Challenges in the Heterologous Production of Furanocoumarins in Escherichia coli
Abstract
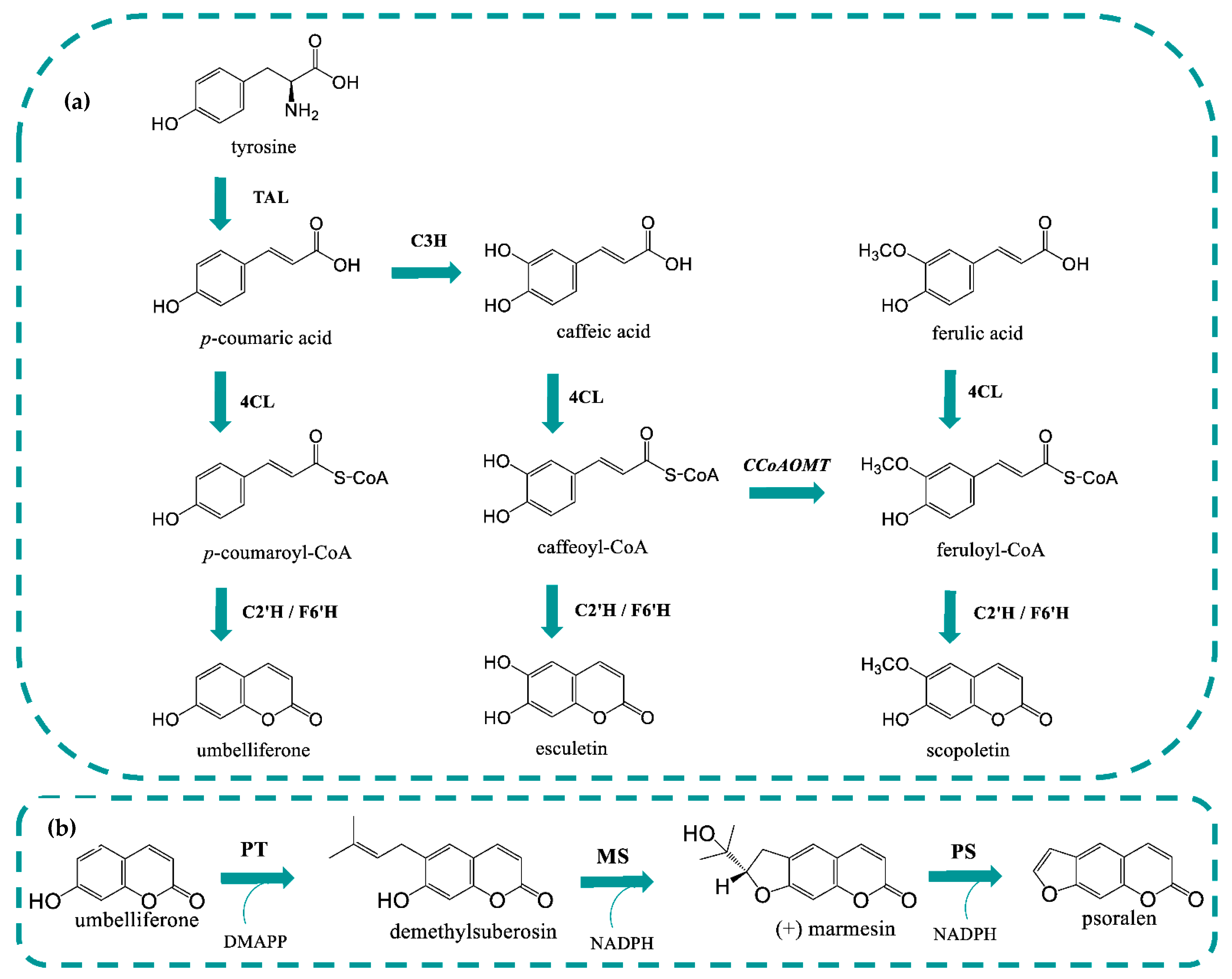
1. Introduction
2. Results and Discussion
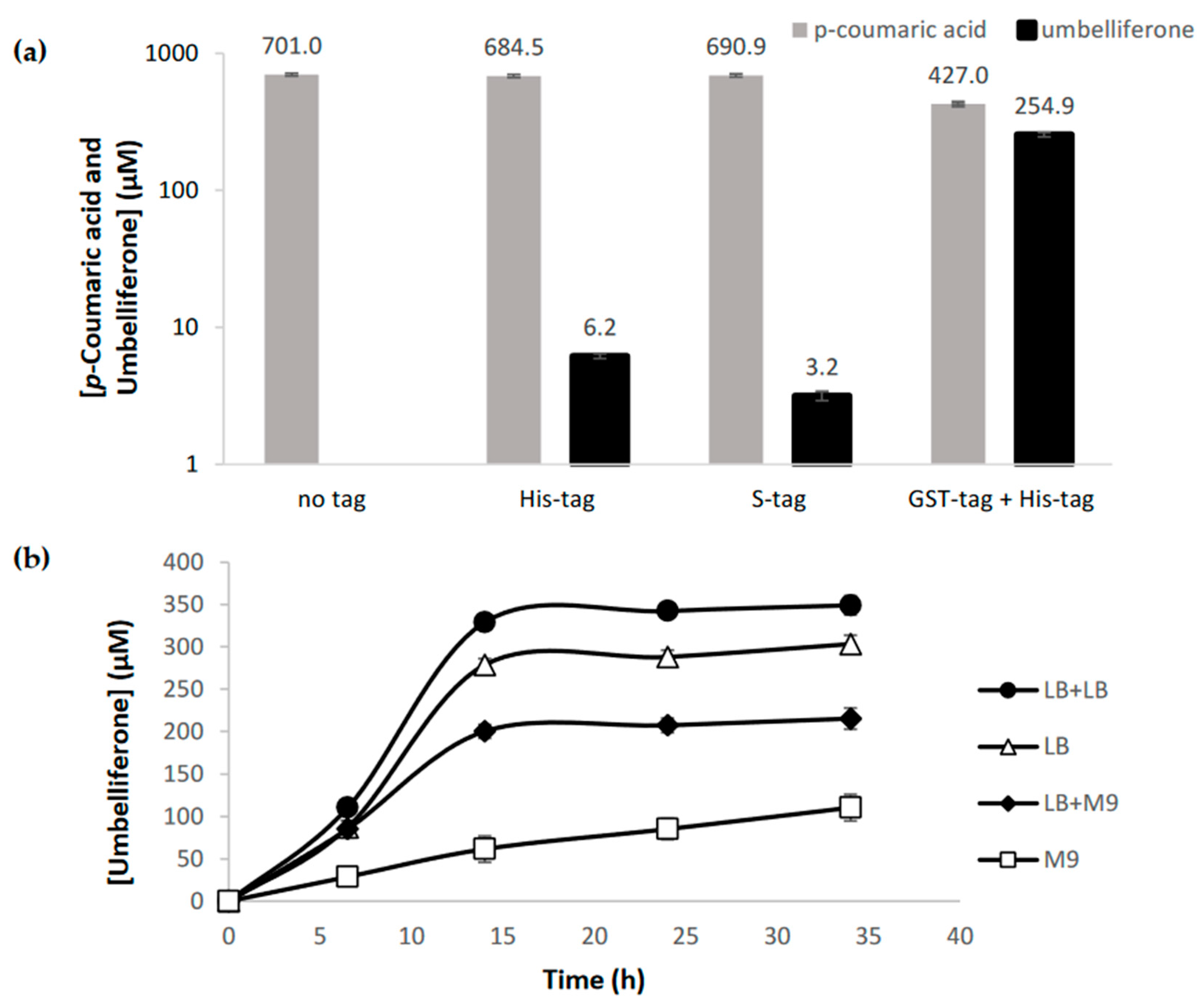
2.1. Heterologous Production of Umbelliferone using C2′H
2.2. C2′H substrate Specificity
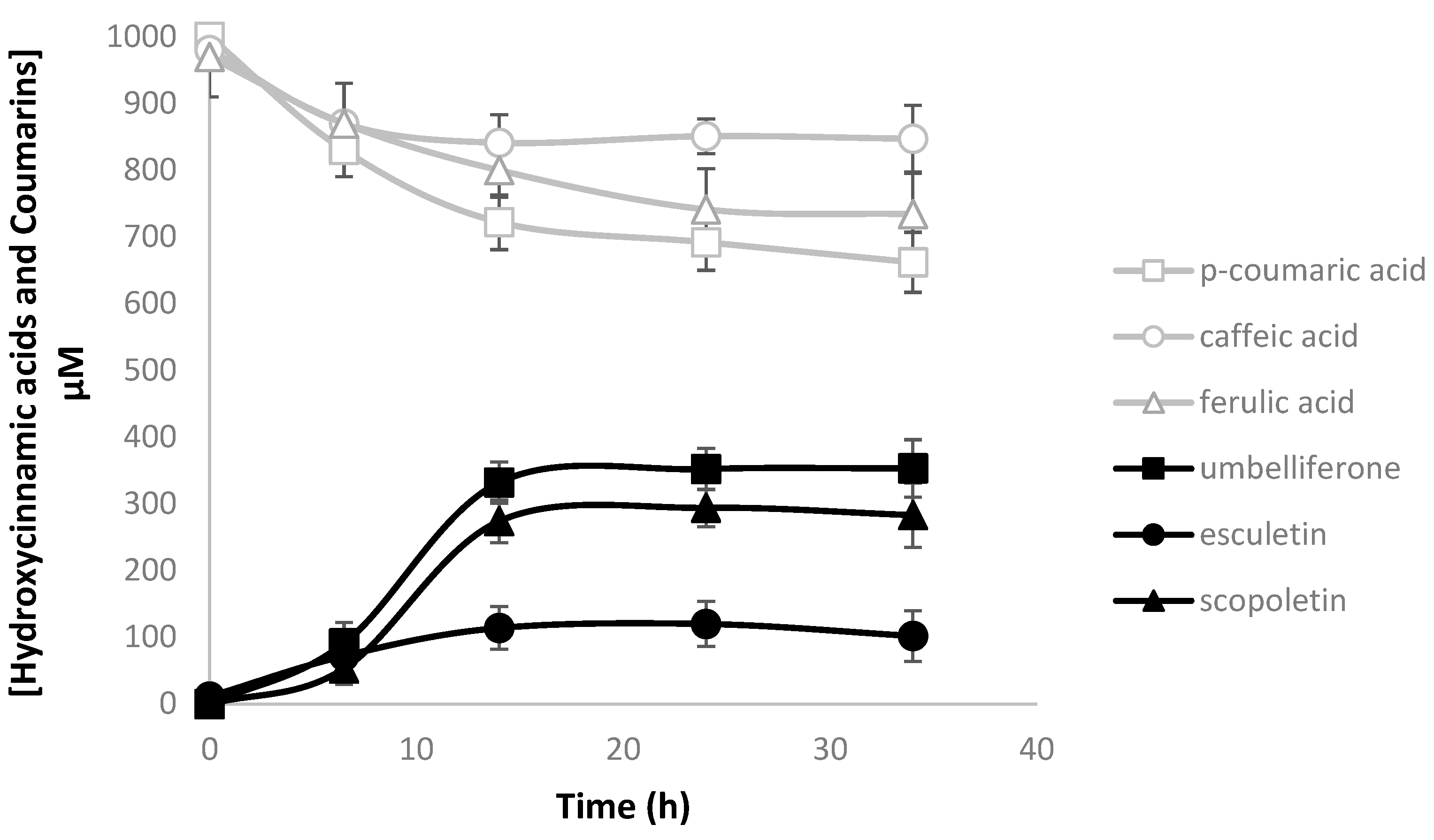
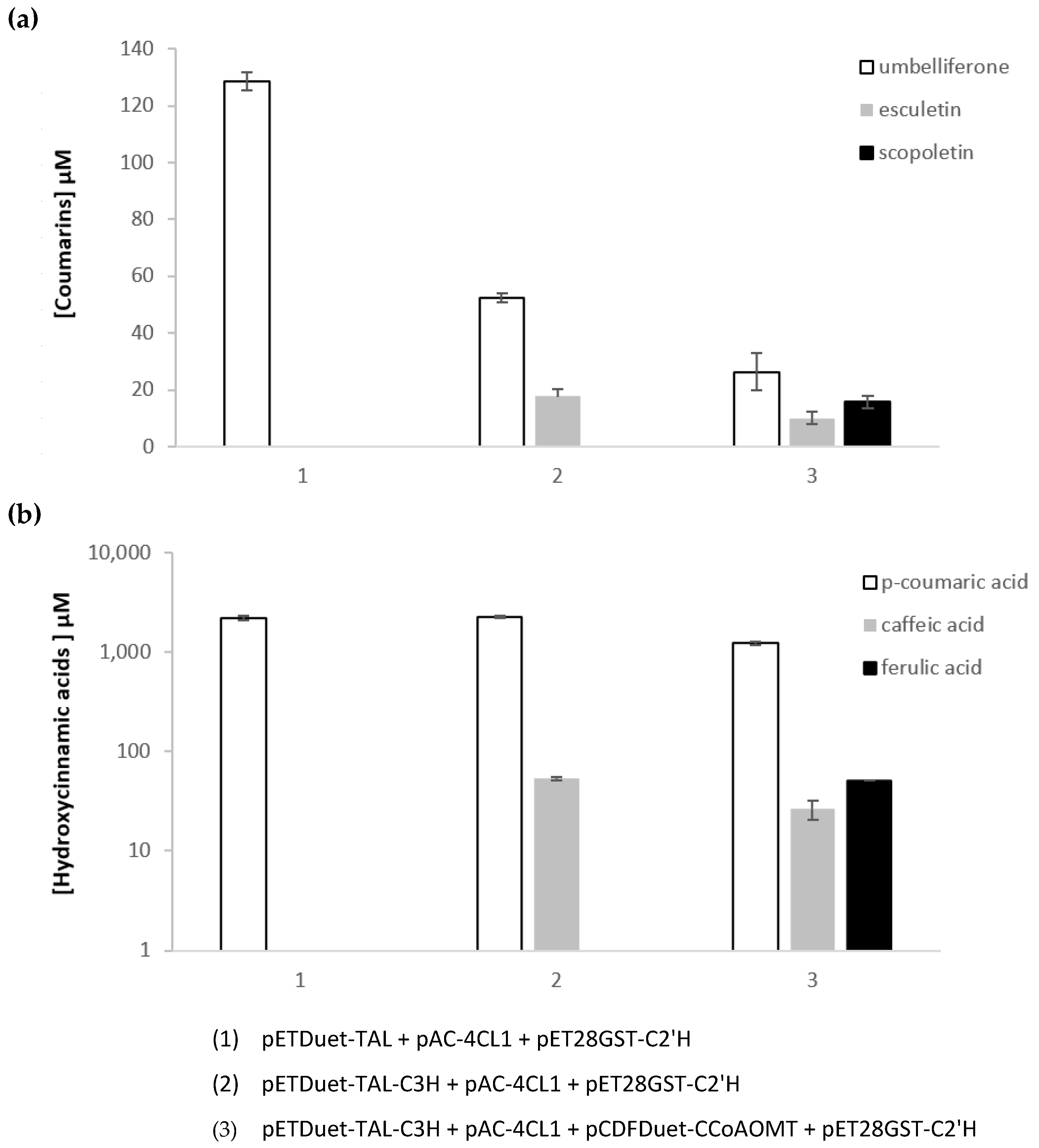
2.3. Heterologous Production of Coumarins from Tyrosine
2.4. The Prenyltransferase (PT) Step
2.5. The Psoralen Synthase (PS) Step
2.6. The Marmesin Synthase (MS) Step
3. Conclusions
4. Materials and Methods
4.1. Strains, Plasmids, and Chemicals
4.2. Construction of Plasmids
4.3. Coumarins/Furanocoumarins Production
4.4. Coumarin/Furanocoumarin Extraction
4.5. UHPLC Analysis
4.6. Protein Analysis
4.7. Enzymatic Assays
4.7.1. PT In Vitro Reactions
4.7.2. PS In Vitro Reactions
4.7.3. MS In Vitro Reactions
4.8. Bioinformatics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.-J.; Jin, B.-R.; An, H.-J. Umbelliferone ameliorates benign prostatic hyperplasia by inhibiting cell proliferation and G1/S phase cell cycle progression through regulation of STAT3/E2F1 axis. Int. J. Mol. Sci. 2021, 22, 9019. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.F.; de Figueiredo, G.F.; Pedro, L.P.; Amorin, Y.M.; Andrade, J.T.; Passos, T.F.; Rodrigues, F.F.; Souza, I.L.A.; Gonçalves, T.P.R.; Lima, L.A.R.D.S.; et al. Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother. 2020, 129, 110432. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hozayen, W.G.; Hasan, I.H.; Shaban, E.; Bin-Jumah, M. Umbelliferone ameliorates CCl4-induced liver fibrosis in rats by upregulating PPARγ and attenuating oxidative stress, inflammation, and TGF-β1/Smad3 signaling. Inflammation 2019, 42, 1103–1116. [Google Scholar] [CrossRef]
- Zagaja, M.; Zagaja, A.; Szala-Rycaj, J.; Szewczyk, A.; Lemieszek, M.K.; Raszewski, G.; Andres-Mach, M. Influence of umbelliferone on the anticonvulsant and neuroprotective activity of selected antiepileptic drugs: An in vivo and in vitro study. Int. J. Mol. Sci. 2022, 23, 3492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phyther. Res. 2022, 36, 279–298. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Qin, S.-W.; Jiang, J.-G. Improvement effects of esculetin on the formation and development of atherosclerosis. Biomed. Pharmacother. 2022, 150, 113001. [Google Scholar] [CrossRef]
- Lemos, A.S.O.; Florêncio, J.R.; Pinto, N.C.C.; Campos, L.M.; Silva, T.P.; Grazul, R.M.; Pinto, P.F.; Tavares, G.D.; Scio, E.; Apolônio, A.C.M.; et al. Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Front. Microbiol. 2020, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Napiroon, T.; Bacher, M.; Balslev, H.; Tawaitakham, K.; Santimaleeworagun, W.; Vajrodaya, S. Scopoletin from Lasianthus lucidus Blume (Rubiaceae): A potential antimicrobial against multidrug-resistant Pseudomonas aeruginosa. J. Appl. Pharm. Sci. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Parama, D.; Girisa, S.; Khatoon, E.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacol. Res. 2022, 179, 106202. [Google Scholar] [CrossRef]
- Kokron, O.; Maca, S.; Gasser, G.; Schmidt, P.R. Cimetidine and coumarin therapy of renal cell carcinoma. Oncology 1991, 48, 102–106. [Google Scholar] [CrossRef]
