Magnetically Driven Muco-Inert Janus Nanovehicles for Enhanced Mucus Penetration and Cellular Uptake
Abstract
1. Introduction
2. Results and Discussion
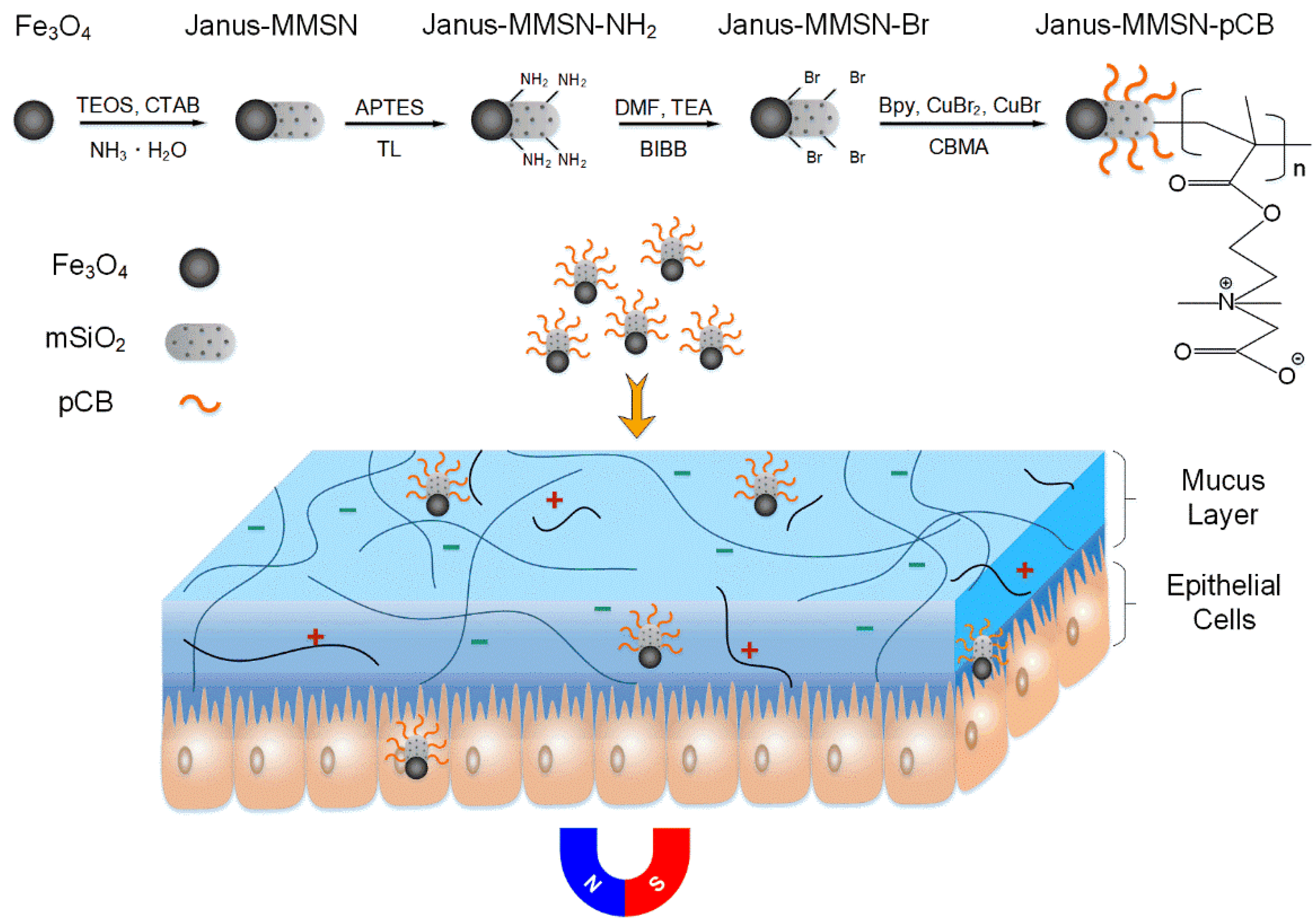
2.1. Preparation and Characterizations of the Nanovehicles
2.2. Mucus Permeation Analysis
2.2.1. Multiple-Particle Tracking
2.2.2. Mucus Diffusion Analysis
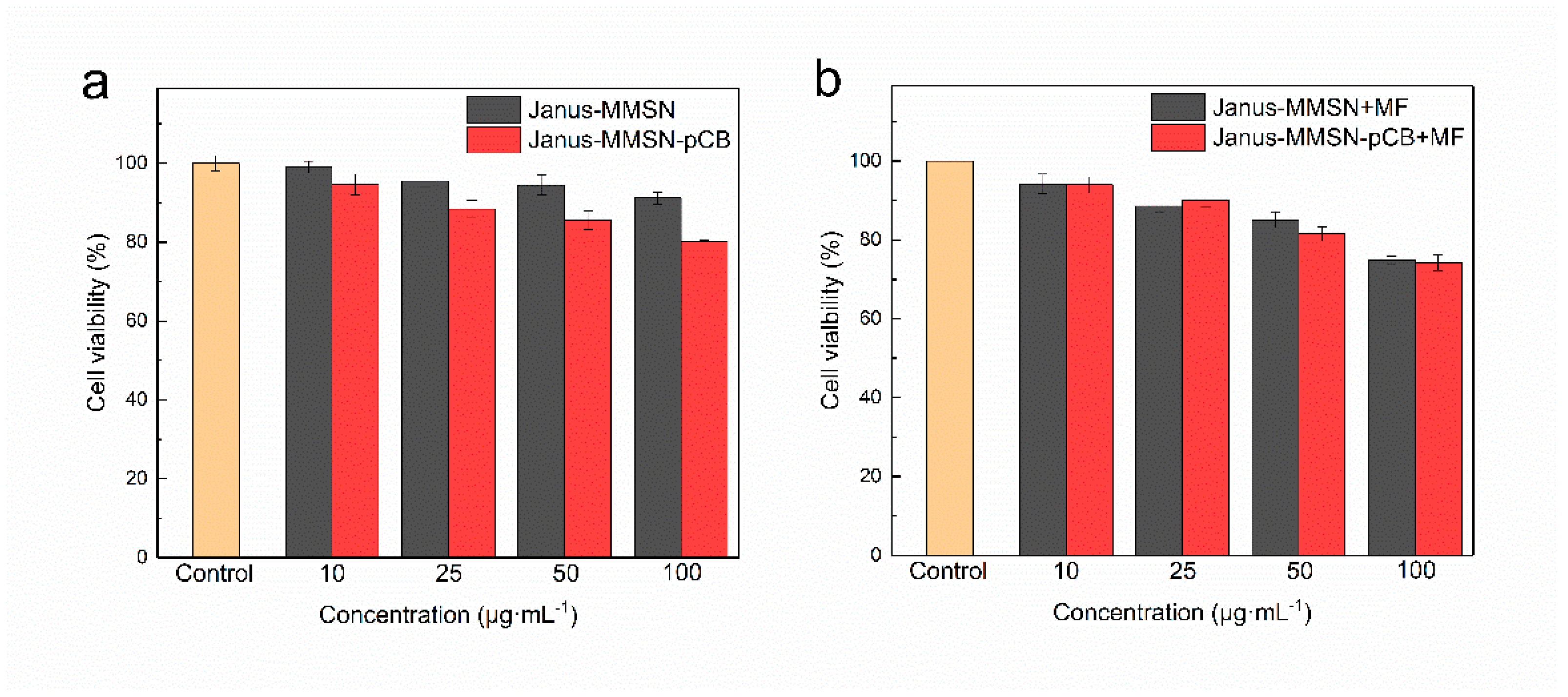
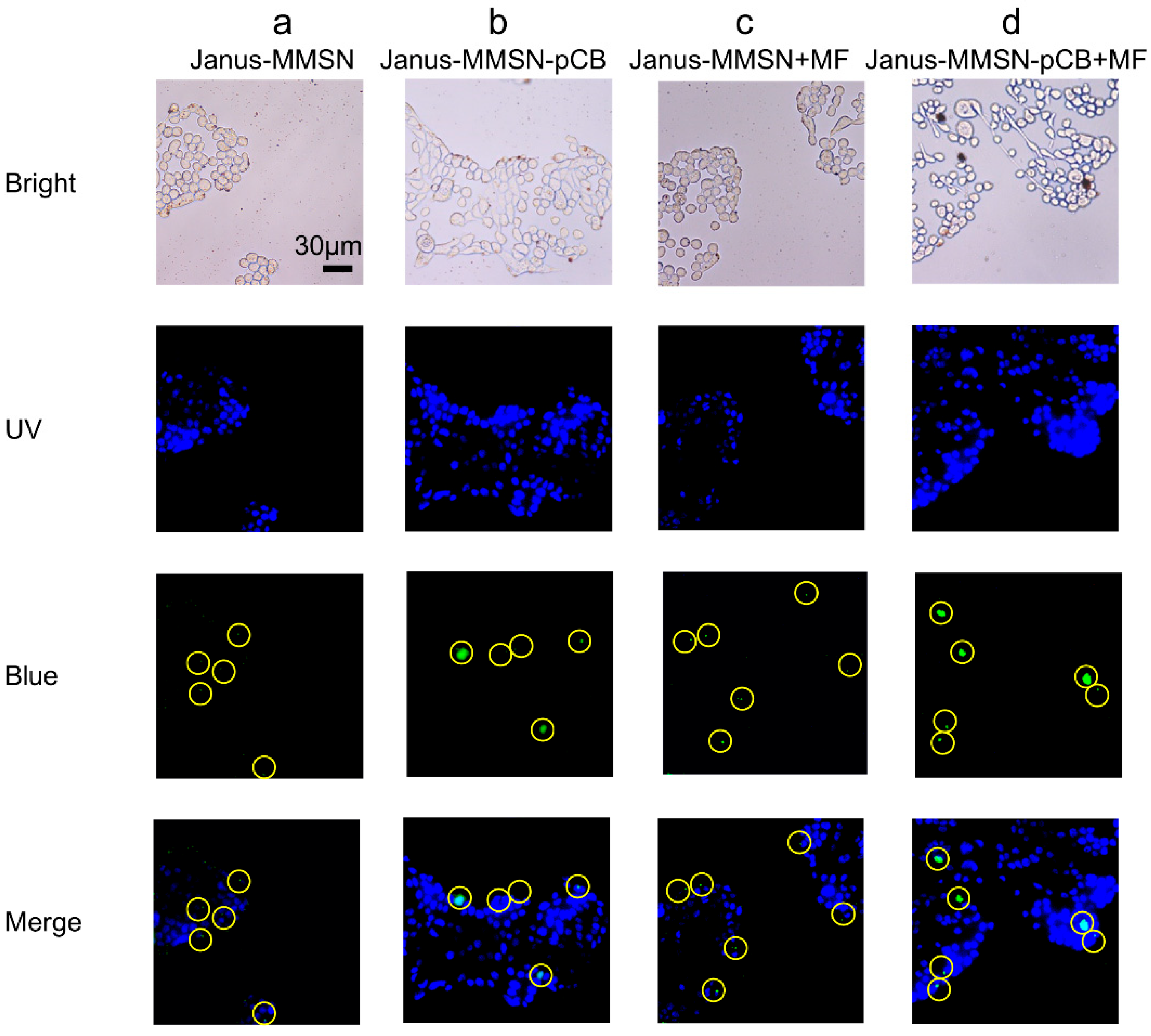
2.3. In Vitro Cytotoxicity and Cellular Uptake
3. Materials and Methods
3.1. Materials and Cells
3.2. Synthesis of Magnetic Mesoporous Janus Nanovehicles (Janus-MMSN)
3.3. Preparation of Muco-Inert Nanovehicles (Janus-MMSN-pCB)
3.4. Characterizations of the Nanoparticles
3.5. Multiparticle Tracking of the Nanovehicles
3.6. Transwell® System Diffusion Analysis
3.7. Cell Viability Study
3.8. Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Witten, J.; Tahoura, S.; Ribbeck, K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar]
- Farhan, T.; Felipe, V.; Abdul, W.B. A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 2018, 124, 16–33. [Google Scholar]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Control. Release 2017, 264, 247–275. [Google Scholar] [CrossRef]
- das Neves, J.; Samento, B. Technological strategies to overcome the mucus barrier in mucosal drug delivery. Adv. Drug Deliv. Rev. 2018, 124, 1–2. [Google Scholar] [CrossRef]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021, 119, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef]
- Mert, O.; Lai, S.K.; Ensign, L.; Yang, M.; Wang, Y.; Wood, J.; Hanes, J. A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. J. Control. Release 2012, 157, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, S.K.; Yu, T.; Wang, Y.; Happe, C.; Zhong, W.; Zhang, M.; Anonuevo, A.; Fridley, C.; Hung, A.; et al. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J. Control. Release 2014, 192, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Schneider, C.; Suk, J.S.; Cone, R.; Hanes, J. Mucus penetrating nanoparticles: Biophysical tool and method of drug and gene delivery. Adv. Mater. 2012, 24, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Vigani, B.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent advances in the mucus-interacting approach for vaginal drug delivery: From mucoadhesive to mucus-penetrating nanoparticles. Expert Opin. Drug Deliv. 2019, 16, 777–781. [Google Scholar] [CrossRef]
