Anthocyanin Content of Crackers and Bread Made with Purple and Blue Wheat Varieties
Abstract
1. Introduction
2. Results and Discussion
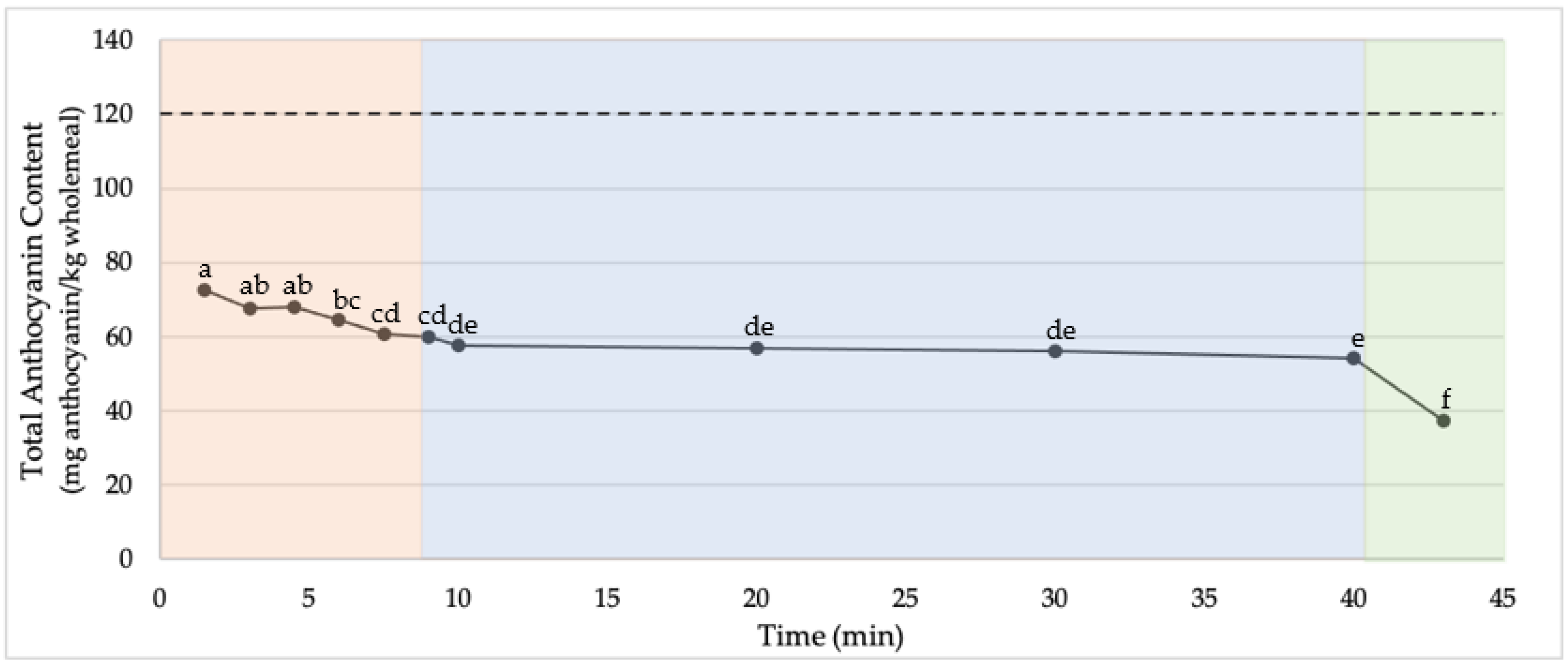
2.1. Stability of Endogenous Wheat Anthocyanins during Cracker Processing
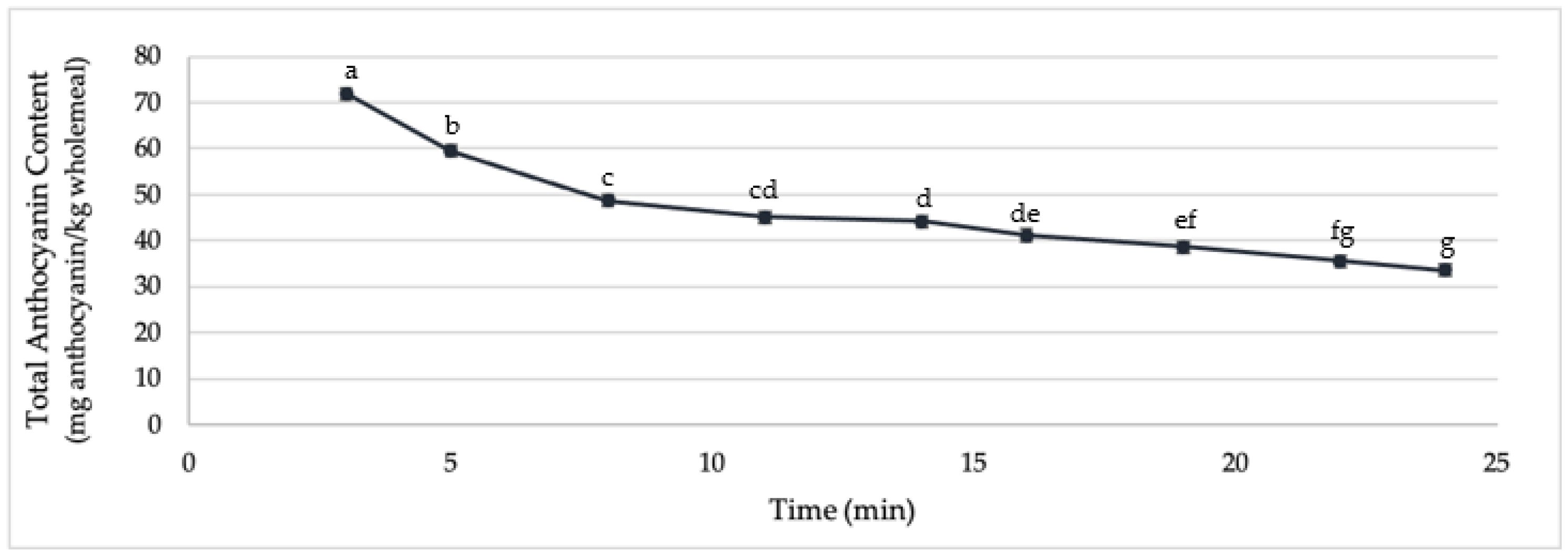
2.2. Stability of Endogenous Wheat Anthocyanins during Bread Processing
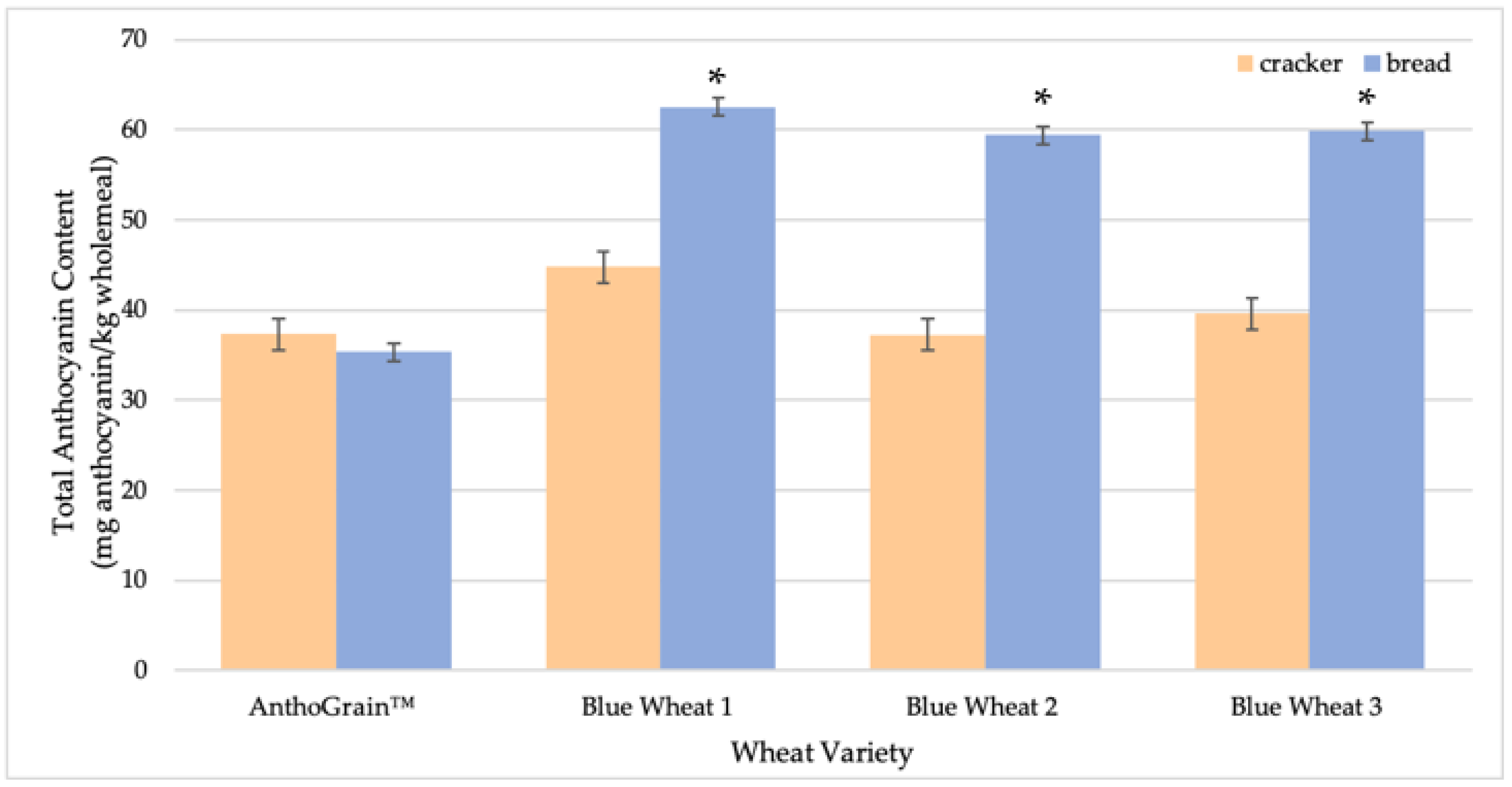
2.3. Comparison of Anthocyanin Stability in Different Cereal Processing Conditions
3. Materials and Methods
3.1. Materials
3.2. Production of Crackers
3.3. Production of Bread
3.4. Anthocyanin Extraction
3.5. Anthocyanin Quantification
3.6. Anthocyanin Identification
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abdel-Aal, E.S.M. Anthocyanin-Pigmented Grain Products. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.M., Piironen, V., Bean, S., Eds.; Oxford University Press, Inc.: Washington, DC, USA, 2011; pp. 76–109. ISBN 9780841226364. [Google Scholar]
- Arendt, E.K.; Zannini, E. Cereal Grains for the Food and Beverage Industries, 1st ed.; Woodhead Publishing: Philidelphia, PA, USA, 2013; ISBN 9780857094131. [Google Scholar]
- Moore, J.; Hao, J. Antioxidant and Health Promoting Properties of Wheat (Triticum spp.). Cereals Pulses Nutraceutical Prop. Health Benefits 2012, 1, 113–130. [Google Scholar] [CrossRef]
- Gani, A.; Wani, S.M.; Masoodi, F.A.; Hameed, G. Whole-Grain Cereal Bioactive Compounds and Their Health Benefits: A Review. J. Food Process. Technol. 2012, 3, 146–156. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E.; Rémésy, C. Is the in Vitro Antioxidant Potential of Whole-Grain Cereals and Cereal Products Well Reflected in Vivo? J. Cereal Sci. 2008, 48, 258–276. [Google Scholar] [CrossRef]
- Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Damodoran, S.; Parkin, K.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; Srinivasan, D., Parkin, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 3540408185. [Google Scholar]
