Structural Analysis of the Complex of Human Transthyretin with 3′,5′-Dichlorophenylanthranilic Acid at 1.5 Å Resolution
Abstract
1. Introduction
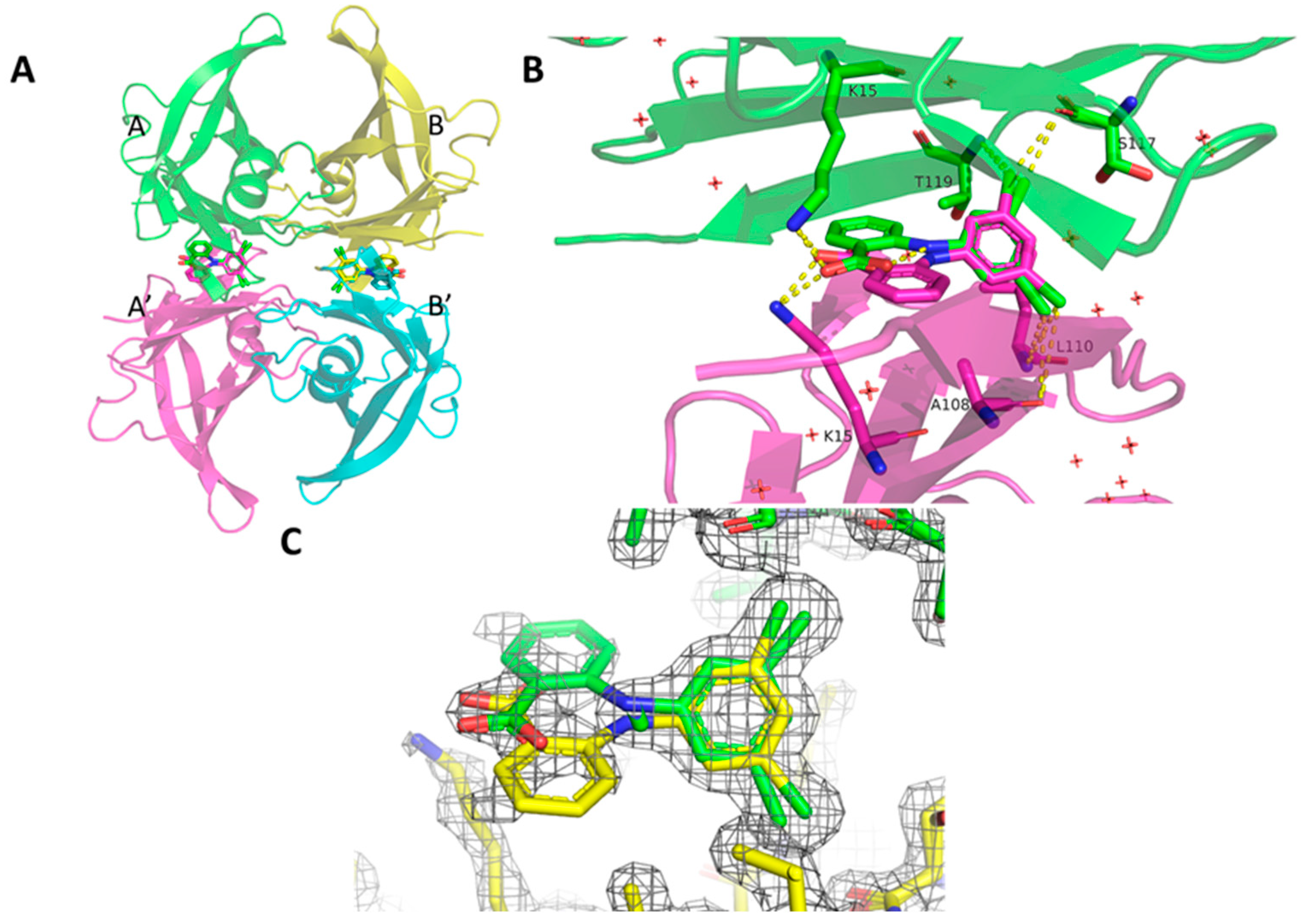
2. Results
3. Discussion
4. Materials and Methods
| Wavelength (Å) | 0.9537 |
| Resolution range (Å) | 51.06–1.52 (1.55–1.52) |
| Space group | I 2 2 2 |
| Unit cell dimensions | |
| a (Å) | 41.20 |
| b (Å) | 64.08 |
| c (Å) | 84.53 |
| α (Å) | 90 |
| β (Å) | 90 |
| γ (Å) | 90 |
| Total reflections | 222,685 (11,136) |
| Unique reflections | 17632 (859) |
| Multiplicity | 12.6 (13.0) |
| Completeness (%) | 99.93 (100.0) |
| Mean I/σ(I) | 11.0 (1.0) |
| Wilson B-factor (Å2) | 22.79 |
| Rmergea | 0.1185 (3.067) |
| Rmeasb | 0.1235 (3.19) |
| Rpimc | 0.0346 (0.8693) |
| CC1/2d | 0.999 (0.337) |
| PDB accession | 8dw5 |
| Resolution range (Å) | 51.06–1.52 (1.574–1.52) |
| Reflections used in refinement | 17630 (1736) |
| Reflections used for R-free | 1763 (174) |
| Rworka | 0.1971 (0.3369) |
| Rfreeb | 0.2353 (0.3582) |
| CCwork | 0.965 (0.641) |
| CCfree | 0.923 (0.657) |
| Number of non-hydrogen atoms | 957 |
| macromolecules | 902 |
| ligands | 18 |
| solvent | 37 |
| Protein residues | 116 |
| RMS (bonds) | 0.014 |
| RMS (angles) | 1.66 |
| Ramachandran favored (%) | 98.25 |
| Ramachandran allowed (%) | 0.88 |
| Ramachandran outliers (%) | 0.88 |
| Rotamer outliers (%) | 1.02 |
| Clashscore | 6.09 |
| Average B-factor (Å2) | 30.57 |
| Macromolecules (Å2) | 30.51 |
| Ligands (Å2) | 22.80 |
| Solvent (Å2) | 35.71 |
| Number of TLS groups | 8 |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braverman, L.E.; Utiger, R.D. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text, 7th ed.; J.B.Lippincott: Philadelphia, PA, USA, 1996. [Google Scholar]
- Benson, M.D. Genetics: Clinical implications of TTR amyloidosis. In Recent Advances in Transthyretin Evolution, Structure and Biological Functions; Richardson, S.J., Cody, V., Eds.; Springer: Berlin, Germany, 2009; pp. 173–189. [Google Scholar]
- Calkins, E. Amyloidosis. Harrison’s Principles of Internal Medicine; McGraw-Hill: Tokyo, Japan, 1974; pp. 644–647. [Google Scholar]
- Munro, S.L.; Lim, C.F.; Hall, J.G.; Barlow, J.W.; Craik, D.J.; Topliss, D.J.; Stockigt, J.R. Drug competition for thyroxine binding to transthyretin (prealbumin): Comparison with effects on thyroxine-binding globulin. J. Clin. Endocrinol. Metab. 1989, 68, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.L. Studies of Thyroid Hormone Binding of Transthyretin. Ph.D. Thesis, Monash University, Melbourne, Australia, 1990. [Google Scholar]
