Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities
Abstract
1. Introduction
2. Results and Discussion
2.1. EPS Production
2.2. Chemical Composition of EPS
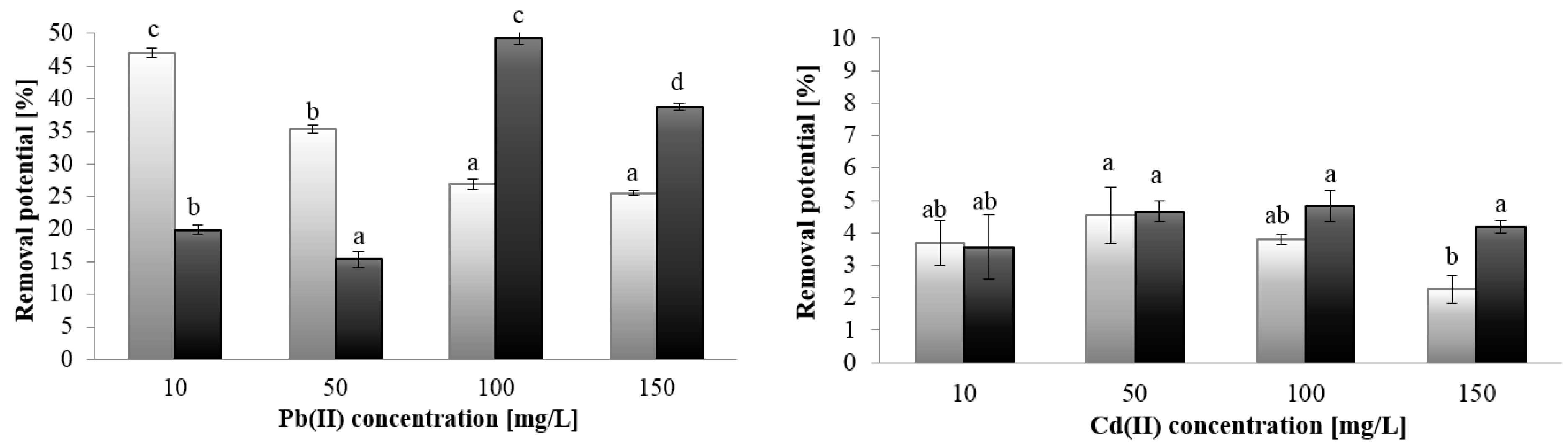
2.3. Metal Sorption
2.3.1. ICP-OES Analysis
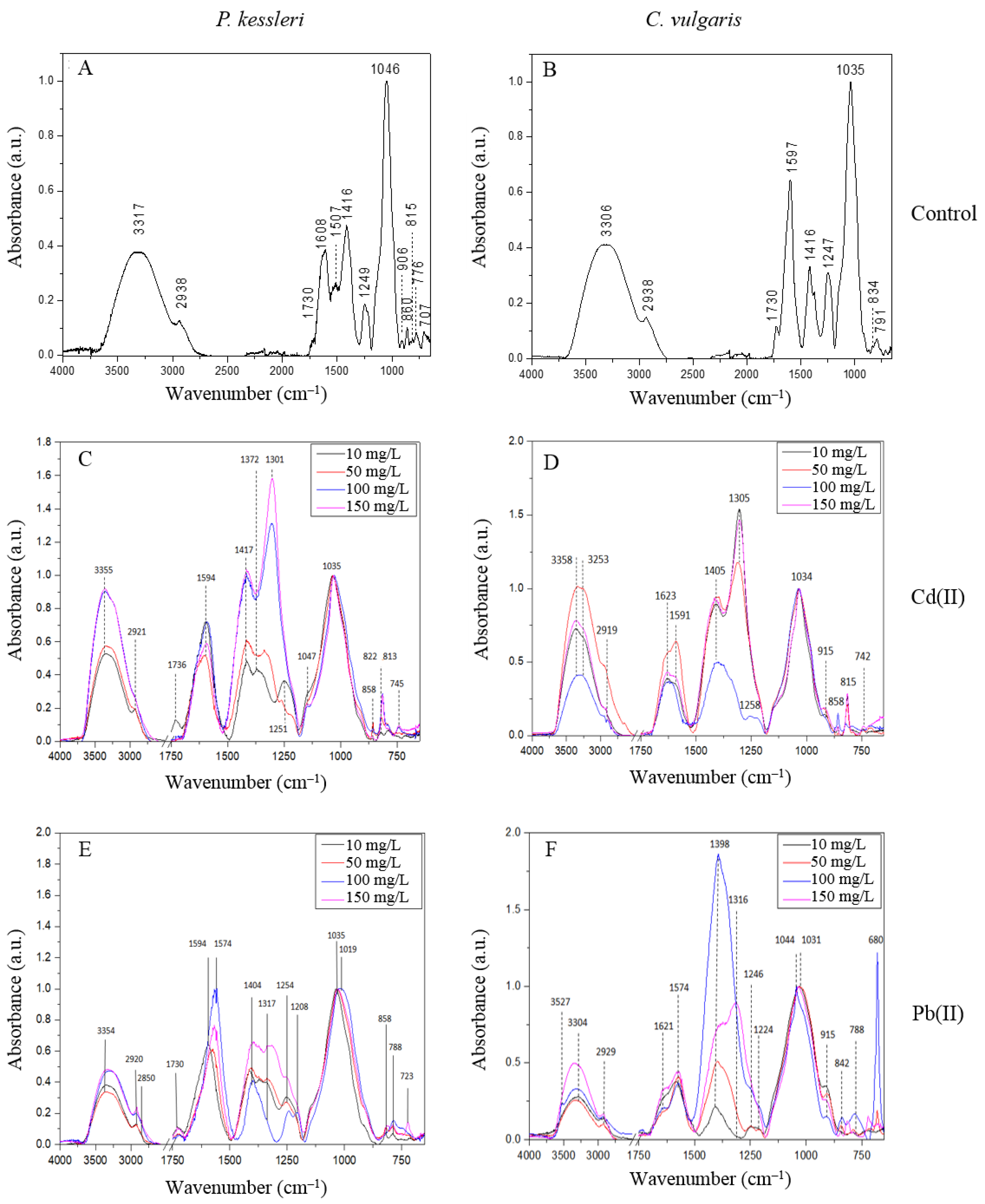
2.3.2. FTIR Analysis
| Species | Assignment | Interpretation | References | |
|---|---|---|---|---|
| P. kessleri | C. vulgaris | |||
| Wavenumber (cm−1) | ||||
| 776, 815, 860 | 791, 834 | (CO), δ(CH) | furanose and pyranose rings of saccharides α- and β-glycosidic bonds | [32] [31] |
| 1046 | 1035 | v(CO), δ(COH) | β(1,4) glycosidic bond | [31] |
| 1249 | 1247 | νas(PO), νas(SO) | phosphate group, sulfate group | [29] [30] |
| 1416 | 1416 | νs(COO) | carboxymethyl groups | [31] |
| 1608 | 1597 | νas(COO) | carboxymethyl groups | [31] |
| 1730 | 1730 | ν(C=O) | esterified carboxyl groups, uronic acid | [28] [31] |
| 2919 | 2938 | νas(CH) | hydrocarbon bond | [27] |
| 3358 | 3306 | ν(OH) | hydroxyl groups | [27] |
3. Materials and Methods
3.1. Culture Strains and Pre-Cultivation
3.2. Experimental Set-Up
3.3. Isolation of EPS
3.4. EPS Production
3.5. Analysis of Chemical Composition of EPS
3.5.1. Biochemical Composition
3.5.2. Monosaccharide Composition
3.5.3. Elemental Composition
3.6. Metal Sorption
3.6.1. Preparation of Solutions and Sorption Experiment
3.6.2. ICP-OES
3.6.3. Fourier Transform Infrared Spectroscopy
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released Polysaccharides (RPS) from Cyanothece sp. CCY 0110 as Biosorbent for Heavy Metals Bioremediation: Interactions between Metals and RPS Binding Sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef] [PubMed]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Katti, S.; Her, B.; Srivastava, A.K.; Taylor, A.B.; Lockless, S.W.; Igumenova, T.I. High Affinity Interactions of Pb2+ with Synaptotagmin I. Metallomics 2018, 10, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, M.S.; Amin, F. Accumulation and Distribution of Lead (Pb) in Plant Tissues of Guar (Cyamopsis tetragonoloba L.) and Sesame (Sesamum indicum L.): Profitable Phytoremediation with Biofuel Crops. Geol. Ecol. Landsc. 2018, 2, 51–60. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Capek, P.; Matulová, M.; Šutovská, M.; Barboríková, J.; Molitorisová, M.; Kazimierová, I. Chlorella vulgaris α-L-Arabino-α-L-Rhamno-α,β-D-Galactan Structure and Mechanisms of Its Anti-Inflammatory and Anti-Remodelling Effects. Int. J. Biol. Macromol. 2020, 162, 188–198. [Google Scholar] [CrossRef]