- Knievel, D.C.; Abdel-Aal, E.S.M.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain Color Development and the Inheritance of High Anthocyanin Blue Aleurone and Purple Pericarp in Spring Wheat (Triticum Aestivum L.). J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Blesso, C.N. Dietary Anthocyanins and Human Health. Nutrients 2019, 11, 2107. [Google Scholar] [CrossRef]
- Seo, Y.; Moon, Y.; Kweon, M. Effect of Purple-Colored Wheat Bran Addition on Quality and Antioxidant Property of Bread and Optimization of Bread-Making Conditions. Appl. Sci. 2021, 11, 4034. [Google Scholar] [CrossRef]
- Bartl, P.; Albreht, A.; Skrt, M.; Tremlova, B.; Ostadlova, M.; Smejkal, K.; Vovk, I.; Ulrih, N.P.N.P.; Tremlová, B.; Oš͗ádalová, M.; et al. Anthocyanins in Purple and Blue Wheat Grains and in Resulting Bread: Quantity, Composition, and Thermal Stability. Int. J. Food Sci. Nutr. 2015, 66, 514–519. [Google Scholar] [CrossRef]
- Eliášová, M.; Kotíková, Z.; Lachman, J.; Orsák, M.; Martinek, P. Influence of Baking on Anthocyanin Content in Coloured-Grain Wheat Bread. Plant Soil Environ. 2020, 66, 381–386. [Google Scholar] [CrossRef]
- Sui, X.; Yap, P.Y.; Zhou, W. Anthocyanins During Baking: Their Degradation Kinetics and Impacts on Color and Antioxidant Capacity of Bread. Food Bioprocess Technol. 2015, 8, 983–994. [Google Scholar] [CrossRef]
- Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pickard, M.D.; Beta, T. Food Chemistry Effect of Thermal Processing on Antioxidant Properties of Purple Wheat Bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Li, J.; Walker, C.E.; Faubion, J.M. Acidulant and Oven Type Affect Total Anthocyanin Content of Blue Corn Cookies. J. Sci. Food Agric. 2011, 91, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.B.M.; de Simone, V.; de Leonardis, A.M.; Giovanniello, V.; del Nobile, M.A.; Padalino, L.; Lecce, L.; Borrelli, G.M.; de Vita, P. Use of Purple Durum Wheat to Produce Naturally Functional Fresh and Dry Pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef]
