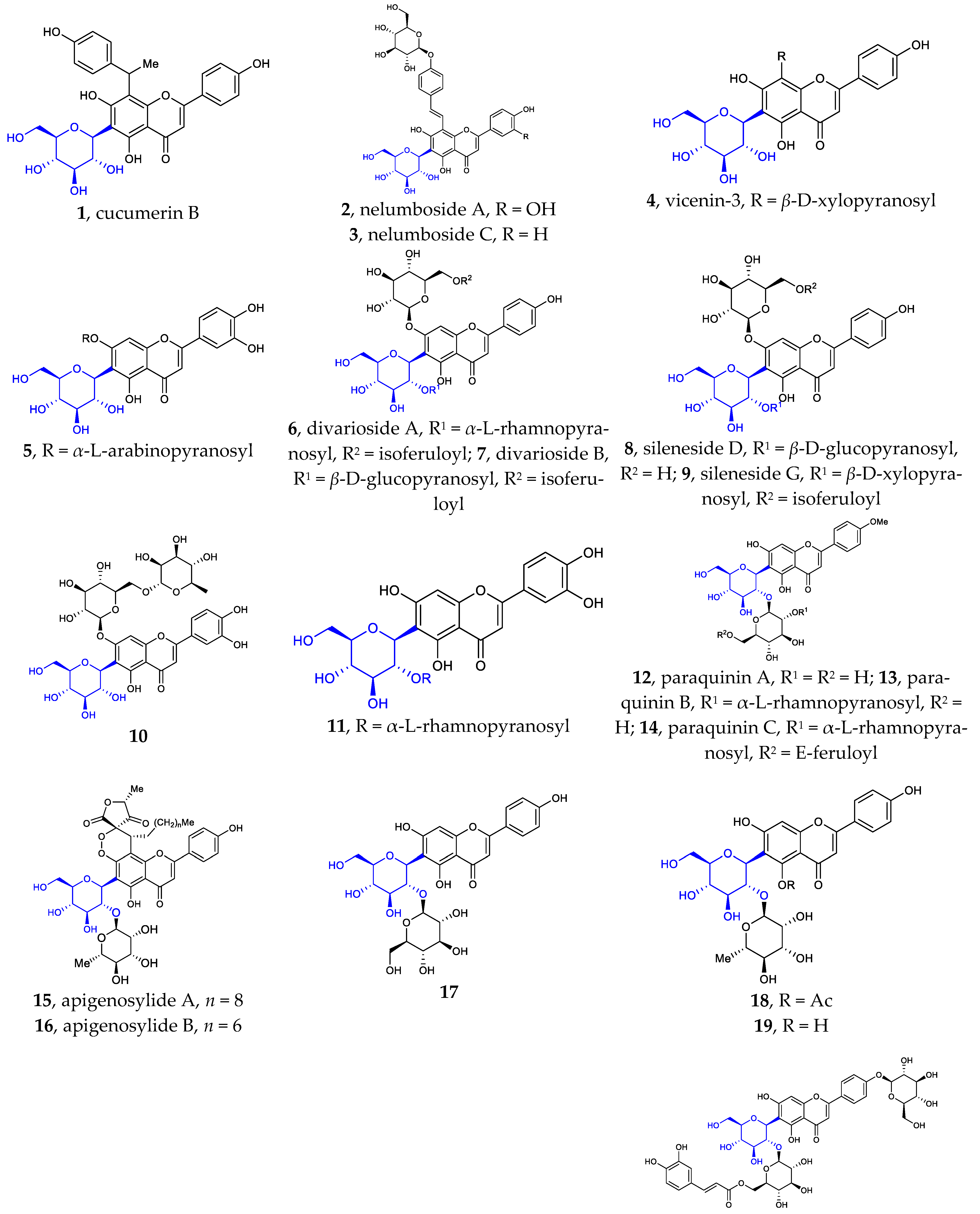
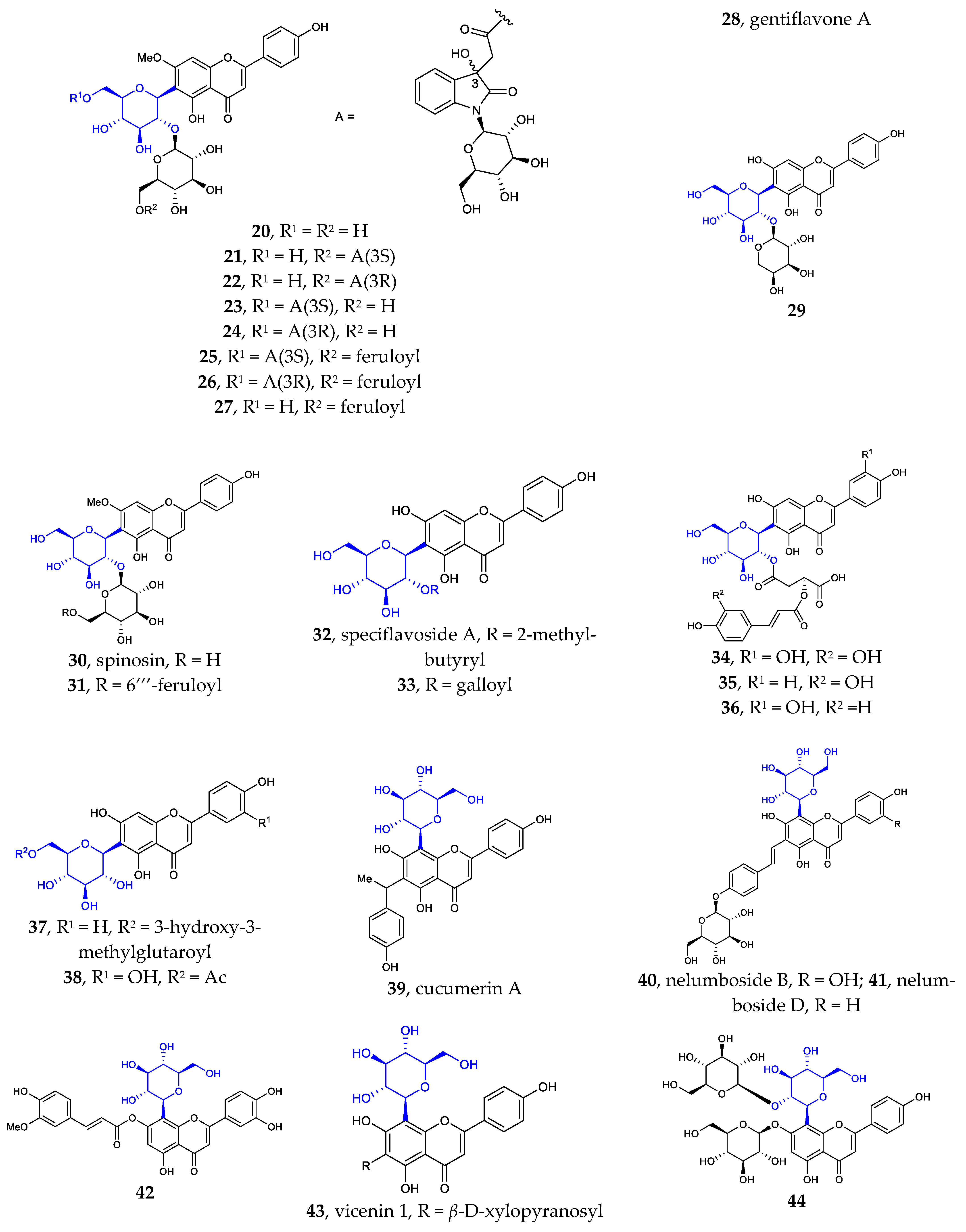
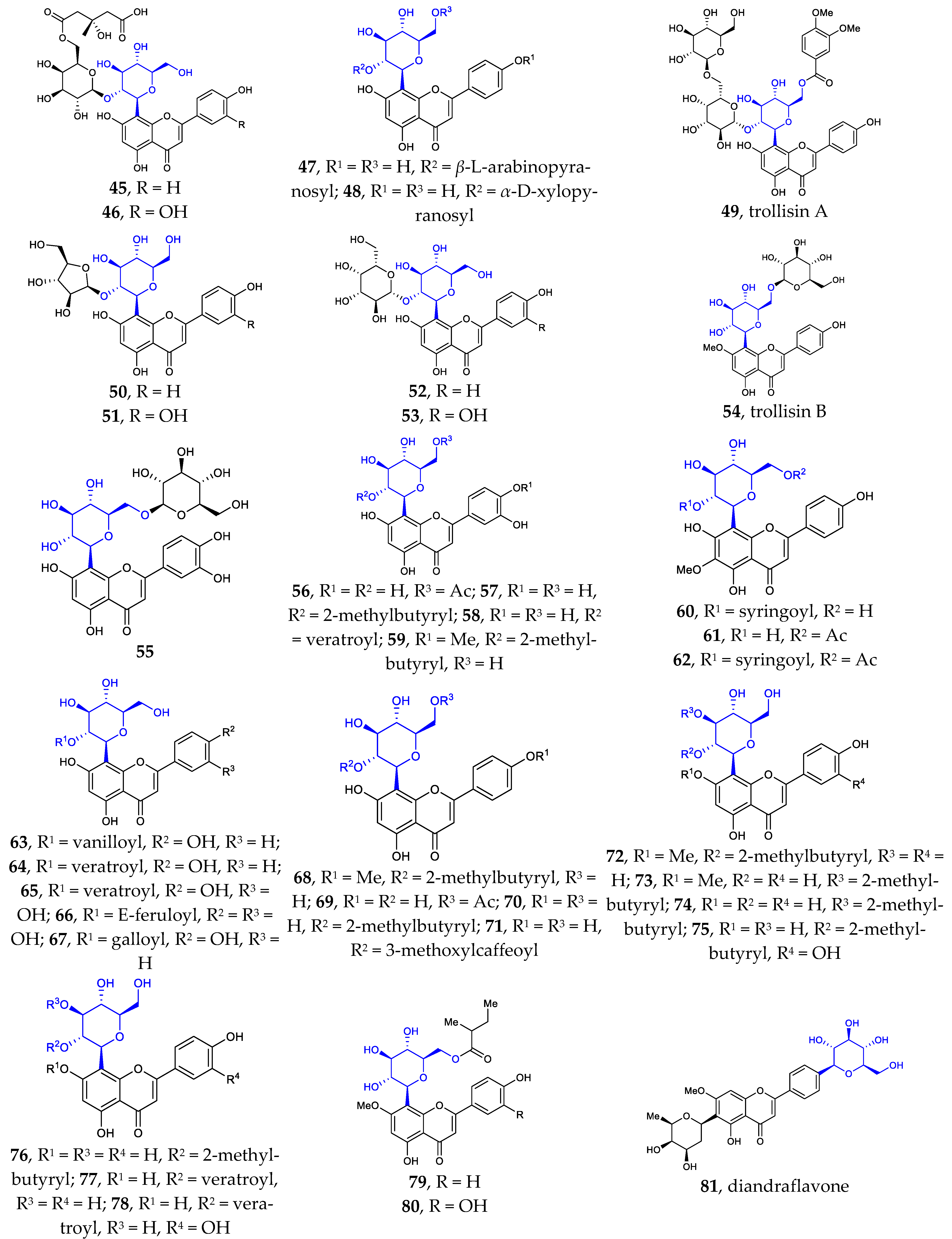
To the best of my knowledge, Flavonoid C-α-D-glucopyranosides, Flavonoid C-α-L-glucopyranosides, and Flavonoid C-β-L-glucopyranosides have not been isolated and identified from natural source. Flavonoid C-β-D-glucopyranosides are the largest group of isolated aryl-C-glycosides. Since 2002, more than 80 new compounds have been isolated and identified. It was found that the D-glucose unit is directly attached to the flavonoid or flavonoid derivatives in β configuration. Compared with the known aryl-C-glycosides previously reported, some of the new ones differ in their core structure, while others differ in the number and/or position of their substituents. The sites of glycosylation in the flavonoids are usually C6 and/or C8. Very few examples are known of C-glycosylation occurring at position C4′.
Figure 1 illustrates the new aryl-C-glycosides with varied core structures or special substituents. These structures differ in the aglycon and or glucoside portions. The core structures are often abundantly decorated with substituents such as aromatic acids (e.g., benzoic acid, 2-methyl butyric acid, gallic acid, veratric acid, sinapic acid, ferulic acid, and acetic acid) and various saccharides (e.g., L-rhamnose, D-xylose, D-glucose, D-galactose, and L-arabinose) through ester or glycosidic linkages, respectively. These isolated structures are often associated with other known compounds, such as flavonoids, lignan, sesquiterpene, steroid, alkaloids, O-glycosides, etc.
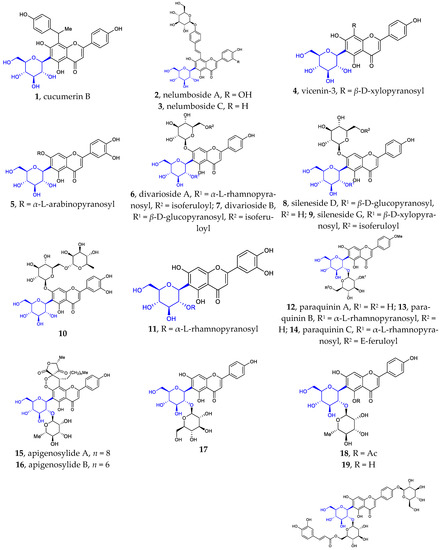
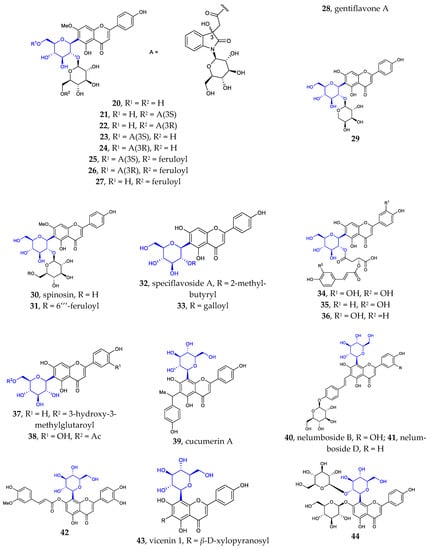
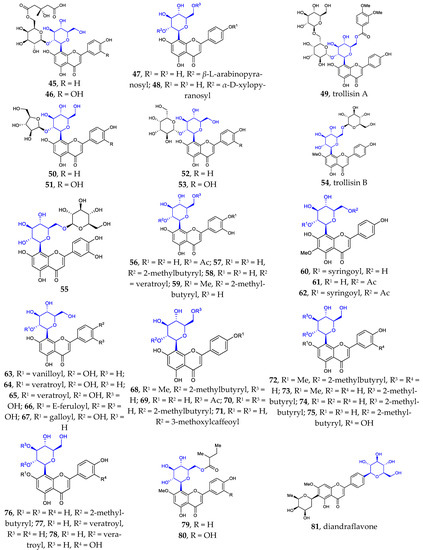
Figure 1.
Chemical structures of flavonoid 6- or 8-C-β-D-glucopyranosides (1-80) and flavonoid 4′-C-β-D-glucopyranoside (81).
Besides the well-known flavonoid C-glycosides vitexin, isovitexin, orientin, isoorientin, schaftoside, isoshaftoside, neoshaftoside, and their O-glycosylated and or O-acylated derivatives, more and more flavonoid C-glycosides were disclosed. These 6- or 8-C-β-D-glucopyranosides can be divided into five groups: (1) mono-C-glycosylated flavonoids 1–3 [12,37], 39–42 [12,13,37]; (2) 6, 8-di-C-glycosylated flavonoids 4 [38], 43 [39]; (3) flavonoid 6- or 8-C-β-D-glucopyranoside-7-O-β-D-glucopyranosides 5–10 [40,41,42,43], 44 [44]; (4) 2″- or 6″-O-glycosylated flavonoid 6- or 8-C-β-D-glucopyranosides 11–31 [9,38,45,46,47,48,49,50], 45–55 [14,51,52,53,54,55,56,57]; (5) 2″- or 6″-O-acylated flavonoid 6- or 8-C-β-D-glucopyranosides 32–38 [10,11,55,56], 56–80 [57,58,59,60,61]. Some of the above compounds are glycosylated with β-D-glucose, a disaccharide consisting of β-D-glucose glycosylated with a monosaccharide at position C2 or C6, or β-D-glucose acylated at C2 or C6. Such glycosylation with various sugars and/or acylation with various acids is a common strategy used by nature to introduce structural diversity and different biological activities in natural products.
It is noteworthy that flavonoid di-C-glycoside 81 features the β-D-glucopyranosyl and β-D-oliopyranosyl groups attached to C-4′ and C-6, respectively [16]. This kind of compound is very rare in nature.