- Fang, L.; Wang, L.; Yao, Y.; Zhang, J.; Wu, X.; Li, X.; Wang, H.; Zhang, X.; Gong, X.; Chang, J. Micro- and nano-carrier systems: The non-invasive and painless local administration strategies for disease therapy in mucosal tissues. Nanomedicine 2017, 13, 153–171. [Google Scholar] [CrossRef]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schoen, A.; Gong, X.; Yang, J.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [PubMed]
- Freese, C.; Gibson, M.I.; Klok, H.-A.; Unger, R.E.; Kirkpatrick, C.J. Size- and coating-dependent uptake of polymer-coated gold nanoparticles in primary human dermal microvascular endothelial cells. Biomacromolecules 2012, 13, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.E.; Wang, Y.; Yang, Q.; Hoang, T.; Chattopadhyay, S.; Hoen, T.; Ensign, L.M.; Nunn, K.L.; Schroeder, H.; McCallen, J.; et al. Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus. Acta Biomater. 2016, 43, 61–70. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Lin, C.; Wang, L.; Yuan, S. Molecular dynamics simulations of surface hydration layers near non-fouling polymer membranes. Acta Chim. Sin. 2013, 71, 649–656. [Google Scholar] [CrossRef]
- Shan, W.; Zhu, X.; Tao, W.; Cui, Y.; Liu, M.; Wu, L.; Li, L.; Zheng, Y.; Huang, Y. Enhanced oral delivery of protein drugs using zwitterion-functionalized nanoparticles to overcome both the diffusion and absorption barriers. ACS Appl. Mater. Interfaces 2016, 8, 25444–25453. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular understanding and design of zwitterionic materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Jiang, S. Dual-functional biomimetic materials: Nonfouling poly(carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules 2006, 7, 3311–3315. [Google Scholar] [CrossRef]
- Yang, W.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Pursuing “zero” protein adsorption of poly(carboxybetaine) from undiluted blood serum and plasma. Langmuir 2009, 25, 11911–11916. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherova, H.; Yang, W.; Zhang, Z.; Cao, Z.; Cheng, G.; Piliarik, M.; Homola, J.; Jiang, S. Ultralow fouling and functionalizable surface chemistry based on a zwitterionic polymer enabling sensitive and specific protein detection in undiluted blood plasma. Anal. Chem. 2008, 80, 7894–7901. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Lu, B.; Wu, W.; Zhang, L.; Liang, J.; Yi, J.; Li, X. Novel polyzwitterion shell with adaptable surface chemistry engineered to enhance anti-fouling and intracellular imaging of detonation nanodiamonds under tumor pHe. Front. Mater. Sci. 2020, 14, 402–412. [Google Scholar] [CrossRef]