- Davidson, I. Dough Mixing. In Biscuit, Cookie, and Cracker Production: Process, Production, and Packaging Equipment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–37. [Google Scholar]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of Thermal Processing on Anthocyanin Stability in Foods; Mechanisms and Kinetics of Degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Brauch, J.E.; Kroner, M.; Schweiggert, R.M.; Carle, R. Studies into the Stability of 3-O-Glycosylated and 3,5-O-Diglycosylated Anthocyanins in Differently Purified Liquid and Dried Maqui (Aristotelia Chilensis (Mol.) Stuntz) Preparations during Storage and Thermal Treatment. J. Agric. Food Chem. 2015, 63, 8705–8714. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P.; Shipp, J.; Rabalski, I. Compositional Differences in Anthocyanins from Blue- and Purple-Grained Spring Wheat Grown in Four Environments in Central Saskatchewan. Cereal Chem. 2016, 93, 32–38. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. Composition and Stability of Anthocyanins in Blue-Grained Wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin Composition in Black, Blue, Pink, Purple, and Red Cereal Grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Bánhidy, J.; Cseuz, L.; Matuz, J. The Anthocyanin Content of Blue and Purple Coloured Wheat Cultivars and Their Hybrid Generations. Cereal Res. Commun. 2013, 41, 284–292. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Decamps, K.; Joye, I.J.; de Vos, D.E.; Courtin, C.M.; Delcour, J.A. Molecular Oxygen and Reactive Oxygen Species in Bread-Making Processes: Scarce, but Nevertheless Important. Crit. Rev. Food Sci. Nutr. 2016, 56, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Draganski, A.; Delcour, J.A.; Ludescher, R.D. Monitoring Molecular Oxygen Depletion in Wheat Flour Dough Using Erythrosin B Phosphorescence: A Biophysical Approach. Food Biophys. 2012, 7, 138–144. [Google Scholar] [CrossRef]