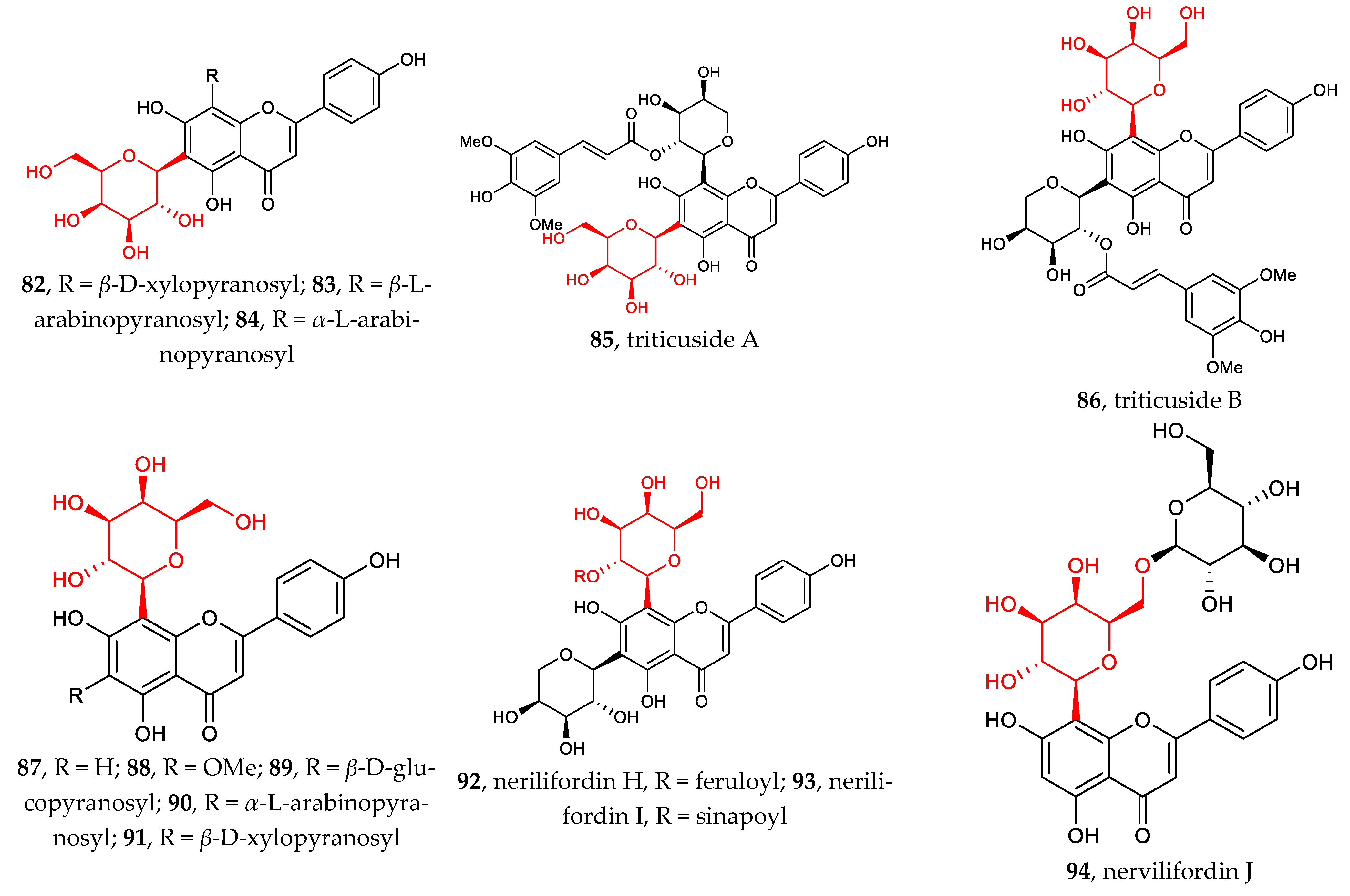
2.1.2. Flavonoid C-Galactosides, C-Arabinosides, C-Xylosides, C-Mannosides, C-Fucosides, C-Boivinosides, and C-Riboside
Compared with flavonoid C-glucosides, flavonoid C-galactosides are less common in nature. Compounds 82–94 have diversified structures which can be classified into two groups: (1) flavonoid 6-C-β-D-galactopyranosides, 82–85 [62,63,64]; (2) flavonoid 8-C-β-D-galactopyranosides, 86–94 [64,65,66]. To date, no flavonoids galactosylated at other sites than C6 and C8 have been discovered. The hydroxyl groups of D-galactose or other sugars are often acylated or glycosylated, which leads to multiple complex structures with unique functions.
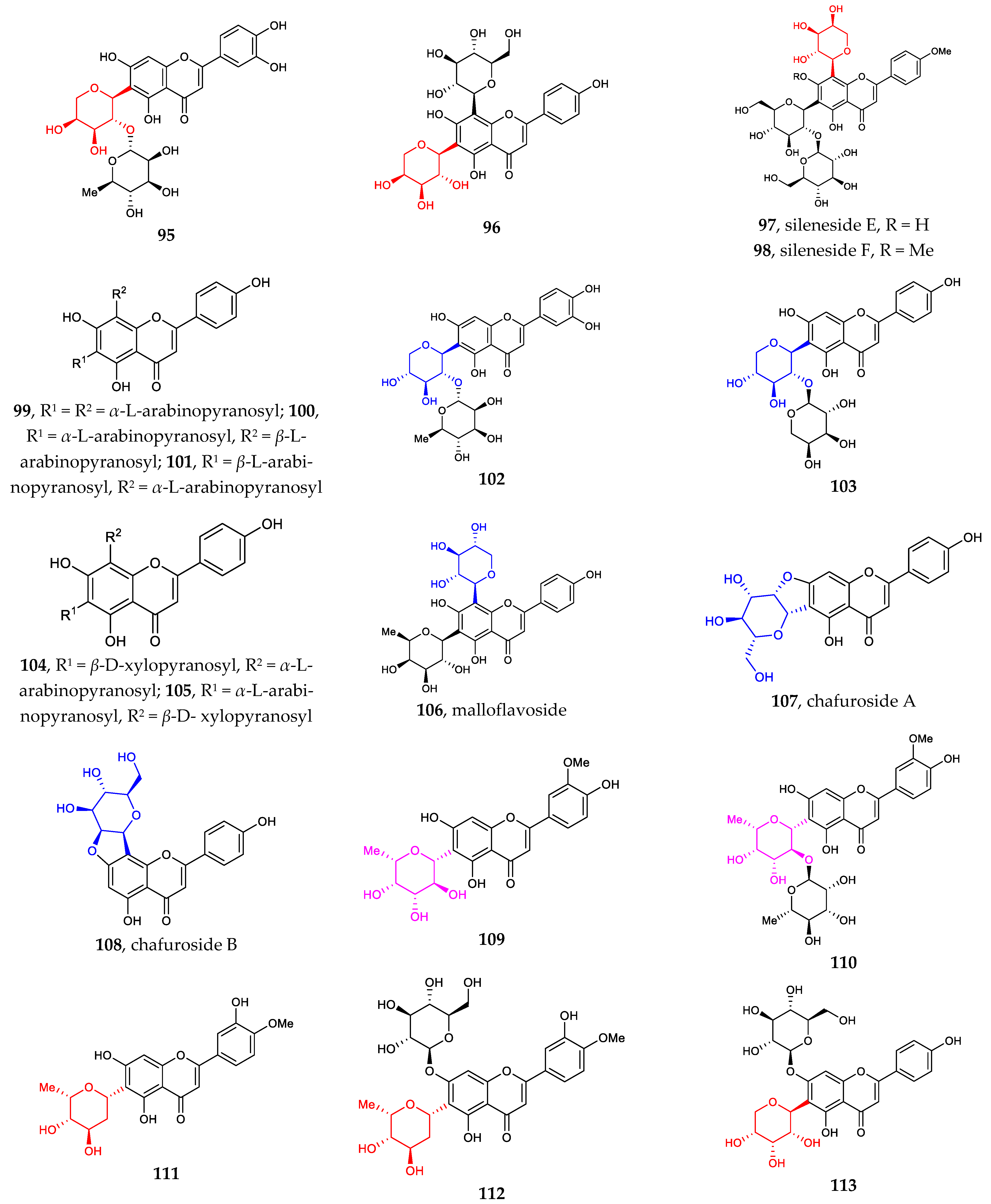
Figure 2 illustrates the recently discovered structures of flavonoid C-arabinosides 95–101 [67,68,69]. Di-C-glycosylation in flavonoids 96–101 usually takes place at positions C6 and C8. Both C-α-L-arabinosides and C-β-L-arabinosides are associated with β-D-glucose, β-D-galactose, and β-D-Xylose, with glycosylation sites shifting between C6 and C8.
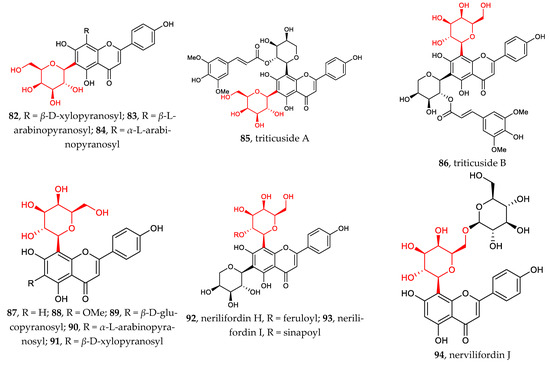
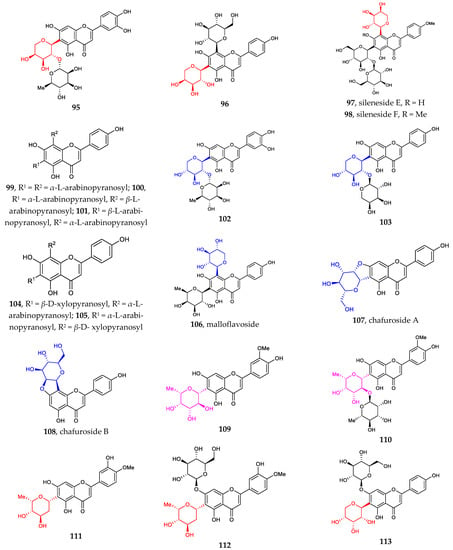
Figure 2.
Chemical structures of flavonoid C-galactosides (82–94), C-arabinosides (95–101), C-xylosides (102–106), C-mannosides (107–108), C-fucosides (109–110), C-boivinosides (111–112), and C-riboside (113).
The sites of C-glycosylation of flavonoid C-xylosides are usually C6 and or C8. Compounds 102–106 can be divided into two groups: (1) flavonoid mono-C-xylopyranosides 102–103 and (2) di-C-glycosylflavonoids 4, 43, 82, 91, 104–106 [38,39,62,69,70]. All discovered flavonoid C-xylosides are in β configuration.
Other flavonoid C-glycosides, including flavonoid C-mannosides 107–108, flavonoid C-fucosides 109–110, flavonoid C-boivinosides 111–112, and flavonoid C-riboside 113, are rarely found in the nature [71]. The site of C-glycosylation is normally C6.
2.2. Other Flavonoid C-Glycosides
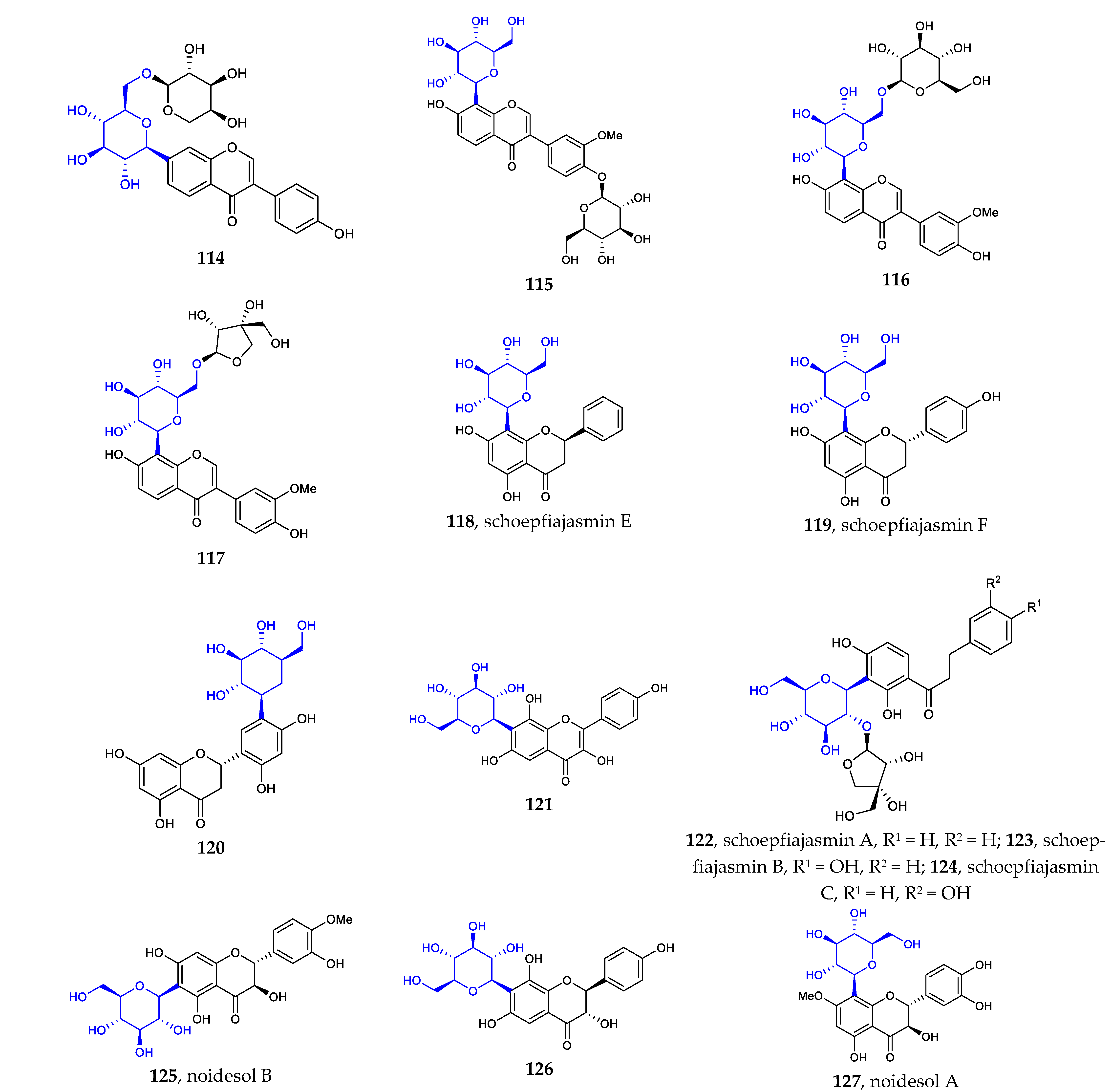
Other flavonoid C-glycosides include isoflavone [72,73], flavanone [74,75], dihydrochalcone [74], flavanonol [76,77], and flavonol C-glycosides [76]. The newly isolated structures are illustrated in Figure 3. Among them, Compound 120 possesses a unique scaffold featuring a C-β-D-glucose core linked to the flavanone at position C5′. This type of aryl-C-glycoside is a rare example of a natural aryl C-glycoside. It is very clear that all compounds are C-β-D-glucopyranosides (114–127), some of which are glycosylated with a monosaccharide at position C2 or C6 (Figure 3).
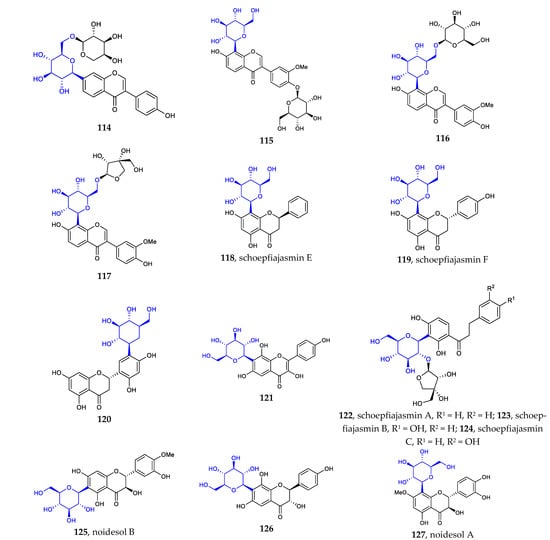
Figure 3.
Chemical structures of isoflavone C-β-D-glycosides (114–117), flavanone C-glycosides (118–120), flavonol C-glycoside 121, dihydrochalcone C-glycosides (122–124), and flavanonol C-glycosides (125–127).
