Effect of Ginger on Inflammatory Diseases
Abstract
1. Introduction
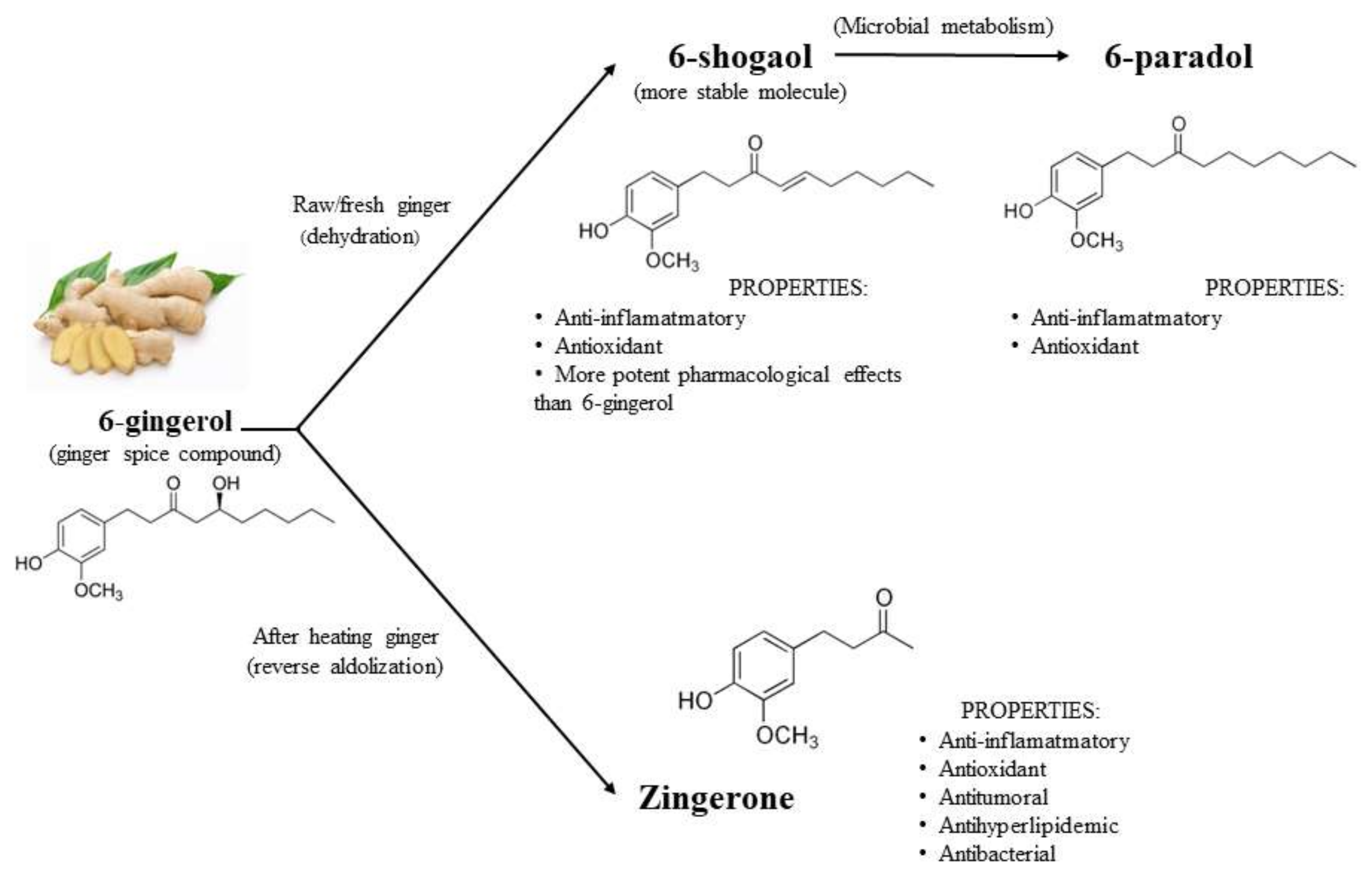
2. Bioactive Compounds in Ginger
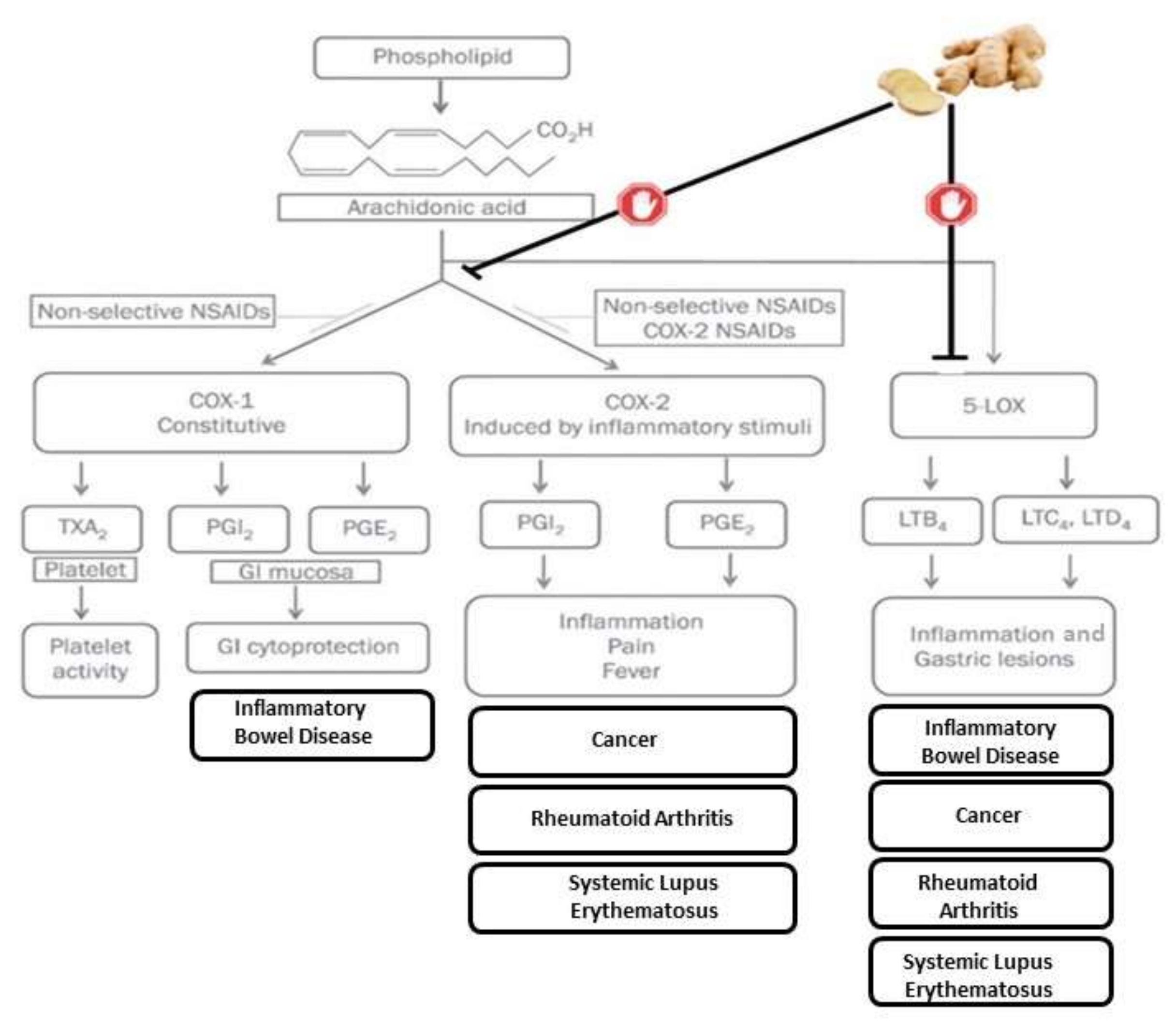
3. Antioxidant and Anti-Inflammatory Properties
4. Effect of Ginger on Inflammatory Diseases
4.1. Rheumatoid Arthritis
4.2. Inflammatory Bowel Disease
4.3. Systemic Lupus Erythematosus
4.4. Psoriasis
4.5. Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singletary, K. Ginger: An overview of health benefits. Nutr. Today 2010, 45, 171–183. [Google Scholar] [CrossRef]
- Kubra, I.R.; Rao, L.J.M. An impression on current developments in the technology, chemistry, and biological activities of ginger (Zingiber officinale Roscoe). Crit. Rev. Food Sci. Nutr. 2012, 52, 651–688. [Google Scholar] [CrossRef] [PubMed]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Patra, J.K.; Das, S.K.; Das, G.; Majnooni, M.B.; Farzaei, M.H. Ginger and Heart Health: From Mechanisms to Therapeutics. Curr. Mol. Pharmacol. 2021, 14, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, Y.A. Gingerol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 177–207. [Google Scholar] [PubMed]
- Amer, M.S.; Ibrahim, H.A.H. Chitosan from marine-derived Penicillum spinulosum MH2 cell wall with special emphasis on its antimicrobial and antifouling properties. Egypt J. Aquat. Res. 2019, 45, 359–365. [Google Scholar] [CrossRef]
- Kravchenko, I.; Eberle, L.; Nesterkina, M.; Kobernik, A. Anti-inflammatory and analgesic activity of ointment based on dense ginger extract (Zingiber officinale). J. Herbmed. Pharmacol. 2019, 8, 126–132. [Google Scholar] [CrossRef]
- Kravchenko, I.A.; Eberle, L.V.; Nesterkina, M.V.; Kobernik, A.O. Pharmacotherapy of inflammatory process by ginger extract (Zingiber officinale) ointment. J. Herb. Med. 2019, 8, 101–107. [Google Scholar] [CrossRef]
- Drozdov, V.N.; Kim, V.A.; Tkachenko, E.V.; Varvanina, G.G. Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J. Altern. Complement. Med. 2012, 18, 583–588. [Google Scholar] [CrossRef]
- Black, C.D.; Herring, M.P.; Hurley, D.J.; O’Connor, P.J. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. J. Pain 2010, 11, 894–903. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Jafarzadeh, S.; Nemati, M. Therapeutic potential of ginger against COVID-19: Is there enough evidence? J. Tradit. Chin. Med. Sci. 2021, 8, 267–279. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef]
- Santos Braga, S. Ginger: Panacea or consumer’s hype? Appl. Sci. 2019, 9, 1570. [Google Scholar] [CrossRef]
