Plasma Metabolites and Liver Composition of Broilers in Response to Dietary Ulva lactuca with Ulvan Lyase or a Commercial Enzyme Mixture
Abstract
1. Introduction
2. Results
2.1. Feed Intake and Animal Growth Performance
2.2. Plasma Biochemical Profile
2.3. Hepatic Total Lipids, Cholesterol Contents and Fatty Acid Composition
2.4. Hepatic Vitamin E, Pigments and Mineral Profile
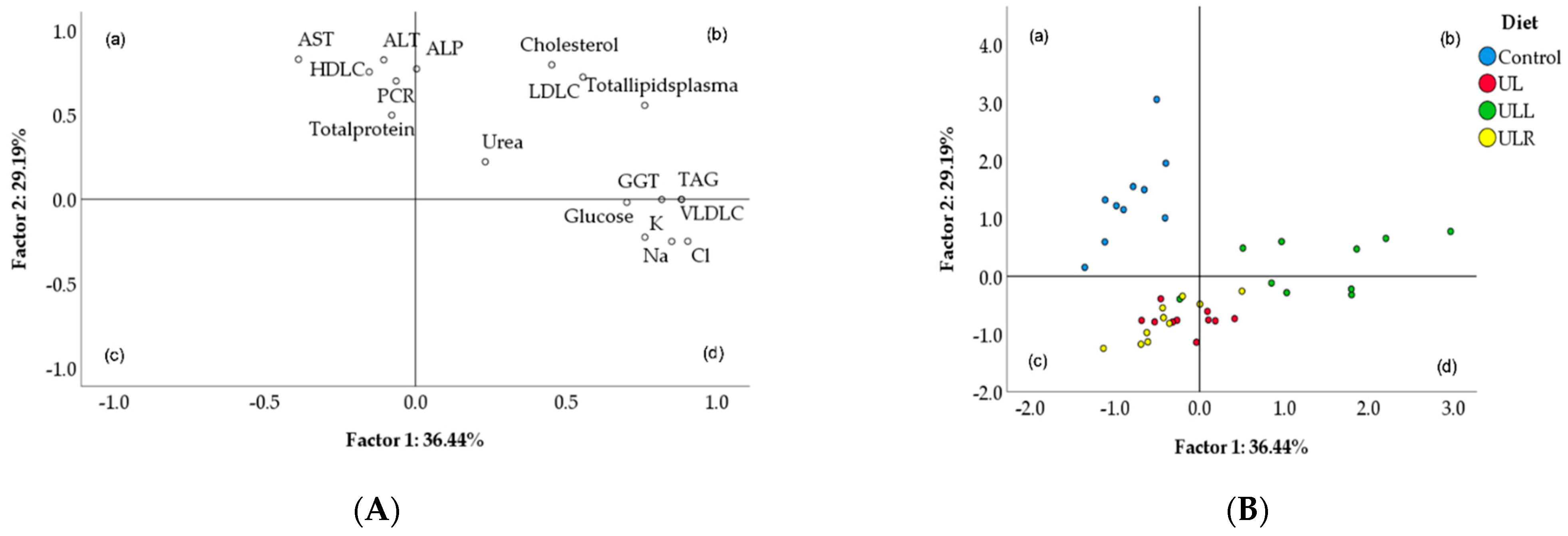
2.5. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Experimental Design, Diets and Samples Collection
4.2. Recombinant Ulvan Lyase Production
4.3. Chemical Analysis of U. lactuca and Diets
4.4. Assessment of Hepatic Diterpenes, Pigments and Mineral Profile
4.5. Assessment of Hepatic Total Lipid Content and Fatty Acid Profile
4.6. Analysis of Plasma Biochemical Parameters
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Meat. In OECD-FAO Agricultural Outlook 2021–2030; OECD: Paris, France, 2021.
