The Antibacterial Activity of Egyptian Wasp Chitosan-Based Nanoparticles against Important Antibiotic-Resistant Pathogens
Abstract
1. Introduction
2. Experimental Procedures
2.1. Extraction of Wasp Chitosan (WCH)
2.2. Characterization of Wasp Chitosan (WCS)
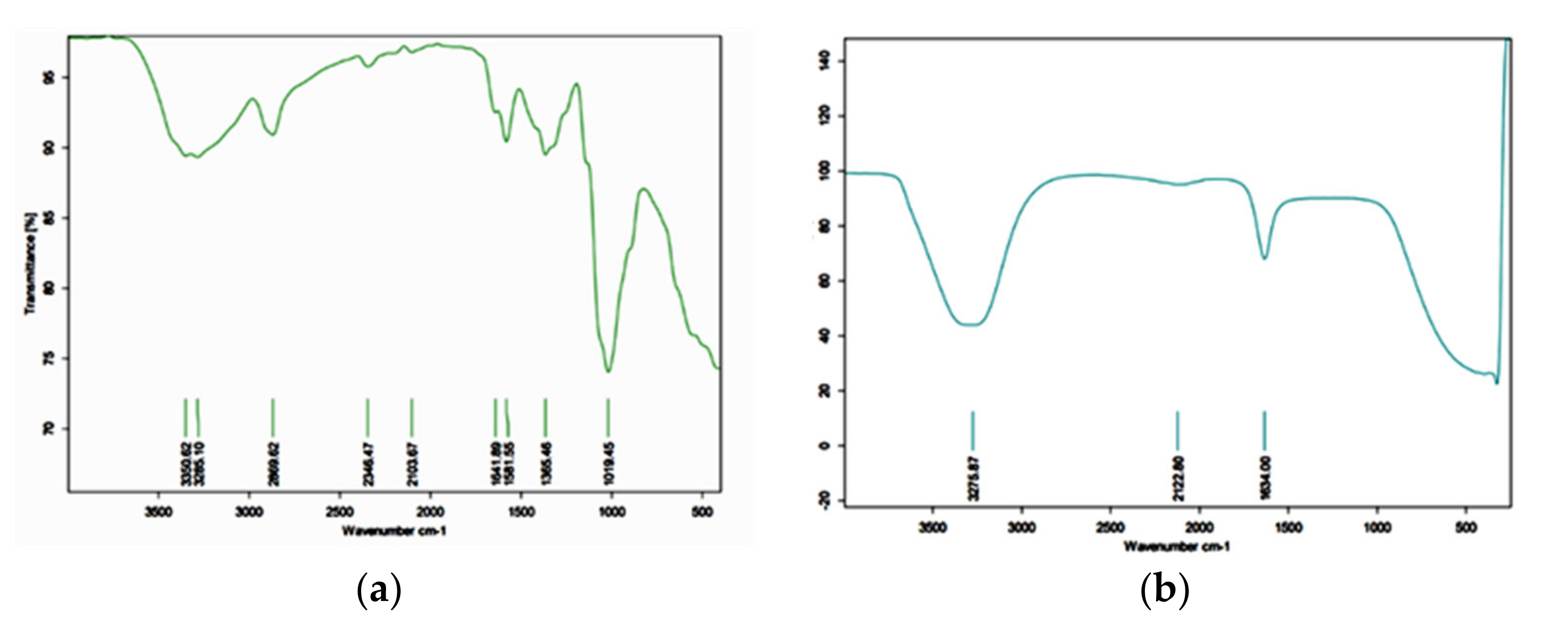
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. The Viscosity
2.3. Conversion of Wasp Chitosan (WCS) into Wasp Chitosan Nanoparticles (WCSNPs)
2.4. Characterization of Wasp Chitosan Nanoparticles (WCSNPs)
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
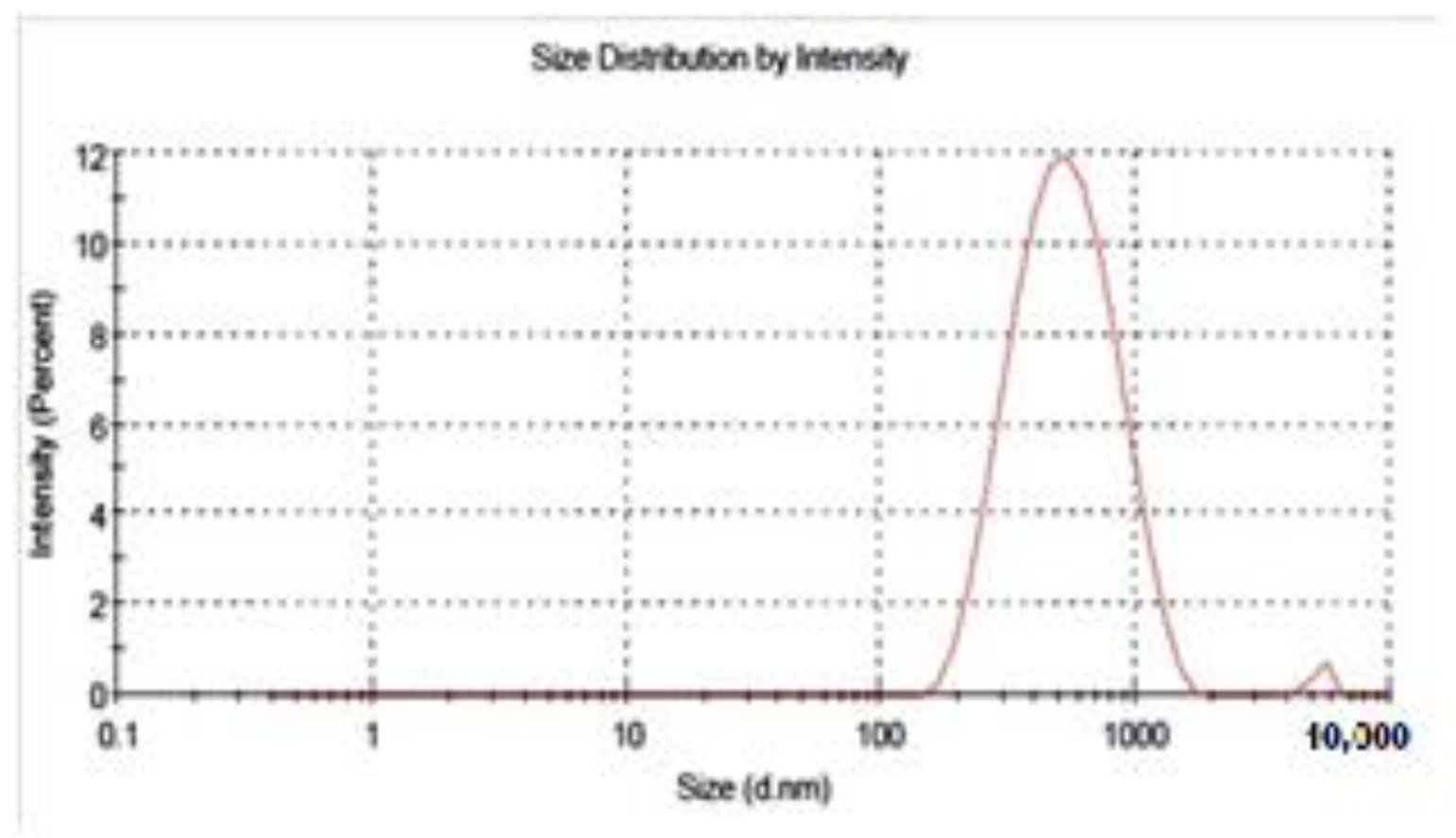
2.4.2. Droplet Size Distribution
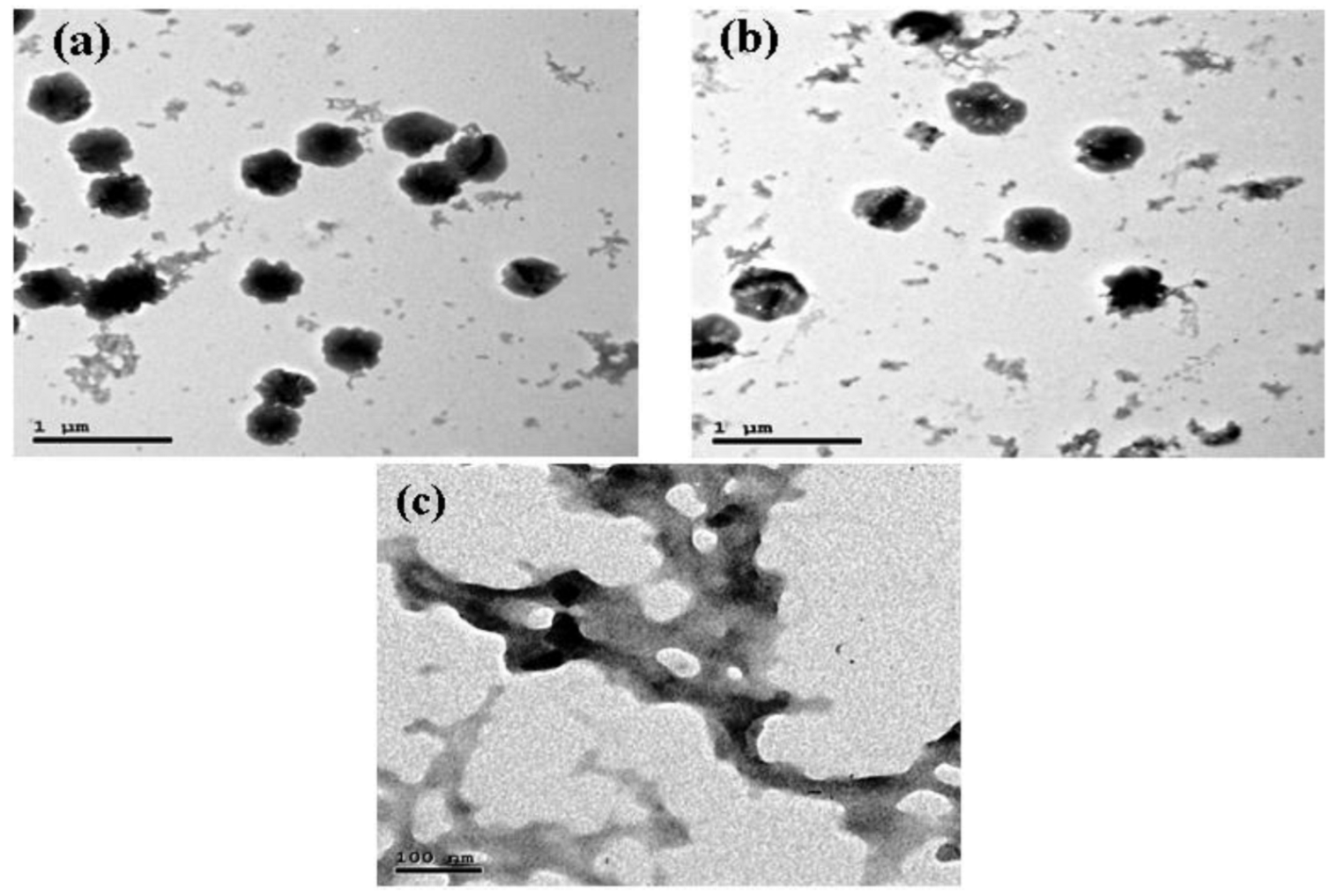
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. Stability
2.5. Evaluation of the Antimicrobial Activity of WCSNPs
2.5.1. Microbial Strains
2.5.2. Bacterial Growth Inhibition
2.5.3. Percent of Inhibition of Bacterial Growth
3. Results
3.1. Characterization of Extracted Chitosan
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR) of Wasp Chitosan
3.1.2. Viscosity Analysis
3.2. Characterization of Wasp Chitosan Nanoparticles (WCSNPs)
3.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.2. Dynamic Light Scattering (DLS) & Polydispersity Index (PDI)
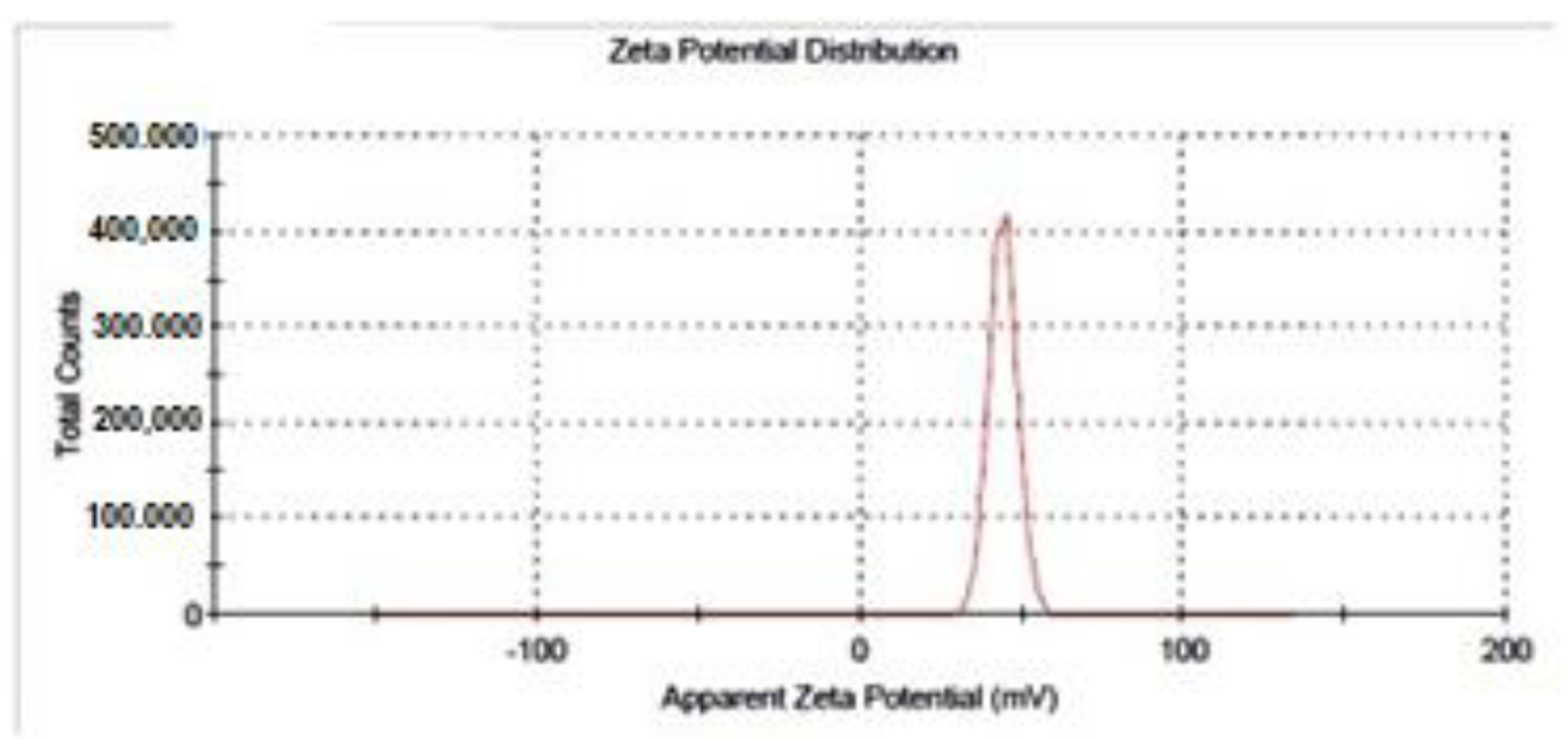
3.2.3. The Surface Charge of the Nanoparticles
3.2.4. Transmission Electron Microscopy (TEM)
3.3. Stability
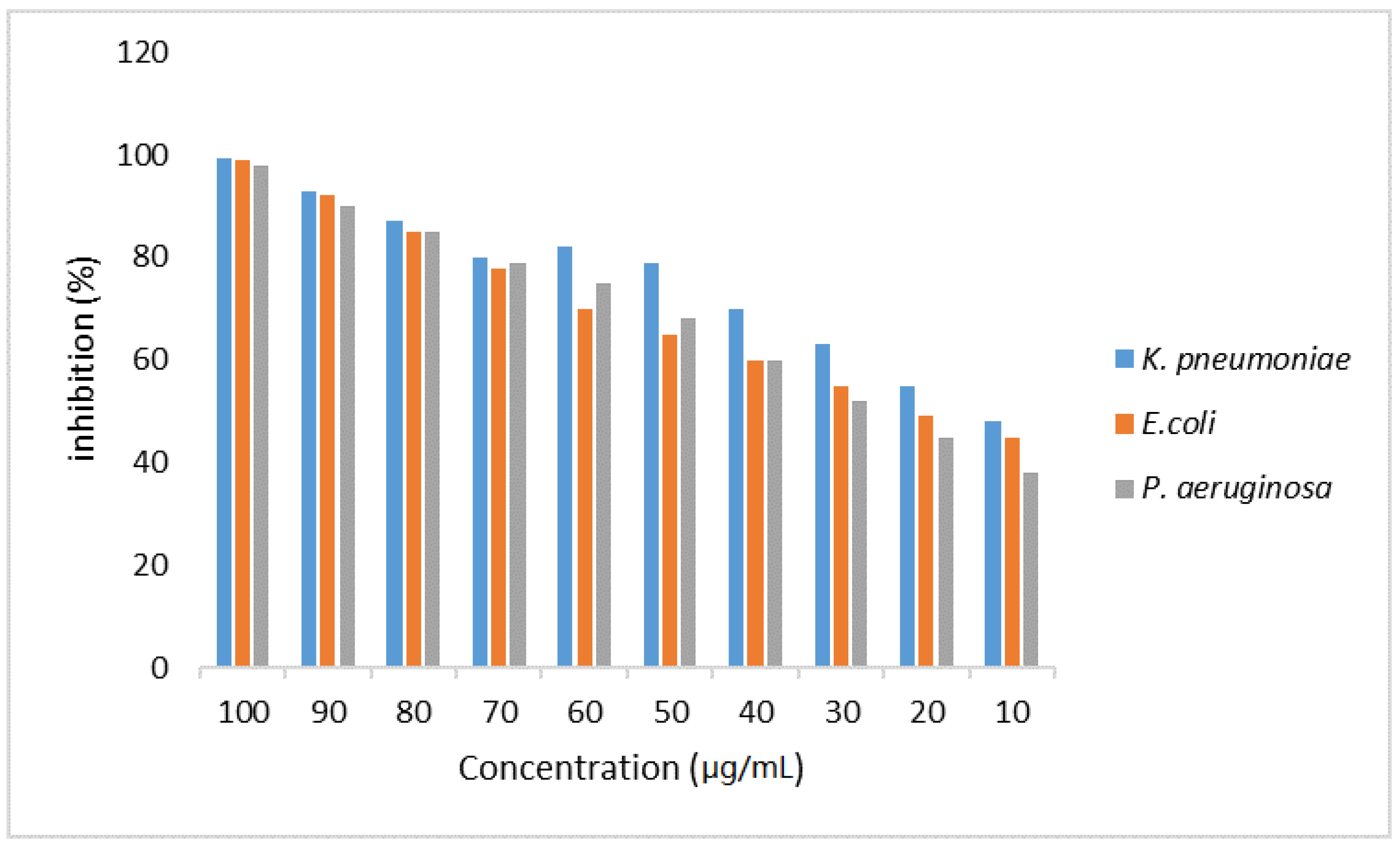
3.4. Evaluation of the Antimicrobial Activity of WCSNPs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis. Clin. N. Am. 2016, 30, 77–390. [Google Scholar] [CrossRef] [PubMed]
