Aerial Oxygen-Driven Selenocyclization of O-Vinylanilides Mediated by Coupled Fe3+/Fe2+ and I2/I− Redox Cycles
Abstract
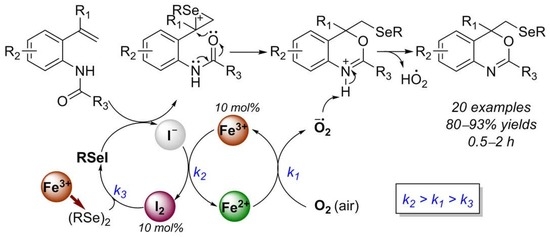
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Synthetic Procedure and the Characterization of Selenylated Benzoxazines (3a–3t)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mugesh, G.; Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B. Organoseleniumand organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Masayuki, N.; Dinesh, R.; Mamoru, K. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011, 255, 2968–2990. [Google Scholar]
- Bhabak, K.P.; Mugesh, G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, Y.; Sugiyama, N.; Horiuchi, T.; Furusawa, M.; Furuhama, K. Ebselen, a seleno-organic compound, protects against ethanol-induced murine gastric mucosal injury in both in vivo and in vitro systems. Eur. J. Pharmacol. 1995, 272, 195–201. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. A simple and efficient strategy to enhance the antioxidant activities of amino-substituted glutathione peroxidase mimics. Chem. Eur. J. 2008, 14, 8640–8651. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Glutathione peroxidase (GPx)-like antioxidant activity of the organoselenium drug ebselen: Unexpected complications with thiol exchange reactions. J. Am. Chem. Soc. 2005, 127, 11477–11485. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Synthesis, characterization, and antioxidant activity of some ebselen analogues. Chem. Eur. J. 2007, 13, 4594–4601. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Huffman, J.H.; Call, E.W.; Alaghamandan, H.; Cook, P.D.; Robins, R.K. Effect of selenazofurin on influenza A and B virus infections of mice. Antivir. Res. 1986, 6, 343–353. [Google Scholar] [CrossRef]
- Koketsu, M.; Ishihara, H.; Hatsu, M. Novel compounds, 1,3-selenazine derivatives, as antibacterial agents against Escherichia coli and Staphylococcus aureus. Res. Commun. Mol. Path. Pharmacol. 1998, 101, 179–180. [Google Scholar]
- Bien, M.; Blaszczyk, B.; Kalinowska, K.; Mlochowski, J. Antifungal activity of 2-(4-chlorophenyl)-1,2-benzisoselenazol-3 (2H)-one, the analog of ebselen. Arch. Immunol. Ther. Exp. 1999, 47, 185–193. [Google Scholar]
- Cho, S.I.; Koketsu, M.; Ishihara, H.; Matsushita, M.; Nairn, A.C.; Fukazawa, H.; Uehara, Y. Novel compounds, 1,3-selenazine derivatives’ as specific inhibitors of eukaryotic elongation factor-2 kinase. BBA Gen. Subj. 2000, 1475, 207–215. [Google Scholar] [CrossRef]
- Palmarisa, F.; Loredana, C.; Ghassan, A.S.; Hiremagalur, N.J.; Vivek, V.G.; Thaw, S.; Bryan, P.S.; William, D.J.; Barry, M.G.; Graziella, P.; et al. Synthesis, structure, and antiproliferative activity of selenophenfurin, an inosine 5-monophosphate dehydrogenase inhibitor analogue of selenazofurin. J. Med. Chem. 1997, 40, 1731–1737. [Google Scholar]
- Guan, Q.; Han, C.; Zuo, D.; Li, Z.; Zhang, Q.; Zhai, Y.; Zhang, W. Synthesis and evaluation of benzimidazole carbamates bearing indole moieties for antiproliferative and antitubulin activities. Eur. J. Med. Chem. 2014, 87, 306–315. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, J.; Wang, Z.; Qi, H.; Xu, Q.; Bai, Z.; Zhang, W. 3-(3,4,5-Trimethoxyphenylselenyl)-1H-indoles and their selenoxides as combretastatin A-4 analogs: Microwave-assisted synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 90, 184–194. [Google Scholar] [CrossRef]
