Abstract
Citral chemotypes Cinnamomum camphora (C. camphora) and Cinnamomum bodinieri (C. bodinieri) are promising industrial plants that contain abundant citral. For a more in-depth study, their significant biological effect, the chemical composition and antioxidant capacity of essential oils of citral-rich chemotype C. camphora and C. bodinieri (EOCC) were determined in the present study. The EOCC yield, obtained by hydro-distillation and analyzed by gas chromatography–mass spectrometry (GC-MS), ranged from 1.45–2.64%. Forty components more than 0.1% were identified and represented, mainly by a high content of neral (28.6–39.2%), geranial (31.8–54.1%), Z-isocitral (1.8–3.2%), E-isocitral (3.2–4.7%), geraniol (1.3–2.6%) and caryophyllene (0.6–2.4%). The antioxidant properties of EOCC were estimated by DPPH, ABTS and FRAP methods. As our results indicated, the antioxidant activity was significantly correlated to oxygenated monoterpenes. The variety of C. bodinieri (N7) presented the best antioxidant profile, given its highest inhibition of DPPH radical (IC50 = 6.887 ± 0.151 mg/mL) and ABTS radical scavenging activity (IC50 = 19.08 ± 0.02 mg/mL). To the best of our knowledge, more than 88% citral of C. bodinieri was investigated and the antioxidant properties described for the first time. Considering high essential oil yield, rich citral content and high antioxidant activity, the N7 variety will be a good candidate for pharmaceutical and cosmetic development of an improved variety.
1. Introduction
Cinnamomum camphora and Cinnamomum bodinieri, from the Lauraceae family, are evergreen broad-leaf trees indigenous to southern China. The chemical polymorphism had been discovered in C. camphora and C. bodinieri, including linalool-, borneol-, camphor-, cineole-, nerolidol- and citral-types. The citral chemotype C. camphora and C. bodinieri were so named for the large amount of citral in its root barks, stem barks and leaves. Citral, 3,7-dimethyl-2,6-octadienal, is a precise monoterpenoid widely used in the pharmaceutical and cosmetic industries [1]. It is generally recognized as safe status (GRAS) and listed by the United States Food and Drug Administration (FDA) and hence, when added to food, is considered safe by experts [2]. It is an important chemical raw material for other components’ synthesis, such as ionone, vitamin A, vitamin E, citronitrile, methyl ionone, hydroxyl-citronellal and isohu menthol [3]. As synthetic citral produced highly concentrated waste water, the essential oils extracted from plants meet the demand of people for green natural products [4] and have become commercially popular due to their impression as a “well-being” life style product [5]. Citral, which is a key component of natural plant essential oils and natural antioxidant substances, can inhibit the oxidation of linoleic acid and protect IEC-6 cells against aspirin-induced oxidative stress [6]. It has been increasingly cultivated during the last few years and the world’s interest in citral as an aromatic plant is still increasing.
In the past decade, a considerable body of literature has grown up around the key technologies of the whole industry chain for high-efficiency planting and the intensive processing of C. camphora and C. bodinieri, including phylogenetic analysis based on the genome of camphor tree [7,8,9], transcriptome analysis and the identification of genes [10,11,12], metabolic pathways and regulatory mechanisms of essential oil biosynthesis [13,14], the effect of exogenous substances during tissue culture [15], comparative extraction method analysis [16], antibacterial, nematicidal and antioxidant activity of essential oil [17,18]; thus, the superior individuals of citral chemotype Sect. Camphora species were screened in Nanchang Institute of Technology over the last five years [19] and the optimal rooting medium for the C. bodinieri citral type was identified [20]. Nevertheless, the citral chemotype has been a largely under explored domain. The antioxidation of natural plant essential oils is very important for their application in the fields of medicine, food and spices, which overcome the deleterious effect of chemically synthesized antioxidants. The 2,2-diphenyl-1 picrylhydrazyl (DPPH) free radical scavenging test, scavenging 2,2′-azinobis(3-ethylbenzo thiazoline-6-sulfonic acid) diammonium salt radical (ABTS) and the ability of Ferric reducing antioxidant power (FRAP) are the most common methods used to evaluate the antioxidant activity of compounds, and various phytoconstituents and their potential antioxidant activities have been reported previously [21,22,23,24,25,26,27,28]. In terms of the C. camphora’s antioxidant activities, linalool, eucalyptol, camphor and borneol chemotypes with strong scavenging activity against 2,2-diphenyl-1 picrylhydrazyl (DPPH) were proved [17]. It is worth noting that the antioxidation of the citral chemotype of C. camphora and C. bodinieri are still unexplored and need to be clarified.
The objectives of this study are: (1) to select C. camphora and C. bodinieri with abundant citral accumulation and high essential oil yield under the same growing conditions, which were screened from different geographical regions by our research group in the early stages; (2) to determine the antioxidant activities of the EOCC by DPPH, ABTS and FRAP methods; and (3) to explore the relationship between the terpenoids and antioxidant properties of the EOCC. The results will provide theoretical basis for subsequent plant breeding and intensive utilization of the EOCC.
2. Results
2.1. Essential Oil Yield
The density of the EOCC was 0.882 ± 0.008 (25 °C) g/cm3 and the colors were yellowish (Table 1). The EOCC extracted from N1 and N2 varieties contained cloudy components, the others were transparent. The oil yield of fresh weight and dry weight ranged from 0.6 to 1.11% (w/w) and from 1.45 to 2.64% (w/w), respectively. The essential oil yield of different varieties had significant differences according to Duncan’s test with 1% significance (p ≤ 0.01) and the C. camphora leaves implied a higher essential oil yield than the C. bodinieri leaves. The essential oil yields from different geographical origins had no significant difference.

Table 1.
The essential oil yield and characteristics of citral chemotype C. camphora and C. bodinieri.
2.2. Chemical Constituents of Essential Oil
After integration of the chromatograms and identification of components of seven EOCC with its concentration more than 0.1%, the components were classified by terpene groups (Table 2). The GC-MS experiment identified the N5 variety 94.9% (11 constituents) and N3 variety 94.9% (28 constituents), followed by N7 variety 94.6% (8 constituents), N2 variety 93% (21 constituents), N4 variety 89.2% (22 constituents), N1 variety 88.0% (27 constituents) and N6 variety 87.5% (13 constituents). Monoterpenes (hydrocarbon and oxygenated) dominated in the chemical composition of the N1~N7 EOCC with proportions of 71.7%, 83.2%, 76.3%, 76.7%, 86.1%, 81.8% and 93.9%, respectively.

Table 2.
Essential oil composition of citral chemotype C. camphora and C. bodinieri.