- Madec, J.Y.; Haenni, M.; Nordmann, P.; Poirel, L. Extended-spectrum β-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: A threat for humans? Clin. Microbiol. Infect. 2017, 23, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi. J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Rajivgandhi, G.; Muneeswaran, T.; Anand, M.; Quero, F. Highly efficient antibacterial activity of graphene/chitosan/magnetite nanocomposites against ESBL-producing Pseudomonas aeruginosa and Klebsiella pneumoniae. Colloids Surf. B Biointerfaces 2021, 202, 111690. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, S.C.; Casellato, S.; Rossini, M.; Paola, G.; Tejada, M.; Costa, E.; Carmignani, L. Extended-spectrum beta-lactamase-positive Escherichia coli causing complicated upper urinary tract infection: Urologist should act in time. Urol. Ann. 2014, 6, 107–112. [Google Scholar] [CrossRef]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.; Essa, E.; Ma’moun, S.; Mo’men, S.; Salama, M.; Barakat, E.; Abou-Elmagd, W. Characterization and solubilization of chitosan from the oriental hornet (vespa orientalis). J. Egypt. Soc. Parasitol. 2018, 48, 669–676. [Google Scholar] [CrossRef]
- Pal, P.; Roy, S. Edible insects: Future of human food–A review. Int. Lett. Nat. Sci. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Essa, E.E.; Mo’men, S.A.A.; Rady, M.H.; Ma’moun, S.A.M.; Barakat, E.; Salama, M.S. Eucalyptus oil nano-emulsion encapsulated in chitosan beads as a new approach in control of Culex pipiens larvae. Int. J. Mosq. Res. 2019, 6, 63–69. [Google Scholar]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Ortiz, J.C.; Ruiz, A.T.; Morales-Ramos, J.; Thomas, M.; Rojas, M.; Tomberlin, J.; Yi, L.; Han, R.; Giroud, L.; Jullien, R. Insects as Sustainable Food