An Integrated Experimental and Theoretical Studies on the Corrosion Inhibition of Carbon Steel by Harmal Extracts
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Preparation of Harmal Parts Extracts
2.1.2. Working Electrodes
2.1.3. Electrolytes
2.2. Methods
2.2.1. Electrochemical Experiment
2.2.2. Computational Details
3. Results and Discussions
3.1. Corrosion Inhibition Study
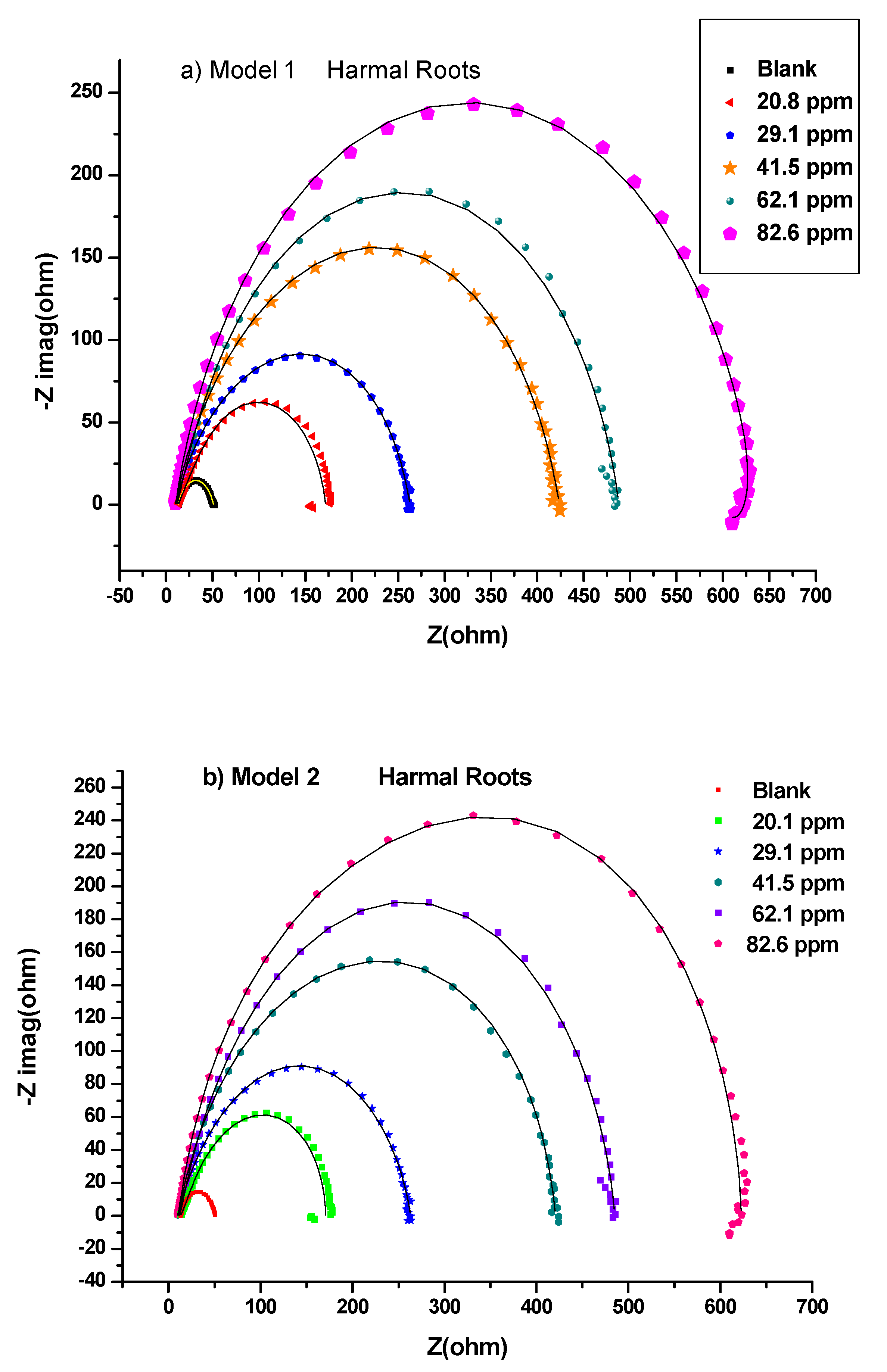
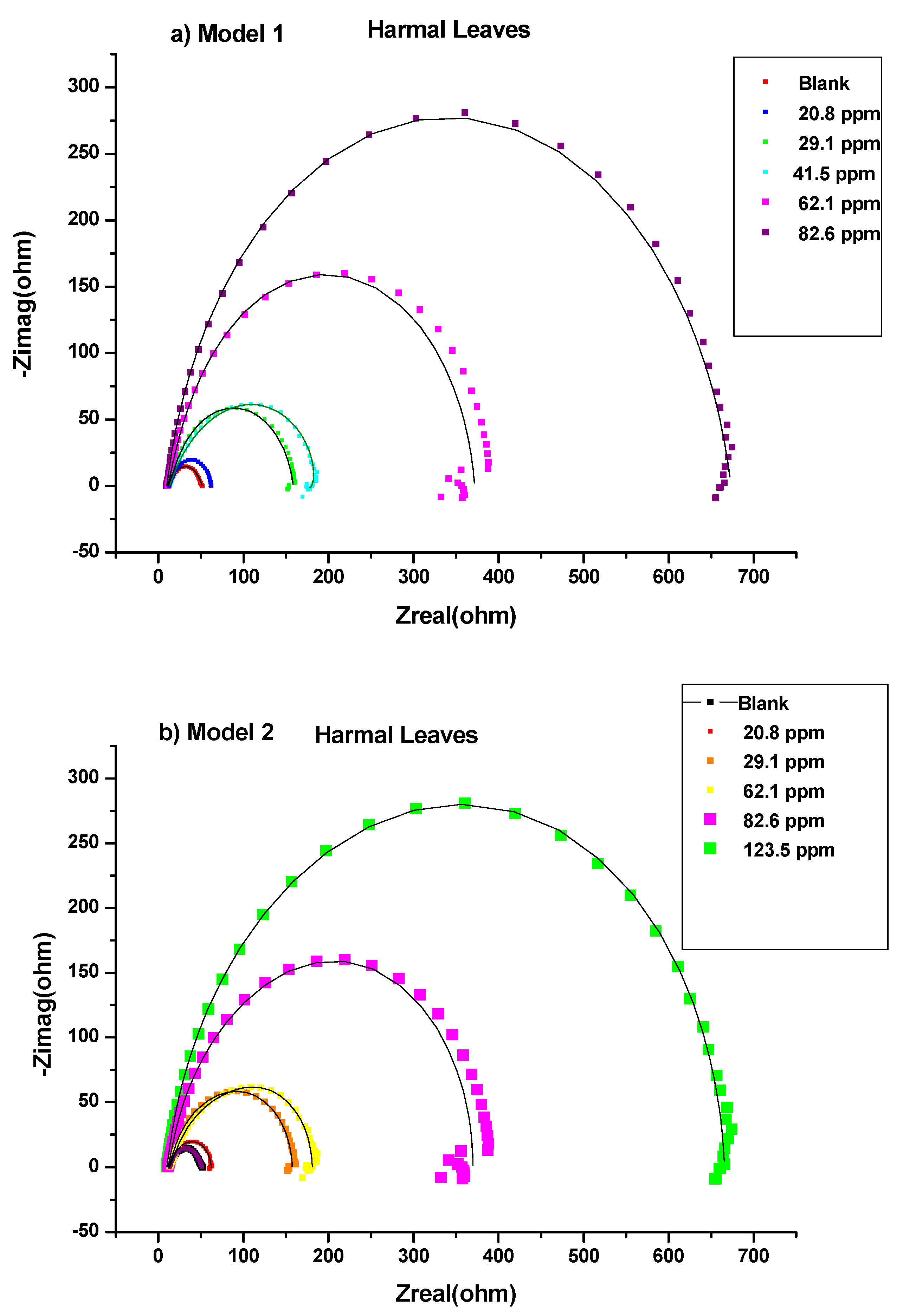
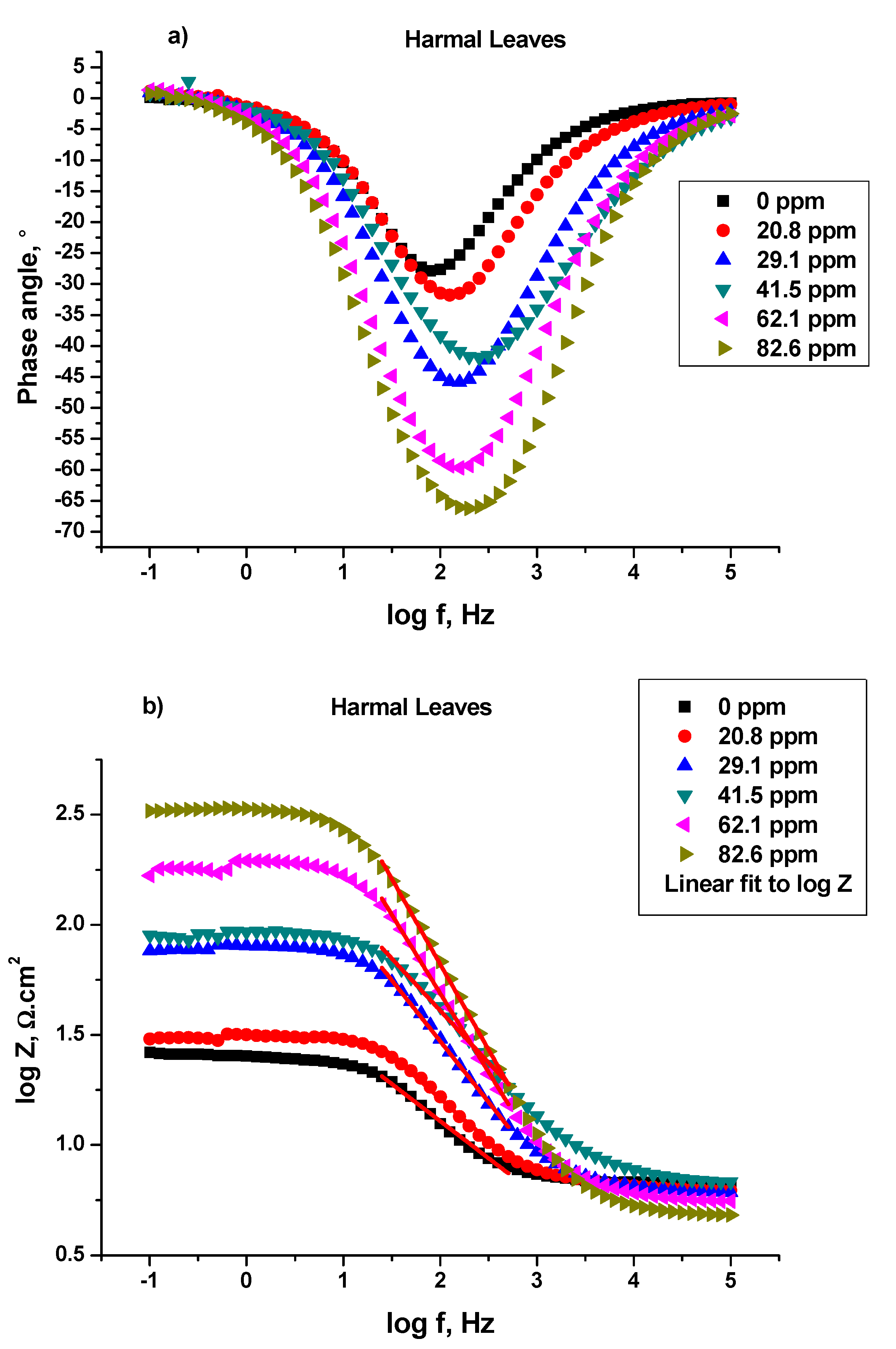
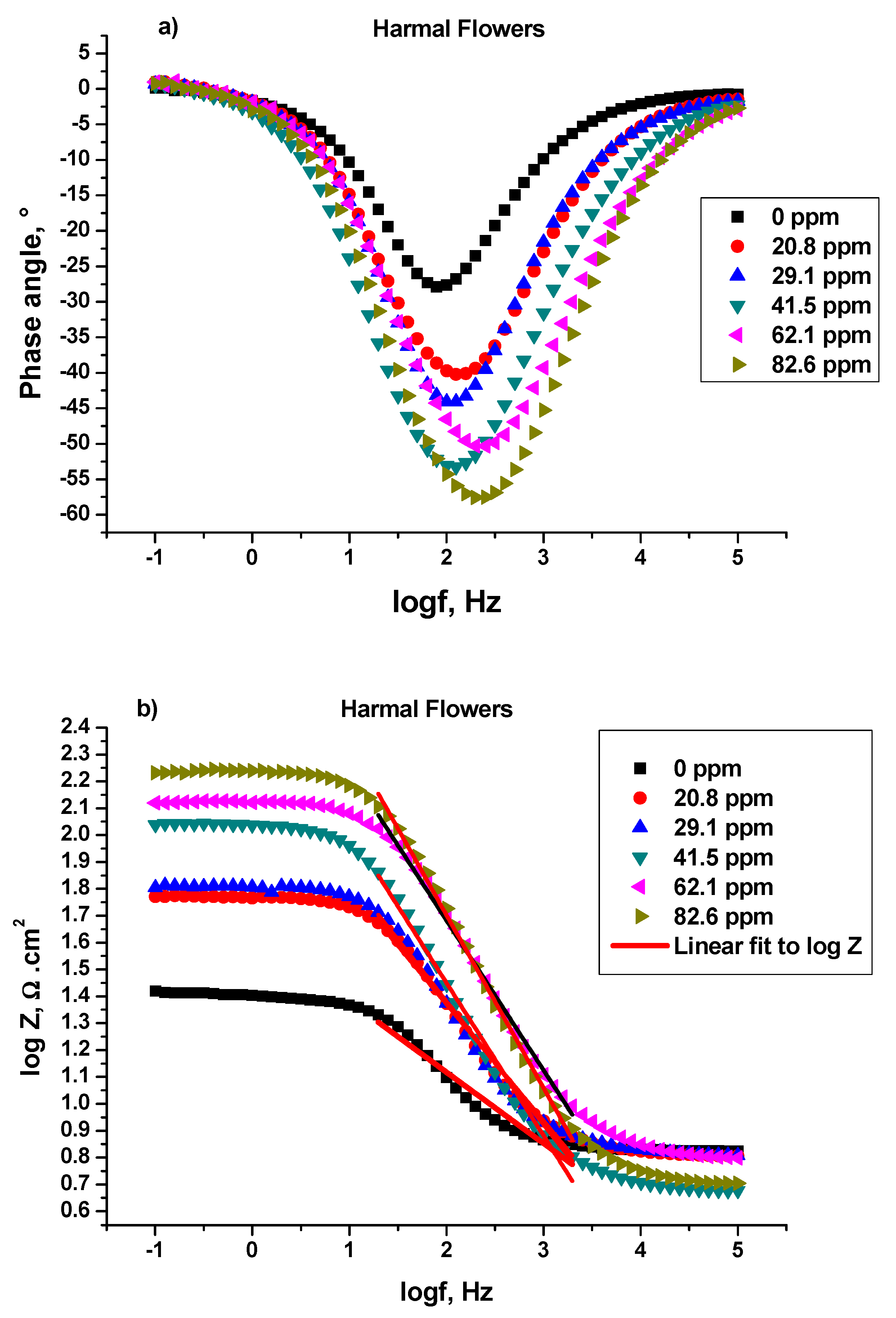
3.1.1. Electrochemical Impedance Study (EIS)
Bode and Bode Phase Angle
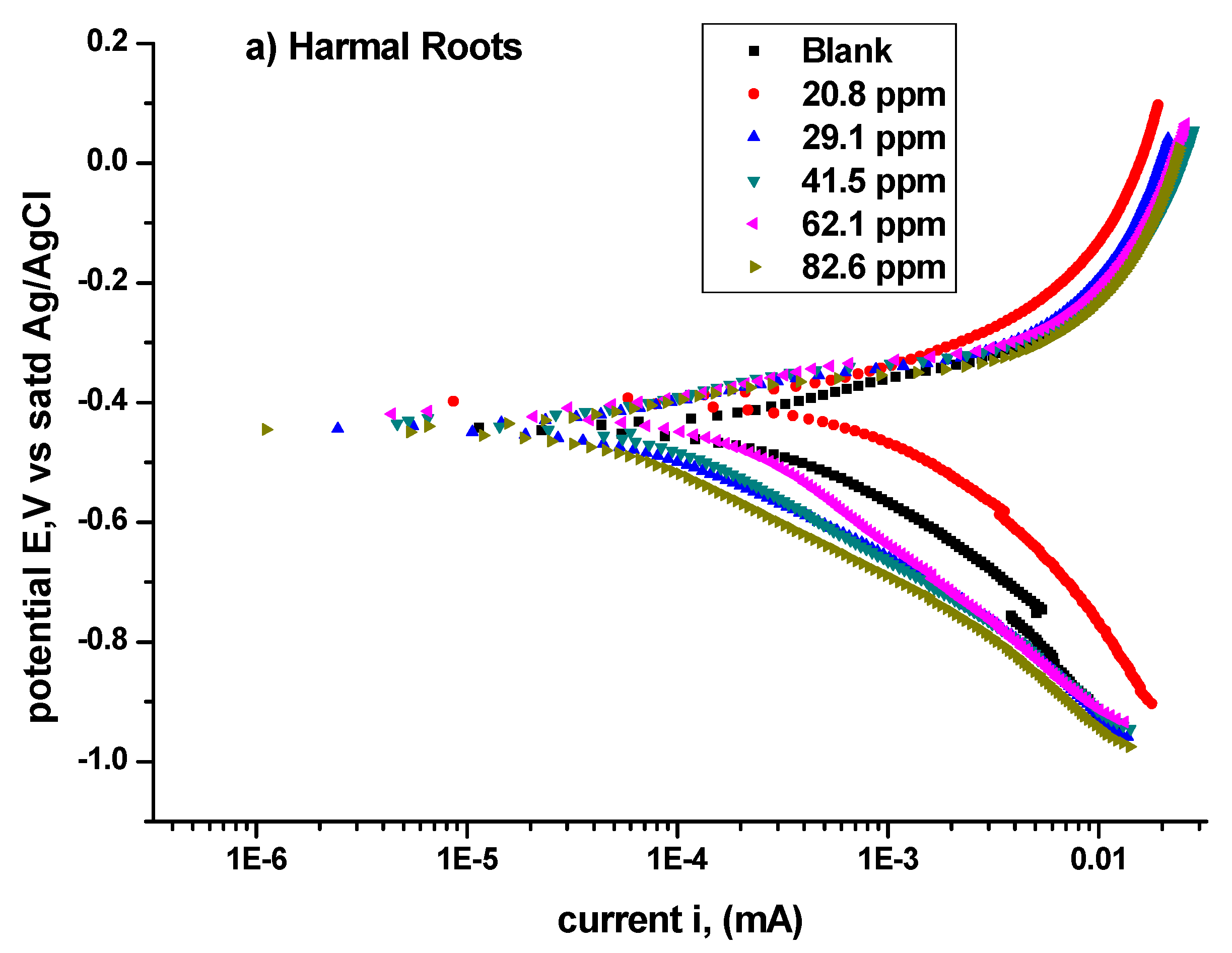
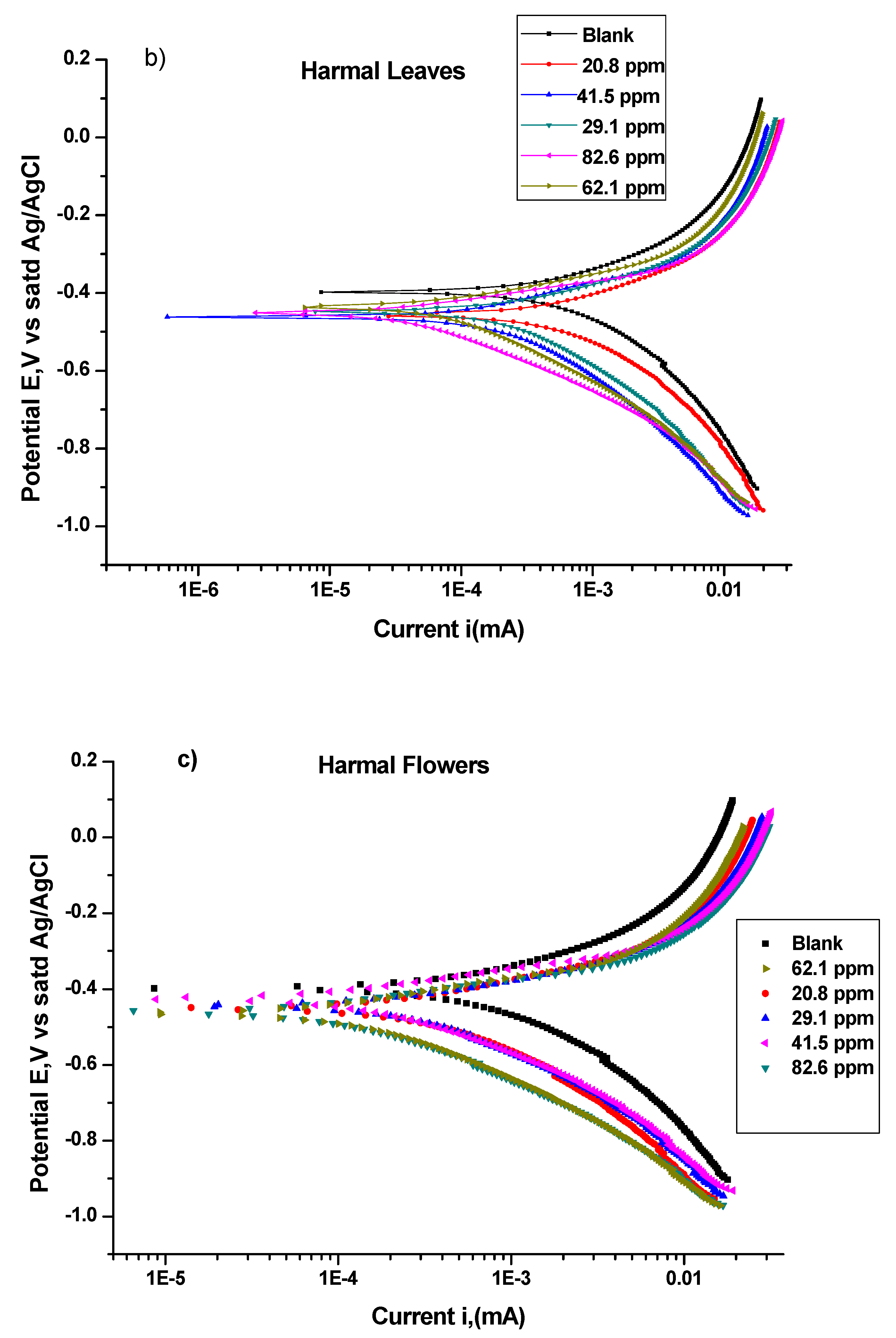
3.1.2. Potentiodynamic Polarization Study (PDP)
3.2. Adsorption Isotherms
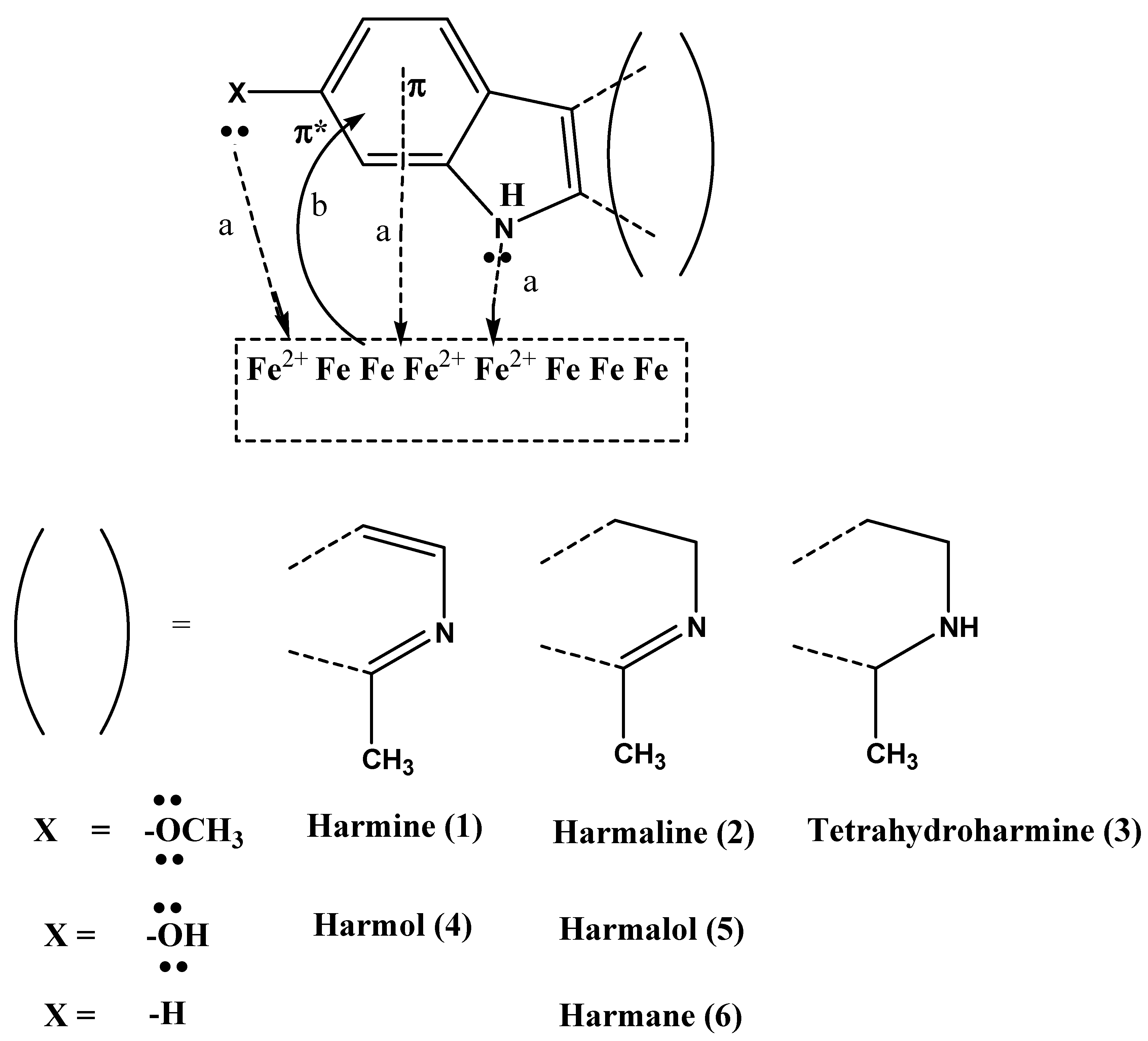
3.3. Inhibition Mechanism
3.4. Comparative Studies of Inhibition Efficiency IE%
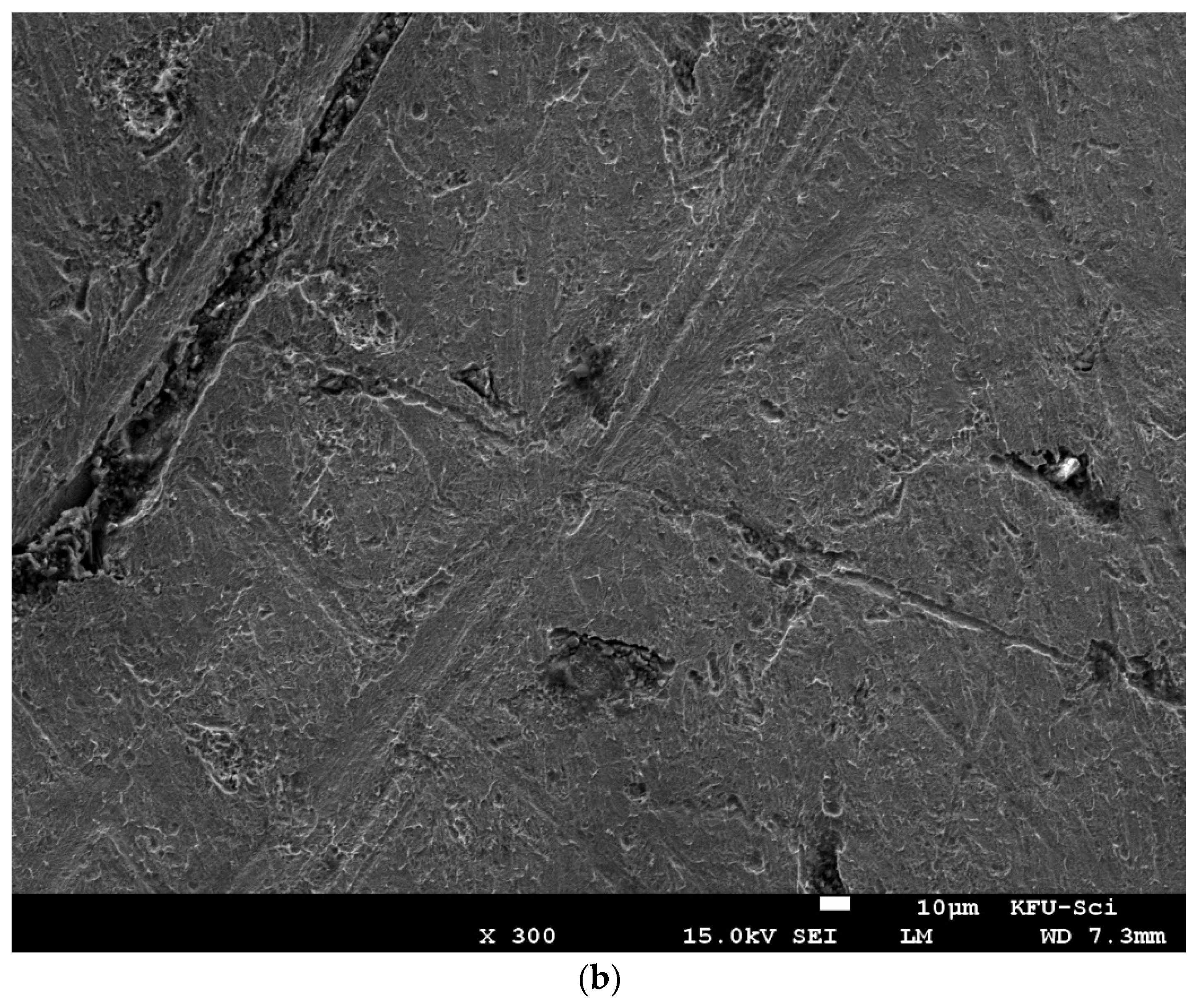
3.5. Surface Morphology
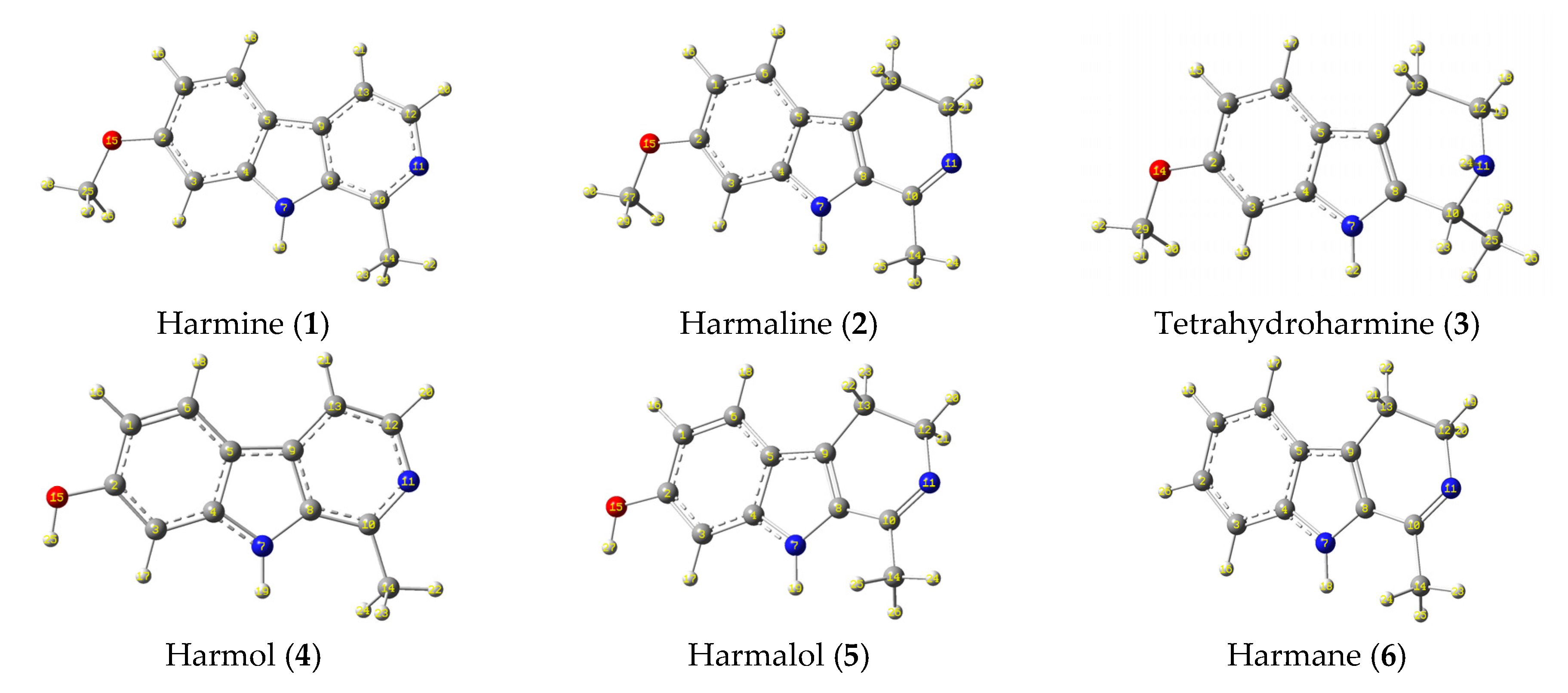
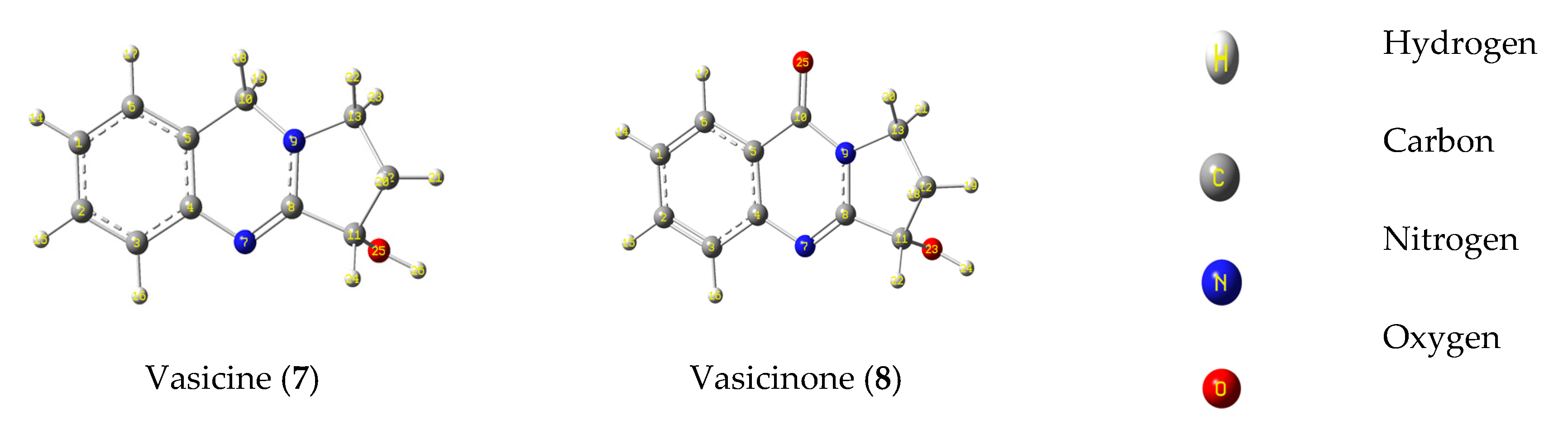
3.6. Computational Studies
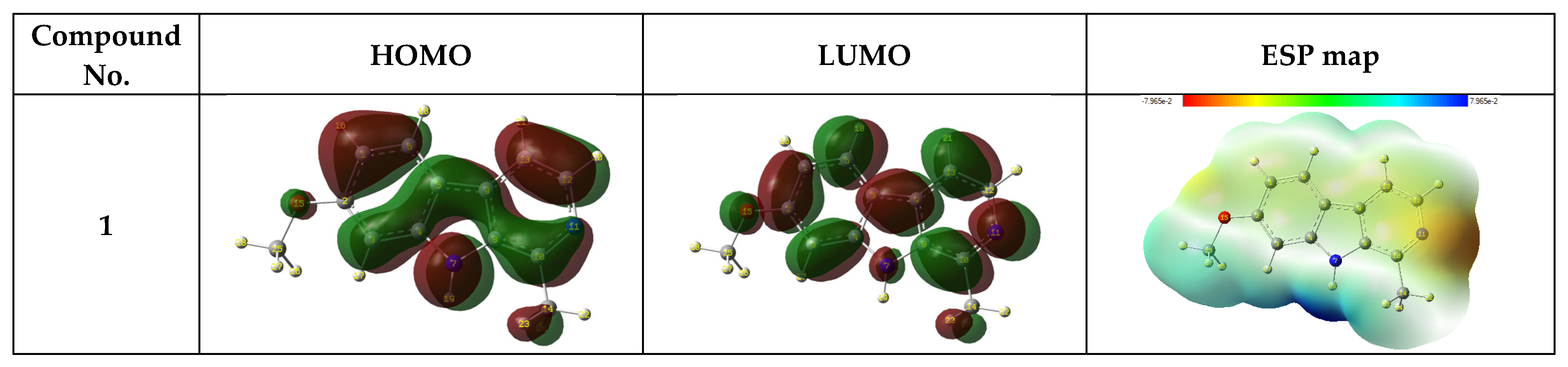
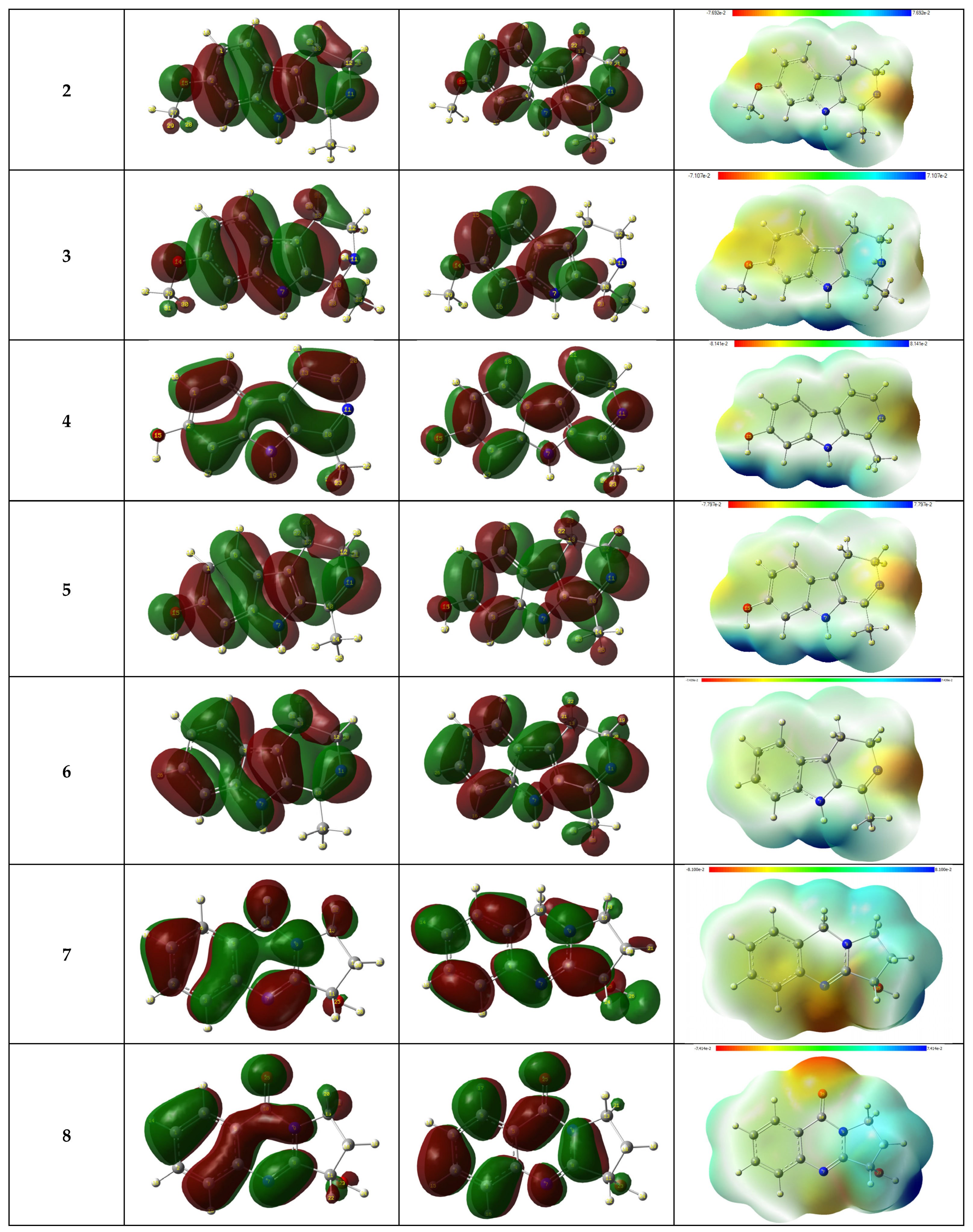
3.6.1. Frontier Molecular Orbital Analysis and Electrostatic Potential Map
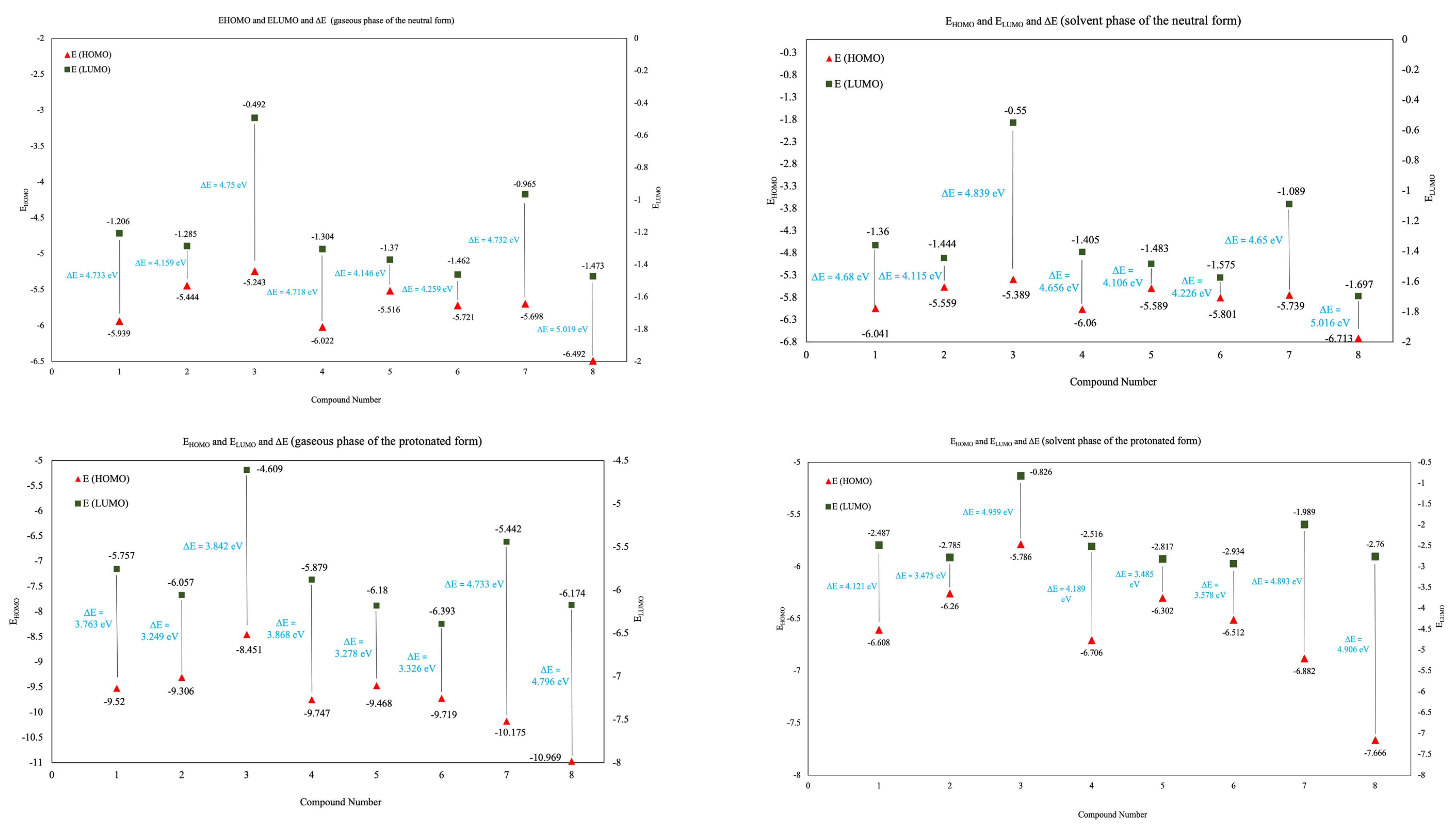
3.6.2. EHOMO and ELUMO and ΔE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zehra, S.; Mobin, M.; Aslam, J. An overview of the corrosion chemistry. In Environmentally Sustainainable Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–23. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China, Npj. Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Cost of corrosion in the United States. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2005; pp. 3–24. [Google Scholar] [CrossRef]
- Alamri, A.H. Localized corrosion and mitigation approach of steel materials used in oil and gas pipelines—An overview. Eng. Fail. Anal. 2020, 116, 104735. [Google Scholar] [CrossRef]
- Panchal, J.; Shah, D.; Patel, R.; Shah, S.; Prajapati, M.; Shah, M. Comprehensive Review and Critical Data Analysis on Corrosion and Emphasizing on Green Eco-friendly Corrosion Inhibitors for Oil and Gas Industries. J. Bio-Tribo-Corros. 2021, 7, 107. [Google Scholar] [CrossRef]
- Kaur, J.; Daksh, N.; Saxena, A. Corrosion Inhibition Applications of Natural and Eco-Friendly Corrosion Inhibitors on Steel in the Acidic Environment: An Overview. Arab. J. Sci. Eng. 2022, 47, 57–74. [Google Scholar] [CrossRef]
- Radha, K.V.; Patel, D.; Kumar, N.; Devasena, T. Investigation of Eco-friendly Corrosion Inhibitor for Low Carbon Steel Using Extract of Physalis Minima Leaves. J. Bio-Tribo-Corros. 2022, 8, 47. [Google Scholar] [CrossRef]
- Zomorodian, A.; Bagonyi, R.; Al-Tabbaa, A. The efficiency of eco-friendly corrosion inhibitors in protecting steel reinforcement. J. Build. Eng. 2021, 38, 102171. [Google Scholar] [CrossRef]
- Verma, C.; Verma, D.K.; Ebenso, E.E.; Quraishi, M.A. Quraishi, Sulfur and phosphorus heteroatom-containing compounds as corrosion inhibitors: An overview. Heteroat. Chem. 2018, 29, e21437. [Google Scholar] [CrossRef]