- Wang, C.-J.; Hsieh, Y.-J.; Chu, C.-Y.; Lin, Y.-L.; Tseng, T.-H. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 2002, 183, 163–168. [Google Scholar] [CrossRef]
- Mohler, J.L.; Gomella, L.G.; Crawford, E.D.; Glode, L.M.; Zippe, C.D.; Fair, W.R.; Marshall, M.E. Phase II evaluation of coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma. Prostate 1992, 20, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Warfarin: From rat poison to clinical use. Nat. Rev. Cardiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Rodrigues, L.R. Biosynthesis and heterologous production of furanocoumarins: Perspectives and current challenges. Nat. Prod. Rep. 2021, 38, 869–879. [Google Scholar] [CrossRef]
- Ling, T.C.; Clayton, T.H.; Crawley, J.; Exton, L.S.; Goulden, V.; Ibbotson, S.; McKenna, K.; Mohd Mustapa, M.F.; Rhodes, L.E.; Sarkany, R. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen–ultraviolet A therapy 2015. Br. J. Dermatol. 2016, 174, 24–55. [Google Scholar] [CrossRef]
- Jagasia, M.; Scheid, C.; Socié, G.; Ayuk, F.A.; Tischer, J.; Donato, M.L.; Bátai, Á.; Chen, H.; Chen, S.-C.; Chin, T.; et al. Randomized controlled study of ECP with methoxsalen as first-line treatment of patients with moderate to severe cGVHD. Blood Adv. 2019, 3, 2218–2229. [Google Scholar] [CrossRef]
- Issa, M.Y.; Elshal, M.F.; Fathallah, N.; Abdelkawy, M.A.; Bishr, M.; Salama, O.; Abulfadl, Y.S. Potential anticancer activity of the furanocoumarin derivative xanthotoxin isolated from Ammi majus L. fruits: In vitro and in silico studies. Molecules 2022, 27, 943. [Google Scholar] [CrossRef]
- Steinack, C.; Robinson, C.A.; Nägeli, M.; Inci, I.; Benden, C. ECP as additional immunomodulation in idiopathic hyperammonemia and recurrent hypercapnic respiratory failure early post lung transplantation. J. Clin. Apher. 2021, 36, 186–188. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Bracken, A.K.; Fronczek, F.R.; Johnson, R.D.; Wedge, D.E.; Duke, S.O. Furanocoumarin with phytotoxic activity from the leaves of Amyris elemifera (Rutaceae). ACS Omega 2021, 6, 401–407. [Google Scholar] [CrossRef]
- Britto, I.O.; Araújo, S.H.C.; Toledo, P.F.S.; Lima, G.D.A.; Salustiano, I.V.; Alves, J.R.; Mantilla-Afanador, J.G.; Kohlhoff, M.; Oliveira, E.E.; Leite, J.P. V Potential of Ficus carica extracts against Euschistus heros: Toxicity of major active compounds and selectivity against beneficial insects. Pest Manag. Sci. 2021, 77, 4638–4647. [Google Scholar] [CrossRef]
- Nebo, L.; Varela, R.M.; Molinillo, J.M.G.; Sampaio, O.M.; Severino, V.G.P.; Cazal, C.M.; Fernandes, M.F.D.G.; Fernandes, J.B.; Macías, F.A. Phytotoxicity of alkaloids, coumarins and flavonoids isolated from 11 species belonging to the Rutaceae and Meliaceae families. Phytochem. Lett. 2014, 8, 226–232. [Google Scholar] [CrossRef]
- Rainha, J.; Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Synthetic biology approaches to engineer Saccharomyces cerevisiae towards the industrial production of valuable polyphenolic compounds. Life 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Heterologous production of curcuminoids. Microbiol. Mol. Biol. Rev. 2015, 79, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Ferreira, D.; Rodrigues, L.R. Synthetic biology strategies towards the development of new bioinspired technologies for medical applications. In Bioinspired Materials for Medical Applications; Mohapatra, S., Ranjan, S., Dasgupta, N., Mishra, R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 451–497. ISBN 9780081007464. [Google Scholar]