2.3. Xanthone C-Glycosides
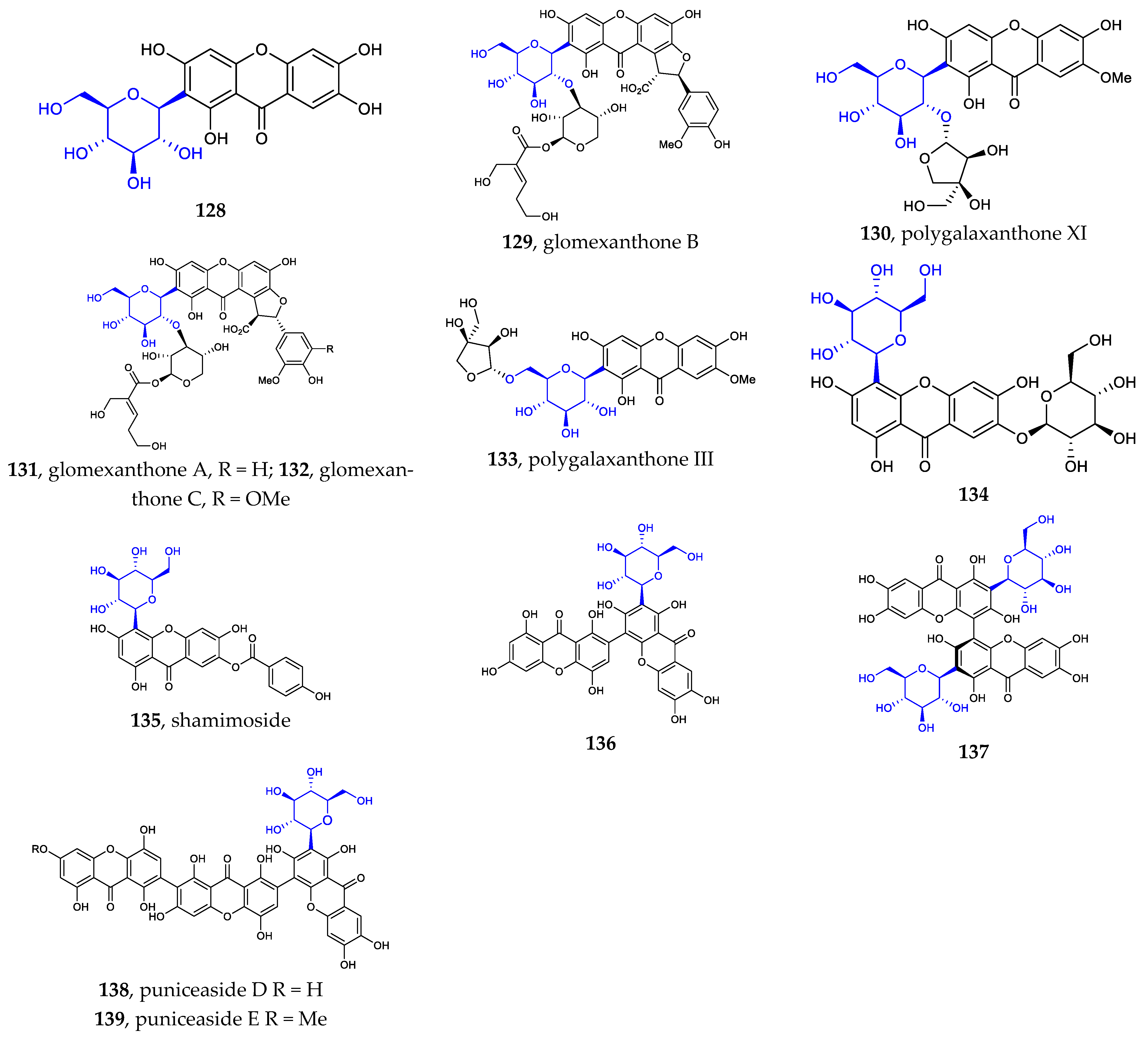
The sites where the sugar is linked to the xanthone are usually C2 (128–133, 136–139) and C4 (134–135) [21,22,23,78,79]. It seems that only C-β-D-glucosides have been isolated from nature. This kind of aryl C-glycosides include mono-xanthone C-glycosides, di-xanthone C-glycosides, and tri-xanthone C-glycosides (Figure 4).
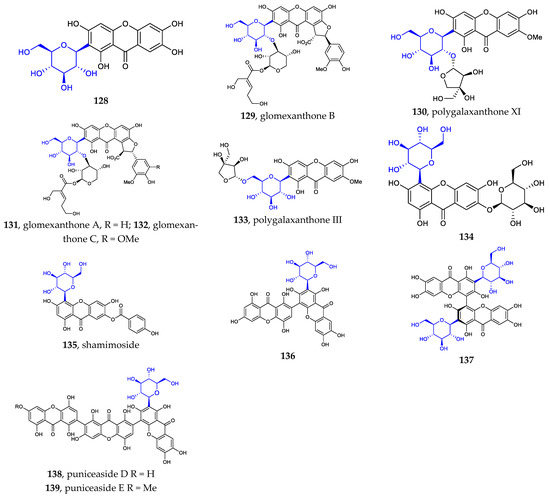
Figure 4.
Chemical structures (128–139) of xanthone C-glycosides.
2.4. Phenyl C-Glycosides
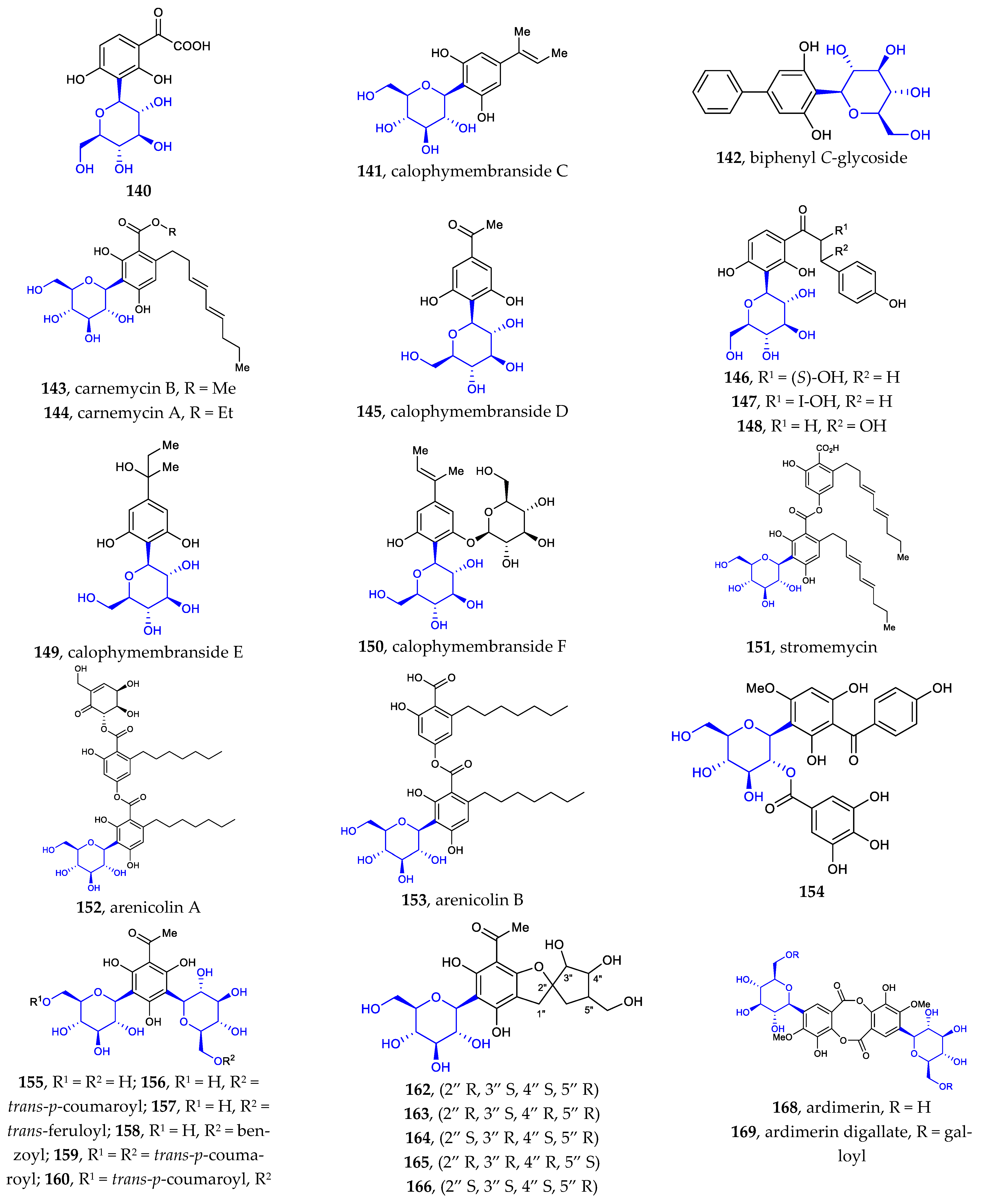
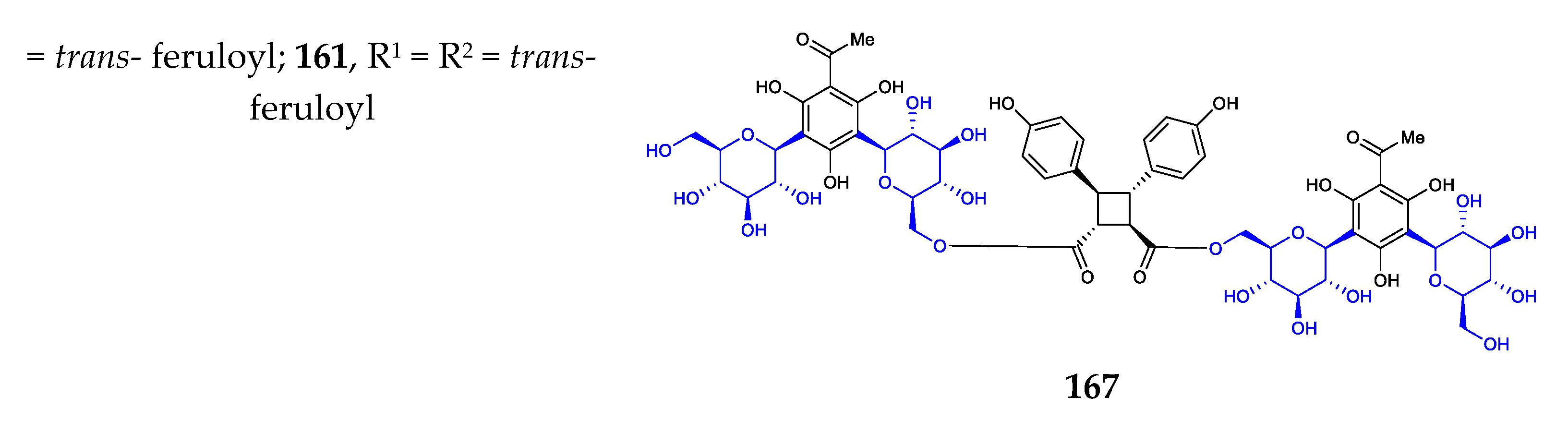
Phenyl C-glycosides were considered the simplest aryl-C-glycosides. The disclosed structures 140–169 feature C-β-D-glucopyranosides, with variations in the substitution on the benzene ring (Figure 5) [24,25,26,27,80,81,82,83,84]. The site of C-glycosylation is usually the ortho position of the phenolic hydroxyl group. The substitution groups of benzene include acyl, alkenyl, hydroxyl, alkyl, and alkoxyl moieties, providing aryl ketone, aryl vinyl, phenol, alkylbenzene, and aryl ether, respectively. The 2′-hydroxylation and 6′-hydroxylation of D-glucose are often accompanied by esterification by gallic acid (compounds 154, 169), trans-p-coumaric acid (compounds 156, 159–160), ferulic acid (compounds 157, 160–161), and benzoic acid (compound 158).
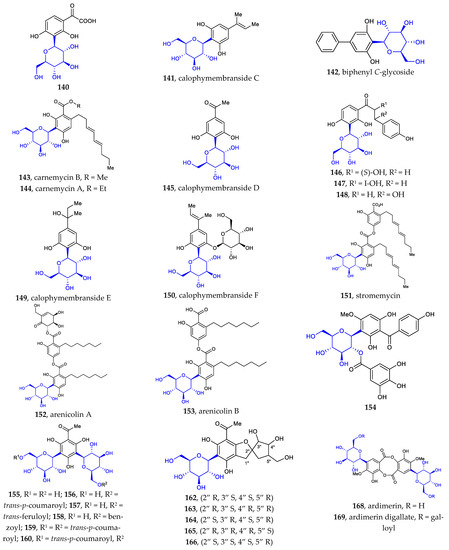
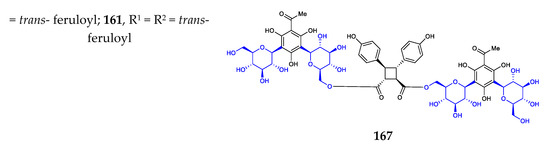
Figure 5.
Chemical structures (140–169) of phenyl C-glycosides.
2.5. Heteroaryl C-Glycosides
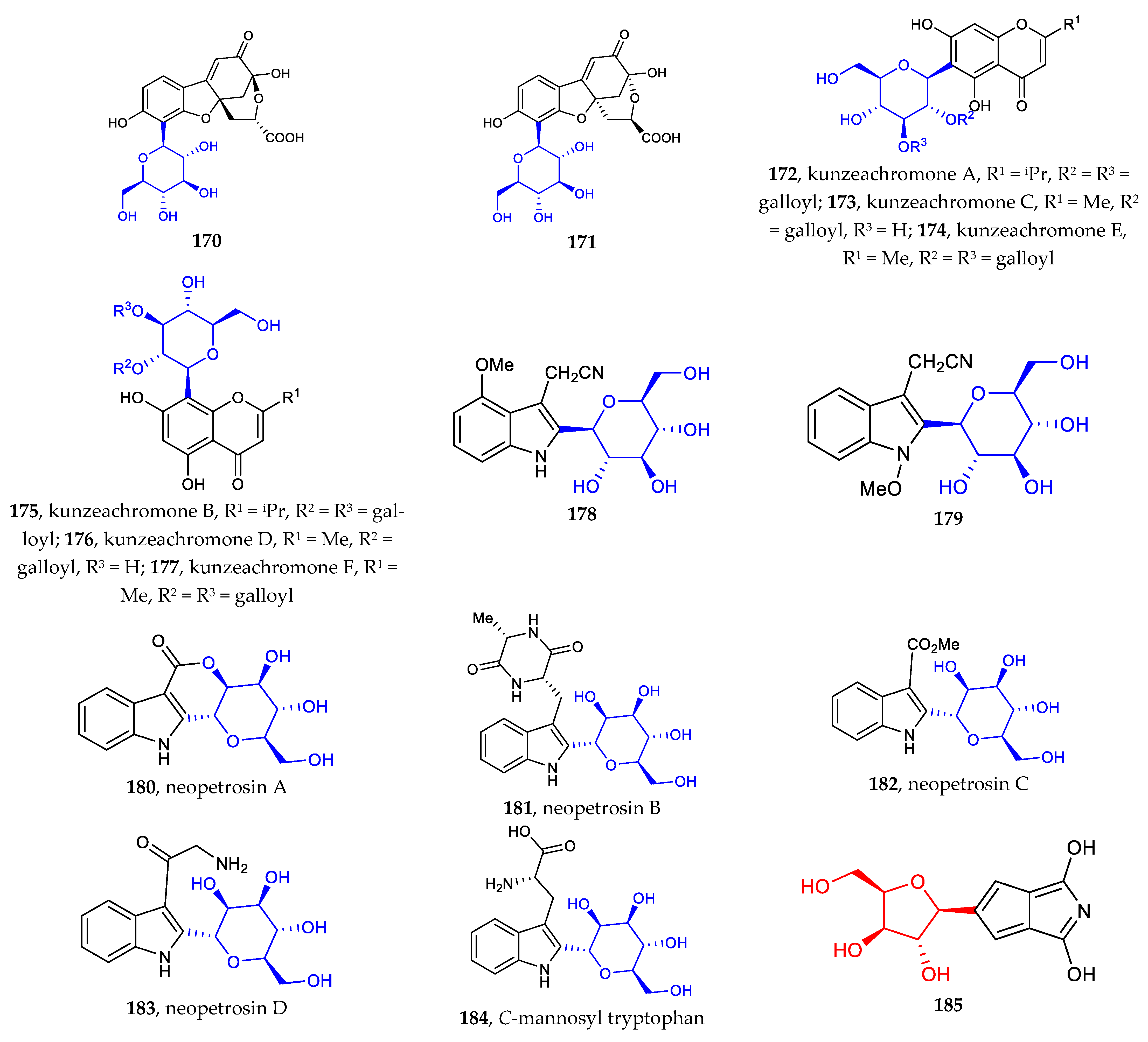
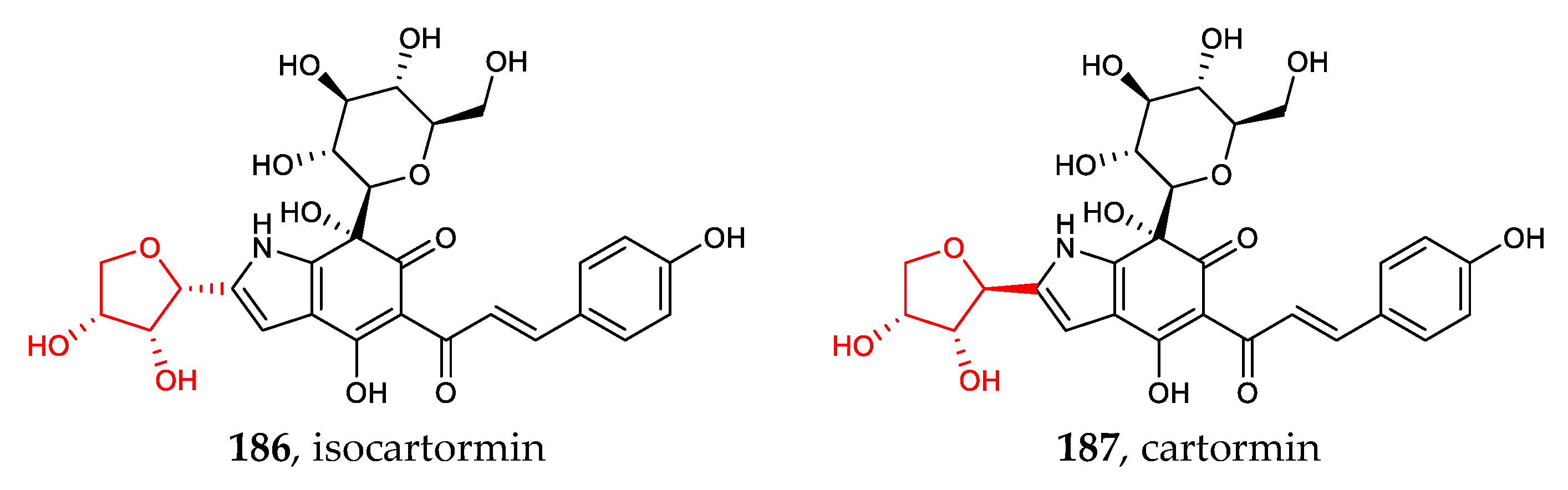
The recently discovered heteroaryl C-glycosides include dihydrobenzofuran C-glycosides (170–171), chromone C-glycosides (172–177), indole C-glycosides (178–184), and other heteroaryl C-glycosides (185–187) [28,29,85,86]. Except for the common C-β-glucosides, C-α-D-mannopyranosides and those containing erythrose are rare examples of aryl-C-glycosides (Figure 6).