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C.-T. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018, 9, 1310–1327. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Nemati, M. Therapeutic potentials of ginger for treatment of Multiple sclerosis: A review with emphasis on its immunomodulatory, anti-inflammatory and anti-oxidative properties. J. Neuroimmunol. 2018, 324, 54–75. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Peng, S.; Yao, J.; Liu, Y.; Duan, D.; Zhang, X.; Fang, J. Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol. Food Funct. 2015, 6, 2813–2823. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Carnuta, M.G.; Deleanu, M.; Barbalata, T.; Toma, L.; Raileanu, M.; Sima, A.V.; Stancu, C.S. Zingiber officinale extract administration diminishes steroyl-CoA desaturase gene expression and activity in hyperlipidemic hamster liver by reducing the oxidative and endoplasmic reticulum stress. Phytomedicine 2018, 48, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Prakash, U.N.S.; Srinivasan, K. Gastrointestinal protective effect of dietary spices during ethanol-induced oxidant stress in experimental rats. Appl. Physiol. Nutr. Metab. 2010, 35, 134–141. [Google Scholar] [CrossRef]
- Kwiatkowska, S.; Piasecka, G.; Zieba, M.; Piotrowski, W.; Nowak, D. Increased serum concentrations of conjugated diens and malondialdehyde in patients with pulmonary tuberculosis. Respir. Med. 1999, 93, 272–276. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Murthy, S.V.; Krishna, D.R.; Prabhakar, M.C. Role of free radicals and antioxidants in tuberculosis patients. Indian J. Tuberc. 2004, 51, 213–218. [Google Scholar]
- Raja, D.A. Immunology of Tuberculosis. Indian J. Med. Res. 2004, 120, 213–232. [Google Scholar]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Marahatha, R.; Basnet, S.; Bhattarai, B.R.; Budhathoki, P.; Aryal, B.; Adhikari, B.; Lamichhane, G.; Poudel, D.K.; Parajuli, N. Potential natural inhibitors of xanthine oxidase and HMG-CoA reductase in cholesterol regulation: In silico analysis. BMC Complement. Med. Ther. 2021, 21, 1. [Google Scholar] [CrossRef]
- Joshi, D.; Srivastav, S.K.; Belemkar, S.; Dixit, V.A. Zingiber officinale and 6-gingerol alleviate liver and kidney dysfunctions and oxidative stress induced by mercuric chloride in male rats: A protective approach. Biomed. Pharmacother. 2017, 91, 645–655. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, N.; Gao, Y.; Sun, L.; Zhang, J. Therapeutic effects of 6-gingerol, 8-gingerol, and 10-gingerol on dextran sulfate sodium-induced acute ulcerative colitis in rats. Phyther. Res. 2017, 31, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Shirdel, Z.; Mirbadalzadeh, R.; Madani, H. Antidiabetic and antilipidemic effect of ginger in alloxan monohydrate diabetic rats in comparison with glibenclamide. Iran J. Diabetes Lipid Disord. 2009, 9, 7–15. [Google Scholar]