- Magierek, E.; Krzemińska, I.; Tys, J. Stimulatory Effect of Indole-3-Acetic Acid and Continuous Illumination on the Growth of Parachlorella kessleri. Int. Agrophysics 2017, 31, 483–489. [Google Scholar] [CrossRef][Green Version]
- Lombardi, A.T.; Hidalgo, T.M.R.; Vieira, A.A.H. Copper Complexing Properties of Dissolved Organic Materials Exuded by the Freshwater Microalgae Scenedesmus acuminatus (Chlorophyceae). Chemosphere 2005, 60, 453–459. [Google Scholar] [CrossRef]
- Lombardi, A.T.; Vieira, A.A.H. Lead- and Copper-Complexing Extracellular Ligands Released by Kirchneriella aperta (Chloroccocales, Chlorophyta). Phycologia 1999, 38, 283–288. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, N.; Lin, D.; Qu, R.; Zhou, Q.; Ge, F. The Complexation with Proteins in Extracellular Polymeric Substances Alleviates the Toxicity of Cd (II) to Chlorella vulgaris. Environ. Pollut. 2020, 263, 114102. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Liu, Y.; Huang, F.; Zhang, C. Effect of Extracellular Polymeric Substances on Arsenic Accumulation in Chlorella pyrenoidosa. Sci. Total Environ. 2020, 704, 135368. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, Q.; Chen, C.; Yang, F.; Cai, Z.; Li, D.; Geng, Q.; Feng, Y.; Wang, H. Role of Extracellular Polymeric Substances on the Behavior and Toxicity of Silver Nanoparticles and Ions to Green Algae Chlorella vulgaris. Sci. Total Environ. 2019, 660, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
- Halaj, M.; Chválová, B.; Cepák, V.; Lukavský, J.; Capek, P. Searching for Microalgal Species Producing Extracellular Biopolymers. Chem. Pap. 2018, 72, 2673–2678. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, Extraction and Characterization of Microalgal and Cyanobacterial Exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Ogawa, K.; Ikeda, Y.; Kondo, S. A New Trisaccharide, a-D-Glucopyranuronosyl-(1→ 3)-a-L-Rhamnopyranosyl-(1→ 2)-a-L-Rhamnopyranose From Chlorella Vulgaris. Carbohydr. Res. 1999, 321, 128–131. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, Extraction and Characterization of Chlorella vulgaris Soluble Polysaccharides and Their Applications in AgNPs Biosynthesis and Biostimulation of Plant Growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef]
- Halaj, M.; Paulovičová, E.; Paulovičová, L.; Jantová, S.; Cepák, V.; Lukavský, J.; Capek, P. Extracellular Biopolymers Produced by Dictyosphaerium Family—Chemical and Immunomodulative Properties. Int. J. Biol. Macromol. 2019, 121, 1254–1263. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef]