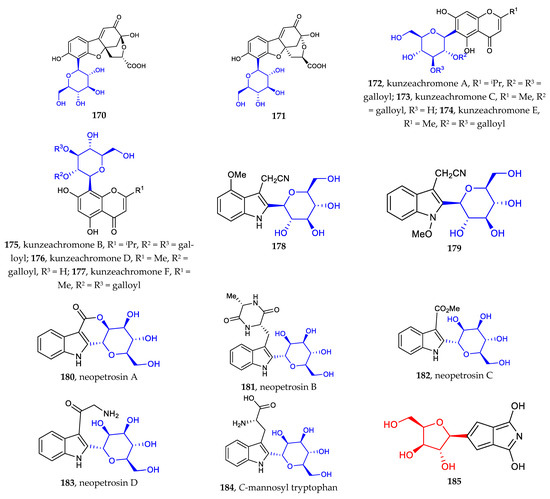
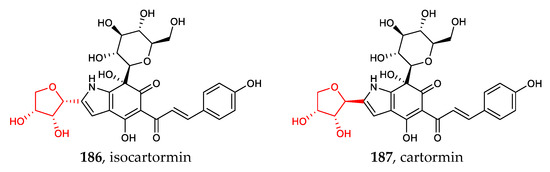
Figure 6.
Chemical structures (170–187) of heteroaryl C-glycosides.
2.6. Other Aryl-C-Glycosides
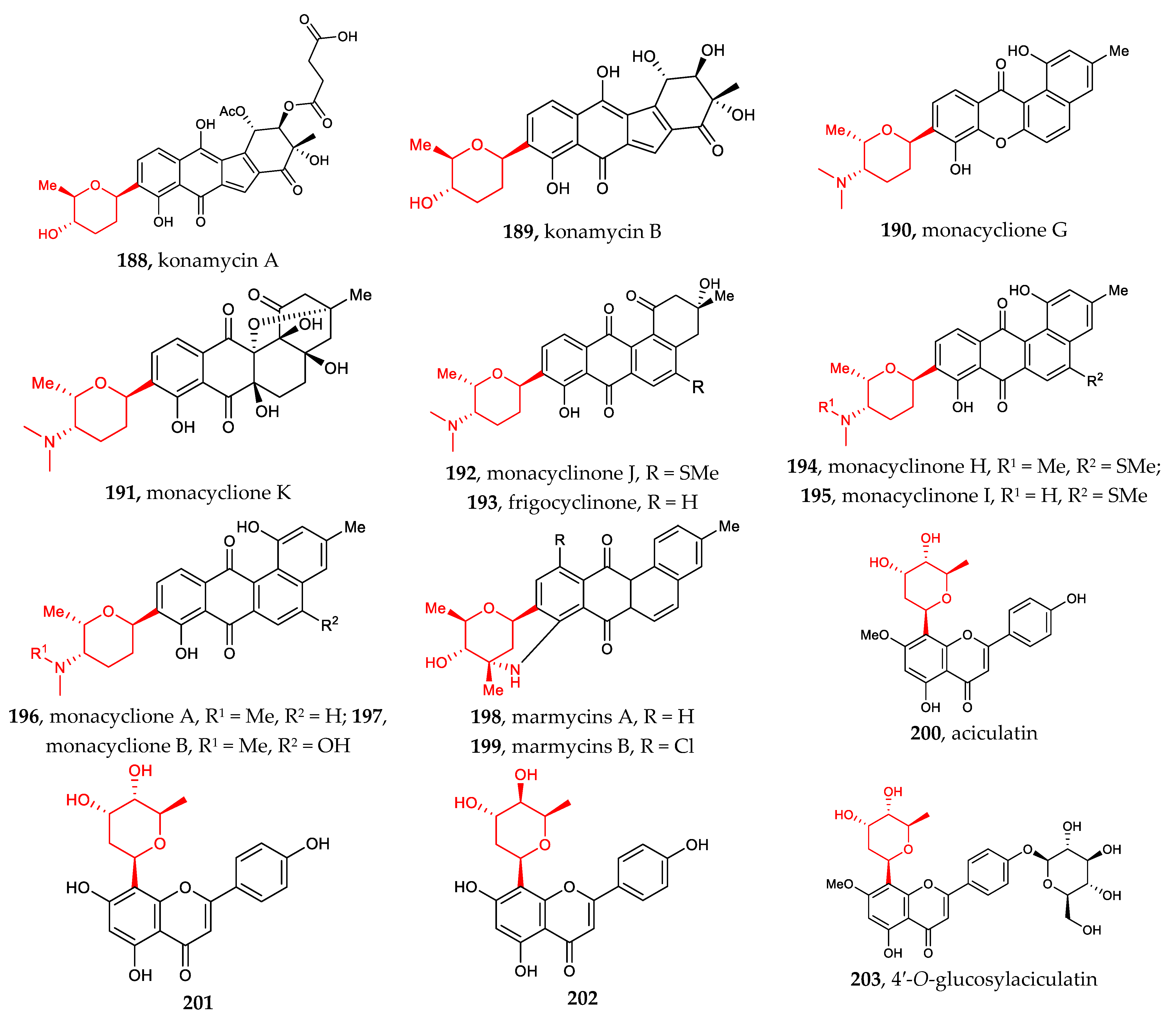
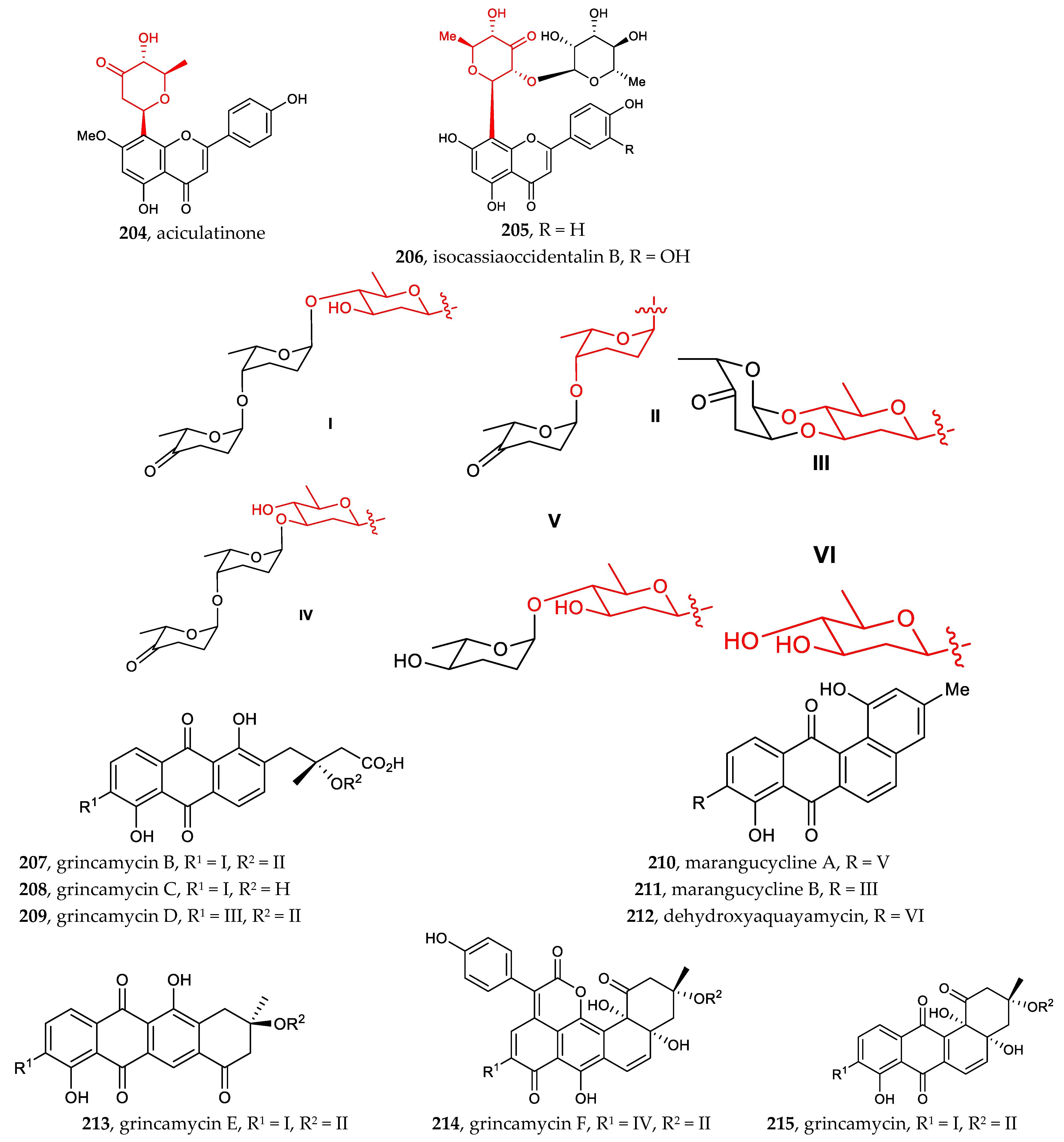
Other aryl-C-glycosides include aryl-C-glycosides of amino sugars and other rare sugars [30,31,32,33,34,35,36]. Compounds 190–197 (4-aminosugar) and compound 198–199 (3-aminosugar), which are 2-deoxylaminoglycoside antibiotics, resemble the well-known pluramycins. Among them, monacyclione G (190) possesses a unique scaffold featuring a xanthone core linked to the aminodeoxysugar ossamine, and monacycliones H−J (194–195, 192) are rare examples of natural angucyclines with an S-methyl group (Figure 7).
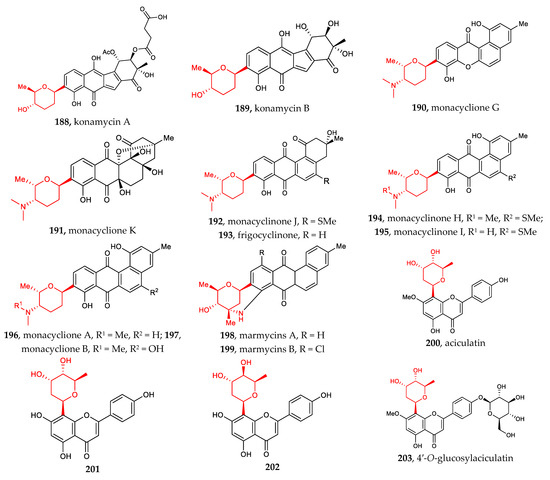
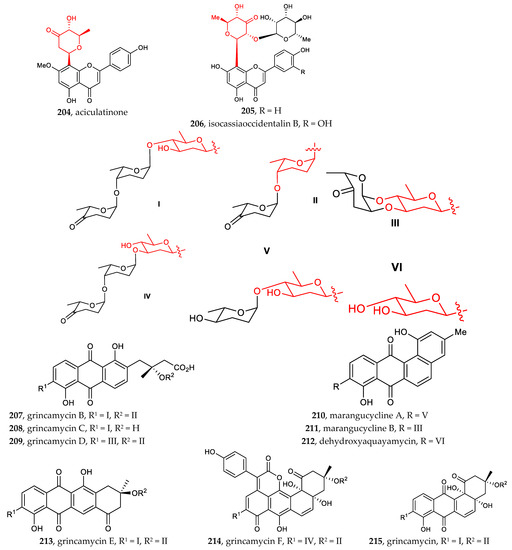
Figure 7.
Chemical structures (188–215) of aryl C-glycosides.
3. Pharmacological Activity
Numerous researchers have investigated the pharmacological activities of various aryl-C-glycosides. Table 1 summarizes the pharmacological features of recently discovered aryl-C-glycoside molecules. They include, but are not limited to, anticancer, anti-inflammatory, antioxidant, antiviral activities, glycation inhibitory activity, other pharmacological activities such as neuroprotective effects, hepatoprotective activity, and antipyretic activity. These pharmacological activities are summarized below.
3.1. Anticancer Activity
The indole C-glucopyranoside 178 exhibited significant cytotoxic activity against human myeloid leukemia cells HL-60 and human liver cancer cells HepG2, with IC50 of 1.3 ± 0.1 and 2.1 ± 0.3 μM, respectively. The indole C-glucopyranoside 179 showed potential cytotoxic activity against HL-60 and human myeloid leukemia Mata cells, with IC50 of 5.1 ± 0.4 and 12.1 ± 0.8 μM, respectively [85]. Monacycliones I (195) and J (192), isolated from the marine-derived Streptomyces sp. HDN15129, showed cytotoxic activity against multiple human cancer cell lines, with IC50 values ranging from 3.5 to 10 μM [31]. Marmycin A (198), isolated from the culture broth of a marine sediment-derived actinomycete related to the genus Streptomyces, displayed significant cytotoxicity against several cancer cell lines, some at nanomolar concentrations, while marmycin B (199) was less potent. For marmycin A (198), tumor cell cytotoxicity appeared to coincide with a modest induction of apoptosis and the arrest in the G1 phase of the cell cycle [32]. Li et al. reported that compounds 34–36, isolated from the small flowering aquatic plant Lemna japonica, exhibited weak cytotoxicity against HepG-2, SW-620, and A-549 cell lines, with IC50 values between 42.5 and 19.2 μg/mL [11]. Isoorientin, recently isolated from leaf and root methanolic extracts of Petrorhagia Velutina, a Mediterranean herbaceous plant, significantly reduced the proliferation of HepG2 cells, as determined by the complete conversion of a tetrazolium probe into formazan after 48 h of exposure [67]. Aciculatin 200, 201, 202, 204, isolated from an ethanolic extract of Chrysopogon aciculatis, showed differential potency on different cancer cell lines. Noticeably, aciculatin and 201 indicated specificity of cytotoxicity in MCF-7 and CEM cell lines [33]. Grincamycins B-E (207–209, 213), isolated from Streptomyces lusitanus SCSIO LR32, exhibited in vitro cytotoxicity against the human cancer cell lines HepG2, SW-1990, HeLa, NCI-H460, and MCF-7 and the mouse melanoma cell line B16, with IC50 values ranging from 1.1 to 31 μM [35]. Marangucycline B (211), isolated from the deep-sea-derived Streptomyces sp. SCSIO 11594, displayed in vitro cytotoxicity against four cancer cell lines, i.e., A594, CNE2, HepG2, and MCF-7, superior to that obtained with cisplatin, used as a positive control. Notably, marangucycline B bearing a keto-sugar displayed significant cytotoxicity against various cancer cell lines, with IC50 values ranging from 0.24 to 0.56 μM. An IC50 value of 3.67 μM was found when using the non-cancerous hepatic cell line HL7702, demonstrating the cancer cell selectivity of marangucycline B [36].