- Berrino, F.; Villarini, A.; Traina, A.; Bonanni, B.; Panico, S.; Mano, M.P.; Mercandino, A.; Galasso, R.; Barbero, M.; Simeoni, M.; et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res. Treat. 2014, 147, 159–165. [Google Scholar] [CrossRef]
- Kart, L.; Buyukoglan, H.; Tekin, I.O.; Altin, R.; Senturk, Z.; Gulmez, I.; Demir, R.; Ozesmi, M. Correlation of serum tumor necrosis factor-α, interleukin-4 and soluble interleukin-2 receptor levels with radiologic and clinical manifestations in active pulmonary tuberculosis. Mediators Inflamm. 2003, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Hong, J.; Wu, H.; Liu, J.; Yang, C.S.; Pan, M.-H.; Badmaev, V.; Ho, C.-T. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009, 57, 10645–10650. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Imunologi Dasar Abbas Edisi ke-5. Singapura: Elsevier gingerol and [6]-shogaol. J. Ethnopharmacol. 2016, 127, 515–520. [Google Scholar]
- Manasa, D.; Srinivas, P.; Sowbhagya, H.B. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 2013, 139, 509–514. [Google Scholar]
- Petrelli, F.; Mariani, F.M.; Alunno, A.; Puxeddu, I. Pathogenesis of rheumatoid arthritis: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 475–482. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Management of rheumatoid arthritis: An overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid arthritis: A brief overview of the treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative stress in rheumatoid arthritis: What the future might hold regarding novel biomarkers and add-on therapies. Oxid. Med. Cell Longev. 2019, 2019, 7536805. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Timmermann, B.N. Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J. Nat. Prod. 2009, 72, 403–407. [Google Scholar] [CrossRef]
- Bischoff-Kont, I.; Primke, T.; Niebergall, L.S.; Zech, T.; Fürst, R. Ginger Constituent 6-Shogaol Inhibits Inflammation- and Angiogenesis-Related Cell Functions in Primary Human Endothelial Cells. Front. Pharmacol. 2022, 13, 844767. [Google Scholar] [CrossRef]
- Ilic, N.M.; Dey, M.; Poulev, A.A.; Logendra, S.; Kuhn, P.E.; Raskin, I. Anti-inflammatory activity of grains of paradise (Aframomum melegueta Schum) extract. J. Agric. Food Chem. 2014, 62, 10452–10457. [Google Scholar] [CrossRef]
- Nonaka, K.; Bando, M.; Sakamoto, E.; Inagaki, Y.; Naruishi, K.; Yumoto, H.; Kido, J.-I. 6-Shogaol Inhibits Advanced Glycation End-Products-Induced IL-6 and ICAM-1 Expression by Regulating Oxidative Responses in Human Gingival Fibroblasts. Molecules 2019, 24, 3705. [Google Scholar] [CrossRef]