- Assad, H.; Kumar, A. Understanding functional group effect on corrosion inhibition efficiency of selected organic compounds. J. Mol. Liq. 2021, 344, 117755. [Google Scholar] [CrossRef]
- Chaubey, N.; Qurashi, A.; Chauhan, D.S.; Quraishi, M. Frontiers and advances in green and sustainable inhibitors for corrosion applications: A critical review. J. Mol. Liq. 2021, 321, 114385. [Google Scholar] [CrossRef]
- Serdaroğlu, G.; Kaya, S. Organic and Inorganic Corrosion Inhibitors. In Organic Corrosion Inhibitors; Wiley: Hoboken, NJ, USA, 2021; pp. 59–73. [Google Scholar] [CrossRef]
- Al Hashmi, S.; Al Zadjali, S.; Rajput, N.S.; Mohammedture, M.; Gutierrez, M.; Esawi, A.M.K. Polymers and Graphene-Based Materials as Barrier Coatings. In Handbook of Research on Green Synthesis and Applications of Nanomaterials; IGI Global: Hershey, PA, USA, 2022; pp. 129–151. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Aslam, J. Nanomaterials as corrosion inhibitors. In Inorganic Anticorrosive Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–20. [Google Scholar] [CrossRef]
- Murungi, P.I.; Sulaimon, A.A. Ideal corrosion inhibitors: A review of plant extracts as corrosion inhibitors for metal surfaces. Corros. Rev. 2022, 40, 127–136. [Google Scholar] [CrossRef]
- Fazal, B.R.; Becker, T.; Kinsella, B.; Lepkova, K. A review of plant extracts as green corrosion inhibitors for CO2 corrosion of carbon steel. Npj Mater. Degrad. 2022, 6, 5. [Google Scholar] [CrossRef]
- Ansari, F.A.; Chauhan, D.S.; Quraishi, M.A. Oleochemicals as Corrosion Inhibitors. In Organic Corrosion Inhibitors: Synthesis, Characterization, Mechanism, and Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 343–369. [Google Scholar] [CrossRef]
- Vaidya, N.R.; Aklujkar, P.; Rao, A.R. Modification of natural gums for application as corrosion inhibitor: A review. J. Coatings Technol. Res. 2022, 19, 223–239. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Alfakeer, M.; Altass, H.M.; Althagafi, I.I.; El-Ossaily, Y.A. Performance of unprecedented synthesized biosurfactants as green inhibitors for the corrosion of mild steel-37-2 in neutral solutions: A mechanistic approach. Green Chem. Lett. Rev. 2021, 14, 488–499. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T.; Wang, J.; Qian, H.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: A review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef]
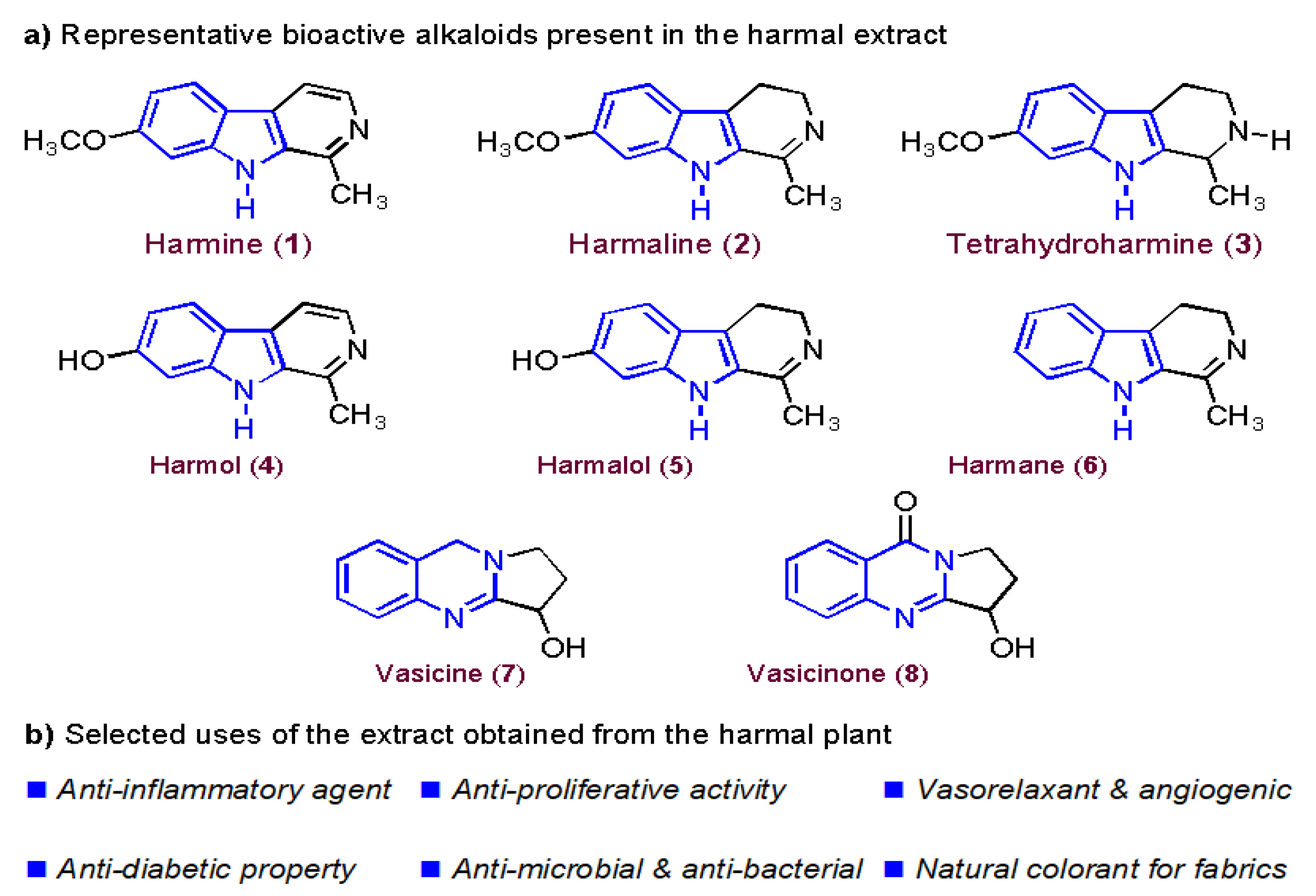
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.H.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, M.C.; Farzaei, M.H.; Amin, G. Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: A review. J. Tradit. Chinese Med. 2015, 35, 104–109. [Google Scholar] [CrossRef]
- Filban, F.; Ravanbakhsh, M.; Poormohammadi, A.; Khaghani, S.; Sadeghi-Nejad, B.; Neisi, A.; Goudarzi, G. Antimicrobial properties of Peganum harmala L. seeds’ smoke in indoors: Applications and prospects. Environ. Monit. Assess. 2022, 194, 17. [Google Scholar] [CrossRef]