- Dong, C.; Wang, H.; Zhang, Z.; Zhang, T.; Liu, B. Carboxybetaine methacrylate oligomer modified nylon for circulating tumor cells capture. J. Colloid Interface Sci. 2014, 432, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, F.; Gu, H.; Xu, H. Tuning of surface protein adsorption by spherical mixed charged silica brushes (MCB) with zwitterionic carboxybetaine component. J. Mater. Chem. B 2017, 5, 435–443. [Google Scholar] [CrossRef]
- Walker, D.; Kaesdorf, B.T.; Jeong, H.-H.; Lieleg, O.; Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 2015, 1, e1500501. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lin, Z.; Zhou, C.; Wang, D.; He, Q. Acoustophoretic motion of erythrocyte-mimicking hemoglobin micromotors. Chin. J. Chem. 2020, 38, 1589–1594. [Google Scholar] [CrossRef]
- Zhao, R.; Han, T.; Sun, D.; Shan, D.; Liu, Z.; Liang, F. Multifunctional Fe3O4@SiO2 Janus particles. Acta Chim. Sin. 2020, 78, 954–960. [Google Scholar] [CrossRef]
- Ye, H.; Wang, Y.; Liu, X.; Xu, D.; Yuan, H.; Sun, H.; Wang, S.; Ma, X. Magnetically steerable iron oxides-manganese dioxide core-shell micromotors for organic and microplastic removals. J. Colloid Interface Sci. 2021, 588, 510–521. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, Y.; Xu, D.; Pan, X.; Chen, Y.; Zhou, D.; Wang, B.; Feng, H.; Ma, X. Magnetic nanomotor-based maneuverable SERS probe. Research 2020, 2020, 7962024. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Z.; Deng, G.; Jiang, K.; Wang, H.; Zhang, X.; Han, H. Gastric acid powered nanomotors release antibiotics for in vivo treatment of Helicobacter pylori infection. Small 2021, 17, e2006877. [Google Scholar] [CrossRef]
- Ismagilov, R.F.; Schwartz, A.; Bowden, N.; Whitesides, G.M. Autonomous movement and self-assembly. Angew. Chem. Int. Ed. 2002, 41, 652–654. [Google Scholar] [CrossRef]
- Su, P.; Wu, H.; Chen, Y.; Peng, F. Micro/Nanomotors as drug delivery agent. Prog. Chem. 2019, 31, 63–69. [Google Scholar]
