Bioanalytical Method Development, Validation and Stability Assessment of Xanthohumol in Rat Plasma
Abstract
1. Introduction
2. Results and Discussion
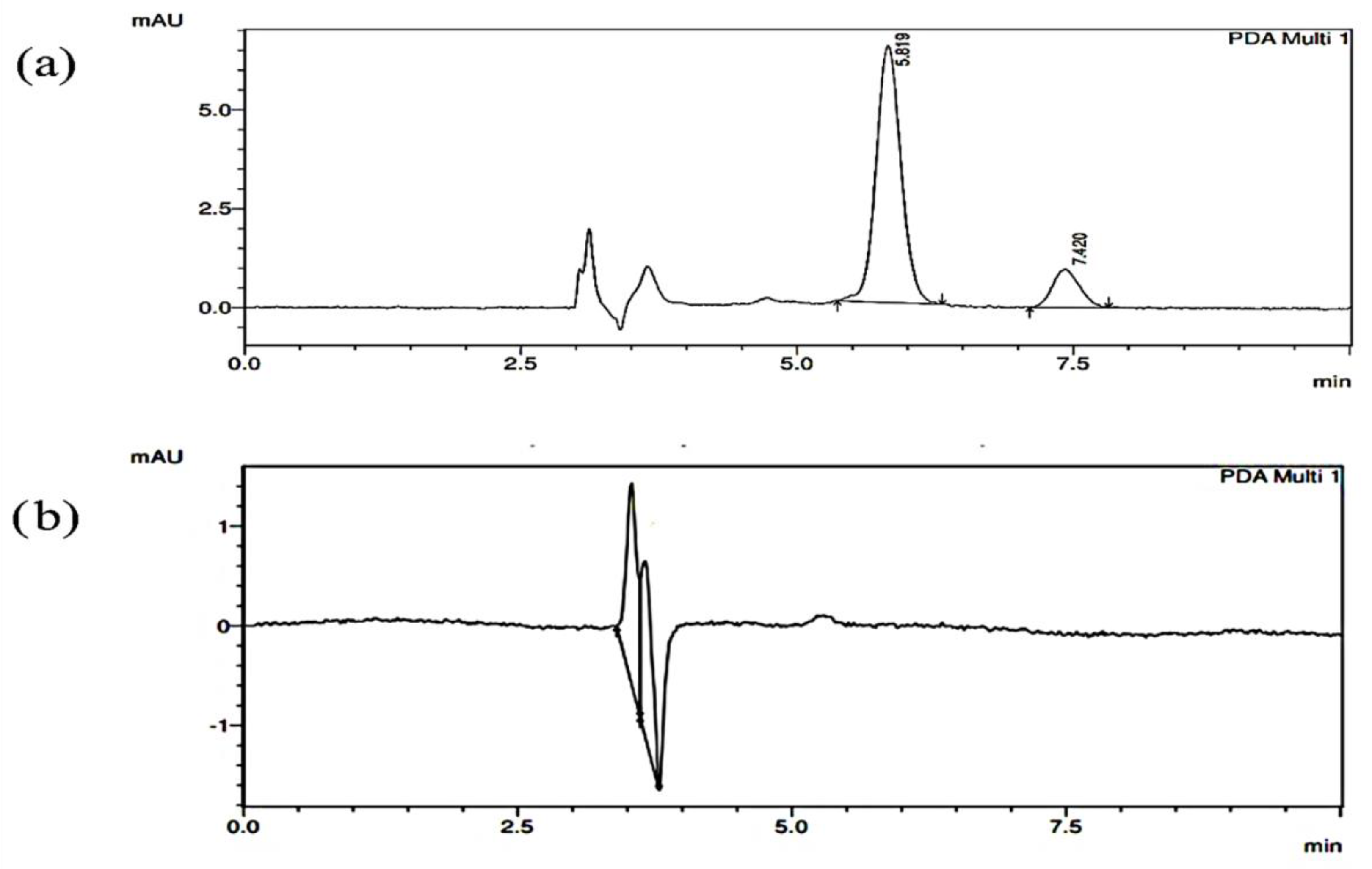
2.1. Chromatograms of Mixture Containing Curcumin and XH
2.2. Specificity Studies
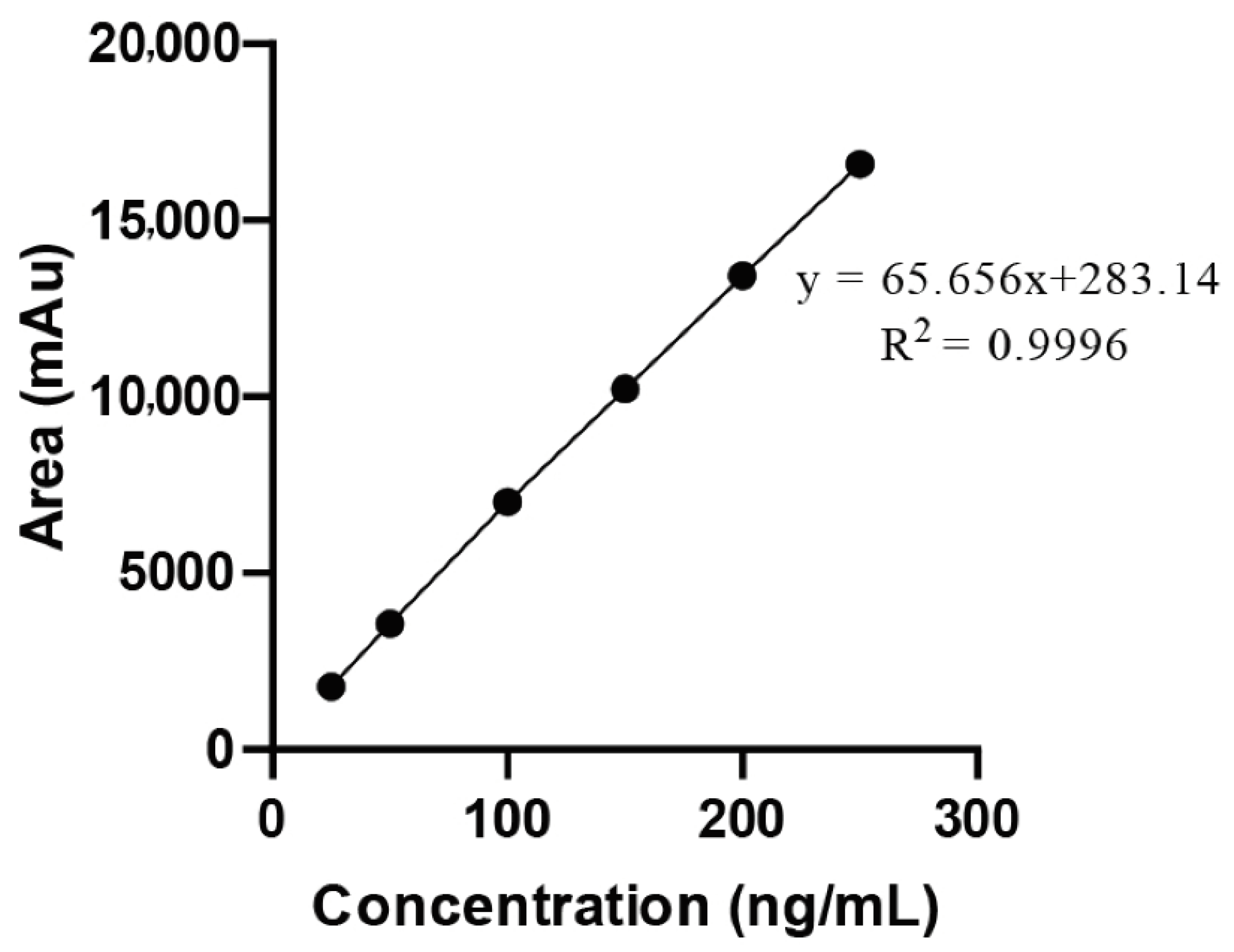
2.3. Development of Calibration Curve (Linearity and Range)
2.4. Matrix Effect
2.5. Accuracy Studies
2.6. Precision Studies
2.7. LOD and LLOQ
2.8. Carry-Over and Dilution Integrity
2.9. System Suitability
2.10. Stability Study of Plasma Samples
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals and Equipment
3.1.2. Animals
3.2. Methods
3.2.1. Chromatographic Conditions
3.2.2. Collection of Blood and Extraction of Plasma
3.2.3. Preparation of Blank Plasma
3.2.4. Preparation of Standard Stock Solution
3.2.5. Preparation of Internal Standard (IS)
3.2.6. Method Specificity
3.2.7. Development of Calibration Curve
3.2.8. Method Validation
3.2.9. Linearity and Range
3.2.10. Matrix Effect
3.2.11. Accuracy
3.2.12. Precision Studies
3.2.13. Determination of LOD and LLOQ
3.2.14. Carry-Over
3.2.15. Dilution Integrity
3.2.16. System Suitability
3.2.17. Stability Study of XH Spiked Plasma Samples
3.2.18. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated Chalcones and Flavonoids for the Prevention and Treatment of Cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for human malignancies: Chemistry, pharmacokinetics and molecular targets. Int. J. Mol. Sci. 2021, 22, 4478. [Google Scholar] [CrossRef] [PubMed]
- Venè, R.; Benelli, R.; Minghelli, S.; Astigiano, S.; Tosetti, F.; Ferrari, N. Xanthohumol Impairs Human Prostate Cancer Cell Growth and Invasion and Diminishes the Incidence and Progression of Advanced Tumors in TRAMP Mice. Mol. Med. 2012, 18, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for Cancer Prevention and Treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 639665, Xanthohumol; National Center for Biotechnology Information: Bethesda, MD, USA, 2021. [Google Scholar]