- Rodrigues, J.L.; Rodrigues, L.R. Synthetic biology: Perspectives in industrial biotechnology. In Current Developments in Biotechnology and Bioengineering: Foundations of Biotechnology and Bioengineering; Pandey, A., Teixeira, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–269. ISBN 9780444636799. [Google Scholar]
- Sun, J.; Sun, W.; Zhang, G.; Lv, B.; Li, C. High efficient production of plant flavonoids by microbial cell factories: Challenges and opportunities. Metab. Eng. 2022, 70, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lyv, Y.; Zhou, S.; Yu, S.; Zhou, J. Microbial cell factories for the production of flavonoids–barriers and opportunities. Bioresour. Technol. 2022, 360, 127538. [Google Scholar] [CrossRef]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef]
- Rainha, J.; Rodrigues, J.L.; Faria, C.; Rodrigues, L.R. Curcumin biosynthesis from ferulic acid by engineered Saccharomyces cerevisiae. Biotechnol. J. 2021, 17, 2100400. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Yuan, Q.; Yan, Y. Combinatorial biosynthesis of plant-specific coumarins in bacteria. Metab. Eng. 2013, 18, 69–77. [Google Scholar] [CrossRef]
- Zhao, Y.; Jian, X.; Wu, J.; Huang, W.; Huang, C.; Luo, J.; Kong, L. Elucidation of the biosynthesis pathway and heterologous construction of a sustainable route for producing umbelliferone. J. Biol. Eng. 2019, 13, 44. [Google Scholar] [CrossRef]
- Yang, S.-M.; Shim, G.Y.; Kim, B.-G.; Ahn, J.-H. Biological synthesis of coumarins in Escherichia coli. Microb. Cell Fact. 2015, 14, 65. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Zhang, R.-K.; Qiao, B.; Li, B.-Z.; Yuan, Y.-J. Engineering budding yeast for the production of coumarins from lignin. Biochem. Eng. J. 2020, 160, 107634. [Google Scholar] [CrossRef]
- Bu, X.; He, B.; Weng, J.; Jiang, C.; Zhao, Y.-L.; Li, S.-M.; Xu, J.; Xu, M. Constructing microbial hosts for the production of benzoheterocyclic derivatives. ACS Synth. Biol. 2020, 9, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Munakata, R.; Olry, A.; Karamat, F.; Courdavault, V.; Sugiyama, A.; Krieger, C.; Silie, P.; Foureau, E.; Papon, N.; Grosjean, J. Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 2016, 211, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Karamat, F.; Olry, A.; Munakata, R.; Koeduka, T.; Sugiyama, A.; Paris, C.; Hehn, A.; Bourgaud, F.; Yazaki, K. A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley. Plant J. 2014, 77, 627–638. [Google Scholar] [CrossRef]