- Yang, C.; Long, D.; Sung, J.; Alghoul, Z.; Merlin, D. Orally Administered Natural Lipid Nanoparticle-Loaded 6-Shogaol Shapes the Anti-Inflammatory Microbiota and Metabolome. Pharmaceutics 2021, 13, 1355. [Google Scholar] [CrossRef]
- Bischoff-Kont, I.; Fürst, R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef] [PubMed]
- Bashir, N.; Ahmad, S.B.; Rehman, M.U.; Muzamil, S.; Bhat, R.R.; Mir, M.U.R.; Shazly, G.A.; Ibrahim, M.A.; Elossaily, G.M.; Sherif, A.Y.; et al. Zingerone (4-(four-hydroxy-3-methylphenyl) butane-two-1) modulates adjuvant-induced rheumatoid arthritis by regulating inflammatory cytokines and antioxidants. Redox Rep. 2021, 26, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Samarpita, S.; Lee, J.S.; Lee, Y.J.; Son, J.E.; Jeong, M.; Kim, J.H.; Hong, S.; Yoo, S.-A.; Kim, W.-U.; et al. 8-Shogaol inhibits rheumatoid arthritis through targeting TAK1. Pharmacol. Res. 2022, 178, 106176. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Mahmoudi, M.; Shahram, F.; Poursani, S.; Jamshidi, F.; Tavakoli, H. The effect of ginger supplementation on IL2, TNFα, and IL1β cytokines gene expression levels in patients with active rheumatoid arthritis: A randomized controlled trial. Med. J. Islam Repub. Iran 2019, 33, 154. [Google Scholar] [CrossRef] [PubMed]
- Argüelles Martín, F.; Argüelles Arias, F. Enfermedad inflamatoria intestinal. Pediatr. Integr. 2003, 7, 115–124. [Google Scholar]
- Masoodi, I.; Alshanqeeti, A.S.; Ahmad, S.; Alyamani, E.J.; A Al-Lehibi, A.; Qutub, A.N.; Alsayari, K.N.; O Alomair, A. Microbial dysbiosis in inflammatory bowel diseases: Results of a metagenomic study in Saudi Arabia. Minerva Gastroenterol. Dietol. 2019, 65, 177–186. [Google Scholar] [CrossRef]
- Guo, S.; Geng, W.; Chen, S.; Wang, L.; Rong, X.; Wang, S.; Wang, T.; Xiong, L.; Huang, J.; Pang, X.; et al. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front. Pharmacol. 2021, 12, 632569. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like receptors and inflammatory bowel disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Dejban, P.; Nikravangolsefid, N.; Chamanara, M.; Dehpour, A.; Rashidian, A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF-kappaB pathway. Phyther. Res. 2021, 35, 835–845. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Kane, S. What physicians don’t know about patient dietary beliefs and behavior can make a difference. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, D.; Polukort, S.H.; Gelzinis, J.; Rovatti, J.; Kaczenski, E.; Galinski, C.; Pantos, M.; Shah, N.N.; Schneider, S.S.; Kennedy, D.R.; et al. Protein Disulfide Isomerases Regulate IgE-Mediated Mast Cell Responses and Their Inhibition Confers Protective Effects During Food Allergy. Front. Immunol. 2020, 11, 606837. [Google Scholar] [CrossRef]