- Jiao, Y.; Cody, G.D.; Harding, A.K.; Wilmes, P.; Schrenk, M.; Wheeler, K.E.; Banfield, J.F.; Thelen, M.P. Characterization of Extracellular Polymeric Substances from Acidophilic Microbial Biofilms. Appl. Environ. Microbiol. 2010, 76, 2916–2922. [Google Scholar] [CrossRef]
- Kehr, J.-C.; Dittmann, E. Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life 2015, 5, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.; De Morais, R.; Bernardo de Morais, A. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.A.M.; Garcıa-Ribera, R.; Quesada, T.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sanchez, M. Biosorption of Heavy Metals by the Exopolysaccharide Produced by Paenibacillus jamilae. World J. Microbiol. Biotechnol. 2008, 24, 2699–2704. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Szcześ, A.; Czemierska, M.; Jarosz-Wikołazka, A. Studies of Cadmium(II), Lead(II), Nickel(II), Cobalt(II) and Chromium(VI) Sorption on Extracellular Polymeric Substances Produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour. Technol. 2017, 225, 113–120. [Google Scholar] [CrossRef]
- Ouyang, D.; Zhuo, Y.; Hu, L.; Zeng, Q.; Hu, Y.; He, Z. Research on the Adsorption Behavior of Heavy Metal Ions by Porous Material Prepared with Silicate Tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef]
- Reddad, Z.; Ge, C.; Cloirec, P.L. Cadmium and Lead Adsorption by a Natural Polysaccharide in MF Membrane Reactor: Experimental Analysis and Modelling. Water Res. 2003, 37, 3983–3991. [Google Scholar] [CrossRef]
- Cybulska, J.; Halaj, M.; Cepák, V.; Lukavský, J.; Capek, P. Nanostructure Features of Microalgae Biopolymer: Nanostructure of Biopolymer. Starch Stärke 2016, 68, 629–636. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Cross-Linking of Sodium Carbonate-Soluble Pectins from Apple by Zinc Ions. Carbohydr. Polym. 2018, 196, 1–7. [Google Scholar] [CrossRef]
- Alam, M.d.A.; Wan, C.; Guo, S.-L.; Zhao, X.-Q.; Huang, Z.-Y.; Yang, Y.-L.; Chang, J.-S.; Bai, F.-W. Characterization of the Flocculating Agent from the Spontaneously Flocculating Microalga Chlorella vulgaris JSC-7. J. Biosci. Bioeng. 2014, 118, 29–33. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; de Silva, E.D.; et al. FTIR Characterization and Antioxidant Activity of Water Soluble Crude Polysaccharides of Sri Lankan Marine Algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→ 3)-β-D-Glucans, Their Preparation, Physico-Chemical Characterization and Immunomodulatory Activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Molaei, H.; Jahanbin, K. Structural Features of a New Water-Soluble Polysaccharide from the Gum Exudates of Amygdalus scoparia Spach (Zedo Gum). Carbohydr. Polym. 