- Gerhauser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Neumann, I.; Scherf, H.-R.; Frank, N.; Bartsch, H.; et al. Cancer Chemopreventive Activity of Xanthohumol, a Natural Product Derived from Hop. Mol. Cancer Ther. 2002, 1, 959–969. [Google Scholar] [PubMed]
- Avula, B.; Ganzera, M.; Warnick, J.E.; Feltenstein, M.W.; Sufka, K.J.; Khan, I.A. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J. Chromatogr. Sci. 2004, 42, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Sus, N.; Schlienz, J.; Calvo-Castro, L.A.; Burkard, M.; Venturelli, S.; Busch, C.; Frank, J. Validation of a rapid and sensitive reversed-phase liquid chromatographic method for the quantification of prenylated chalcones and flavanones in plasma and urine. NFS J. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Nowak, B.; Pozniak, B.; Poplonski, J. Pharmacokinetics of xanthohumol in rats of both sexes after oral and intravenous administration of pure xanthohumol and prenylflavonoid extract. Adv. Clin. Exp. Med. 2020, 29, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Loureiro, P.V.; Jiménez, I.H.; Sendón, R.; de Quirós, A.R.-B.; Barbosa-Pereira, L. Determination of Xanthohumol in Hops, Food Supplements and Beers by HPLC. Foods 2019, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Wadhwa, S.; Kumar, B.; Gulati, M.; Gupta, S.; Chaitanya, M.; Kumar, D.; Jha, N.K.; Gupta, G.; Prasher, P. Development and validation of RP-HPLC based bioanalytical method for simultaneous estimation of curcumin and quercetin in rat’s plasma. S. Afr. J. Bot. 2022, 149, 870–877. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Khursheed, R.; Awasthi, A.; Khurana, N.; Singh, S.K.; Khurana, S.; Sharma, N.; Gunjal, P.; Kaur, J. Development and validation of RP-HPLC method for estimation of fisetin in rat plasma. S. Afr. J. Bot. 2021, 140, 284–289. [Google Scholar] [CrossRef]
