Extending the Scope of the New Variant of the Castagnoli–Cushman Cyclocondensation onto o-Methyl Benzoic Acids Bearing Various Electron-Withdrawing Groups in the α-Position
Abstract
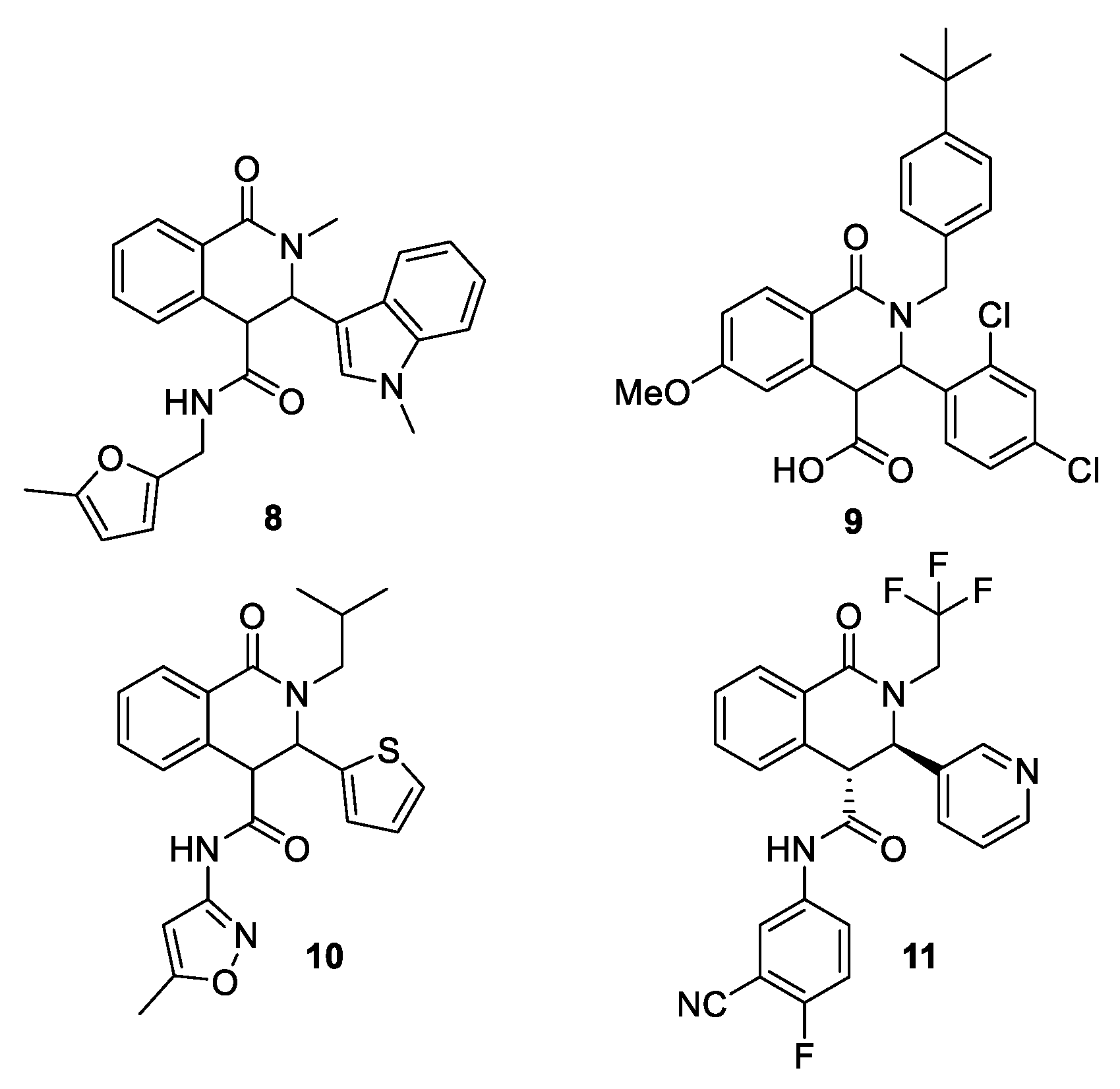
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
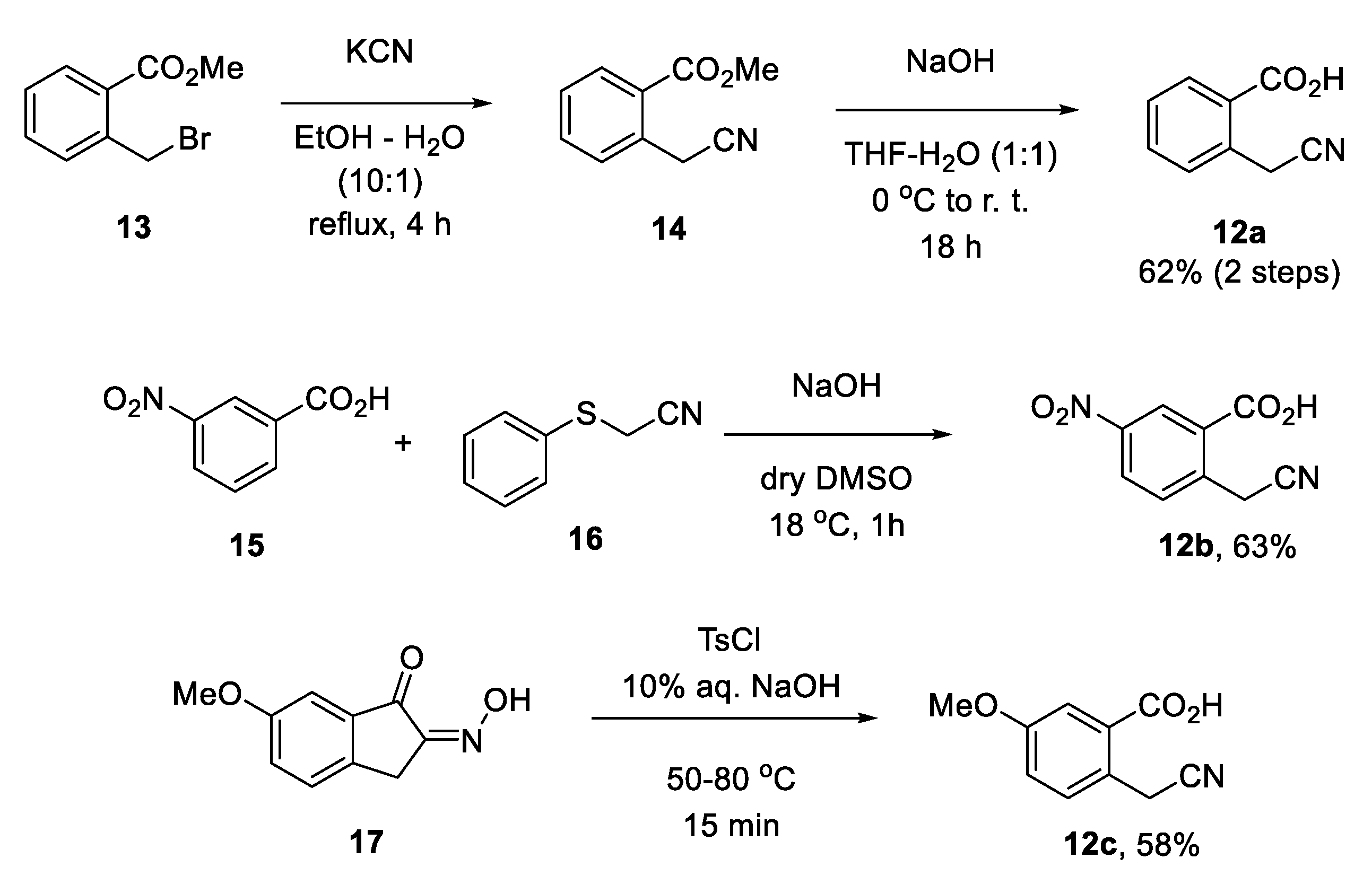
3.2. Synthesis of Compounds 12a–c
3.2.1. 2-(Cyanomethyl)benzoic Acid (12a)
3.2.2. 2-(Cyanomethyl)-5-nitrobenzoic Acid (12b)
3.2.3. 2-(Cyanomethyl)-5-methoxybenzoic Acid (12c)
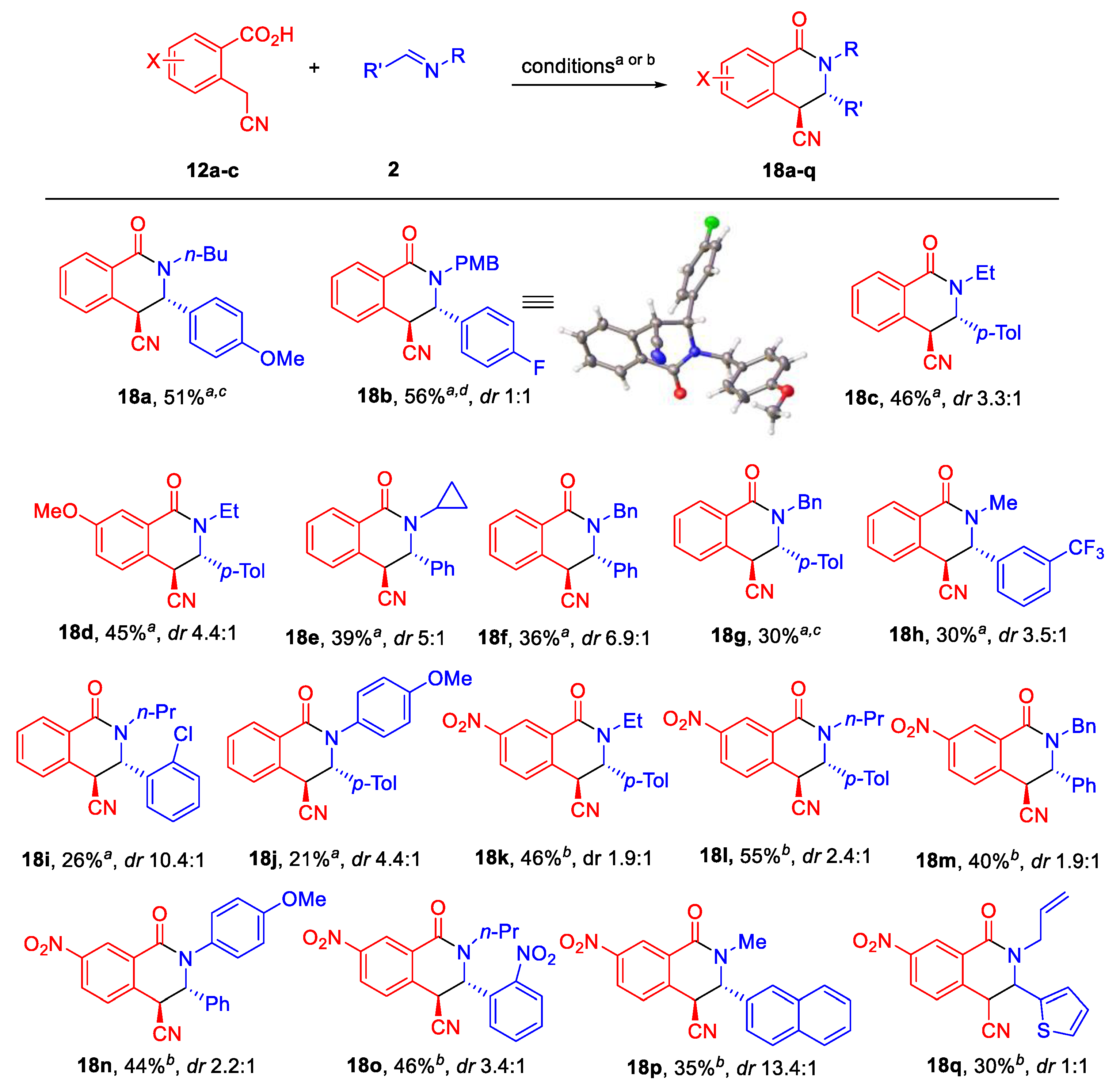
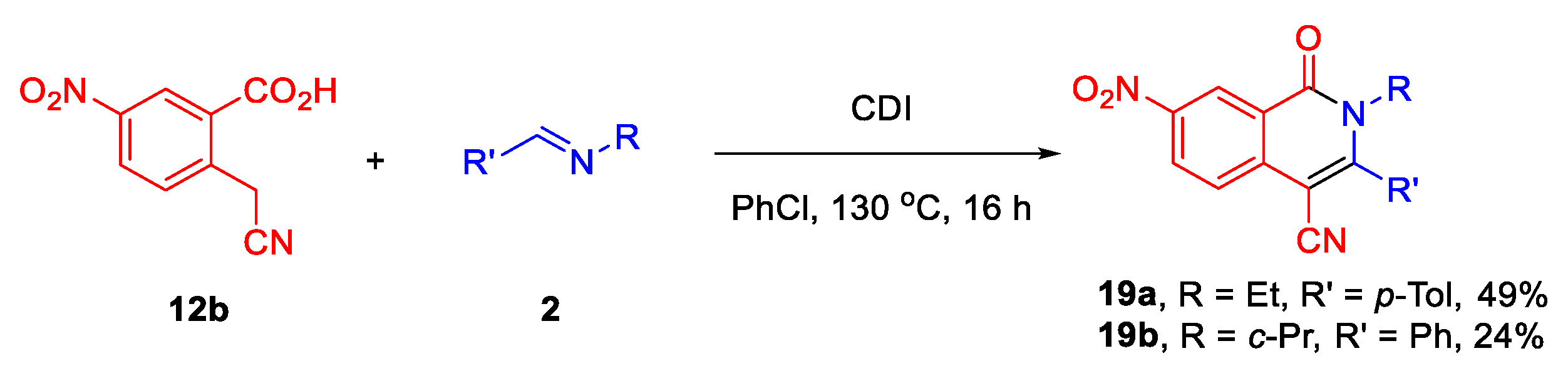
3.3. General Procedure for the Synthesis of Tetrahydroisoquinolone Carbonitriles 18a–j and Dihydroisoquinolines 19a,b
3.3.1. (±)-(3S,4S)-2-Butyl-3-(4-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18a)
3.3.2. (±)-3-(4-Fluorophenyl)-2-(4-methoxybenzyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18b)
3.3.3. (±)-(3S,4S)-2-Ethyl-1-oxo-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18c)
3.3.4. (±)-(3S,4S)-2-Ethyl-7-methoxy-1-oxo-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18d)
3.3.5. (±)-(3S,4S)-2-Cyclopropyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18e)
3.3.6. (±)-(3S,4S)-2-Benzyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18f)
3.3.7. (±)-(3S,4S)-2-Benzyl-1-oxo-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18g)
3.3.8. (±)-(3S,4S)-2-Methyl-1-oxo-3-(3-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18h)
3.3.9. (±)-(3S,4S)-3-(2-Chlorophenyl)-1-oxo-2-propyl-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18i)