- Cody, V.; Wojtczak, A.; Ciszak, E.; Luft, J.R. Differences in inhibitor and substrate binding in transthyretin crystal complexes. In Progress in Thyroid Research; Gordon, A.H., Gross, J., Hennemann, G., Eds.; Routledge: Oxfordshire, UK, 1991; pp. 793–796. [Google Scholar]
- Wojtczak, A.; Luft, J.; Cody, V. Mechanism of molecular recognition. Structural aspects of 3,3′-diiodo-L-thyronine binding to human serum transthyretin. J. Biol. Chem. 1992, 267, 353–357. [Google Scholar] [CrossRef]
- Ciszak, E.; Cody, V.; Luft, J.R. Crystal structure determination at 2.3-A resolution of human transthyretin-3′,5′-dibromo-2′,4,4′,6-tetrahydroxyaurone complex. Proc. Natl. Acad. Sci. USA 1992, 89, 6644–6648. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, D.K.; Scholz, G.H.; Topliss, D.J.; Kolliniatis, E.; Munro, S.L.; Craik, D.J.; Iskander, M.N.; Stockigt, J.R. Thyroid hormone uptake by hepatocytes: Structure-activity relationships of phenylanthranilic acids with inhibitory activity. J. Med. Chem. 1993, 36, 1272–1277. [Google Scholar] [CrossRef]
- Wojtczak, A.; Luft, J.R.; Cody, V. Structural aspects of inotropic bipyridine binding. Crystal structure determination to 1.9 A of the human serum transthyretin-milrinone complex. J. Biol. Chem. 1993, 268, 6202–6206. [Google Scholar] [CrossRef]
- Cody, V. Thyroid hormone structure-function relationships. In Werner & Ingbar’s The Thyroid: A Fundemental and Clinical Text, 8th ed.; Braverman, L.E., Utiger, R.D., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2000; pp. 196–201. [Google Scholar]
- Cody, V.; Wojtczak, A. Mechanisms of molecular recognition: Structural characteristics of transthyretin ligand interactions. In Recent Advances in Transthyretin Evolution, Structure and Biological Functions; Richardson, S.J., Cody, V., Eds.; Springer: Berlin, Germany, 2009; pp. 1–22. [Google Scholar]
- Bulawa, C.E.; Connelly, S.; Devit, M.; Wang, L.; Weigel, C.; Fleming, J.A.; Packman, J.; Powers, E.T.; Wiseman, R.L.; Foss, T.R.; et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc. Natl. Acad. Sci. USA 2012, 109, 9629–9634. [Google Scholar] [CrossRef]
- Grimster, N.P.; Connelly, S.; Baranczak, A.; Dong, J.; Krasnova, L.B.; Sharpless, K.B.; Powers, E.T.; Wilson, I.A.; Kelly, J.W. Aromatic sulfonyl fluorides covalently kinetically stabilize transthyretin to prevent amyloidogenesis while affording a fluorescent conjugate. J. Am. Chem. Soc. 2013, 135, 5656–5668. [Google Scholar] [CrossRef]