- Jayaram, V.B.; Cuyvers, S.; Lagrain, B.; Verstrepen, K.J.; Delcour, J.A.; Courtin, C.M. Mapping of Saccharomyces Cerevisiae Metabolites in Fermenting Wheat Straight-Dough Reveals Succinic Acid as PH-Determining Factor. Food Chem. 2013, 136, 301–308. [Google Scholar] [CrossRef]
| Time Point | AnthoGrain™ | Blue 1 | Blue 2 | Blue 3 |
|---|---|---|---|---|
| Wholemeal | 121.19 ± 4.77 a | 168.62 ± 10.50 a | 194.36 ± 5.51 a | 135.64 ± 6.95 a |
| Cracker Dough (40 min) | 54.30 ± 1.19 b | 94.77 ± 1.60 b | 89.14 ± 0.60 b | 87.13 ± 0.44 b |
| Baked Cracker | 37.31 ± 0.72 c | 44.76 ± 3.29 c | 37.22 ± 1.15 c | 39.61 ± 0.87 c |
| Process. | AnthoGrain™ | Blue 1 | Blue 2 | Blue 3 |
|---|---|---|---|---|
| Mixing + | 52.34 ± 0.92 d | n.d. | n.d. | n.d. |
| Resting ++ | 5.87 ± 4.56 a | n.d. | n.d. | n.d. |
| Mixing and Resting + | n.d. | 43.64 ± 3.91 c | 54.11 ± 1.51 d | 35.65 ± 3.26 b |
| Baking ++ | 31.06 ± 2.80 b | 52.79 ± 3.00 d | 58.25 ± 1.25 d | 54.55 ± 0.92 d |
| Overall + | 69.11 ± 0.72 e | 73.31 ± 3.51 ef | 80.83 ± 1.13 f | 70.73 ± 2.00 e |
| Wholemeal | Cracker Dough | Baked Cracker | ||||
| Aglycone | Substituent | Aglycone | Substituent | Aglycone | Substituent | |
| Antho GrainTM | Cyanidin | Acetylated hexose | ||||
| Cyanidin | Hexose+ malonic acid | Cyanidin | Hexose+ malonic acid | |||
| Cyanidin | Hexose | |||||
| Cyanidin | ||||||
| Peonidin | Hexose | Peonidin | Hexose | Peonidin | Hexose | |
| Peonidin | Hexose+ malonic acid | Peonidin | Hexose+ malonic acid | Peonidin | Hexose+ malonic acid | |
| Peonidin | Acetylated hexose | |||||
| Peonidin | Succinyl hexose | |||||
| Peonidin | Hexose+ malonic acid+ acetic acid | |||||
| Peonidin | Hexose+ deoxy hexose | Peonidin | Hexose+ deoxy hexose | Peonidin | Hexose+ deoxy hexose | |
| Peonidin | Hexose | |||||
| Peonidin | Peonidin | Peonidin | ||||
| Malvidin | Hexose+ deoxy hexose | Malvidin | Hexose+ deoxy hexose | |||
| Malvidin | Malvidin | Malvidin | ||||
