Sensitive and Facile HCOOH Fluorescence Sensor Based on Highly Active Ir Complexes’ Catalytic Transfer Hydrogen Reaction
Abstract
1. Introduction
2. Results
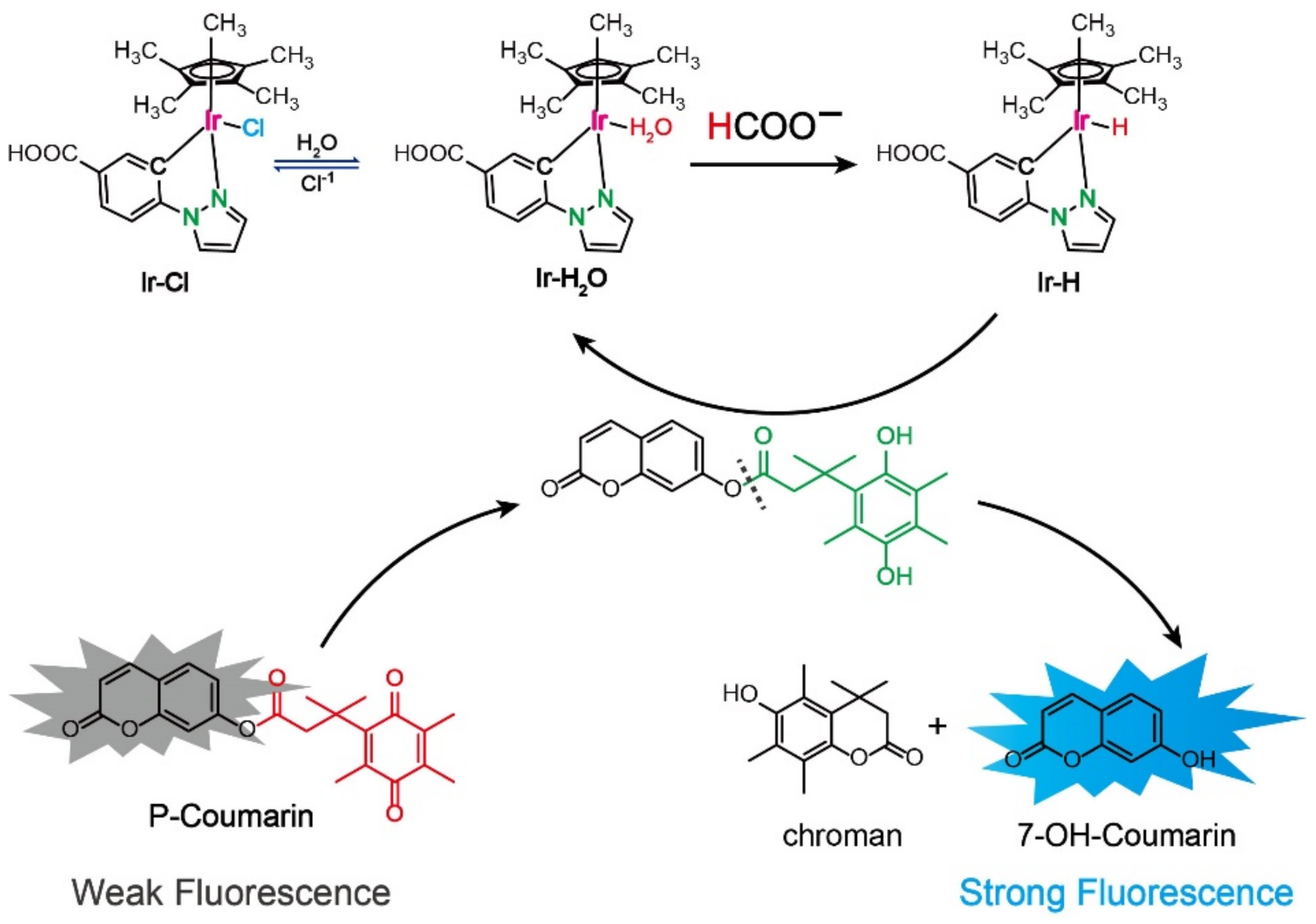
2.1. Detection Principle of the Ir-Complex Catalytic Fluorescence Sensor for HCOO−
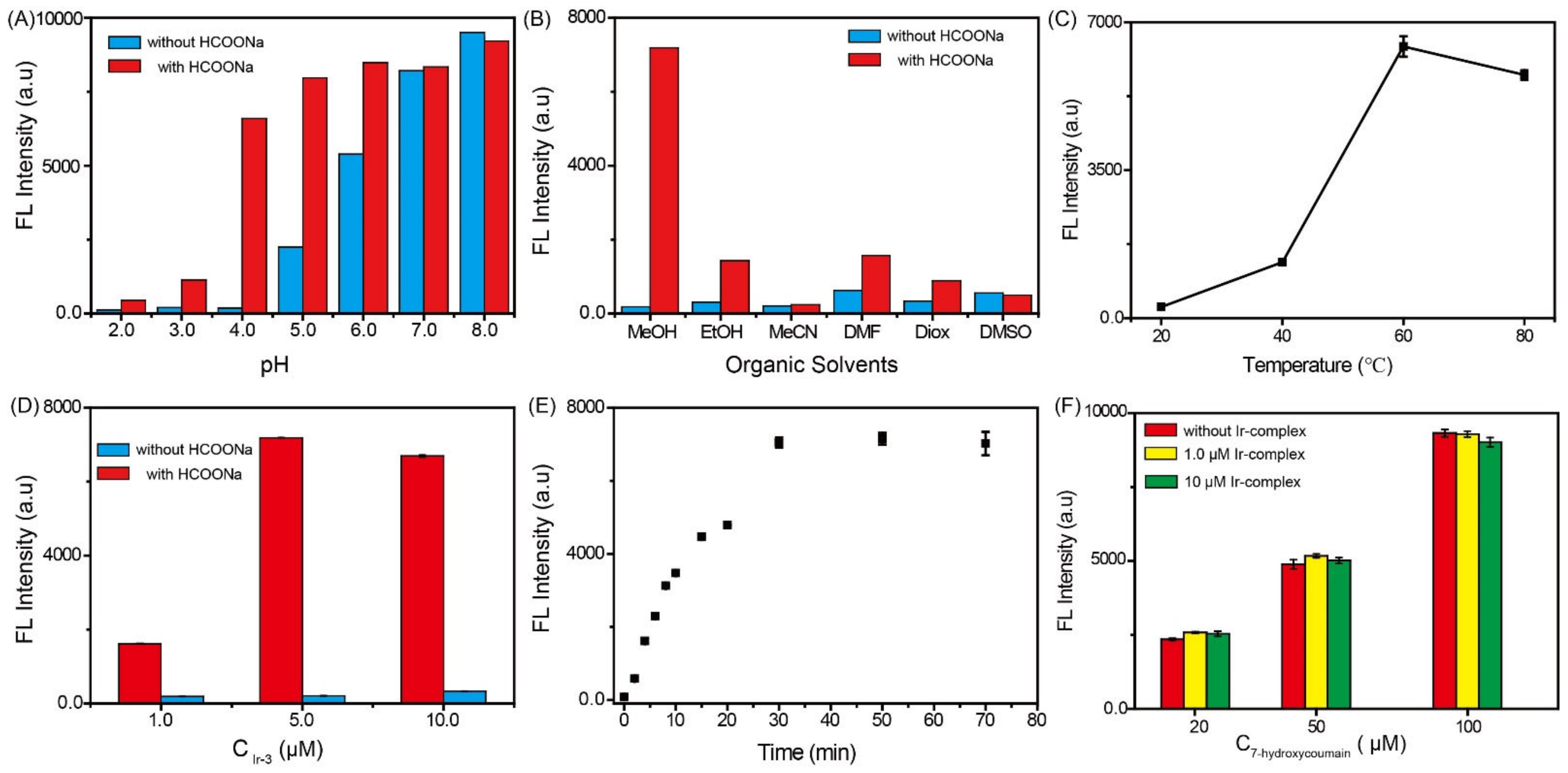
2.2. Optimization of Experimental Parameters
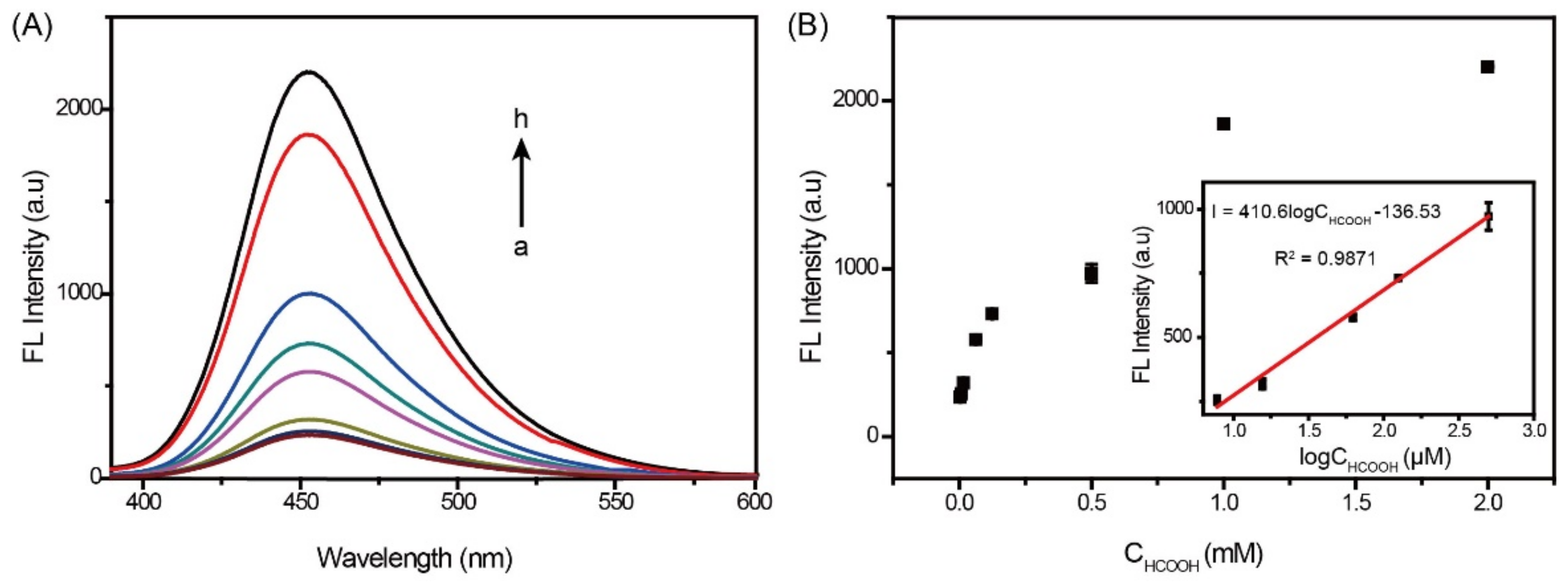
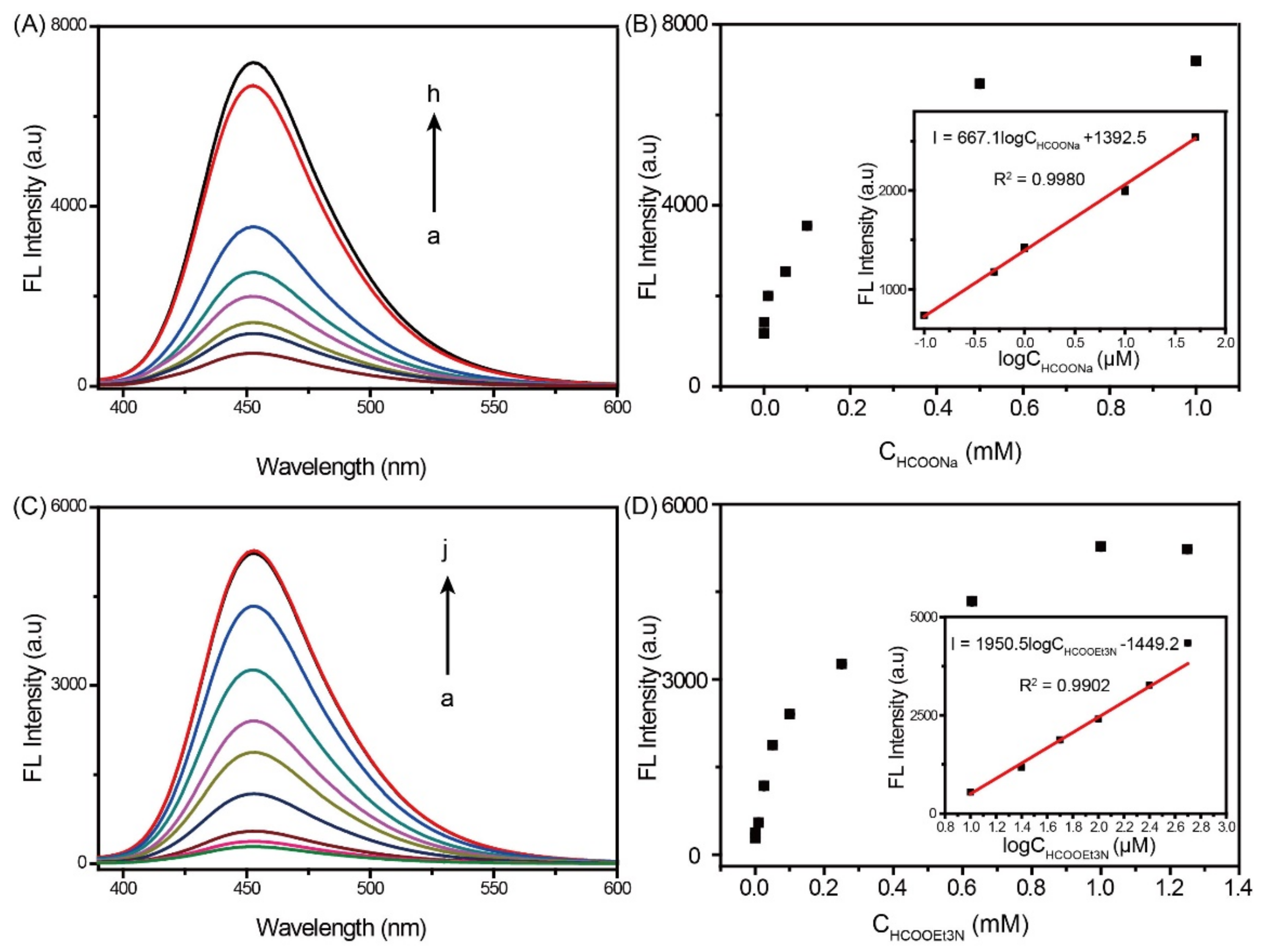
2.3. Ir-Complex Catalytic Fluorescence Sensor for the Detection of HCOOH and Formates (HCOONa and HCOOEt3N)
2.4. The Selectivity and Repeatability of the Ir-Complex Catalytic Fluorescence Sensor
2.5. Detection of Actual Samples
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Detection Protocol and Spectrophotometric Analysis
3.3. Detection of Actual Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, L.; Zhao, N.; Wei, W.; Sun, Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel 2013, 108, 112–130. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, B.; Chen, F.; Liu, C.; Lu, S.; Yu, Y.; Zhang, B. Thermally-assisted photocatalytic CO2 reduction to fuels. Chem. Eng. J. 2021, 408, 127280. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Z.; Wei, X.G.; Gallenkamp, C.; Bonin, J.; Anxolabehere-Mallart, E.; Lau, K.C.; Lau, T.C.; Robert, M. Molecular Catalysis of the Electrochemical and Photochemical Reduction of CO2 with Earth-Abundant Metal Complexes. Selective Production of CO vs. HCOOH by Switching of the Metal Center. J. Am. Chem. Soc. 2015, 137, 10918–10921. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nat. Commun. 2019, 10, 2807. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dutta, I.; Lum, Y.; Lai, Z.; Huang, K.-W. Enabling storage and utilization of low-carbon electricity: Power to formic acid. Energy Environ. Sci. 2021, 14, 1194–1246. [Google Scholar] [CrossRef]
- Hao, L.; Benxian, S.; Xiaolong, Z. An Improved Desulfurization Process Based on H2O2 /Formic Acid Oxidation and Liquid-Liquid Extraction 2. FCC Diesel Oil Feedstocks. Pet. Sci. Technol. 2006, 24, 1043–1054. [Google Scholar] [CrossRef]
- Fernandes, I.P.; Amaral, J.S.; Pinto, V.; Ferreira, M.J.; Barreiro, M.F. Development of chitosan-based antimicrobial leather coatings. Carbohydr. Polym. 2013, 98, 1229–1235. [Google Scholar] [CrossRef]
- Seabra, I.J.; Chim, R.B.; Salgueiro, P.; Braga, M.E.M.; de Sousa, H.C. Influence of solvent additives on the aqueous extraction of tannins from pine bark: Potential extracts for leather tanning. J. Chem. Technol. Biotechnol. 2018, 93, 1169–1182. [Google Scholar] [CrossRef]
- Li, X.; Bian, R.; Wang, J.; Wang, X.; Ma, J.; Ma, G.; Sui, H.; He, L. Recovery of extra-heavy oil and minerals from carbonate asphalt rocks by reactive extraction. RSC Adv. 2019, 9, 14372–14381. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Zhang, T.; Das, S. Oxidative Transformation of Biomass into Formic Acid. Eur. J. Org. Chem. 2021, 2021, 1331–1343. [Google Scholar] [CrossRef]
- Garai, P.; Banerjee, P.; Sharma, P.; Chatterjee, A.; Bhattacharya, R.; Saha, N.C. Mechanistic insights to lactic and formic acid toxicity on benthic oligochaete worm Tubifex tubifex. Environ. Sci. Pollut. Res. Int. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Dojo, M.; Yamashita, H. Pd and Pd–Ag Nanoparticles within a Macroreticular Basic Resin: An Efficient Catalyst for Hydrogen Production from Formic Acid Decomposition. ACS Catal. 2013, 3, 1114–1119. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-Nanoparticle-Catalyzed Hydrogen Generation from Formic Acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef]
- Preuster, P.; Albert, J. Biogenic Formic Acid as a Green Hydrogen Carrier. Energy Technol. 2018, 6, 501–509. [Google Scholar] [CrossRef]
- Landmesser, A.; Scherer, G.; Pluym, N.; Niessner, R.; Scherer, M. A novel quantification method for sulfur-containing biomarkers of formaldehyde and acetaldehyde exposure in human urine and plasma samples. Anal. Bioanal. Chem. 2020, 412, 7535–7546. [Google Scholar] [CrossRef]