- Navaneethan, U.; Lashner, B.A. Effects of Immunosuppression and Liver Transplantation on Inflammatory Bowel Disease in Patients With Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2013, 11, 524–525. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, B.O.; Adedara, I.A.; Farombi, E.O. Protective mechanisms of 6-gingerol in dextran sulfate sodium-induced chronic ulcerative colitis in mice. Hum. Exp. Toxicol. 2018, 37, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J. Crohn’s Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; McGrath, K.C.-Y.; Nammi, S.; Heather, A.K.; Roufogalis, B.D. Attenuation of liver pro-inflammatory responses by Zingiber officinale via inhibition of NF-kappa B activation in high-fat diet-fed rats. Basic Clin. Pharmacol. Toxicol. 2012, 110, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Lashgari, N.; Roudsari, N.M.; Khayatan, D.; Shayan, M.; Momtaz, S.; Roufogalis, B.D.; Abdolghaffari, A.H.; Sahebkar, A. Ginger and its constituents: Role in treatment of inflammatory bowel disease. BioFactors 2022, 48, 7–21. [Google Scholar] [CrossRef]
- Van Tilburg, M.A.L.; Palsson, O.S.; Levy, R.L.; Feld, A.D.; Turner, M.J.; Drossman, D.A.; Whitehead, W.E. Complementary and alternative medicine use and cost in functional bowel disorders: A six month prospective study in a large HMO. BMC Complement Altern Med. 2008, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qing, P.; Yang, H.; Wu, Y.; Liu, Y.; Luo, Y. Gut Microbiome and Metabolites in Systemic Lupus Erythematosus: Link, Mechanisms and Intervention. Front Immunol. 2021, 12, 686501. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khandker, S.S.; Kotyla, P.J.; Hassan, R. Immunomodulatory Effects of Diet and Nutrients in Systemic Lupus Erythematosus (SLE): A Systematic Review. Front. Immunol. 2020, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.E.; Rus, V.; Szeto, G.L. Leveraging Heterogeneity in Systemic Lupus Erythematosus for New Therapies. Trends Mol. Med. 2021, 27, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Illescas-Montes, R.; Corona-Castro, C.C.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Infectious processes and systemic lupus erythematosus. Immunology 2019, 158, 153–160. [Google Scholar] [CrossRef]
- Ali, R.A.; Gandhi, A.A.; Dai, L.; Weiner, J.; Estes, S.K.; Yalavarthi, S.; Gockman, K.; Sun, D.; Knight, J.S. Antineutrophil properties of natural gingerols in models of lupus. JCI Insight 2021, 6, e138385. [Google Scholar] [CrossRef]
- Chen, Y.; Lyga, J. Brain-skin connection: Stress, inflammation and skin aging. Inflamm. Allergy-Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ung, T.T.; Li, S.; Lian, S.; Xia, Y.; Park, S.Y.; Do Jung, Y. Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ke, W.; Bao, R.; Hu, X.; Chen, F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: A review. Ann. N. Y. Acad. Sci. 2017, 1398, 83–98. [Google Scholar] [CrossRef]