- Casaril, A.M.; Ignasiak, M.T.; Chuang, C.Y.; Vieira, B.; Padilha, N.B.; Carroll, L.; Davies, M.J. Selenium-containing indolyl compounds: Kinetics of reaction with inflammation-associated oxidants and protective effect against oxidation of extracellular matrix proteins. Free Radic. Bio. Med. 2017, 113, 395–405. [Google Scholar] [CrossRef]
- Mamgain, R.; Singh, F.V. Selenium-based fluorescence probes for the detection of bioactive molecules. ACS Org. Inorg. Au. 2022, 4, 262–288. [Google Scholar] [CrossRef]
- Halle, M.B.; Yudhistira, T.; Lee, K.J.; Choi, J.H.; Kim, Y.; Park, H.S.; Churchill, D.G. Overriding phthalate decomposition when exploring mycophenolic acid intermediates as selenium-based ROS biological probes. ACS Omega 2018, 3, 13474–13483. [Google Scholar] [CrossRef] [PubMed]
- Madibone, K.S.; Deshmukh, P.P.; Navalkar, A.; Maji, S.K.; Badani, P.M.; Manjare, S.T. Cyclic organoselenide BODIPY-based probe: Targeting superoxide in MCF-7 cancer cells. ACS Omega 2020, 5, 14186–14193. [Google Scholar] [CrossRef]
- Debnath, S.; Chithiravel, S.; Sharma, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Selenium-containing fused bicyclic heterocycle diselenolodiselenole: Field effect transistor study and structure-property relationship. ACS Appl. Mater. Interfaces 2016, 8, 18222–18230. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, G.K.; Saleem, F.; Singh, A.K. Organoselenium ligands in catalysis. Dalton Trans. 2012, 41, 11949–11977. [Google Scholar] [CrossRef] [PubMed]
- Spell, M.; Wang, X.; Wahba, A.E.; Conner, E.; Ragains, J. An α-selective, visible light photocatalytic glycosylation of alcohols with selenoglycosides. Carbohyd. Res. 2013, 369, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Win, K.M.N.; Sonawane, A.D.; Koketsu, M. Iodine mediated in situ generation of R-Se–I: Application towards the construction of pyrano [4,3-b] quinoline heterocycles and fluorescence properties. Org. Biomol. Chem. 2019, 17, 9039–9049. [Google Scholar] [CrossRef]
- Goulart, T.A.; Kazmirski, J.A.; Back, D.F.; Zeni, G. Iron(III)-promoted synthesis of 3-(organoselanyl)-1,2-dihydroquinolines from diorganyl diselenides and N-arylpropargylamines by sequential carbon-carbon and carbon-selenium bond formation. Adv. Synth. Catal. 2018, 361, 96–104. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Lu, J.; Jiang, X. Recent progress in selenium-catalyzed organic reactions. Org. Chem. Front. 2019, 6, 2999–3041. [Google Scholar] [CrossRef]
- Jurinic, C.K.; Belladona, A.L.; Schumacher, R.F.; Godoi, B. Diorganyl dichalcogenides and copper/iron salts: Versatile cyclization system to achieve carbo- and heterocycles from alkynes. Synthesis 2021, 53, 2545–2558. [Google Scholar]
- Sonawane, A.D.; Sonawane, R.A.; Ninomiya, M.; Koketsu, M. Synthesis of seleno-heterocycles via electrophilic/radical cyclization of alkyne containing heteroatoms. Adv. Synth. Catal. 2020, 362, 3485–3515. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Li, C.; Wang, H.; Li, L. Recent advances in tandem selenocyclization and tellurocyclization with alkenes and alkynes. Org. Chem. Front. 2020, 7, 3100–3119. [Google Scholar] [CrossRef]
- Kumar, N.; Yadav, N.; Amarnath, N.; Sharma, V.; Shukla, S.; Srivastava, A.; Lochab, B. Integrative natural medicine inspired graphene nanovehicle-benzoxazine derivatives as potent therapy for cancer. Mol. Cell. Biochem. 2018, 454, 123–138. [Google Scholar] [CrossRef]
- Tanabe, J.; Sue, M.; Ishihara, A.; Iwamura, H. Occurrence and characterization of 2-hydroxy-1,4-benzoxazin-3-one and indole hydroxylases in juvenile wheat. Biosci. Biotechol. Biochem. 1999, 63, 1614–1617. [Google Scholar] [CrossRef][Green Version]