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Eismann, A.I.; Reis, R.P.; da Silva, A.F.; Cavalcanti, D.N. Ulva spp. carotenoids: Responses to environmental conditions. Algal Res. 2020, 48, 101916. [Google Scholar] [CrossRef]
- Taboada, C.; Millán, R.; Míguez, I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010, 90, 445–449. [Google Scholar] [PubMed]
- Cardoso, C.; Ripol, A.; Afonso, C.; Freire, M.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Sci. Nutr. 2017, 5, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, H.; Loret, E.P. Ulva lactuca, a source of troubles and potential riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef]
- Alagan, V.T.; Vatsala, R.N.; Sagadevan, I.; Subbiah, V.; Ragothaman, V. Effect of dietary supplementation of seaweed (Ulva lactuca) and Azolla on growth performance, haematological and serum biochemical parameters of Aseel chicken. Beni-Suef. Univ. J. Basic Appl. Sci. 2020, 9, 58. [Google Scholar] [CrossRef]
- Cañedo-Castro, B.; Piñón-Gimate, A.; Carrillo, S.; Ramos, D.; Valdez, C. Prebiotic effect of Ulva rigida meal on the intestinal integrity and serum cholesterol and triglyceride content in broilers. J. Appl. Phycol. 2019, 31, 3265–3273. [Google Scholar] [CrossRef]
- Matshogo, T.B.; Mnisi, C.M.; Mlambo, V. Dietary green seaweed compromises overall feed conversion efficiency but not blood parameters and meat quality and stability in broiler chickens. Agriculture 2020, 10, 547. [Google Scholar] [CrossRef]
- Nhlane, L.T.; Mnisi, C.M.; Mlambo, V.; Madibana, M.J. Effect of seaweed-containing diets on visceral organ sizes, carcass characteristics, and meat quality and stability of Boschveld indigenous hens. Poult. Sci. 2021, 100, 949–956. [Google Scholar] [CrossRef]
- Ventura, M.R.; Castanon, J.I.R.; Mcnab, J.M. Nutritional value of seaweed (Ulva rigida) for poultry. Anim. Feed Sci. Technol. 1994, 49, 87–92. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Pestana, J.M.; Puerta, B.; Santos, H.; Madeira, M.S.; Alfaia, C.M.; Lopes, P.A.; Pinto, R.M.A.; Lemos, J.P.C.; Fontes, C.M.G.A.; Lordelo, M.M.; et al. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020, 99, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Alfaia, C.M.; Pestana, J.M.; Rodrigues, M.; Aires, M.J.; Ribeiro, D.M.; Major, V.T.; Martins, C.F.; Santos, H.; Lopes, P.A.; Lemos, J.P.C.; et al. Influence of dietary Chlorella vulgaris and carbohydrate-active enzymes on growth performance, meat quality and lipid composition of broiler chickens. Poult. Sci. 2021, 100, 926–937. [Google Scholar] [CrossRef]
- Costa, M.M.; Pestana, J.M.; Osório, D.; Alfaia, C.M.; Martins, C.F.; Mourato, M.; Gueifão, S.; Rego, A.M.; Coelho, I.; Coelho, D.; et al. Effect of dietary Laminaria digitata with carbohydrases on broiler production performance and meat quality, lipid profile and mineral composition. Animals 2022, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Lopes, P.A.; Assunção, J.M.P.; Alfaia, C.M.R.P.M.; Coelho, D.F.M.; Mourato, M.P.; Pinto, R.M.A.; Lordelo, M.M.; Prates, J.A.M. Combined effects of dietary Laminaria digitata with alginate lyase on plasma metabolites and hepatic lipid, pigment and mineral composition of broilers. BMC Vet. Res. 2022, 18, 153. [Google Scholar] [CrossRef]