2018, 182, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR Spectra of Alginic Acid Block Fractions in Three Species of Brown Seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT-Raman and 13C-NMR Spectroscopy for Identification of Some Seaweed Phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandrika, J.U.; Mathimani, T.; Rajaram, R.; Annadurai, G.; Yin, H. Production and Functionality of Exopolysaccharides in Bacteria Exposed to a Toxic Metal Environment. Ecotoxicol. Environ. Saf. 2021, 208, 111567. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.-P.; Peng, M.-W.; Guo, J.-S.; Shen, Y.; Yan, P.; Zhou, Q.-H.; Jiang, J.; Fang, F. Extracellular Polymeric Substances Dependence of Surface Interactions of Bacillus subtilis with Cd2+ and Pb2+: An Investigation Combined with Surface Plasmon Resonance and Infrared Spectra. Colloids Surf. B Biointerfaces 2017, 154, 357–364. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Dahryn Trivedi, A.B. Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment. J. Chromatogr. Sep. Tech. 2015, 06, 5. [Google Scholar] [CrossRef]
- Arulmozhi, K.T.; Mythili, N. Studies on the Chemical Synthesis and Characterization of Lead Oxide Nanoparticles with Different Organic Capping Agents. AIP Adv. 2013, 3, 122122. [Google Scholar] [CrossRef]
- Senvaitiene, J.; Smirnova, J.; Beganskiene, A.; Kareiva, A. XRD and FTIR Characterisation of Lead Oxide-Based Pigments and Glazes. Acta Chim. Slov. 2007, 54, 185–193. [Google Scholar]
- Mota, R.; Guimarães, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al. Production and Characterization of Extracellular Carbohydrate Polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef]
- Allard, B.; Casadevall, E. Carbohydrate Composition and Characterization of Sugars from the Green Microalga Botryococcus braunii. Phytochemistry 1990, 29, 1875–1878. [Google Scholar] [CrossRef]
- Czemierska, M.; Szcześ, A.; Hołysz, L.; Wiater, A.; Jarosz-Wilkołazka, A. Characterisation of Exopolymer R-202 Isolated from Rhodococcus rhodochrous and Its Flocculating Properties. Eur. Polym. J. 2017, 88, 21–33. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hope, C.F.A.; Burns, R.G. Activity, Origins and Location of Cellulases in a Silt Loam Soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A Modified Uronic Acid Carbazole Reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Belcher, R.; Nutten, A.J.; Sambrook, C.M. The Determination of Glucosamine. Analyst 1954, 79, 201–208. [Google Scholar] [CrossRef]