- He, Y.; Dong, S.; Wang, L.; Rong, W.; Sun, L. Bipedal microwalkers actuated by oscillating magnetic fields. Soft Matter 2020, 16, 7927–7934. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ma, N.; Yu, H.; Sun, H.; Chang, X.; Wu, Z.; Deng, J.; Zhao, S.; Wang, W.; Zhang, G.; et al. Self-propelled Janus microdimer swimmers under a rotating magnetic field. Nanomaterials 2019, 9, 1672. [Google Scholar] [CrossRef] [PubMed]
- Öndeş, B.; Uygun, M.; Evli, S.; Aktaş Uygun, D. Immobilization of urokinase onto magnetically directed micromotors. Appl. Biochem. Biotechnol. 2022, 194, 3351–3364. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, F.; Piao, H.; Huang, X.; Cong, J.; Luo, Z.; Pan, L.; Liu, Y. Rod-shaped nanomotor powered by magnetic field gradients and its application to surface-enhanced Raman-scattering-based detection. Appl. Phys. Express 2017, 10, 045202. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, K.; Ji, F.; Zhang, L. Enhanced removal of toxic heavy Metals using swarming biohybrid adsorbents. Adv. Funct. Mater. 2018, 28, 1806340. [Google Scholar] [CrossRef]
- Chikazumi, S. Physics of Ferromagnetism; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Shao, D.; Li, J.; Zheng, X.; Pan, Y.; Wang, Z.; Zhang, M.; Chen, Q.; Dong, W.; Chen, L. Janus “nano-bullets” for magnetic targeting liver cancer chemotherapy. Biomaterials 2016, 100, 118–133. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Liu, J.; Xu, Q.; Xiao, H.; Wang, X.; Xu, H.; Zhou, J. Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem. Eng. J. 2013, 226, 30–38. [Google Scholar] [CrossRef]
- Majumder, S.; Sardar, M.; Satpati, B.; Kumar, S.; Banerjee, S. Magnetization enhancement of Fe3O4 by attaching onto graphene oxide: An interfacial effect. J. Phys. Chem. C 2018, 122, 21356–21365. [Google Scholar] [CrossRef]
- Mao, Y.; Feng, S.; Zhang, X.; Zhao, Q.; Fang, Y.; Wang, S. Thiolated polymer and cell-penetrating peptide dual-surface functionalization of mesoporous silicon nanoparticles to overcome intestinal barriers. J. Drug Deliv. Sci. Technol. 2019, 53, 101184. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Tian, M.; Deng, C.; Cao, L.; Yuan, H. Synthesis of worm-like mesoporous silica and enzymatic activity of the immobilized laccase. Acta Chim. Sin. 2013, 71, 602–612. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Zhang, L.; Wang, S.; White, A.D.; Jiang, S. Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials 2009, 30, 5617–5621. [Google Scholar] [CrossRef]