- Costa, M.M.; Pestana, J.M.; Carvalho, P.; Alfaia, C.M.; Martins, C.F.; Carvalho, D.; Mourato, M.; Gueifão, S.; Delgado, I.; Coelho, I.; et al. Effect on broiler production performance and meat quality of feeding Ulva lactuca supplemented with carbohydrases. Animals 2022, 12, 1720. [Google Scholar] [CrossRef]
- Matshogo, T.B.; Mlambo, V.; Mnisi, C.M.; Manyeula, F. Effect of pre-treating dietary green seaweed with fibrolytic enzymes on growth performance, blood indices, and meat quality parameters of Cobb 500 broiler chickens. Livest. Sci. 2021, 251, 104652. [Google Scholar] [CrossRef]
- Matshogo, T.B.; Mnisi, C.M.; Mlambo, V. Effect of pre-treating dietary green seaweed with proteolytic and fibrolytic enzymes on physiological and meat quality parameters of broiler chickens. Foods 2021, 10, 1862. [Google Scholar] [CrossRef]
- Costa, M.; Fernandes, V.O.; Ribeiro, T.; Serrano, L.; Cardoso, V.; Santos, H.; Lordelo, M.; Ferreira, L.M.A.; Fontes, C.M.G.A. Construction of GH16 beta-glucanase mini-cellulosomes to improve the nutritive value of barley-based diets for broilers. J. Agric. Food Chem. 2014, 62, 7496–7506. [Google Scholar] [CrossRef]
- Fernandes, V.O.; Costa, M.; Ribeiro, T.; Serrano, L.; Cardoso, V.; Santos, H.; Lordelo, M.; Ferreira, L.M.A.; Fontes, C.M.G.A. 1,3-1,4-beta-glucanases and not 1,4-beta-glucanases improve the nutritive value of barley-based diets for broilers. Anim. Feed Sci. Technol. 2016, 211, 153–163. [Google Scholar] [CrossRef]
- Costa, M.M.; Pio, L.B.; Bule, P.; Cardoso, V.A.; Duarte, M.; Alfaia, C.M.; Coelho, D.F.; Brás, J.A.; Fontes, C.M.G.A.; Prates, J.A.M. Recalcitrant cell wall of Ulva lactuca seaweed is degraded by a single ulvan lyase from family 25 of polysaccharide lyases. Anim. Nutr. 2022, 9, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Stokvis, L.; van Krimpen, M.M.; Kwakkel, R.P.; Bikker, P. Evaluation of the nutritional value of seaweed products for broiler chickens’ nutrition. Anim. Feed Sci. Technol. 2021, 280, 115061. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 5th ed.; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Kuttappan, V.A.; Huff, G.R.; Huff, W.E.; Hargis, B.M.; Apple, J.K.; Coon, C.; Owens, C.M. Comparison of hematologic and serologic profiles of broiler birds with normal and severe degrees of white striping in breast fillets. Poult. Sci. 2013, 92, 339–345. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Okab, A.B.; Aljumaah, R.S.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A. Nutritional value of green seaweed (Ulva lactuca) for broiler chickens. Ital. J. Anim. Sci. 2013, 12, e28. [Google Scholar] [CrossRef]
- Song, D.; Wang, Y.W.; Hou, Y.J. The effects of dietary supplementation of microencapsulated Enterococcus faecalis and the extract of Camellia oleifera seed on growth performance, immune functions, and serum biochemical parameters in broiler chickens. J. Anim. Sci. 2016, 94, 3271–3277. [Google Scholar] [CrossRef]
- Lai, W.; Huang, W.; Dong, B. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens. Poult. Sci. 2018, 97, 196–202. [Google Scholar] [CrossRef]