Among these compounds, geranial ((2E)-3,7-dimethylocta-2,6-dienal) and neral ((2Z)-3,7-dimethylocta-2,6-dienal) known as citral a and citral b, two geometric isomers of citral (Figure 1), were dominated in the seven EOCCs, which ranged from 60.5% to 88.7%. In particular, the citral of N7 variety was 88.7% (34.6% neral and 54.1% geranial), followed by N5 variety 78.8% (34.1% neral and 44.7% geranial). In addition, the EOCC contained high contents of eucalyptol, sabinene, Z-isocitral, E-isocitral, geraniol, geranic acid, geranyl acetate, caryophyllene, humulene, cyclogermacrene, bicyclogermacrene, 10′-apocarotenonal, humulene epoxide II, etc.
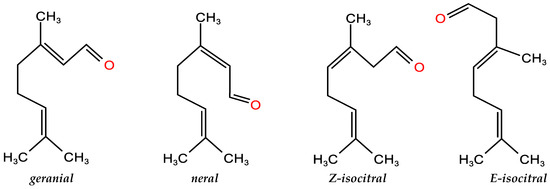
Figure 1.
Chemical structures of the main compounds of the essential oils from citral chemotype C. camphora and C. bodinieri.
2.3. Antioxidant of the Essential Oil
2.3.1. DPPH Radical Scavenging Activity
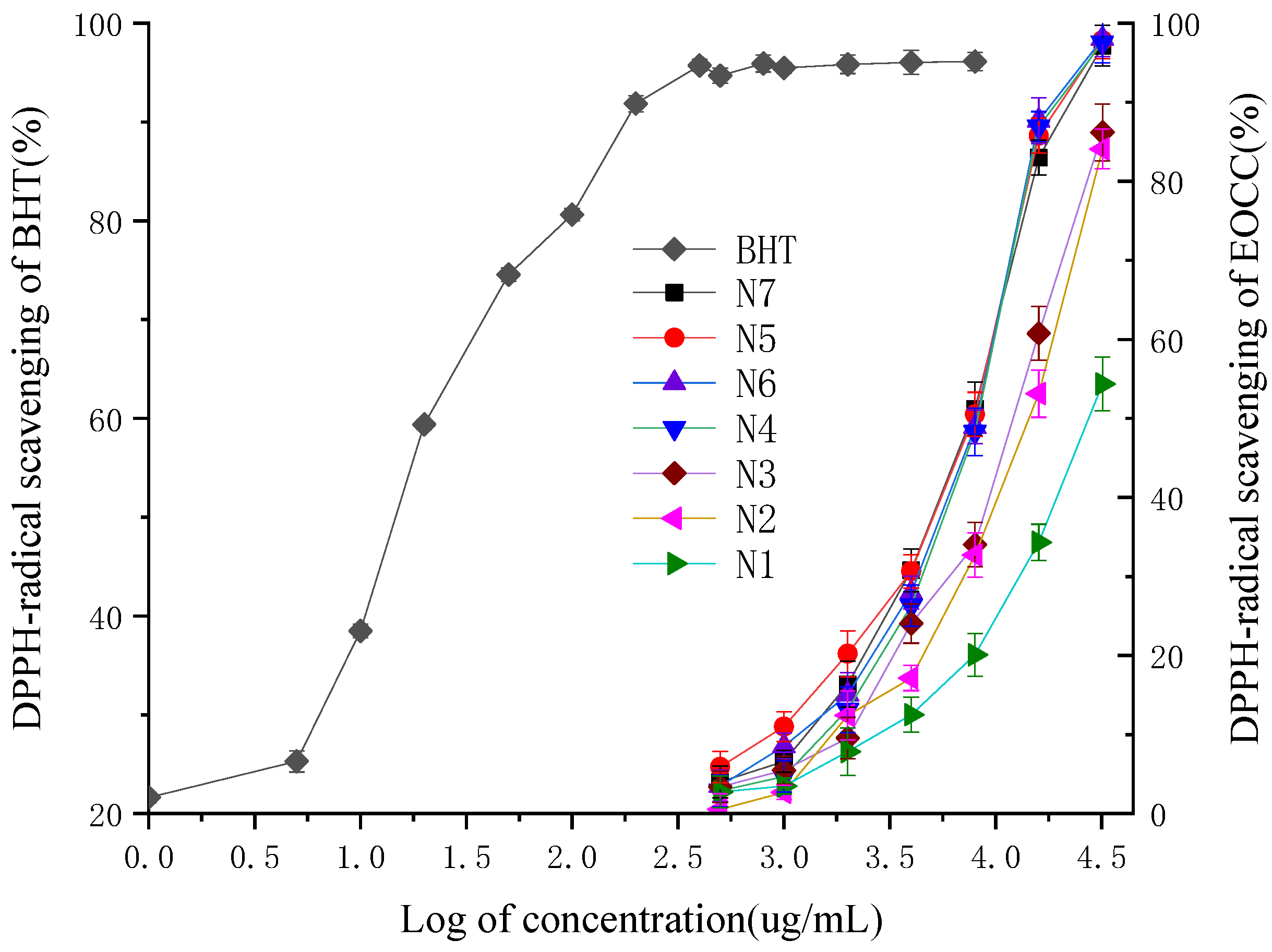
On the basis of the analysis of the different radical scavenging activity of the EOCC against DPPH between different varieties, the four-parameter logistic curve equation could be established for these relationships to predict the IC50 based on log of the EOCC concentration (Table 3), with the 3,5-ditertiobutyl-4-hydroxytoluène (BHT) as a positive control (Figure 2). The coefficients of determination of the logistic curve models of N1, N2, N3, N4, N5, N6, N7 and BHT were elevated to 0.997, 0.996, 0.990, 0.989, 0.987, 0.985, 0.996 and 0.994, respectively, and all F-test values were less than 0.001. The anti-radical activity fell into the following descending order: BHT > N7 > N5 > N6 > N4 > N3 > N2 > N1, with significant differences according to Duncan’s test with 1% significance. The IC50 values of seven EOCC were ranged from 6.887 ± 0.151 mg/mL to 28.133 ± 0.44 mg/mL. The most active DPPH radical scavenging activity was N7 (IC50 = 6.887 ± 0.151 mg/mL), followed by N5 (IC50 = 7.065 ± 0.086 mg/mL) and the lowest DPPH radical scavenging activity was N1 (IC50 = 28.133 ± 0.44 mg/mL). The scavenging activity of BHT (IC50 = 0.015 ± 0.007 mg/mL) for the DPPH radical was superior to that of EOCC.

Table 3.
Scavenging activity of the EOCC against DPPH and ABTS free radicals.