- Ji, Y.; Wei, Y.; Liu, X.; Wang, J.; Ren, K.; Ji, J. Zwitterionic polycarboxybetaine coating functionalized with REDV peptide to improve selectivity for endothelial cells. J. Biomed. Mater. Res. A 2012, 100A, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Y. Poly(carboxybetaine methacrylate)-grafted silica nanoparticle: A novel carrier for enzyme immobilization. Biochem. Eng. J. 2018, 132, 122–129. [Google Scholar] [CrossRef]
- Wang, W.; Casrto, L.A.; Hoyos, M.; Mallouk, T.E. Autonomous motion of metallic microrods propelled by ultrasound. ACS Nano 2012, 6, 6122–6132. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Soto, F.; Gao, W.; Garcia-Gradilla, V.; Li, J.; Zhang, X.; Wang, J. Ultrasound-modulated bubble propulsion of chemically powered microengines. J. Am. Chem. Soc. 2014, 136, 8552–8555. [Google Scholar] [CrossRef] [PubMed]
- Dames, P.; Gleich, B.; Flemmer, A.; Hajek, K.; Seidl, N.; Wiekhorst, F.; Eberbeck, D.; Bittmann, I.; Bergemann, C.; Weyh, T.; et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat. Nanotechnol. 2007, 2, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Manshadi, M.K.D.; Mohammadi, M.; Zare, M.J.; Zarei, M.; Kamali, R.; Sanati-Nezhadbc, A. Magnetic particle targeting for diagnosis and therapy of lung cancers. J. Control. Release 2020, 328, 776–791. [Google Scholar] [CrossRef]
- Luo, Z.; Paunović, N.; Leroux, J.C. Physical methods for enhancing drug absorption from the gastrointestinal tract. Adv. Drug Deliv. Rev. 2021, 175, 113814. [Google Scholar] [CrossRef]
- Gong, X.; Xu, Q.; Song, X.; Yu, T.; Schuster, B.; Yang, J.; Lamb, N.W.; Chang, J.; Hanes, J. Magnetic mucus-penetrating nanoparticles for drug delivery through human cervicovaginal mucus. Nanomed.-Nanotechnol. 2016, 12, 521. [Google Scholar] [CrossRef]
- Lubbe, A.S.; Alexiou, C.; Bergemann, C. Clinical applications of magnetic drug targeting. J. Surg. Res. 2001, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Griessinger, J.; Duennhaupt, S.; Cattoz, B.; Griffiths, P.; Oh, S.; Borros i Gomez, S.; Wilcox, M.; Pearson, J.; Gumbleton, M.; Abdulkarim, M.; et al. Methods to determine the interactions of micro- and nanoparticles with mucus. Eur. J. Pharm. Biopharm. 2015, 96, 464–476. [Google Scholar] [CrossRef]