- Miller, G.I.; Miller, N.E. Plasma high-density lipoprotein concentration and development of ischaemic heart disease. Lancet I 1975, 305, 16–19. [Google Scholar] [CrossRef]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef]
- Silva, P.R.L.; Freitas Neto, O.C.; Laurentiz, A.C.; Junqueira, O.M.; Fagliari, J.J. Blood serum components and serum protein test of Hybro-PG broilers of different ages. Braz. J. Poult. Sci. 2007, 9, 229–232. [Google Scholar] [CrossRef]
- Shrimanker, I.; Bhattarai, S. Electrolytes. Treasure Island (FL); Stat Pearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, S.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Costa, M.M.; Pinto, R.M.A.; Almeida, J.M.; Moreira, O.; Fontes, C.M.G.A.; Prates, J.A.M. Impact of Chlorella vulgaris as feed ingredient and carbohydrases on the health status and hepatic lipid metabolism of finishing pigs. Res. Vet. Sci. 2022, 44, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, Y.; Nishikawa, A.; Imazawa, T.; Kitamura, Y.; Kanki, K.; Ishii, Y.; Umemura, T.; Hirose, M. A subchronic toxicity study of dunaliella carotene in F344 rats. Food Chem. Toxicol. 2006, 44, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Murillo, E. Hypercholesterolemic effect of canthaxanthin and astaxanthin in rats. Arch. Latinoam. Nutr. 1992, 42, 409–413. [Google Scholar]
- Airanthi, M.K.W.-A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef]
- Kouba, M.; Mourot, J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie 2011, 93, 13–17. [Google Scholar] [CrossRef]
- Tao, L.; Sun, T.; Magnuson, A.D.; Qamar, T.R.; Lei, X.G. Defatted microalgae mediated enrichment of n–3 polyunsaturated fatty acids in chicken muscle is not affected by dietary selenium, vitamin E., or corn oil. J. Nutr. 2018, 148, 1547–1555. [Google Scholar] [CrossRef]
- Smink, W.; Gerrits, W.J.J.; Gloaguen, M.; Ruiter, A.; Van Baal, J. Linoleic and α-linolenic acid as precursor and inhibitor for the synthesis of long-chain polyunsaturated fatty acids in liver and brain of growing pigs. Animal 2012, 6, 262–270. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- İrkin, L.C.; Erduğan, H. Chemical composition of Ulva rigida C. Agardh from the Çanakkale Strait (Dardanelles), Turkey. J. Black Sea/Mediterr. Environ. 2014, 20, 114–121. [Google Scholar]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbø, R.; Amlund, H.; Heesch, S.; Lock, E.J. Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health benefits of carotenoids and potential application in poultry industry: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.M.; Alfaia, C.M.; Pestana, J.M.; Carvalho, D.F.P.; Costa, M.; Martins, C.F.; Lemos, J.P.C.; Mourato, M.; Gueifão, S.; Delgado, I.; et al. Influence of feeding weaned piglets with Laminaria digitata on the quality and nutritional value of meat. Foods 2022, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, R. Recent data on iodine intake in Germany and Europe. J. Trace Elem. Med. Biol. 2016, 37, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.J.; Ashmore, E. Risk assessment of antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium concentrations in the New Zealand diet. Food Addit. Contam. Part A 2020, 37, 451–464. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Prates, J.A.M.; Quaresma, M.A.G.; Bessa, R.J.B.; Fontes, C.M.G.A.; Alfaia, C.M.P.M. Simultaneous HPLC quantification of total cholesterol, tocopherols and [beta]-carotene in Barrosã-PDO veal. Food Chem. 2006, 94, 469–477. [Google Scholar] [CrossRef]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of Spirulina platensis meal as feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 396, 14–19. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Covaci, A.; Voorspoels, S.; Thomsen, C.; van Bavel, B.; Neels, H. Evaluation of total lipids using enzymatic methods for the normalization of persistent organic pollutant levels in serum. Sci. Total Environ. 2006, 366, 361–366. [Google Scholar] [CrossRef] [PubMed]
| Parameters/Diets | Control | UL | ULR | ULL | SEM | p-Value |
|---|---|---|---|---|---|---|
| Initial weight (g) | 767 | 748 | 754 | 727 | 82.5 | 0.763 |
| Final weight (g) | 1763 a | 1621 a,b | 1679 a,b | 1523 b | 159.7 | 0.016 |
| Average daily gain (g/d) | 77.4 a | 68.1 a,b | 72.1 a,b | 61.8 b | 3.38 | 0.018 |
| Average daily feed intake (g/pen) | 371 | 310 | 318 | 320 | 49.5 | 0.057 |
| Feed conversion ratio | 1.68 | 1.73 | 1.63 | 1.78 | 0.139 | 0.211 |
| Parameters/Diets | Control | UL | ULR | ULL | SEM | p-Value |
|---|---|---|---|---|---|---|
| Plasma lipids | ||||||
| Total lipids 1 (mg/dL) | 429 b | 413 b,c | 400 c | 457 a | 7.35 | <0.001 |
| TAG (mg/dL) | 37.5 b | 48.2 b | 43.6 b | 65.6 a | 3.78 | <0.001 |
| Total cholesterol (mg/dL) | 121 a | 107 b | 103 b | 121 a | 2.45 | <0.001 |
| HDL-cholesterol (mg/dL) | 82.4 a | 74.4 b | 75.2 b | 75.4 b | 1.24 | 0.001 |
| LDL-cholesterol (mg/dL) | 19.7 a | 15.2 b | 14.7 b | 21.7 a | 0.591 | <0.001 |
| VLDL-cholesterol 2 (mg/dL) | 7.50 b | 9.64 b | 8.72 b | 13.1 a | 0.757 | <0.001 |
| Other plasma metabolites | ||||||
| Glucose (mg/dL) | 249 b | 254 a,b | 249 b | 258 a | 2.13 | 0.013 |
| Urea (mg/dL) | 2.00 | 1.57 | 2.09 | 1.92 | 0.152 | 0.102 |
| Creatinine (mg/dL) | <0.001 | <0.001 | <0.001 | <0.001 | - | - |
| Total protein (g/dL) | 2.37 b | 2.20 c | 2.34 b | 2.66 a | 0.028 | <0.001 |
| C-reactive protein (mg/dL) | 0.03 a | 0.01 d | 0.01 c | 0.02 b | 0.001 | <0.001 |
| Ionogram | ||||||
| Chloride (mEq/L) | 109 c | 115 b | 116 b | 124 a | 1.07 | <0.001 |
| Sodium (mEq/L) | 146 b | 151 a | 151 a | 155 a | 1.28 | <0.001 |
| Potassium (mEq/L) | 7.13 c | 8.25 b | 8.15 b | 9.41 a | 0.171 | <0.001 |
| Hepatic markers | ||||||
| ALT (U/L) | 5.10 a | 3.20 b | 2.60 b | 3.50 b | 0.305 | <0.001 |
| AST (U/L) | 531 a | 206 b | 183 b | 196 b | 23.9 | <0.001 |
| ALP (U/L) | 1853 a | 1346 b | 1432 b | 1589 a,b | 70.6 | <0.001 |
| GGT (U/L) | 18.5 c | 21.4 b | 18.2 c | 30.4 a | 0.589 | <0.001 |
| Parameters/Diets | Control | UL | ULR | ULL | SEM | p-Value |
|---|---|---|---|---|---|---|
| Total lipids (g/100 g) | 2.69 | 2.44 | 2.47 | 2.43 | 0.079 | 0.086 |
| Cholesterol (g/100 g) | 3.07 b | 3.62 a | 3.55 a | 3.60 a | 0.114 | 0.004 |
| Fatty acid composition (g/100 g FA) | ||||||
| 14:0 | 0.11 | 0.10 | 0.09 | 0.09 | 0.008 | 0.105 |