- Karasawa, M.M.G.; Mohan, C. Fruits as Prospective Reserves of bioactive Compounds: A Review. Nat. Products Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef]
- Shi, C.-C.; Liao, J.-F.; Chen, C.-F. Comparative Study on the Vasorelaxant Effects of Three Harmala Alkaloids In Vitro. Jpn. J. Pharmacol. 2001, 85, 299–305. [Google Scholar] [CrossRef]
- Berrougui, H.; Cordero, C.M.; Khalil, A.; Hmamouchi, M.; Ettaib, A.; Marhuenda, E.; Herrera, M. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seed’s in isolated rat aorta. Pharmacol. Res. 2006, 54, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Improving health benefits with considering traditional and modern health benefits of Peganum harmala. Clin. Phytosci. 2021, 7, 18. [Google Scholar] [CrossRef]
- Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.; Guillén, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Farouk, L.; Laroubi, A.; Aboufatima, R.; Benharref, A.; Chait, A. Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L.: Possible mechanisms involved. J. Ethnopharmacol. 2008, 115, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zan, Y.-H.; Yang, H.-G.; Yang, M.-Y.; Liu, F.-S.; Li, S.-G.; Peng, X.-H.; Lin, B.; Li, Z.-L.; Li, D.-H.; et al. Anti-tumor alkaloids from Peganum harmala. Phytochemistry 2022, 197, 113107. [Google Scholar] [CrossRef]
- Elkady, A.I.; A Hussein, R.; El-Assouli, S.M. Harmal Extract Induces Apoptosis of HCT116 Human Colon Cancer Cells, Mediated by Inhibition of Nuclear Factor-κB and Activator Protein-1 Signaling Pathways and Induction of Cytoprotective Genes. Asian Pacific J. Cancer Prev. 2016, 17, 1947–1959. [Google Scholar] [CrossRef]
- Khadraoui, N.; Essid, R.; Jallouli, S.; Damergi, B.; Ben Takfa, I.; Abid, G.; Jedidi, I.; Bachali, A.; Ayed, A.; Limam, F.; et al. Antibacterial and antibiofilm activity of Peganum harmala seed extract against multidrug-resistant Pseudomonas aeruginosa pathogenic isolates and molecular mechanism of action. Arch. Microbiol. 2022, 204, 133. [Google Scholar] [CrossRef]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264, 108940. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Zhang, Z.-J.; Chou, G.-X.; Wu, T.; Cheng, X.-M.; Wang, C.-H.; Wang, Z.-T. Acetylcholinesterase inhibitive activity-guided isolation of two new alkaloids from seeds of Peganum nigellastrum Bunge by an in vitro TLC- bioautographic assay. Arch. Pharm. Res. 2009, 32, 1245–1251. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; He, D.-D.; Li, S.-P.; Zhu, Y.-D.; Jiang, B.; Cheng, X.-M.; Wang, Z.-T.; Wang, C.-H. Antitussive, expectorant, and bronchodilating effects of quinazoline alkaloids (±)-vasicine, deoxyvasicine, and (±)-vasicinone from aerial parts of Peganum harmala L. Phytomedicine 2015, 22, 1088–1095. [Google Scholar] [CrossRef]
- Adeel, S.; Rehman, F.-U.; Zuber, M.; Batool, F.; Habib, N.; Hussaan, M.; Amin, N. Environmental friendly application of ultrasonic rays for extraction of natural colorant from Harmal (P. harmala) for dyeing of bio-mordanted silk. J. Eng. Fiber. Fabr. 2021, 16, 155892502110638. [Google Scholar] [CrossRef]