3.3.10. (±)-(3S,4S)-2-(4-Methoxyphenyl)-1-oxo-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18j)
3.3.11. (±)-(3S,4S)-2-Ethyl-7-nitro-1-oxo-3-(p-tolyl)-1,2-dihydroisoquinoline-4-carbonitrile (19a)
3.3.12. (±)-(3S,4S)-2-Cyclopropyl-7-nitro-1-oxo-3-phenyl-1,2-dihydroisoquinoline-4-carbonitrile (19b)
3.4. General Procedure for the Synthesis of 7-Nitro Tetrahydroisoquinolone Carbonitriles (18k−q)
3.4.1. (±)-(3S,4S)-2-Ethyl-7-nitro-1-oxo-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18k)
3.4.2. (±)-(3S,4S)-7-Nitro-1-oxo-2-propyl-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18l)
3.4.3. (±)-(3S,4S)-2-Benzyl-7-nitro-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18m)
3.4.4. (±)-(3S,4S)-2-(4-Methoxyphenyl)-7-nitro-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18n)
3.4.5. (±)-(3S,4S)-7-Nitro-3-(2-nitrophenyl)-1-oxo-2-propyl-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18o)
3.4.6. (±)-(3S,4S)-2-Methyl-3-(naphthalen-2-yl)-7-nitro-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18p)
3.4.7. (±)-(3S,4S)-2-Allyl-7-nitro-1-oxo-3-(thiophen-2-Yl)-1,2,3,4-tetrahydroisoquinoline-4-carbonitrile (18q)
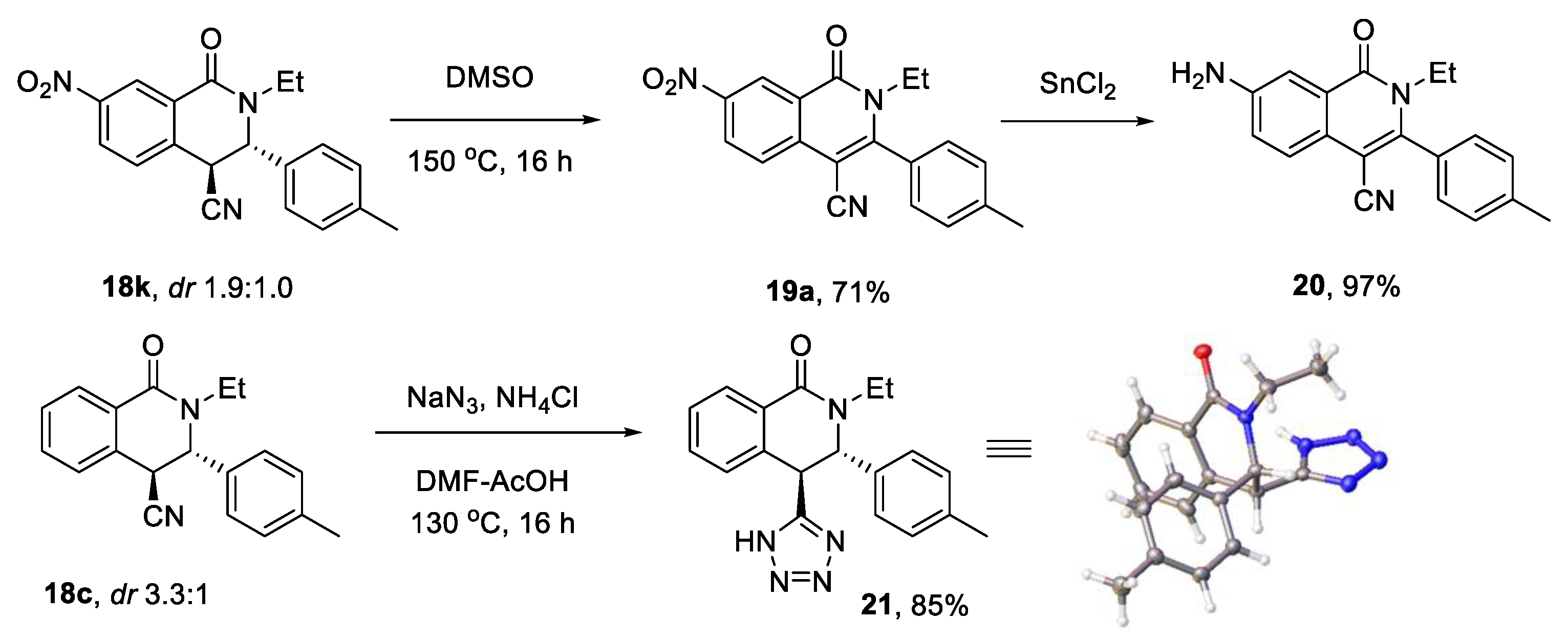
3.5. Post-Condensational Modifications
3.5.1. Oxidation Protocol (Scheme 4)
3.5.2. Reduction Protocol (Scheme 4). 7-Amino-2-ethyl-1-oxo-3-(p-tolyl)-1,2-dihydroisoquinoline-4-carbonitrile (20)
3.5.3. Tetrazole Synthesis. (3S,4S)-2-Ethyl-4-(1H-tetrazol-5-Yl)-3-(p-tolyl)-3,4-dihydroisoquinolin-1(2H)-one (21)
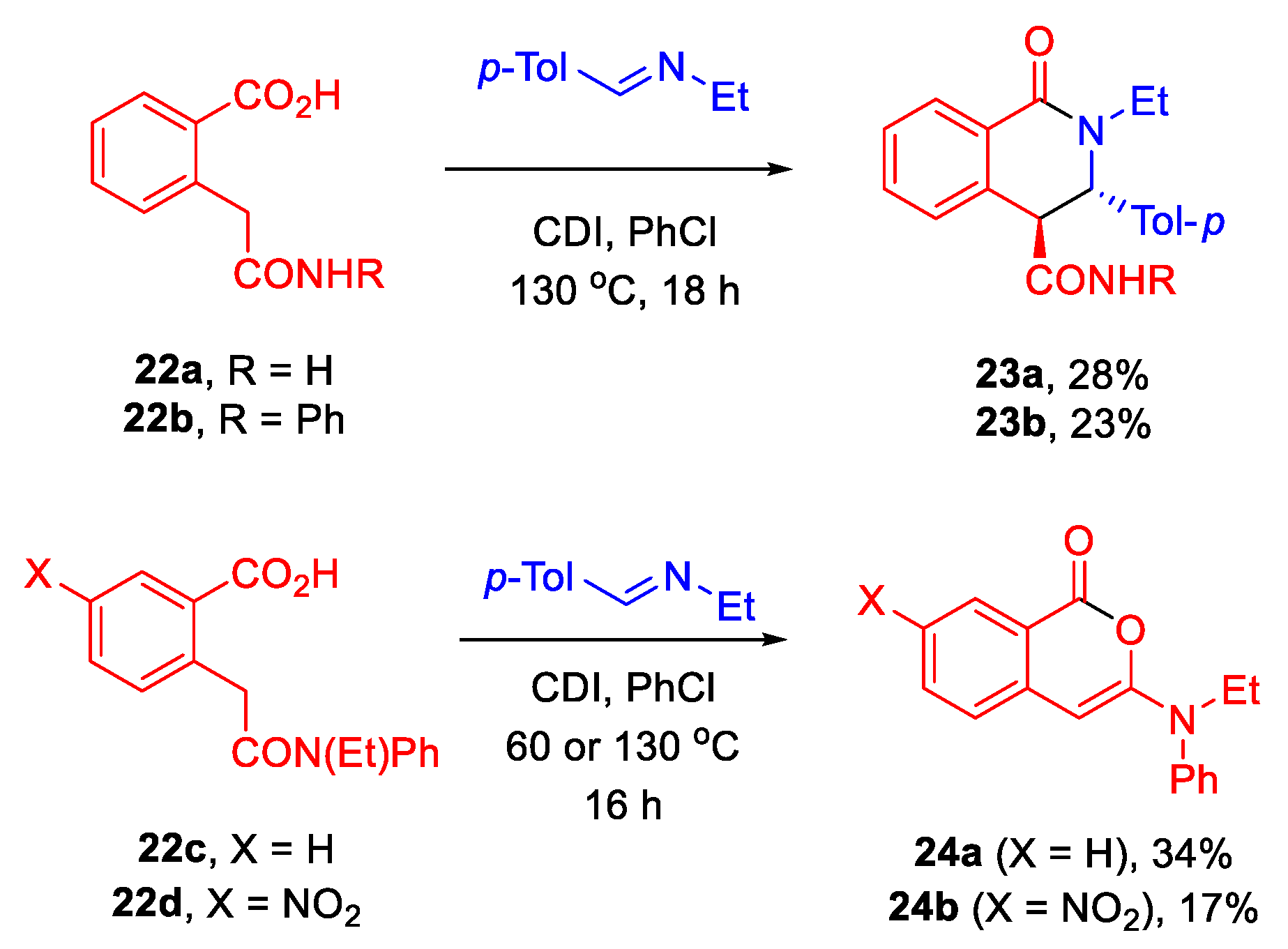
3.6. 2-(2-Amino-2-oxoethyl)benzoic Acid (22a)
3.7. Synthesis of Amides 22b–d