| 15:0 | 0.05 c | 0.09 a | 0.08 ab | 0.07 b | 0.003 | <0.001 |
| 16:0 | 16.6 a | 14.5 b | 13.9 b | 14.4 b | 0.29 | <0.001 |
| 16:1c7 | 0.29 b | 0.40 a | 0.38 a | 0.28 b | 0.022 | 0.001 |
| 16:1c9 | 0.14 a | 0.10 ab | 0.10 ab | 0.08 b | 0.010 | 0.009 |
| 17:0 | 0.33 b | 0.50 a | 0.45 a | 0.43 a | 0.018 | <0.001 |
| 17:1c9 | 0.01 b | 0.02 a | 0.02 ab | 0.02 ab | 0.003 | 0.007 |
| 18:0 | 27.0 | 25.9 | 26.9 | 27.2 | 0.37 | 0.060 |
| 18:1c9 | 7.59 a | 7.09 ab | 6.36 bc | 5.78 c | 0.288 | 0.001 |
| 18:1c11 | 0.83 c | 1.42 a | 1.23 b | 1.12 b | 0.043 | <0.001 |
| 18:2n-6 | 20.6 b | 24.1 a | 23.9 a | 23.8 a | 0.32 | <0.001 |
| 18:3n-6 | 0.05 b | 0.08 a | 0.07 ab | 0.07 ab | 0.004 | 0.001 |
| 18:2t9t12 | 0.20 | 0.22 | 0.19 | 0.15 | 0.016 | 0.053 |
| 18:3n-3 | 0.07 b | 0.14 a | 0.14 a | 0.13 a | 0.006 | <0.001 |
| 18:4n-3 | 0.04 b | 0.06 a | 0.06 a | 0.05 ab | 0.004 | 0.007 |
| 20:0 | 0.07 c | 0.21 a | 0.13 b | 0.13 b | 0.013 | <0.001 |
| 20:1c11 | 0.20 ab | 0.25 a | 0.20 ab | 0.19 b | 0.014 | 0.012 |
| 20:2n-6 | 1.39 | 1.37 | 1.50 | 1.52 | 0.060 | 0.177 |
| 20:3n-6 | 1.98 a | 1.08 b | 1.32 b | 1.27 b | 0.121 | <0.001 |
| 20:4n-6 | 15.4 | 15.7 | 16.1 | 16.2 | 0.39 | 0.434 |
| 20:3n-3 | 0.02 b | 0.04 a | 0.03 a | 0.03 a | 0.002 | 0.001 |
| 20:5n-3 | 0.03 b | 0.12 a | 0.13 a | 0.13 a | 0.012 | <0.001 |
| 22:0 | 0.06 b | 0.14 a | 0.09 b | 0.08 b | 0.011 | <0.001 |
| 22:1n-9 | 0.01 | 0.03 | 0.03 | 0.02 | 0.007 | 0.119 |
| 22:5n-3 | 0.25 b | 1.04 a | 1.16 a | 0.99 a | 0.063 | <0.001 |
| 22:6n-3 | 0.86 b | 2.40 a | 2.52 a | 2.80 a | 0.167 | <0.001 |
| Others | 5.88 a | 2.91 b | 2.99 b | 2.92 b | 0.247 | <0.001 |
| Partial sums of fatty acids (g/100 g FA) | ||||||
| SFA 1 | 44.2 a | 41.4 b | 41.6 b | 42.4 b | 0.35 | <0.001 |
| cis-MUFA 2 | 9.07 a | 9.31 a | 8.31 ab | 7.49 b | 0.329 | 0.003 |
| PUFA 3 | 40.8 b | 46.4 a | 47.1 a | 47.2 a | 0.34 | <0.001 |
| n-3 PUFA 4 | 1.27 b | 3.78 a | 4.04 a | 4.13 a | 0.206 | <0.001 |
| n-6 PUFA 5 | 39.4 b | 42.4 a | 42.9 a | 42.9 a | 0.29 | <0.001 |
| Ratios of fatty acids | ||||||
| PUFA/SFA | 0.92 b | 1.12 a | 1.13 a | 1.11 a | 0.014 | <0.001 |
| n-6/n-3 | 31.7 a | 11.6 b | 10.9 b | 10.6 b | 1.04 | <0.001 |
| Parameters/Diets | Control | UL | ULR | ULL | SEM | p-Value |
|---|---|---|---|---|---|---|
| Diterpene profile (µg/g) | ||||||
| α-Tocopherol | 18.6 a | 16.3 ab | 15.1 ab | 12.6 b | 1.22 | 0.013 |
| γ-Tocopherol | 0.33 a | 0.27 ab | 0.30 ab | 0.22 b | 0.022 | 0.009 |
| Pigments (µg/100 g) | ||||||
| β-Carotene | 0.26 b | 14.5 a | 14.7 a | 14.4 a | 1.67 | <0.001 |
| Chlorophyll-a 1 | 0.09 b | 0.46 a | 0.50 a | 0.43 a | 0.044 | <0.001 |
| Chlorophyll-b 2 | 0.14 b | 0.79 a | 0.78 a | 0.80 a | 0.069 | <0.001 |
| Total carotenoids 3 | 5.52 b | 143 a | 146 a | 146 a | 12.8 | <0.001 |
| Mineral profile (mg/100 g) | ||||||
| Calcium | 32.8 | 34.3 | 32.2 | 34.3 | 1.02 | 0.390 |
| Magnesium | 20.6 | 19.5 | 20.4 | 21.4 | 1.29 | 0.771 |
| Phosphorous | 353 | 347 | 350 | 353 | 3.90 | 0.699 |
| Potassium | 424 | 425 | 430 | 423 | 7.30 | 0.927 |
| Sodium | 99.2 | 94.6 | 92.7 | 98.3 | 2.93 | 0.385 |
| Sulphur | 231 | 242 | 240 | 240 | 4.00 | 0.284 |