- Challa, B.R.; Boddu, S.H.; Awen, B.Z.; Chandu, B.R.; Bannoth, C.K.; Khagga, M.; Kanala, K.; Shaik, R.P. Development and validation of a Sensitive bioanalytical method for the quantitative estimation of Pantoprazole in human plasma samples by LC–MS/MS: Application to bioequivalence study. J. Chromatogr. B 2010, 878, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.K.; Swamy, N.; Rathod, R.; Sengupta, P. A Bioanalytical Method for Eliglustat Quantification in Rat Plasma. J. Chromatogr. Sci. 2019, 57, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Barfield, M.; Wheller, R. Use of dried plasma spots in the determination of pharmacokinetics in clinical studies: Validation of a quantitative bioanalytical method. Anal. Chem. 2011, 83, 118–124. [Google Scholar] [CrossRef] [PubMed]

| Level | Actual Conc. of XH (ng/mL) | Amount of Drug Recovered in Plasma Sample (ng/mL), (n = 6) | Recovery in Plasma (%) | % RSD |
|---|---|---|---|---|
| LLOQ | 25.73 | 25.87 ± 0.079 | 100.50 | 0.307 |
| HQC | 180 | 175.0 ± 0.18 | 97.22 | 0.102 |
| Level | Actual Conc. of XH (ng/mL) | Amount of Drug Recovered in Plasma Sample (ng/mL), (n = 6) | Recovery in Plasma (%) | %RSD |
|---|---|---|---|---|
| LLOQ | 25.73 | 24.6 ± 0.05 | 95.60 | 0.203 |
| LQC | 120 | 120.9 ± 0.02 | 100.75 | 0.556 |
| MQC | 150 | 153.1 ± 0.08 | 102.00 | 1.069 |
| HQC | 180 | 179.0 ± 0.09 | 99.40 | 1.111 |
| Levels | Concentration (ng/mL) | Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interday Precision (Repeatability) (Mean Area ± SD) (n = 6) | %RSD | Interanalyst (Mean Area ± SD) (n = 6) | %RSD | Intraday Precision (Mean Area ± SD) (n = 6) | %RSD | ||||||
| Analyst 1 | Analyst 2 | Analyst 3 | Day 1 | Day 2 | Day 3 | ||||||
| LLOQ | 25.73 | 1779.20 ± 15.01 | 0.84 | 1786.41 ± 16.01 | 0.89 | 1775.95 ± 16.95 | 0.95 | ||||