- Kokalj, A. Molecular modeling of organic corrosion inhibitors: Calculations, pitfalls, and conceptualization of molecule–surface bonding. Corros. Sci. 2021, 193, 109650. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Verma, D.K.; Lgaz, H.; Guo, L.; Kaya, S.; Quraishi, M.A. Molecular modelling of compounds used for corrosion inhibition studies: A review. Phys. Chem. Chem. Phys. 2021, 23, 19987–20027. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Dahlous, K.A.; Al Othman, Z.A.; Al-Lohedan, H.A.; El-Mahdy, G.A. sym-Trisubstituted 1,3,5-Triazine Derivatives as Promising Organic Corrosion Inhibitors for Steel in Acidic Solution. Molecules 2016, 21, 436. [Google Scholar] [CrossRef] [PubMed]
- Al Otaibi, N.; Hammud, H.H. Corrosion Inhibition Using Harmal Leaf Extract as an Eco-Friendly Corrosion Inhibitor. Molecules 2021, 26, 7024. [Google Scholar] [CrossRef]
- Ali, A.I.; Mahrous, Y.S. Corrosion inhibition of C-steel in acidic media from fruiting bodies of Melia azedarach L. extract and a synergistic Ni 2+ additive. RSC Adv. 2017, 7, 23687–23698. [Google Scholar] [CrossRef]
- Parr, R.G.; Weitao, Y. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2016; Volume 16. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci. 2010, 52, 657–660. [Google Scholar] [CrossRef]
- Mennucci, B.; Cancès, E.; Tomasi, J. Evaluation of Solvent Effects in Isotropic and Anisotropic Dielectrics and in Ionic Solutions with a Unified Integral Equation Method: Theoretical Bases, Computational Implementation, and Numerical Applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Faiz, M.; Zahari, A.; Awang, K.; Hussin, H. Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv. 2020, 10, 6547–6562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coatings 2004, 49, 293–301. [Google Scholar] [CrossRef]
- Meng, F.; Liu, L.; Tian, W.; Wu, H.; Li, Y.; Zhang, T.; Wang, F. The influence of the chemically bonded interface between fillers and binder on the failure behaviour of an epoxy coating under marine alternating hydrostatic pressure. Corros. Sci. 2015, 101, 139–154. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta. 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2015, 90, 107–117. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Adindu, C.B.; Enenebeaku, C.K.; Ogukwe, C.E.; Chidiebere, M.A.; Oguzie, K.L. Natural Products for Materials Protection: Mechanism of Corrosion Inhibition of Mild Steel by Acid Extracts of Piper guineense. J. Phys. Chem. C. 2012, 116, 13603–13615. [Google Scholar] [CrossRef]
- Mohammed, N.; Othman, N.; Taib, M.; Samat, M.; Yahya, S. Experimental and Theoretical Studies on Extract of Date Palm Seed as a Green Anti-Corrosion Agent in Hydrochloric Acid Solution. Molecules 2021, 26, 3535. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Shin, I.; Byeon, J.-W. Corrosion Inhibition of Mild Steel and 304 Stainless Steel in 1 M Hydrochloric Acid Solution by Tea Tree Extract and Its Main Constituents. Materials 2021, 14, 5016. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. A green Brassica oleracea L. extract as a novel corrosion inhibitor for Q235 steel in two typical acid media Colloids and Surfaces A. Physicochem. Eng. Asp. 2021, 616, 126077. [Google Scholar] [CrossRef]