- Tang, Z.; Tan, Y.; Chen, H.; Wan, Y. Benzoxazine: A privileged scaffold in medicinal chemistry. Curr. Med. Chem. 2022, 29, 8670–8673. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, D.; Wu, J. Synthesis of 3-((arylsulfonyl) methyl) indolin-2-ones via insertion of sulfur dioxide using anilines as the aryl source. Org. Chem. Front. 2017, 4, 1079–1083. [Google Scholar] [CrossRef]
- Tian, Y.; Ge, Y.; Zheng, L.; Yan, Q.; Ren, Y.; Wang, Z.; Zhang, K.; Wang, Z.; Zhao, J.; Li, Z. A free radical cascade difunctionalization of o-vinylanilides with simple ketones and esters. Asian J. Org. Chem. 2019, 8, 2188–2191. [Google Scholar] [CrossRef]
- Wu, J.; Zong, Y.; Zhao, C.; Yan, Q.; Sun, L.; Li, Y.; Zhao, J.; Ge, Y.; Li, Z. Silver or cerium-promoted free radical cascade difunctionalization of o-vinylanilides with sodium aryl-oralkylsulfinates. Org. Biomol. Chem. 2018, 17, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-F.; Duan, X.-H.; Yang, H.; Guo, L.-N. Transition-metal-free oxyfluorination of olefinic amides for the synthesis of fluorinated heterocycles. J. Org. Chem. 2015, 80, 11149–11155. [Google Scholar] [CrossRef]
- Okuma, K.; Seto, J.I. Synthesis of indoles, 3,1-benzoxazines, and quinolines from 2-alkenylanilides and active seleniums. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1014–1020. [Google Scholar] [CrossRef]
- Chaitanya, M.; Anbarasan, P. Acid-mediated oxychalcogenation of o-vinylanilides with N-(arylthio/arylseleno) succinimides. Org. Lett. 2018, 20, 1183–1186. [Google Scholar] [CrossRef]
- Speranca, A.; Godoi, B.; Pinton, S.; Back, D.F.; Menezes, P.H.; Zeni, G. Regioselective synthesis of isochromenones by iron (III)/PhSeSePh-mediated cyclization of 2-alkynylaryl esters. J. Org. Chem. 2011, 76, 6789–6797. [Google Scholar] [CrossRef]
- Speranca, A.; Godoi, B.; Zeni, G. Iron(III) chloride/diorganyl diselenides: A tool for intramolecular cyclization of alkynone o-methyloximes. J. Org. Chem. 2013, 78, 1630–1637. [Google Scholar] [CrossRef]
- Mantovani, A.C.; Goulart, T.A.; Back, D.F.; Menezes, P.H.; Zeni, G. Iron(III) chloride and diorganyl diselenides-mediated 6-endo-dig cyclization of arylpropiolates and arylpropiolamides leading to 3-organoselenyl-2H-coumarins and 3-organoselenyl-quinolinones. J. Org. Chem. 2014, 79, 10526–10536. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-F.; Li, F.-H.; Li, J.; Wang, S.-Y.; Ji, S.-J. Iron(III) chloride-promoted cyclization of α,β-alkynic tosylhydrazones with diselenides: Synthesis of 4-(arylselanyl)-1H-pyrazoles. Org. Biomol. Chem. 2020, 18, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-J.; Hu, B.-L.; Deng, C.-L.; Zhang, X.-G. Iron-promoted electrophilic annulation of aryl enynes with disulfides or diselenides leading to polysubstituted naphthalenes. Adv. Synth. Catal. 2014, 356, 1962–1966. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, L.-L.; Mao, Y.-H.; Dong, Y.-X.; Liu, B.; Zhu, G.-F.; Zhang, J.-Q. Iron-catalyzed radical annulation of unsaturated carboxylic acids with disulfides for the synthesis of γ-lactones. J. Org. Chem. 2021, 86, 8620–8629. [Google Scholar] [CrossRef]
- Win, K.M.N.; Sonawane, A.D.; Koketsu, M. Synthesis of selenated tetracyclic indoloazulenes via iodine and diorganyl diselenides. Org. Biomol. Chem. 2021, 19, 3199–3206. [Google Scholar] [CrossRef]