- Munakata, R.; Kitajima, S.; Nuttens, A.; Tatsumi, K.; Takemura, T.; Ichino, T.; Galati, G.; Vautrin, S.; Bergès, H.; Grosjean, J. Convergent evolution of the UbiA prenyltransferase family underlies the independent acquisition of furanocoumarins in plants. New Phytol. 2020, 225, 2166–2182. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Kellner, S.; Specker, S.; Hehn, A.; Gontier, E.; Hans, J.; Bourgaud, F.; Matern, U. Molecular cloning and functional characterization of psoralen synthase, the first committed monooxygenase of furanocoumarin biosynthesis. J. Biol. Chem. 2007, 282, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Hehn, A.; Hans, J.; Schneider, S.; Jugdé, H.; Schneider, B.; Matern, U.; Bourgaud, F. Isolation and functional characterization of CYP71AJ4 encoding for the first P450 monooxygenase of angular furanocoumarin biosynthesis. J. Biol. Chem. 2009, 284, 4776–4785. [Google Scholar] [CrossRef]
- Villard, C.; Munakata, R.; Kitajima, S.; Velzen, R.; Schranz, M.E.; Larbat, R.; Hehn, A. A new P450 involved in the furanocoumarin pathway underlies a recent case of convergent evolution. New Phytol. 2021, 231, 1923–1939. [Google Scholar] [CrossRef]
- Matsumoto, S.; Mizutani, M.; Sakata, K.; Shimizu, B.-I. Molecular cloning and functional analysis of the ortho-hydroxylases of p-coumaroyl coenzyme A/feruloyl coenzyme A involved in formation of umbelliferone and scopoletin in sweet potato, Ipomoea batatas (L.) Lam. Phytochemistry 2012, 74, 49–57. [Google Scholar] [CrossRef]
- Kai, K.; Mizutani, M.; Kawamura, N.; Yamamoto, R.; Tamai, M.; Yamaguchi, H.; Sakata, K.; Shimizu, B. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 2008, 55, 989–999. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Production of curcuminoids from tyrosine by a metabolically engineered Escherichia coli using caffeic acid as an intermediate. Biotechnol. J. 2015, 10, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Gomes, D.; Rodrigues, L.R. A combinatorial approach to optimize the production of curcuminoids from tyrosine in Escherichia coli. Front. Bioeng. Biotechnol. 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Heterologous production of caffeic acid from tyrosine in Escherichia coli. Enzyme Microb. Technol. 2015, 71, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Couto, M.R.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.; Rodrigues, L.R. Hydroxycinnamic acids and curcumin production in engineered Escherichia coli using heat shock promoters. Biochem. Eng. J. 2017, 125, 41–49. [Google Scholar] [CrossRef]
- Couto, M.R.; Rodrigues, J.L.; Rodrigues, L.R. Optimization of fermentation conditions for the production of curcumin by engineered Escherichia coli. J. R. Soc. Interface 2017, 14, 20170470. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, W.J.C.; Levisson, M.; Beekwilder, J.; van Berkel, W.J.H.; Vincken, J.-P. Plant aromatic prenyltransferases: Tools for microbial cell factories. Trends Biotechnol. 2020, 38, 917–934. [Google Scholar] [CrossRef]
- Munakata, R.; Inoue, T.; Koeduka, T.; Karamat, F.; Olry, A.; Sugiyama, A.; Takanashi, K.; Dugrand, A.; Froelicher, Y.; Tanaka, R. Molecular cloning and characterization of a geranyl diphosphate-specific aromatic prenyltransferase from lemon. Plant Physiol. 2014, 166, 80–90. [Google Scholar] [CrossRef]
- Looman, A.C.; Bodlaender, J.; Comstock, L.J.; Eaton, D.; Jhurani, P.; de Boer, H.A.; van Knippenberg, P.H. Influence of the codon following the AUG initiation codon on the expression of a modified lacZ gene in Escherichia coli. EMBO J. 1987, 6, 2489–2492. [Google Scholar] [CrossRef]