Ingredients: Production, Processing and Food Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–201. [Google Scholar]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Bikiaris, D.; Avgoustakis, K.; Karavas, E.; Georgarakis, M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr. Polym. 2008, 73, 44–54. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef]
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 907–916. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods–A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Kumar, D.; Verma, A. Isolation and degree of deacetylation of chitin from cultured biomass of diatoms. Bionotes 2012, 14, 116–117. [Google Scholar]
- Kittur, F.S.; Kumar, A.B.V.; Tharanathan, R.N. Low molecular weight chitosans—Preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr. Res. 2003, 338, 1283–1290. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Formulation and characterization of plant essential oil based nanoemulsion: Evaluation of its larvicidal activity against Aedes aegypti. Asian J. Chem. 2013, 25, S321–S323. [Google Scholar]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Elshafiee, E.A.; Nader, S.M.; Dorgham, S.M.; Hamza, D.A. Carbapenem-resistant Pseudomonas Aeruginosa Originating from Farm Animals and People in Egypt. J. Vet. Res. 2019, 63, 333–337. [Google Scholar] [CrossRef]
- Hamza, D.; Dorgham, S.; Ismael, E.; El-Moez, S.I.A.; Elhariri, M.; Elhelw, R.; Hamza, E. Emergence of β-lactamase- and carbapenemase- producing Enterobacteriaceae at integrated fish farms. Antimicrob. Resist. Infect. Control. Vol. 2020, 9, 67. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Rajivgandhi, G.; Muneeswaran, T.; Song, J.M.; Manoharan, N. Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microb. Pathog. 2018, 121, 224–231. [Google Scholar] [CrossRef]