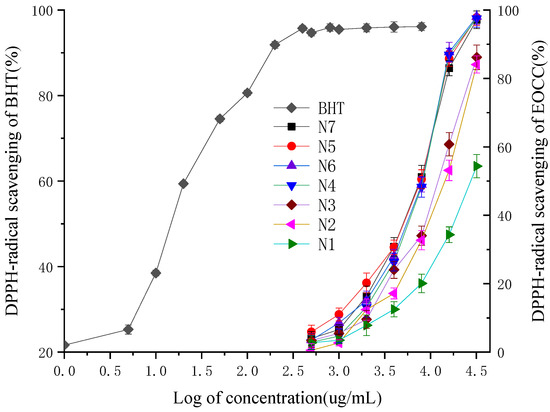
Figure 2.
DPPH-radical scavenging activities of the BHT and EOCC.
The Spearman test revealed a significant negative correlation (p ≤ 0.01) between the IC50 DPPH and oxygenated monoterpenes (OM) in the EOCC (Table 4). The oxygenated monoterpenes (OM) chemical families that are opposed to the IC50 DPPH promote radical scavenging activity against DPPH. The HS (p ≤ 0.01) and OS (p ≤ 0.05) chemical groups showed high positive correlation coefficients. The HM and NT chemical groups had no effect on the radical scavenging activity against DPPH.

Table 4.
Matrix of correlations between IC50 DPPH and chemical composition of citral chemotype C. camphora and C. bodinieri.
2.3.2. ABTS Radical Scavenging Activity
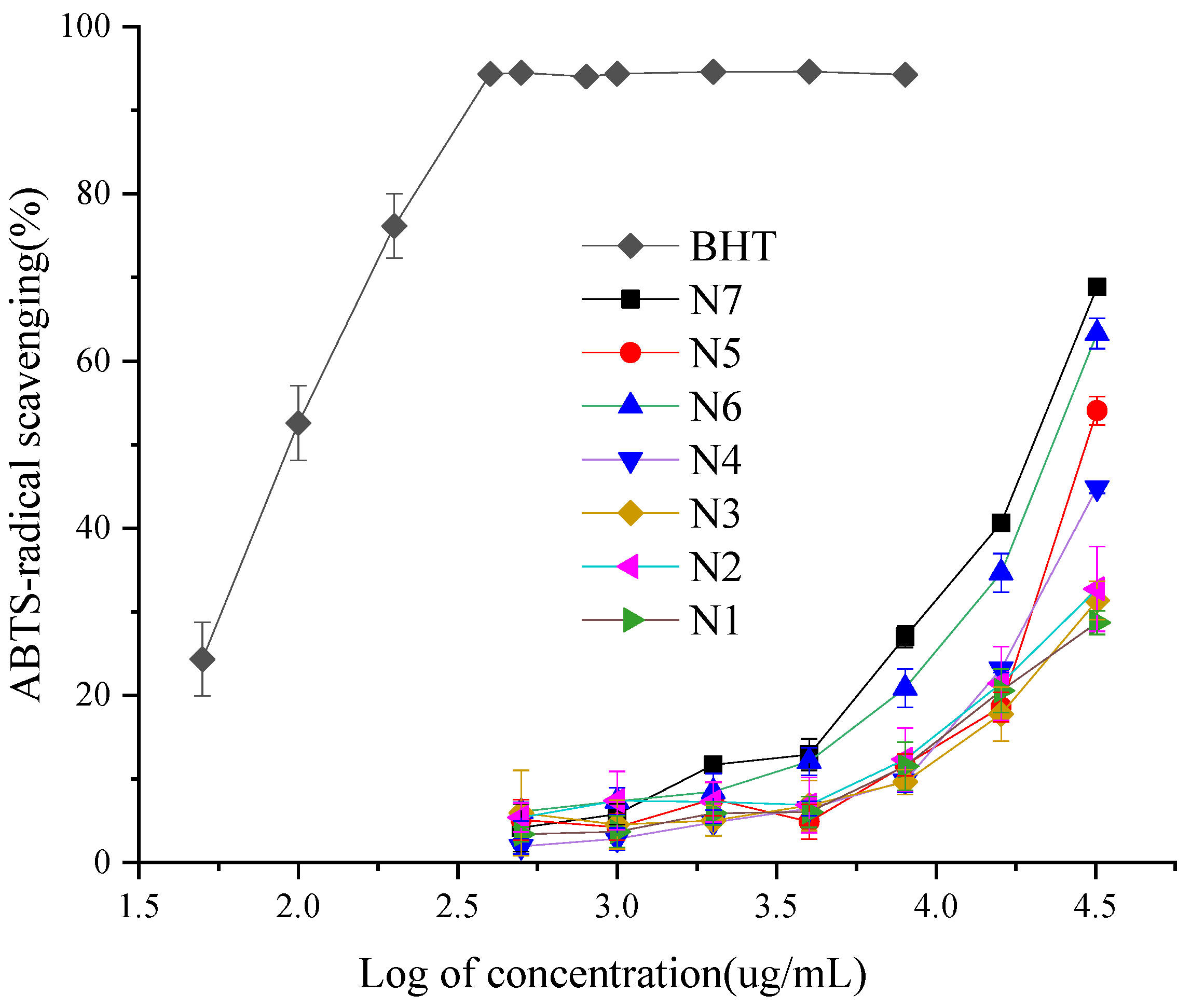
The analysis of the different ABTS radical scavenging activity of the EOCC, with the 3,5-ditertiobutyl-4-hydroxytoluène (BHT) as a positive control (Figure 3), showed some ABTS radical scavenging activity was dependent on EOCC concentration. The EOCC ABTS radical scavenging capacity fell into the following descending order: BHT > N7 > N6 > N5 > N4 > N2 > N3 > N1, with significant differences according to Duncan’s test with 1% significance (Table 3). The highest ABTS radical scavenging activity was N7 (IC50 = 19.08 ± 0.02 mg/mL), followed by N6 (IC50 =22.53 ± 0.04 mg/mL) and the lowest ABTS radical scavenging activity was N1 (IC50 =117.22 ± 5.4 mg/mL). The scavenging activity of BHT (IC50 = 0.10 ± 0.004 mg/mL) for the ABTS radical was superior to that of EOCC. As with the DPPH test, the Spearman test revealed the same rules between ABTS IC50 and chemical groups; the ABTS-radical scavenging activities were significantly correlated to oxygenated monoterpenes (Table 4).
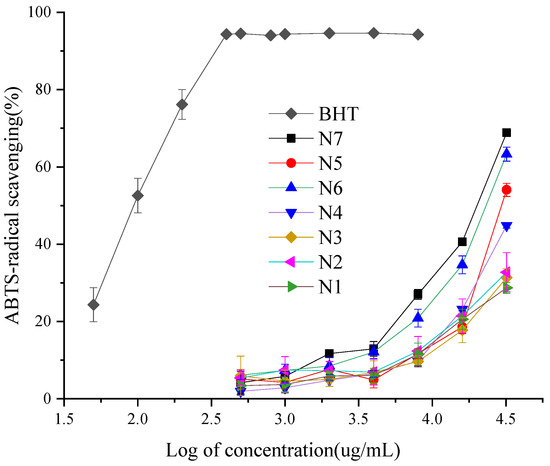
Figure 3.