- Palhano, F.L.; Lee, J.; Grimster, N.P.; Kelly, J.W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013, 135, 7503–7510. [Google Scholar] [CrossRef]
- Alshehri, B.; D’Souza, D.G.; Lee, J.Y.; Petratos, S.; Richardson, S.J. The diversity of mechanisms influenced by transthyretin in neurobiology: Development, disease and endocrine disruption. J. Neuroendocrinol. 2015, 27, 303–323. [Google Scholar] [CrossRef]
- Dessì, A.; Peluso, P.; Dallocchio, R.; Weiss, R.; Andreotti, G.; Allocca, M.; Aubert, E.; Pale, P.; Mamane, V.; Cossu, S. Rational design, synthesis, characterization and evaluation of iodinated 4,4′-bipyridines as new transthyretin fibrillogenesis inhibitors. Molecules 2020, 25, 2213. [Google Scholar] [CrossRef]
- Yokoyama, T.; Mizuguchi, M. Transthyretin amyloidogenesis inhibitors: From discovery to current developments. J. Med. Chem. 2020, 63, 14228–14242. [Google Scholar] [CrossRef]
- McLean, T.R.; Rank, M.M.; Smooker, P.M.; Richardson, S.J. Evolution of thyroid hormone distributor proteins. Mol. Cell. Endocrinol. 2017, 459, 43–52. [Google Scholar] [CrossRef]
- Blake, C.C.; Geisow, M.J.; Oatley, S.J.; Rerat, B.; Rerat, C. Structure of prealbumin: Secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J. Mol. Biol. 1978, 121, 339–356. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Pages, R.A.; Saroff, H.A.; Edelhoch, H.; Robbins, J. Analysis of thyroid hormone binding to human serum prealbumin by 8-anilinonaphthalene-1-sulfonate fluorescence. Biochemistry 1977, 16, 3707–3713. [Google Scholar] [CrossRef]
- Robbins, J. Thyroid Hormone Transport Proteins and the Physiology of Hormone Binding. In The Thyroid—A Fundamental and Clinical Text, 8th ed.; Braverman, L.E., Utiger, R.D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; pp. 105–120. [Google Scholar]
- Chalmers, D.K. The Design and Synthesis of Novel Thyroid Hormone Analogs. Ph.D. Thesis, University of Melbourne, Melbourne, Australia, 1993. [Google Scholar]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Wiseman, R.L.; Johnson, S.M.; Kelker, M.S.; Foss, T.; Wilson, I.A.; Kelly, J.W. Kinetic stabilization of an oligomeric protein by a single ligand binding event. J. Am. Chem. Soc. 2005, 127, 5540–5551. [Google Scholar] [CrossRef]