- Fernando, S.S.N.; Gunasekara, T.D.C.P.; Holton, J. Antimicrobial nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug. Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Kumar, M.; Curtis, A.; Hoskins, C. Application of Nanoparticle Technologies in the Combat against Anti-Microbial Resistance. Pharmaceutics 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.H.; Yarce, C.J.; Roman, Y.; Davalos, A.F.; Rivera, G.R. Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex. Pharmaceuticals 2018, 11, 19. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I: Crystallographic properties. Polym. Int. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Özüsağlam, M.A.; Çakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 72–81. [Google Scholar] [CrossRef]
- Kim, M.-W.; Song, Y.-S.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Park, Y.-K.; Kang, S.H.; Kim, S.-A.; Choi, C.; Jung, W.-J. Production of chitin and chitosan from the exoskeleton of adult two-spotted field crickets (Gryllus bimaculatus). Entomol. Res. 2017, 47, 279–285. [Google Scholar] [CrossRef]
- Zivanovic, S.; Basurto, C.C.; Chi, S.; Davidson, P.M.; Weiss, J. Molecular weight of chitosan influences antimicrobial activity in oil-in-water emulsions. J. Food Prot. 2004, 67, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Kumar, A.B.V.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391, 167–175. [Google Scholar]
- Mohamed, N.M.; Hamouda, A.S.; Abdel Kader, S.; El Kady, M.Y.; Khalil, M.M.H. Removal of cadmium from aqueous solution using modified magnetic glycine modified cross-linked chitosan resin. Egypt. J. Aquat. Biol. Fish. 2021, 25, 550–570. [Google Scholar] [CrossRef]