ABTS-radical scavenging activities of the BHT and EOCC.
2.3.3. Ferric Reducing Antioxidant Power (FRAP)
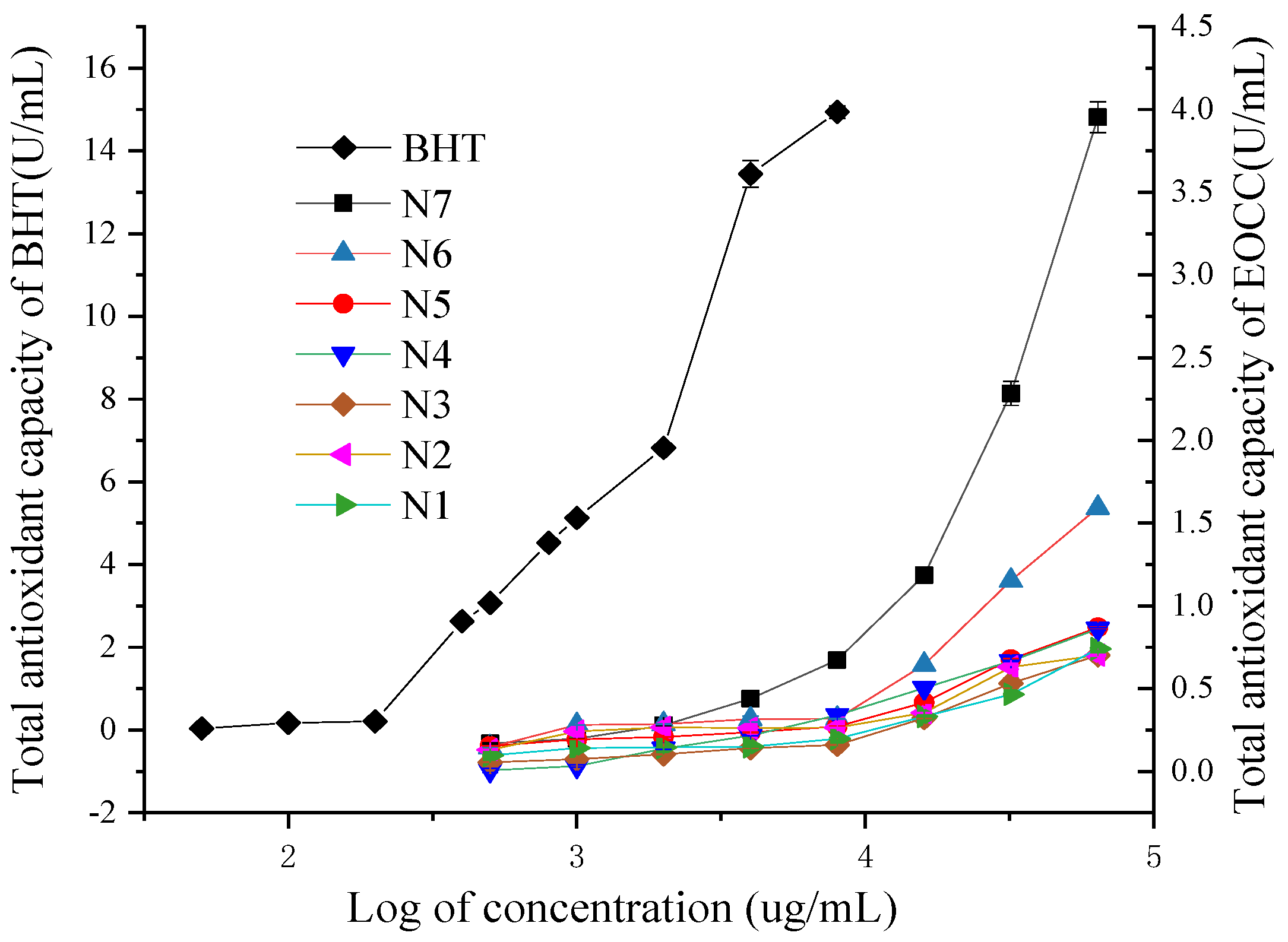
All EOCCs had some Fe3+ reducing capacity and the reduction capacity for Fe3+ increased gradually when the concentration of the essential oils increased (Figure 4). Among seven EOCCs, the N7 variety had the highest total antioxidant capacity (T-AOC).
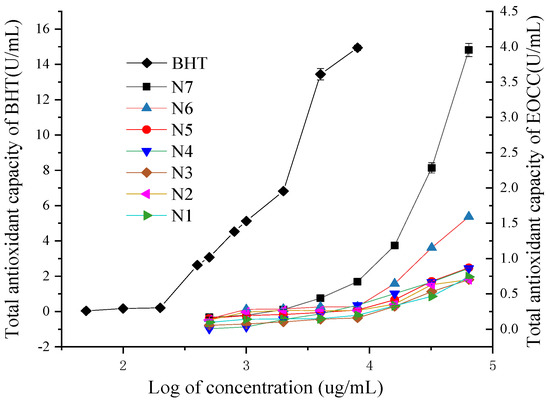
Figure 4.
Total antioxidant capacities of the BHT and EOCC.
At the same concentration, the T-AOC of the BHT and EOCC fell into the following descending order: BHT > N7 > N6 > N5 > N4 > N1 > N2 > N3. When the EOCC concentration was 64.0 mg/mL, the T-AOCs of N1~N7 were 0.74, 0.71, 0.70, 0.86, 0.87, 1.59, 4.0 U/mL, respectively.
3. Discussion
The EOCC oil yields of fresh weight ranging from 0.6 to 1.11% were lower when compared to those identified in the literature for C. camphora linalool chemotype (1.3%) [13]; approximately the same result was found in the Sect. Camphor (Trew.) Meissn. citral chemotype (0.8%) [19], significantly higher than C. camphora ordinary varieties (0.212–0.480%) [16]. The yield of essential oils depends on the genotype [32], geographical origin [33,34], the time of harvest [35], the temperature [36], the humidity level [37], the nature of the soil [38], the organ of the used plant [22,23], the organ’s age [39], plant density [40], nutrient application [32] and the extraction method [16,27,41]. The samples tested in the experiment were collected from the same cutting orchard and the environmental conditions were similar when their leaves were picked. The essential oil yield of different varieties had significant differences (p ≤ 0.01) and the C. camphora leaves conferred a higher essential oil yield than the C. bodinieri. Variations might result from changes in the expression of related genes [32]. C. bodinieri leaves are thicker than those of C. camphora; thus, in the same process of steam distillation extraction of essential oil, the residual amount of essential oil in the residue is larger, leading to a low oil yield. Therefore, it is necessary to apply other effective technologies for C. bodinieri.