| Blue 2 | Cyanidin | Hexose+ deoxy hexose | Cyanidin | Hexose+ hexose | ||
| Cyanidin | Hexose+ deoxy hexose | Cyanidin | Hexose+ deoxy hexose | |||
| Delphinidin | Hexose+ deoxy hexose | Delphinidin | Hexose+ deoxy hexose | Delphinidin | Hexose+ deoxy hexose | |
| Delphinidin | Hexose | Delphinidin | Hexose | |||
| Delphinidin | Acetylated hexose | Delphinidin | Hexose+ acetic acid | |||
| Peonidin | Hexose+ deoxy hexose+ hexose | Peonidin | Hexose+ deoxy hexose+ hexose | Peonidin | Hexose+ deoxy hexose+ hexose | |
| Peonidin | Hexose+ hexose | |||||
| Peonidin | Hexose+ deoxy hexose | Peonidin | Hexose+ deoxy hexose | |||
| Malvidin | Hexose+ hexose | |||||
| Malvidin | Hexose+ deoxy hexose | Malvidin | Hexose+ deoxy hexose | Malvidin | Hexose+ deoxy hexose | |
| Time Point | AnthoGrain™ | Blue 1 | Blue 2 | Blue 3 |
|---|---|---|---|---|
| Wholemeal | 121.19 ± 4.77 a | 168.62 ± 10.50 a | 194.36 ± 5.51 a | 135.64 ± 6.95 a |
| Dough (0 min) | 75.22 ± 0.24 b | 141.28 ± 0.81 b | 125.57 ± 1.76 b | 125.48 ± 2.04 b |
| Fermented Dough (52 min) | 73.31 ± 1.17 bc | 139.87 ± 1.73 b | 124.98 ± 1.86 b | 124.93 ± 0.61 b |
| Fermented Dough (77 min) | 71.99 ± 1.05 c | 137.035 ± 0.42 b | 126.90 ± 4.20 b | 122.82 ± 3.15 b |
| Fermented Dough (90 min) | 71.89 ± 0.26 c | 138.26 ± 1.68 b | 129.00 ± 0.78 b | 124.27 ± 2.21 b |
| Fermented Dough (123 min) | 71.79 ± 0.90 c | 141.56 ± 1.29 b | 131.06 ± 1.58 b | 125.40 ± 0.56 b |
| Bread | 35.53 ± 0.35 d | 62.56 ± 0.31 c | 59.38 ± 0.47 c | 59.86 ± 2.31 c |
| Process | AnthoGrain™ | Blue 1 | Blue 2 | Blue 3 |
|---|---|---|---|---|
| Mixing + | 38.01 ± 2.81 d | 16.01 ± 4.90 c | 35.35 ± 2.45 d | 7.12 ± 6.10 b |
| Fermentation ++ | 4.56 ± 1.45 b | −0.20 ± 1.48 ab | −4.38 ± 0.23 a | 1.15 ± 0.45 ab |
| Baking ++ | 46.64 ± 3.40 e | 55.81 ± 0.56 fg | 54.69 ± 0.55 ef | 52.26 ± 1.85 ef |
| Overall + | 68.35 ± 2.63 e | 62.81 ± 2.31 ge | 69.44 ± 0.73 e | 55.81 ± 2.27 fg |
| Wholemeal | Bread Dough | Bread | ||||