- Oza, V.B.; Smith, C.; Raman, P.; Koepf, E.K.; Lashuel, H.A.; Petrassi, H.M.; Chiang, K.P.; Powers, E.T.; Sachettinni, J.; Kelly, J.W. Synthesis, structure, and activity of diclofenac analogues as transthyretin amyloid fibril formation inhibitors. J. Med. Chem. 2002, 45, 321–332. [Google Scholar] [CrossRef]
- Wojtczak, A.; Cody, V.; Luft, J.R.; Pangborn, W. Structures of human transthyretin complexed with thyroxine at 2.0 angstrom resolution and 3′,5′-dinitro-N-acetyl-L-thyronine at 2.2 angstrom resolution. Acta Crystallogr. Sect. D—Biol. Crystallogr. 1996, 52, 758–765. [Google Scholar] [CrossRef]
- Azevedo, E.P.C.; Pereira, H.M.; Garratt, R.C.; Kelly, J.W.; Foguel, D.; Palhano, F.L. Dissecting the Structure, Thermodynamic Stability, and Aggregation Properties of the A25T Transthyretin (A25T-TTR) Variant Involved in Leptomeningeal Amyloidosis: Identifying Protein Partners That Co-Aggregate during A25T-TTR Fibrillogenesis in Cerebrospinal Fluid. Biochemistry 2011, 50, 11070–11083. [Google Scholar]
- Zanotti, G.; Vallese, F.; Ferrari, A.; Menozzi, I.; Saldano, T.E.; Berto, P.; Fernandez-Alberti, S.; Berni, R. Structural and dynamics evidence for scaffold asymmetric flexibility of the human transthyretin tetramer. PLoS ONE 2017, 12, e0187716. [Google Scholar] [CrossRef]
- Sant’Anna, R.; Gallego, P.; Robinson, L.Z.; Pereira-Henriques, A.; Ferreira, N.; Pinheiro, F.; Esperante, S.; Pallares, I.; Huertas, O.; Rosário Almeida, M.; et al. Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nat. Commun. 2016, 7, 10787. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, T.; Petrassi, H.M.; Oza, V.B.; Raman, P.; Kelly, J.W.; Sacchettini, J.C. Rational design of potent human transthyretin amyloid disease inhibitors. Nat. Struct. Biol. 2000, 7, 312–321. [Google Scholar] [PubMed]
- Wojtczak, A.; Cody, V.; Luft, J.R.; Pangborn, W. Structure of rat transthyretin (rTTR) complex with thyroxine at 2.5 A resolution: First non-biased insight into thyroxine binding reveals different hormone orientation in two binding sites. Acta Crystallogr. Sect. D—Biol. Crystallogr. 2001, 57, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Cody, V.; Wojtczak, A. Structural basis of negative cooperativity in transthyretin. Acta Biochim. Pol. 2001, 48, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, A.; Neumann, P.; Cody, V. Structure of a new polymorphic monoclinic form of human transthyretin at 3 A resolution reveals a mixed complex between unliganded and T4-bound tetramers of TTR. Acta Crystallogr. Sect. D—Biol. Crystallogr. 2001, 57, 957–967. [Google Scholar] [CrossRef]