3.2. Anti-Inflammatory Activity
Compound 68, isolated from the rhizomes of Cyperus rotundus, showed moderate inhibitory activity against MRB, with an IC50 value of approximately 56.03 µM [15]. Nervilifordin J (94), isolated from a 60% EtOH extract of the aerial parts of Nervilia fordii, showed interesting inhibitory effects on nitric oxide production in lipopolysaccharide-activated RAW264.7 macrophages, with EC50 values of 14.80 µM [17]. Vicenin-2 was isolated and identified from an ethanol extract of the aerial parts of Urtica circularis. This crude extract was found to possess significant anti-inflammatory activity in a carrageenan-induced rat hind paw edema model (41.5% inhibition at a dose of 300 mg/kg). In cultured murine macrophages, this compound modified LPS-induced total nitrite and TNF-α production, in addition to promoting the LPS-induced translocation of nuclear factor NF-κB [87].
3.3. Antioxidant Activity
Shamimoside (135), isolated from a methanolic extract of the leaves of Bombax ceiba, showed antioxidant potential (IC50 = 150 µg/mL) [22]. Nelumboside B (40) exhibited strong scavenging activity (SC50 = 14.12 µM, ABTS assay), compared with the positive control L-ascorbic acid (SC50 = 26.15 µM, ABTS assay) [12]. Isocassiaoccidentalin B (206), isolated from whole Cassia nomame (SIEBER) HONDA plants, showed significant free-radical scavenging activity [34]. Compound 42, isolated from Gentiana piasezkii, showed significant free-radical scavenging activity (IC20 = 5.20 ± 0.10 µM) in the DPPH assay [13]. Compound 113, isolated from the methanolic extracts of Dtps. Tinny Ribbon × Dtps. Plum Rose (Phalaenopsis hybrids), exhibited moderate α, α-diphenyl-β-picrylhydrazyl free-radical scavenging activity, with half-maximal inhibitory concentration (IC50) values of 27.3 µM, compared to the reference compound vitamin E (IC50 12.5 µM) [71].
3.4. Antiviral Activity
Speciflavoside A (32), isolated from a 70% methanolic extract of Lilium speciosum var. gloriosoides Baker, showed potent antiviral activity against RSV, with an IC50 value of 2.9 μg/mL, comparable to that of ribavirin, an approved drug for the treatment of RSV infections in humans [10].
3.5. Glycation Inhibitory Activity
In 2003, Okuyama T. et al. reported that chrysoeriol 6-C-β-fucopyranoside 109, isolated from the style of Zea mays L. showed an inhibitory effect on glycation, with a percent inhibition value greater than that of aminoguanidine, a known glycation inhibitor [19]. Compounds 130–131, which contains the rare sugar boivinose, exhibited a glycation inhibitory activity similar to that of aminoguanidine [20].
3.6. Other Pharmacological Effects
Other pharmacological effects include neuroprotective effects, hepatoprotective activity, HIV inhibitory activity, antipyretic activity, transcriptional inhibitory activity of RXRα, cytotoxicity activity, and other activities.
Glomexanthones A–C (131, 129, 132), isolated from an ethanol extract of Polygala glomerata, showed moderate neuroprotective effects on L-glutamic acid-induced cellular damage in human neuroblastoma SK-N-SH cells [21]. Compound 136, isolated from the entire plant of Swertia punicea, exhibited potent neuroprotective activity against H2O2-induced PC12 cell damage [23]. Neopetrosins A, B, D (180–181, 183) were isolated from the marine sponge Neopetrosia chaliniformis collected off Xisha Island in the South China Sea. They exhibited in vivo hepatoprotective activity in a zebrafish model at a concentration of 20 μM [29]. Ardimerin digallate (169) was isolated from the whole plant of Ardisia japonica and was shown to inhibit HIV-1 and HIV-2 RNase H in vitro, with IC50 values of 1.5 and 1.1 µM, respectively [27]. The compound 3,5-di-C-β-D-glucopyranosyl phloroacetophenone (155), isolated from the edible leaves of Melicope pteleifolia, was found to be responsible for the antipyretic activity of M. pteleifolia based on in vivo experiments [26]. Calophymembranside C (141), isolated from the stems of Calophyllum membranaceum, showed transcriptional inhibitory activity towards RXRα, with 50% inhibitory concentration (IC50) values of 29.95 ± 1.08 [24]. Arenicolin A (152), isolated from Penicillium arenicola, exhibited cytotoxicity toward mammalian cell lines, including colorectal carcinoma (HCT-116), neuroblastoma (IMR-32), and ductal carcinoma (BT-474) cells, with IC50 values of 7.3, 6.0, and 9.7 μM, respectively [73]. Apigenosylide B (16), isolated from an EtOH extract of the leaves of Machilus japonica var. kusanoi, possesses moderate inhibitory activity against α-glucosidase [9]. Compounds 45–46 inhibited complement activation in the classic pathway in vitro, with IC50 values ranging from 0.88 to 4.02 mM. This may suggest the application of the herb for the treatment of acute respiratory distress syndrome, etc. [14]. Diandraflavone (81), isolated from Drymaria diandra, showed significantly selective inhibition on superoxide anion generation from human neutrophils stimulated by fMLP/CB, with an IC50 value of 10.0 µg/mL [16].
4. Synthesis
In nature, most C-glycosides are derived from plants. Aryl C-glycosides are biosynthetically prepared by the catalysis of C-glycosyltransferases (CGTs). However, while a large family of O-glycosyltransferases (OGTs) is known, a limited number of CGTs have been discovered in plants [88]. Many detailed reviews on the chemical synthesis of aryl-C-glycoside have been published [8,89,90,91,92,93]. Herein, a brief historical review of the total synthesis of natural aryl-C-glycosides is presented. From a retrosynthetic viewpoint, the strategy of synthesis of aryl-C-glycoside usually includes two protocols, as described below.
4.1. C-Glycosylation of Arenes and De Novo Construction of the Aromatic Moiety
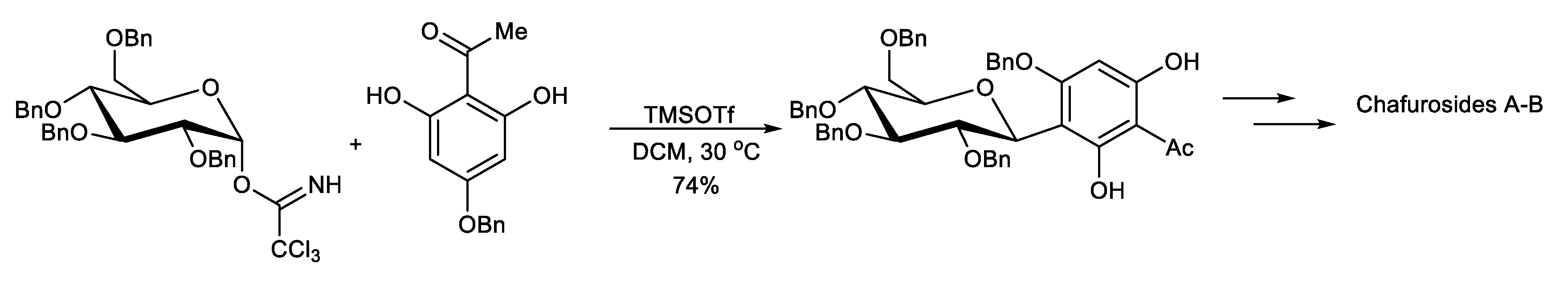
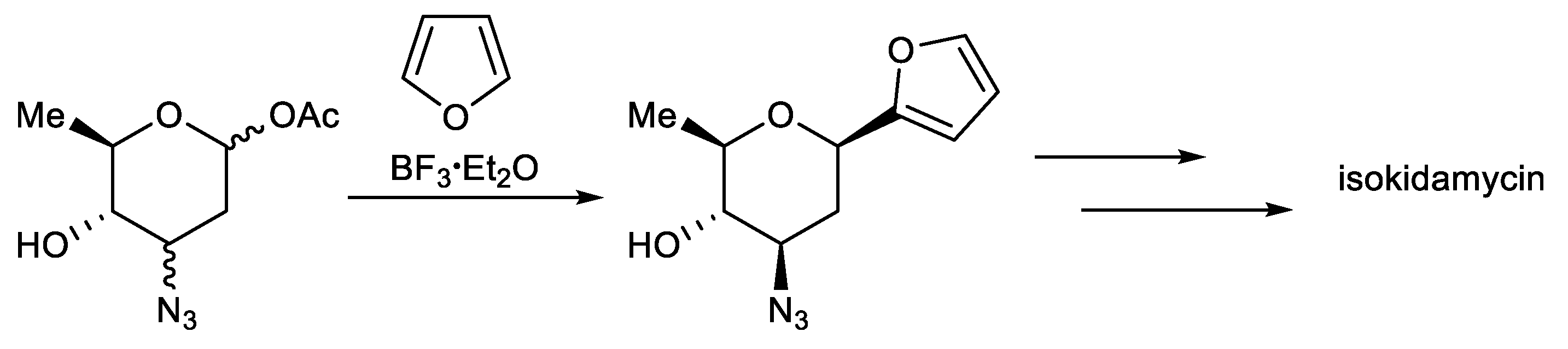
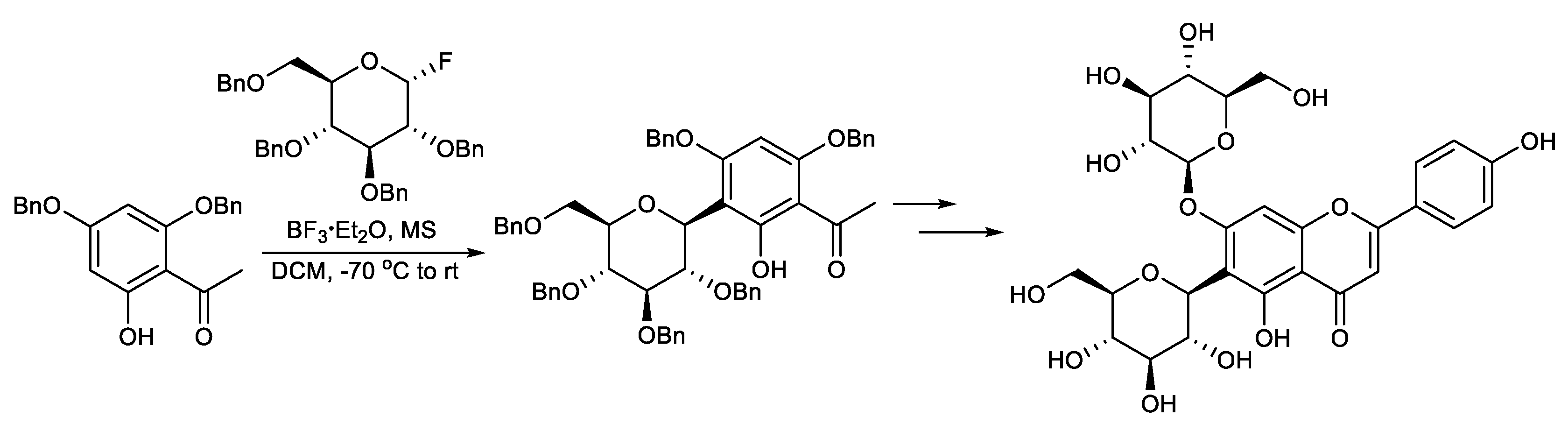
This protocol exploits the installation of a sugar moiety on a full or partial aromatic structure. For a partial aryl-C-glycoside structure, it uses a simple C-glycoside as the starting material, which is functionalized, providing a complex aryl unit. The classical electrophilic aromatic substitution approach was often applied. The sugar portion includes glycosyl trichloroacetimidate (Scheme 1) [94], glycosyl acetate (Scheme 2) [95], glycosyl fluoride (Scheme 3) [96], glycosyl lactone (Scheme 4) [97], glycosyl thioglycoside (Scheme 5) [98], and other sugars. This strategy was successfully applied to the total synthesis of pluramycins [99], aciculatin [98], vineomycin B2 and its methyl ester [100,101,102], angucycline C5 glycosides [103], paecilomycin B [104,105], aspalathin [106], nothofagin [106], chrysomycin A [107], vicenin-2 [108], 3,3′-Di-O-methyl Ardimerin [109], deacetylravidomycin M [110], aquayamycin [111], a precursor of kendomycin [112], and anthraquinone-based aryl-C-glycosides [113].
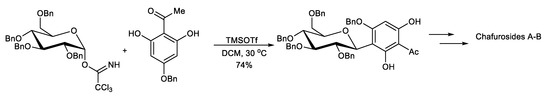
Scheme 1.
Concise synthesis of chafurosides A and B.
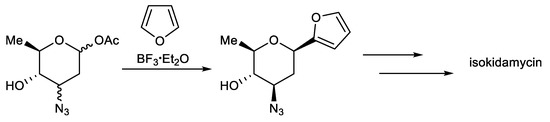
Scheme 2.
Total synthesis of isokidamycin.
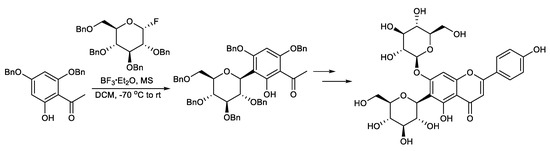
Scheme 3.
Total synthesis of saponarin.
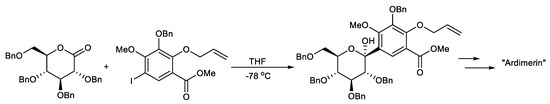
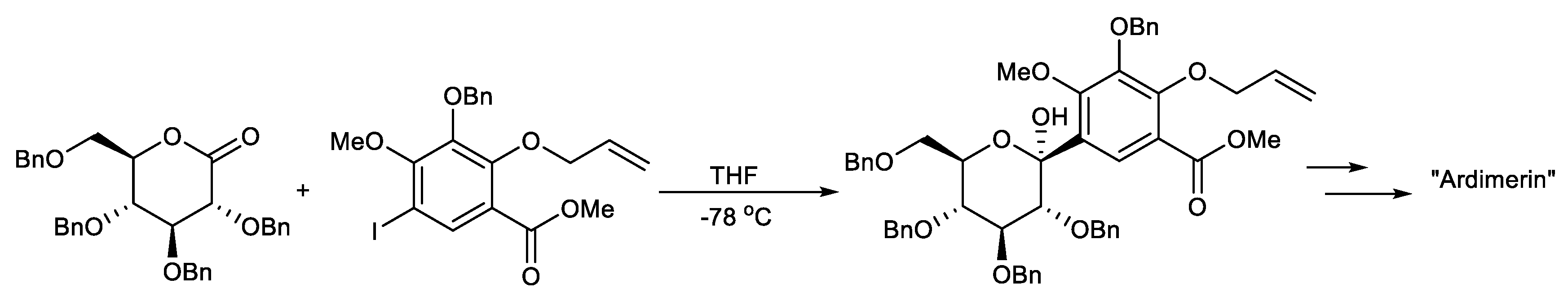
Scheme 4.
Total synthesis of the proposed structure of ardimerin.
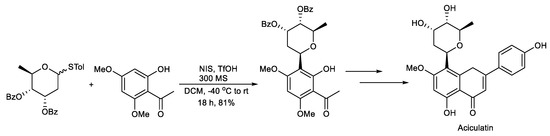
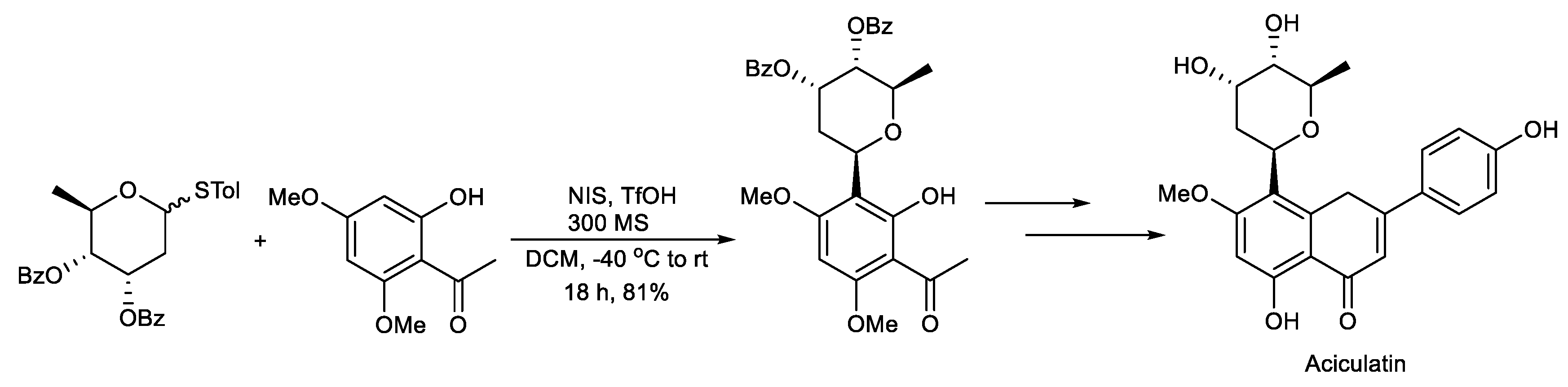
Scheme 5.