- Marahatta, A.; Marahatta, A.B. A DFT Analysis for the Electronic Structure, Mulliken Charges Distribution and Frontier Molecular Orbitals of Monolayer Graphene Sheet. Int. J. Progress. Sci. Technol. 2019, 16, 51–65. [Google Scholar]
- Efil, K.; Obot, I.B. Quantum Chemical Investigation of the Relationship Between Molecular Structure and Corrosion Inhibition Efficiency of Benzotriazole and its Alkyl-Derivatives on Iron. Prot. Met. Phys. Chem. Surfaces 2017, 53, 1139–1149. [Google Scholar] [CrossRef]
- Harismah, K.; Dhumad, A.M.; Ibraheem, H.S.; Zandi, H.; Majeed, H.J. A DFT approach on tioguanine: Exploring tio-tiol tautomers, frontier molecular orbitals, IR and UV spectra, and quadrupole coupling constants. J. Mol. Liq. 2021, 334, 116018. [Google Scholar] [CrossRef]
- Benhiba, F.; Hsissou, R.; Abderrahim, K.; Serrar, H.; Rouifi, Z.; Boukhris, S.; Kaichouh, G.; Bellaouchou, A.; Guenbour, A.; Oudda, H.; et al. Development of New Pyrimidine Derivative Inhibitor for Mild Steel Corrosion in Acid Medium. J. Bio- Tribo-Corros. 2022, 8, 36. [Google Scholar] [CrossRef]
- Nazir, U.; Akhter, Z.; Ali, N.Z.; Hussain, R.; Liaqat, F.; Tahir, A.; Qamar, S. Corrosion inhibition studies of ferrocenyl Schiff bases in a mild acidic medium through experimental methods and DFT calculations. New J. Chem. 2022, 46, 3925–3938. [Google Scholar] [CrossRef]
- Oyeneyin, O.E.; Ojo, N.D.; Ipinloju, N.; James, A.C.; Agbaffa, E.B. Investigation of Corrosion Inhibition Potentials of Some Aminopyridine Schiff Bases Using Density Functional Theory and Monte Carlo Simulation. Chem. Africa 2022, 5, 319–332. [Google Scholar] [CrossRef]
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. WIREs Comput. Mol. Sci. 2022, 12, e1601. [Google Scholar] [CrossRef]
- El-Hendawy, M.M.; Kamel, A.M.; Mohamed, M.M.A. The anti-corrosive behavior of benzo-fused N-heterocycles: An in silico study towards developing organic corrosion inhibitors. Phy. Chem. Chem. Phy. 2022, 24, 743. [Google Scholar] [CrossRef]
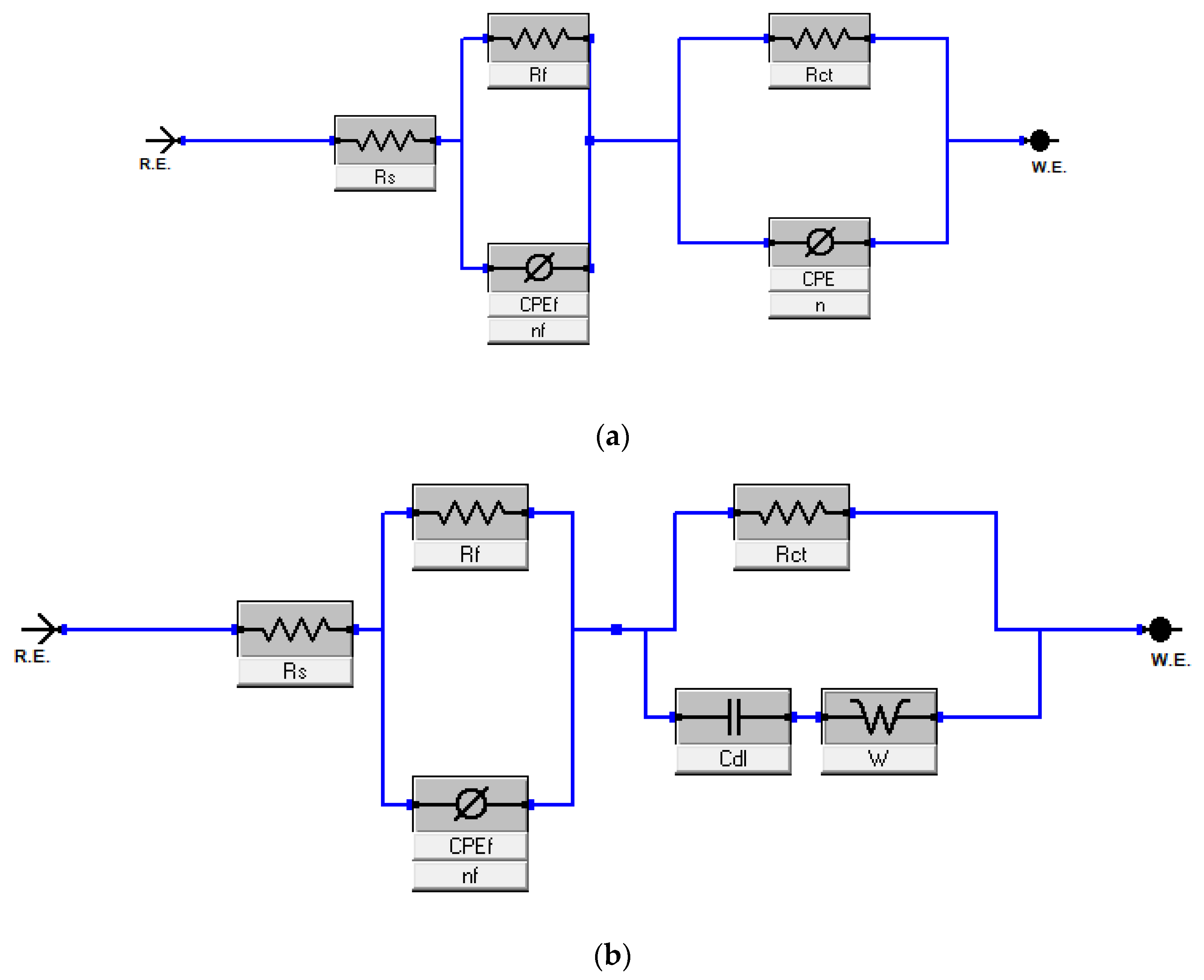


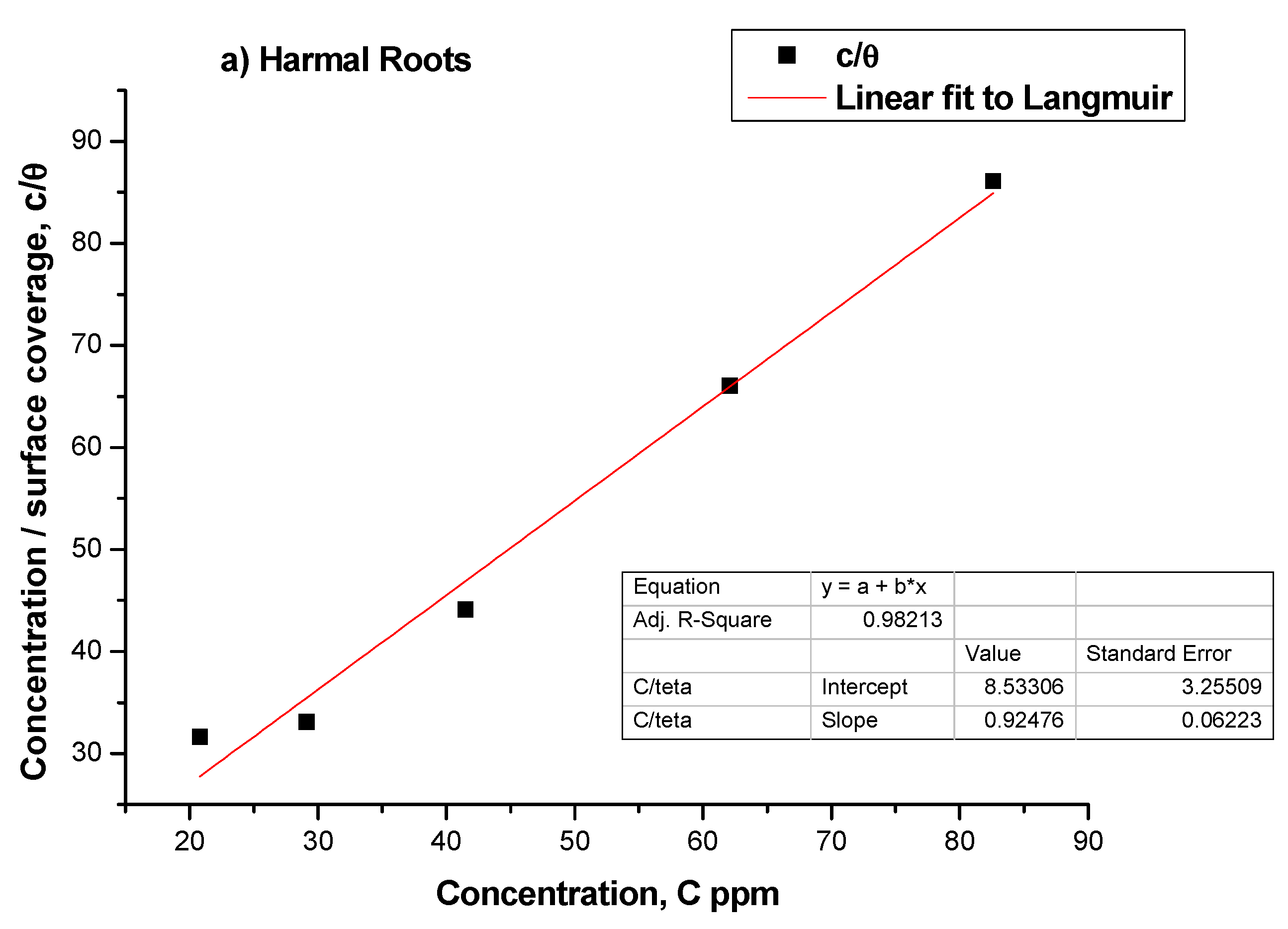
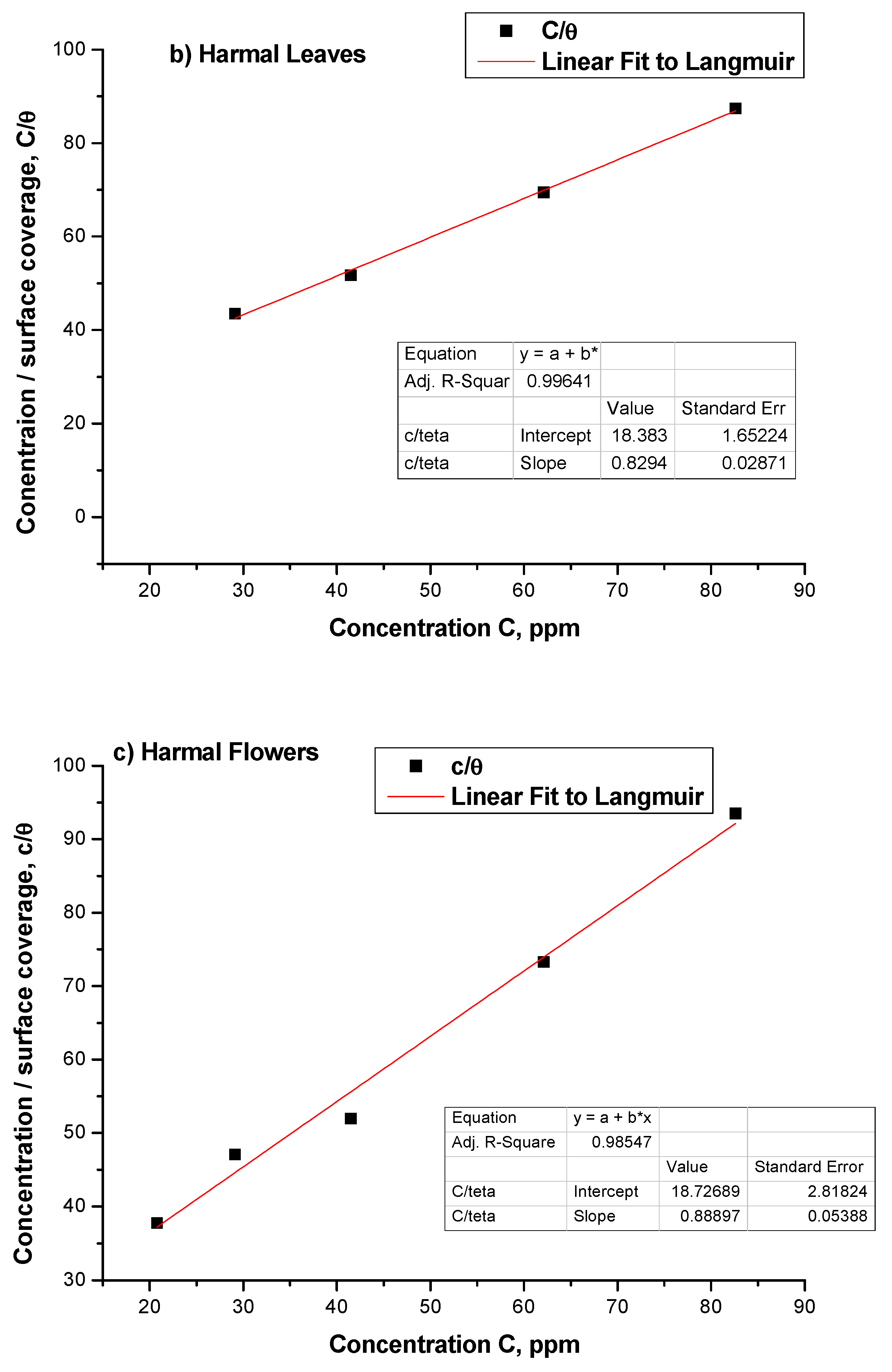



| Harmal Roots | Rs | Rf | nf | ZCPEf | Rct | n | ZCPE | Cdl | Rf + Rct | θ | IE% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Ω cm2 | Ω cm2 | µΩ−1s n cm−2 | Ω cm2 | µΩ−1s n cm−2 | μF/cm2 | Ω cm2 | ||||
| 0 ppm | 6.63 | 9.23 | 0.659 | 2936.14 | 9.94 | 0.976 | 4.30 × 102 | 375.66 | 19.17 | 0.000 | 0.00 |
| 20.8 ppm | 6.68 | 64.25 | 0.924 | 131.37 | 15.53 | 0.614 | 8.76 × 102 | 58.62 | 79.78 | 0.760 | 75.97 |
| 29.1 ppm | 5.27 | 55.85 | 0.923 | 171.53 | 71.03 | 0.738 | 1.87 × 102 | 40.25 | 126.88 | 0.849 | 84.89 |
| 41.5 ppm | 4.94 | 85.11 | 0.937 | 131.41 | 123.01 | 0.761 | 1.37 × 102 | 37.78 | 208.12 | 0.908 | 90.79 |
| 62.1 ppm | 5.87 | 222.14 | 0.878 | 67.83 | 17.37 | 0.589 | 1.65 × 103 | 138.10 | 239.52 | 0.920 | 92.00 |