- Muziol, T.; Cody, V.; Luft, J.R.; Pangborn, W.; Wojtczak, A. Complex of rat transthyretin with tetraiodothyroacetic acid refined at 2.1 and 1.8 A resolution. Acta Biochim. Pol. 2001, 48, 877–884. [Google Scholar] [CrossRef]
- Muziol, T.; Cody, V.; Wojtczak, A. Comparison of binding interactions of dibromoflavonoids with transthyretin. Acta Biochim. Pol. 2001, 48, 885–892. [Google Scholar] [CrossRef]
- Cody, V. Mechanisms of molecular recognition: Crystal structure analysis of human and rat transthyretin inhibitor complexes. Clin. Chem. Lab. Med. 2002, 40, 1237–1243. [Google Scholar] [CrossRef]
- Kohrle, J.; Fang, S.L.; Yang, Y.; Irmscher, K.; Hesch, R.D.; Pino, S.; Alex, S.; Braverman, L.E. Rapid effects of the flavonoid EMD 21388 on serum thyroid- hormone binding and thyrotropin regulation in the rat. Endocrinology 1989, 125, 532–537. [Google Scholar] [CrossRef]
- Rosen, H.N.; Murrell, J.R.; Liepnieks, J.J.; Benson, M.D.; Cody, V.; Moses, A.C. Threonine for alanine substitution at position 109 of transthyretin differentially alters human transthyretin’s affinity for iodothyronines. Endocrinology 1994, 134, 27–34. [Google Scholar] [CrossRef]
- Cody, V. Mechanisms of molecular recognition: Crystallographic evidence for multiple inhibitor binding modes in two protein classes. J. Mol. Graph. 1994, 12, 77–78. [Google Scholar] [CrossRef]
- Aragao, D.; Aishima, J.; Cherukuvada, H.; Clarken, R.; Clift, M.; Cowieson, N.P.; Ericsson, D.J.; Gee, C.L.; Macedo, S.; Mudie, N.; et al. MX2: A high-flux undulator microfocus beamline serving both the chemical and macromolecular crystallography communities at the Australian Synchrotron. J. Synchro. Rad. 2018, 25, 885–891. [Google Scholar] [CrossRef]
- Battye, T.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. Sect. D—Biol. Crystallogr. 2011, 67, 271–281. [Google Scholar] [CrossRef]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. Sect. D—Biol. Crystallogr. 2013, 69, 1204–1214. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Adams, P.D.; Moriarty, N.W.; Cohn, J.D. Ligand identification using electron-density map correlations. Acta Crystallogr. Sect. D—Biol. Crystallogr. 2007, 63, 101–107. [Google Scholar] [CrossRef]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D—Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D—Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Arndt, U.W.; Crowther, R.A.; Mallett, J.F. A computer-linked cathode-ray tube microdensitometer for x-ray crystallography. J. Sci. Instrum. 1968, 1, 510–516. [Google Scholar] [CrossRef]