Synthesis of aciculatin.
Toshiyuki Kan et al. reported the regioselective synthesis of chafurosides A (107) and B (108) using a novel protecting-group strategy. The construction of the dihydrofuran ring was achieved via an intramolecular Mitsunobu reaction. The key step in the C-glycosylation is the O→C rearrangement of the phenolic glycoside formed by TMSOTf-catalyzed glycosylation (Scheme 1) [94].
Martin et al. reported the total synthesis of isokidamycin, which features the use of a silicon tether as a disposable regiocontrol element in an intramolecular Diels–Alder reaction between a substituted naphthyne and a glycosyl furan and a subsequent O→C-glycoside rearrangement (Scheme 2) [95].
Sato et al. reported the total synthesis of saponarin. Saponarin was efficiently synthesized via 11 steps from 2, 4-O-dibenzylphloroacetophenone, with an overall yield of 37%. The key step also features a glycosylation involving per-O-benzylglucosyl α-fluoride and 2, 4-O-dibenzylphloroacetophenone, applying the O→C glycoside rearrangement method (Scheme 3) [96].
Suzuki K. et al. reported the total synthesis of the proposed structure of ardimerin, whose key step includes the β-selective formation of the crucial C-glycoside linkage by the reaction between aryl iodide, through a halogen–metal exchange reaction, and lactone (Scheme 4) [97].
Lee et al. reported the total synthesis of aciculatin. The key step is the glycosylation of the digitoxosyl thioglycoside with an electron-rich phenol activated by NIS/TfOH, which afforded the β-D-digitoxopyranoside (Scheme 5) [98].
Besides the nucleophilic attack reaction of the aryl portions to provide aryl-C-glycoside molecules, the transition metal-catalyzed cross-coupling reactions to form the Csp3-Csp2 bond of aryl-C-glycosides are becoming more and more powerful. The Negeshi [114], Kumada [115], Stille [116,117,118], Heck [119,120], Sonogashira [121], Hiyama [122], and radical [123,124,125] cross-coupling reactions with different metals such as Pd [126,127], Ni [128,129], Fe [124,125], Co [115], Ir [130] as catalysts, as well as C-H activation [131,132] were successfully applied to the construction of aryl-C-glycoside scaffolds.
4.2. De Novo Construction of the Sugar Moiety
This approach uses the reactions of assembled aryl units based on methods for the construction of a sugar, including, but not limited to (a) the hetero-Diels–Alder reaction, (b) the 1,3-dipolar cycloaddition of nitrile oxides, (c) the ring-closing olefin metathesis [8], the de novo asymmetric approach [133,134,135], and the ring opening-ring closure strategy [136]. It was successfully applied to the synthesis of O-spiro-C-aryl glycosides [137] and 2-deoxy-β-C-aryl glycosides [138].
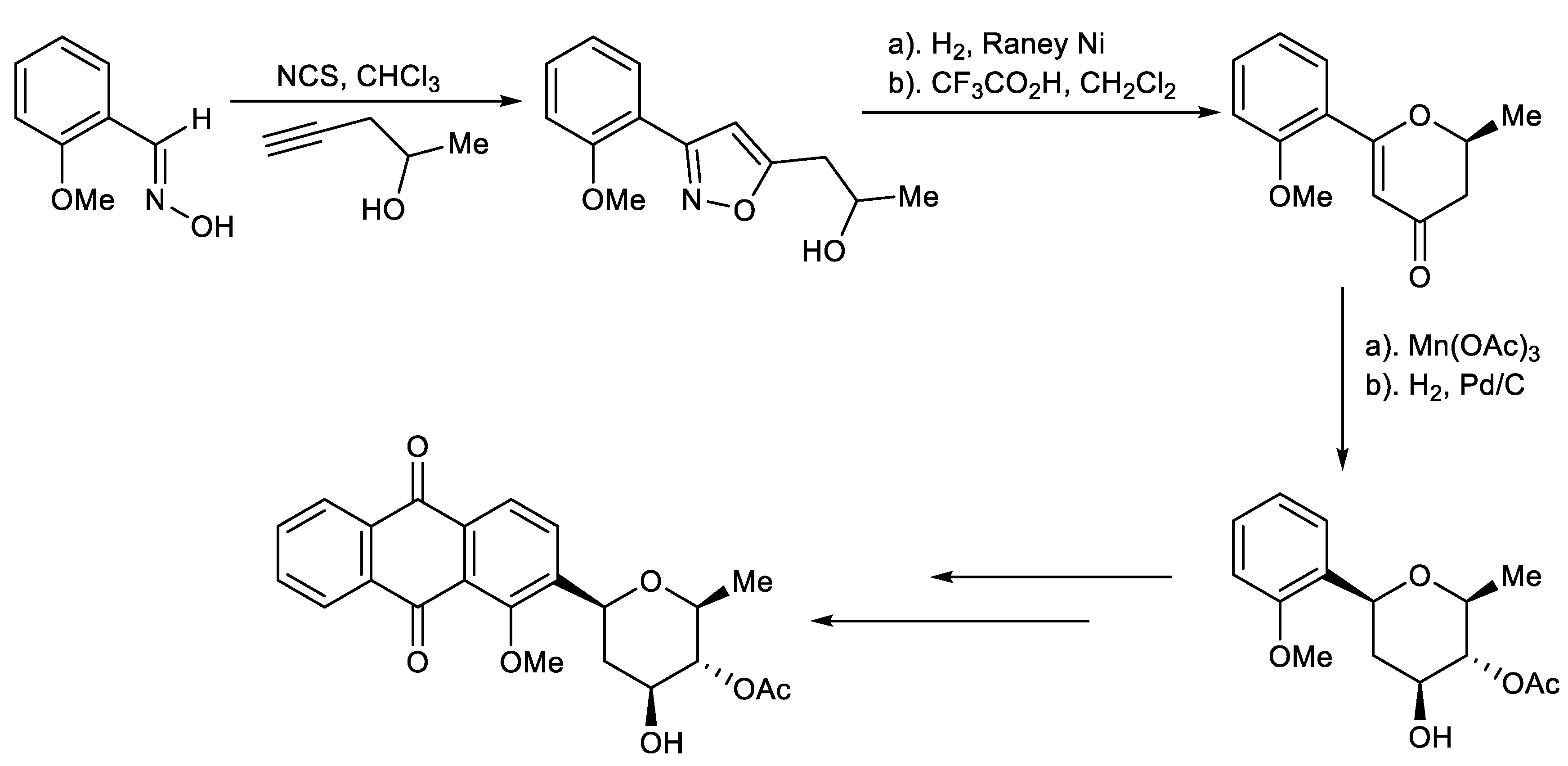
Hauser et al. reported that de novo synthesis of C-aryl glycosides based on cycloaddition of an aryl nitrile oxide with 4-pentyn-2-ol, which was straightforwardly converted to the pyranone through sequential hydrogenolysis of the N-O bond of the isoxazole followed by acid-catalyzed intramolecular cyclization. (Scheme 6) [139].
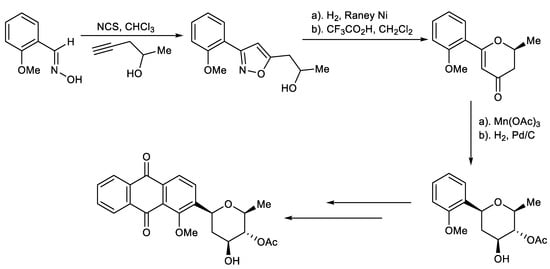
Scheme 6.
Total synthesis of naturally occurring C-aryl glycosides.
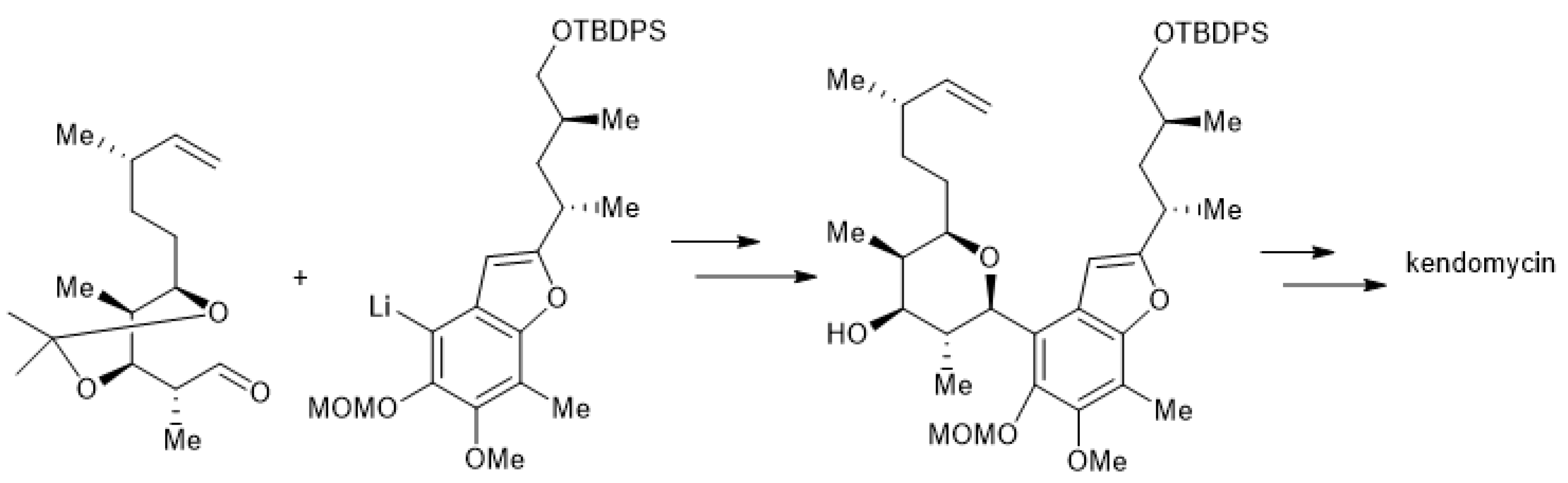
Johann Mulzer et al. reported the development of a convergent and concise route to an advanced precursor of kendomycin by applying an SN1 ring cyclization as a key step. The sugar moiety formed by the acid-catalyzed intramolecular etherification reaction of aryl-substituted 1, 3, 5-triol (Scheme 7) [140].
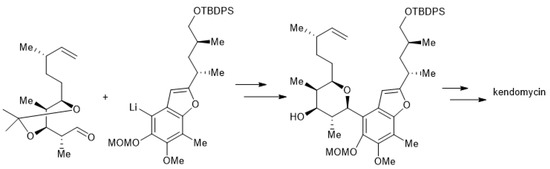
Scheme 7.
Concise synthesis of kendomycin.
5. Conclusions and Perspectives
In this review, the recently discovered aryl-C-glycoside structures were listed and their biological activities as well as their synthetical approaches were summarized. The diverse structures of natural aryl-C-glycosides and their multiple pharmacological effects suggest significant medicinal applications. This review presents a summary of studies published from 2002 to date on this promising compounds. The core structure of aryl-C-glycosides, which is glycosylated and/or acylated, exhibits a great number of molecular entities and possesses various pharmaceutical effects. It is expected that more and more aryl-C-glycoside scaffolds including natural and synthetical molecules will be disclosed, and their medicinal application will come true in the near future to benefit human health.
Funding
This research was funded by National Natural Science Foundation of China, grant number [21762004].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The research was financially supported by the National Natural Science Foundation of China (21762004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levy, D.E.; Tang, C. The Chemistry of C-Glycosides; Pergamon: Tarrytown, NY, USA, 1995. [Google Scholar]
- Pałasz, A.; Cież, D.; Trzewik, B.; Miszczak, K.; Tynor, G.; Bazan, B. In the Search of Glycoside-Based Molecules as Antidiabetic Agents. Top. Curr. Chem. 2019, 37, 19. [Google Scholar] [CrossRef] [PubMed]
- Bokor, É.; Kun, S.; Goyard, D.; Tóth, M.; Praly, J.-P.; Vidal, S.; Somsák, L. C-Glycopyranosyl Arenes and Hetarenes: Synthetic Methods and Bioactivity Focused on Antidiabetic Potential. Chem. Rev. 2017, 117, 1687–1764. [Google Scholar] [CrossRef] [PubMed]
- Sezaki, M.; Kondo, S.; Maeda, K.; Umezawa, H.; Ohno, M. The structure of aquayamycin. Tetrahedron 1970, 26, 5171–5190. [Google Scholar] [CrossRef]
- Bililign, T.; Griffith, B.R.; Thorson, J.S. Structure, activity, synthesis and biosynthesis of aryl-C-glycosides. Nat. Prod. Rep. 2005, 22, 742–760. [Google Scholar] [CrossRef]
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325. [Google Scholar] [CrossRef]
- Bajracharya, G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: Potential materials for therapeutic usages. Fitoterapia 2015, 101, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Total Synthesis of Aryl C-Glycoside Natural Products: Strategies and Tactics. Chem. Rev. 2018, 118, 1495–1598. [Google Scholar] [CrossRef]
- Lee, S.-S.; Lin, Y.-S.; Chen, C.-K. Three Adducts of Butenolide and Apigenin Glycoside from the Leaves of Machilus japonica. J. Nat. Prod. 2009, 72, 1249–1252. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Wang, J.; Hu, X. Flavonoid Glycosides from the Bulbs of Lilium speciosum var. gloriosoides and their Potential Antiviral Activity Against RSV. Chem. Nat. Compd. 2019, 55, 461–464. [Google Scholar]
- Bai, H.-H.; Wang, N.-N.; Mi, J.; Yang, T.; Fang, D.-M.; Wu, L.-W.; Zhao, H.; Li, G.-Y. Hydroxycinnamoylmalated flavone C-glycosides from Lemna japonica. Fitoterapia 2018, 124, 211–216. [Google Scholar] [CrossRef]
- Jiang, X.-L.; Wang, L.; Wang, E.-J.; Zhang, G.-L.; Chen, B.; Wang, M.-K.; Li, F. Flavonoid glycosides and alkaloids from the embryos of Nelumbo nucifera seeds and their antioxidant activity. Fitoterapia 2018, 125, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-X.; Li, Y.; Shi, Y.-P. Antioxidant phenolic glucosides from Gentiana piasezkii. J. Asian Nat. Prod. Res. 2006, 8, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y.; Li, S.-Y.; Feng, J.-Y.; Sun, Y.; Cai, J.-N.; Sun, X.-F.; Yang, S.-L. Flavone C-glycosides from the flowers of Trollius chinensis and their anti-complementary activity. J. Asian Nat. Prod. Res. 2013, 15, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, T.; Xiao, H.; Zhou, X.; Wu, H. A New C-Glycosylflavone from the Rhizomes of Cyperus rotundus. Chem. Nat. Compd. 2015, 51, 640–642. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Chang, F.-R.; Lee, K.-H.; Hwang, T.-L.; Chang, S.-M.; Wu, Y.-C. A New Anti-HIV Alkaloid, Drymaritin, and a New C-Glycoside Flavonoid, Diandraflavone, from Drymaria diandra. J. Nat. Prod. 2004, 67, 1175–1177. [Google Scholar] [CrossRef]
- Qiu, L.; Jiao, Y.; Xie, J.-Z.; Huang, G.-K.; Qiu, S.-L.; Miao, J.-H.; Yao, X.-S. Five new flavonoid glycosides from Nervilia fordii. J. Asian Nat. Prod. Res. 2013, 15, 589–599. [Google Scholar] [CrossRef]
- Iwao, Y.; Ishida, H.; Kimura, S.I.; Wakimoto, T.; Kondo, H.; Itai, S.; Noguchic, S. Crystal Structures of Flavone C-Glycosides from Oolong Tea Leaves: Chafuroside A Dihydrate and Chafuroside B Monohydrate. Chem. Pharm. Bull. 2019, 67, 935–939. [Google Scholar] [CrossRef]
- Suzuki, R.; Okada, Y.; Okuyama, T. A New Flavone C-Glycoside from the Style of Zea mays L. with Glycation Inhibitory Activity. Chem. Pharm. Bull. 2003, 51, 1186–1188. [Google Scholar] [CrossRef]
- Suzuki, R.; Okada, Y.; Okuyama, T. Two Flavone C-Glycosides from the Style of Zea mays with Glycation Inhibitory Activity. J. Nat. Prod. 2003, 66, 564–565. [Google Scholar] [CrossRef]
- Li, C.-J.; Yang, J.-Z.; Yu, S.-S.; Zhao, C.-Y.; Peng, Y.; Wang, X.-L.; Zhang, D.-M. Glomexanthones A-C, three xanthonolignoid C-glycosides from Polygala glomerata Lour. Fitoterapia 2014, 93, 175–181. [Google Scholar]
- Faizi, S.; Zikr-ur-Rehman, S.; Naz, A.; Versiani, M.A.; Dar, A.; Naqvi, S. Bioassay-guided studies on Bombax ceiba leaf extract: Isolation of shamimoside, a new antioxidant xanthone C-glucoside. Chem. Nat. Compd. 2012, 48, 774–779. [Google Scholar] [CrossRef]
- Du, X.-G.; Wang, W.; Zhang, S.-P.; Pu, X.-P.; Zhang, Q.-Y.; Ye, M.; Zhao, Y.-Y.; Wang, B.-R.; Khan, I.A.; Guo, D.-A. Neuroprotective Xanthone Glycosides from Swertia punicea. J. Nat. Prod. 2010, 73, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Zhang, X.; Chen, H.-F.; Zhu, L.-J.; Zeng, D.-Q.; Yang, J.; Wu, G.-X.; Wu, Y.-Z.; Yao, X.-S. RXR alpha transcriptional inhibitors from the stems of Calophyllum membranaceum. Fitoterapia 2016, 108, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Perlatti, B.; Lan, N.; Earp, C.E.; AghaAmiri, S.; Vargas, S.H.; Azhdarinia, A.; Bills, G.F.; Gloer, J.B. Arenicolins: C-Glycosylated Depsides from Penicillium arenicola. J. Nat. Prod. 2020, 83, 668–674. [Google Scholar] [CrossRef]
- Lee, B.-W.; Park, J.-G.; Ha, T.K.Q.; Pham, H.T.T.; An, J.-P.; Noh, J.-R.; Lee, C.-H.; Oh, W.-K. Constituents of the Edible Leaves of Melicope pteleifolia with Potential Analgesic Activity. J. Nat. Prod. 2019, 82, 2201–2210. [Google Scholar] [CrossRef]
- Dat, N.T.; Bae, K.; Wamiru, A.; McMahon, J.B.; Grice, S.F.J.L.; Bona, M.; Beutler, J.A.; Kim, Y.H. A Dimeric Lactone from Ardisia japonica with Inhibitory Activity for HIV-1 and HIV-2 Ribonuclease H. J. Nat. Prod. 2007, 70, 839–841. [Google Scholar] [CrossRef]
- Ito, H.; Kasajima, N.; Tokuda, H.; Nishino, H.; Yoshida, T. Dimeric Flavonol Glycoside and Galloylated C-Glucosylchromones from Kunzea ambigua. J. Nat. Prod. 2004, 67, 411–415. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Li, X.; Han, X.; Wang, Z.; Zhang, W.; Dou, B.; Lu, Z.; Li, P.; Li, G. Neopetrosins A–D and Haliclorensin D, Indole-C-Mannopyranosides and a Diamine Alkaloid Isolated from the South China Sea Marine Sponge Neopetrosia chaliniformis. J. Nat. Prod. 2022, 85, 1626–1633. [Google Scholar] [CrossRef]
- Harunari, E.; Imada, C.; Igarashi, Y. Konamycins A and B and Rubromycins CA1 and CA2, Aromatic Polyketides from the Tunicate-Derived Streptomyces hyaluromycini MB-PO13T. J. Nat. Prod. 2019, 82, 1609–1615. [Google Scholar] [CrossRef]
- Chang, Y.; Xing, L.; Sun, C.; Liang, S.; Liu, T.; Zhang, X.; Zhu, T.; Pfeifer, B.A.; Che, Q.; Zhang, G.; et al. Monacycliones G–K and ent-Gephyromycin A, Angucycline Derivatives from the Marine-Derived Streptomyces sp. HDN15129. J. Nat. Prod. 2020, 83, 2749–2755. [Google Scholar] [CrossRef]
- Martin, G.D.A.; Tan, L.T.; Jensen, P.R.; Dimayuga, R.E.; Fairchild, C.R.; Raventos-Suarez, C.; Fenical, W. Marmycins A and B, Cytotoxic Pentacyclic C-Glycosides from a Marine Sediment-Derived Actinomycete Related to the Genus Streptomyces. J. Nat. Prod. 2007, 70, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-C.; Cheng, J.-J.; Lay, H.-L.; Wu, S.-Y.; Ni, C.-L.; Teng, C.-M.; Chen, C.-C. Cytotoxic Apigenin Derivatives from Chrysopogon aciculatis. J. Nat. Prod. 2012, 75, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.S.; Akram, M.; Bae, O.-N.; Kim, C.Y. Isocassiaoccidentalin B, A New C-Glycosyl Flavone Containing a 3-Keto Sugar, and Other Constituents from Cassia nomame. Helv. Chim. Acta 2016, 99, 691–695. [Google Scholar] [CrossRef]