- Maeda, T.; Wood, T. Formate detection by potassium permanganate for enhanced hydrogen production in Escherichia coli. Int. J. Hydrog. Energy 2008, 33, 2409–2412. [Google Scholar] [CrossRef]
- Batista, E.A.; Malpass, G.R.P.; Motheo, A.J.; Iwasita, T. New mechanistic aspects of methanol oxidation. J. Electroanal. Chem. 2004, 571, 273–282. [Google Scholar] [CrossRef]
- Hu, Z.; Tan, S.; Mi, R.; Li, X.; Bai, J.; Guo, X.; Hu, G.; Hang, P.; Li, J.; Li, D.; et al. Formic Acid or Formate Derivatives as the In Situ Hydrogen Source in Au-Catalyzed Reduction of para-Chloronitrobenzene. ChemistrySelect 2018, 3, 2850–2853. [Google Scholar] [CrossRef]
- Pérez-Zúñiga, C.; Negrete-Vergara, C.; Guerchais, V.; Le Bozec, H.; Moya, S.A.; Aguirre, P. Hydrogenation of N-benzylideneaniline by palladium (II) catalysts with phosphorus-nitrogen ligands using formic acid as a renewable hydrogen source. Mol. Catal. 2019, 462, 126–131. [Google Scholar] [CrossRef]
- Zhao, L.-J.; Zhang, C.; Zhang, W.; Shi, Y.; Xia, L.; Zhang, W.; Peng, B.; Jin, X.; Tian, C.; Luan, F.; et al. Iridium-Complex-Functionalized Magnetic Nanoparticles for Fluorescent Detection of Mercapto Drugs. ACS Appl. Nano Mater. 2021, 4, 12920–12925. [Google Scholar] [CrossRef]
- Zhao, L.J.; Yin, Z.Q.; Shi, Y.S.; Sun, W.; Sun, L.B.; Su, H.J.; Sun, X.; Zhang, W.L.; Xia, L.Y.; Qi, C.X. A highly active Cp*Ir complex with an anionic N,N-donor chelate ligand catalyzes the robust regeneration of NADH under physiological conditions. Catal. Sci. Technol. 2021, 11, 7982–7991. [Google Scholar] [CrossRef]