- Sahoo, H.; Grandhi, G.S.; Ramakrishna, I.; Baidya, M. Metal-free switchable ortho/ipso-cyclization of N-arylalkynamides: Divergent synthesis of 3-selenyl quinolin-2-ones and azaspiro[4,5]trienones. Org. Biomol. Chem. 2019, 17, 10163–10166. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, H.; Yang, D.; Yue, H.; He, C.; Zhang, Y.; Wang, H. Visible-light-enabled spirocyclization of alkynes leading to 3-sulfonyl and 3-sulfenyl azaspiro[4,5]trienones. Green Chem. 2017, 19, 5608–5613. [Google Scholar] [CrossRef]
- Yu, L.; Ren, L.; Yi, R.; Wu, Y.; Chen, T.; Guo, R. Iron salt, a cheap, highly efficient and environment-friendly metal catalyst for Se-Se bond cleavage and the further reaction with methylenecyclopropanes under mild conditions. J. Org. Chem. 2011, 696, 2228–2233. [Google Scholar] [CrossRef]
- Guo, J.; Hao, Y.; Li, G.; Wang, Z.; Liu, Y.; Li, Y.; Wang, Q. Efficient synthesis of SCF3-substituted tryptanthrins by a radical tandem cyclization. Org. Biomol. Chem. 2020, 18, 1994–2001. [Google Scholar] [CrossRef]
- Nishio, T. Reaction of (1,ω)-N-acylamino alcohols with Lawesson’s reagent: Synthesis of sulfur-containing heterocycles. J. Org. Chem. 1997, 62, 1106–1111. [Google Scholar] [CrossRef]
- Jana, S.; Ashokan, A.; Kumar, S.; Verma, A.; Kumar, S. Copper-catalyzed trifluoromethylation of alkenes: Synthesis of trifluoromethylated benzoxazines. Org. Biomol. Chem. 2015, 13, 8411–8415. [Google Scholar] [CrossRef]
- Cooper, P.; Crisenza, G.E.; Feron, L.J.; Bower, J.F. Iridium-catalyzed α-selective arylation of styrenes by dual C−H functionalization. Angew. Chem. Int. Ed. 2018, 130, 14394–14398. [Google Scholar] [CrossRef]
- Lu, F.; Xu, J.; Li, H.; Wang, K.; Ouyang, D.; Sun, L.; Huang, M.; Jiang, J.; Hu, J.; Alhumade, H.; et al. Electrochemical oxidative radical cascade cyclization of olefinic amides and thiophenols towards the synthesis of sulfurated benzoxazines, oxazolines and iminoisobenzofurans. Green Chem. 2021, 23, 7982–7986. [Google Scholar] [CrossRef]





 | ||||
| Entry | Mn+ | Solvent | Reaction Time (h) | Isolated Yield (%) |
|---|---|---|---|---|
| 1 | Cu(acac)2 | CH3CN | 8 | 55 |
| 2 | Co(acac)3 | CH3CN | 8 | 51 |
| 3 | VO(acac)2 | CH3CN | 12 | 45 |
| 4 | Ce(NH4)2(NO3)6 | CH3CN | 2 | 52 |
| 5 | Ni(acac)2 | CH3CN | 8 | 22 |
| 6 | MoO2(acac)2 | CH3CN | 8 | 27 |
| 7 | PMA | CH3CN | 5 | 28 |
| 8 | Fe(OTf)3 | CH3CN | 9 | 92 |
| 9 | Fe(acac)3 | CH3CN | 1 | 94 |
| 10 | FeCl3 | CH3CN | 0.5 | 93 |
| 11 | FeCl3 | DCE | 3 | 55 |
| 12 | FeCl3 | THF | 4 | 73 |
| 13 | FeCl3 | DMSO | 3 | 80 |
| 14 | FeCl3 | EtOH | 6 | 81 |
 |
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-Y.; Zeng, T.-T.; Xie, Z.-B.; Dong, Y.-Y.; Ma, C.; Gong, S.-S.; Sun, Q. Aerial Oxygen-Driven Selenocyclization of O-Vinylanilides Mediated by Coupled Fe3+/Fe2+ and I2/I− Redox Cycles. Molecules 2022, 27, 7386. https://doi.org/10.3390/molecules27217386
Zhang H-Y, Zeng T-T, Xie Z-B, Dong Y-Y, Ma C, Gong S-S, Sun Q. Aerial Oxygen-Driven Selenocyclization of O-Vinylanilides Mediated by Coupled Fe3+/Fe2+ and I2/I− Redox Cycles. Molecules. 2022; 27(21):7386. https://doi.org/10.3390/molecules27217386
Chicago/Turabian StyleZhang, Hao-Yuan, Tong-Tong Zeng, Zhen-Biao Xie, Ying-Ying Dong, Cha Ma, Shan-Shan Gong, and Qi Sun. 2022. "Aerial Oxygen-Driven Selenocyclization of O-Vinylanilides Mediated by Coupled Fe3+/Fe2+ and I2/I− Redox Cycles" Molecules 27, no. 21: 7386. https://doi.org/10.3390/molecules27217386
APA StyleZhang, H.-Y., Zeng, T.-T., Xie, Z.-B., Dong, Y.-Y., Ma, C., Gong, S.-S., & Sun, Q. (2022). Aerial Oxygen-Driven Selenocyclization of O-Vinylanilides Mediated by Coupled Fe3+/Fe2+ and I2/I− Redox Cycles. Molecules, 27(21), 7386. https://doi.org/10.3390/molecules27217386