- Huang, H.; Yang, T.; Ren, X.; Liu, J.; Song, Y.; Sun, A.; Ma, J.; Wang, B.; Zhang, Y.; Huang, C.; et al. Cytotoxic Angucycline Class Glycosides from the Deep-Sea Actinomycete Streptomyces lusitanus SCSIO LR32. J. Nat. Prod. 2012, 75, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, G.; Li, J.; Huang, H.; Zhang, X.; Zhang, H.; Ju, J. Cytotoxic and Antibacterial Angucycline- and Prodigiosin- Analogues from the Deep-Sea Derived Streptomyces sp. SCSIO 11594. Mar. Drugs 2015, 13, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- McNally, D.J.; Wurms, K.V.; Labbé, C.; Quideau, S.; Bélanger, R.R. Complex C-Glycosyl Flavonoid Phytoalexins from Cucumis sativus. J. Nat. Prod. 2003, 66, 1280–1283. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Muharini, R.; Lin, W.H.; Teslov, L.; Proksch, P. Flavonoids from Stellaria nemorum and Stellaria holostea. Nat. Prod. Commun. 2015, 10, 437–440. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Luo, J.-G.; Han, C.; Xu, J.-F.; Kong, L.-Y. Bioassay-guided preparative separation of angiotensin-converting enzyme inhibitory C-flavone glycosides from Desmodium styracifolium by recycling complexation high-speed counter-current chromatography. J. Pharmaceut. Biomed. 2015, 102, 276–281. [Google Scholar] [CrossRef]
- Elbandy, M.; Miyamoto, T.; Lacaille-Dubois, M.-A. Sulfated Lupane Triterpene Derivatives and a Flavone C-Glycoside from Gypsophila repens. Chem. Pharm. Bull. 2007, 55, 808–811. [Google Scholar] [CrossRef][Green Version]
- Olennikov, D.N.; Chirikova, N.K. New C, O-Glycosylflavones from Melandrium divaricatum. Chem. Nat. Compd. 2019, 55, 1032–1038. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New C, O-Glycosylflavones from the Genus Silene. Chem. Nat. Compd. 2020, 56, 1026–1034. [Google Scholar] [CrossRef]
- Obmann, A.; Werner, I.; Presser, A.; Zehl, M.; Swoboda, Z.; Purevsuren, S.; Narantuya, S.; Kletter, C.; Glasl, S. Flavonoid C- and O-glycosides from the Mongolian medicinal plant Dianthus versicolor Fisch. Carbohyd. Res. 2011, 346, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-M.; Wei, L.-B.; Lu, C.-L.; Zhou, G.-X. A flavonoid 8-C-glycoside and a triterpenoid cinnamate from Nervilia fordii. J. Asian Nat. Prod. Res. 2013, 15, 1088–1093. [Google Scholar] [CrossRef]
- Devkota, H.P.; Fukusako, K.; Ishiguro, K.; Yahara, S. Flavone C-Glycosides from Lychnis senno and their Antioxidative Activity. Nat. Prod. Commun. 2013, 8, 1413–1414. [Google Scholar] [CrossRef]
- Xu, K.-J.; Xu, X.-M.; Deng, W.-L.; Zhang, L.; Wang, M.-K.; Ding, L.-S. Three new flavone C-glycosides from the aerial parts of Paraquilegia microphylla. J. Asian Nat. Prod. Res. 2011, 13, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.; Formisano, C.; Chianese, G.; Luciano, P.; Stornaiuolo, M.; Perveen, S.; Taglialatela-Scafati, O. Glycosylated Phenols and an Unprecedented Diacid from the Saudi Plant Cissus rotundifolia. J. Nat. Prod. 2020, 83, 3298–3304. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Tsoi, B.; Jin, X.-J.; He, R.-R.; Yao, X.-J.; Dai, Y.; Kurihara, H.; Yao, X.-S. Indoleacetic acid derivatives from the seeds of Ziziphus jujuba var. spinosa. Fitoterapia 2014, 99, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K. New Compounds from Siberian Gentiana Species. II. Xanthone and C, O-Glycosylflavone. Chem. Nat. Compd. 2021, 57, 681–684. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Xu, Z.-L.; Wang, H.; Kano, Y.; Yuan, D. A novel spinosin derivative from Semen Ziziphi Spinosae. J. Asian Nat. Prod. Res. 2011, 13, 1151–1157. [Google Scholar] [CrossRef]
- Song, Z.; Hashi, Y.; Sun, H.; Liang, Y.; Lan, Y.; Wang, H.; Chen, S. Simultaneous determination of 19 flavonoids in commercial trollflowers by using high-performance liquid chromatography and classification of samples by hierarchical clustering analysis. Fitoterapia 2013, 91, 272–279. [Google Scholar] [CrossRef]
- Wu, L.-Z.; Wu, H.-F.; Xu, X.-D.; Yang, J.-S. Two New Flavone C-Glycosides from Trollius ledebourii. Chem. Pharm. Bull. 2011, 59, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-H.; Yang, J.-S.; Dong, Y.-S.; Zhou, L.; Lin, G. Flavone C-glycosides from flowers of Trollius ledebouri. Phytochemistry 2005, 66, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, H.; Ren, B.; Zhang, B.; Hashi, Y.; Chen, S. On-line study of flavonoids of Trollius chinensis Bunge binding to DNA with ethidium bromide using a novel combination of chromatographic, mass spectrometric and fluorescence techniques. J. Chromatogr. A 2013, 1282, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapoom, T. Aromatic diglycosides from Cladogynos orientalis. Phytochemistry 2007, 68, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Le Moullec, A.; Juvik, O.J.; Fossen, T. First identification of natural products from the African medicinal plant Zamioculcas zamiifolia—A drought resistant survivor through millions of years. Fitoterapia 2015, 106, 280–285. [Google Scholar] [CrossRef]
- Tang, L.; Xu, X.-M.; Rinderspacher, K.A.; Cai, C.-Q.; Ma, Y.; Long, C.-L.; Feng, J.-C. Two new compounds from Comastoma pedunlulatum. J. Asian Nat. Prod. Res. 2011, 13, 895–900. [Google Scholar] [CrossRef]
- Ebrahimi, S.N.; Gafner, F.; Dell’Acqua, G.; Schweikert, K.; Hamburger, M. Flavone 8-C-Glycosides from Haberlea rhodopensis Friv. (Gesneriaceae). Helv. Chim. Acta 2011, 94, 38–45. [Google Scholar] [CrossRef]
- Li, Z.-L.; Li, D.-Y.; Hua, H.-M.; Chen, X.-H.; Kim, C.-S. Three new acylated flavone C-glycosides from the flowers of Trollius chinensis. J. Asian Nat. Prod. Res. 2009, 11, 426–432. [Google Scholar] [CrossRef]
- Zou, J.-H.; Yang, J.; Zhou, L. Acylated Flavone C-Glycosides from Trollius ledebouri. J. Nat. Prod. 2004, 67, 664–667. [Google Scholar] [CrossRef]
- Dong, F.-Y.; Guan, L.-N.; Zhang, Y.-H.; Cui, Z.-H.; Wang, L.; Wang, W. Acylated flavone C-glycosides from Hemistepta lyrate. J. Asian Nat. Prod. Res. 2010, 12, 776–780. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, X.; Lu, F.; Jiang, X.; Friesen, J.B.; Pauli, G.F.; Chen, S.-N.; Li, D.-P. Preparation of flavone di-C-glycoside isomers from Jian-Gu injection (Premna fulva Craib.) using recycling counter-current chromatography. J. Chromatogr. A 2019, 1599, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Huang, Y.; Qiao, S.-Y. Studies on chemical constituents from Stellaria media I. China J. Chin. Mater. Med. 2007, 32, 1048–1051. [Google Scholar]
- Feng, X.; Jiang, D.; Shan, Y.; Dai, T.; Dong, Y.; Cao, W. New flavonoid-C-Glycosides from Triticum aestivum. Chem. Nat. Compd. 2008, 44, 171–173. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Zhou, C.-X.; Zhang, S.-L.; Zheng, X.-X.; Zhao, Y. Constituents from Ranunculus sieboldii Miq. J. Chin. Pharm. Sci. 2004, 13, 92–96. [Google Scholar]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Giresser, U. A Validated HPLC Method for Simultaneous Determination of Caffeoyl Phenylethanoid Glucosides and Flavone 8-C-glycosides in Haberlea rhodopensis. Nat. Prod. Commun. 2016, 11, 791–792. [Google Scholar] [CrossRef]
- Pacifico, S.; Scognamiglio, M.; D’Abrosca, B.; Piccolella, S.; Tsafantakis, N.; Gallicchio, M.; Ricci, A.; Fiorentino, A. Spectroscopic Characterization and Antiproliferative Activity on HepG2 Human Hepatoblastoma Cells of Flavonoid C-Glycosides from Petrorhagia velutina. J. Nat. Prod. 2010, 73, 1973–1978. [Google Scholar] [CrossRef]
- Zheng, J.-X.; Zheng, Y.; Dai, Y.; Wang, N.-L.; Fang, Y.-X.; Du, Z.-Y.; Zhao, S.-Q.; Zhang, K.; Wu, L.-Y.; Fan, M. Flavone Di-C-Glycosides from Selaginella uncinate and Their Antioxidative Activities. Chem. Nat. Compd. 2016, 52, 306–308. [Google Scholar] [CrossRef]
- Xie, C.; Veitch, N.C.; Houghton, P.J.; Simmonds, M.S.J. Flavone C-Glycosides from Viola yedoensis MAKINO. Chem. Pharm. Bull. 2003, 51, 1204–1207. [Google Scholar] [CrossRef]
- Anh, N.H.; Yen, D.T.H.; Cuong, N.T.; Tai, B.H.; Yen, P.H.; Chinh, P.T.; Cuong, P.V.; Nam, N.H.; Kiem, P.V.; Cho, S.-H.; et al. Three new chromanes and one new flavone C-glycoside from Mallotus apelta. J. Asian Nat. Prod. Res. 2022, 24, 1–9. [Google Scholar] [CrossRef]
- Lam, S.-H.; Hung, H.-Y.; Yang, M.-L.; Chen, H.-H.; Kuo, P.-C.; Wu, T.-S. Chemical Constituents from Phalaenopsis Hybrids and Their Bioactivities. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Hu, H.-B.; Zhu, J.-H. Flavonoid Constituents from the Roots of Acanthopanax brachypus. Chem. Pharm. Bull. 2011, 59, 135–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, G.-H.; Zhang, Q.-W.; Wang, L.; Zhang, X.-Q.; Ye, W.-C.; Wang, Y.-T. New Isoflavone C-Glycosides from Pueraria lobata. Helv. Chim. Acta 2011, 94, 423–428. [Google Scholar] [CrossRef]
- Ukida, K.; Doi, T.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Takeda, Y. Schoepfiajasmins A–H: C-Glycosyl Dihydrochalcones, Dihydrochalcone Glycoside, C-Glucosyl Flavanones, Flavanone Glycoside and Flavone Glycoside from the Branches of Schoepfia jasminodora. Chem. Pharm. Bull. 2013, 61, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-K.; Cao, Y.-G.; Ke, Y.-Y.; Zhang, Y.-L.; Li, F.; Gong, J.-H.; Zhao, X.; Kuang, H.-X.; Feng, W.-S. Phenolic constituents from the root bark of Morus alba L. and their cardioprotective activity in vitro. Phytochemistry 2017, 135, 128–134. [Google Scholar] [CrossRef]
- Ateba, S.B.; Njamen, D.; Gatterer, C.; Scherzer, T.; Zehl, M.; Kählig, H.; Krenn, L. Rare phenolic structures found in the aerial parts of Eriosema laurentii De Wild. Phytochemistry 2016, 128, 5–11. [Google Scholar] [CrossRef]
- Shimokawa, Y.; Akao, Y.; Hirasawa, Y.; Awang, K.; Hadi, A.H.A.; Sato, S.; Aoyama, C.; Takeo, J.; Shiro, M.; Morita, H. Gneyulins A and B, Stilbene Trimers, and Noidesols A and B, Dihydroflavonol-C-Glucosides, from the Bark of Gnetum gnemonoides. J. Nat. Prod. 2010, 73, 763–767. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Nishihara, M.; Osumi, Y.; Hakamatsuka, T.; Goda, Y.; Uchiyama, N.; Ozekia, Y. Structural Analysis of Polygalaxanthones, C-Glucosyl Xanthones of Polygala tenuifolia Roots. Chem. Pharm. Bull. 2019, 67, 1242–1247. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Bayoumi, S.A.H.; Chen, C.; Vavricka, C.J.; Li, L.; Malik, A.; Dai, H.; Song, F.; Wang, L.; Zhang, J.; et al. Benzophenone C-glucosides and gallotannins from mango tree stem bark with broad-spectrum anti-viral activity. Bioorg. Med. Chem. 2014, 22, 2236–2243. [Google Scholar] [CrossRef]
- Achari, B.; Dutta, P.K.; Roy, S.K.; Chakraborty, P.; Sengupta, J.; Bandyopadhyay, D.; Maity, J.K.; Khan, I.A.; Ding, Y.; Ferreira, D. Fluorescent Pigment and Phenol Glucosides from the Heartwood of Pterocarpus marsupium. J. Nat. Prod. 2012, 75, 655–660. [Google Scholar] [CrossRef]
- Zou, J.; Jin, D.; Chen, W.; Wang, J.; Liu, Q.; Zhu, X.; Zhao, W. Selective Cyclooxygenase-2 Inhibitors from Calophyllum membranaceum. J. Nat. Prod. 2005, 68, 1514–1518. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Denisenko, V.A.; Ermakova, S.P.; Slinkina, N.N.; Dmitrenok, P.S.; Kim, N.Y. Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz. Phytochemistry 2012, 80, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-J.; Yi, S.; Li, X.; Chen, H.-F.; Ming, M.; Zhang, X.; Yao, X.-S. C-glycosides from the stems of Calophyllum membranaceum. J. Asian Nat. Prod. Res. 2018, 20, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Steffens, S.; Günther, E.; Schaumann, K. Evariquinone, isoemericellin, and stromemycin from a sponge derived strain of the fungus Emericella variecolor. Phytochemistry 2003, 63, 437–443. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z.-X.; Hu, H.; Li, D.; Qiu, G.; Hu, X.; He, X. Novel indole C-glycosides from Isatis indigotica and their potential cytotoxic activity. Fitoterapia 2011, 82, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, Z.; Ye, Y. Isocartormin, a novel quinochalcone C-glycoside from Carthamus tinctorius. Acta Pharm. Sin. B 2017, 7, 527–531. [Google Scholar] [CrossRef]