| Aglycone | Substituent | Aglycone | Substituent | Aglycone | Substituent | |
| Antho GrainTM | Cyanidin | Acetylated hexose | ||||
| Cyanidin | Hexose+ malonic acid | Cyanidin | Hexose+ malonic acid | |||
| Cyanidin | Hexose | Cyanidin | Hexose | |||
| Cyanidin | ||||||
| Peonidin | Hexose | Peonidin | Hexose | Peonidin | Hexose | |
| Peonidin | Hexose+ malonic acid | Peonidin | Hexose+ malonic acid | Peonidin | Hexose+ malonic acid | |
| Peonidin | Acetylated hexose | |||||
| Peonidin | Succinyl hexose | |||||
| Peonidin | Hexose+ malonic acid+ acetic acid | |||||
| Peonidin | Hexose+ deoxy hexose | Peonidin | Hexose+ deoxy hexose | |||
| Peonidin | Hexose | |||||
| Peonidin | Peonidin | Peonidin | ||||
| Peonidin | Deoxy hexose+ formic acid | |||||
| Malvidin | Hexose+ deoxy hexose | |||||
| Malvidin | Malvidin | Malvidin | ||||
| Blue 2 | Cyanidin | Hexose+ deoxy hexose | Cyanidin | Hexose+ deoxy hexose | ||
| Cyanidin | Hexose | |||||
| Delphinidin | Hexose+ deoxy hexose | Delphinidin | Hexose+ deoxy hexose | |||
| Delphinidin | Hexose | Delphinidin | Hexose | Delphinidin | Hexose | |
| Delphinidin | Acetylated hexose | |||||
| Delphinidin | Di-rutinose | |||||
| Peonidin | Hexose+ deoxy hexose+ hexose | |||||
| Peonidin | Hexose+ hexose | Peonidin | Hexose+ hexose | |||
| Peonidin | Hexose+ deoxy hexose | Peonidin | Hexose+ deoxy hexose | |||
| Peonidin | Di-rutinose | |||||
| Peonidin | Deoxy hexose+ Formic acid | |||||
| Malvidin | Hexose+ hexose | |||||
| Malvidin | Hexose+ deoxy hexose | Malvidin | Hexose+ deoxy hexose | |||
| Process | Cracker | Bread |
|---|---|---|
| Process Time | Short (45 min) | Long (147 min) |
| Baking | ||
| Temperature | High (249 °C) | Low (205 °C) |
| Time | 3 min | 24 min |
| Leavening System | Chemical | Biological |
| Dough pH | 7.0 | 5.45 |
| Moisture | Low | High |
| 46% (dough) | 59% (dough) | |
| 2% (baked product) | 35% (baked product) |
| Time Point | Control | No Sodium Bicarbonate | No Sodium Bicarbonate No SAPP-40 |