- Groo, A.C.; Mircheva, K.; Bejaud, J.; Aihas, C.; Panaiotov, I.; Saulnier, P.; Ivanova, T.; Lagarce, F. Development of 2D and 3D mucus models and their interactions with mucus-penetrating paclitaxel-loaded lipid nanocapsules. Pharm. Res. 2014, 31, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Friedl, H.; Dünnhaupt, S.; Hintzen, F.; Waldner, C.; Parikh, S.; Pearson, J.P.; Wilcox, M.D.; Bernkop-Schnürch, A. Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. J. Pharm. Sci. 2013, 102, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Xu, Y.; Zhao, L.; Xu, J.; Li, S.; Wen, C.; Xia, X.; Dong, Q.; Hu, X.; Wang, X.; et al. In situ pepsin-assisted needle assembly of magnetic-graphitic-nanocapsules for enhanced gastric retention and mucus penetration. Nano Today 2021, 36, 101032. [Google Scholar] [CrossRef]
- Gao, Y.; He, Y.; Zhang, H.; Zhang, Y.; Gao, T.; Wang, J.-H.; Wang, S. Zwitterion-functionalized mesoporous silica nanoparticles for enhancing oral delivery of protein drugs by overcoming multiple gastrointestinal barriers. J. Colloid Interface Sci. 2021, 582, 364–375. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, X.; Liu, W.; Zhang, F.; Zheng, X.; Qiao, P.; Li, J.; Dong, W.; Chen, L. Janus silver-mesoporous silica nanocarriers for SERS traceable and pH-sensitive drug delivery in cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 4303–4308. [Google Scholar] [CrossRef]
- Mohammadzadehab, V.; Zirakc, M.R.; Khah, S.M.H.; Kamali, H.; Jaafari, M.R. pH-sensitive nanocarriers for curcumin delivery in cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 66, 102879. [Google Scholar] [CrossRef]
- Ji, F.; Zhang, K.; Li, J.; Gu, Y.; Zhao, J.; Zhang, J. A dual pH/magnetic responsive nanocarrier based on PEGylated Fe3O4 nanoparticles for doxorubicin delivery. J. Naonosci. Nanotechnol. 2018, 18, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sun, Y.; Dong, X. Zwitterionic polymer micelles with dual conjugation of doxorubicin and curcumin: Synergistically enhanced efficacy against multidrug-resistant tumor cells. Langmuir 2020, 36, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.L.; Smyth, H.D.C. Disruption of the mucus barrier by topically applied exogenous particles. Mol. Pharm. 2010, 7, 2280–2288. [Google Scholar] [CrossRef]
- Dawson, M.; Krauland, E.; Wirtz, D.; Hanes, J. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnol. Progress 2004, 20, 851–857. [Google Scholar] [CrossRef]