- Marrassini, C.; Davicino, R.; Acevedo, C.; Anesini, C.; Gorzalczany, S.; Ferraro, G. Vicenin-2, a Potential Anti-inflammatory Constituent of Urtica circularis. J. Nat. Prod. 2011, 74, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.-D.; Li, K.; Wang, Z.-L.; Wang, Y.-X.; He, J.-B.; Su, H.-F.; Zhang, Z.-Y.; Chi, C.-B.; Shi, X.-M.; et al. Functional Characterization and Structural Basis of an Efficient Di-C-glycosyltransferase from Glycyrrhiza glabra. J. Am. Chem. Soc. 2020, 142, 3506–3512. [Google Scholar] [CrossRef]
- Bennett, C.S.; Galan, M.C. Methods for 2-Deoxyglycoside Synthesis. Chem. Rev. 2018, 118, 7931–7985. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, B. Recent Advances in the Chemical Synthesis of C-Glycosides. Chem. Rev. 2017, 117, 12281–12356. [Google Scholar] [CrossRef]
- Liao, H.; Ma, J.; Yao, H.; Liu, X.-W. Recent progress of C-glycosylation methods in the total synthesis of natural products and pharmaceuticals. Org. Biomol. Chem. 2018, 16, 1791–1806. [Google Scholar] [CrossRef]
- Lee, D.Y.W.; He, M. Recent Advances in Aryl C-Glycoside Synthesis. Curr. Top. Med. Chem. 2005, 5, 1333–1350. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Lessons from Total Synthesis of Hybrid Natural Products. Chem. Rec. 2010, 10, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Nakayama, M.; Suzuki, H.; Tajimi, H.; Inai, M.; Nukaya, H.; Wakimoto, T.; Kan, T. Concise Synthesis of Chafurosides A and B. Org. Lett. 2009, 11, 2233–2236. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.M.; Mans, D.M.; Kaelin, D.E.; Martin, S.F. Total Synthesis of Isokidamycin. J. Am. Chem. Soc. 2010, 132, 15528–15530. [Google Scholar] [CrossRef] [PubMed]
- Misawa, K.; Takahashi, Y.; Sato, S. First Synthesis of Saponarin, 6-C- and 7-O-Di-β-d-glucosylapigenin. Chem. Pharm. Bull. 2013, 61, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Tanzer, E.-M.; Kusumi, T.; Ohmori, K.; Suzuki, K. Total Synthesis of the Proposed Structure of Ardimerin, and Proposal for its Structural Revision. Helv. Chim. Acta 2016, 99, 944–960. [Google Scholar] [CrossRef]
- Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Synthesis of the Pluramycins 1: Two Designed Anthrones as Enabling Platforms for Flexible Bis-C-Glycosylation. Angew. Chem. Int. Ed. 2014, 53, 1258–1261. [Google Scholar] [CrossRef]
- Yao, C.-H.; Tsai, C.-H.; Lee, J.-C. Total Synthesis of the Naturally Occurring Glycosylflavone Aciculatin. J. Nat. Prod. 2016, 79, 1719–1723. [Google Scholar] [CrossRef]
- Kusumi, S.; Tomono, S.; Okuzawa, S.; Kaneko, E.; Ueda, T.; Sasaki, K.; Takahashi, D.; Toshima, K. Total Synthesis of Vineomycin B2. J. Am. Chem. Soc. 2013, 135, 15909–15912. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, Y.; O’Doherty, G.A. Convergent de novo synthesis of vineomycinone B2 methyl ester. Chem. Commun. 2013, 49, 6806–6808. [Google Scholar] [CrossRef]
- Chen, C.-L.; Sparks, S.M.; Martin, S.F. C-Aryl Glycosides via Tandem Intramolecular Benzyne–Furan Cycloadditions. Total Synthesis of Vineomycinone B2 Methyl Ester. J. Am. Chem. Soc. 2006, 128, 13696–13697. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Behera, B.; Maiti, T.K.; Mal, D. Angucycline C5 Glycosides: Regio- and Stereocontrolled Synthesis and Cytotoxicity. J. Org. Chem. 2013, 78, 9748–9757. [Google Scholar] [CrossRef]
- Ohba, K.; Nakata, M. Total Synthesis of Paecilomycin B. Org. Lett. 2015, 17, 2890–2893. [Google Scholar] [CrossRef] [PubMed]
- Ohba, K.; Nataka, M. Convergent Total Synthesis of Paecilomycin B and 6′-epi-Paecilomycin B by a Barbier-Type Reaction Using 2,4,6-Triisopropylphenyllithium. J. Org. Chem. 2018, 83, 7019–7032. [Google Scholar] [CrossRef] [PubMed]
- Yepremyan, A.; Salehani, B.; Minehan, T.G. Concise Total Syntheses of Aspalathin and Nothofagin. Org. Lett. 2010, 12, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, J.; Song, F.; Wang, S.; Guo, H.; Wei, Q.; Dai, H.; Chen, X.; Xia, X.; Liu, X.; et al. Chrysomycin A Derivatives for the Treatment of Multi-Drug-Resistant Tuberculosis. ACS Cent. Sci. 2020, 6, 928–938. [Google Scholar] [CrossRef]
- Ho, T.C.; Kamimura, H.; Ohmori, K.; Suzuki, K. Total Synthesis of (+)-Vicenin-2. Org. Lett. 2016, 18, 4488–4490. [Google Scholar] [CrossRef]
- Mavlan, M.; Ng, K.; Panesar, H.; Yepremyan, A.; Minehan, T.G. Synthesis of 3,3′-Di-O-methyl Ardimerin and Exploration of Its DNA Binding Properties. Org. Lett. 2014, 16, 2212–2215. [Google Scholar] [CrossRef]
- Ben, A.; Hsu, D.-S.; Matsumoto, T.; Suzuki, K. Total synthesis and structure revision of deacetylravidomycin M. Tetrahedron 2011, 67, 6460–6468. [Google Scholar] [CrossRef]
- Acharya, P.P.; Khatri, H.R.; Janda, S.; Zhu, J. Synthesis and antitumor activities of aquayamycin and analogues of derhodinosylurdamycin A. Org. Biomol. Chem. 2019, 17, 2691–2704. [Google Scholar] [CrossRef]
- Yuan, Y.; Men, H.; Lee, C. Total Synthesis of Kendomycin: A Macro-C-Glycosidation Approach. J. Am. Chem. Soc. 2004, 126, 14720–14721. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Upadhyaya, K.; Ajay, A.; Mahar, R.; Shukla, S.K.; Kumar, B.; Tripathi, R.P. A Strategy for the Synthesis of Anthraquinone-Based Aryl-C-glycosides. J. Org. Chem. 2013, 78, 4685–4696. [Google Scholar] [CrossRef]
- Gong, H.; Gagné, M.R. Diastereoselective Ni-Catalyzed Negishi Cross-Coupling Approach to Saturated, Fully Oxygenated C-Alkyl and C-Aryl Glycosides. J. Am. Chem. Soc. 2008, 130, 12177–12183. [Google Scholar] [CrossRef]
- Nicolas, L.; Angibaud, P.; Stansfield, I.; Bonnet, P.; Meerpoel, L.; Reymond, S.; Cossy, J. Diastereoselective Metal-Catalyzed Synthesis of C-Aryl and C-Vinyl Glycosides. Angew. Chem. Int. Ed. 2012, 51, 11101–11104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rourke, M.J.; Yang, T.; Rodriguez, J.; Walczak, M.A. Highly Stereospecific Cross-Coupling Reactions of Anomeric Stannanes for the Synthesis of C-Aryl Glycosides. J. Am. Chem. Soc. 2016, 138, 12049–12052. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rodriguez, J.; Yang, T.; Kevlishvili, I.; Miller, E.; Yi, D.; O’Neill, S.; Rourke, M.J.; Liu, P.; Walczak, M.A. Glycosyl Cross-Coupling of Anomeric Nucleophiles: Scope, Mechanism, and Applications in the Synthesis of Aryl C-Glycosides. J. Am. Chem. Soc. 2017, 139, 17908–17922. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Zhu, F.; Walczak, M.A. Glycosyl Cross-Coupling with Diaryliodonium Salts: Access to Aryl C-Glycosides of Biomedical Relevance. Org. Lett. 2018, 20, 1936–1940. [Google Scholar] [CrossRef]
- Xiong, D.-C.; Zhang, L.-H.; Ye, X.-S. Oxidant-Controlled Heck-Type C-Glycosylation of Glycals with Arylboronic Acids: Stereoselective Synthesis of Aryl 2-Deoxy-C-glycosides. Org. Lett. 2009, 11, 1709–1712. [Google Scholar] [CrossRef]
- Li, H.-H.; Ye, X.-S. Regio- and stereo-selective synthesis of aryl 2-deoxy-C-glycopyranosides by palladium-catalyzed Heck coupling reactions of glycals and aryl iodides. Org. Biomol. Chem. 2009, 7, 3855–3861. [Google Scholar] [CrossRef]
- Yepremyan, A.; Minehan, T.G. Total synthesis of indole-3-acetonitrile-4-methoxy-2-C-β-D-glucopyranoside. Proposal for structural revision of the natural product. Org. Biomol. Chem. 2012, 10, 5194–5196. [Google Scholar] [CrossRef]
- Denmark, S.E.; Regens, C.S.; Kobayashi, T. Total Synthesis of Papulacandin D. J. Am. Chem. Soc. 2007, 129, 2774–2776. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ben-zvi, B.; Diao, T. Diastereoselective Synthesis of Aryl C-Glycosides from Glycosyl Esters via C-O Bond Homolysis. Angew. Chem. Int. Ed. 2021, 60, 9433–9438. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Jiang, Y.; Zhang, H.; Yu, L.; Tian, C.; Chen, G.; Koh, M.J. Iron-catalysed reductive cross-coupling of glycosyl radicals for the stereoselective synthesis of C-glycosides. Nat. Synth. 2022, 1, 235–244. [Google Scholar] [CrossRef]
- Adak, L.; Kawamura, S.; Toma, G.; Takenaka, T.; Isozaki, K.; Takaya, H.; Orita, A.; Li, H.C.; Shing, T.K.M.; Nakamura, M. Synthesis of Aryl C-Glycosides via Iron-Catalyzed Cross Coupling of Halosugars: Stereoselective Anomeric Arylation of Glycosyl Radicals. J. Am. Chem. Soc. 2017, 139, 10693–10701. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, Q.; Xiong, D.-C.; Jiang, S.; Li, Q.; Ye, X.-S. Stereocontrolled Synthesis of 2-Deoxy-C-glycopyranosyl Arenes Using Glycals and Aromatic Amines. Org. Lett. 2018, 20, 3079–3082. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kanaujiya, V.K.; Tiwari, V.; Sabiah, S.; Kandasamy, J. Development of Routes for the Stereoselective Preparation of β-Aryl-C-glycosides via C-1 Aryl Enones. Org. Lett. 2020, 22, 7650–7655. [Google Scholar] [CrossRef]
- Liu, J.; Gong, H. Stereoselective Preparation of α-C-Vinyl/Aryl Glycosides via Nickel-Catalyzed Reductive Coupling of Glycosyl Halides with Vinyl and Aryl Halides. Org. Lett. 2018, 20, 7991–7995. [Google Scholar] [CrossRef]
- Mou, Z.-D.; Wang, J.-X.; Zhang, X.; Niu, D. Stereoselective Preparation of C-Aryl Glycosides via Visible-Light-Induced Nickel-Catalyzed Reductive Cross-Coupling of Glycosyl Chlorides and Aryl Bromides. Adv. Synth. Catal. 2021, 363, 3025–3029. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Y.; Xie, X.; Hu, S.; Zhang, S.; Zeng, M.; Zhang, D.; Wang, J.; Liu, H. Ir(I)-Catalyzed C-H Glycosylation for Synthesis of 2-Indolyl-C-Deoxyglycosides. Adv. Synth. Catal. 2021, 363, 4926–4931. [Google Scholar] [CrossRef]
- Ghouilem, J.; Tran, C.; Grimblat, N.; Retailleau, P.; Alami, M.; Gandon, V.; Messaoudi, S. Diastereoselective Pd-Catalyzed Anomeric C(sp3) −H Activation: Synthesis of α-(Hetero)aryl C-Glycosides. ACS Catal. 2021, 11, 1818–1826. [Google Scholar] [CrossRef]
- Liu, M.; Niu, Y.; Wu, Y.-F.; Ye, X.-S. Ligand-Controlled Monoselective C-Aryl Glycoside Synthesis via Palladium-Catalyzed C–H Functionalization of N-Quinolyl Benzamides with 1-Iodoglycals. Org. Lett. 2016, 18, 1836–1839. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; O’Doherty, G.A. De novo synthesis of a galacto-papulacandin moiety via an iterative dihydroxylation strategy. Tetrahedron Lett. 2005, 46, 4151–4155. [Google Scholar] [CrossRef]
- Balachari, D.; O’Doherty, G.A. Enantioselective Synthesis of the Papulacandin Ring System: Conversion of the Mannose Diastereoisomer into a Glucose Stereoisomer. Org. Lett. 2000, 2, 4033–4036. [Google Scholar] [CrossRef] [PubMed]
- Balachari, D.; O’Doherty, G.A. Sharpless Asymmetric Dihydroxylation of 5-Aryl-2-vinylfurans: Application to the Synthesis of the Spiroketal Moiety of Papulacandin D. Org. Lett. 2000, 2, 863–866. [Google Scholar] [CrossRef]
- Liu, C.-F.; Xiong, D.-C.; Ye, X.-S. “Ring Opening–Ring Closure” Strategy for the Synthesis of Aryl-C-glycosides. J. Org. Chem. 2014, 79, 4676–4686. [Google Scholar] [CrossRef]
- Mainkar, P.S.; Johny, K.; Rao, T.P.; Chandrasekhar, S. Synthesis of O-Spiro-C-Aryl Glycosides Using Organocatalysis. J. Org. Chem. 2012, 77, 2519–2525. [Google Scholar] [CrossRef]
- Moral, J.A.; Moon, S.-J.; Rodriguez-Torres, S.; Minehan, T.G. A Sequential Indium-Mediated Aldehyde Allylation/Palladium-Catalyzed Cross-Coupling Reaction in the Synthesis of 2-Deoxy-β-C-Aryl Glycosides. Org. Lett. 2009, 11, 3734–3737. [Google Scholar] [CrossRef]
- Hauser, F.M.; Hu, X. A New Route to C-Aryl Glycosides. Org. Lett. 2002, 4, 977–978. [Google Scholar] [CrossRef]
- Pichlmair, S.; Marques, M.M.B.; Green, M.P.; Martin, H.J.; Mulzer, J. A Novel Approach toward the Synthesis of Kendomycin: Selective Synthesis of a C-Aryl Glycoside as a Single Atropisomer. Org. Lett. 2003, 5, 4657–4659. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).