- Bailey, J.L. Techniques in Protein Chemistry; Elsevier Publishing Company: Amsterdam, The Netherlands, 1967; ISBN 978-0-444-40369-8. [Google Scholar]
- Ghebregzabeier, M.; Rufini, S.; Monaldi, B.; Lato, M. Thin-Layer Chromatography of Carbohydrates. Chromatogr. Rev. 1976, 127, 133–162. [Google Scholar] [CrossRef]
- Proc, K.; Bulak, P.; Wiącek, D.; Bieganowski, A. Hermetia illucens Exhibits Bioaccumulative Potential for 15 Different Elements—Implications for Feed and Food Production. Sci. Total Environ. 2020, 723, 138125. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Sarkar, B.; Sarkar, D.; Sarkar, S.K.; Rai, M.; Vithanage, M. Exploration of an Extracellular Polymeric Substance from Earthworm Gut Bacterium (Bacillus Licheniformis) for Bioflocculation and Heavy Metal Removal Potential. Appl. Sci. 2020, 10, 349. [Google Scholar] [CrossRef]
| P. kessleri | C. vulgaris | |
|---|---|---|
| Biomass yield [g/L] | 0.77 ± 0.08 a | 0.86 ± 0.09 a |
| EPS yield [mg/L] | 12.49 ± 1.21 b | 10.42 ± 1.20 a |
| EPS specific productivity [mg/g DW] | 16.18 ± 1.09 b | 12.73 ± 2.09 a |
| Compound [µg/mg EPS] | ||
| Carbohydrates 1 | 635.0 ± 15.3 a | 577.3 ± 19.34 b |
| Proteins 1 | 5.5 ± 0.41 b | 7.5 ± 0.15 a |
| Reducing sugars 2 | 158.2 ± 7.30 a | 206.4 ± 4.17 b |
| Uronic acids 2 | 123.0 ± 2.46 a | 134.1 ± 5.02 b |
| Amino acids 2 | 14.4 ± 0.84 a | 35.4 ± 1.32 b |
| Amino sugars 2 | 4.2 ± 0.20 a | 4.4 ± 0.32 a |
| Rf | P. kessleri | C. vulgaris |
|---|---|---|
| Rha | 0.77 | 0.80 |
| Xyl | 0.68 | - |
| Man | 0.56 | 0.56 |
| Gal | 0.43 | 0.41 |
| Element | P. kessleri | C. vulgaris |
|---|---|---|
| Ca | 45.30 ± 0.55 | 86.73 ± 0.09 |
| Mg | 34.12 ± 0.17 | 20.02 ± 0.10 |
| Mn | 3.49 ± 0.02 | 0.76 ± 0.00 |
| P | 4.85 ± 0.58 | 18.00 ± 0.91 |
| S | 27.16 ± 0.11 | 44.61 ± 0.14 |
| Zn | 2.34 ± 0.01 | 0.54 ± 0.01 |
| Metal Ion Concentration [mg/L] | Sorption Capacity [mg/g] | |||
|---|---|---|---|---|
| Cd (II) | Pb (II) | |||
| P. kessleri | C. vulgaris | P. kessleri | C. vulgaris | |
| 10 | 3.85 ± 1.06 c | 3.73 ± 1.07 a | 50.96 ± 1.55 a | 20.74 ± 0.85 a |
| 50 | 15.37 ± 2.34 a | 22.73 ± 1.33 b | 157.27 ± 1.59 b | 79.43 ± 2.38 b |
| 100 | 35.35 ± 3.18 b | 41.1 ± 4.15 c | 264.1 ± 10.32 c | 490.85 ± 1.06 c |
| 150 | 26.33 ± 4.93 ab | 48.67 ± 2.08 d | 263.0 ± 20.0 c | 573.6 ± 10.61 d |
| Element [mg/mL] | Cd (II) [mg/L] | Pb (II) [mg/L] | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 100 | 150 | 10 | 50 | 100 | 150 | ||
| EPS P. kessleri | |||||||||