3.7.1. 2-(2-Oxo-2-(phenylamino)ethyl)benzoic Acid (22b)
3.7.2. 2-(2-(Ethyl(phenyl)amino)-2-oxoethyl)benzoic Acid (22c)
3.7.3. 2-((Ethyl(phenyl)amino)-2-oxoethyl)-5-nitrobenzoic Acid (22d)
3.8. Synthesis of Tetrahydroisoquinolonecarboamides 23a,b
3.8.1. (±)-(3S,4S)-2-Ethyl-1-oxo-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carboxamide (23a)
3.8.2. (±)-(3S,4S)-2-Ethyl-1-oxo-N-phenyl-3-(p-tolyl)-1,2,3,4-tetrahydroisoquinoline-4-carboxamide (23b)
3.9. Synthesis of Isochromenones 24
3.9.1. 3-(Ethyl(phenyl)amino)-1H-isochromen-1-one (24a)
3.9.2. 3-(Ethyl(phenyl)amino)-7-nitro-1H-isochromen-1-one (24b)
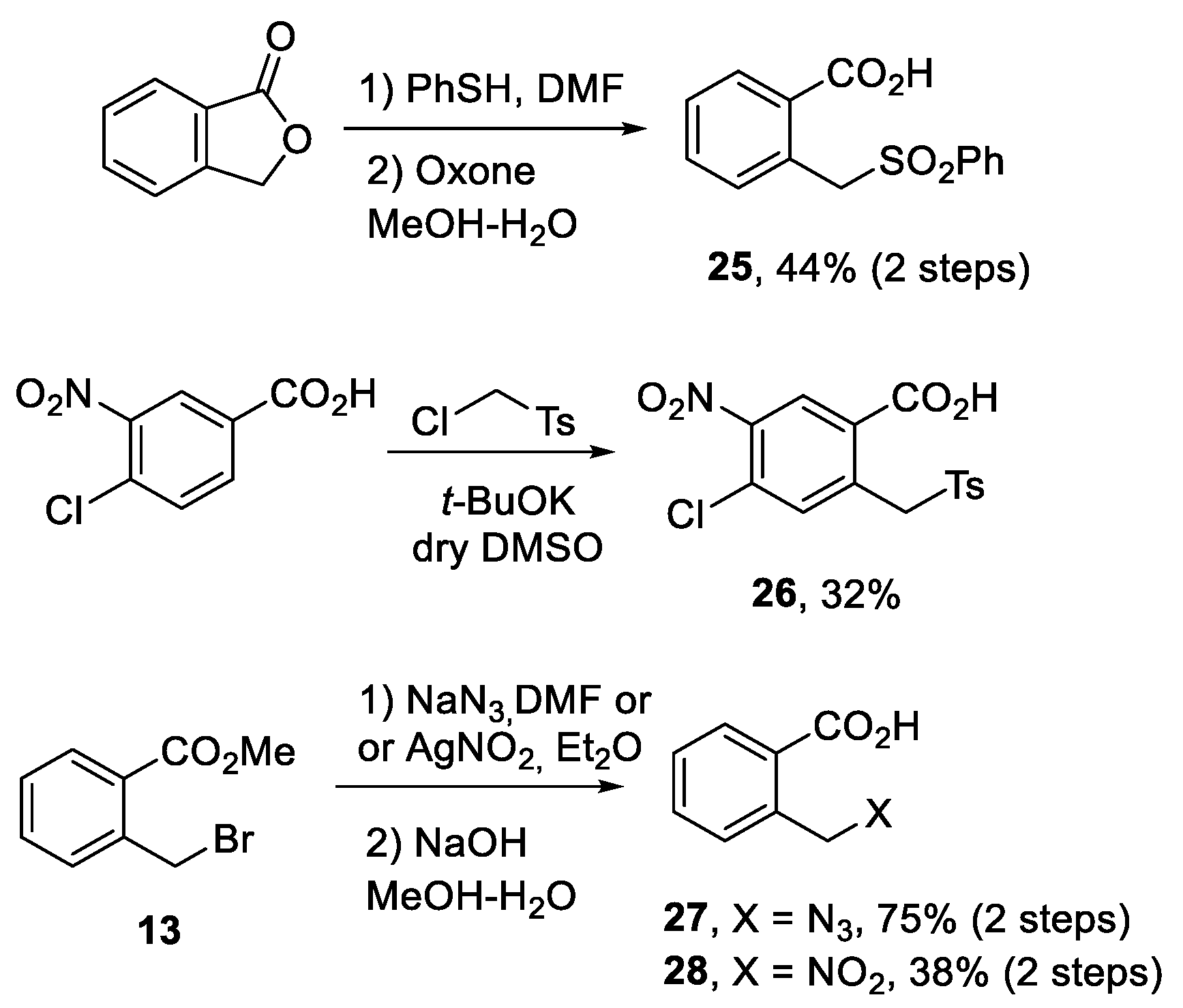
3.10. Synthesis of 4-Chloro-5-nitro-2-(tosylmethyl)benzoic Acid (26)
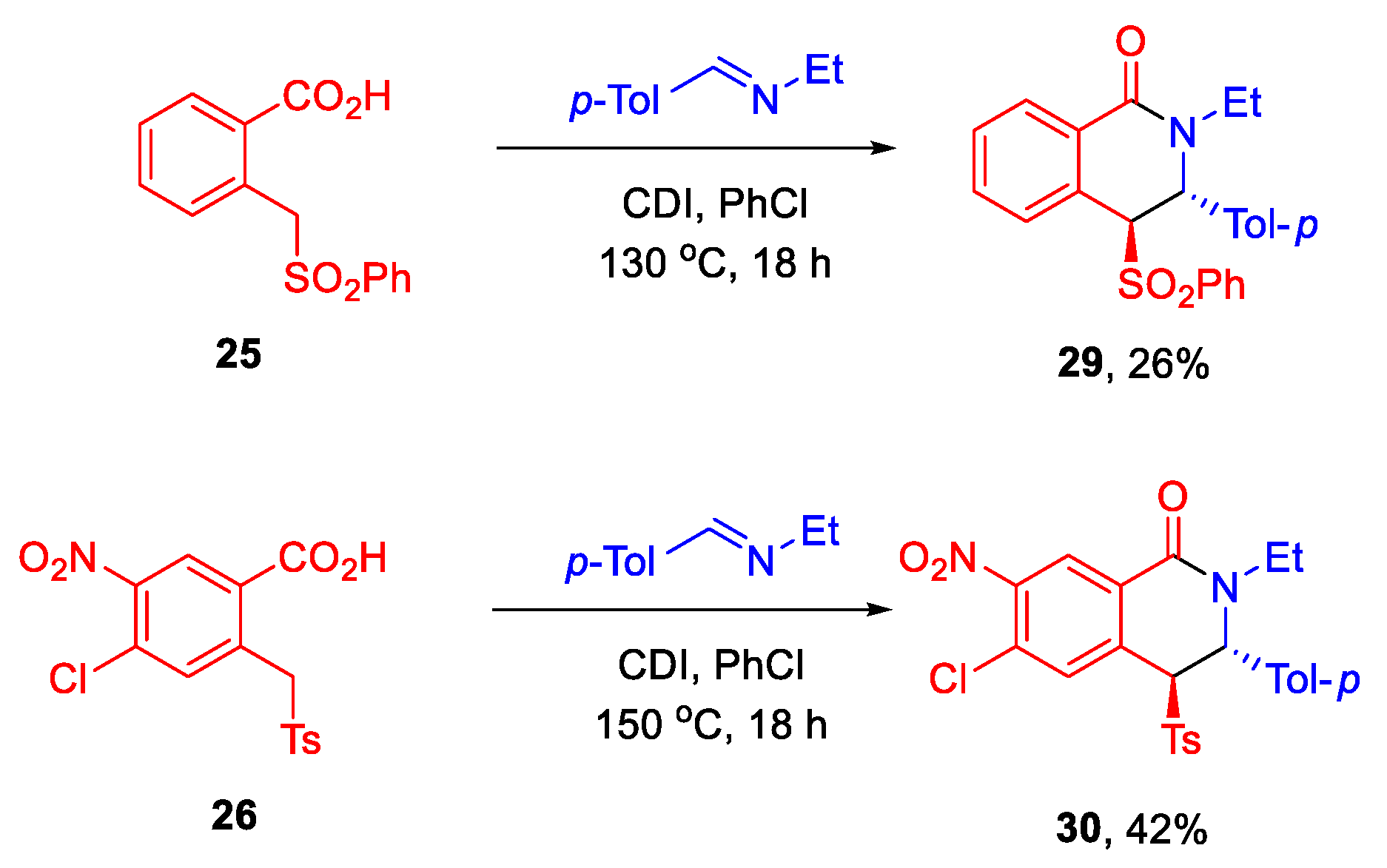
3.11. Synthesis of 3-Aryl Sulphonyl Tetrahydroisoquinolones 29 and 30
3.11.1. (±)-(3R,4S)-2-Ethyl-4-(phenylsulfonyl)-3-(p-tolyl)-3,4-dihydroisoquinolin-1(2H)-one (29)
3.11.2. (±)-(3R,4S)-6-Chloro-2-ethyl-7-nitro-3-(p-tolyl)-4-tosyl-3,4-dihydroisoquinolin-1(2H)-one (30)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Howard, S.Y.; Di Maso, M.J.; Shimabukuro, K.; Burlow, N.P.; Tan, D.Q.; Fettinger, J.C.; Malig, T.C.; Hein, J.E.; Shaw, J.T. Mechanistic Investigation of Castagnoli–Cushman Multicomponent Reactions Leading to a Three-Component Synthesis of Dihydroisoquinolones. J. Org. Chem. 2021, 86, 11599–11607. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Z.; Chen, J.; Ni, S.; Zhang, Y.; Shi, F. Rational Design of Axially Chiral Styrene-Based Organocatalysts and Their Application in Catalytic Asymmetric (2+4) Cyclizations. Angew. Chem. Int. Ed. 2021, 61, e202112226. [Google Scholar] [CrossRef]