| 82.6 ppm | 7.37 | 22.48 | 0.800 | 62.44 | 319.37 | 0.840 | 4.42 × 101 | 19.29 | 341.84 | 0.944 | 94.39 |
| Harmal Roots | Rs | Rf | nf | ZCPEf | Rct | Cdl | Rf + Rct | θ | IE% | W |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 2 | Ω cm2 | Ω cm2 | µΩ−1s n cm−2 | Ω cm2 | μF/cm2 | Ω cm2 | Ss1/2 | |||
| 0 ppm | 6.58 | 15.11 | 0.902 | 430.08 | 4.12 | 30475.43 | 19.23 | 0.000 | 0.00 | 0.00873 |
| 20.8 ppm | 6.42 | 28.59 | 1.000 | 305.35 | 50.92 | 91.25 | 79.44 | 0.758 | 75.79 | 0.00127 |
| 29.1 ppm | 5.17 | 118.79 | 0.824 | 105.79 | 8.46 | 82.38 | 127.25 | 0.849 | 84.89 | 0.00296 |
| 41.5 ppm | 4.80 | 119.49 | 0.815 | 95.86 | 87.22 | 128.09 | 206.71 | 0.907 | 90.70 | 0.00325 |
| 62.1 ppm | 5.73 | 210.78 | 0.900 | 70.06 | 27.425 | 99.36 | 238.20 | 0.919 | 91.93 | 0.00178 |
| 82.6 ppm | 4.91 | 131.96 | 0.88 | 44.86 | 176.15 | 57.11 | 308.10 | 0.938 | 93.76 | 0.00254 |
| Harmal Leaves | Rs | Rf | nf | ZCPEf | Rct | n | ZCPE | Cdl | Rf + Rct | θ | IE% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Ω cm2 | Ω cm2 | µΩ−1s n cm−2 | Ω cm2 | µΩ−1s n cm−2 | μF/cm2 | Ω cm2 | ||||
| 0 ppm | 6.6 | 9.23 | 0.6588 | 2936.14 | 9.94 | 0.976 | 4.30 × 102 | 375.66 | 19.17 | 0.000 | 0.00 |
| 20.8 ppm | 6.2 | 22.64 | 0.8894 | 265.76 | 2.45 | 0.613 | 3.18 × 103 | 147.48 | 25.10 | 0.236 | 23.61 |
| 29.1 ppm | 6.0 | 66.26 | 0.8968 | 123.87 | 7.48 | 0.632 | 1.55 × 103 | 115.54 | 73.73 | 0.740 | 74.00 |
| 41.5 ppm | 5.9 | 65.65 | 0.6606 | 99.52 | 147.84 | 0.698 | 1.87 × 102 | 39.54 | 213.50 | 0.910 | 91.02 |
| 62.1 ppm | 5.5 | 164.28 | 0.9515 | 53.01 | 17.23 | 0.666 | 8.83 × 102 | 107.89 | 181.51 | 0.894 | 89.44 |
| 82.6 ppm | 4.5 | 333.09 | 0.8875 | 47.05 | 0.90 | 0.265 | 5.49 × 104 | 12.80 | 333.99 | 0.943 | 94.26 |
| Harmal Leaves | Rs | Rf | nf | ZCPEf | Rct | Cdl | Rf + Rct | θ | IE% | W |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 2 | Ω cm2 | Ω cm2 | µΩ−1s n cm−2 | Ω cm2 | μF/cm2 | Ω cm2 | Ss1/2 | |||
| 0 ppm | 6.6 | 15.11 | 0.9024 | 430.08 | 4.12 | 30,475.43 | 19.23 | 0.000 | 0.00 | 8.73 × 10−3 |
| 20.8 ppm | 6.1 | 2.10 | 1.0000 | 559.18 | 22.92 | 185.20 | 25.01 | 0.231 | 23.11 | 3.95 × 10−3 |
| 29.1 ppm | 5.8 | 41.16 | 0.9701 | 196.00 | 32.13 | 84.76 | 73.29 | 0.738 | 73.76 | 2.27 × 10−3 |
| 41.5 ppm | 6.3 | 45.08 | 0.9751 | 151.38 | 39.64 | 43.86 | 84.72 | 0.773 | 77.30 | 1.10 × 10−3 |
| 62.1 ppm | 5.3 | 132.16 | 1.0000 | 63.88 | 48.51 | 62.88 | 180.67 | 0.894 | 89.36 | 2.06 × 10−3 |
| 82.6 ppm | 4.7 | 143.77 | 0.8924 | 68.09 | 186.15 | 71.61 | 329.92 | 0.942 | 94.17 | 5.10 × 10−3 |
| Harmal Flowers | Rs | Rf | nf | ZCPEf | Rct | n | ZCPE | Cdl | Rf + Rct | θ | IE% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Ω cm2 | Ω cm2 | µΩ−1s n cm−2 | Ω cm2 | µΩ−1s n cm−2 | μF/cm2 | Ω cm2 | ||||
| 0 ppm | 6.63 | 9.23 | 0.659 | 2936.14 | 9.94 | 0.976 | 4.30 × 102 | 375.66 | 19.17 | 0.000 | −0.01 |
| 20.8 ppm | 6.38 | 32.73 | 0.937 | 238.71 | 20.29 | 0.734 | 5.72 × 102 | 114.15 | 53.02 | 0.638 | 63.85 |
| 29.1 ppm | 6.29 | 55.65 | 0.895 | 154.82 | 2.99 | 0.538 | 4.73 × 103 | 121.90 | 58.64 | 0.673 | 67.31 |