- Cortizo, M.S.; Berghoff, C.F.; Alessandrini, J.L. Characterization of chitin from Illex argentinus squid pen. Carbohydr. Polym. 2008, 74, 10–15. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. AAPS Pharm. Sci. Tech. 2006, 7, E50. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Farrugia, B.L.; Dargaville, T.R.; Dhara, S. Physico-chemical/biological properties of tripolyphosphate cross-linked chitosan based nanofibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1446–1454. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug. Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Nasti, A.; Zaki, N.M.; de Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef]
- Tsai, M.L.; Bai, S.W.; Chen, R.H. Cavitation effects versus stretch effects resulted in different size and polydispersity of ionotropic gelation chitosan–sodium tripolyphosphate nanoparticle. Carbohydr. Polym. 2008, 71, 448–457. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Hu, B.; Pan, C.; Sun, Y.; Hou, Z.; Ye, H.; Zeng, X. Optimization of fabrication parameters to produce chitosan-tripolyphosphate nanoparticles for delivery of tea catechins. J. Agric. Food Chem. 2008, 56, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, Y.; Lapitsky, Y. Factors affecting the stability of chitosan/tripolyphosphate micro-and nanogels: Resolving the opposing findings. J. Mater. Chem. B 2015, 3, 5957–5970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Oh, M.; Allen, C.; Kumacheva, E. Monodisperse chitosan nanoparticles for mucosal drug delivery. Biomacromolecules 2004, 5, 2461–2468. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjøniksen, A.L.; Hiorth, M. Effects of ionic strength on the size and compactness of chitosan nanoparticles. Colloid Polym. Sci. 2012, 290, 919–929. [Google Scholar] [CrossRef]