- Alp, G.; Aydogan, N. Enhancing the spreading behavior on pulmonary mucus mimicking subphase via catanionic surfactant solutions: Toward effective drug delivery through the lungs. Mol. Pharm. 2018, 15, 1361–1370. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew. Chem. Int. Ed. 2009, 48, 5875–5879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Dong, W.; Song, J.; Huo, Q.; Sun, H. Magnetic-mesoporous Janus nanoparticles. Chem. Commun. 2011, 47, 1225–1227. [Google Scholar] [CrossRef]
- Panahian, P.; Salami-Kalajahi, M.; Hosseini, M.S. Synthesis of dual thermosensitive and pH-sensitive hollow nanospheres based on poly(acrylic acid-b-2-hydroxyethyl methacrylate) via an atom transfer reversible addition-fragmentation radical process. Ind. Eng. Chem. Res. 2014, 53, 8079–8086. [Google Scholar] [CrossRef]
- Mou, F.; Pan, D.; Chen, C.; Gao, Y.; Xu, L.; Guan, J. Magnetically modulated pot-like MnFe2O4 micromotors: Nanoparticle assembly fabrication and their capability for direct oil removal. Adv. Funct. Mater. 2015, 25, 6173–6181. [Google Scholar] [CrossRef]
- Jia, Y. 3D Magnetic Manipulation System for Actuation of Magnetic Microrobot. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- Zhou, X.; Li, Z.; Tan, L.; Zhang, Y.; Jiao, Y. Near-infrared light-steered graphene aerogel micromotor with high speed and precise navigation for active transport and microassembly. ACS Appl. Mater. Interfaces 2020, 12, 23134–23144. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Cardoso, L.; Rosa da Costa, A.M.; Grenha, A. Biocompatibility and stability of polysaccharide polyelectrolyte complexes aimed at respiratory delivery. Materials 2015, 8, 5647–5670. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Bai, S.; Yu, L.; Sun, Y. Magnetically Driven Muco-Inert Janus Nanovehicles for Enhanced Mucus Penetration and Cellular Uptake. Molecules 2022, 27, 7291. https://doi.org/10.3390/molecules27217291
Hao Y, Bai S, Yu L, Sun Y. Magnetically Driven Muco-Inert Janus Nanovehicles for Enhanced Mucus Penetration and Cellular Uptake. Molecules. 2022; 27(21):7291. https://doi.org/10.3390/molecules27217291
Chicago/Turabian StyleHao, Yue, Shu Bai, Linling Yu, and Yan Sun. 2022. "Magnetically Driven Muco-Inert Janus Nanovehicles for Enhanced Mucus Penetration and Cellular Uptake" Molecules 27, no. 21: 7291. https://doi.org/10.3390/molecules27217291
APA StyleHao, Y., Bai, S., Yu, L., & Sun, Y. (2022). Magnetically Driven Muco-Inert Janus Nanovehicles for Enhanced Mucus Penetration and Cellular Uptake. Molecules, 27(21), 7291. https://doi.org/10.3390/molecules27217291