- He, J.; Hu, Z.; Dong, Z.; Li, B.; Chen, K.; Shang, Z.; Zhang, M.; Qiao, X.; Ye, M. Enzymatic O-prenylation of diverse phenolic compounds by a permissive O-prenyltransferase from the medicinal mushroom Antrodia camphorata. Adv. Synth. Catal. 2020, 362, 528–532. [Google Scholar] [CrossRef]
- Levisson, M.; Araya-Cloutier, C.; De Bruijn, W.J.C.; Van Der Heide, M.; López, J.M.S.; Daran, J.-M.; Vincken, J.-P.; Beekwilder, J. Toward developing a yeast cell factory for the production of prenylated flavonoids. J. Agric. Food Chem. 2019, 67, 13478–13486. [Google Scholar] [CrossRef]
- Shirai, A.; Matsuyama, A.; Yashiroda, Y.; Hashimoto, A.; Kawamura, Y.; Arai, R.; Komatsu, Y.; Horinouchi, S.; Yoshida, M. Global analysis of gel mobility of proteins and its use in target identification. J. Biol. Chem. 2008, 283, 10745–10752. [Google Scholar] [CrossRef] [PubMed]
- Rath, A.; Glibowicka, M.; Nadeau, V.G.; Chen, G.; Deber, C.M. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-D.; Kemper, B. Different structural requirements at specific proline residue positions in the conserved proline-rich region of cytochrome P450 2C2. J. Biol. Chem. 1996, 271, 28607–28611. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.J.; Arlotto, M.P.; Waterman, M.R. Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proc. Natl. Acad. Sci. USA 1991, 88, 5597–5601. [Google Scholar] [CrossRef]
- Stahlhut, S.G.; Siedler, S.; Malla, S.; Harrison, S.J.; Maury, J.; Neves, A.R.; Forster, J. Assembly of a novel biosynthetic pathway for production of the plant flavonoid fisetin in Escherichia coli. Metab. Eng. 2015, 31, 84–93. [Google Scholar] [CrossRef]
- Tamaki, S.; Yagi, M.; Nishihata, Y.; Yamaji, H.; Shigeri, Y.; Uno, T.; Imaishi, H. Modification of N-terminal amino acids of fungal benzoate hydroxylase (CYP53A15) for the production of p-hydroxybenzoate and optimization of bioproduction conditions in Escherichia coli. J. Microbiol. Biotechnol. 2018, 28, 439–447. [Google Scholar] [CrossRef]
- Li, J.; Tian, C.; Xia, Y.; Mutanda, I.; Wang, K.; Wang, Y. Production of plant-specific flavones baicalein and scutellarein in an engineered E. coli from available phenylalanine and tyrosine. Metab. Eng. 2019, 52, 124–133. [Google Scholar] [CrossRef]
- Dimaano, N.G.; Yamaguchi, T.; Fukunishi, K.; Tominaga, T.; Iwakami, S. Functional characterization of cytochrome P450 CYP81A subfamily to disclose the pattern of cross-resistance in Echinochloa phyllopogon. Plant Mol. Biol. 2020, 102, 403–416. [Google Scholar] [CrossRef]
- Christensen, U.; Vazquez-Albacete, D.; Søgaard, K.M.; Hobel, T.; Nielsen, M.T.; Harrison, S.J.; Hansen, A.H.; Møller, B.L.; Seppälä, S.; Nørholm, M.H.H. De-bugging and maximizing plant cytochrome P450 production in Escherichia coli with C-terminal GFP fusions. Appl. Microbiol. Biotechnol. 2017, 101, 4103–4113. [Google Scholar] [CrossRef]
- Rouck, J.E.; Biggs, B.W.; Kambalyal, A.; Arnold, W.R.; De Mey, M.; Ajikumar, P.K.; Das, A. Heterologous expression and characterization of plant Taxadiene-5α-Hydroxylase (CYP725A4) in Escherichia coli. Protein Expr. Purif. 2017, 132, 60–67. [Google Scholar] [CrossRef][Green Version]