|---|---|---|---|
| pH-6.96 | 5.24 | 5.77 | |
| Wholemeal | 121.19 ± 4.77 a | 121.19 ± 4.77 a | 121.19 ± 4.77 a |
| 10 min | 66.70 ± 2.95 bcd | 69.79 ± 0.93 bc | 68.04 ± 1.43 bc |
| 20 min | 56.88 ± 0.33 e | 68.48 ± 0.75 bc | 65.20 ± 2.71 cd |
| 30 min | 56.02 ± 0.44 e | 69.87 ± 3.32 bc | 58.45 ± 0.54 e |
| 40 min | 54.30 ± 1.19 e | 69.97 ± 0.17 b | 63.21 ± 0.30 d |
| Baked Cracker | 37.41 ± 0.72 fg | 42.00 ± 1.24 f | 36.03 ± 1.29 g |
| Temperature (°C) | Time (min) | Total Anthocyanin Content |
|---|---|---|
| 215 | 24 | 38.99 ± 2.18 a |
| 240 | 20 | 56.18 ± 4.81 b |
| Ingredients | Weight (g) | Percentage (% w/w) |
|---|---|---|
| Wholemeal (13.0% moisture) | 100 | 60.24 |
| Water | 40 | 24.10 |
| Oil | 12 | 7.23 |
| Corn Syrup | 4 | 2.41 |
| Sucrose | 6 | 3.61 |
| Salt (NaCl) | 1.5 | 0.90 |
| Sodium Bicarbonate | 1.25 | 0.75 |
| Sodium Acid Pyrophosphate 40 (SAPP-40) | 1.25 | 0.75 |
| Ingredients | Weight (g) | Percentage (% w/w) |
|---|---|---|
| Wholemeal (14.0% moisture) | 100 | 55.93 |
| Water | 63 | 35.23 |
| Yeast | 5.3 | 2.96 |
| Sucrose | 6 | 3.36 |
| Salt (NaCl) | 1.5 | 0.84 |
| Shortening | 3 | 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francavilla, A.; Joye, I.J. Anthocyanin Content of Crackers and Bread Made with Purple and Blue Wheat Varieties. Molecules 2022, 27, 7180. https://doi.org/10.3390/molecules27217180
Francavilla A, Joye IJ. Anthocyanin Content of Crackers and Bread Made with Purple and Blue Wheat Varieties. Molecules. 2022; 27(21):7180. https://doi.org/10.3390/molecules27217180
Chicago/Turabian StyleFrancavilla, Alyssa, and Iris J. Joye. 2022. "Anthocyanin Content of Crackers and Bread Made with Purple and Blue Wheat Varieties" Molecules 27, no. 21: 7180. https://doi.org/10.3390/molecules27217180
APA StyleFrancavilla, A., & Joye, I. J. (2022). Anthocyanin Content of Crackers and Bread Made with Purple and Blue Wheat Varieties. Molecules, 27(21), 7180. https://doi.org/10.3390/molecules27217180