| Ca | 3.06 ± 0.02 | 2.65 ± 0.03 | 3.74 ± 0.02 | 2.85 ± 0.02 | 7.98 ± 0.04 | 7.47 ± 0.02 | 7.05 ± 0.05 | 5.36 ± 0.04 | 7.12 ± 0.13 |
| Mg | 2.53 ± 0.01 | 2.54 ± 0.02 | 3.56 ± 0.02 | 2.92 ± 0.01 | 3.50 ± 0.01 | 3.13 ± 0.01 | 3.18 ± 0.01 | 3.39 ± 0.02 | 3.83 ± 0.04 |
| Mn | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | <0.01 | 0.55 ± 0.01 |
| P | 0.91 ± 0.03 | 0.70 ± 0.01 | 0.73 ± 0.04 | 0.57 ± 0.07 | 0.29 ± 0.04 | 0.36 ± 0.08 | 0.18 ± 0.10 | 0.02 ± 0.01 | 1.94 ± 0.08 |
| S | 1.65 ± 0.01 | 1.39 ± 0.01 | 2.16 ± 0.02 | 1.81 ± 0.02 | 2.45 ± 0.01 | 1.96 ± 0.01 | 1.31 ± 0.01 | 2.20 ± 0.01 | 3.21 ± 0.06 |
| Zn | 0.58 ± 0.00 | 0.49 ± 0.01 | 0.34 ± 0.00 | 0.36 ± 0.00 | 0.22 ± 0.00 | 0.65 ± 0.00 | 0.93 ± 0.00 | 0.13 ± 0.00 | 0.69 ± 0.06 |
| EPS C. vulgaris | |||||||||
| Ca | 6.58 ± 0.01 | 5.86 ± 0.05 | 6.47 ± 0.02 | 6.42 ± 0.01 | 4.05 ± 0.03 | 8.39 ± 0.04 | 5.49 ± 0.08 | 5.63 ± 0.07 | 7.94 ± 0.02 |
| Mg | 1.53 ± 0.00 | 1.45 ± 0.01 | 1.63 ± 0.01 | 1.60 ± 0.00 | 1.01 ± 0.01 | 2.20 ± 0.01 | 1.29 ± 0.10 | 1.16 ± 0.02 | 1.96 ± 0.03 |
| Mn | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | <0.01 |
| P | 1.05 ± 0.06 | 0.90 ± 0.05 | 0.93 ± 0.12 | 0.81 ± 0.09 | 0.60 ± 0.03 | 0.73 ± 0.16 | 0.61 ± 0.09 | 0.65 ± 0.04 | 0.01 ± 0.00 |
| S | 3.08 ± 0.01 | 2.69 ± 0.02 | 2.85 ± 0.02 | 2.96 ± 0.01 | 1.94 ± 0.01 | 3.92 ± 0.05 | 2.25 ± 0.05 | 1.50 ± 0.07 | 3.17 ± 0.02 |
| Zn | 0.70 ± 0.00 | 0.86 ± 0.00 | 0.56 ± 0.01 | 1.04 ± 0.01 | 0.84 ± 0.00 | 1.09 ± 0.00 | 1.51 ± 0.05 | 0.55 ± 0.01 | 0.20 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciempiel, W.; Czemierska, M.; Szymańska-Chargot, M.; Zdunek, A.; Wiącek, D.; Jarosz-Wilkołazka, A.; Krzemińska, I. Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities. Molecules 2022, 27, 7153. https://doi.org/10.3390/molecules27217153
Ciempiel W, Czemierska M, Szymańska-Chargot M, Zdunek A, Wiącek D, Jarosz-Wilkołazka A, Krzemińska I. Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities. Molecules. 2022; 27(21):7153. https://doi.org/10.3390/molecules27217153
Chicago/Turabian StyleCiempiel, Wioleta, Magdalena Czemierska, Monika Szymańska-Chargot, Artur Zdunek, Dariusz Wiącek, Anna Jarosz-Wilkołazka, and Izabela Krzemińska. 2022. "Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities" Molecules 27, no. 21: 7153. https://doi.org/10.3390/molecules27217153
APA StyleCiempiel, W., Czemierska, M., Szymańska-Chargot, M., Zdunek, A., Wiącek, D., Jarosz-Wilkołazka, A., & Krzemińska, I. (2022). Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities. Molecules, 27(21), 7153. https://doi.org/10.3390/molecules27217153