- López-León, T.; Carvalho, E.L.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-González, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lapitsky, Y. Ionically crosslinked polyelectrolyte nanocarriers: Recent advances and open problems. Curr. Opin. Colloid Interface Sci. 2014, 19, 122–130. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Salt-assisted mechanistic analysis of chitosan/tripolyphosphate micro- and nanogel formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug. Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Jamil, B.; Habib, H.; Abbasi, S.; Nasir, H.; Rahman, A.; Rehman, A.; Bokhari, H.; Imran, M. Cefazolin loaded chitosan nanoparticles to cure multi drug resistant Gram-negative pathogens. Carbohydr. Polym. 2016, 136, 682–691. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Samir, A.; Abdel-Moein, K.A.; Zaher, H.M. The Public Health Burden of Virulent Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae Strains Isolated from Diseased Horses. Vector Borne Zoonotic Dis. 2022, 22, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.P.; Andrade, C.T.; Del Aguila, E.M.; Alexander, C.; Paschoalin, V.M.F. Assessing the antimicrobial activity of chitosan nanoparticles by fluorescence-labeling. Int. J. Biotechnol. Bioeng. 2018, 12, 111–117. [Google Scholar]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.L.; Ho, Y.C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2017, 43, 3527–3537. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.d.; Assis, O.B.J. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Ngan, L.T.K.; Wang, S.-L.; Hiep, D.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