- Kage, S.; Kudo, K.; Ikeda, H.; Ikeda, N. Simultaneous determination of formate and acetate in whole blood and urine from humans using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 805, 113–117. [Google Scholar] [CrossRef]
- Kuban, P.; Foret, F.; Bocek, R. Capillary electrophoresis with contactless conductometric detection for rapid screening of formate in blood serum after methanol intoxication. J. Chromatogr. A 2013, 1281, 142–147. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, C.; Gao, H.; Qin, W.; Meng, F. High Response Formic Acid Gas Sensor Based on MoS2 Nanosheets. IEEE Trans. Nanotechnol. 2021, 20, 177–184. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, Z.; Gu, S. Determination of formic acid and propionic acid contents in feed acidifer by high performance liquid chromatography. Acta Agric. Shanghai 2010, 26, 64–66. [Google Scholar]

| Methods | Linear Range (ppm) | Detection Limit (ppm) | Ref. |
|---|---|---|---|
| GC-MS | 2.3–230 | 0.92 | [25] |
| CE | 0.23–9.2 | 0.10 | [26] |
| Resistance | 10–100 | 10.00 | [27] |
| HPLC | 100–2000 | 5.00 | [28] |
| Fluorescence | 0.078–5.000 | 0.05 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RDS (%) |
|---|---|---|---|---|
| 1 | 50 | 49.96 | 99.92 | 0.4 |