- Burdzhiev, N.T.; Baramov, T.I.; Stanoeva, E.R.; Yanev, S.G.; Stoyanova, T.D.; Dimitrova, D.H.; Kostadinova, K.A. Synthesis of novel trans-4-(phthalimidomethyl)- and 4-(imidazol-1-ylmethyl)-3-indolyl-tetrahydroisoquinolinones as possible aromatase inhibitors. Chem. Pap. 2019, 73, 1263–1277. [Google Scholar] [CrossRef]
- Safrygin, A.; Zhmurov, P.; Dar’In, D.; Silonov, S.; Kasatkina, M.; Zonis, Y.; Gureev, M.; Krasavin, M. Three-component Castagnoli-Cushman reaction with ammonium acetate delivers 2-unsubstituted isoquinol-1-ones as potent inhibitors of poly(ADP-ribose) polymerase (PARP). J. Enzym. Inhib. Med. Chem. 2021, 36, 1916–1921. [Google Scholar] [CrossRef]
- Garcia, J.; Eichwald, J.; Zesiger, J.; Beng, T.K. Leveraging the 1,3-azadiene-anhydride reaction for the synthesis of functionalized piperidines bearing up to five contiguous stereocenters. RSC Adv. 2021, 12, 309–318. [Google Scholar] [CrossRef]
- Pashev, A.; Burdzhiev, N.; Stanoeva, E. One-step route to tricyclic fused 1,2,3,4-tetrahydroisoquinoline systems via the Castagnoli–Cushman protocol. Beilstein J. Org. Chem. 2020, 16, 1456–1464. [Google Scholar] [CrossRef]
- Krasavin, M.; Dar’In, D. Current diversity of cyclic anhydrides for the Castagnoli–Cushman-type formal cycloaddition reactions: Prospects and challenges. Tetrahedron Lett. 2016, 57, 1635–1640. [Google Scholar] [CrossRef]
- Lepikhina, A.; Dar’In, D.; Bakulina, O.; Chupakhin, E.; Krasavin, M. Skeletal Diversity in Combinatorial Fashion: A New Format for the Castagnoli–Cushman Reaction. ACS Comb. Sci. 2017, 19, 702–707. [Google Scholar] [CrossRef]