The citral (60.5–88.7%) was the main component in the EOCC, equal to or better than the citral-rich plants, including Backhousia citriodra (85–95%) [4,26]; Litsea cubeba (70–90%) [42]; Cymbopogan flexuosus (65–85%) [43]; Ocimum gratissimum (65–75%) [44]; Lippia citriodor (30–60%) [1,45]; Citrus aurantium bigarade (25–30%) [46]. The citral chemotype C. camphora and C. bodinieri, as the evergreen tree, had more advantages for extracting citral in terms of biomass, oil yield of essential oil and citral content; moreover, their tending and harvesting can be mechanized. Unsaturated aldehydes of the citral structure were quite labile, and iso-citrals as reaction products frequently existed. Z-isocitral and E-isocitral were identified in our research, meanwhile, exo-isocitral was not detected. These marked differences in the citral isomers of Backhousia citriodra determined by Southwell et al. [4] from that of the present study could be attributed to species or isomer content difference. After repeated GC-MS experiments to identify the composition of citral chemotype C. camphora and C. bodinieri essential oil, exo-isocitral was not detected; this result ties in well with previous studies [29,31,47]. We speculated that exo-isocitral content might be less than the GC-MS minimum threshold for detection, resulting in not being identified.
Four chemotypes of C. camphora extracts showed high scavenging activity against DPPH free radicals in July, due to the seasonal variations in the terpenoid content [17], So we chose to conduct this study in July. The four-parameter logistic curve equation could be established for these relationships to predict the IC50 and the coefficients of determination of DPPH and ABTS radical scavenging activity were greater than 0.95. The model was well fitted to understand intuitively and predict accurately the IC50 value of seven EOCCs. The IC50 of BHT in the DPPH test was about 0.015 mg/mL, which is consistent with other reported results (0.012 mg/mL) [48]. The EOCC IC50 values were consistent with the IC50 of Cinnamomum parthenoxylon (4.528 mg/mL) [24], but less than Cinnamomum iners Reinw. ex Blume (0.015 mg/mL) [49] and Lindera pulcherrima (0.087 mg/mL) [50]. This might be due to the absence of strong biologically active components such as phenols and polyphenols, which have remarkable activity against free radicals. The antioxidant activity of EOCC is significantly inferior to that of BHT, but the plant essential oil is a natural substance with the advantage of being green, clean, environmentally friendly and of good potential application.
The essential oil exhibited strong concentration dependency in a sigmoidal dose-response curve over the concentration range. Other studies on the antioxidants of essential oils have proved that the DPPH and ABTS radical scavenging ability of essential oils exhibits a significant positive correlation with the concentration of essential oils and has a close connection with its chemical components, especially its main components [28]. In the DPPH, ABTS and FRAP assays, although their ranking differed slightly, all assays identified the top three varieties according to their antioxidant capacities as N7, N5 and N6 varieties. This could be due to the synergetic effects of the identified essential oil components. DPPH radicals can be scavenged because essential oils donate a hydrogen atom to DPPH and give rise to the reduced DPPH-H with the loss of this violet color [51]. The main components of the EOCC are oxygenated terpenoids such as neral and geranial, which have a great impact on the antioxidant activity of essential oil. According to the classification and analysis of the main components of the EOCC, the antioxidant activity of the essential oils is positively correlated with the content of oxygenated terpenoids (oxygenated monoterpenes and sesquiterpenes), due to terpenoid antioxidant activity depending on the numbers and positions of C=C double bonds [52], which can easily react with free radicals and ROS to serve their antioxidant function. Terpenoids have also been found to possess chain-breaking antioxidant activity [17,53], which are similar to phenols. A previous study found that the strongest scavenging activity was mainly detected in the C. camphora extracts, which had the highest terpenoid content among the four chemotypes [17]. The molecular mechanism of the EOCC radical scavenging activity had been a largely under-explored domain. We wish to extend this study to the relationship between the counterpart compositions of C. camphora and C. bodinieri and their radical scavenging activity.
4. Materials and Methods
4.1. Plant Material and Reagent
Healthy pest-free mature leaves (200 g) of C. camphora and C. bodinieri were harvested from 5-year-old clones grown at the cuttings orchard of NanChang Institute of Technology in July 2022 (Latitude: 28°41′47″ N, Longitude: 116°1′49″ W). The clones were propagated from mother trees through cutting propagation. For each biological replicate (n = 3), leaves from at least six tree clones, which were cloned from the same mother tree, were collected from the east, south, west and north of the canopy, and mixed. Afterward, the leaves were stored at 4 °C until isolation. The citral-rich asexual mother plants were collected from 40,000 C. camphora and C. bodinieri in their natural geographic distributions, included Jiangxi, Guangxi, Hubei and Guizhou, ranging from 2011 to 2017, and propagated into the cuttings orchard of NanChang Institute of Technology in 2017. The plants were authenticated by Professor Zhinong Jin. The voucher specimens were deposited in the Gene Bank of the Camphor Tree laboratory, Jiangxi Provincial Engineering Research Center For Seed-Breeding and Utilization of Camphor Trees, and the voucher numbers were for C. camphora-GX/ZS/004 (N1 variety); C. bodinieri-GX/QZ/003 (N2 variety); C. camphora-GX/ZS/003 (N3 variety); C. camphora-JX/NC/002 (N4 variety); C. camphora-JX/NC/001 (N5 variety); C. bodinieri-HB/CY/021 (N6 variety); C. bodinieri-GZ/TZ/028 (N7 variety).
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH), Methanol and 6-Di-tert-butyl-4-methyl phenol (BHT) were purchased from Macklin Reagent Co., (Shanghai, China, www.macklin.cn, accessed on 14 January 2022). CO2 (99.5 wt.%) was purchased from Hongqing Gas Co., (Nanchang, China). Citral, 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid ammonium salt) (ABTS), potassium persulfate and C7-C40 saturated alkanes standard were obtained from Shanghai Aladdin (Shanghai, China, www.aladdin-e.com, accessed on 8 July 2022). Ethanol was procured by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, www.reagent.com.cn, accessed on 24 February 2022). Total antioxidant capacity (T-AOC) diagnostic kits were obtained from Sangon Biotech (Shanghai, China). All reagents were AR grade.
4.2. Isolation of Essential Oil
Leaves (200 g) were placed into a 1000 mL extraction stainless steel cell for oil extraction immediately after harvesting. Leaf samples were hydro-distilled in a modified Clevenger apparatus (the patent application number: 201710158988.8) for 90 min. The essential oil was dried over anhydrous sodium sulphate separately and kept in a refrigerator (4 °C) for GC-MS [19].