| 2 | 100 | 94.77 | 94.77 | 0.2 |
| 3 | 150 | 149.95 | 99.97 | 4.9 |
| 4 | 200 | 215.45 | 107.73 | 2.7 |
| 5 | 300 | 311.14 | 103.72 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, W.; Wu, Y.; Peng, B.; Tian, C.; Luan, F.; Sun, W.; Zhuang, X.; Zhao, L. Sensitive and Facile HCOOH Fluorescence Sensor Based on Highly Active Ir Complexes’ Catalytic Transfer Hydrogen Reaction. Molecules 2022, 27, 7431. https://doi.org/10.3390/molecules27217431
Zhang C, Zhang W, Wu Y, Peng B, Tian C, Luan F, Sun W, Zhuang X, Zhao L. Sensitive and Facile HCOOH Fluorescence Sensor Based on Highly Active Ir Complexes’ Catalytic Transfer Hydrogen Reaction. Molecules. 2022; 27(21):7431. https://doi.org/10.3390/molecules27217431
Chicago/Turabian StyleZhang, Caimei, Wenjuan Zhang, Yiran Wu, Bo Peng, Chunyuan Tian, Feng Luan, Wen Sun, Xuming Zhuang, and Lijun Zhao. 2022. "Sensitive and Facile HCOOH Fluorescence Sensor Based on Highly Active Ir Complexes’ Catalytic Transfer Hydrogen Reaction" Molecules 27, no. 21: 7431. https://doi.org/10.3390/molecules27217431
APA StyleZhang, C., Zhang, W., Wu, Y., Peng, B., Tian, C., Luan, F., Sun, W., Zhuang, X., & Zhao, L. (2022). Sensitive and Facile HCOOH Fluorescence Sensor Based on Highly Active Ir Complexes’ Catalytic Transfer Hydrogen Reaction. Molecules, 27(21), 7431. https://doi.org/10.3390/molecules27217431