- Biggs, B.W.; Lim, C.G.; Sagliani, K.; Shankar, S.; Stephanopoulos, G.; De Mey, M.; Ajikumar, P.K. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- García-Fruitós, E.; González-Montalbán, N.; Morell, M.; Vera, A.; Ferraz, R.M.; Arís, A.; Ventura, S.; Villaverde, A. Aggregation as bacterial inclusion bodies does not imply inactivation of enzymes and fluorescent proteins. Microb. Cell Fact. 2005, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Nimtz, M.; Rinas, U. Global proteome response of Escherichia coli BL21 to production of human basic fibroblast growth factor in complex and defined medium. Eng. Life Sci. 2017, 17, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Rodrigues, L.R. Potential applications of the Escherichia coli heat shock response in synthetic biology. Trends Biotechnol. 2018, 36, 186–198. [Google Scholar] [CrossRef]
- Lee, A.R.; Li, L.; Shin, S.-Y.; Moon, J.S.; Eom, H.-J.; Han, N.S. Soluble expression of the fucosyltransferase gene from Helicobacter pylori in Escherichia coli by co-expression of molecular chaperones. Microbiol. Biotechnol. Lett. 2015, 43, 212–218. [Google Scholar] [CrossRef]
- Vallejo, L.F.; Rinas, U. Strategies for the recovery of active proteins through refolding of bacterial inclusion body proteins. Microb. Cell Fact. 2004, 3, 11. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Sousa, M.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Selection of Escherichia coli heat shock promoters toward their application as stress probes. J. Biotechnol. 2014, 188, 61–71. [Google Scholar] [CrossRef]
- Hamerski, D.; Matern, U. Elicitor-induced biosynthesis of psoralens in Ammi majus L. suspension cultures. Eur. J. Biochem. 1988, 171, 369–375. [Google Scholar] [CrossRef]
- Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Perspectives on the design of microbial cell factories to produce prenylflavonoids. Int. J. Food Microbiol. 2022, 367, 109588. [Google Scholar] [CrossRef]
- Chen, R.; Gao, B.; Liu, X.; Ruan, F.; Zhang, Y.; Lou, J.; Feng, K.; Wunsch, C.; Li, S.-M.; Dai, J.; et al. Molecular insights into the enzyme promiscuity of an aromatic prenyltransferase. Nat. Chem. Biol. 2017, 13, 226–234. [Google Scholar] [CrossRef]
- Ni, W.; Zheng, Z.; Liu, H.; Wang, P.; Wang, H.; Sun, X.; Yang, Q.; Fang, Z.; Tang, H.; Zhao, G. Combining mutagenesis on Glu281 of prenyltransferase NovQ and metabolic engineering strategies for the increased prenylated activity towards menadione. Appl. Microbiol. Biotechnol. 2020, 104, 4371–4382. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Jiang, Y.; Yang, H.; Yun, Z.; Rong, W.; Yang, B. Regiospecific synthesis of prenylated flavonoids by a prenyltransferase cloned from Fusarium oxysporum. Sci. Rep. 2016, 6, 24819. [Google Scholar] [CrossRef] [PubMed]
- He, B.-B.; Bu, X.-L.; Zhou, T.; Li, S.-M.; Xu, M.-J.; Xu, J. Combinatory biosynthesis of prenylated 4-hydroxybenzoate derivatives by overexpression of the substrate-promiscuous prenyltransferase XimB in engineered E. coli. ACS Synth. Biol. 2018, 7, 2094–2104. [Google Scholar] [CrossRef]
- Rainha, J.; Rodrigues, J.L.; Rodrigues, L.R. CRISPR-Cas9: A powerful tool to efficiently engineer Saccharomyces cerevisiae. Life 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Rainha, J.; Rodrigues, L.R.; Rodrigues, J.L. Yeast synthetic biology approaches for the production of valuable polyphenolic compounds. In Synthetic Biology of Yeasts; Harzevili, F., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 119–156. [Google Scholar]