Extracted essential oil was weighed, and the rate of water content was measured by MA150 rapid moisture analyzer (Sartorius, Germany), which was repeated three times. Finally, the oil yield was calculated using the formula:
where W1 is the weight of extracted essential oil; W2 is the weight of fresh leaves; and M is rate of water content.
Fresh leaf essential oil yield (%) = W1/W2 × 100
Dry leaf essential oil yield (%) = W1/(W2 × (100% − M)) × 100
4.3. Gas Chromatography-Mass Spectrometry (GC-MS)
Analyses of essential oils were performed on a gas chromatography system (Agilent 7890B-5975C GC-MS; USA) equipped with a Agilent J&W HP-5MS column (30 m × 250 μm × 0.25 μm). Referring to the experimental conditions of our previous study, the mass spectra electronic impact was taken at 70 eV, the scanned mass range was set at 50 to 650 m/z, the scanned rate was set at 0.5 scans/s, the conductor temperature was 250 °C, the ion source temperature was 230 °C, the quadrupole temperature was 150 °C and the multiplier voltage was 1200 V. Helium was the carrier gas (flow rate of 2.6953 mL/min) and an injection volume of 0.1 µL was employed (split ratio 20:1). Oven temperature program conditions were as follows: initial temperature of 80 °C for 5 min with a solvent delay of 3 min, then gradually increased to 120 °C at a 2.5 °C/min rate, where it remained for 1 min, then ramped at 20 °C/min to 240 °C for 5 min, total run time 60 min. Essential oils were diluted with methanol (1%), filtered and injected manually.
The chemical compounds’ data of the essential oils were exported using the supplied enhanced data analysis software, selecting the Wiley7n.l /NIST17.L library of spectra; the citral standards were used as controls to find the corresponding compounds according to the comparison of their relative retention time (RT). Retention indices (RI) were measured with respect to C7-C40 saturated alkanes standard.
4.4. Antioxidant Activity DPPH Test
The effects of 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging potentials of the essential oils were determined on the basis of the method described by Brand-Williams et al. [54], prepared with some modifications. A total of 3.94 mg (0.01 mmol) of DPPH were dissolved in 100 mL of ethanol. The 0.1 mmol/L DPPH solution (2.0 mL) was mixed with 2 mL of essential oils of 32.0, 16.0, 8.0, 4.0, 2.0, 1.0 and 0.5 mg/mL. The absorbance reading for each concentration was taken at 517 nm after 30 min of incubation in the dark at room temperature. The 6-Di-tert-butyl-4-methylphenol (BHT) was used as a positive control and ethanol was measured as a negative control. All spectrophotometric data were acquired using a Molecular Devices SpctraMax 190 (USA). The analyses were performed in 3 replications.
The antioxidant activity linked to inhibition percentage of DPPH was calculated by the equation: Inhibition (%) = (A0 − A1)/A0 × 100%, where A0 is ethanol DPPH blank absorbance, A1 is sample DPPH absorbance.
The radical scavenging activity of the studied samples was expressed as IC50, defined as the concentration of the essential oil necessary to reduce or inhibit 50% of DPPH radical solution. The best activity against the DPPH radical was obtained with the lowest value of IC50. IC50 were estimated from the inhibition percentage versus concentration plots using a non-linear regression algorithm.
4.5. ABTS Radical Scavenging Activity
The ABTS+ was produced by reacting 1:1 substance ratio 7 mmol/L stock solution of ABTS with 2.45 mmol/L potassium persulfate and allowing the mixture to stand in the dark for 12–16 h at room temperature. After incubation, the solution ABTS+ was diluted with methanol to obtain an absorbance of 0.70 ± 0.02 at 734 nm. A volume of 0.2 mL of essential oil at the tested concentration (64.0, 32.0, 16.0, 8.0, 4.0, 2.0, 1.0 and 0.5 mg/mL) was added to 3.8 mL of the ABTS+ solution. Absorbance was measured at 734 nm. The percentage inhibition of the radical cation ABTS+ was determined using the following formula: Inhibition of ABTS (%) = (A0 − A1)/A0 × 100%, where A0 is ethanol ABTS+ blank absorbance, A1 is the essential oil absorbance.
4.6. Ferric Reducing Antioxidant Power (FRAP)
FRAP was measured by total antioxidant capacity (T-AOC) diagnostic kits (Shanghai, China). The FRAP reagent 1, 2, 3 were mixed daily at the volume ratio of 7:1:1. A total of 180 uL FRAP reagent, 18 uL double distilled water were mixed in 1 mL centrifugal tube with 6 uL of essential oil solution (64.0, 32.0, 16.0, 8.0, 4.0, 2.0, 1.0 and 0.5 mg/mL). The mixture was vigorously shaken, and absorbance was measured at 593 nm after 10 min.
Ferrous sulfate standard solution (40 umol/mL) was produced by reacting 10 mg ferrous sulfate heptahydrate, 0.9 mL distilled water and 20 uL concentrated sulfuric acid. The standard solution was diluted to 0.15, 0.1, 0.05, 0.025, 0.0125, 0.00625, 0.003125, 0.00156 umol/mL, then mixed with 100 uL standard solution and 100 uL TPTZ solution. Absorbance was measured at 593 nm after 10 min. All measurements were repeated 3 times. Total antioxidant capacity in the measuring systems, expressed as ferrous sulfate equivalents, was calculated. Correlation coefficient (R2) for the calibration curve was 0.9982.
The total antioxidant capacity (U/mL) = X × Vt/Vs, where X is the sample antioxidant capacity expressed as the concentration of the FeSO4 solution when the absorbance of the sample is equal to the absorbance of the FeSO4 standard solution (umol/mL), Vt is 0.204 mL, Vs is 0.006 mL.
4.7. Statistical Analysis
All data represent the mean of 3 tests ± standard deviations (SD). Analysis of variance (ANOVA) test was conducted using SPSS 22.0. Origin 2018 software (Origin Lab, Northampton, MA, USA) was used for graphical analysis. GraphPad Prism (GraphPad Software 8.0.1) was used for IC50. KingDraw chemical structure editor software was used to depict the chemical structure.
5. Conclusions
In this paper, we studied the oil yield, essential oil composition and antioxidant activities of seven citral chemotype C. camphora and C. bodinieri of different origins and conducted a comparative analysis to explore the relationship between their antioxidant activities and their main components. The main component of the essential oil was citral (neral and geranial), with GC-MS concentrations ranging from 60.5–88.7%. The N7 variety had the highest citral content in seven EOCCs and the antioxidant activity was significantly stronger than other varieties in the DPPH, ABTS and FRAP assays, therefore, it could be preferentially selected as the raw material for the extraction of citral. The seven essential oils had a moderate antioxidant capacity, showing a positive correlation with the content of oxygenated terpenoids in the EOCC. This study made a major contribution by identifying that the citral chemotype C. bodinieri is an unrivalled source of citral by demonstrating large biomass, high oil yield and rich citral content.