| LQC | 120 | 9567.10 ± 38.65 | 0.93 | 9749.33 ± 57.56 | 0.59 | 9617.05 ± 28.84 | 0.35 | ||||
| MQC | 150 | 10,629.0 ± 52.11 | 0.95 | 10,710.33 ± 76.64 | 0.72 | 10,584.89 ± 37.04 | 0.35 | ||||
| HQC | 180 | 11,425.3 ± 140.17 | 1.09 | 11,620.33 ± 73.60 | 0.63 | 11,526.50 ± 114.69 | 0.98 | ||||
| Parameter | Value | Limit |
|---|---|---|
| Tailing factor | 1.187 | <2 |
| Theoretical plate | 4160.691 | >2000 |
| HETP | 36.052 | Depends on theoretical plate |
| Actual Concentration of XH (ng/mL) | Mean Area ± SD (n = 3) | % RSD | Actual Amount of XH Recovered in Plasma (ng/mL) | % Recovery |
|---|---|---|---|---|
| Cycle 1 | ||||
| LQC | 8183.00 ± 60.46 | 0.73 | 119.52 | 99.52 |
| MQC | 9999.00 ± 17.04 | 0.17 | 147.54 | 98.34 |
| HQC | 12,022.67 ± 54.10 | 0.44 | 178.63 | 99.27 |
| Cycle 2 | ||||
| LQC | 8205.33 ± 108.32 | 1.32 | 119.84 | 99.93 |
| MQC | 10,121.00 ± 24.12 | 0.23 | 149.31 | 99.58 |
| HQC | 12,019.67 ± 75.21 | 0.62 | 178.68 | 99.25 |
| Cycle 3 | ||||
| LQC | 8226.00 ± 15.82 | 1.88 | 120.27 | 100.13 |
| MQC | 10,043.33 ± 38.87 | 0.38 | 148.26 | 98.87 |
| HQC | 12,089.33 ± 40.45 | 0.33 | 179.78 | 99.85 |
| Actual Concentration of XH (ng/mL) | Mean Area ± SD | % RSD | Actual Amount of XH Recovered in Plasma (ng/mL) | % Recovery |
|---|---|---|---|---|
| 1 h | ||||
| LQC | 8231.66 ± 90.63 | 1.11 | 120.30 | 100.25 |
| MQC | 10,125.33 ± 85.64 | 0.85 | 149.46 | 99.64 |
| HQC | 12,114.00 ± 83.83 | 0.69 | 180.08 | 100.04 |
| 2nd h | ||||
| LQC | 8190.66 ± 81.32 | 0.99 | 119.67 | 99.72 |
| MQC | 10,081.00 ± 124.49 | 1.23 | 148.78 | 99.18 |
| HQC | 12,161.00 ± 94.32 | 0.78 | 180.81 | 100.45 |
| 3rd h | ||||
| LQC | 8124.00 ± 38.19 | 0.47 | 118.64 | 98.86 |
| MQC | 10,158.67 ± 44.73 | 0.44 | 149.97 | 99.98 |
| HQC | 12,094.33 ± 137.55 | 1.14 | 179.78 | 99.88 |
| Actual Concentration of XH (ng/mL) | Mean Area ± SD | % RSD | Actual Amount of XH Recovered in Plasma (ng/mL) | % Recovery |