| Total macrominerals (M) | 1161 | 1163 | 1188 | 1170 | 10.9 | 0.340 |
| Copper | 0.38 | 0.39 | 0.42 | 0.40 | 0.018 | 0.339 |
| Iron | 55.6 b | 88.3 a | 68.6 ab | 45.8 b | 6.76 | 0.001 |
| Manganese | 0.36 b | 0.47 a | 0.49 a | 0.46 a | 0.021 | 0.001 |
| Zinc | 2.54 | 2.67 | 2.49 | 2.59 | 0.094 | 0.565 |
| Total microminerals (m) | 53.3 b | 91.8 a | 72.0 ab | 49.3 b | 7.14 | 0.001 |
| Total minerals | 1214 | 1255 | 1257 | 1219 | 15.5 | 0.112 |
| Variables | Factor 1 | Factor 2 |
|---|---|---|
| Cholesterol | 0.453 | 0.796 |
| LDL-C | 0.556 | 0.724 |
| HDL-C | −0.154 | 0.754 |
| VLDL-C | 0.884 | −0.002 |
| TAG | 0.884 | −0.002 |
| Total lipids | 0.762 | 0.555 |
| Glucose | 0.702 | −0.019 |
| Urea | 0.232 | 0.221 |
| Total protein | −0.079 | 0.498 |
| C-reactive protein | −0.064 | 0.700 |
| Chloride | 0.905 | −0.250 |
| Sodium | 0.851 | −0.251 |
| Potassium | 0.762 | −0.226 |
| ALT | −0.105 | 0.826 |
| AST | −0.389 | 0.828 |
| GGT | 0.818 | −0.002 |
| ALP | 0.004 | 0.772 |
| Diet Composition | Control | UL | ULR | ULL |
|---|---|---|---|---|
| Ingredients (% as fed basis) | ||||
| Corn | 50.4 | 43.7 | 43.7 | 43.7 |
| Soybean meal | 41.2 | 33.2 | 33.2 | 33.2 |
| Sunflower oil | 4.80 | 5.98 | 5.98 | 5.98 |
| Sodium chloride | 0.38 | 0.00 | 0.00 | 0.00 |
| Calcium carbonate | 1.10 | 0.00 | 0.00 | 0.00 |
| Dicalcium phosphate | 1.60 | 1.40 | 1.40 | 1.40 |
| DL-Methionine | 0.12 | 0.17 | 0.17 | 0.17 |
| L-Lysine | 0.00 | 0.12 | 0.12 | 0.12 |
| Vitamin-mineral premix 1 | 0.40 | 0.40 | 0.40 | 0.40 |
| Ulva lactuca powder | - | 15.0 | 15.0 | 15.0 |
| Rovabio® Excel AP | - | - | 0.005 | - |
| Recombinant CAZyme | - | - | - | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaia, C.M.; Costa, M.M.; Pinto, R.M.A.; Pestana, J.M.; Mourato, M.; Carvalho, P.; Martins, C.F.; Lopes, P.A.; Lordelo, M.M.; Prates, J.A.M. Plasma Metabolites and Liver Composition of Broilers in Response to Dietary Ulva lactuca with Ulvan Lyase or a Commercial Enzyme Mixture. Molecules 2022, 27, 7425. https://doi.org/10.3390/molecules27217425
Alfaia CM, Costa MM, Pinto RMA, Pestana JM, Mourato M, Carvalho P, Martins CF, Lopes PA, Lordelo MM, Prates JAM. Plasma Metabolites and Liver Composition of Broilers in Response to Dietary Ulva lactuca with Ulvan Lyase or a Commercial Enzyme Mixture. Molecules. 2022; 27(21):7425. https://doi.org/10.3390/molecules27217425
Chicago/Turabian StyleAlfaia, Cristina M., Mónica M. Costa, Rui M. A. Pinto, José M. Pestana, Miguel Mourato, Patrícia Carvalho, Cátia F. Martins, Paula A. Lopes, Madalena M. Lordelo, and José A. M. Prates. 2022. "Plasma Metabolites and Liver Composition of Broilers in Response to Dietary Ulva lactuca with Ulvan Lyase or a Commercial Enzyme Mixture" Molecules 27, no. 21: 7425. https://doi.org/10.3390/molecules27217425
APA StyleAlfaia, C. M., Costa, M. M., Pinto, R. M. A., Pestana, J. M., Mourato, M., Carvalho, P., Martins, C. F., Lopes, P. A., Lordelo, M. M., & Prates, J. A. M. (2022). Plasma Metabolites and Liver Composition of Broilers in Response to Dietary Ulva lactuca with Ulvan Lyase or a Commercial Enzyme Mixture. Molecules, 27(21), 7425. https://doi.org/10.3390/molecules27217425