Author Contributions
Conceptualization, Q.L. and B.Z.; methodology, Q.L. and Z.J.; software, Q.L., B.Z. and Z.J.; validation, Z.X., J.H. and C.X.; formal analysis, Q.L. and B.Z.; investigation, Q.L., B.Z., Y.W., Z.X., J.H., C.X. and Z.J.; resources, Q.L. and Z.J.; data curation, Q.L. and Y.W.; writing—original draft preparation, Q.L., writing—review and editing, Q.L., Y.L. and Z.J.; visualization, Z.X., J.H. and C.X.; supervision, Y.L. and Z.J.; super project administration, Y.L. and Z.J.; funding acquisition, Y.L. and Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the major program of JiangXi Science and Technology Department (grant no. 20203ABC28W016), Camphor innovative R&D project of JiangXi provincial department of forestry (grant no. [2020]07), Forestry science and technology innovation project of Jiangxi province department forestry (grant no. [2019]04) and Key R&D Programmes of JiangXi Science and Technology Department (grant no. 2017ACH80016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Beiqi Jiang, Gang Li and Xiaodan Ning for their assistance in sample harvesting, essential oil extraction and gas chromatography–mass spectrometry (GC-MS). We would also like to thank Junfei Jiang, Bangliang Deng and reviewers for their help in improving the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hirai, M.; Ota, Y.; Ito, M. Diversity in principal constituents of plants with a lemony scent and the predominance of citral. J. Nat. Med. 2022, 76, 254–258. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, H.; Wen, Z.; Zhang, B.; Chen, G. Toward the Efficient Synthesis of Pseudoionone from Citral in a Continuous-Flow Microreactor. Ind. Eng. Chem. Res. 2018, 57, 11288–11298. [Google Scholar] [CrossRef]
- Southwell, I. Backhousia citriodora F. Muell. (Lemon Myrtle), an Unrivalled Source of Citral. Foods 2021, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Shim, C.-H.; Lee, I.-S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Biological properties of citral and its potential protective effects against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 87, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Qi, H.; Luan, X.; Xu, W.; Yu, F.; Zhong, Y.; Xu, M. The chromosome-level genome sequence of the camphor tree provides insights into Lauraceae evolution and terpene biosynthesis. Plant Biotechnol. J. 2022, 20, 244–246. [Google Scholar] [CrossRef]
- Sun, W.-H.; Xiang, S.; Zhang, Q.-G.; Xiao, L.; Zhang, D.; Zhang, P.; Chen, D.-Q.; Hao, Y.; Liu, D.-K.; Ding, L.; et al. The camphor tree genome enhances the understanding of magnoliid evolution. J. Genet. Genom. 2021, 49, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mao, Y.; Wang, L. A Chromosome-Level Genome of the Camphor Tree and the Underlying Genetic and Climatic Factors for Its Top-Geoherbalism. Front. Plant Sci. 2022, 13, 17. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhong, Y.; Wu, Y.; Li, Z.; Xu, L.-A.; Xu, M. Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genom. 2018, 19, 550. [Google Scholar] [CrossRef]
- Xiangmei, J.; Yanfang, W.; Fuming, X.; Zhenyu, X.; Haining, X. Transcriptome analysis for leaves of five chemical types in Cinnamomum camphora. Hereditas 2014, 36, 58–68. [Google Scholar] [CrossRef]
- Yang, T.; Li, J.; Wang, H.; Zeng, Y. A geraniol-synthase gene from Cinnamomum tenuipilum. Phytochemistry 2005, 66, 285–293. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Zhang, B.; Jin, X.; Zhang, H.; Jin, Z. Transcriptional Analysis of Metabolic Pathways and Regulatory Mechanisms of Essential Oil Biosynthesis in the Leaves of Cinnamomum camphora (L.) Presl. Front. Genet. 2020, 11, 598714. [Google Scholar] [CrossRef]
- Qiu, F.; Wang, X.; Zheng, Y.; Wang, H.; Liu, X.; Su, X. Full-Length Transcriptome Sequencing and Different Chemotype Expression Profile Analysis of Genes Related to Monoterpenoid Biosynthesis in Cinnamomum porrectum. Int. J. Mol. Sci. 2019, 20, 6230. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, B.; Wang, Y.; Li, F.; Jin, Z.; Lü, X.; Zhang, H.; Zhang, J.; Zhao, J. Transcriptomic Analysis Reveals That Exogenous Indole-3-Butyric Acid Affects the Rooting Process during Stem Segment Culturing of Cinnamomum camphora Linalool Type. Plant Mol. Biol. Report. 2022, 40, 1–13. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, T.; Liao, X.; Zhou, Y.; Chen, S.; Chen, J.; Xiong, W. Extraction of Camphor Tree Essential Oil by Steam Distillation and Supercritical CO2 Extraction. Molecules 2022, 27, 5385. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Tian, Z.; Zheng, T.; Xu, S.; Ma, Y.; Zou, S.; Zuo, Z. Terpenoid composition and antioxidant activity of extracts from four chemotypes of Cinnamomum camphora and their main antioxidant agents. Biofuels Bioprod. Biorefining 2022, 16, 510–522. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Meng, Y.; Zu, Y.; Han, F.; Zhao, X. Isolation, Antibacterial, Nematicidal and Anxiolytic Activities of Essential Oil from Cinnamomum longepaniculatum (Gamble) N. Chao ex H. W. Li Leaves. J. Essent. Oil Bear. Plants 2022, 25, 581–600. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, C.; Xiao, Z.; Zhang, H.; Cao, M.; Liu, Y.; Jin, Z. Chemical Constituents and Chemotypes of Fresh Leaf Essential Oil of Wild Species Belonging to Sect. Camphor (Trew.) Meissn. in Southeastern China. J. Essent. Oil Bear. Plants 2019, 22, 1115–1122. [Google Scholar] [CrossRef]
- Xiao, Z. Effects of IBA on rooting ability of Cinnamomum bodinieri citral type micro-shoots from transcriptomics analysis. Plant Biotechnol. Rep. 2020, 14, 467–477. [Google Scholar] [CrossRef]
- Ksouda, G.; Sellimi, S.; Merlier, F.; Falcimaigne-cordin, A.; Thomasset, B.; Nasri, M.; Hajji, M. Composition, antibacterial and antioxidant activities of Pimpinella saxifraga essential oil and application to cheese preservation as coating additive. Food Chem. 2019, 288, 47–56. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A. Composition of the Essential Oils of the Leaves and Flowers of Thymus pallescens de Noé and Origanum floribundum Munby From Algeria. J. Essent. Oil Res. 2009, 21, 267–270. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Q.; Yuan, G.; Xu, F.; Li, B.; Wang, J.; Ouyang, J. Comparison of the Essential Oil Composition of Wild Rhododendron tomentosum Stems, Leaves, and Flowers in Bloom and Non-bloom Periods from Northeast China. J. Essent. Oil Bear. Plants 2016, 19, 1216–1223. [Google Scholar] [CrossRef]