| Container and Volume | Sample Volume (mL) | Temperature °C | Spindle | RPM | Torque% | Average c.P | Mwt |
|---|---|---|---|---|---|---|---|
| Beaker 250 | 250 | 22 | S001 | 100 | 13.8 | 13.8 ± 0.057 | 1990 Da |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essa, E.E.; Hamza, D.; Khalil, M.M.H.; Zaher, H.; Salah, D.; Alnemari, A.M.; Rady, M.H.; Mo`men, S.A.A. The Antibacterial Activity of Egyptian Wasp Chitosan-Based Nanoparticles against Important Antibiotic-Resistant Pathogens. Molecules 2022, 27, 7189. https://doi.org/10.3390/molecules27217189
Essa EE, Hamza D, Khalil MMH, Zaher H, Salah D, Alnemari AM, Rady MH, Mo`men SAA. The Antibacterial Activity of Egyptian Wasp Chitosan-Based Nanoparticles against Important Antibiotic-Resistant Pathogens. Molecules. 2022; 27(21):7189. https://doi.org/10.3390/molecules27217189
Chicago/Turabian StyleEssa, Eman E., Dalia Hamza, Mostafa M. H. Khalil, Hala Zaher, Dina Salah, Ashwaq M. Alnemari, Magda H. Rady, and Shimaa A. A. Mo`men. 2022. "The Antibacterial Activity of Egyptian Wasp Chitosan-Based Nanoparticles against Important Antibiotic-Resistant Pathogens" Molecules 27, no. 21: 7189. https://doi.org/10.3390/molecules27217189
APA StyleEssa, E. E., Hamza, D., Khalil, M. M. H., Zaher, H., Salah, D., Alnemari, A. M., Rady, M. H., & Mo`men, S. A. A. (2022). The Antibacterial Activity of Egyptian Wasp Chitosan-Based Nanoparticles against Important Antibiotic-Resistant Pathogens. Molecules, 27(21), 7189. https://doi.org/10.3390/molecules27217189