- Javadi, B. Diet Therapy for Cancer Prevention and Treatment Based on Traditional Persian Medicine. Nutr. Cancer 2018, 70, 376–403. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.H.M.; Makpol, S.; Abdul Hamid, N.A.; Das, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. [Google Scholar] [CrossRef] [PubMed]
- De Lima, R.M.T.; dos Reis, A.C.; de Menezes, A.-A.P.M.; de Oliveira Santos, J.V.; de Oliveira Filho, J.W.G.; de Oliveira Ferreira, J.R.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Yang, G.; Yang, Y. Biological properties of 6-gingerol: A brief review. Nat. Prod. Commun. 2014, 9, 1027–1030. [Google Scholar] [CrossRef]
- Agnoli, C.; Grioni, S.; Sieri, S.; Sacerdote, C.; Ricceri, F.; Tumino, R.; Frasca, G.; Pala, V.; Mattiello, A.; Chiodini, P.; et al. Metabolic syndrome and breast cancer risk: A case-cohort study nested in a multicentre italian cohort. PLoS ONE 2015, 10, e0128891. [Google Scholar] [CrossRef]
- Karimi, N.; Roshan, V.D.; Bayatiyani, Z.F. Individually and combined water-based exercise with ginger supplement, on systemic inflammation and metabolic syndrome indices, among the obese women with breast neoplasms. Iran. J. Cancer Prev. 2015, 8, e3856. [Google Scholar] [CrossRef]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.-O.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef]
| Phenolic Compounds-Gingerol Analogues | Other Phenolic Compounds | Terpens | ||
|---|---|---|---|---|
| GINGEROLS | SHOGAOLS | PARADOLS | ||
| 6-gingerol 8-gingerol 10-gingerol 12-gingerol | 6- shogaol 8-shogaol 10- shogaol dehydro-14-gingerdione 6-gingerdione 10-gingerdione | 6-paradol 8-paradol 10-paradol | Zingerone Quercetin Gingerenone-A 6-dehydrogingerdione | β-bisabolene α-curcumene α-farnesene β-sesquiphellandrene Zingiberene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. https://doi.org/10.3390/molecules27217223
Ballester P, Cerdá B, Arcusa R, Marhuenda J, Yamedjeu K, Zafrilla P. Effect of Ginger on Inflammatory Diseases. Molecules. 2022; 27(21):7223. https://doi.org/10.3390/molecules27217223
Chicago/Turabian StyleBallester, Pura, Begoña Cerdá, Raúl Arcusa, Javier Marhuenda, Karen Yamedjeu, and Pilar Zafrilla. 2022. "Effect of Ginger on Inflammatory Diseases" Molecules 27, no. 21: 7223. https://doi.org/10.3390/molecules27217223
APA StyleBallester, P., Cerdá, B., Arcusa, R., Marhuenda, J., Yamedjeu, K., & Zafrilla, P. (2022). Effect of Ginger on Inflammatory Diseases. Molecules, 27(21), 7223. https://doi.org/10.3390/molecules27217223