| 41.5 ppm | 4.59 | 102.30 | 0.876 | 140.74 | 4.04 | 0.542 | 4.56 × 103 | 155.72 | 106.34 | 0.820 | 81.97 |
| 62.1 ppm | 6.09 | 126.38 | 0.832 | 86.06 | 2.50 | 0.586 | 2.05 × 103 | 49.32 | 128.88 | 0.851 | 85.13 |
| 82.6 ppm | 4.90 | 3.89 | 0.540 | 4887.61 | 166.59 | 0.87 | 6.94 × 101 | 1.56 | 170.49 | 0.888 | 88.76 |
| Harmal Flowers | Rs | Rf | nf | ZCPEf | Rct | Cdl | Rf + Rct | θ | IE% | W |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 2 | Ω cm2 | Ω cm2 | µΩ−1s n cm−2 | Ω cm2 | μF/cm2 | Ω cm2 | Ss1/2 | |||
| 0 ppm | 6.58 | 15.11 | 0.902 | 430.08 | 4.12 | 30,475.43 | 19.23 | 0.000 | 0.00 | 8.73 × 10−3 |
| 20.8 ppm | 6.23 | 39.07 | 0.924 | 229.16 | 13.83 | 127.97 | 52.89 | 0.636 | 63.64 | 3.61 × 10−3 |
| 29.1 ppm | 6.25 | 15.20 | 0.954 | 277.10 | 43.04 | 163.82 | 58.23 | 0.670 | 66.98 | 3.01 × 10−3 |
| 41.5 ppm | 4.54 | 43.97 | 0.884 | 239.31 | 61.53 | 190.31 | 105.50 | 0.818 | 81.77 | 2.98 × 10−3 |
| 62.1 ppm | 5.91 | 84.91 | 0.918 | 115.56 | 42.77 | 49.33 | 127.68 | 0.849 | 84.94 | 1.49 × 10−3 |
| 82.6 ppm | 4.82 | 75.36 | 0.87 | 99.82 | 93.55 | 97.23 | 168.91 | 0.886 | 88.62 | 2.77 × 10−3 |
| Harmal Extract | |||||||
|---|---|---|---|---|---|---|---|
| 0 ppm | 20.8 ppm | 29.1 ppm | 41.5 ppm | 62.1 ppm | 82.6 ppm | ||
| Harmal Roots | Phase angle ° | −27.87 | −41.30 | −49.06 | −56.00 | −57.16 | −62.52 |
| Frequency Hz | 79.00 | 125.60 | 252.40 | 252.40 | 158.40 | 315.50 | |
| Slope α | −0.3635 | −0.4804 | −0.5491 | −0.6417 | −0.6619 | −0.7190 | |
| R2 | 0.9646 | 0.9955 | 0.9973 | 0.9982 | 0.9999 | 0.9968 | |
| Harmal Flowers | Phase angle ° | −27.87 | −40.23 | −44.08 | −53.23 | −50.23 | −57.58 |
| Frequency Hz | 75.00 | 125.60 | 125.60 | 125.60 | 252.40 | 198.60 | |
| Slope α | −0.3635 | −0.4945 | −0.5492 | −0.6324 | −0.5995 | −0.6571 | |
| R2 | 0.9492 | 0.9875 | 0.9776 | 0.9886 | 0.9652 | 0.9964 | |
| Harmal Leaves | Phase angle ° | −27.87 | −31.78 | −45.74 | −41.88 | −59.68 | −66.28 |
| Frequency Hz | 79.00 | 125.60 | 125.60 | 252.40 | 158.40 | 198.60 | |
| Slope α | −0.3635 | −0.3995 | −0.5557 | −0.4851 | −0.7075 | −0.7671 | |
| R2 | 0.9892 | 0.9958 | 0.9969 | 0.9930 | 0.9982 | 0.9984 | |
| Harmal Roots | Icorr μA | Ecorr mV | βa V/Decade | βc V/Decade | Corrosion Rate mpy | χ2 | Icorr μA/cm2 | θ | IE% |
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm | 1710.0 | −397.0 | 0.4128 | 0.4970 | 1554.00 | 6.532 | 3401.63 | 0.000 | 0.0 |
| 20.8 ppm | 585.0 | −444.0 | 0.2681 | 0.3553 | 532.00 | 21.51 | 1163.72 | 0.658 | 65.8 |
| 29.1 ppm | 206.6 | −417.0 | 0.2066 | 0.3011 | 187.40 | 33.64 | 410.98 | 0.879 | 87.9 |
| 41.5 ppm | 102.2 | −432.0 | 0.1808 | 0.2369 | 92.47 | 40.54 | 203.30 | 0.940 | 94.0 |
| 62.1 ppm | 102.0 | −443.0 | 0.1886 | 0.2333 | 92.73 | 38.78 | 202.90 | 0.940 | 94.0 |
| 82.6 ppm | 69.7 | −446.0 | 0.1687 | 0.2244 | 63.34 | 43.41 | 138.65 | 0.959 | 95.9 |
| Harmal Leaves | Icorr μA | Ecorr mV | βa V/Decade | βc V/Decade | Corrosion Rate mpy | χ2 | Icorr μA/cm2 | θ | IE% |
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm | 1710.0 | −397.0 | 0.4128 | 0.4970 | 1554.0 | 6.532 | 3401.63 | 0.000 | 0.0 |