- Yuan, S.-F.; Yi, X.; Johnston, T.G.; Alper, H.S. De novo resveratrol production through modular engineering of an Escherichia coli–Saccharomyces cerevisiae co-culture. Microb. Cell Fact. 2020, 19, 143. [Google Scholar] [CrossRef]
- Häser, K.; Wenk, H.H.; Schwab, W. Biocatalytic production of dihydrocoumarin from coumarin by Saccharomyces cerevisiae. J. Agric. Food Chem. 2006, 54, 6236–6240. [Google Scholar] [CrossRef]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol. 2006, 6, 22. [Google Scholar] [CrossRef]
- Bannai, H.; Tamada, Y.; Maruyama, O.; Nakai, K.; Miyano, S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 2002, 18, 298–305. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Salvatore, M.; Emanuelsson, O.; Winther, O.; von Heijne, G.; Elofsson, A.; Nielsen, H. Detecting sequence signals in targeting peptides using deep learning. Life Sci. Alliance 2019, 2, e201900429. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Hecht, M.; Hamp, T.; Karl, T.; Yachdav, G.; Ahmed, N.; Altermann, U.; Angerer, P.; Ansorge, S.; Balasz, K.; et al. LocTree3 prediction of localization. Nucleic Acids Res. 2014, 42, W350–W355. [Google Scholar] [CrossRef] [PubMed]
- Pierleoni, A.; Martelli, P.L.; Fariselli, P.; Casadio, R. BaCelLo: A balanced subcellular localization predictor. Bioinformatics 2006, 22, e408–e416. [Google Scholar] [CrossRef] [PubMed]
| Plasmids | Construct | Source |
|---|---|---|
| pRSFDuet-1 | RSF1030 ori, lacI, double PT7lac, KanR | Novagen |
| pCDFDuet-1 | CloDF13 ori, lacI, double PT7lac, SpecR | Novagen |
| pNZY29-C2′H | ColE1(pBR322) ori, AmpR; pNZY29 carrying codon-optimized p-coumaroyl-CoA 2′-hydroxylase (C2′H) from Ipomoea batatas | NZYTech |
| pHTP0-PsPT1 | ColE1(pBR322) ori, AmpR; pHTP0 carrying codon-optimized prenyltransferase 1 from Pastinaca sativa (PsPT1) | NZYTech |
| pHTP0-PS | ColE1(pBR322) ori, AmpR; pHTP0 carrying codon-optimized psoralen synthase (PS) from Ammi majus | NZYTech |
| pET28GST-LIC | ColE1(pBR322) ori, lacI, PT7lac, KanR; carrying levansucrase (SacB) from Bacillus subtilis and glutathione S-transferase (GST) | Addgene (#26101) |
| pAC-4CL1 | p15A ori, Plac, CmR, pACYC184-derived plasmid carrying 4-coumarate-CoA ligase 1 (4CL1) from Arabidopsis thaliana | Addgene (#35947) |
| pETDuet-PcPT-XimD-XimE | pBR322 ori, lacI, double PT7lac, AmpR; pETDuet-1 carrying codon-optimized prenyltransferase from Petroselinum crispum (PcPT) and marmesin synthase (XimD) and snoaL-like cyclase (XimE) from Streptomyces xiamenensis | Addgene (#172654) |
| pMVA | pCDFDuet-1 carrying hydroxymethylglutaryl-CoA synthase (MvaS) and bifunctional acetoacetyl-CoA thiolase and 3-hydroxy-3-methylglutaryl-CoA reductase (MvaE) from Enterococcus faecalis, isopentenyl pyrophosphate—dimethylallylpyrophosphate isomerase (IDI) from E. coli BL21 and mevalonate (MVA) kinase (ERG12), phospho-MVA kinase (ERG8) and MVA pyrophosphate decarboxylase (ERG19) from Saccharomyces cerevisiae | Addgene (#121149) |
| pETDuet-CPR2 | pBR322 ori, lacI, double PT7lac, AmpR; pETDuet-1 carrying cytochrome P450 reductase 2 (CPR2) from A. thaliana | [59] |
| pYeDP60-CYP76F112 | pUC ori, 2 µ ori, URA3, TetR, AmpR; pYeDP60 carrying marmesin synthase from Ficus carica (CYP76F112) | [40] |