|---|---|---|---|---|
| Week 1 | ||||
| LQC | 8131.67 ± 110.21 | 1.36 | 118.76 | 98.92 |
| MQC | 10,092.00 ± 90.80 | 0.89 | 148.94 | 99.24 |
| HQC | 12,080.67 ± 37.20 | 0.31 | 179.57 | 99.75 |
| Week 2 | ||||
| LQC | 8165.00 ± 112.21 | 1.38 | 119.27 | 99.38 |
| MQC | 10,158.67 ± 44.73 | 0.44 | 149.97 | 99.97 |
| HQC | 12,147.33 ± 77.35 | 0.64 | 180.60 | 100.35 |
| Week 3 | ||||
| LQC | 8185.00 ± 66.67 | 0.82 | 119.58 | 99.63 |
| MQC | 10,042.00 ± 72.84 | 0.73 | 148.17 | 98.78 |
| HQC | 12,064.00 ± 75.90 | 0.63 | 179.31 | 99.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harish, V.; Almalki, W.H.; Alshehri, A.; Alzahrani, A.; Alzarea, S.I.; Kazmi, I.; Gulati, M.; Tewari, D.; Chellappan, D.K.; Gupta, G.; et al. Bioanalytical Method Development, Validation and Stability Assessment of Xanthohumol in Rat Plasma. Molecules 2022, 27, 7117. https://doi.org/10.3390/molecules27207117
Harish V, Almalki WH, Alshehri A, Alzahrani A, Alzarea SI, Kazmi I, Gulati M, Tewari D, Chellappan DK, Gupta G, et al. Bioanalytical Method Development, Validation and Stability Assessment of Xanthohumol in Rat Plasma. Molecules. 2022; 27(20):7117. https://doi.org/10.3390/molecules27207117
Chicago/Turabian StyleHarish, Vancha, Waleed Hassan Almalki, Ahmed Alshehri, Abdulaziz Alzahrani, Sami I. Alzarea, Imran Kazmi, Monica Gulati, Devesh Tewari, Dinesh Kumar Chellappan, Gaurav Gupta, and et al. 2022. "Bioanalytical Method Development, Validation and Stability Assessment of Xanthohumol in Rat Plasma" Molecules 27, no. 20: 7117. https://doi.org/10.3390/molecules27207117
APA StyleHarish, V., Almalki, W. H., Alshehri, A., Alzahrani, A., Alzarea, S. I., Kazmi, I., Gulati, M., Tewari, D., Chellappan, D. K., Gupta, G., Dua, K., & Singh, S. K. (2022). Bioanalytical Method Development, Validation and Stability Assessment of Xanthohumol in Rat Plasma. Molecules, 27(20), 7117. https://doi.org/10.3390/molecules27207117