- Bakulina, O.; Chizhova, M.; Dar’In, D.; Krasavin, M. A General Way to Construct Arene-Fused Seven-Membered Nitrogen Heterocycles. Eur. J. Org. Chem. 2018, 2018, 362–371. [Google Scholar] [CrossRef]
- Firsov, A.; Chupakhin, E.; Dar’In, D.; Bakulina, O.; Krasavin, M. Three-Component Castagnoli–Cushman Reaction of 3-Arylglutaconic Acids with Aromatic Aldehydes and Amines Delivers Rare 4,6-Diaryl-1,6-dihydropyridin-2(3H)-ones. Org. Lett. 2019, 21, 1637–1640. [Google Scholar] [CrossRef]
- Guranova, N.; Bakulina, O.; Dar’In, D.; Kantin, G.; Krasavin, M. Homophthalic Esters: A New Type of Reagents for the Castagnoli-Cushman Reaction. Eur. J. Org. Chem. 2022, 2022, e202101281. [Google Scholar] [CrossRef]
- Ivashchenko, A.V.; Tkachenko, S.Y.; Okun, I.M.; Rivkees, S.A.; Kravchenko, D.V.; Khvat, A.V. Pharmaceutical Composition, Method for the Production and the Use Thereof. Int. Pat. Appl. WO 2007133108, 22 November 2007. [Google Scholar]
- Okun, I.; Balakin, K.; Tkachenko, S.; Ivachtchenko, A. Caspase Activity Modulators as Anticancer Agents. Anti-Cancer Agents Med. Chem. 2008, 8, 322–341. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.A.; da Silva, E.B.; Braga, S.F.; Leite, P.G.; Martins, L.C.; Vieira, R.P.; Soh, W.T.; Villela, F.S.; Costa, F.M.; Ray, D.; et al. Discovery and characterization of trypanocidal cysteine protease inhibitors from the ‘malaria box’. Eur. J. Med. Chem. 2019, 179, 765–778. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, F.; Hammill, J.; Holbrook, G.; Yang, L.; Freeman, B.; White, K.L.; Shackleford, D.M.; O’Loughlin, K.G.; Charman, S.A.; et al. Selecting an anti-malarial clinical candidate from two potent dihydroisoquinolones. Malar. J. 2021, 20, 1–15. [Google Scholar] [CrossRef]