| pETDuet-TAL | pBR322 ori, lacI, double PT7lac, AmpR; pETDuet-1 carrying tyrosine ammonia lyase (TAL) from Rhodotorula glutinis | [45] |
| pETDuet-TAL-C3H | pETDuet-TAL carrying 4-coumarate 3-hydroxylase (C3H) from Saccharothrix espanaensis | [45] |
| pCDFDuet-CCoAOMT | pCDFDuet-1 carrying caffeoyl-CoA 3-O-methyltransferase (CCoAOMT) from Medicago sativa | [43] |
| pET28GST-C2′H | pET28GST-LIC without SacB and carrying C2′H in frame with GST and His-tag | This study |
| pRSFDuet-C2′H | pRSFduet-1 carrying C2′H without His-tag or S-tag in frame | This study |
| pRSFDuet-His-tag-C2′H | pRSFDuet-1 carrying C2′H with His-tag in frame | This study |
| pRSFDuet-C2′H-S-tag | pRSFDuet-1 carrying C2′H with S-tag in frame | This study |
| pETDuet-TAL-PcPT | pETDuet-TAL carrying PcPT | This study |
| pCDFDuet-PcPT | pCDFDuet-1 carrying PcPT | This study |
| pRSFDuet-PcPT | pRSFDuet-1 carrying PcPT | This study |
| pET28GST-PcPT | pET28GST-LIC without SacB and carrying PcPT | This study |
| pRSFDuet-∆48PcPT | pRSFDuet-1 carrying truncated PcPT | This study |
| pET28-∆48PcPT | pET28GST-LIC without SacB and carrying truncated PcPT | This study |
| pRSFDuet-PsPT1 | pRSFDuet-1 carrying PsPT1 | This study |
| pET28GST-PsPT1 | pET28GST-LIC without SacB and carrying PsPT1 | This study |
| pRSFDuet-∆48PsPT1 | pRSFDuet-1 carrying truncated PsPT1 | This study |
| pET28-∆48PsPT1 | pET28GST-LIC without SacB and carrying truncated PsPT1 | This study |
| pRSFDuet-PS | pRSFDuet-1 carrying PS | This study |
| pET28GST-PS | pET28GST-LIC without SacB and carrying PS | This study |
| pRSFDuet-MA-∆37PS | pRSFDuet-1 carrying MA-∆37PS | This study |
| pET28-MA-∆37PS | pET28GST-LIC without SacB and GST and carrying MA-∆37PS | This study |
| pRSFDuet-8RP-∆37PS | pRSFDuet-1 carrying 8RP-∆37PS | This study |
| pET28-8RP-∆37PS | pET28GST-LIC without SacB and GST and carrying 8RP-∆37PS | This study |
| pRSFDuet-2C3-∆37PS | pRSFDuet-1 carrying 2C3-∆37PS | This study |
| pET28-2C3-∆37PS | pET28GST-LIC without SacB and GST and carrying 2C3-∆37PS | This study |
| pRSFDuet-28tag-∆37PS | pRSFDuet-1 carrying 28tag-∆37PS | This study |
| pET28-28tag-∆37PS | pET28GST-LIC without SacB and GST and carrying 28tag-∆37PS | This study |
| pCDFDuet-GroESL | pCDFDuet-1 carrying chaperones GroES and GroEL from E. coli BL21 in an operon | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, J.L.; Gomes, D.; Rodrigues, L.R. Challenges in the Heterologous Production of Furanocoumarins in Escherichia coli. Molecules 2022, 27, 7230. https://doi.org/10.3390/molecules27217230
Rodrigues JL, Gomes D, Rodrigues LR. Challenges in the Heterologous Production of Furanocoumarins in Escherichia coli. Molecules. 2022; 27(21):7230. https://doi.org/10.3390/molecules27217230
Chicago/Turabian StyleRodrigues, Joana L., Daniela Gomes, and Lígia R. Rodrigues. 2022. "Challenges in the Heterologous Production of Furanocoumarins in Escherichia coli" Molecules 27, no. 21: 7230. https://doi.org/10.3390/molecules27217230
APA StyleRodrigues, J. L., Gomes, D., & Rodrigues, L. R. (2022). Challenges in the Heterologous Production of Furanocoumarins in Escherichia coli. Molecules, 27(21), 7230. https://doi.org/10.3390/molecules27217230