| 20.8 ppm | 1550.0 | −457.0 | 0.3539 | 0.4447 | 1412.0 | 11.01 | 3083.35 | 0.094 | 9.4 |
| 29.1 ppm | 567.0 | −449.0 | 0.2669 | 0.3593 | 515.7 | 22.63 | 1127.91 | 0.668 | 66.8 |
| 41.5 ppm | 338.0 | −462.0 | 0.2423 | 0.3060 | 307.2 | 30.61 | 672.37 | 0.802 | 80.2 |
| 62.1 ppm | 180.0 | −436.0 | 0.2186 | 0.2565 | 163.7 | 38.45 | 358.07 | 0.895 | 89.5 |
| 82.6 ppm | 94.7 | −452.0 | 0.1823 | 0.2136 | 86.1 | 51.29 | 188.38 | 0.945 | 94.5 |
| Harmal Flowers | Icorr μA | Ecorr mV | βa V/Decade | βc V/Decade | Corrosion Rate mpy | χ2 | Icorr μA/cm2 | θ | IE% |
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm | 1710.0 | −397.0 | 0.4128 | 0.4970 | 1554.00 | 6.532 | 3401.63 | 0.000 | 0.0 |
| 20.8 ppm | 767.0 | −451.0 | 0.2897 | 0.3984 | 696.90 | 16.57 | 1525.76 | 0.551 | 55.1 |
| 29.1 ppm | 653.0 | −444.0 | 0.2684 | 0.3540 | 593.80 | 23.17 | 1298.99 | 0.618 | 61.8 |
| 41.5 ppm | 344.0 | −425.0 | 0.2239 | 0.2850 | 313.00 | 33.80 | 684.30 | 0.799 | 79.9 |
| 62.1 ppm | 261.0 | −464.0 | 0.2281 | 0.2783 | 237.10 | 28.18 | 519.20 | 0.847 | 84.7 |
| 82.6 ppm | 199.0 | −460.0 | 0.2007 | 0.2584 | 180.70 | 37.51 | 395.86 | 0.884 | 88.4 |
| Harmal (ppm) This Work | HRE IE% EIS 0.25 M H2SO4 C-Steel | HLE IE% EIS 0.25 M H2SO4 C-Steel | HFE IE% EIS 0.25 M H2SO4 C-Steel | Date Palm Seed (ppm) | IE% WLS 0.5 M HCl C-Steel | Tea Tree (ppm) | IE% PDP 1 M HCl Mild Steel | Ginkgo Leaf Extract (ppm) | IE% PDP 1 M HCl X-70 Steel | Brassica Oleracea L (ppm) | IE% PDP 0.50 M H2SO4 Q235 Steel |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20.8 | 65.8 | 9.4 | 55.1 | 800 | 71 | 150 | 56.5 | 25 | 62.0 | 50 | 81.2 |
| 29.1 | 87.9 | 66.8 | 61.8 | 900 | 82 | 300 | 64.0 | 50 | 70.8 | 100 | 86.0 |
| 41.5 | 94.0 | 80.2 | 79.9 | 1200 | 88 | 750 | 75.6 | 100 | 86.3 | 200 | 90.0 |
| 62.1 | 94.0 | 89.5 | 84.7 | 1400 | 95 | 2250 | 78.6 | 200 | 90.0 | 300 | 92.7 |
| 82.6 | 95.9 | 94.5 | 88.4 | 2000 | 91 | ||||||
| This work | [58] | [59] | [60] | [61] | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammud, H.H.; Maache, S.A.; Al Otaibi, N.; Sheikh, N.S. An Integrated Experimental and Theoretical Studies on the Corrosion Inhibition of Carbon Steel by Harmal Extracts. Molecules 2022, 27, 7250. https://doi.org/10.3390/molecules27217250
Hammud HH, Maache SA, Al Otaibi N, Sheikh NS. An Integrated Experimental and Theoretical Studies on the Corrosion Inhibition of Carbon Steel by Harmal Extracts. Molecules. 2022; 27(21):7250. https://doi.org/10.3390/molecules27217250
Chicago/Turabian StyleHammud, Hassan H., Sarah A. Maache, Nasreen Al Otaibi, and Nadeem S. Sheikh. 2022. "An Integrated Experimental and Theoretical Studies on the Corrosion Inhibition of Carbon Steel by Harmal Extracts" Molecules 27, no. 21: 7250. https://doi.org/10.3390/molecules27217250
APA StyleHammud, H. H., Maache, S. A., Al Otaibi, N., & Sheikh, N. S. (2022). An Integrated Experimental and Theoretical Studies on the Corrosion Inhibition of Carbon Steel by Harmal Extracts. Molecules, 27(21), 7250. https://doi.org/10.3390/molecules27217250