- Cánepa, A.S.; Bravo, R.D. A Convenient Synthesis of 2-Acylphenylacetonitriles. Synth. Commun. 2004, 34, 579–588. [Google Scholar] [CrossRef]
- Makosza, M.; Ludwiczak, S. Vicarious Nucleophilic Substitution of Hydrogen in Nitrobenzoic Acids. Synthesis 1986, 1986, 50–52. [Google Scholar] [CrossRef]
- Hill, R.; Newkome, G. The absolute configuration of physostigmine. Tetrahedron 1969, 25, 1249–1260. [Google Scholar] [CrossRef]
- Lyapustin, D.; Ulomsky, E.; Balyakin, I.; Shchepochkin, A.; Rusinov, V.; Chupakhin, O. Oxidative Aromatization of 4,7-Dihydro-6-nitroazolo[1,5-a]pyrimidines: Synthetic Possibilities and Limitations, Mechanism of Destruction, and the Theoretical and Experimental Substantiation. Molecules 2021, 26, 4719. [Google Scholar] [CrossRef]
- Kantin, G.; Dar’In, D.; Krasavin, M. RhII -Catalyzed Cycloaddition of α-Diazo Homophthalimides and Nitriles Delivers Oxazolo[5,4-c]isoquinolin-5(4H )-one Scaffold. Eur. J. Org. Chem. 2018, 2018, 4857–4859. [Google Scholar] [CrossRef]
- Himo, F.; Demko, Z.P.; Noodleman, L.; Sharpless, K.B. Mechanisms of Tetrazole Formation by Addition of Azide to Nitriles. J. Am. Chem. Soc. 2002, 124, 12210–12216. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Huryn, D.; Smith, A.B. Carboxylic Acid (Bio)Isosteres in Drug Design. ChemMedChem 2013, 8, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Floyd, D.M.; Stein, P.; Wang, Z.; Liu, J.; Castro, S.; Clark, J.A.; Connelly, M.; Zhu, F.; Holbrook, G.; Matheny, A.; et al. Hit-to-Lead Studies for the Antimalarial Tetrahydroisoquinolone Carboxanilides. J. Med. Chem. 2016, 59, 7950–7962. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Fuchs, N.; Grathwol, C.; Hellmich, U.A.; Wagner, A.; Diehl, E.; Willmes, T.; Sotriffer, C.; Schirmeister, T. New peptidomimetic rhodesain inhibitors with improved selectivity towards human cathepsins. Eur. J. Med. Chem. 2022, 238. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.L.; Rodriguez-Aristegui, S.; Bardos, J.; Cano, C.; Golding, B.T.; Hardcastle, I.R.; Peacock, M.; Parveen, N.; Griffin, R.J. Mapping the ATP-binding domain of DNA-dependent protein kinase (DNA-PK) with coumarin- and isocoumarin-derived inhibitors. Bioorganic Med. Chem. Lett. 2010, 20, 3649–3653. [Google Scholar] [CrossRef] [PubMed]
- Sreedharan, D.T.; Clive, D.L.J. Asymmetric synthesis of carbocycles: Use of intramolecular conjugate displacement. Org. Biomol. Chem. 2013, 11, 3128–3144. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Demaegdt, H.; Vauquelin, G.; Lindeberg, G.; Karlén, A.; Hallberg, M. Ligands to the (IRAP)/AT4 receptor encompassing a 4-hydroxydiphenylmethane scaffold replacing Tyr2. Bioorg. Med. Chem. 2008, 16, 6924–6935. [Google Scholar] [CrossRef] [PubMed]
- Schneken, B. Pharm. Zentr. 1968; 107, 284–288. [Google Scholar]
- Sorto, N.A.; Di Maso, M.J.; Muñoz, M.A.; Dougherty, R.J.; Fettinger, J.C.; Shaw, J.T. Diastereoselective Synthesis of γ- and δ-Lactams from Imines and Sulfone-Substituted Anhydrides. J. Org. Chem. 2014, 79, 2601–2610. [Google Scholar] [CrossRef]