- Tangjitjaroenkun, J.; Tangchitcharoenkhul, R.; Yahayo, W.; Supabphol, S.; Sappapan, R.; Supabphol, R. Chemical compositions of essential oils of Amomum verum and Cinnamomum parthenoxylon and their in vitro biological properties. J. Herbmed Pharmacol. 2020, 9, 223–231. [Google Scholar] [CrossRef]
- Anifalaje, E.O.; Ibok, M.G. Chemical Compositions and Antioxidant Activities of Albizia lebbeck L. Essential Oils. J. Essent. Oil Bear. Plants 2020, 23, 810–820. [Google Scholar] [CrossRef]
- Lim, A.C.; Tang, S.G.H.; Zin, N.M.; Maisarah, A.M.; Ariffin, I.A.; Ker, P.J.; Mahlia, T.M.I. Chemical Composition, Antioxidant, Antibacterial, and Antibiofilm Activities of Backhousia citriodora Essential Oil. Molecules 2022, 27, 4895. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Yang, Z.; Zhang, N. Chemical Composition of Essential Oils from the Heartwood of Pterocarpus macrocarpus by Different Extraction Methods in Southern China. J. Essent. Oil Bear. Plants 2017, 20, 110–115. [Google Scholar] [CrossRef]
- Lu, C.; Li, H.; Li, C.; Chen, B.; Shen, Y. Chemical composition and radical scavenging activity of Amygdalus pedunculata Pall leaves’ essential oil. Food Chem. Toxicol. 2018, 119, 368–374. [Google Scholar] [CrossRef]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical Composition of Essential Oils of Litsea cubeba Harvested from Its Distribution Areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Pokharel, K.K.; Setzer, W.N. Bioactivities and Compositional Analyses of Cinnamomum Essential Oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013, 8, 1934578X1300801. [Google Scholar] [CrossRef]
- Southwell, I.A.; Russell, M.; Smith, R.L.; Archer, D.W. Backhousia citriodora F. Muell. (Myrtaceae), A Superior Source of Citral. J. Essent. Oil Res. 2000, 12, 735–741. [Google Scholar] [CrossRef]
- Alhasan, A.S.; Abbas, M.K.; Al-Ameri, D.T. Response of Two Purple basil (Ocimum basilicum L.) Cultivars Grown Under Field Conditions to Different Rates of NPK Foliar Fertilization. IOP Conf. Ser. Earth Environ. Sci. 2021, 735, 012053. [Google Scholar] [CrossRef]
- Čeh, B.; Štraus, S.; Hladnik, A.; Kušar, A. Impact of Linseed Variety, Location and Production Year on Seed Yield, Oil Content and Its Composition. Agronomy 2020, 10, 1770. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Yousefzadeh, S.; Sabaghnia, N. Chemical Comparison of Essential Oils in Dragonhead (Dracocephalum moldavica L.) Samples Grown in Different Areas. J. Essent. Oil Bear. Plants 2018, 21, 950–962. [Google Scholar] [CrossRef]
- Shokrgoo, A.; Madandoust, M. Effect of Harvest Time on Essential Oil Content and Chemical Composition of Origanum vulgare (L.) from Iran. J. Essent. Oil Bear. Plants 2018, 21, 1682–1686. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, Q.; Hu, Q.; Liu, P.; Yang, Y.; Li, G.; Xie, H.; Tang, H. Drying temperature affects essential oil yield and composition of black cardamom (Amomum tsao-ko). Ind. Crops Prod. 2021, 168, 113580. [Google Scholar] [CrossRef]
- Sarrazin, S.; da Silva, L.; de Assunção, A.; Oliveira, R.; Calao, V.; da Silva, R.; Stashenko, E.; Maia, J.; Mourão, R. Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules 2015, 20, 1860–1871. [Google Scholar] [CrossRef]
- Kazemi, S.Y.; Nabavi, J.; Zali, H.; Ghorbani, J. Effect of Altitude and Soil on the Essential Oils Composition of Juniperus communis. J. Essent. Oil Bear. Plants 2017, 20, 1380–1390. [Google Scholar] [CrossRef]
- Norouzi, M.; Maboud, H.E.; Seyedi, S.M.; Niknam, V. Changes in Pistachios Essential Oil Composition during Fruit Ripening. J. Essent. Oil Bear. Plants 2019, 22, 1481–1487. [Google Scholar] [CrossRef]
- Agha Mohammad Reza, M.; Paknejad, F.; Shirani Rad, A.H.; Ardakani, M.R.; Kashani, A. Change in plant densities combined with zinc application affects rapeseed seed oil and fatty acid composition. J. Plant Nutr. 2022, 45, 471–481. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Z.; Yang, L.; Yang, R.; Ji, Y.; Zeng, Q.; Xiao, F.; Huang, P. Optimization extraction of rosemary essential oils using hydrodistillation with extraction kinetics analysis. Food Sci. Nutr. 2021, 9, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2022, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lal, R.K.; Chanotiya, C.S.; Dwivedi, A. The pre-eminence of agro-parameters and chemical constituents in the influence of harvest interval by traits × environment interaction over the years in lemon-scented basil (Ocimum africanum Lour.). Ind. Crops Prod. 2021, 172, 113989. [Google Scholar] [CrossRef]
- Kaskoos, R.A. Essential Oil Analysis by GC-MS and Analgesic Activity of Lippia citriodora and Citrus limon. J. Essent. Oil Bear. Plants 2019, 22, 273–281. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: Limonene and citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Hammid, S.A.; Ahmad, F. Chemotype of Litsea cubeba Essential Oil and Its Bioactivity. Nat. Prod. Commun. 2015, 10, 1934578X1501000. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghosh, B.; Panda, P.C.; Nayak, S.; Mahapatra, N. Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind. Crops Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Udayaprakash, N.K.; Ranjithkumar, M.; Deepa, S.; Sripriya, N.; Al-Arfaj, A.A.; Bhuvaneswari, S. Antioxidant, free radical scavenging and GC–MS composition of Cinnamomum iners Reinw. ex Blume. Ind. Crops Prod. 2015, 69, 175–179. [Google Scholar] [CrossRef]
- Mathela, C.; Joshi, S. Antioxidant and antibacterial activities of the leaf essential oil and its constituents furanodienone and curzerenone from Lindera pulcherrima (Nees.) Benth. ex hook. f. Pharmacogn. Res. 2012, 4, 80. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model Studies on the Antioxidant Activity of Common Terpenoid Constituents of Essential Oils by Means of the 2,2-Diphenyl-1-picrylhydrazyl Method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. CMC 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).