- Diederichs, K.; Karplus, P.A. Improved R-factors for diffraction data analysis in macromolecular crystallography. Nat. Struct. Biol. 1997, 4, 269–275. [Google Scholar] [CrossRef]
- Weiss, M.S.; Hilgenfeld, R. On the use of the merging R factor as a quality indicator for X-ray data. J. Appl. Crystallogr. 1997, 30, 203–205. [Google Scholar] [CrossRef]
- Karplus, P.A.; Diederichs, K. Linking crystallographic model and data quality. Science 2012, 336, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Brunger, A.T. Free R value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 1992, 355, 472–475. [Google Scholar] [CrossRef] [PubMed]

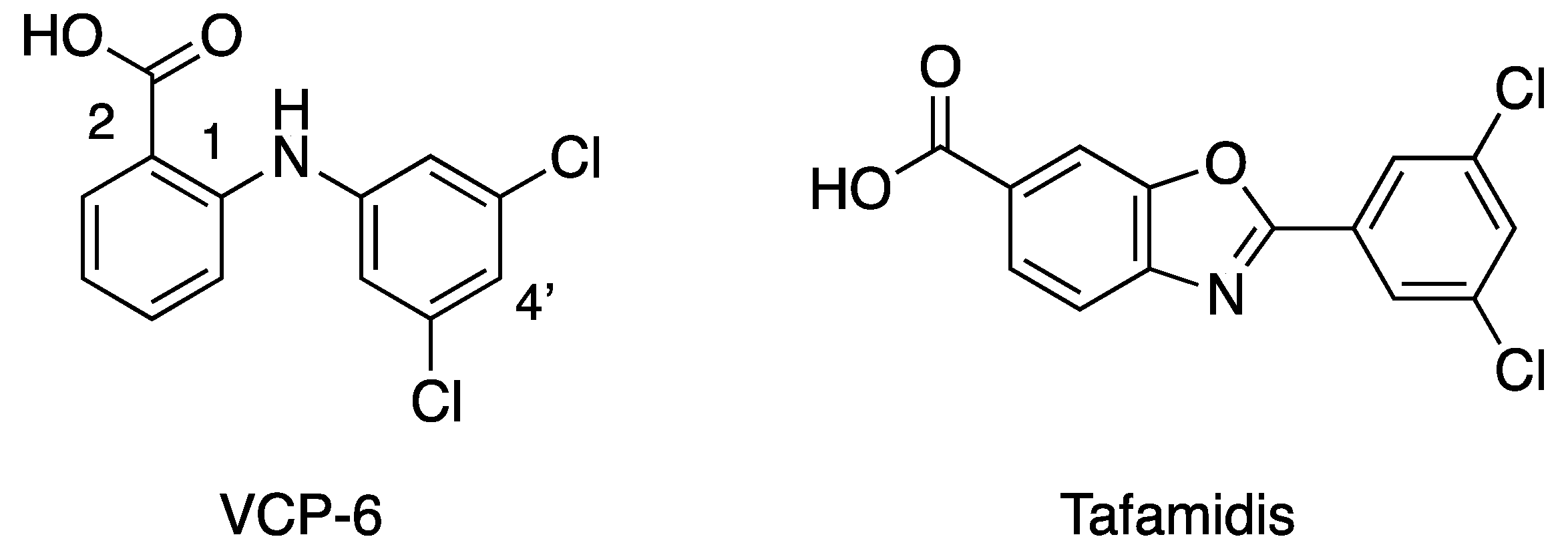

 | |||
|---|---|---|---|
| Compound | R3′ | R5′ | pIC50 |
| T4 | - | - | 6.43 |
| VCP-2 | Cl | H | 6.9 |
| VCP-5 | CF3 | CF3 | 7.09 |
| VCP-6 | Cl | Cl | 7.14 |
| VCP-16 | I | I | 6.89 |
| VCP-17 | Br | Br | 6.73 |
| VCP-19 | Br | H | 6.80 |
| VCP-20 | I | H | 7.07 |
| VCP-21 | CH3 | H | 6.32 |
| VCP-25 | CN | H | 6.24 |
| Flufenamic acid | CF3 | H | 6.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cody, V.; Truong, J.Q.; Holdsworth, B.A.; Holien, J.K.; Richardson, S.J.; Chalmers, D.K.; Craik, D.J. Structural Analysis of the Complex of Human Transthyretin with 3′,5′-Dichlorophenylanthranilic Acid at 1.5 Å Resolution. Molecules 2022, 27, 7206. https://doi.org/10.3390/molecules27217206
Cody V, Truong JQ, Holdsworth BA, Holien JK, Richardson SJ, Chalmers DK, Craik DJ. Structural Analysis of the Complex of Human Transthyretin with 3′,5′-Dichlorophenylanthranilic Acid at 1.5 Å Resolution. Molecules. 2022; 27(21):7206. https://doi.org/10.3390/molecules27217206
Chicago/Turabian StyleCody, Vivian, Jia Q. Truong, Bruce A. Holdsworth, Jessica K. Holien, Samantha J. Richardson, David K. Chalmers, and David J. Craik. 2022. "Structural Analysis of the Complex of Human Transthyretin with 3′,5′-Dichlorophenylanthranilic Acid at 1.5 Å Resolution" Molecules 27, no. 21: 7206. https://doi.org/10.3390/molecules27217206
APA StyleCody, V., Truong, J. Q., Holdsworth, B. A., Holien, J. K., Richardson, S. J., Chalmers, D. K., & Craik, D. J. (2022). Structural Analysis of the Complex of Human Transthyretin with 3′,5′-Dichlorophenylanthranilic Acid at 1.5 Å Resolution. Molecules, 27(21), 7206. https://doi.org/10.3390/molecules27217206