- Wegscheider, R.; Glogau, A. Üntersuchungen über die Veresterung unsymmetrischer zwei- und mehrbasischer Säuren. Mon. Chem. 1903, 24, 915–958. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guranova, N.; Yakovleva, L.; Bakulina, O.; Dar’in, D.; Krasavin, M. Extending the Scope of the New Variant of the Castagnoli–Cushman Cyclocondensation onto o-Methyl Benzoic Acids Bearing Various Electron-Withdrawing Groups in the α-Position. Molecules 2022, 27, 7211. https://doi.org/10.3390/molecules27217211
Guranova N, Yakovleva L, Bakulina O, Dar’in D, Krasavin M. Extending the Scope of the New Variant of the Castagnoli–Cushman Cyclocondensation onto o-Methyl Benzoic Acids Bearing Various Electron-Withdrawing Groups in the α-Position. Molecules. 2022; 27(21):7211. https://doi.org/10.3390/molecules27217211
Chicago/Turabian StyleGuranova, Natalia, Lyudmila Yakovleva, Olga Bakulina, Dmitry Dar’in, and Mikhail Krasavin. 2022. "Extending the Scope of the New Variant of the Castagnoli–Cushman Cyclocondensation onto o-Methyl Benzoic Acids Bearing Various Electron-Withdrawing Groups in the α-Position" Molecules 27, no. 21: 7211. https://doi.org/10.3390/molecules27217211
APA StyleGuranova, N., Yakovleva, L., Bakulina, O., Dar’in, D., & Krasavin, M. (2022). Extending the Scope of the New Variant of the Castagnoli–Cushman Cyclocondensation onto o-Methyl Benzoic Acids Bearing Various Electron-Withdrawing Groups in the α-Position. Molecules, 27(21), 7211. https://doi.org/10.3390/molecules27217211






